User login
Fewer Side Effects With Adenosine Analogue
CHICAGO — A selective adenosine receptor agonist was as effective as conventional adenosine for cardiac stress imaging and produced fewer adverse events in results from two pivotal, controlled trials with a total of about 800 patients.
Treating patients with binodenoson “provides similar clinical information on the extent and severity of ischemia as adenosine and is associated with a significant reduction in the incidence and intensity of many side effects,” Dr. James E. Udelson said at the annual meeting of the American College of Cardiology. A major feature of the improved safety profile was that treatment with binodenoson led to no episodes of second- or third-degree atrioventricular block in about 800 treated patients, compared with about a 2% rate of this complication when the same patients were treated with adenosine, reported Dr. Udelson, professor and acting chief of cardiology at Tufts Medical Center in Boston.
Binodenoson is being developed as CorVue by King Pharmaceuticals Inc. Dr. Udelson said that he received research grants and consulting honoraria from the company, as did several of his collaborators.
Results from the two studies reported by Dr. Udelson showed that the diagnostic accuracy of stress perfusion imaging with single-photon emission CT was comparable in binodenoson- and adenosine-treated patients, with binodenoson causing fewer adverse events and having easier, bolus dosing, commented Dr. Jagat Narula, professor and chief of cardiology at the University of California, Irvine. “It will all boil down to dollars. If we can reduce or contain the cost then [binodenoson] will become the preferred agent,” he said.
Dr. Udelson reported data from two separate arms of the Vasodilator Induced Stress in Concordance with Adenosine (VISION) study, conducted at 79 U.S. centers. Both studies enrolled patients aged 30 years or older who were scheduled to undergo pharmacologic stress imaging of their hearts because of typical or atypical angina and suspected ischemia. The study excluded patients with a very low pretest likelihood of disease, a contraindication for adenosine, or severe left-ventricular dysfunction. The two studies enrolled a total of 842 patients, with an average age of 63 years and an average BMI of 31 kg/m
In both studies, patients were randomized to initial imaging with either binodenoson or adenosine and also received a placebo dose in place of the other agent. Imaging was repeated 2–7 days later with the same protocol, but the active and placebo agents were reversed. Binodenoson (and its matched placebo) was administered at a dose of 1.5 mcg/kg as a bolus injection that lasted 30 seconds; adenosine (and its matching placebo) was given at a dose of 140 mcg/kg per minute administered as an intravenous infusion for 6 minutes. The imaging aspect of the study was done identically both times each patient was tested.
Efficacy was assessed by comparing the extent of myocardial ischemia diagnosed by blinded readers using the two stress methods. The study's prespecified criteria said that the two stress agents would be considered identical in performance if the average summed difference in stress ischemia between the two methods was less than 1.5 U, and if fewer than 10% of all tested patients had highly discordant findings.
The average summed difference in stress scores was 0.09 in one study and 0.68 in the other, which meant that the results from both trials fulfilled the criterion that the average difference between the summed stress scores was less than 1.5.
The safety analysis showed that binodenoson consistently produced fewer adverse events in both studies. In addition to causing no episodes of atrioventricular block, treatment with binodenoson was associated with significantly fewer and less intense episodes of flushing, chest pain, and dyspnea, Dr. Udelson said.
Binodenoson provides similar clinical information to adenosine, with a significant reduction in many side effects. DR. UDELSON
CHICAGO — A selective adenosine receptor agonist was as effective as conventional adenosine for cardiac stress imaging and produced fewer adverse events in results from two pivotal, controlled trials with a total of about 800 patients.
Treating patients with binodenoson “provides similar clinical information on the extent and severity of ischemia as adenosine and is associated with a significant reduction in the incidence and intensity of many side effects,” Dr. James E. Udelson said at the annual meeting of the American College of Cardiology. A major feature of the improved safety profile was that treatment with binodenoson led to no episodes of second- or third-degree atrioventricular block in about 800 treated patients, compared with about a 2% rate of this complication when the same patients were treated with adenosine, reported Dr. Udelson, professor and acting chief of cardiology at Tufts Medical Center in Boston.
Binodenoson is being developed as CorVue by King Pharmaceuticals Inc. Dr. Udelson said that he received research grants and consulting honoraria from the company, as did several of his collaborators.
Results from the two studies reported by Dr. Udelson showed that the diagnostic accuracy of stress perfusion imaging with single-photon emission CT was comparable in binodenoson- and adenosine-treated patients, with binodenoson causing fewer adverse events and having easier, bolus dosing, commented Dr. Jagat Narula, professor and chief of cardiology at the University of California, Irvine. “It will all boil down to dollars. If we can reduce or contain the cost then [binodenoson] will become the preferred agent,” he said.
Dr. Udelson reported data from two separate arms of the Vasodilator Induced Stress in Concordance with Adenosine (VISION) study, conducted at 79 U.S. centers. Both studies enrolled patients aged 30 years or older who were scheduled to undergo pharmacologic stress imaging of their hearts because of typical or atypical angina and suspected ischemia. The study excluded patients with a very low pretest likelihood of disease, a contraindication for adenosine, or severe left-ventricular dysfunction. The two studies enrolled a total of 842 patients, with an average age of 63 years and an average BMI of 31 kg/m
In both studies, patients were randomized to initial imaging with either binodenoson or adenosine and also received a placebo dose in place of the other agent. Imaging was repeated 2–7 days later with the same protocol, but the active and placebo agents were reversed. Binodenoson (and its matched placebo) was administered at a dose of 1.5 mcg/kg as a bolus injection that lasted 30 seconds; adenosine (and its matching placebo) was given at a dose of 140 mcg/kg per minute administered as an intravenous infusion for 6 minutes. The imaging aspect of the study was done identically both times each patient was tested.
Efficacy was assessed by comparing the extent of myocardial ischemia diagnosed by blinded readers using the two stress methods. The study's prespecified criteria said that the two stress agents would be considered identical in performance if the average summed difference in stress ischemia between the two methods was less than 1.5 U, and if fewer than 10% of all tested patients had highly discordant findings.
The average summed difference in stress scores was 0.09 in one study and 0.68 in the other, which meant that the results from both trials fulfilled the criterion that the average difference between the summed stress scores was less than 1.5.
The safety analysis showed that binodenoson consistently produced fewer adverse events in both studies. In addition to causing no episodes of atrioventricular block, treatment with binodenoson was associated with significantly fewer and less intense episodes of flushing, chest pain, and dyspnea, Dr. Udelson said.
Binodenoson provides similar clinical information to adenosine, with a significant reduction in many side effects. DR. UDELSON
CHICAGO — A selective adenosine receptor agonist was as effective as conventional adenosine for cardiac stress imaging and produced fewer adverse events in results from two pivotal, controlled trials with a total of about 800 patients.
Treating patients with binodenoson “provides similar clinical information on the extent and severity of ischemia as adenosine and is associated with a significant reduction in the incidence and intensity of many side effects,” Dr. James E. Udelson said at the annual meeting of the American College of Cardiology. A major feature of the improved safety profile was that treatment with binodenoson led to no episodes of second- or third-degree atrioventricular block in about 800 treated patients, compared with about a 2% rate of this complication when the same patients were treated with adenosine, reported Dr. Udelson, professor and acting chief of cardiology at Tufts Medical Center in Boston.
Binodenoson is being developed as CorVue by King Pharmaceuticals Inc. Dr. Udelson said that he received research grants and consulting honoraria from the company, as did several of his collaborators.
Results from the two studies reported by Dr. Udelson showed that the diagnostic accuracy of stress perfusion imaging with single-photon emission CT was comparable in binodenoson- and adenosine-treated patients, with binodenoson causing fewer adverse events and having easier, bolus dosing, commented Dr. Jagat Narula, professor and chief of cardiology at the University of California, Irvine. “It will all boil down to dollars. If we can reduce or contain the cost then [binodenoson] will become the preferred agent,” he said.
Dr. Udelson reported data from two separate arms of the Vasodilator Induced Stress in Concordance with Adenosine (VISION) study, conducted at 79 U.S. centers. Both studies enrolled patients aged 30 years or older who were scheduled to undergo pharmacologic stress imaging of their hearts because of typical or atypical angina and suspected ischemia. The study excluded patients with a very low pretest likelihood of disease, a contraindication for adenosine, or severe left-ventricular dysfunction. The two studies enrolled a total of 842 patients, with an average age of 63 years and an average BMI of 31 kg/m
In both studies, patients were randomized to initial imaging with either binodenoson or adenosine and also received a placebo dose in place of the other agent. Imaging was repeated 2–7 days later with the same protocol, but the active and placebo agents were reversed. Binodenoson (and its matched placebo) was administered at a dose of 1.5 mcg/kg as a bolus injection that lasted 30 seconds; adenosine (and its matching placebo) was given at a dose of 140 mcg/kg per minute administered as an intravenous infusion for 6 minutes. The imaging aspect of the study was done identically both times each patient was tested.
Efficacy was assessed by comparing the extent of myocardial ischemia diagnosed by blinded readers using the two stress methods. The study's prespecified criteria said that the two stress agents would be considered identical in performance if the average summed difference in stress ischemia between the two methods was less than 1.5 U, and if fewer than 10% of all tested patients had highly discordant findings.
The average summed difference in stress scores was 0.09 in one study and 0.68 in the other, which meant that the results from both trials fulfilled the criterion that the average difference between the summed stress scores was less than 1.5.
The safety analysis showed that binodenoson consistently produced fewer adverse events in both studies. In addition to causing no episodes of atrioventricular block, treatment with binodenoson was associated with significantly fewer and less intense episodes of flushing, chest pain, and dyspnea, Dr. Udelson said.
Binodenoson provides similar clinical information to adenosine, with a significant reduction in many side effects. DR. UDELSON
Infection May Increase Risks From MRI Contrast Agents
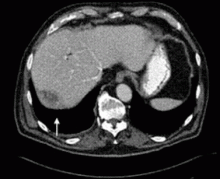
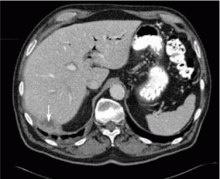
The presence of infection at the time of magnetic resonance imaging using gadolinium contrast may predispose patients with renal failure to nephrogenic systemic fibrosis, according to a hospital analysis.
The estimated NSF development rate for infected patients with renal failure was 6.7%, compared with 0.3% for uninfected patients with renal failure—a 33-fold difference that was highly significant.
“If the presence of infection indeed proves to be a risk factor for the development of NSF, then some renal failure patients presently judged to be acceptable risks for MR contrast administration on the basis of the degree of renal failure might be reconsidered as high-risk patients,” wrote Dr. Lauren Goldberg of Moses H. Cone Memorial Hospital, Greensboro, N.C., and Dr. James Provenzale, a radiologist at Duke University, Durham, N.C. (Am. J. Roentgenol. 2008;190:1069–75).
Eight patients with symptoms consistent with NSF between 2002 and 2006 were prospectively identified by the nephrology group. Seven biopsy-proven cases were identified, along with another case involving strong clinical signs and symptoms of NSF without biopsy confirmation.
Seven patients had MRI contrast with gadodiamide (Omniscan, GE Healthcare), at a dose from 0.1 mmol/kg to 0.3 mmol/kg. NSF symptoms began 2 days to 5 months after contrast administration. The sole patient not exposed to gadolinium contrast had end-stage renal disease, breast carcinoma, and calciphylaxis.
All eight patients with NSF noted stiffening and thickening of the hands and lower extremities—often described as woody changes of the skin. Five patients had severe chronic pain, the authors reported.
No single medication was common to all patients with NSF. None had vascular thrombosis. Only one patient had undergone major surgery. Five of the patients had documented infection, including catheter infection, urinary tract infection, bacteremia, pneumonia, cellulitis, and osteomyelitis. Two patients who received gadolinium contrast did not have proinflammatory conditions. The one patient who did not receive gadolinium contrast was also considered not to have a proinflammatory condition.
The researchers estimated that 750 renal-failure patients without documented infection and 75 renal-failure patients with documented infection underwent contrast-enhanced MRI between 2002 and 2006.
“All six patients who were hemodialysis dependent at the time of contrast-enhanced MRI were dialyzed 1 day after gadodiamide administration, suggesting that prompt hemodialysis may not be protective against the development of NSF,” the authors wrote.
The investigators disclosed no conflicts of interest.
The presence of infection at the time of magnetic resonance imaging using gadolinium contrast may predispose patients with renal failure to nephrogenic systemic fibrosis, according to a hospital analysis.
The estimated NSF development rate for infected patients with renal failure was 6.7%, compared with 0.3% for uninfected patients with renal failure—a 33-fold difference that was highly significant.
“If the presence of infection indeed proves to be a risk factor for the development of NSF, then some renal failure patients presently judged to be acceptable risks for MR contrast administration on the basis of the degree of renal failure might be reconsidered as high-risk patients,” wrote Dr. Lauren Goldberg of Moses H. Cone Memorial Hospital, Greensboro, N.C., and Dr. James Provenzale, a radiologist at Duke University, Durham, N.C. (Am. J. Roentgenol. 2008;190:1069–75).
Eight patients with symptoms consistent with NSF between 2002 and 2006 were prospectively identified by the nephrology group. Seven biopsy-proven cases were identified, along with another case involving strong clinical signs and symptoms of NSF without biopsy confirmation.
Seven patients had MRI contrast with gadodiamide (Omniscan, GE Healthcare), at a dose from 0.1 mmol/kg to 0.3 mmol/kg. NSF symptoms began 2 days to 5 months after contrast administration. The sole patient not exposed to gadolinium contrast had end-stage renal disease, breast carcinoma, and calciphylaxis.
All eight patients with NSF noted stiffening and thickening of the hands and lower extremities—often described as woody changes of the skin. Five patients had severe chronic pain, the authors reported.
No single medication was common to all patients with NSF. None had vascular thrombosis. Only one patient had undergone major surgery. Five of the patients had documented infection, including catheter infection, urinary tract infection, bacteremia, pneumonia, cellulitis, and osteomyelitis. Two patients who received gadolinium contrast did not have proinflammatory conditions. The one patient who did not receive gadolinium contrast was also considered not to have a proinflammatory condition.
The researchers estimated that 750 renal-failure patients without documented infection and 75 renal-failure patients with documented infection underwent contrast-enhanced MRI between 2002 and 2006.
“All six patients who were hemodialysis dependent at the time of contrast-enhanced MRI were dialyzed 1 day after gadodiamide administration, suggesting that prompt hemodialysis may not be protective against the development of NSF,” the authors wrote.
The investigators disclosed no conflicts of interest.
The presence of infection at the time of magnetic resonance imaging using gadolinium contrast may predispose patients with renal failure to nephrogenic systemic fibrosis, according to a hospital analysis.
The estimated NSF development rate for infected patients with renal failure was 6.7%, compared with 0.3% for uninfected patients with renal failure—a 33-fold difference that was highly significant.
“If the presence of infection indeed proves to be a risk factor for the development of NSF, then some renal failure patients presently judged to be acceptable risks for MR contrast administration on the basis of the degree of renal failure might be reconsidered as high-risk patients,” wrote Dr. Lauren Goldberg of Moses H. Cone Memorial Hospital, Greensboro, N.C., and Dr. James Provenzale, a radiologist at Duke University, Durham, N.C. (Am. J. Roentgenol. 2008;190:1069–75).
Eight patients with symptoms consistent with NSF between 2002 and 2006 were prospectively identified by the nephrology group. Seven biopsy-proven cases were identified, along with another case involving strong clinical signs and symptoms of NSF without biopsy confirmation.
Seven patients had MRI contrast with gadodiamide (Omniscan, GE Healthcare), at a dose from 0.1 mmol/kg to 0.3 mmol/kg. NSF symptoms began 2 days to 5 months after contrast administration. The sole patient not exposed to gadolinium contrast had end-stage renal disease, breast carcinoma, and calciphylaxis.
All eight patients with NSF noted stiffening and thickening of the hands and lower extremities—often described as woody changes of the skin. Five patients had severe chronic pain, the authors reported.
No single medication was common to all patients with NSF. None had vascular thrombosis. Only one patient had undergone major surgery. Five of the patients had documented infection, including catheter infection, urinary tract infection, bacteremia, pneumonia, cellulitis, and osteomyelitis. Two patients who received gadolinium contrast did not have proinflammatory conditions. The one patient who did not receive gadolinium contrast was also considered not to have a proinflammatory condition.
The researchers estimated that 750 renal-failure patients without documented infection and 75 renal-failure patients with documented infection underwent contrast-enhanced MRI between 2002 and 2006.
“All six patients who were hemodialysis dependent at the time of contrast-enhanced MRI were dialyzed 1 day after gadodiamide administration, suggesting that prompt hemodialysis may not be protective against the development of NSF,” the authors wrote.
The investigators disclosed no conflicts of interest.
Duplex Ultrasound Looks Safe For Post-EVAR Surveillance
SAN DIEGO — Postendovascular aneurysm repair surveillance, with color flow duplex ultrasound only, is a safe alternative to the current standard practice of follow-up CT with contrast, results from a single-center study showed.
After endovascular aneurysm repair (EVAR), “CT follow-up is associated with significant risk, including increased cost, contrast nephropathy, contrast allergy, and radiation exposure,” Dr. Rabih A. Chaer said at the Vascular Annual Meeting. “Alternative follow-up methods have been proposed, including color flow duplex ultrasound, MRI, and contrast-enhanced ultrasound. Of all these modalities, it's clear that simple color flow duplex ultrasound is the most readily available, the cheapest, and the least invasive.”
He and his associates in the division of vascular surgery at the University of Pittsburgh Medical Center studied 184 patients who were switched to color flow duplex ultrasound (CDU) surveillance in 2003 as an alternative to CT. Selective CT scanning was used only for new endoleaks or for patients who presented with an enlarging abdominal aortic aneurysm (AAA) sac. Only patients with at least 1 year of follow-up were included.
Criteria for switch to CDU included patients with a residual AAA sac of 4 cm or less anytime after the first year of follow-up, patients with a stable AAA sac size for 2 years, or patients with a stable type II endoleak for 2 years. The average CDU study duration was 20 minutes. The researchers used a GE Logiq 9 machine with a 3.5-MHz curve probe.
Of the 184 patients, 13 had an active stable type II endoleak, 23 had a prior endoleak that was treated or that resolved spontaneously. The mean follow-up on CDU was 24 months. Of the 184 grafts, 76 were Ancure, 58 were Zenith, 39 were Excluder, 7 were AneuRx, and 4 were Lifepath.
Dr. Chaer reported that there were three new endoleaks detected on CDU follow-up, all in patients who received an Ancure graft. Only one patient presented with sac enlargement. “One type II endoleak was detected, but this spontaneously resolved at 3 months,” he said. “There were two distal type I endoleaks that were treated with limb extension.”
CDU identified two patients (one with an Ancure and one with an AneruRx graft) who had an increase in their AAA sac size, yet no endoleak was detected. No endoleak was seen on CT scan, but when both patients underwent angiograms, a distal type I endoleak was detected in one patient.
There were no ruptures or graft occlusions observed during the follow-up period. Eight patients died. One was an aneurysm-related death following an Ancure explantation for infection that occurred 4 years post EVAR; two were related to malignancy, and five were related to acute myocardial infarctions.
The cumulative freedom from secondary intervention after the switch to CDU was 98% at 4 years.
In order to determine the applicability of the switch criteria for a full cohort of EVAR patients, the researchers examined 196 consecutive EVAR patients in 2004. Of these, 86 (44%) had been switched to CDU surveillance, whereas the remaining 110 were still followed with CT scan. At the 6-month follow-up, only 1.5% of patients followed with CT scan met the current criteria for the switch to CDU-only surveillance. But the proportion at 1, 2, and 3 years was 55%, 86%, and 97%, respectively.
“CDU-only surveillance is safe and can be initiated early after treatment on patients with a shrinking or a stable AAA sac,” he concluded. “Most patients treated with EVAR are eligible for this modality. After the 1 year follow-up, we do recommend that CT scanning should only be selectively utilized in patients treated with EVAR. This policy should result in cost-saving advantages and avoid the complications associated with CT.”
Dr. Chaer disclosed he had no relevant conflicts.
SAN DIEGO — Postendovascular aneurysm repair surveillance, with color flow duplex ultrasound only, is a safe alternative to the current standard practice of follow-up CT with contrast, results from a single-center study showed.
After endovascular aneurysm repair (EVAR), “CT follow-up is associated with significant risk, including increased cost, contrast nephropathy, contrast allergy, and radiation exposure,” Dr. Rabih A. Chaer said at the Vascular Annual Meeting. “Alternative follow-up methods have been proposed, including color flow duplex ultrasound, MRI, and contrast-enhanced ultrasound. Of all these modalities, it's clear that simple color flow duplex ultrasound is the most readily available, the cheapest, and the least invasive.”
He and his associates in the division of vascular surgery at the University of Pittsburgh Medical Center studied 184 patients who were switched to color flow duplex ultrasound (CDU) surveillance in 2003 as an alternative to CT. Selective CT scanning was used only for new endoleaks or for patients who presented with an enlarging abdominal aortic aneurysm (AAA) sac. Only patients with at least 1 year of follow-up were included.
Criteria for switch to CDU included patients with a residual AAA sac of 4 cm or less anytime after the first year of follow-up, patients with a stable AAA sac size for 2 years, or patients with a stable type II endoleak for 2 years. The average CDU study duration was 20 minutes. The researchers used a GE Logiq 9 machine with a 3.5-MHz curve probe.
Of the 184 patients, 13 had an active stable type II endoleak, 23 had a prior endoleak that was treated or that resolved spontaneously. The mean follow-up on CDU was 24 months. Of the 184 grafts, 76 were Ancure, 58 were Zenith, 39 were Excluder, 7 were AneuRx, and 4 were Lifepath.
Dr. Chaer reported that there were three new endoleaks detected on CDU follow-up, all in patients who received an Ancure graft. Only one patient presented with sac enlargement. “One type II endoleak was detected, but this spontaneously resolved at 3 months,” he said. “There were two distal type I endoleaks that were treated with limb extension.”
CDU identified two patients (one with an Ancure and one with an AneruRx graft) who had an increase in their AAA sac size, yet no endoleak was detected. No endoleak was seen on CT scan, but when both patients underwent angiograms, a distal type I endoleak was detected in one patient.
There were no ruptures or graft occlusions observed during the follow-up period. Eight patients died. One was an aneurysm-related death following an Ancure explantation for infection that occurred 4 years post EVAR; two were related to malignancy, and five were related to acute myocardial infarctions.
The cumulative freedom from secondary intervention after the switch to CDU was 98% at 4 years.
In order to determine the applicability of the switch criteria for a full cohort of EVAR patients, the researchers examined 196 consecutive EVAR patients in 2004. Of these, 86 (44%) had been switched to CDU surveillance, whereas the remaining 110 were still followed with CT scan. At the 6-month follow-up, only 1.5% of patients followed with CT scan met the current criteria for the switch to CDU-only surveillance. But the proportion at 1, 2, and 3 years was 55%, 86%, and 97%, respectively.
“CDU-only surveillance is safe and can be initiated early after treatment on patients with a shrinking or a stable AAA sac,” he concluded. “Most patients treated with EVAR are eligible for this modality. After the 1 year follow-up, we do recommend that CT scanning should only be selectively utilized in patients treated with EVAR. This policy should result in cost-saving advantages and avoid the complications associated with CT.”
Dr. Chaer disclosed he had no relevant conflicts.
SAN DIEGO — Postendovascular aneurysm repair surveillance, with color flow duplex ultrasound only, is a safe alternative to the current standard practice of follow-up CT with contrast, results from a single-center study showed.
After endovascular aneurysm repair (EVAR), “CT follow-up is associated with significant risk, including increased cost, contrast nephropathy, contrast allergy, and radiation exposure,” Dr. Rabih A. Chaer said at the Vascular Annual Meeting. “Alternative follow-up methods have been proposed, including color flow duplex ultrasound, MRI, and contrast-enhanced ultrasound. Of all these modalities, it's clear that simple color flow duplex ultrasound is the most readily available, the cheapest, and the least invasive.”
He and his associates in the division of vascular surgery at the University of Pittsburgh Medical Center studied 184 patients who were switched to color flow duplex ultrasound (CDU) surveillance in 2003 as an alternative to CT. Selective CT scanning was used only for new endoleaks or for patients who presented with an enlarging abdominal aortic aneurysm (AAA) sac. Only patients with at least 1 year of follow-up were included.
Criteria for switch to CDU included patients with a residual AAA sac of 4 cm or less anytime after the first year of follow-up, patients with a stable AAA sac size for 2 years, or patients with a stable type II endoleak for 2 years. The average CDU study duration was 20 minutes. The researchers used a GE Logiq 9 machine with a 3.5-MHz curve probe.
Of the 184 patients, 13 had an active stable type II endoleak, 23 had a prior endoleak that was treated or that resolved spontaneously. The mean follow-up on CDU was 24 months. Of the 184 grafts, 76 were Ancure, 58 were Zenith, 39 were Excluder, 7 were AneuRx, and 4 were Lifepath.
Dr. Chaer reported that there were three new endoleaks detected on CDU follow-up, all in patients who received an Ancure graft. Only one patient presented with sac enlargement. “One type II endoleak was detected, but this spontaneously resolved at 3 months,” he said. “There were two distal type I endoleaks that were treated with limb extension.”
CDU identified two patients (one with an Ancure and one with an AneruRx graft) who had an increase in their AAA sac size, yet no endoleak was detected. No endoleak was seen on CT scan, but when both patients underwent angiograms, a distal type I endoleak was detected in one patient.
There were no ruptures or graft occlusions observed during the follow-up period. Eight patients died. One was an aneurysm-related death following an Ancure explantation for infection that occurred 4 years post EVAR; two were related to malignancy, and five were related to acute myocardial infarctions.
The cumulative freedom from secondary intervention after the switch to CDU was 98% at 4 years.
In order to determine the applicability of the switch criteria for a full cohort of EVAR patients, the researchers examined 196 consecutive EVAR patients in 2004. Of these, 86 (44%) had been switched to CDU surveillance, whereas the remaining 110 were still followed with CT scan. At the 6-month follow-up, only 1.5% of patients followed with CT scan met the current criteria for the switch to CDU-only surveillance. But the proportion at 1, 2, and 3 years was 55%, 86%, and 97%, respectively.
“CDU-only surveillance is safe and can be initiated early after treatment on patients with a shrinking or a stable AAA sac,” he concluded. “Most patients treated with EVAR are eligible for this modality. After the 1 year follow-up, we do recommend that CT scanning should only be selectively utilized in patients treated with EVAR. This policy should result in cost-saving advantages and avoid the complications associated with CT.”
Dr. Chaer disclosed he had no relevant conflicts.
New Cardiac Pacemaker Avoids MRI Interference
MUNICH — A new type of cardiac pacemaker was safe in patients undergoing an MRI examination, with no need for a special imaging protocol in a randomized study with 151 evaluable patients.
“The functionality of the new pacemaker is the same as a conventional pacemaker, but one is MRI safe and the other is not,” Dr. Torsten Sommer said at the annual congress of the European Society of Cardiology. There is no apparent downside to the new pacemaker, except that it may cost more than current units do, he said.
Patients with a standard pacemaker face “a huge limitation” by not being able to easily undergo MRI imaging, which has become a practice staple, said Dr. Sommer, chief of the cardiovascular imaging section at the University of Bonn (Germany). He estimated that an average pacemaker patient today has a 50%–75% lifetime chance of needing at least one MRI exam.
The major danger that MRI poses to a patient with a conventional pacemaker (or other implanted cardiac device) is heating of the lead tips by radiofrequency radiation. Other concerns are interference with pacemaker function and risk of an electrical reset of the device.
A special MRI protocol has been reported that avoids these problems, but the method is complicated and available only at a limited number of experienced imaging centers, commented Dr. Christopher Cannon, a cardiologist at Brigham and Women's Hospital and Harvard Medical School, Boston. Having a new pacemaker without the MRI limitation would be “very helpful,” he said in an interview.
The new pacemaker, called EnRhythm, was developed by Medtronic Inc., which funded the current study. Dr. Sommer is a consultant to Medtronic, but he and his associates in Bonn do not hold any patents on the new device or technology. The data he reported came from the first phase of a study designed to eventually place the new pacemaker in 470 patients. When the final results are available, Medtronic will apply for marketing approval from the Food and Drug Administration, a step that the company hopes to take in 2010, said Tracy McNulty, a Medtronic spokeswoman.
The new, dual-chamber pacemaker features a reduced number of ferromagnetic parts, better protection of internal circuits, and altered software. But the implantation technique is the same as for a standard pacemaker, Dr. Sommer said.
The study is being done at 53 centers in the United States, Canada, and Europe. So far, 245 patients with a class I or II indication for a pacemaker have been implanted with the EnRhythm device. Ninety patients were randomized to undergo MRI imaging and 101 were randomized as controls who received no imaging.
The MRI exam was done on a 1.5-T unit and involved 15 clinically relevant head- and lumbar spine-imaging sequences. To maximize safety during the first phase of testing, no magnetic coils were placed directly above a spinal region that extended from the C1 to the T12 level. But even with this limitation, full imaging of the chest region was obtained, Dr. Sommer said.
Follow-up data collected 1 month after the MRI exam were available for 70 patients and for 81 of the matched controls. The results showed no adverse events or complications triggered by the MRI exam, and no electrical resets of the pacemakers. During follow-up, no patients had sustained arrhythmias and the pacemakers did not show any changes in their rhythm-capture thresholds or any other performance changes.
'The functionality … is the same as a conventional pacemaker, but one is MRI safe and the other is not.' DR. SOMMER
MUNICH — A new type of cardiac pacemaker was safe in patients undergoing an MRI examination, with no need for a special imaging protocol in a randomized study with 151 evaluable patients.
“The functionality of the new pacemaker is the same as a conventional pacemaker, but one is MRI safe and the other is not,” Dr. Torsten Sommer said at the annual congress of the European Society of Cardiology. There is no apparent downside to the new pacemaker, except that it may cost more than current units do, he said.
Patients with a standard pacemaker face “a huge limitation” by not being able to easily undergo MRI imaging, which has become a practice staple, said Dr. Sommer, chief of the cardiovascular imaging section at the University of Bonn (Germany). He estimated that an average pacemaker patient today has a 50%–75% lifetime chance of needing at least one MRI exam.
The major danger that MRI poses to a patient with a conventional pacemaker (or other implanted cardiac device) is heating of the lead tips by radiofrequency radiation. Other concerns are interference with pacemaker function and risk of an electrical reset of the device.
A special MRI protocol has been reported that avoids these problems, but the method is complicated and available only at a limited number of experienced imaging centers, commented Dr. Christopher Cannon, a cardiologist at Brigham and Women's Hospital and Harvard Medical School, Boston. Having a new pacemaker without the MRI limitation would be “very helpful,” he said in an interview.
The new pacemaker, called EnRhythm, was developed by Medtronic Inc., which funded the current study. Dr. Sommer is a consultant to Medtronic, but he and his associates in Bonn do not hold any patents on the new device or technology. The data he reported came from the first phase of a study designed to eventually place the new pacemaker in 470 patients. When the final results are available, Medtronic will apply for marketing approval from the Food and Drug Administration, a step that the company hopes to take in 2010, said Tracy McNulty, a Medtronic spokeswoman.
The new, dual-chamber pacemaker features a reduced number of ferromagnetic parts, better protection of internal circuits, and altered software. But the implantation technique is the same as for a standard pacemaker, Dr. Sommer said.
The study is being done at 53 centers in the United States, Canada, and Europe. So far, 245 patients with a class I or II indication for a pacemaker have been implanted with the EnRhythm device. Ninety patients were randomized to undergo MRI imaging and 101 were randomized as controls who received no imaging.
The MRI exam was done on a 1.5-T unit and involved 15 clinically relevant head- and lumbar spine-imaging sequences. To maximize safety during the first phase of testing, no magnetic coils were placed directly above a spinal region that extended from the C1 to the T12 level. But even with this limitation, full imaging of the chest region was obtained, Dr. Sommer said.
Follow-up data collected 1 month after the MRI exam were available for 70 patients and for 81 of the matched controls. The results showed no adverse events or complications triggered by the MRI exam, and no electrical resets of the pacemakers. During follow-up, no patients had sustained arrhythmias and the pacemakers did not show any changes in their rhythm-capture thresholds or any other performance changes.
'The functionality … is the same as a conventional pacemaker, but one is MRI safe and the other is not.' DR. SOMMER
MUNICH — A new type of cardiac pacemaker was safe in patients undergoing an MRI examination, with no need for a special imaging protocol in a randomized study with 151 evaluable patients.
“The functionality of the new pacemaker is the same as a conventional pacemaker, but one is MRI safe and the other is not,” Dr. Torsten Sommer said at the annual congress of the European Society of Cardiology. There is no apparent downside to the new pacemaker, except that it may cost more than current units do, he said.
Patients with a standard pacemaker face “a huge limitation” by not being able to easily undergo MRI imaging, which has become a practice staple, said Dr. Sommer, chief of the cardiovascular imaging section at the University of Bonn (Germany). He estimated that an average pacemaker patient today has a 50%–75% lifetime chance of needing at least one MRI exam.
The major danger that MRI poses to a patient with a conventional pacemaker (or other implanted cardiac device) is heating of the lead tips by radiofrequency radiation. Other concerns are interference with pacemaker function and risk of an electrical reset of the device.
A special MRI protocol has been reported that avoids these problems, but the method is complicated and available only at a limited number of experienced imaging centers, commented Dr. Christopher Cannon, a cardiologist at Brigham and Women's Hospital and Harvard Medical School, Boston. Having a new pacemaker without the MRI limitation would be “very helpful,” he said in an interview.
The new pacemaker, called EnRhythm, was developed by Medtronic Inc., which funded the current study. Dr. Sommer is a consultant to Medtronic, but he and his associates in Bonn do not hold any patents on the new device or technology. The data he reported came from the first phase of a study designed to eventually place the new pacemaker in 470 patients. When the final results are available, Medtronic will apply for marketing approval from the Food and Drug Administration, a step that the company hopes to take in 2010, said Tracy McNulty, a Medtronic spokeswoman.
The new, dual-chamber pacemaker features a reduced number of ferromagnetic parts, better protection of internal circuits, and altered software. But the implantation technique is the same as for a standard pacemaker, Dr. Sommer said.
The study is being done at 53 centers in the United States, Canada, and Europe. So far, 245 patients with a class I or II indication for a pacemaker have been implanted with the EnRhythm device. Ninety patients were randomized to undergo MRI imaging and 101 were randomized as controls who received no imaging.
The MRI exam was done on a 1.5-T unit and involved 15 clinically relevant head- and lumbar spine-imaging sequences. To maximize safety during the first phase of testing, no magnetic coils were placed directly above a spinal region that extended from the C1 to the T12 level. But even with this limitation, full imaging of the chest region was obtained, Dr. Sommer said.
Follow-up data collected 1 month after the MRI exam were available for 70 patients and for 81 of the matched controls. The results showed no adverse events or complications triggered by the MRI exam, and no electrical resets of the pacemakers. During follow-up, no patients had sustained arrhythmias and the pacemakers did not show any changes in their rhythm-capture thresholds or any other performance changes.
'The functionality … is the same as a conventional pacemaker, but one is MRI safe and the other is not.' DR. SOMMER
The painful knee: Choosing the right imaging test
Radiography plays a key role in the initial evaluation of acute knee pain in adults. Yet conflicting studies and the absence of clear guidelines may leave the primary care physician uncertain as to which imaging test to order—ie, whether radiography is sufficient, and when computed tomography (CT) or magnetic resonance imaging (MRI) is needed. This article reviews the indications for radiologic examination of the knee and discusses indications for cross-sectional imaging studies. Imaging in oncology patients is not discussed here.
ACUTE KNEE PAIN: A TYPICAL SCENARIO
A 47-year-old woman presents to the emergency department with left knee pain after a motor vehicle accident that occurred the day before. The car she was driving hit a tree, and she hit her knee on the dashboard. She was wearing a seatbelt at the time of the accident. She says she was unable to walk immediately after the accident because of knee pain.
The initial examination in the emergency room reveals swelling and pain throughout the range of motion. The anterior drawer test and the Lachman test are negative (see below).
The patient is discharged home with a knee immobilizer, pain medication, and crutches, with instructions for a follow-up visit in the orthopedics clinic.
Five days later, she returns to the emergency department complaining of continuing knee pain. She says the knee gives way when she puts weight on it. The physical findings are unchanged, and she is discharged home with a follow-up appointment with orthopedics in 3 days.
At the follow-up visit, she complains of persistent knee pain in the medial aspect of the knee joint. Physical examination is difficult because of pain and swelling, and it reveals mild joint effusion with no gross instability. She has pain on the medial side with valgus stress, but there appears to be a hard end point. There is no posterior sag, and the Lachman test is negative.
Based on the physical examination and the patient’s complaints, she receives a diagnosis of medial collateral ligament strain and injury. She is given a hinged brace and is instructed to undergo a physical rehabilitation program.
Three weeks after the initial evaluation, she returns to the orthopedics clinic with continuing knee problems. Mild knee effusion persists, but she has less pain and swelling, allowing a more complete examination. The examination reveals less limitation of range of motion and a hint of positivity on the Lachman test. The knee is diffusely tender, and the pain seems out of proportion with the maneuvers used during the examination. She requests more pain medication. You suspect internal derangement of the knee. Which imaging test should you order to further evaluate this patient?
A SYSTEMATIC AND COST-EFFECTIVE APPROACH IS NEEDED
The case presented above represents a typical scenario for the presentation of acute knee pain and illustrates the diagnostic challenges.
Knee pain is a common reason for emergency room visits, and it accounts for approximately 1.9 million visits to primary care clinics annually.1 In the emergency department, most patients undergo plain radiography to assess for fracture, yet approximately 92% of radiographic studies do not show a fracture.2 Clearly, the evaluation of knee pain requires a systematic, accurate, and cost-effective approach.
Key elements of the physical examination
In acute knee pain, accurate diagnosis begins with a detailed history and physical examination.
The anterior drawer test is done to evaluate the anterior cruciate ligament. With the relaxed knee flexed to approximately 80° and the foot stabilized in a neutral position, the examiner grasps the proximal tibia in a firm yet gentle grip, and then applies anterior force, noting the degree of anterior displacement compared with the other knee.
The Lachman test, a variation of the anterior drawer test, is more definitive for the anterior cruciate ligament and is carried out with the knee in 15° of flexion and external rotation, in order to relax the iliotibial band. The upper hand grasps the distal thigh, and the lower hand, with the thumb on the tibial tubercle, pulls the tibia forward. The degree of anterior motion in millimeters is noted and compared with that on the other side, and the end point is graded as “soft” or “hard.” An end point is considered hard when a ligament abruptly halts the motion of the bone being tested against the stabilized bone. An end point is considered soft when the ligament is disrupted and the restraints are the more elastic secondary stabilizers.
Debate continues
Some authors contend that in skilled hands a thorough history, physical examination, and radiographic examination are sufficient to diagnose trauma-related intra-articular knee disorders.3 Others contend that MRI plays a key role in the initial evaluation. A number of studies4–8 have shown that using MRI in the initial evaluation not only identifies key lesions, but also may eliminate the need for an invasive diagnostic procedure (ie, arthroscopy).
For example, MRI can reveal fracture, stress fracture, insufficiency fracture, and transient patellar dislocation—conditions that may satisfactorily explain knee symptoms.
PLAIN RADIOGRAPHY STILL THE FIRST STEP IN KNEE EVALUATION
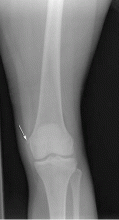
Radiography is the first step in the evaluation of knee pain. It is quick and inexpensive and can yield many diagnostic clues. It can readily reveal fractures, osteochondral defects, bony lesions, joint effusions, joint space narrowing, and bone misalignment.
In patients with knee trauma, supine anteroposterior and cross-table lateral radiographic images are generally obtained. In patients whose knee pain is not due to trauma, standing projections are done, as well as dedicated projection of the patellofemoral articulation. A standing series is most helpful for evaluating joint space and alignment.
Applying the Ottawa rules
When a patient presents to the emergency room with acute knee pain, the immediate concern is whether he or she has a fracture. The Ottawa knee rules9 for when to order radiography in adults with knee pain are highly sensitive for detecting a clinically important fracture. If any one of the five Ottawa criteria applies—ie, the patient is age 55 or older, has tenderness at the head of the fibula, has patellar tenderness, is unable to flex the knee to 90°, or is unable to bear weight—then radiography is indicated.
While studies have validated the ability of the Ottawa rules to detect important fractures in acute knee injury,2,10 fracture is the cause of only a small percentage of knee complaints in the primary care setting. More common causes include osteoarthritis, meniscal injury, ligamental injury, and crystal arthropathy, and these account for approximately half of all diagnoses. Sprain and strain account for most of the rest of knee injuries.1
Acute exacerbations of osteoarthritis
Osteoarthritis is a chronic problem, yet it is not unusual for a patient to present to the primary care physician with an acute exacerbation of joint pain. The clinical hallmarks include age over 50, stiffness lasting less than 30 minutes, bony enlargement and tenderness, and crepitus. The radiographic hallmarks, according to the Kellgren-Lawrence grading scale, are joint space narrowing, osteophytes, subchondral cysts, and sclerosis. These radiographic findings correlate well with clinical findings in these patients.11
Situations in which radiography is less helpful
In some cases the radiographic findings may not explain the patient’s clinical signs and symptoms. For example, in suspected crystalline and septic arthritis, the clinical presentation may include warmth, erythema, and effusion. Arthrocentesis would be indicated in such a patient. Indeed, in the case of suspected pseudogout, chondrocalcinosis may be radiographically evident. However, it is also present in many patients without symptoms or with osteoarthritis, so radiographic evidence does not provide a definite diagnosis.
While radiography may not always identify the cause of knee pain, it is useful in excluding serious problems such as fractures, advanced degenerative changes, and neoplasms, and it may help direct further management. Radiography is not useful in the evaluation of the cruciate and collateral ligaments, the menisci, and the hyaline cartilage of the knee and may fail to show an insufficiency or stress fracture. To evaluate these structures and associated soft tissues, MRI is preferable.
COMPUTED TOMOGRAPHY IN ACUTE KNEE PAIN
CURRENT USES OF MRI TO EVALUATE ACUTE KNEE PAIN
As mentioned above, MRI is useful in evaluating suspected meniscal and ligamentous injuries.
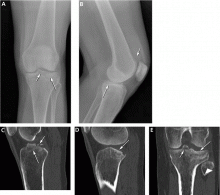
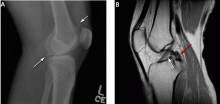
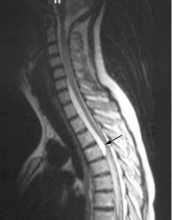
Figure 3 shows how T2-weighted MRI was used to evaluate for suspected meniscal injury in our 47-year-old female patient with left knee pain after a motor vehicle accident.
Still a matter of debate
MRI’s role in the diagnosis of knee pain is still a contentious issue.
Advantages of MRI are that it is noninvasive, it does not use ionizing radiation, it gives multiplanar images, and it provides images of soft-tissue structures, which other imaging methods cannot.12 It is a well-proven and widely accepted test. Its sensitivity for detecting meniscal and cruciate ligament injury ranges from 75% to 88%,1 and it can help in the evaluation of other injuries for which radiography is not useful, including synovitis, bone bruise, stress or insufficiency fracture, osteochondral defects, and osteonecrosis.
In addition, several studies show that using MRI to establish the diagnosis in acute knee pain can mean that 22% to 42% fewer arthroscopic procedures need to be performed.4–8 Authors of a prospective double-blind study8 recommended that MRI be used in patients with acute knee injury when the findings of the clinical history and examination by orthopedic surgeons prove equivocal.8 MRI evaluation is especially desirable for young, active patients who wish to resume activity as soon as possible.
A routine MRI examination consists of T1- and T2-weighted images in three planes, although the number of sequences and planes varies from hospital to hospital. The use of gadolinium contrast is indicated only when osteomyelitis, septic arthritis, or a mass is suspected.
Disadvantages of MRI include its cost: Medicare reimbursement for knee MRI is around $400, compared with $200 for knee CT and $50 for knee radiography with four views. Also, while studies have shown MRI to have a high sensitivity and specificity in the diagnosis of acute knee injury, some have reported a high false-positive rate for the detection of meniscal tear.13,14 MRI has also been shown to have a lower sensitivity than arthroscopy for lesions of the articular cartilage.13 Furthermore, MRI has been shown to reveal cartilage lesions, osteophytes, and meniscal abnormalities in asymptomatic study volunteers with no history of pain, trauma or knee disease.14 Therefore, findings on MRI must closely correlate with findings on the history and physical examination.
Additional indications for knee MRI
Cartilage can be assessed on routine MRI sequences of the knee. Since closed MRI systems have more powerful magnets than open systems, closed MRI systems provide greater anatomic detail.
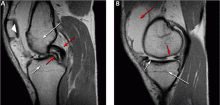
MRI can identify other lesions, such as spontaneous osteonecrosis of the knee, usually seen in elderly women who may present with sudden knee pain. In such patients, MRI findings of focal replacement of the bone marrow and surrounding edema are specific for osteonecrosis.
Opinions vary as to whether bone marrow edema is always associated with pain. Sequential MRI studies have shown persistence of bone marrow edema for 2 years in patients with degenerative arthritis whose symptoms have waned. Bone marrow edema may be associated with pain but may be absent or inconsequential in the presence of pain.
Because fluid-sensitive T2-weighted MRI is exquisitely sensitive for mobile water protons (ie, in bone marrow edema), it is important that a cause for the edema-like signal be sought on the MRI scan, since this finding is nonspecific and may be associated with articular disease, trauma, osteonecrosis, infection, or bone tumors. Additionally, clinicians need to be aware that the findings on MRI depend on the quality of the study, and are influenced by technical factors such as magnet strength, imaging planes, and use of surface coils.
MRI should be used in patients in whom surgical treatment, ie, arthroscopy, is being considered. As discussed above, several studies have shown that a significant number of unnecessary arthroscopies may be prevented when preceded by an MRI examination.
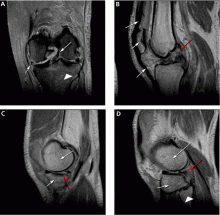
Figure 5 shows the use of MRI in the evaluation of a 45-year-old man with left knee pain after a motorcycle accident.
ULTRASONOGRAPHY HAS ONLY A LIMITED ROLE
Ultrasonography does not play a major role in the evaluation of acute knee pain in the United States, in part because the accuracy of the results depend much on the technical skills and experience of the operator.
Ultrasonography can be useful in evaluating for rupture of the quadriceps and patellar tendon, or to assess a repaired tendon after surgery,15 and it is a quick and reliable way to determine the presence of joint effusion and popliteal cyst. It is also used to guide needle placement for joint aspiration and injection.
- Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 2003; 139:575–588.
- Steill IG, Greenberg GH, Wells GA, et al. Prospective validation of a decision rule for the use of radiographs in acute knee injuries. JAMA 1996; 275:611–615.
- O’Shea KJ, Murphy DP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med 1996; 24:164–167.
- Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? J Bone Joint Surg 1993; 75:49–52.
- Bui-Mansfield LT, Youngberg RA, Warme W, Pitcher JD, Nguyen PL. Potential cost savings of MR imaging obtained before arthroscopy of the knee: evaluation of 50 consecutive patients. AJR Am J Roentgenol 1997; 168:913–918.
- Rangger C, Klestil T, Kathrein A, Inderster A, Hamid L. Influence of magnetic resonance imaging on indications for arthroscopy of the knee. Clin Orthop Rel Res 1996; 330:133–142.
- Mackenzie R, Dixon AK, Keene GS, Hollingsworth W, Lomas DJ, Villar RN. Magnetic resonance imaging of the knee: assessment of effectiveness. Clin Radiol 1996; 51:245–250.
- Munshi M, Davidson M, MacDonald PB, Froese W, Sutherland K. The efficacy of magnetic resonance imaging in acute knee injuries. Clin J Sport Med 2000; 10:34–39.
- Steill IG, Wells GA, Hoag RH, et al. Implementation of the Ottawa knee rule for the use of radiography in acute knee injuries. JAMA 1997; 278:2075–2079.
- Tigges S, Pitts S, Mukundan S, Morrison D, Olson M, Shahriara A. External validation of the Ottawa knee rules in an urban trauma center in the United States. AJR Am J Roentgenol 1999; 172:1069–1071.
- Claessens AA, Schouten JS, van den Ouweland FA, Valkenburg HA. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis 1990; 49:771–774.
- Gries PE, Bardana DE, Holmstrom MC, Burks RT. Meniscal injury: basic science and evaluation. J Am Acad Orthop Surg 2002; 10:168–176.
- Gelb HJ, Glasgow SG, Sapega AA, Torg JS. Magnetic resonance imaging of knee disorders. Clinical value and cost-effectiveness in a sports medicine practice. Am J Sports Med 1996; 24:99–103.
- Beattie KA, Boulos P, Pui M, et al. Abnormalities identified in the knees of asymptomatic volunteers using peripheral magnetic resonance imaging. Osteoarthritis Cartilage 2005; 13:181–186.
- Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg 2003; 11:192–200.
Radiography plays a key role in the initial evaluation of acute knee pain in adults. Yet conflicting studies and the absence of clear guidelines may leave the primary care physician uncertain as to which imaging test to order—ie, whether radiography is sufficient, and when computed tomography (CT) or magnetic resonance imaging (MRI) is needed. This article reviews the indications for radiologic examination of the knee and discusses indications for cross-sectional imaging studies. Imaging in oncology patients is not discussed here.
ACUTE KNEE PAIN: A TYPICAL SCENARIO
A 47-year-old woman presents to the emergency department with left knee pain after a motor vehicle accident that occurred the day before. The car she was driving hit a tree, and she hit her knee on the dashboard. She was wearing a seatbelt at the time of the accident. She says she was unable to walk immediately after the accident because of knee pain.
The initial examination in the emergency room reveals swelling and pain throughout the range of motion. The anterior drawer test and the Lachman test are negative (see below).
The patient is discharged home with a knee immobilizer, pain medication, and crutches, with instructions for a follow-up visit in the orthopedics clinic.
Five days later, she returns to the emergency department complaining of continuing knee pain. She says the knee gives way when she puts weight on it. The physical findings are unchanged, and she is discharged home with a follow-up appointment with orthopedics in 3 days.
At the follow-up visit, she complains of persistent knee pain in the medial aspect of the knee joint. Physical examination is difficult because of pain and swelling, and it reveals mild joint effusion with no gross instability. She has pain on the medial side with valgus stress, but there appears to be a hard end point. There is no posterior sag, and the Lachman test is negative.
Based on the physical examination and the patient’s complaints, she receives a diagnosis of medial collateral ligament strain and injury. She is given a hinged brace and is instructed to undergo a physical rehabilitation program.
Three weeks after the initial evaluation, she returns to the orthopedics clinic with continuing knee problems. Mild knee effusion persists, but she has less pain and swelling, allowing a more complete examination. The examination reveals less limitation of range of motion and a hint of positivity on the Lachman test. The knee is diffusely tender, and the pain seems out of proportion with the maneuvers used during the examination. She requests more pain medication. You suspect internal derangement of the knee. Which imaging test should you order to further evaluate this patient?
A SYSTEMATIC AND COST-EFFECTIVE APPROACH IS NEEDED
The case presented above represents a typical scenario for the presentation of acute knee pain and illustrates the diagnostic challenges.
Knee pain is a common reason for emergency room visits, and it accounts for approximately 1.9 million visits to primary care clinics annually.1 In the emergency department, most patients undergo plain radiography to assess for fracture, yet approximately 92% of radiographic studies do not show a fracture.2 Clearly, the evaluation of knee pain requires a systematic, accurate, and cost-effective approach.
Key elements of the physical examination
In acute knee pain, accurate diagnosis begins with a detailed history and physical examination.
The anterior drawer test is done to evaluate the anterior cruciate ligament. With the relaxed knee flexed to approximately 80° and the foot stabilized in a neutral position, the examiner grasps the proximal tibia in a firm yet gentle grip, and then applies anterior force, noting the degree of anterior displacement compared with the other knee.
The Lachman test, a variation of the anterior drawer test, is more definitive for the anterior cruciate ligament and is carried out with the knee in 15° of flexion and external rotation, in order to relax the iliotibial band. The upper hand grasps the distal thigh, and the lower hand, with the thumb on the tibial tubercle, pulls the tibia forward. The degree of anterior motion in millimeters is noted and compared with that on the other side, and the end point is graded as “soft” or “hard.” An end point is considered hard when a ligament abruptly halts the motion of the bone being tested against the stabilized bone. An end point is considered soft when the ligament is disrupted and the restraints are the more elastic secondary stabilizers.
Debate continues
Some authors contend that in skilled hands a thorough history, physical examination, and radiographic examination are sufficient to diagnose trauma-related intra-articular knee disorders.3 Others contend that MRI plays a key role in the initial evaluation. A number of studies4–8 have shown that using MRI in the initial evaluation not only identifies key lesions, but also may eliminate the need for an invasive diagnostic procedure (ie, arthroscopy).
For example, MRI can reveal fracture, stress fracture, insufficiency fracture, and transient patellar dislocation—conditions that may satisfactorily explain knee symptoms.
PLAIN RADIOGRAPHY STILL THE FIRST STEP IN KNEE EVALUATION
Radiography is the first step in the evaluation of knee pain. It is quick and inexpensive and can yield many diagnostic clues. It can readily reveal fractures, osteochondral defects, bony lesions, joint effusions, joint space narrowing, and bone misalignment.
In patients with knee trauma, supine anteroposterior and cross-table lateral radiographic images are generally obtained. In patients whose knee pain is not due to trauma, standing projections are done, as well as dedicated projection of the patellofemoral articulation. A standing series is most helpful for evaluating joint space and alignment.
Applying the Ottawa rules
When a patient presents to the emergency room with acute knee pain, the immediate concern is whether he or she has a fracture. The Ottawa knee rules9 for when to order radiography in adults with knee pain are highly sensitive for detecting a clinically important fracture. If any one of the five Ottawa criteria applies—ie, the patient is age 55 or older, has tenderness at the head of the fibula, has patellar tenderness, is unable to flex the knee to 90°, or is unable to bear weight—then radiography is indicated.
While studies have validated the ability of the Ottawa rules to detect important fractures in acute knee injury,2,10 fracture is the cause of only a small percentage of knee complaints in the primary care setting. More common causes include osteoarthritis, meniscal injury, ligamental injury, and crystal arthropathy, and these account for approximately half of all diagnoses. Sprain and strain account for most of the rest of knee injuries.1
Acute exacerbations of osteoarthritis
Osteoarthritis is a chronic problem, yet it is not unusual for a patient to present to the primary care physician with an acute exacerbation of joint pain. The clinical hallmarks include age over 50, stiffness lasting less than 30 minutes, bony enlargement and tenderness, and crepitus. The radiographic hallmarks, according to the Kellgren-Lawrence grading scale, are joint space narrowing, osteophytes, subchondral cysts, and sclerosis. These radiographic findings correlate well with clinical findings in these patients.11
Situations in which radiography is less helpful
In some cases the radiographic findings may not explain the patient’s clinical signs and symptoms. For example, in suspected crystalline and septic arthritis, the clinical presentation may include warmth, erythema, and effusion. Arthrocentesis would be indicated in such a patient. Indeed, in the case of suspected pseudogout, chondrocalcinosis may be radiographically evident. However, it is also present in many patients without symptoms or with osteoarthritis, so radiographic evidence does not provide a definite diagnosis.
While radiography may not always identify the cause of knee pain, it is useful in excluding serious problems such as fractures, advanced degenerative changes, and neoplasms, and it may help direct further management. Radiography is not useful in the evaluation of the cruciate and collateral ligaments, the menisci, and the hyaline cartilage of the knee and may fail to show an insufficiency or stress fracture. To evaluate these structures and associated soft tissues, MRI is preferable.
COMPUTED TOMOGRAPHY IN ACUTE KNEE PAIN
CURRENT USES OF MRI TO EVALUATE ACUTE KNEE PAIN
As mentioned above, MRI is useful in evaluating suspected meniscal and ligamentous injuries.
Figure 3 shows how T2-weighted MRI was used to evaluate for suspected meniscal injury in our 47-year-old female patient with left knee pain after a motor vehicle accident.
Still a matter of debate
MRI’s role in the diagnosis of knee pain is still a contentious issue.
Advantages of MRI are that it is noninvasive, it does not use ionizing radiation, it gives multiplanar images, and it provides images of soft-tissue structures, which other imaging methods cannot.12 It is a well-proven and widely accepted test. Its sensitivity for detecting meniscal and cruciate ligament injury ranges from 75% to 88%,1 and it can help in the evaluation of other injuries for which radiography is not useful, including synovitis, bone bruise, stress or insufficiency fracture, osteochondral defects, and osteonecrosis.
In addition, several studies show that using MRI to establish the diagnosis in acute knee pain can mean that 22% to 42% fewer arthroscopic procedures need to be performed.4–8 Authors of a prospective double-blind study8 recommended that MRI be used in patients with acute knee injury when the findings of the clinical history and examination by orthopedic surgeons prove equivocal.8 MRI evaluation is especially desirable for young, active patients who wish to resume activity as soon as possible.
A routine MRI examination consists of T1- and T2-weighted images in three planes, although the number of sequences and planes varies from hospital to hospital. The use of gadolinium contrast is indicated only when osteomyelitis, septic arthritis, or a mass is suspected.
Disadvantages of MRI include its cost: Medicare reimbursement for knee MRI is around $400, compared with $200 for knee CT and $50 for knee radiography with four views. Also, while studies have shown MRI to have a high sensitivity and specificity in the diagnosis of acute knee injury, some have reported a high false-positive rate for the detection of meniscal tear.13,14 MRI has also been shown to have a lower sensitivity than arthroscopy for lesions of the articular cartilage.13 Furthermore, MRI has been shown to reveal cartilage lesions, osteophytes, and meniscal abnormalities in asymptomatic study volunteers with no history of pain, trauma or knee disease.14 Therefore, findings on MRI must closely correlate with findings on the history and physical examination.
Additional indications for knee MRI
Cartilage can be assessed on routine MRI sequences of the knee. Since closed MRI systems have more powerful magnets than open systems, closed MRI systems provide greater anatomic detail.
MRI can identify other lesions, such as spontaneous osteonecrosis of the knee, usually seen in elderly women who may present with sudden knee pain. In such patients, MRI findings of focal replacement of the bone marrow and surrounding edema are specific for osteonecrosis.
Opinions vary as to whether bone marrow edema is always associated with pain. Sequential MRI studies have shown persistence of bone marrow edema for 2 years in patients with degenerative arthritis whose symptoms have waned. Bone marrow edema may be associated with pain but may be absent or inconsequential in the presence of pain.
Because fluid-sensitive T2-weighted MRI is exquisitely sensitive for mobile water protons (ie, in bone marrow edema), it is important that a cause for the edema-like signal be sought on the MRI scan, since this finding is nonspecific and may be associated with articular disease, trauma, osteonecrosis, infection, or bone tumors. Additionally, clinicians need to be aware that the findings on MRI depend on the quality of the study, and are influenced by technical factors such as magnet strength, imaging planes, and use of surface coils.
MRI should be used in patients in whom surgical treatment, ie, arthroscopy, is being considered. As discussed above, several studies have shown that a significant number of unnecessary arthroscopies may be prevented when preceded by an MRI examination.
Figure 5 shows the use of MRI in the evaluation of a 45-year-old man with left knee pain after a motorcycle accident.
ULTRASONOGRAPHY HAS ONLY A LIMITED ROLE
Ultrasonography does not play a major role in the evaluation of acute knee pain in the United States, in part because the accuracy of the results depend much on the technical skills and experience of the operator.
Ultrasonography can be useful in evaluating for rupture of the quadriceps and patellar tendon, or to assess a repaired tendon after surgery,15 and it is a quick and reliable way to determine the presence of joint effusion and popliteal cyst. It is also used to guide needle placement for joint aspiration and injection.
Radiography plays a key role in the initial evaluation of acute knee pain in adults. Yet conflicting studies and the absence of clear guidelines may leave the primary care physician uncertain as to which imaging test to order—ie, whether radiography is sufficient, and when computed tomography (CT) or magnetic resonance imaging (MRI) is needed. This article reviews the indications for radiologic examination of the knee and discusses indications for cross-sectional imaging studies. Imaging in oncology patients is not discussed here.
ACUTE KNEE PAIN: A TYPICAL SCENARIO
A 47-year-old woman presents to the emergency department with left knee pain after a motor vehicle accident that occurred the day before. The car she was driving hit a tree, and she hit her knee on the dashboard. She was wearing a seatbelt at the time of the accident. She says she was unable to walk immediately after the accident because of knee pain.
The initial examination in the emergency room reveals swelling and pain throughout the range of motion. The anterior drawer test and the Lachman test are negative (see below).
The patient is discharged home with a knee immobilizer, pain medication, and crutches, with instructions for a follow-up visit in the orthopedics clinic.
Five days later, she returns to the emergency department complaining of continuing knee pain. She says the knee gives way when she puts weight on it. The physical findings are unchanged, and she is discharged home with a follow-up appointment with orthopedics in 3 days.
At the follow-up visit, she complains of persistent knee pain in the medial aspect of the knee joint. Physical examination is difficult because of pain and swelling, and it reveals mild joint effusion with no gross instability. She has pain on the medial side with valgus stress, but there appears to be a hard end point. There is no posterior sag, and the Lachman test is negative.
Based on the physical examination and the patient’s complaints, she receives a diagnosis of medial collateral ligament strain and injury. She is given a hinged brace and is instructed to undergo a physical rehabilitation program.
Three weeks after the initial evaluation, she returns to the orthopedics clinic with continuing knee problems. Mild knee effusion persists, but she has less pain and swelling, allowing a more complete examination. The examination reveals less limitation of range of motion and a hint of positivity on the Lachman test. The knee is diffusely tender, and the pain seems out of proportion with the maneuvers used during the examination. She requests more pain medication. You suspect internal derangement of the knee. Which imaging test should you order to further evaluate this patient?
A SYSTEMATIC AND COST-EFFECTIVE APPROACH IS NEEDED
The case presented above represents a typical scenario for the presentation of acute knee pain and illustrates the diagnostic challenges.
Knee pain is a common reason for emergency room visits, and it accounts for approximately 1.9 million visits to primary care clinics annually.1 In the emergency department, most patients undergo plain radiography to assess for fracture, yet approximately 92% of radiographic studies do not show a fracture.2 Clearly, the evaluation of knee pain requires a systematic, accurate, and cost-effective approach.
Key elements of the physical examination
In acute knee pain, accurate diagnosis begins with a detailed history and physical examination.
The anterior drawer test is done to evaluate the anterior cruciate ligament. With the relaxed knee flexed to approximately 80° and the foot stabilized in a neutral position, the examiner grasps the proximal tibia in a firm yet gentle grip, and then applies anterior force, noting the degree of anterior displacement compared with the other knee.
The Lachman test, a variation of the anterior drawer test, is more definitive for the anterior cruciate ligament and is carried out with the knee in 15° of flexion and external rotation, in order to relax the iliotibial band. The upper hand grasps the distal thigh, and the lower hand, with the thumb on the tibial tubercle, pulls the tibia forward. The degree of anterior motion in millimeters is noted and compared with that on the other side, and the end point is graded as “soft” or “hard.” An end point is considered hard when a ligament abruptly halts the motion of the bone being tested against the stabilized bone. An end point is considered soft when the ligament is disrupted and the restraints are the more elastic secondary stabilizers.
Debate continues
Some authors contend that in skilled hands a thorough history, physical examination, and radiographic examination are sufficient to diagnose trauma-related intra-articular knee disorders.3 Others contend that MRI plays a key role in the initial evaluation. A number of studies4–8 have shown that using MRI in the initial evaluation not only identifies key lesions, but also may eliminate the need for an invasive diagnostic procedure (ie, arthroscopy).
For example, MRI can reveal fracture, stress fracture, insufficiency fracture, and transient patellar dislocation—conditions that may satisfactorily explain knee symptoms.
PLAIN RADIOGRAPHY STILL THE FIRST STEP IN KNEE EVALUATION
Radiography is the first step in the evaluation of knee pain. It is quick and inexpensive and can yield many diagnostic clues. It can readily reveal fractures, osteochondral defects, bony lesions, joint effusions, joint space narrowing, and bone misalignment.
In patients with knee trauma, supine anteroposterior and cross-table lateral radiographic images are generally obtained. In patients whose knee pain is not due to trauma, standing projections are done, as well as dedicated projection of the patellofemoral articulation. A standing series is most helpful for evaluating joint space and alignment.
Applying the Ottawa rules
When a patient presents to the emergency room with acute knee pain, the immediate concern is whether he or she has a fracture. The Ottawa knee rules9 for when to order radiography in adults with knee pain are highly sensitive for detecting a clinically important fracture. If any one of the five Ottawa criteria applies—ie, the patient is age 55 or older, has tenderness at the head of the fibula, has patellar tenderness, is unable to flex the knee to 90°, or is unable to bear weight—then radiography is indicated.
While studies have validated the ability of the Ottawa rules to detect important fractures in acute knee injury,2,10 fracture is the cause of only a small percentage of knee complaints in the primary care setting. More common causes include osteoarthritis, meniscal injury, ligamental injury, and crystal arthropathy, and these account for approximately half of all diagnoses. Sprain and strain account for most of the rest of knee injuries.1
Acute exacerbations of osteoarthritis
Osteoarthritis is a chronic problem, yet it is not unusual for a patient to present to the primary care physician with an acute exacerbation of joint pain. The clinical hallmarks include age over 50, stiffness lasting less than 30 minutes, bony enlargement and tenderness, and crepitus. The radiographic hallmarks, according to the Kellgren-Lawrence grading scale, are joint space narrowing, osteophytes, subchondral cysts, and sclerosis. These radiographic findings correlate well with clinical findings in these patients.11
Situations in which radiography is less helpful
In some cases the radiographic findings may not explain the patient’s clinical signs and symptoms. For example, in suspected crystalline and septic arthritis, the clinical presentation may include warmth, erythema, and effusion. Arthrocentesis would be indicated in such a patient. Indeed, in the case of suspected pseudogout, chondrocalcinosis may be radiographically evident. However, it is also present in many patients without symptoms or with osteoarthritis, so radiographic evidence does not provide a definite diagnosis.
While radiography may not always identify the cause of knee pain, it is useful in excluding serious problems such as fractures, advanced degenerative changes, and neoplasms, and it may help direct further management. Radiography is not useful in the evaluation of the cruciate and collateral ligaments, the menisci, and the hyaline cartilage of the knee and may fail to show an insufficiency or stress fracture. To evaluate these structures and associated soft tissues, MRI is preferable.
COMPUTED TOMOGRAPHY IN ACUTE KNEE PAIN
CURRENT USES OF MRI TO EVALUATE ACUTE KNEE PAIN
As mentioned above, MRI is useful in evaluating suspected meniscal and ligamentous injuries.
Figure 3 shows how T2-weighted MRI was used to evaluate for suspected meniscal injury in our 47-year-old female patient with left knee pain after a motor vehicle accident.
Still a matter of debate
MRI’s role in the diagnosis of knee pain is still a contentious issue.
Advantages of MRI are that it is noninvasive, it does not use ionizing radiation, it gives multiplanar images, and it provides images of soft-tissue structures, which other imaging methods cannot.12 It is a well-proven and widely accepted test. Its sensitivity for detecting meniscal and cruciate ligament injury ranges from 75% to 88%,1 and it can help in the evaluation of other injuries for which radiography is not useful, including synovitis, bone bruise, stress or insufficiency fracture, osteochondral defects, and osteonecrosis.
In addition, several studies show that using MRI to establish the diagnosis in acute knee pain can mean that 22% to 42% fewer arthroscopic procedures need to be performed.4–8 Authors of a prospective double-blind study8 recommended that MRI be used in patients with acute knee injury when the findings of the clinical history and examination by orthopedic surgeons prove equivocal.8 MRI evaluation is especially desirable for young, active patients who wish to resume activity as soon as possible.
A routine MRI examination consists of T1- and T2-weighted images in three planes, although the number of sequences and planes varies from hospital to hospital. The use of gadolinium contrast is indicated only when osteomyelitis, septic arthritis, or a mass is suspected.
Disadvantages of MRI include its cost: Medicare reimbursement for knee MRI is around $400, compared with $200 for knee CT and $50 for knee radiography with four views. Also, while studies have shown MRI to have a high sensitivity and specificity in the diagnosis of acute knee injury, some have reported a high false-positive rate for the detection of meniscal tear.13,14 MRI has also been shown to have a lower sensitivity than arthroscopy for lesions of the articular cartilage.13 Furthermore, MRI has been shown to reveal cartilage lesions, osteophytes, and meniscal abnormalities in asymptomatic study volunteers with no history of pain, trauma or knee disease.14 Therefore, findings on MRI must closely correlate with findings on the history and physical examination.
Additional indications for knee MRI
Cartilage can be assessed on routine MRI sequences of the knee. Since closed MRI systems have more powerful magnets than open systems, closed MRI systems provide greater anatomic detail.
MRI can identify other lesions, such as spontaneous osteonecrosis of the knee, usually seen in elderly women who may present with sudden knee pain. In such patients, MRI findings of focal replacement of the bone marrow and surrounding edema are specific for osteonecrosis.
Opinions vary as to whether bone marrow edema is always associated with pain. Sequential MRI studies have shown persistence of bone marrow edema for 2 years in patients with degenerative arthritis whose symptoms have waned. Bone marrow edema may be associated with pain but may be absent or inconsequential in the presence of pain.
Because fluid-sensitive T2-weighted MRI is exquisitely sensitive for mobile water protons (ie, in bone marrow edema), it is important that a cause for the edema-like signal be sought on the MRI scan, since this finding is nonspecific and may be associated with articular disease, trauma, osteonecrosis, infection, or bone tumors. Additionally, clinicians need to be aware that the findings on MRI depend on the quality of the study, and are influenced by technical factors such as magnet strength, imaging planes, and use of surface coils.
MRI should be used in patients in whom surgical treatment, ie, arthroscopy, is being considered. As discussed above, several studies have shown that a significant number of unnecessary arthroscopies may be prevented when preceded by an MRI examination.
Figure 5 shows the use of MRI in the evaluation of a 45-year-old man with left knee pain after a motorcycle accident.
ULTRASONOGRAPHY HAS ONLY A LIMITED ROLE
Ultrasonography does not play a major role in the evaluation of acute knee pain in the United States, in part because the accuracy of the results depend much on the technical skills and experience of the operator.
Ultrasonography can be useful in evaluating for rupture of the quadriceps and patellar tendon, or to assess a repaired tendon after surgery,15 and it is a quick and reliable way to determine the presence of joint effusion and popliteal cyst. It is also used to guide needle placement for joint aspiration and injection.
- Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 2003; 139:575–588.
- Steill IG, Greenberg GH, Wells GA, et al. Prospective validation of a decision rule for the use of radiographs in acute knee injuries. JAMA 1996; 275:611–615.
- O’Shea KJ, Murphy DP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med 1996; 24:164–167.
- Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? J Bone Joint Surg 1993; 75:49–52.
- Bui-Mansfield LT, Youngberg RA, Warme W, Pitcher JD, Nguyen PL. Potential cost savings of MR imaging obtained before arthroscopy of the knee: evaluation of 50 consecutive patients. AJR Am J Roentgenol 1997; 168:913–918.
- Rangger C, Klestil T, Kathrein A, Inderster A, Hamid L. Influence of magnetic resonance imaging on indications for arthroscopy of the knee. Clin Orthop Rel Res 1996; 330:133–142.
- Mackenzie R, Dixon AK, Keene GS, Hollingsworth W, Lomas DJ, Villar RN. Magnetic resonance imaging of the knee: assessment of effectiveness. Clin Radiol 1996; 51:245–250.
- Munshi M, Davidson M, MacDonald PB, Froese W, Sutherland K. The efficacy of magnetic resonance imaging in acute knee injuries. Clin J Sport Med 2000; 10:34–39.
- Steill IG, Wells GA, Hoag RH, et al. Implementation of the Ottawa knee rule for the use of radiography in acute knee injuries. JAMA 1997; 278:2075–2079.
- Tigges S, Pitts S, Mukundan S, Morrison D, Olson M, Shahriara A. External validation of the Ottawa knee rules in an urban trauma center in the United States. AJR Am J Roentgenol 1999; 172:1069–1071.
- Claessens AA, Schouten JS, van den Ouweland FA, Valkenburg HA. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis 1990; 49:771–774.
- Gries PE, Bardana DE, Holmstrom MC, Burks RT. Meniscal injury: basic science and evaluation. J Am Acad Orthop Surg 2002; 10:168–176.
- Gelb HJ, Glasgow SG, Sapega AA, Torg JS. Magnetic resonance imaging of knee disorders. Clinical value and cost-effectiveness in a sports medicine practice. Am J Sports Med 1996; 24:99–103.
- Beattie KA, Boulos P, Pui M, et al. Abnormalities identified in the knees of asymptomatic volunteers using peripheral magnetic resonance imaging. Osteoarthritis Cartilage 2005; 13:181–186.
- Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg 2003; 11:192–200.
- Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 2003; 139:575–588.
- Steill IG, Greenberg GH, Wells GA, et al. Prospective validation of a decision rule for the use of radiographs in acute knee injuries. JAMA 1996; 275:611–615.
- O’Shea KJ, Murphy DP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med 1996; 24:164–167.
- Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? J Bone Joint Surg 1993; 75:49–52.
- Bui-Mansfield LT, Youngberg RA, Warme W, Pitcher JD, Nguyen PL. Potential cost savings of MR imaging obtained before arthroscopy of the knee: evaluation of 50 consecutive patients. AJR Am J Roentgenol 1997; 168:913–918.
- Rangger C, Klestil T, Kathrein A, Inderster A, Hamid L. Influence of magnetic resonance imaging on indications for arthroscopy of the knee. Clin Orthop Rel Res 1996; 330:133–142.
- Mackenzie R, Dixon AK, Keene GS, Hollingsworth W, Lomas DJ, Villar RN. Magnetic resonance imaging of the knee: assessment of effectiveness. Clin Radiol 1996; 51:245–250.
- Munshi M, Davidson M, MacDonald PB, Froese W, Sutherland K. The efficacy of magnetic resonance imaging in acute knee injuries. Clin J Sport Med 2000; 10:34–39.
- Steill IG, Wells GA, Hoag RH, et al. Implementation of the Ottawa knee rule for the use of radiography in acute knee injuries. JAMA 1997; 278:2075–2079.
- Tigges S, Pitts S, Mukundan S, Morrison D, Olson M, Shahriara A. External validation of the Ottawa knee rules in an urban trauma center in the United States. AJR Am J Roentgenol 1999; 172:1069–1071.
- Claessens AA, Schouten JS, van den Ouweland FA, Valkenburg HA. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis 1990; 49:771–774.
- Gries PE, Bardana DE, Holmstrom MC, Burks RT. Meniscal injury: basic science and evaluation. J Am Acad Orthop Surg 2002; 10:168–176.
- Gelb HJ, Glasgow SG, Sapega AA, Torg JS. Magnetic resonance imaging of knee disorders. Clinical value and cost-effectiveness in a sports medicine practice. Am J Sports Med 1996; 24:99–103.
- Beattie KA, Boulos P, Pui M, et al. Abnormalities identified in the knees of asymptomatic volunteers using peripheral magnetic resonance imaging. Osteoarthritis Cartilage 2005; 13:181–186.
- Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg 2003; 11:192–200.
KEY POINTS
- In the emergency department, most patients undergo plain radiography to assess for fracture, yet more than 90% of these studies do not show a fracture.
- CT is useful in patients with knee trauma but normal radiographs.
- MRI is the imaging modality for internal derangement of the knee.
- Ultrasonography’s role in the evaluation of acute knee pain is generally limited to assessment of the extensor mechanism, joint effusion, and popliteal cyst.
Dropped gallstones disguised as a liver abscess
A 67-year-old retired man presents to his internist with a 3-month history of abdominal discomfort in the right upper quadrant on deep breathing. He has no other abdominal complaints, but he mentions that he underwent laparoscopic cholecystectomy 3 months ago for gallstone pancreatitis.
A biopsy specimen obtained with CT guidance shows chronic inflammation but is sterile on aerobic culture. There is no evidence of malignancy. Because of concern for underlying infection, the infectious disease staff recommends empirical treatment with a 4-week course of ampicillin-sulbactam (Unasyn). At completion of the antibiotic course, the patient’s symptoms have resolved.
LAPAROSCOPY’S DRAWBACKS
Complications of dropped stones, though rare, can include localized or systemic infection, inflammation, fibrosis, adhesion, cutaneous sinus formation, ileus, and abscess.1,6 Lohan et al1 estimated that dropped stones produce an intra-abdominal abscess in 0.6% to 2.9% of cases of dropped stones and bile spillage, based on reports by Rice et al4 and Morrin et al.7 Dropped stones should be recognized as a potential cause of intra-abdominal abscess in any cholecystectomy patient months or even years after the surgery. Also, these abscesses are not necessarily confined to the right upper quadrant: they can occur anywhere in the abdominal cavity.5,7
Given the ever-increasing popularity of laparoscopic cholecystectomy, the problem of intra-abdominal abscess due to dropped gallstones will only become a more common problem. Early diagnosis is the key to avoiding long and unnecessary treatment.
If dropped gallstones do become infected and eventually cause symptoms, they may require surgical or percutaneous removal in conjunction with antimicrobial therapy.8
- Lohan D, Walsh S, McLoughlin R, Murphy J. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol 2005; 15:904–912.
- Casillas S, Kittur DS. Late abscess formation after spilled gallstones masquerading as a liver mass. Surg Endosc 2003; 17:833.
- Tumer AR, Yuksek YN, Yasti AC, Gozalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg 2005; 29:437–440.
- Rice DC, Memon MA, Jamison RL, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1997; 1:85–91.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004; 80:77–79.
- Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 1998; 175:375–379.
- Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol 2000; 174:1441–1445.
- Akyar G, Aytac S, Yagci C, Akyar S. Abscess formation due to dropped gallstone after laparoscopic cholecystectomy. Eur J Radiol 1997; 25:242–245.
A 67-year-old retired man presents to his internist with a 3-month history of abdominal discomfort in the right upper quadrant on deep breathing. He has no other abdominal complaints, but he mentions that he underwent laparoscopic cholecystectomy 3 months ago for gallstone pancreatitis.
A biopsy specimen obtained with CT guidance shows chronic inflammation but is sterile on aerobic culture. There is no evidence of malignancy. Because of concern for underlying infection, the infectious disease staff recommends empirical treatment with a 4-week course of ampicillin-sulbactam (Unasyn). At completion of the antibiotic course, the patient’s symptoms have resolved.
LAPAROSCOPY’S DRAWBACKS
Complications of dropped stones, though rare, can include localized or systemic infection, inflammation, fibrosis, adhesion, cutaneous sinus formation, ileus, and abscess.1,6 Lohan et al1 estimated that dropped stones produce an intra-abdominal abscess in 0.6% to 2.9% of cases of dropped stones and bile spillage, based on reports by Rice et al4 and Morrin et al.7 Dropped stones should be recognized as a potential cause of intra-abdominal abscess in any cholecystectomy patient months or even years after the surgery. Also, these abscesses are not necessarily confined to the right upper quadrant: they can occur anywhere in the abdominal cavity.5,7
Given the ever-increasing popularity of laparoscopic cholecystectomy, the problem of intra-abdominal abscess due to dropped gallstones will only become a more common problem. Early diagnosis is the key to avoiding long and unnecessary treatment.
If dropped gallstones do become infected and eventually cause symptoms, they may require surgical or percutaneous removal in conjunction with antimicrobial therapy.8
A 67-year-old retired man presents to his internist with a 3-month history of abdominal discomfort in the right upper quadrant on deep breathing. He has no other abdominal complaints, but he mentions that he underwent laparoscopic cholecystectomy 3 months ago for gallstone pancreatitis.
A biopsy specimen obtained with CT guidance shows chronic inflammation but is sterile on aerobic culture. There is no evidence of malignancy. Because of concern for underlying infection, the infectious disease staff recommends empirical treatment with a 4-week course of ampicillin-sulbactam (Unasyn). At completion of the antibiotic course, the patient’s symptoms have resolved.
LAPAROSCOPY’S DRAWBACKS
Complications of dropped stones, though rare, can include localized or systemic infection, inflammation, fibrosis, adhesion, cutaneous sinus formation, ileus, and abscess.1,6 Lohan et al1 estimated that dropped stones produce an intra-abdominal abscess in 0.6% to 2.9% of cases of dropped stones and bile spillage, based on reports by Rice et al4 and Morrin et al.7 Dropped stones should be recognized as a potential cause of intra-abdominal abscess in any cholecystectomy patient months or even years after the surgery. Also, these abscesses are not necessarily confined to the right upper quadrant: they can occur anywhere in the abdominal cavity.5,7
Given the ever-increasing popularity of laparoscopic cholecystectomy, the problem of intra-abdominal abscess due to dropped gallstones will only become a more common problem. Early diagnosis is the key to avoiding long and unnecessary treatment.
If dropped gallstones do become infected and eventually cause symptoms, they may require surgical or percutaneous removal in conjunction with antimicrobial therapy.8
- Lohan D, Walsh S, McLoughlin R, Murphy J. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol 2005; 15:904–912.
- Casillas S, Kittur DS. Late abscess formation after spilled gallstones masquerading as a liver mass. Surg Endosc 2003; 17:833.
- Tumer AR, Yuksek YN, Yasti AC, Gozalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg 2005; 29:437–440.
- Rice DC, Memon MA, Jamison RL, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1997; 1:85–91.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004; 80:77–79.
- Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 1998; 175:375–379.
- Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol 2000; 174:1441–1445.
- Akyar G, Aytac S, Yagci C, Akyar S. Abscess formation due to dropped gallstone after laparoscopic cholecystectomy. Eur J Radiol 1997; 25:242–245.
- Lohan D, Walsh S, McLoughlin R, Murphy J. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol 2005; 15:904–912.
- Casillas S, Kittur DS. Late abscess formation after spilled gallstones masquerading as a liver mass. Surg Endosc 2003; 17:833.
- Tumer AR, Yuksek YN, Yasti AC, Gozalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg 2005; 29:437–440.
- Rice DC, Memon MA, Jamison RL, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1997; 1:85–91.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004; 80:77–79.
- Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 1998; 175:375–379.
- Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol 2000; 174:1441–1445.
- Akyar G, Aytac S, Yagci C, Akyar S. Abscess formation due to dropped gallstone after laparoscopic cholecystectomy. Eur J Radiol 1997; 25:242–245.
A review of spinal arachnoid cysts
Many patients with spinal arachnoid cysts complain of symptoms suggesting spinal cord compression, and are often initially evaluated by their primary physicians. However, these cysts are often discovered incidentally.
This article discusses how to manage spinal arachnoid cysts, whether found incidentally or during an evaluation for symptoms of spinal cord compression.
PRESENTATIONS CAN VARY WIDELY
A patient with a clinically relevant spinal arachnoid cyst is most likely to be a boy in his teens, but these cysts can occur in either sex and have been reported in patients as young as a few months and as old as nearly 80 years.1–6
In their typical presentation, spinal arachnoid cysts cause progressive signs and symptoms suggesting spinal cord compression. But because a cyst can occur at any spinal level and in a patient of any age, no one clinical presentation is pathognomonic, and the clinical sequelae can differ drastically from patient to patient. Nevertheless, we can make certain generalizations: a spinal arachnoid cyst that compresses the spinal cord typically causes waxing and waning pain and progressive spastic or flaccid paraparesis, which often are exacerbated by Valsalva maneuvers.1,6 Spinal arachnoid cysts can also present with symptoms suggestive of an isolated radiculopathy.
Less typical presentations include noncardiac chest pain, isolated gait difficulty, and isolated urinary urgency.2–4
Missed diagnosis is common
Because the symptoms are so variable and nonspecific, the diagnosis of spinal arachnoid cysts is often missed. For example, a sacral extradural arachnoid cyst can cause pain in the low back and perineal region, which is often relieved by lying flat and aggravated by Valsalva maneuvers.7
Complicating the picture, spinal arachnoid cysts can also coexist with other disorders of the central nervous system. Cases have been reported of sacral extradural arachnoid cysts coexisting with lumbar disk prolapse7 and of spinal arachnoid cysts located near a syrinx (a tube-shaped cavity in the spinal cord).3,8 A patient can have more than one spinal arachnoid cyst, or both a spinal arachnoid cyst and a concurrent intracranial arachnoid cyst or a tumor.9
EXTRADURAL VS INTRADURAL CYSTS
Like other types of spinal meningeal cysts, spinal arachnoid cysts can be broadly characterized as either extradural or intradural.10
Extradural cysts are extradural outpouchings of arachnoid that are contiguous with the spinal subarachnoid space via a small dural defect. They typically occur in the thoracic spine dorsal to the spinal cord, although they may be found elsewhere.
Intradural cysts are outpouchings of arachnoid that, regardless of size, lie entirely within the dural space. Intradural arachnoid cysts are more common than extradural cysts.
Either type of cyst may or may not communicate with the subarachnoid space.1–3
Other cystic lesions of the spine exist. One of the most common is the Tarlov cyst, which may look similar to a spinal arachnoid cyst, as both types of cysts are collections of cerebrospinal fluid. But, unlike typical spinal arachnoid cysts, Tarlov cysts occur only in the sacral spine and appear solely within the sacral root on radiographic imaging.
HOW DO CYSTS FORM?
How spinal arachnoid cysts start to form is open to conjecture, and several theories exist.1,2,7 They are often attributed to congenital defects. Another possibility is that arachnoid adhesions develop secondary to inflammation, which may arise from infection (meningitis), hemorrhage, or an iatrogenic cause such as injected contrast media or anesthetics or from the intraoperative contaminants of fibrin glue.11 Some cysts are due to trauma from lumbar puncture, anesthetic procedures, or intradural surgery. Other cysts are idiopathic.
WHY DO CYSTS ENLARGE?
Several mechanisms have been proposed to explain why spinal arachnoid cysts enlarge.2 The cells in the cyst wall probably do not secrete fluid: many spinal arachnoid cyst walls are composed primarily of simple connective tissue, and many completely lack an inner arachnoid lining—the cells that normally secrete spinal fluid—or have only a sparse lining.6 A unidirectional “valve” might let fluid in but not out. Another mechanism is pathologic distribution of arachnoid trabeculae, leading to fluid shifts within the cyst, thereby causing an increase in size.
DIAGNOSIS IS OFTEN INCIDENTAL
Spinal arachnoid cysts are rare, so an algorithm to diagnose them solely on the basis of common presenting symptoms would be impractical.
Whenever an arachnoid cyst is discovered, one must determine whether the cyst—or another problem—is actually causing the symptoms. If treatment is to succeed, the clinical presentation must correspond to the radiographic findings. For example, removing a cervical arachnoid cyst is unlikely to relieve low back pain.
Imaging studies help evaluate pain from suspected nerve compression
Although most arachnoid cysts are found by MRI, it is inappropriate to initially order MRI to evaluate a cyst’s common presenting symptoms (eg, back pain, radiculopathy).
Plain radiography should be done first. Although arachnoid cysts are composed of fluid and soft tissue, which are not easily detectable on plain films, subtle and indirect signs of a chronic, large cyst may be visible.5
MRI is the next step if plain radiographs do not reveal bony abnormalities that could explain a patient’s symptoms.
Further studies help characterize the lesion
Diffusion-weighted MRI can help differentiate an epidermoid cyst from an arachnoid cyst. It may also help differentiate a cyst from an abscess or tumor: abscesses have areas of restricted diffusion, and tumors tend to lack cerebrospinal fluid signal in their central core. Diffusion-weighted MRI can also help evaluate spinal cord atrophy and inflammatory changes.1,6,12 If an arachnoid cyst accompanies a nerve root as it enters the neural foramen, this would also appear on MRI.
Myelography or computed tomographic (CT) myelography were used to further characterize the form and structure of spinal arachnoid cysts discovered on MRI in most reported cases, and most authors advocate these studies.1,3,8,12 Specifically, CT myelography has been used to look for a communication between the intraspinal subarachnoid space and the spinal arachnoid cyst, and it is sensitive in determining whether a communication exists, although it does not pinpoint the location of the communication very well.12 CT myelography is also invaluable for imaging the spine of patients who have contraindications to MRI.
Kinematic MRI (cine-MRI) is now widely available and can help evaluate for the presence of communications between the cyst and the subarachnoid space. Dural defects may be located by carefully scrutinizing cine-MRI images for pulsating turbulent flow voids, facilitating a more focused and minimally invasive treatment strategy.13
Neo et al12 used cine-MRI to evaluate and plan the surgical resection of a giant spinal extradural arachnoid cyst. MRI helped determine the initial diagnosis, and a pulsating turbulent flow void was observed by cine-MRI in the area later confirmed surgically to contain the communication between the cyst and the spinal subarachnoid space.
Cine-MRI is not necessary as part of the initial diagnostic evaluation for spinal arachnoid cysts. It is of particular value only to the surgeon, who can request it if needed.
HISTOPATHOLOGY
With hematoxylin and eosin staining, the walls of spinal arachnoid cysts are typically seen as fibrous and lined by meningothelial cells.
TREATMENT
Observe asymptomatic cysts
For incidentally discovered spinal arachnoid cysts that cause no symptoms—ie, most of them—surgery is not recommended. No correlation exists between the size of a cyst and the need for treatment. Yearly imaging should be done to detect any new abnormality and determine whether the cyst is truly benign.
If symptoms arise, reevaluation of the cyst with MRI should be immediately undertaken.
Remove symptomatic cyst if possible
For a patient with symptoms, treatment offers an excellent chance of neurologic recovery.
Aspiration of the cyst is not routinely advised. Although aspiration may intuitively seem like the best initial approach to management, it only temporarily improves symptoms. However, percutaneous aspiration under fluoroscopic guidance may be appropriate for determining whether a cyst is causing a patient’s symptoms and thereby predicting whether surgery can help. Surgery should be undertaken only after careful consideration, as postoperative complications, though uncommon, may be very troublesome for both the patient and the surgeon.
Complete resection is ideal treatment. The standard treatment of an isolated spinal arachnoid cyst is complete surgical removal of the cyst.1 Surgery typically results in excellent outcomes in terms of resolution of symptoms, and is effective across a large range of cyst sizes.
Drain cysts that cannot be resected. Unfortunately, not all isolated spinal arachnoid cysts can be fully resected, owing to their location or to intraoperative findings such as extensive adhesion of a cyst to the spinal cord. In such cases, fenestration of the cyst wall, percutaneous drainage, or shunting the cyst into the peritoneal cavity may relieve symptoms.1–3,6
Minimally invasive surgical techniques have also met with some success. Neo et al12 reported that they successfully treated a giant spinal extradural arachnoid cyst by selectively closing the dural defect with clips. Cine-MRI was used to pinpoint the communication, allowing for a focused, limited surgical approach requiring only fenestration. The dural surface of the cyst was examined with an operating microscope.
Endoscopic approaches have also been used to treat sacral extradural arachnoid cysts.7
SOME CASES ARE MORE COMPLEX
Managing spinal arachnoid cysts becomes more complex as cysts become more intricate in morphology and if multiple cysts exist across different vertebral levels. Surgical planning and intraoperative monitoring are also complicated if a spinal arachnoid cyst coexists with another central nervous system problem.
Cases have been reported of patients with coexisting spinal arachnoid cysts and lumbar disk herniation; in many, the latter problem was considered to be the cause of symptoms.7
Holly and Batzdorf3 described patients with both intradural arachnoid cysts and syringomyelia. Cysts were resected with the aid of an operating microscope, and intraoperative ultrasonography confirmed that normal pulsation of the subarachnoid cerebrospinal fluid had returned after resection. The syrinx cavities were not surgically manipulated, yet MRI taken 3 months after surgery revealed that they had significantly diminished in each case.
The best predictor of recovery in patients who undergo surgery for spinal arachnoid cysts is if the clinical presentation correlates with the defect.1,7 Usually the postsurgical prognosis is good, with significant to full neurologic recovery in patients with all cyst types and clinical presentations.
- Choi JY, Kim SH, Lee WS, Sung KH. Spinal extradural arachnoid cyst. Acta Neurochir (Wien) 2006; 148:579–585.
- Kumar K, Malik S, Schulte PA. Symptomatic spinal arachnoid cysts: report of two cases with review of the literature. Spine 2003; 28:E25–E29.
- Holly LT, Batzdorf U. Syringomyelia associated with intradural arachnoid cysts. J Neurosurg Spine 2006; 5:111–116.
- Liu JK, Cole CD, Sherr GT, Kestle JR, Walker ML. Noncommunicating spinal extradural arachnoid cyst causing spinal cord compression in a child. J Neurosurg 2005; 103 3 suppl:266–269.
- Prevo RL, Hageman G, Bruyn RP, Broere G, van de Stadt J. Extended extradural spinal arachnoid cyst: an unusual cause of progressive spastic paraparesis. Clin Neurol Neurosurg 1999; 101:260–263.
- Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol 2003; 60:49–56.
- Muthukumar N. Sacral extradural arachnoid cyst: a rare cause of low back and perineal pain. Eur Spine J 2002; 11:162–166.
- Takeuchi A, Miyamoto K, Sugiyama S, Saitou M, Hosoe H, Shimizu K. Spinal arachnoid cysts associated with syringomyelia: report of two cases and a review of the literature. J Spinal Disord Tech 2003; 16:207–211.
- Kurokawa R, Kawase T. Spinal arachnoid cyst causing paraplegia following skull base surgery. Neurol Med Chir (Tokyo) 2006; 46:309–312.
- Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988; 68:366–377.
- Taguchi Y, Suzuki R, Okada M, Sekino H. Spinal arachnoid cyst developing after surgical treatment of a ruptured vertebral artery aneurysm: a possible complication of topical use of fibrin glue. Case report. J Neurosurg 1996; 84:526–529.
- Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine 2004; 29:E426–E430.
- Doita M, Nishida K, Miura J, Takada T, Kurosaka M, Fujii M. Kinematic magnetic resonance imaging of a thoracic spinal extradural arachnoid cyst: an alternative suggestion for exacerbation of symptoms during straining. Spine 2003; 28:E229–E233.
Many patients with spinal arachnoid cysts complain of symptoms suggesting spinal cord compression, and are often initially evaluated by their primary physicians. However, these cysts are often discovered incidentally.
This article discusses how to manage spinal arachnoid cysts, whether found incidentally or during an evaluation for symptoms of spinal cord compression.
PRESENTATIONS CAN VARY WIDELY
A patient with a clinically relevant spinal arachnoid cyst is most likely to be a boy in his teens, but these cysts can occur in either sex and have been reported in patients as young as a few months and as old as nearly 80 years.1–6
In their typical presentation, spinal arachnoid cysts cause progressive signs and symptoms suggesting spinal cord compression. But because a cyst can occur at any spinal level and in a patient of any age, no one clinical presentation is pathognomonic, and the clinical sequelae can differ drastically from patient to patient. Nevertheless, we can make certain generalizations: a spinal arachnoid cyst that compresses the spinal cord typically causes waxing and waning pain and progressive spastic or flaccid paraparesis, which often are exacerbated by Valsalva maneuvers.1,6 Spinal arachnoid cysts can also present with symptoms suggestive of an isolated radiculopathy.
Less typical presentations include noncardiac chest pain, isolated gait difficulty, and isolated urinary urgency.2–4
Missed diagnosis is common
Because the symptoms are so variable and nonspecific, the diagnosis of spinal arachnoid cysts is often missed. For example, a sacral extradural arachnoid cyst can cause pain in the low back and perineal region, which is often relieved by lying flat and aggravated by Valsalva maneuvers.7
Complicating the picture, spinal arachnoid cysts can also coexist with other disorders of the central nervous system. Cases have been reported of sacral extradural arachnoid cysts coexisting with lumbar disk prolapse7 and of spinal arachnoid cysts located near a syrinx (a tube-shaped cavity in the spinal cord).3,8 A patient can have more than one spinal arachnoid cyst, or both a spinal arachnoid cyst and a concurrent intracranial arachnoid cyst or a tumor.9
EXTRADURAL VS INTRADURAL CYSTS
Like other types of spinal meningeal cysts, spinal arachnoid cysts can be broadly characterized as either extradural or intradural.10
Extradural cysts are extradural outpouchings of arachnoid that are contiguous with the spinal subarachnoid space via a small dural defect. They typically occur in the thoracic spine dorsal to the spinal cord, although they may be found elsewhere.
Intradural cysts are outpouchings of arachnoid that, regardless of size, lie entirely within the dural space. Intradural arachnoid cysts are more common than extradural cysts.
Either type of cyst may or may not communicate with the subarachnoid space.1–3
Other cystic lesions of the spine exist. One of the most common is the Tarlov cyst, which may look similar to a spinal arachnoid cyst, as both types of cysts are collections of cerebrospinal fluid. But, unlike typical spinal arachnoid cysts, Tarlov cysts occur only in the sacral spine and appear solely within the sacral root on radiographic imaging.
HOW DO CYSTS FORM?
How spinal arachnoid cysts start to form is open to conjecture, and several theories exist.1,2,7 They are often attributed to congenital defects. Another possibility is that arachnoid adhesions develop secondary to inflammation, which may arise from infection (meningitis), hemorrhage, or an iatrogenic cause such as injected contrast media or anesthetics or from the intraoperative contaminants of fibrin glue.11 Some cysts are due to trauma from lumbar puncture, anesthetic procedures, or intradural surgery. Other cysts are idiopathic.
WHY DO CYSTS ENLARGE?
Several mechanisms have been proposed to explain why spinal arachnoid cysts enlarge.2 The cells in the cyst wall probably do not secrete fluid: many spinal arachnoid cyst walls are composed primarily of simple connective tissue, and many completely lack an inner arachnoid lining—the cells that normally secrete spinal fluid—or have only a sparse lining.6 A unidirectional “valve” might let fluid in but not out. Another mechanism is pathologic distribution of arachnoid trabeculae, leading to fluid shifts within the cyst, thereby causing an increase in size.
DIAGNOSIS IS OFTEN INCIDENTAL
Spinal arachnoid cysts are rare, so an algorithm to diagnose them solely on the basis of common presenting symptoms would be impractical.
Whenever an arachnoid cyst is discovered, one must determine whether the cyst—or another problem—is actually causing the symptoms. If treatment is to succeed, the clinical presentation must correspond to the radiographic findings. For example, removing a cervical arachnoid cyst is unlikely to relieve low back pain.
Imaging studies help evaluate pain from suspected nerve compression
Although most arachnoid cysts are found by MRI, it is inappropriate to initially order MRI to evaluate a cyst’s common presenting symptoms (eg, back pain, radiculopathy).
Plain radiography should be done first. Although arachnoid cysts are composed of fluid and soft tissue, which are not easily detectable on plain films, subtle and indirect signs of a chronic, large cyst may be visible.5
MRI is the next step if plain radiographs do not reveal bony abnormalities that could explain a patient’s symptoms.
Further studies help characterize the lesion
Diffusion-weighted MRI can help differentiate an epidermoid cyst from an arachnoid cyst. It may also help differentiate a cyst from an abscess or tumor: abscesses have areas of restricted diffusion, and tumors tend to lack cerebrospinal fluid signal in their central core. Diffusion-weighted MRI can also help evaluate spinal cord atrophy and inflammatory changes.1,6,12 If an arachnoid cyst accompanies a nerve root as it enters the neural foramen, this would also appear on MRI.
Myelography or computed tomographic (CT) myelography were used to further characterize the form and structure of spinal arachnoid cysts discovered on MRI in most reported cases, and most authors advocate these studies.1,3,8,12 Specifically, CT myelography has been used to look for a communication between the intraspinal subarachnoid space and the spinal arachnoid cyst, and it is sensitive in determining whether a communication exists, although it does not pinpoint the location of the communication very well.12 CT myelography is also invaluable for imaging the spine of patients who have contraindications to MRI.
Kinematic MRI (cine-MRI) is now widely available and can help evaluate for the presence of communications between the cyst and the subarachnoid space. Dural defects may be located by carefully scrutinizing cine-MRI images for pulsating turbulent flow voids, facilitating a more focused and minimally invasive treatment strategy.13
Neo et al12 used cine-MRI to evaluate and plan the surgical resection of a giant spinal extradural arachnoid cyst. MRI helped determine the initial diagnosis, and a pulsating turbulent flow void was observed by cine-MRI in the area later confirmed surgically to contain the communication between the cyst and the spinal subarachnoid space.
Cine-MRI is not necessary as part of the initial diagnostic evaluation for spinal arachnoid cysts. It is of particular value only to the surgeon, who can request it if needed.
HISTOPATHOLOGY
With hematoxylin and eosin staining, the walls of spinal arachnoid cysts are typically seen as fibrous and lined by meningothelial cells.
TREATMENT
Observe asymptomatic cysts
For incidentally discovered spinal arachnoid cysts that cause no symptoms—ie, most of them—surgery is not recommended. No correlation exists between the size of a cyst and the need for treatment. Yearly imaging should be done to detect any new abnormality and determine whether the cyst is truly benign.
If symptoms arise, reevaluation of the cyst with MRI should be immediately undertaken.
Remove symptomatic cyst if possible
For a patient with symptoms, treatment offers an excellent chance of neurologic recovery.
Aspiration of the cyst is not routinely advised. Although aspiration may intuitively seem like the best initial approach to management, it only temporarily improves symptoms. However, percutaneous aspiration under fluoroscopic guidance may be appropriate for determining whether a cyst is causing a patient’s symptoms and thereby predicting whether surgery can help. Surgery should be undertaken only after careful consideration, as postoperative complications, though uncommon, may be very troublesome for both the patient and the surgeon.
Complete resection is ideal treatment. The standard treatment of an isolated spinal arachnoid cyst is complete surgical removal of the cyst.1 Surgery typically results in excellent outcomes in terms of resolution of symptoms, and is effective across a large range of cyst sizes.
Drain cysts that cannot be resected. Unfortunately, not all isolated spinal arachnoid cysts can be fully resected, owing to their location or to intraoperative findings such as extensive adhesion of a cyst to the spinal cord. In such cases, fenestration of the cyst wall, percutaneous drainage, or shunting the cyst into the peritoneal cavity may relieve symptoms.1–3,6
Minimally invasive surgical techniques have also met with some success. Neo et al12 reported that they successfully treated a giant spinal extradural arachnoid cyst by selectively closing the dural defect with clips. Cine-MRI was used to pinpoint the communication, allowing for a focused, limited surgical approach requiring only fenestration. The dural surface of the cyst was examined with an operating microscope.
Endoscopic approaches have also been used to treat sacral extradural arachnoid cysts.7
SOME CASES ARE MORE COMPLEX
Managing spinal arachnoid cysts becomes more complex as cysts become more intricate in morphology and if multiple cysts exist across different vertebral levels. Surgical planning and intraoperative monitoring are also complicated if a spinal arachnoid cyst coexists with another central nervous system problem.
Cases have been reported of patients with coexisting spinal arachnoid cysts and lumbar disk herniation; in many, the latter problem was considered to be the cause of symptoms.7
Holly and Batzdorf3 described patients with both intradural arachnoid cysts and syringomyelia. Cysts were resected with the aid of an operating microscope, and intraoperative ultrasonography confirmed that normal pulsation of the subarachnoid cerebrospinal fluid had returned after resection. The syrinx cavities were not surgically manipulated, yet MRI taken 3 months after surgery revealed that they had significantly diminished in each case.
The best predictor of recovery in patients who undergo surgery for spinal arachnoid cysts is if the clinical presentation correlates with the defect.1,7 Usually the postsurgical prognosis is good, with significant to full neurologic recovery in patients with all cyst types and clinical presentations.
Many patients with spinal arachnoid cysts complain of symptoms suggesting spinal cord compression, and are often initially evaluated by their primary physicians. However, these cysts are often discovered incidentally.
This article discusses how to manage spinal arachnoid cysts, whether found incidentally or during an evaluation for symptoms of spinal cord compression.
PRESENTATIONS CAN VARY WIDELY
A patient with a clinically relevant spinal arachnoid cyst is most likely to be a boy in his teens, but these cysts can occur in either sex and have been reported in patients as young as a few months and as old as nearly 80 years.1–6
In their typical presentation, spinal arachnoid cysts cause progressive signs and symptoms suggesting spinal cord compression. But because a cyst can occur at any spinal level and in a patient of any age, no one clinical presentation is pathognomonic, and the clinical sequelae can differ drastically from patient to patient. Nevertheless, we can make certain generalizations: a spinal arachnoid cyst that compresses the spinal cord typically causes waxing and waning pain and progressive spastic or flaccid paraparesis, which often are exacerbated by Valsalva maneuvers.1,6 Spinal arachnoid cysts can also present with symptoms suggestive of an isolated radiculopathy.
Less typical presentations include noncardiac chest pain, isolated gait difficulty, and isolated urinary urgency.2–4
Missed diagnosis is common
Because the symptoms are so variable and nonspecific, the diagnosis of spinal arachnoid cysts is often missed. For example, a sacral extradural arachnoid cyst can cause pain in the low back and perineal region, which is often relieved by lying flat and aggravated by Valsalva maneuvers.7
Complicating the picture, spinal arachnoid cysts can also coexist with other disorders of the central nervous system. Cases have been reported of sacral extradural arachnoid cysts coexisting with lumbar disk prolapse7 and of spinal arachnoid cysts located near a syrinx (a tube-shaped cavity in the spinal cord).3,8 A patient can have more than one spinal arachnoid cyst, or both a spinal arachnoid cyst and a concurrent intracranial arachnoid cyst or a tumor.9
EXTRADURAL VS INTRADURAL CYSTS
Like other types of spinal meningeal cysts, spinal arachnoid cysts can be broadly characterized as either extradural or intradural.10
Extradural cysts are extradural outpouchings of arachnoid that are contiguous with the spinal subarachnoid space via a small dural defect. They typically occur in the thoracic spine dorsal to the spinal cord, although they may be found elsewhere.
Intradural cysts are outpouchings of arachnoid that, regardless of size, lie entirely within the dural space. Intradural arachnoid cysts are more common than extradural cysts.
Either type of cyst may or may not communicate with the subarachnoid space.1–3
Other cystic lesions of the spine exist. One of the most common is the Tarlov cyst, which may look similar to a spinal arachnoid cyst, as both types of cysts are collections of cerebrospinal fluid. But, unlike typical spinal arachnoid cysts, Tarlov cysts occur only in the sacral spine and appear solely within the sacral root on radiographic imaging.
HOW DO CYSTS FORM?
How spinal arachnoid cysts start to form is open to conjecture, and several theories exist.1,2,7 They are often attributed to congenital defects. Another possibility is that arachnoid adhesions develop secondary to inflammation, which may arise from infection (meningitis), hemorrhage, or an iatrogenic cause such as injected contrast media or anesthetics or from the intraoperative contaminants of fibrin glue.11 Some cysts are due to trauma from lumbar puncture, anesthetic procedures, or intradural surgery. Other cysts are idiopathic.
WHY DO CYSTS ENLARGE?
Several mechanisms have been proposed to explain why spinal arachnoid cysts enlarge.2 The cells in the cyst wall probably do not secrete fluid: many spinal arachnoid cyst walls are composed primarily of simple connective tissue, and many completely lack an inner arachnoid lining—the cells that normally secrete spinal fluid—or have only a sparse lining.6 A unidirectional “valve” might let fluid in but not out. Another mechanism is pathologic distribution of arachnoid trabeculae, leading to fluid shifts within the cyst, thereby causing an increase in size.
DIAGNOSIS IS OFTEN INCIDENTAL
Spinal arachnoid cysts are rare, so an algorithm to diagnose them solely on the basis of common presenting symptoms would be impractical.
Whenever an arachnoid cyst is discovered, one must determine whether the cyst—or another problem—is actually causing the symptoms. If treatment is to succeed, the clinical presentation must correspond to the radiographic findings. For example, removing a cervical arachnoid cyst is unlikely to relieve low back pain.
Imaging studies help evaluate pain from suspected nerve compression
Although most arachnoid cysts are found by MRI, it is inappropriate to initially order MRI to evaluate a cyst’s common presenting symptoms (eg, back pain, radiculopathy).
Plain radiography should be done first. Although arachnoid cysts are composed of fluid and soft tissue, which are not easily detectable on plain films, subtle and indirect signs of a chronic, large cyst may be visible.5
MRI is the next step if plain radiographs do not reveal bony abnormalities that could explain a patient’s symptoms.
Further studies help characterize the lesion
Diffusion-weighted MRI can help differentiate an epidermoid cyst from an arachnoid cyst. It may also help differentiate a cyst from an abscess or tumor: abscesses have areas of restricted diffusion, and tumors tend to lack cerebrospinal fluid signal in their central core. Diffusion-weighted MRI can also help evaluate spinal cord atrophy and inflammatory changes.1,6,12 If an arachnoid cyst accompanies a nerve root as it enters the neural foramen, this would also appear on MRI.
Myelography or computed tomographic (CT) myelography were used to further characterize the form and structure of spinal arachnoid cysts discovered on MRI in most reported cases, and most authors advocate these studies.1,3,8,12 Specifically, CT myelography has been used to look for a communication between the intraspinal subarachnoid space and the spinal arachnoid cyst, and it is sensitive in determining whether a communication exists, although it does not pinpoint the location of the communication very well.12 CT myelography is also invaluable for imaging the spine of patients who have contraindications to MRI.
Kinematic MRI (cine-MRI) is now widely available and can help evaluate for the presence of communications between the cyst and the subarachnoid space. Dural defects may be located by carefully scrutinizing cine-MRI images for pulsating turbulent flow voids, facilitating a more focused and minimally invasive treatment strategy.13
Neo et al12 used cine-MRI to evaluate and plan the surgical resection of a giant spinal extradural arachnoid cyst. MRI helped determine the initial diagnosis, and a pulsating turbulent flow void was observed by cine-MRI in the area later confirmed surgically to contain the communication between the cyst and the spinal subarachnoid space.
Cine-MRI is not necessary as part of the initial diagnostic evaluation for spinal arachnoid cysts. It is of particular value only to the surgeon, who can request it if needed.
HISTOPATHOLOGY
With hematoxylin and eosin staining, the walls of spinal arachnoid cysts are typically seen as fibrous and lined by meningothelial cells.
TREATMENT
Observe asymptomatic cysts
For incidentally discovered spinal arachnoid cysts that cause no symptoms—ie, most of them—surgery is not recommended. No correlation exists between the size of a cyst and the need for treatment. Yearly imaging should be done to detect any new abnormality and determine whether the cyst is truly benign.
If symptoms arise, reevaluation of the cyst with MRI should be immediately undertaken.
Remove symptomatic cyst if possible
For a patient with symptoms, treatment offers an excellent chance of neurologic recovery.
Aspiration of the cyst is not routinely advised. Although aspiration may intuitively seem like the best initial approach to management, it only temporarily improves symptoms. However, percutaneous aspiration under fluoroscopic guidance may be appropriate for determining whether a cyst is causing a patient’s symptoms and thereby predicting whether surgery can help. Surgery should be undertaken only after careful consideration, as postoperative complications, though uncommon, may be very troublesome for both the patient and the surgeon.
Complete resection is ideal treatment. The standard treatment of an isolated spinal arachnoid cyst is complete surgical removal of the cyst.1 Surgery typically results in excellent outcomes in terms of resolution of symptoms, and is effective across a large range of cyst sizes.
Drain cysts that cannot be resected. Unfortunately, not all isolated spinal arachnoid cysts can be fully resected, owing to their location or to intraoperative findings such as extensive adhesion of a cyst to the spinal cord. In such cases, fenestration of the cyst wall, percutaneous drainage, or shunting the cyst into the peritoneal cavity may relieve symptoms.1–3,6
Minimally invasive surgical techniques have also met with some success. Neo et al12 reported that they successfully treated a giant spinal extradural arachnoid cyst by selectively closing the dural defect with clips. Cine-MRI was used to pinpoint the communication, allowing for a focused, limited surgical approach requiring only fenestration. The dural surface of the cyst was examined with an operating microscope.
Endoscopic approaches have also been used to treat sacral extradural arachnoid cysts.7
SOME CASES ARE MORE COMPLEX
Managing spinal arachnoid cysts becomes more complex as cysts become more intricate in morphology and if multiple cysts exist across different vertebral levels. Surgical planning and intraoperative monitoring are also complicated if a spinal arachnoid cyst coexists with another central nervous system problem.
Cases have been reported of patients with coexisting spinal arachnoid cysts and lumbar disk herniation; in many, the latter problem was considered to be the cause of symptoms.7
Holly and Batzdorf3 described patients with both intradural arachnoid cysts and syringomyelia. Cysts were resected with the aid of an operating microscope, and intraoperative ultrasonography confirmed that normal pulsation of the subarachnoid cerebrospinal fluid had returned after resection. The syrinx cavities were not surgically manipulated, yet MRI taken 3 months after surgery revealed that they had significantly diminished in each case.
The best predictor of recovery in patients who undergo surgery for spinal arachnoid cysts is if the clinical presentation correlates with the defect.1,7 Usually the postsurgical prognosis is good, with significant to full neurologic recovery in patients with all cyst types and clinical presentations.
- Choi JY, Kim SH, Lee WS, Sung KH. Spinal extradural arachnoid cyst. Acta Neurochir (Wien) 2006; 148:579–585.
- Kumar K, Malik S, Schulte PA. Symptomatic spinal arachnoid cysts: report of two cases with review of the literature. Spine 2003; 28:E25–E29.
- Holly LT, Batzdorf U. Syringomyelia associated with intradural arachnoid cysts. J Neurosurg Spine 2006; 5:111–116.
- Liu JK, Cole CD, Sherr GT, Kestle JR, Walker ML. Noncommunicating spinal extradural arachnoid cyst causing spinal cord compression in a child. J Neurosurg 2005; 103 3 suppl:266–269.
- Prevo RL, Hageman G, Bruyn RP, Broere G, van de Stadt J. Extended extradural spinal arachnoid cyst: an unusual cause of progressive spastic paraparesis. Clin Neurol Neurosurg 1999; 101:260–263.
- Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol 2003; 60:49–56.
- Muthukumar N. Sacral extradural arachnoid cyst: a rare cause of low back and perineal pain. Eur Spine J 2002; 11:162–166.
- Takeuchi A, Miyamoto K, Sugiyama S, Saitou M, Hosoe H, Shimizu K. Spinal arachnoid cysts associated with syringomyelia: report of two cases and a review of the literature. J Spinal Disord Tech 2003; 16:207–211.
- Kurokawa R, Kawase T. Spinal arachnoid cyst causing paraplegia following skull base surgery. Neurol Med Chir (Tokyo) 2006; 46:309–312.
- Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988; 68:366–377.
- Taguchi Y, Suzuki R, Okada M, Sekino H. Spinal arachnoid cyst developing after surgical treatment of a ruptured vertebral artery aneurysm: a possible complication of topical use of fibrin glue. Case report. J Neurosurg 1996; 84:526–529.
- Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine 2004; 29:E426–E430.
- Doita M, Nishida K, Miura J, Takada T, Kurosaka M, Fujii M. Kinematic magnetic resonance imaging of a thoracic spinal extradural arachnoid cyst: an alternative suggestion for exacerbation of symptoms during straining. Spine 2003; 28:E229–E233.
- Choi JY, Kim SH, Lee WS, Sung KH. Spinal extradural arachnoid cyst. Acta Neurochir (Wien) 2006; 148:579–585.
- Kumar K, Malik S, Schulte PA. Symptomatic spinal arachnoid cysts: report of two cases with review of the literature. Spine 2003; 28:E25–E29.
- Holly LT, Batzdorf U. Syringomyelia associated with intradural arachnoid cysts. J Neurosurg Spine 2006; 5:111–116.
- Liu JK, Cole CD, Sherr GT, Kestle JR, Walker ML. Noncommunicating spinal extradural arachnoid cyst causing spinal cord compression in a child. J Neurosurg 2005; 103 3 suppl:266–269.
- Prevo RL, Hageman G, Bruyn RP, Broere G, van de Stadt J. Extended extradural spinal arachnoid cyst: an unusual cause of progressive spastic paraparesis. Clin Neurol Neurosurg 1999; 101:260–263.
- Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol 2003; 60:49–56.
- Muthukumar N. Sacral extradural arachnoid cyst: a rare cause of low back and perineal pain. Eur Spine J 2002; 11:162–166.
- Takeuchi A, Miyamoto K, Sugiyama S, Saitou M, Hosoe H, Shimizu K. Spinal arachnoid cysts associated with syringomyelia: report of two cases and a review of the literature. J Spinal Disord Tech 2003; 16:207–211.
- Kurokawa R, Kawase T. Spinal arachnoid cyst causing paraplegia following skull base surgery. Neurol Med Chir (Tokyo) 2006; 46:309–312.
- Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988; 68:366–377.
- Taguchi Y, Suzuki R, Okada M, Sekino H. Spinal arachnoid cyst developing after surgical treatment of a ruptured vertebral artery aneurysm: a possible complication of topical use of fibrin glue. Case report. J Neurosurg 1996; 84:526–529.
- Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine 2004; 29:E426–E430.
- Doita M, Nishida K, Miura J, Takada T, Kurosaka M, Fujii M. Kinematic magnetic resonance imaging of a thoracic spinal extradural arachnoid cyst: an alternative suggestion for exacerbation of symptoms during straining. Spine 2003; 28:E229–E233.
KEY POINTS
- Spinal arachnoid cysts can occur at any age and at any spinal level.
- Symptoms vary widely but typically include waxing and waning pain and spastic or flaccid paraparesis.
- Most spinal arachnoid cysts are asymptomatic when diagnosed and are discovered incidentally on MRI or myelography.
- MRI and computed tomography help characterize spinal arachnoid cysts and differentiate them from abscesses and tumors.
- Symptomatic cysts should be surgically resected. If complete resection is impossible, fenestration of the cyst wall, drainage, or shunting may relieve symptoms.
- An asymptomatic spinal arachnoid cyst should be followed annually with serial imaging.
Distal Biceps Brachii Tendon Tear
Larger Left Atrial Size May Increase Stroke Risk in Blacks
Echocardiographically measured left atrial size was significantly related to ischemic stroke and all-cause mortality in a follow-up analysis of 1,886 blacks in the Atherosclerosis Risk in Communities study.
At a median of 9 years follow-up, there were 103 strokes (6.47/1,000 person-years) and 206 deaths (13.3/1,000 person-years) in participants in the Jackson, Miss., cohort of the study. Their mean age was 59 years; 65% were women.
Left atrial size was significantly related to hypertension, diabetes, and body mass index, Dr. Harsha S. Nagarajarao reported at the American Federation for Medical Research Southern Regional meeting in New Orleans.
In an effort to adjust left atrial (LA) size to body size, LA size was indexed to height and was then divided into quintiles, with 377 patients in the top quintile of LA size (2.57–3.55 cm/m) and 1,509 patients in the bottom four quintiles (1.29–2.56 cm/m). Significantly more patients in the top quintile of LA size were hypertensive (74.3% vs. 57%), diabetic (29% vs. 21.3%), and had a higher mean BMI, compared with those with lower LA size (34.6 vs. 29.4 kg/m
In a multivariate analysis, LA size on echocardiogram was significantly associated with ischemic stroke (hazard ratio 1.58) and all-cause mortality (HR 1.47), even after adjustment for age, sex, cigarette smoking, diabetes, hypertension, BMI, ratio of total cholesterol to HDL cholesterol, and triglyceride levels.
Left atrial size remained significantly related to all-cause mortality (HR 1.40) after further adjustment for left ventricular hypertrophy, Dr. Nagarajarao of the University of Mississippi Medical Center, in Jackson, and colleagues reported.
Non-Hispanic whites also have an increased incidence of stroke with increased LA size, but LA size is more important in blacks because of that population's increased stroke risk, Dr. Nagarajarao said in an interview. Blacks have a twofold higher incidence of stroke when compared with non-Hispanic whites, he added.
“Echocardiography may be a potentially useful noninvasive tool in identifying additional risk factors for stroke, and identifying participants with larger LA size may allow us to take preventive measures in identifying risk factors and treating them,” he said.
Last year, investigators at the University of Mississippi Medical Center also reported that echocardiographically derived left ventricular mass index (LVMI) was an independent predictor of incident ischemic stroke among 1,792 blacks in the Jackson cohort, after adjustment for similar cardiovascular risk factors (Stroke 2007;38:2686–91). In addition, the relation between LVMI and stroke remained significant after adding LA size and mitral annular calcification to the multivariable analysis.
Clinicians at the center determine LA size routinely on echocardiography in all patients at risk of stroke, Dr. Nagarajarao said. When asked which measurement is preferred, he said both LVMI and LA size have the potential to be independent predictors of risk factors, adding that it is important to recognize that each is independent of the other.
Previous studies have shown that the BP medication hydrochlorothiazide has reduced LA size when used along with controlling hypertension, although further study is needed to determine whether this has any effect on reducing stroke incidence, he said.
Echocardiographically measured left atrial size was significantly related to ischemic stroke and all-cause mortality in a follow-up analysis of 1,886 blacks in the Atherosclerosis Risk in Communities study.
At a median of 9 years follow-up, there were 103 strokes (6.47/1,000 person-years) and 206 deaths (13.3/1,000 person-years) in participants in the Jackson, Miss., cohort of the study. Their mean age was 59 years; 65% were women.
Left atrial size was significantly related to hypertension, diabetes, and body mass index, Dr. Harsha S. Nagarajarao reported at the American Federation for Medical Research Southern Regional meeting in New Orleans.
In an effort to adjust left atrial (LA) size to body size, LA size was indexed to height and was then divided into quintiles, with 377 patients in the top quintile of LA size (2.57–3.55 cm/m) and 1,509 patients in the bottom four quintiles (1.29–2.56 cm/m). Significantly more patients in the top quintile of LA size were hypertensive (74.3% vs. 57%), diabetic (29% vs. 21.3%), and had a higher mean BMI, compared with those with lower LA size (34.6 vs. 29.4 kg/m
In a multivariate analysis, LA size on echocardiogram was significantly associated with ischemic stroke (hazard ratio 1.58) and all-cause mortality (HR 1.47), even after adjustment for age, sex, cigarette smoking, diabetes, hypertension, BMI, ratio of total cholesterol to HDL cholesterol, and triglyceride levels.
Left atrial size remained significantly related to all-cause mortality (HR 1.40) after further adjustment for left ventricular hypertrophy, Dr. Nagarajarao of the University of Mississippi Medical Center, in Jackson, and colleagues reported.
Non-Hispanic whites also have an increased incidence of stroke with increased LA size, but LA size is more important in blacks because of that population's increased stroke risk, Dr. Nagarajarao said in an interview. Blacks have a twofold higher incidence of stroke when compared with non-Hispanic whites, he added.
“Echocardiography may be a potentially useful noninvasive tool in identifying additional risk factors for stroke, and identifying participants with larger LA size may allow us to take preventive measures in identifying risk factors and treating them,” he said.
Last year, investigators at the University of Mississippi Medical Center also reported that echocardiographically derived left ventricular mass index (LVMI) was an independent predictor of incident ischemic stroke among 1,792 blacks in the Jackson cohort, after adjustment for similar cardiovascular risk factors (Stroke 2007;38:2686–91). In addition, the relation between LVMI and stroke remained significant after adding LA size and mitral annular calcification to the multivariable analysis.
Clinicians at the center determine LA size routinely on echocardiography in all patients at risk of stroke, Dr. Nagarajarao said. When asked which measurement is preferred, he said both LVMI and LA size have the potential to be independent predictors of risk factors, adding that it is important to recognize that each is independent of the other.
Previous studies have shown that the BP medication hydrochlorothiazide has reduced LA size when used along with controlling hypertension, although further study is needed to determine whether this has any effect on reducing stroke incidence, he said.
Echocardiographically measured left atrial size was significantly related to ischemic stroke and all-cause mortality in a follow-up analysis of 1,886 blacks in the Atherosclerosis Risk in Communities study.
At a median of 9 years follow-up, there were 103 strokes (6.47/1,000 person-years) and 206 deaths (13.3/1,000 person-years) in participants in the Jackson, Miss., cohort of the study. Their mean age was 59 years; 65% were women.
Left atrial size was significantly related to hypertension, diabetes, and body mass index, Dr. Harsha S. Nagarajarao reported at the American Federation for Medical Research Southern Regional meeting in New Orleans.
In an effort to adjust left atrial (LA) size to body size, LA size was indexed to height and was then divided into quintiles, with 377 patients in the top quintile of LA size (2.57–3.55 cm/m) and 1,509 patients in the bottom four quintiles (1.29–2.56 cm/m). Significantly more patients in the top quintile of LA size were hypertensive (74.3% vs. 57%), diabetic (29% vs. 21.3%), and had a higher mean BMI, compared with those with lower LA size (34.6 vs. 29.4 kg/m
In a multivariate analysis, LA size on echocardiogram was significantly associated with ischemic stroke (hazard ratio 1.58) and all-cause mortality (HR 1.47), even after adjustment for age, sex, cigarette smoking, diabetes, hypertension, BMI, ratio of total cholesterol to HDL cholesterol, and triglyceride levels.
Left atrial size remained significantly related to all-cause mortality (HR 1.40) after further adjustment for left ventricular hypertrophy, Dr. Nagarajarao of the University of Mississippi Medical Center, in Jackson, and colleagues reported.
Non-Hispanic whites also have an increased incidence of stroke with increased LA size, but LA size is more important in blacks because of that population's increased stroke risk, Dr. Nagarajarao said in an interview. Blacks have a twofold higher incidence of stroke when compared with non-Hispanic whites, he added.
“Echocardiography may be a potentially useful noninvasive tool in identifying additional risk factors for stroke, and identifying participants with larger LA size may allow us to take preventive measures in identifying risk factors and treating them,” he said.
Last year, investigators at the University of Mississippi Medical Center also reported that echocardiographically derived left ventricular mass index (LVMI) was an independent predictor of incident ischemic stroke among 1,792 blacks in the Jackson cohort, after adjustment for similar cardiovascular risk factors (Stroke 2007;38:2686–91). In addition, the relation between LVMI and stroke remained significant after adding LA size and mitral annular calcification to the multivariable analysis.
Clinicians at the center determine LA size routinely on echocardiography in all patients at risk of stroke, Dr. Nagarajarao said. When asked which measurement is preferred, he said both LVMI and LA size have the potential to be independent predictors of risk factors, adding that it is important to recognize that each is independent of the other.
Previous studies have shown that the BP medication hydrochlorothiazide has reduced LA size when used along with controlling hypertension, although further study is needed to determine whether this has any effect on reducing stroke incidence, he said.
Using CAC Imaging to Track Tx Response and Rule Out Risk
SNOWMASS, COLO. — The most intriguing potential application for coronary artery calcium imaging is as a tool to track atherosclerosis progression over time in response to treatment, Dr. Matthew J. Budoff said at a conference sponsored by the Society for Cardiovascular Angiography and Interventions.
“I'm not suggesting that this is a current application, but the data now emerging are pretty interesting,” according to Dr. Budoff, director of cardiac CT at Harbor-UCLA Medical Center, Torrance, Calif.
He cited an observational study in which investigators tracked the change in coronary artery calcium (CAC) on serial electron-beam CT scans in 495 statin-treated asymptomatic patients. Forty-one subjects had an acute MI during up to 7 years of follow-up. The relative risk of an MI was increased 17-fold in those with at least a 15% per year rise in CAC score (Arterioscler. Thromb. Vasc. Biol.2004;24:1272–7).
“This might be a way, in the future, of monitoring therapy. You're on a statin, your LDL is pretty good, but your CAC is increasing—maybe we should do something more,” Dr. Budoff said at the conference cosponsored by the ACC.
He also described several current uses for CAC imaging:
▸ Screening asymptomatic patients with an intermediate Framingham risk score. Forty percent of asymptomatic adults fall into the Framingham intermediate-risk category, meaning they have an estimated 10%–20% risk of a coronary event within the next 10 years. Most acute MIs occur in this mid-risk group. Dr. Budoff was coauthor of a 2007 ACC/AHA Clinical Expert Consensus Statement that endorsed CAC measurement as a means of further stratifying Framingham intermediate-risk patients in order to identify a higher-risk subgroup in whom aggressive primary preventive measures are warranted (J. Am. Coll. Cardiol. 2007;49:378–402).
The Multi-Ethnic Study of Atherosclerosis (MESA), a National Institutes of Health-sponsored prospective study of 6,814 patients followed for 3.5 years now in press, was merely the most recent of several large studies showing that a CAC score of 100 or more was associated with a 10-fold increased risk of incident coronary heart disease.
And a prospective study sponsored by the NIH of more than 10,700 asymptomatic persons free of known coronary heart disease when they underwent CAC measurement showed that a baseline CAC of 97–409 was linked with an adjusted 9.7-fold greater risk of nonfatal MI or CHD death in the next 3.5 years, compared with subjects with a CAC of 0 (Am. J. Epidemiol. 2005;162:421–9).
“A CAC greater than 100 is more robust as a predictor of future events than Framingham risk factors … and more robust than C-reactive protein or carotid intimal-medial thickness,” observed Dr. Budoff, who is on the speakers bureau for General Electric.
▸ Identification of very-low-risk patients needing no further evaluation for coronary artery disease. Four studies totalling nearly 6,000 patients indicate a CAC of 0 has a 95%–99% negative predictive value for obstructive coronary disease. A fifth study, by Dr. Budoff and coinvestigators, concluded that a CAC score of 0 on an initial scan has at least a 5-year warranty before a repeat scan is appropriate because the likelihood of CAC progression during that first half-decade is so low (Int. J. Cardiol. 2007;117:227–31).
▸ A tool to improve compliance. In a study by Dr. Budoff's group, showing patients their CAC image was tied with 91% adherence to statin therapy over 3 years among those who scored in the top CAC quartile (Atherosclerosis 2006;185:394–9).
SNOWMASS, COLO. — The most intriguing potential application for coronary artery calcium imaging is as a tool to track atherosclerosis progression over time in response to treatment, Dr. Matthew J. Budoff said at a conference sponsored by the Society for Cardiovascular Angiography and Interventions.
“I'm not suggesting that this is a current application, but the data now emerging are pretty interesting,” according to Dr. Budoff, director of cardiac CT at Harbor-UCLA Medical Center, Torrance, Calif.
He cited an observational study in which investigators tracked the change in coronary artery calcium (CAC) on serial electron-beam CT scans in 495 statin-treated asymptomatic patients. Forty-one subjects had an acute MI during up to 7 years of follow-up. The relative risk of an MI was increased 17-fold in those with at least a 15% per year rise in CAC score (Arterioscler. Thromb. Vasc. Biol.2004;24:1272–7).
“This might be a way, in the future, of monitoring therapy. You're on a statin, your LDL is pretty good, but your CAC is increasing—maybe we should do something more,” Dr. Budoff said at the conference cosponsored by the ACC.
He also described several current uses for CAC imaging:
▸ Screening asymptomatic patients with an intermediate Framingham risk score. Forty percent of asymptomatic adults fall into the Framingham intermediate-risk category, meaning they have an estimated 10%–20% risk of a coronary event within the next 10 years. Most acute MIs occur in this mid-risk group. Dr. Budoff was coauthor of a 2007 ACC/AHA Clinical Expert Consensus Statement that endorsed CAC measurement as a means of further stratifying Framingham intermediate-risk patients in order to identify a higher-risk subgroup in whom aggressive primary preventive measures are warranted (J. Am. Coll. Cardiol. 2007;49:378–402).
The Multi-Ethnic Study of Atherosclerosis (MESA), a National Institutes of Health-sponsored prospective study of 6,814 patients followed for 3.5 years now in press, was merely the most recent of several large studies showing that a CAC score of 100 or more was associated with a 10-fold increased risk of incident coronary heart disease.
And a prospective study sponsored by the NIH of more than 10,700 asymptomatic persons free of known coronary heart disease when they underwent CAC measurement showed that a baseline CAC of 97–409 was linked with an adjusted 9.7-fold greater risk of nonfatal MI or CHD death in the next 3.5 years, compared with subjects with a CAC of 0 (Am. J. Epidemiol. 2005;162:421–9).
“A CAC greater than 100 is more robust as a predictor of future events than Framingham risk factors … and more robust than C-reactive protein or carotid intimal-medial thickness,” observed Dr. Budoff, who is on the speakers bureau for General Electric.
▸ Identification of very-low-risk patients needing no further evaluation for coronary artery disease. Four studies totalling nearly 6,000 patients indicate a CAC of 0 has a 95%–99% negative predictive value for obstructive coronary disease. A fifth study, by Dr. Budoff and coinvestigators, concluded that a CAC score of 0 on an initial scan has at least a 5-year warranty before a repeat scan is appropriate because the likelihood of CAC progression during that first half-decade is so low (Int. J. Cardiol. 2007;117:227–31).
▸ A tool to improve compliance. In a study by Dr. Budoff's group, showing patients their CAC image was tied with 91% adherence to statin therapy over 3 years among those who scored in the top CAC quartile (Atherosclerosis 2006;185:394–9).
SNOWMASS, COLO. — The most intriguing potential application for coronary artery calcium imaging is as a tool to track atherosclerosis progression over time in response to treatment, Dr. Matthew J. Budoff said at a conference sponsored by the Society for Cardiovascular Angiography and Interventions.
“I'm not suggesting that this is a current application, but the data now emerging are pretty interesting,” according to Dr. Budoff, director of cardiac CT at Harbor-UCLA Medical Center, Torrance, Calif.
He cited an observational study in which investigators tracked the change in coronary artery calcium (CAC) on serial electron-beam CT scans in 495 statin-treated asymptomatic patients. Forty-one subjects had an acute MI during up to 7 years of follow-up. The relative risk of an MI was increased 17-fold in those with at least a 15% per year rise in CAC score (Arterioscler. Thromb. Vasc. Biol.2004;24:1272–7).
“This might be a way, in the future, of monitoring therapy. You're on a statin, your LDL is pretty good, but your CAC is increasing—maybe we should do something more,” Dr. Budoff said at the conference cosponsored by the ACC.
He also described several current uses for CAC imaging:
▸ Screening asymptomatic patients with an intermediate Framingham risk score. Forty percent of asymptomatic adults fall into the Framingham intermediate-risk category, meaning they have an estimated 10%–20% risk of a coronary event within the next 10 years. Most acute MIs occur in this mid-risk group. Dr. Budoff was coauthor of a 2007 ACC/AHA Clinical Expert Consensus Statement that endorsed CAC measurement as a means of further stratifying Framingham intermediate-risk patients in order to identify a higher-risk subgroup in whom aggressive primary preventive measures are warranted (J. Am. Coll. Cardiol. 2007;49:378–402).
The Multi-Ethnic Study of Atherosclerosis (MESA), a National Institutes of Health-sponsored prospective study of 6,814 patients followed for 3.5 years now in press, was merely the most recent of several large studies showing that a CAC score of 100 or more was associated with a 10-fold increased risk of incident coronary heart disease.
And a prospective study sponsored by the NIH of more than 10,700 asymptomatic persons free of known coronary heart disease when they underwent CAC measurement showed that a baseline CAC of 97–409 was linked with an adjusted 9.7-fold greater risk of nonfatal MI or CHD death in the next 3.5 years, compared with subjects with a CAC of 0 (Am. J. Epidemiol. 2005;162:421–9).
“A CAC greater than 100 is more robust as a predictor of future events than Framingham risk factors … and more robust than C-reactive protein or carotid intimal-medial thickness,” observed Dr. Budoff, who is on the speakers bureau for General Electric.
▸ Identification of very-low-risk patients needing no further evaluation for coronary artery disease. Four studies totalling nearly 6,000 patients indicate a CAC of 0 has a 95%–99% negative predictive value for obstructive coronary disease. A fifth study, by Dr. Budoff and coinvestigators, concluded that a CAC score of 0 on an initial scan has at least a 5-year warranty before a repeat scan is appropriate because the likelihood of CAC progression during that first half-decade is so low (Int. J. Cardiol. 2007;117:227–31).
▸ A tool to improve compliance. In a study by Dr. Budoff's group, showing patients their CAC image was tied with 91% adherence to statin therapy over 3 years among those who scored in the top CAC quartile (Atherosclerosis 2006;185:394–9).