User login
3-D Mammography System Approved
Hologic’s Selenia Dimensions digital breast tomosynthesis system (Dimensions 3-D) is the first three-dimensional mammography system to reach the U.S. market following premarket approval by the Food and Drug Administration on Feb. 11. The low-dose x-ray device provides both 2-D and 3-D images of the breast for breast cancer screening and diagnosis.
Conventional 2-D mammography systems have limitations caused by overlapping tissue in the breast that may hide lesions or cause benign areas to appear suspicious, the company explained. Clinical trials of Dimensions 3-D showed significant gains in specificity and other benefits, including improved lesion and margin visibility and the ability to accurately localize structures in the breast, the firm noted.
The approval follows endorsement of the product’s safety and efficacy data by FDA’s Radiological Devices advisory panel last September.
This coverage is provided courtesy of "The Pink Sheet." "The Pink Sheet" and Internal Medicine News Digital Network are both owned by Elsevier.
Hologic’s Selenia Dimensions digital breast tomosynthesis system (Dimensions 3-D) is the first three-dimensional mammography system to reach the U.S. market following premarket approval by the Food and Drug Administration on Feb. 11. The low-dose x-ray device provides both 2-D and 3-D images of the breast for breast cancer screening and diagnosis.
Conventional 2-D mammography systems have limitations caused by overlapping tissue in the breast that may hide lesions or cause benign areas to appear suspicious, the company explained. Clinical trials of Dimensions 3-D showed significant gains in specificity and other benefits, including improved lesion and margin visibility and the ability to accurately localize structures in the breast, the firm noted.
The approval follows endorsement of the product’s safety and efficacy data by FDA’s Radiological Devices advisory panel last September.
This coverage is provided courtesy of "The Pink Sheet." "The Pink Sheet" and Internal Medicine News Digital Network are both owned by Elsevier.
Hologic’s Selenia Dimensions digital breast tomosynthesis system (Dimensions 3-D) is the first three-dimensional mammography system to reach the U.S. market following premarket approval by the Food and Drug Administration on Feb. 11. The low-dose x-ray device provides both 2-D and 3-D images of the breast for breast cancer screening and diagnosis.
Conventional 2-D mammography systems have limitations caused by overlapping tissue in the breast that may hide lesions or cause benign areas to appear suspicious, the company explained. Clinical trials of Dimensions 3-D showed significant gains in specificity and other benefits, including improved lesion and margin visibility and the ability to accurately localize structures in the breast, the firm noted.
The approval follows endorsement of the product’s safety and efficacy data by FDA’s Radiological Devices advisory panel last September.
This coverage is provided courtesy of "The Pink Sheet." "The Pink Sheet" and Internal Medicine News Digital Network are both owned by Elsevier.
Recurrent abdominal pain after laparoscopic cholecystectomy
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
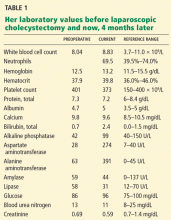
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
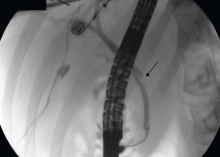
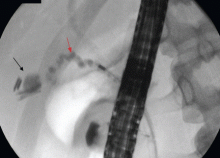
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
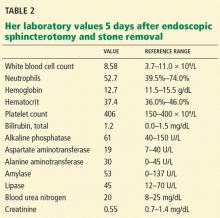
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
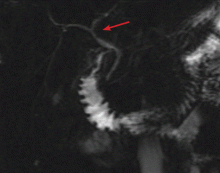
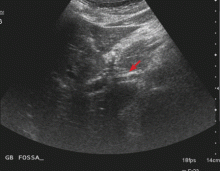
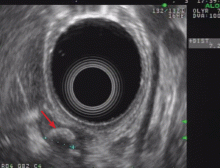
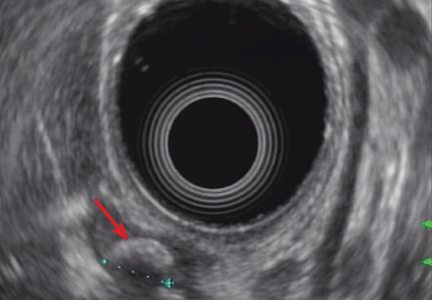
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
Cough and Wheezing
Abdominal Pain and Vomiting
Acute Disorders of the Joints and Bursae: Radiographic Clues to Diagnosis
Imaging Slightly Better at Identifying Early RA Progression
Two imaging modalities independently predicted progressive joint erosion in patients with early rheumatoid arthritis as a group, but the tests performed only slightly better than did clinical and demographic variables for individual prognoses, judging from findings of a 1-year study published in Annals of the Rheumatic Diseases.
Among 79 patients who completed quarterly follow-ups with a battery of imaging and nonimaging measures, 53 (67%) showed erosive progression. On a group level, results of ultrasound grey-scale (USGS) findings of inflammation and magnetic resonance images showing bone marrow edema each were significant predictors that erosive disease progression would be detected by MRI.
Patients with USGS inflammation in the dominant wrist were twice as likely to develop erosive progression and patients with MRI bone marrow edema in the dominant wrist were 28% more likely to develop erosive progression compared with patients without these imaging findings, Dr. Pernille Bøyesen and associates reported (Ann. Rheum. Dis. 2011;70:176-9 [doi: 10.1136/ard.2009.126953]).
On an individual level, however, the imaging modalities were not dramatically better than clinical and demographic variables to predict erosive progression of early RA. USGS inflammation, synovitis on MRI, and bone marrow edema that was visible on MRI performed slightly better than using antibody to cyclic citrullinated protein, rheumatoid factor, and disease activity score based on a 28-joint count, reported Dr. Bøyesen of Diakonhjemmet Hospital, Oslo.
USGS inflammation was the best of 12 imaging modalities and measures of disease severity in identifying patients at risk of developing erosions on MRI, with a sensitivity of 78%, a specificity of 55%, a positive likelihood ration of 1.75, and accuracy of 70%.
Future studies are needed using composite indices of disease progression, including modern imaging modalities, to determine their value as predictors of an individual patient’s likelihood of disease progression, the investigators concluded.
The study appears to be the first to confirm previous data suggesting that measuring inflammation by ultrasound can help predict subsequent joint damage, they noted. The findings also confirmed previous data identifying bone marrow edema on MRI as an independent predictor of joint damage.
Other imaging modalities in the study included digital x-ray radiogrammetry (DXR) of cortical bone mineral density in the hand. Results showed only trends toward higher levels of synovitis on MRI and bone density loss on DXR in patients with erosive progression of disease at 1 year. The findings did not support previous studies that reported cortical hand bone mineral density to be independently predictive of erosive progression, perhaps due to the small size of the study, Dr. Bøyesen added.
Given the comprehensive comparison of imaging modalities in the study, however, 84 patients can be considered a large number, the investigators noted.
The multivariate analyses controlled for the effects of age, sex, and other independent variables.
The investigators declared having no conflicts of interest. The study was funded by the Eastern Norway Regional Health Authority, the Research Council of Norway, the Norwegian Rheumatism Association, the Norwegian Women Public Health Association, the Grethe Harbitz Legacy, and the Marie and Else Mustad Legacy.
Two imaging modalities independently predicted progressive joint erosion in patients with early rheumatoid arthritis as a group, but the tests performed only slightly better than did clinical and demographic variables for individual prognoses, judging from findings of a 1-year study published in Annals of the Rheumatic Diseases.
Among 79 patients who completed quarterly follow-ups with a battery of imaging and nonimaging measures, 53 (67%) showed erosive progression. On a group level, results of ultrasound grey-scale (USGS) findings of inflammation and magnetic resonance images showing bone marrow edema each were significant predictors that erosive disease progression would be detected by MRI.
Patients with USGS inflammation in the dominant wrist were twice as likely to develop erosive progression and patients with MRI bone marrow edema in the dominant wrist were 28% more likely to develop erosive progression compared with patients without these imaging findings, Dr. Pernille Bøyesen and associates reported (Ann. Rheum. Dis. 2011;70:176-9 [doi: 10.1136/ard.2009.126953]).
On an individual level, however, the imaging modalities were not dramatically better than clinical and demographic variables to predict erosive progression of early RA. USGS inflammation, synovitis on MRI, and bone marrow edema that was visible on MRI performed slightly better than using antibody to cyclic citrullinated protein, rheumatoid factor, and disease activity score based on a 28-joint count, reported Dr. Bøyesen of Diakonhjemmet Hospital, Oslo.
USGS inflammation was the best of 12 imaging modalities and measures of disease severity in identifying patients at risk of developing erosions on MRI, with a sensitivity of 78%, a specificity of 55%, a positive likelihood ration of 1.75, and accuracy of 70%.
Future studies are needed using composite indices of disease progression, including modern imaging modalities, to determine their value as predictors of an individual patient’s likelihood of disease progression, the investigators concluded.
The study appears to be the first to confirm previous data suggesting that measuring inflammation by ultrasound can help predict subsequent joint damage, they noted. The findings also confirmed previous data identifying bone marrow edema on MRI as an independent predictor of joint damage.
Other imaging modalities in the study included digital x-ray radiogrammetry (DXR) of cortical bone mineral density in the hand. Results showed only trends toward higher levels of synovitis on MRI and bone density loss on DXR in patients with erosive progression of disease at 1 year. The findings did not support previous studies that reported cortical hand bone mineral density to be independently predictive of erosive progression, perhaps due to the small size of the study, Dr. Bøyesen added.
Given the comprehensive comparison of imaging modalities in the study, however, 84 patients can be considered a large number, the investigators noted.
The multivariate analyses controlled for the effects of age, sex, and other independent variables.
The investigators declared having no conflicts of interest. The study was funded by the Eastern Norway Regional Health Authority, the Research Council of Norway, the Norwegian Rheumatism Association, the Norwegian Women Public Health Association, the Grethe Harbitz Legacy, and the Marie and Else Mustad Legacy.
Two imaging modalities independently predicted progressive joint erosion in patients with early rheumatoid arthritis as a group, but the tests performed only slightly better than did clinical and demographic variables for individual prognoses, judging from findings of a 1-year study published in Annals of the Rheumatic Diseases.
Among 79 patients who completed quarterly follow-ups with a battery of imaging and nonimaging measures, 53 (67%) showed erosive progression. On a group level, results of ultrasound grey-scale (USGS) findings of inflammation and magnetic resonance images showing bone marrow edema each were significant predictors that erosive disease progression would be detected by MRI.
Patients with USGS inflammation in the dominant wrist were twice as likely to develop erosive progression and patients with MRI bone marrow edema in the dominant wrist were 28% more likely to develop erosive progression compared with patients without these imaging findings, Dr. Pernille Bøyesen and associates reported (Ann. Rheum. Dis. 2011;70:176-9 [doi: 10.1136/ard.2009.126953]).
On an individual level, however, the imaging modalities were not dramatically better than clinical and demographic variables to predict erosive progression of early RA. USGS inflammation, synovitis on MRI, and bone marrow edema that was visible on MRI performed slightly better than using antibody to cyclic citrullinated protein, rheumatoid factor, and disease activity score based on a 28-joint count, reported Dr. Bøyesen of Diakonhjemmet Hospital, Oslo.
USGS inflammation was the best of 12 imaging modalities and measures of disease severity in identifying patients at risk of developing erosions on MRI, with a sensitivity of 78%, a specificity of 55%, a positive likelihood ration of 1.75, and accuracy of 70%.
Future studies are needed using composite indices of disease progression, including modern imaging modalities, to determine their value as predictors of an individual patient’s likelihood of disease progression, the investigators concluded.
The study appears to be the first to confirm previous data suggesting that measuring inflammation by ultrasound can help predict subsequent joint damage, they noted. The findings also confirmed previous data identifying bone marrow edema on MRI as an independent predictor of joint damage.
Other imaging modalities in the study included digital x-ray radiogrammetry (DXR) of cortical bone mineral density in the hand. Results showed only trends toward higher levels of synovitis on MRI and bone density loss on DXR in patients with erosive progression of disease at 1 year. The findings did not support previous studies that reported cortical hand bone mineral density to be independently predictive of erosive progression, perhaps due to the small size of the study, Dr. Bøyesen added.
Given the comprehensive comparison of imaging modalities in the study, however, 84 patients can be considered a large number, the investigators noted.
The multivariate analyses controlled for the effects of age, sex, and other independent variables.
The investigators declared having no conflicts of interest. The study was funded by the Eastern Norway Regional Health Authority, the Research Council of Norway, the Norwegian Rheumatism Association, the Norwegian Women Public Health Association, the Grethe Harbitz Legacy, and the Marie and Else Mustad Legacy.
FROM ANNALS OF THE RHEUMATIC DISEASES
Myocardial Perfusion Imaging Often Brings High Radiation Exposure
CHICAGO — Repeated myocardial perfusion imaging is common and associated with high cumulative radiation doses that are well within the range believed to increase cancer risk, according to a large, single-center study.
This retrospective study of 1,097 consecutive patients who underwent MPI at Columbia University Medical Center, New York, in the first 100 days of 2006 showed that 39% of them received more than one MPI during the study period running from 1988 through June 2008; 18% had at least three MPIs, and 5% had five or more, Dr. Andrew J. Einstein reported at the annual scientific sessions of the American Heart Association.
Among patients with multiple MPIs, the median time between the imaging studies was just under 2 years. However, 56% of patients with multiple MPIs had two within 2 years, and 28% had two within 1 year.
Patients with more than one MPI had a median 121-mSv cumulative estimated effective radiation dose from all medical sources. That’s more than in Japanese atomic bomb survivors, as documented in the landmark Life Span Study. By comparison, 1 year’s background radiation exposure is about 3 mSv, noted Dr. Einstein, a cardiologist at Columbia.
The radiation burden accruing from CT scans has drawn much attention in recent years, but in fact MPI entails the highest radiation exposure of all imaging procedures. Moreover, MPI is booming in popularity: The volume in the United States rose from fewer than 3 million of the imaging procedures in 1990 to 9.3 million in 2002.
In the Columbia University series, men, whites, and patients with health insurance had significantly greater likelihood of undergoing multiple MPIs, compared with women, nonwhites, and the uninsured. They also received higher cumulative radiation doses over the 20-year study period. But whether this increased utilization resulted in improved cardiovascular outcomes requires further study.
The great majority of MPIs ordered in the Columbia study were medically justified as an aid to therapeutic decision making, given that more than 80% of initial MPIs and 90% of repeat procedures were performed in patients with known cardiac disease or symptoms consistent with it. But in ordering these imaging studies, physicians often don’t consider that patients with heart disease undergo numerous additional procedures involving radiation exposure, including cardiac catheterizations. Indeed, the 1,097 patients in this study had a median of 15 procedures involving radiation exposure, including 4 high-dose procedures, Dr. Einstein noted.
Alternative tests without radiation exposure include stress MRI, stress echocardiography, and exercise ECG. Lower radiation exposure alternatives to MPI for use in ruling out cardiac causes of atypical symptoms are CT angiography and percutaneous angiography, he said.
In addition to utilizing tests other than MPI when appropriate, another means of reducing cumulative radiation doses is to avoid the dual-isotope MPI imaging protocol, which typically entails more than twice as great a radiation dose than does technetium-99m MPI, the cardiologist added.
Although the high cumulative radiation doses documented in the Columbia study are "certainly a matter of concern and an important target for improvement," in Dr. Einstein’s view it is worth bearing in mind that solid tumors generally don’t develop until at least 5-10 years following radiation exposure. Patients undergoing MPI are typically older than the general population, and they have a shorter-than-average life expectancy for their age because of their cardiac disease. So the risk:benefit ratio of radiation exposure from MPIs isn’t the same as a similar exposure would be in healthy young adults.
The study was published simultaneously with his presentation at the American Heart Association meeting (JAMA 2010;304:2137-44).
Dr. Einstein’s study was supported by the National Institutes of Health and university research grants. He declared having served as a consultant to the International Atomic Energy Agency as well as GE Healthcare.
CHICAGO — Repeated myocardial perfusion imaging is common and associated with high cumulative radiation doses that are well within the range believed to increase cancer risk, according to a large, single-center study.
This retrospective study of 1,097 consecutive patients who underwent MPI at Columbia University Medical Center, New York, in the first 100 days of 2006 showed that 39% of them received more than one MPI during the study period running from 1988 through June 2008; 18% had at least three MPIs, and 5% had five or more, Dr. Andrew J. Einstein reported at the annual scientific sessions of the American Heart Association.
Among patients with multiple MPIs, the median time between the imaging studies was just under 2 years. However, 56% of patients with multiple MPIs had two within 2 years, and 28% had two within 1 year.
Patients with more than one MPI had a median 121-mSv cumulative estimated effective radiation dose from all medical sources. That’s more than in Japanese atomic bomb survivors, as documented in the landmark Life Span Study. By comparison, 1 year’s background radiation exposure is about 3 mSv, noted Dr. Einstein, a cardiologist at Columbia.
The radiation burden accruing from CT scans has drawn much attention in recent years, but in fact MPI entails the highest radiation exposure of all imaging procedures. Moreover, MPI is booming in popularity: The volume in the United States rose from fewer than 3 million of the imaging procedures in 1990 to 9.3 million in 2002.
In the Columbia University series, men, whites, and patients with health insurance had significantly greater likelihood of undergoing multiple MPIs, compared with women, nonwhites, and the uninsured. They also received higher cumulative radiation doses over the 20-year study period. But whether this increased utilization resulted in improved cardiovascular outcomes requires further study.
The great majority of MPIs ordered in the Columbia study were medically justified as an aid to therapeutic decision making, given that more than 80% of initial MPIs and 90% of repeat procedures were performed in patients with known cardiac disease or symptoms consistent with it. But in ordering these imaging studies, physicians often don’t consider that patients with heart disease undergo numerous additional procedures involving radiation exposure, including cardiac catheterizations. Indeed, the 1,097 patients in this study had a median of 15 procedures involving radiation exposure, including 4 high-dose procedures, Dr. Einstein noted.
Alternative tests without radiation exposure include stress MRI, stress echocardiography, and exercise ECG. Lower radiation exposure alternatives to MPI for use in ruling out cardiac causes of atypical symptoms are CT angiography and percutaneous angiography, he said.
In addition to utilizing tests other than MPI when appropriate, another means of reducing cumulative radiation doses is to avoid the dual-isotope MPI imaging protocol, which typically entails more than twice as great a radiation dose than does technetium-99m MPI, the cardiologist added.
Although the high cumulative radiation doses documented in the Columbia study are "certainly a matter of concern and an important target for improvement," in Dr. Einstein’s view it is worth bearing in mind that solid tumors generally don’t develop until at least 5-10 years following radiation exposure. Patients undergoing MPI are typically older than the general population, and they have a shorter-than-average life expectancy for their age because of their cardiac disease. So the risk:benefit ratio of radiation exposure from MPIs isn’t the same as a similar exposure would be in healthy young adults.
The study was published simultaneously with his presentation at the American Heart Association meeting (JAMA 2010;304:2137-44).
Dr. Einstein’s study was supported by the National Institutes of Health and university research grants. He declared having served as a consultant to the International Atomic Energy Agency as well as GE Healthcare.
CHICAGO — Repeated myocardial perfusion imaging is common and associated with high cumulative radiation doses that are well within the range believed to increase cancer risk, according to a large, single-center study.
This retrospective study of 1,097 consecutive patients who underwent MPI at Columbia University Medical Center, New York, in the first 100 days of 2006 showed that 39% of them received more than one MPI during the study period running from 1988 through June 2008; 18% had at least three MPIs, and 5% had five or more, Dr. Andrew J. Einstein reported at the annual scientific sessions of the American Heart Association.
Among patients with multiple MPIs, the median time between the imaging studies was just under 2 years. However, 56% of patients with multiple MPIs had two within 2 years, and 28% had two within 1 year.
Patients with more than one MPI had a median 121-mSv cumulative estimated effective radiation dose from all medical sources. That’s more than in Japanese atomic bomb survivors, as documented in the landmark Life Span Study. By comparison, 1 year’s background radiation exposure is about 3 mSv, noted Dr. Einstein, a cardiologist at Columbia.
The radiation burden accruing from CT scans has drawn much attention in recent years, but in fact MPI entails the highest radiation exposure of all imaging procedures. Moreover, MPI is booming in popularity: The volume in the United States rose from fewer than 3 million of the imaging procedures in 1990 to 9.3 million in 2002.
In the Columbia University series, men, whites, and patients with health insurance had significantly greater likelihood of undergoing multiple MPIs, compared with women, nonwhites, and the uninsured. They also received higher cumulative radiation doses over the 20-year study period. But whether this increased utilization resulted in improved cardiovascular outcomes requires further study.
The great majority of MPIs ordered in the Columbia study were medically justified as an aid to therapeutic decision making, given that more than 80% of initial MPIs and 90% of repeat procedures were performed in patients with known cardiac disease or symptoms consistent with it. But in ordering these imaging studies, physicians often don’t consider that patients with heart disease undergo numerous additional procedures involving radiation exposure, including cardiac catheterizations. Indeed, the 1,097 patients in this study had a median of 15 procedures involving radiation exposure, including 4 high-dose procedures, Dr. Einstein noted.
Alternative tests without radiation exposure include stress MRI, stress echocardiography, and exercise ECG. Lower radiation exposure alternatives to MPI for use in ruling out cardiac causes of atypical symptoms are CT angiography and percutaneous angiography, he said.
In addition to utilizing tests other than MPI when appropriate, another means of reducing cumulative radiation doses is to avoid the dual-isotope MPI imaging protocol, which typically entails more than twice as great a radiation dose than does technetium-99m MPI, the cardiologist added.
Although the high cumulative radiation doses documented in the Columbia study are "certainly a matter of concern and an important target for improvement," in Dr. Einstein’s view it is worth bearing in mind that solid tumors generally don’t develop until at least 5-10 years following radiation exposure. Patients undergoing MPI are typically older than the general population, and they have a shorter-than-average life expectancy for their age because of their cardiac disease. So the risk:benefit ratio of radiation exposure from MPIs isn’t the same as a similar exposure would be in healthy young adults.
The study was published simultaneously with his presentation at the American Heart Association meeting (JAMA 2010;304:2137-44).
Dr. Einstein’s study was supported by the National Institutes of Health and university research grants. He declared having served as a consultant to the International Atomic Energy Agency as well as GE Healthcare.
Scan Neonatal Brain Early to Optimize Results
LONDON – Magnetic resonance imaging should be used as early as possible to scan the neonatal brain if there are any unexplained symptoms, or if hemorrhagic or thrombotic lesions are suspected.
In the case of thrombosis, it’s important to scan early to decide whether anticoagulation is required, neonatal imaging expert Dr. Mary A. Rutherford of Imperial College London said at the Excellence in Paediatrics annual meeting.
However, Dr. Rutherford, who trained as a pediatrician, noted that for acquired injuries, the use of conventional MRI should be delayed 1-4 weeks, as hypoxic-ischemic brain injury is easier to detect 7 days after the insult.
MRI can be used for a variety of diagnostic and prognostic reasons in full-term neonates; it can help to establish the cause of symptoms, identify brain abnormalities, and determine the likely timing of any injury to the developing brain, she said. Neonatal MRI can also help predict outcomes, provide information for risk management and, in these days of increased medicolegal vigilance, perhaps help clinicians avoid the threat of litigation.
Today, most clinical departments will have an MRI scanner that uses field strengths of 1.5-3 Tesla, which are fine for neonatal imaging, Dr. Rutherford said. "What you really need," she noted, "is an interested radiologist or radiographer." Myelination occurs during the first 2 years of life, so an experienced pediatric or radiology image interpreter is also required.
As access to MRI scanners may be limited, it’s important to be ready as soon as a scanner is free, Dr. Rutherford advised. This means that the neonate must be suitably prepared prior to the scan. Choral hydrate, given 15 minutes before the scan, may be used to keep the neonate sedated temporarily, and ear protection must be worn. The neonate’s head and body should then be "wrapped and fixed" as carefully as possibly into position inside the scanner, so there is little chance of any waking or movement during the scan.
Ventilation may be needed during the scan, and a pediatric staff member needs to be with the baby at all times. Pulse oximetry can be used to monitor the neonate while the scan is in progress.
"MRI is an expensive technology, and you really want to make sure that you get the best possible results from all the effort that is put in by trying to put a baby into an MRI scanner," Dr. Rutherford said.
A key element to successful scanning is ensuring that the MRI coil used fits as closely as possibly to the neonate’s head. "Poor coil choice or head positioning results in poor image quality," she warned.
Motion is another common reason for poor quality images, so motion-resistant sequences need to be considered. T1-weighted (transverse/sagittal), T2-weighted (transverse), and diffusion-weighted imaging may all be appropriate sequences to use. Magnetic resonance venography and angiography can provide useful information about thrombosis or stroke, respectively.
"In this day and age, all infants with a neonatal encephalopathy require – and deserve to have – the benefit of an MR scan," Dr. Rutherford said. Other candidates for MRI include neonates with seizures, severe jaundice, abnormal cranial ultrasound findings, unexplained neurologic signs, dysmorphic features, and where a co-twin has died in utero.
Neonates with unexplained symptoms or who are at risk of hemorrhagic/thrombotic lesions require prompt MRI, as soon as access to a scanner can be arranged.
"You will also want to image fairly quickly in cases of severe neonatal encephalopathy, especially if you are considering withdrawing active treatment," Dr. Rutherford said.
By contrast, for acquired injuries, she noted, "If we want to get the best possible detection of any acquired injury, then we should think about imaging probably between 1 and 4 weeks post insult." Diffusion-weighted imaging is best for identifying early ischemic lesions.
With regards to preterm neonates, routine MRI scanning is not recommended, but early scanning is perhaps advisable if there are unexplained symptoms or withdrawal of care is being considered.
Dr. Rutherford is the editor of "MRI of the Neonatal Brain," (Philadelphia: Saunders, Ltd., 2001). More information: MRI of the Neonatal Brain.
LONDON – Magnetic resonance imaging should be used as early as possible to scan the neonatal brain if there are any unexplained symptoms, or if hemorrhagic or thrombotic lesions are suspected.
In the case of thrombosis, it’s important to scan early to decide whether anticoagulation is required, neonatal imaging expert Dr. Mary A. Rutherford of Imperial College London said at the Excellence in Paediatrics annual meeting.
However, Dr. Rutherford, who trained as a pediatrician, noted that for acquired injuries, the use of conventional MRI should be delayed 1-4 weeks, as hypoxic-ischemic brain injury is easier to detect 7 days after the insult.
MRI can be used for a variety of diagnostic and prognostic reasons in full-term neonates; it can help to establish the cause of symptoms, identify brain abnormalities, and determine the likely timing of any injury to the developing brain, she said. Neonatal MRI can also help predict outcomes, provide information for risk management and, in these days of increased medicolegal vigilance, perhaps help clinicians avoid the threat of litigation.
Today, most clinical departments will have an MRI scanner that uses field strengths of 1.5-3 Tesla, which are fine for neonatal imaging, Dr. Rutherford said. "What you really need," she noted, "is an interested radiologist or radiographer." Myelination occurs during the first 2 years of life, so an experienced pediatric or radiology image interpreter is also required.
As access to MRI scanners may be limited, it’s important to be ready as soon as a scanner is free, Dr. Rutherford advised. This means that the neonate must be suitably prepared prior to the scan. Choral hydrate, given 15 minutes before the scan, may be used to keep the neonate sedated temporarily, and ear protection must be worn. The neonate’s head and body should then be "wrapped and fixed" as carefully as possibly into position inside the scanner, so there is little chance of any waking or movement during the scan.
Ventilation may be needed during the scan, and a pediatric staff member needs to be with the baby at all times. Pulse oximetry can be used to monitor the neonate while the scan is in progress.
"MRI is an expensive technology, and you really want to make sure that you get the best possible results from all the effort that is put in by trying to put a baby into an MRI scanner," Dr. Rutherford said.
A key element to successful scanning is ensuring that the MRI coil used fits as closely as possibly to the neonate’s head. "Poor coil choice or head positioning results in poor image quality," she warned.
Motion is another common reason for poor quality images, so motion-resistant sequences need to be considered. T1-weighted (transverse/sagittal), T2-weighted (transverse), and diffusion-weighted imaging may all be appropriate sequences to use. Magnetic resonance venography and angiography can provide useful information about thrombosis or stroke, respectively.
"In this day and age, all infants with a neonatal encephalopathy require – and deserve to have – the benefit of an MR scan," Dr. Rutherford said. Other candidates for MRI include neonates with seizures, severe jaundice, abnormal cranial ultrasound findings, unexplained neurologic signs, dysmorphic features, and where a co-twin has died in utero.
Neonates with unexplained symptoms or who are at risk of hemorrhagic/thrombotic lesions require prompt MRI, as soon as access to a scanner can be arranged.
"You will also want to image fairly quickly in cases of severe neonatal encephalopathy, especially if you are considering withdrawing active treatment," Dr. Rutherford said.
By contrast, for acquired injuries, she noted, "If we want to get the best possible detection of any acquired injury, then we should think about imaging probably between 1 and 4 weeks post insult." Diffusion-weighted imaging is best for identifying early ischemic lesions.
With regards to preterm neonates, routine MRI scanning is not recommended, but early scanning is perhaps advisable if there are unexplained symptoms or withdrawal of care is being considered.
Dr. Rutherford is the editor of "MRI of the Neonatal Brain," (Philadelphia: Saunders, Ltd., 2001). More information: MRI of the Neonatal Brain.
LONDON – Magnetic resonance imaging should be used as early as possible to scan the neonatal brain if there are any unexplained symptoms, or if hemorrhagic or thrombotic lesions are suspected.
In the case of thrombosis, it’s important to scan early to decide whether anticoagulation is required, neonatal imaging expert Dr. Mary A. Rutherford of Imperial College London said at the Excellence in Paediatrics annual meeting.
However, Dr. Rutherford, who trained as a pediatrician, noted that for acquired injuries, the use of conventional MRI should be delayed 1-4 weeks, as hypoxic-ischemic brain injury is easier to detect 7 days after the insult.
MRI can be used for a variety of diagnostic and prognostic reasons in full-term neonates; it can help to establish the cause of symptoms, identify brain abnormalities, and determine the likely timing of any injury to the developing brain, she said. Neonatal MRI can also help predict outcomes, provide information for risk management and, in these days of increased medicolegal vigilance, perhaps help clinicians avoid the threat of litigation.
Today, most clinical departments will have an MRI scanner that uses field strengths of 1.5-3 Tesla, which are fine for neonatal imaging, Dr. Rutherford said. "What you really need," she noted, "is an interested radiologist or radiographer." Myelination occurs during the first 2 years of life, so an experienced pediatric or radiology image interpreter is also required.
As access to MRI scanners may be limited, it’s important to be ready as soon as a scanner is free, Dr. Rutherford advised. This means that the neonate must be suitably prepared prior to the scan. Choral hydrate, given 15 minutes before the scan, may be used to keep the neonate sedated temporarily, and ear protection must be worn. The neonate’s head and body should then be "wrapped and fixed" as carefully as possibly into position inside the scanner, so there is little chance of any waking or movement during the scan.
Ventilation may be needed during the scan, and a pediatric staff member needs to be with the baby at all times. Pulse oximetry can be used to monitor the neonate while the scan is in progress.
"MRI is an expensive technology, and you really want to make sure that you get the best possible results from all the effort that is put in by trying to put a baby into an MRI scanner," Dr. Rutherford said.
A key element to successful scanning is ensuring that the MRI coil used fits as closely as possibly to the neonate’s head. "Poor coil choice or head positioning results in poor image quality," she warned.
Motion is another common reason for poor quality images, so motion-resistant sequences need to be considered. T1-weighted (transverse/sagittal), T2-weighted (transverse), and diffusion-weighted imaging may all be appropriate sequences to use. Magnetic resonance venography and angiography can provide useful information about thrombosis or stroke, respectively.
"In this day and age, all infants with a neonatal encephalopathy require – and deserve to have – the benefit of an MR scan," Dr. Rutherford said. Other candidates for MRI include neonates with seizures, severe jaundice, abnormal cranial ultrasound findings, unexplained neurologic signs, dysmorphic features, and where a co-twin has died in utero.
Neonates with unexplained symptoms or who are at risk of hemorrhagic/thrombotic lesions require prompt MRI, as soon as access to a scanner can be arranged.
"You will also want to image fairly quickly in cases of severe neonatal encephalopathy, especially if you are considering withdrawing active treatment," Dr. Rutherford said.
By contrast, for acquired injuries, she noted, "If we want to get the best possible detection of any acquired injury, then we should think about imaging probably between 1 and 4 weeks post insult." Diffusion-weighted imaging is best for identifying early ischemic lesions.
With regards to preterm neonates, routine MRI scanning is not recommended, but early scanning is perhaps advisable if there are unexplained symptoms or withdrawal of care is being considered.
Dr. Rutherford is the editor of "MRI of the Neonatal Brain," (Philadelphia: Saunders, Ltd., 2001). More information: MRI of the Neonatal Brain.
EXPERT ANALYSIS FROM THE EXCELLENCE IN PAEDIATRICS ANNUAL MEETING
Shortness of Breath
Meta-Analysis Reveals CCTA's Prognostic Value
For symptomatic patients who undergo cardiac computed tomography angiography to evaluate suspected coronary artery disease, a finding of no CAD conveys an excellent prognosis, according to a meta-analysis of 18 studies of diagnostic cardiac computed tomography angiography (CCTA).
The meta-analysis clearly showed that the low (0.16%) annualized event rate that follows such negative test results is comparable to the background event rate among healthy, low-risk individuals in the general population. It is also comparable to the event rates observed after risk-stratification modalities such as stress echocardiography and myocardial perfusion scanning, said Dr. Edward A. Hulten of the cardiology service at Walter Reed Army Medical Center, Washington, and his associates.
The diagnostic accuracy of CCTA has been reported in more than 50 studies, but the technology's prognostic value has been less well established, the investigators observed. This meta-analysis shows that “the concept that CCTA offers anatomic but not prognostic value compared with widely used functional stress testing is no longer accurate,” they wrote.
Dr. Hulten and his colleagues performed a meta-analysis of prospective and retrospective observational studies in which 9,592 patients suspected of having CAD were evaluated using CCTA and followed for a median of 20 months. Seventeen of these studies were rated as good quality.
For the patients whose CCTA results indicated no CAD, the annualized rate of major adverse cardiovascular events (MACE) was 0.16%. There were no coronary revascularizations, myocardial infarctions, or admissions for unstable angina; the only events were from all-cause mortality.
“Considered in concert with the wealth of data regarding the high anatomic accuracy for CCTA, these results are convincing for CCTA to effectively diagnose CAD and convey risk strata for future adverse cardiovascular events,” the researchers said (J. Am. Coll. Cardiol. 2010;57 [doi:10.1016/jacc.2010.10.011]).
Positive results on CCTA correlated with major adverse cardiovascular events. The average annualized MACE rate for a finding of CAD was 8.8% per year (vs. 0.17% per year for negative findings). Revascularization procedures accounted for most of these events; the annualized rate of death or MI was 3.2% vs. 0.15 for positive vs. negative scans. Moreover, the rate of adverse events increased as the severity of coronary artery disease on CCTA exam increased.
Although a negative CCTA scan can be considered strongly predictive of an excellent outcome, the reverse is not true: A positive CCTA scan cannot be considered strongly predictive of future adverse events. With the annualized MACE rate at only 8.8%, the great majority of patients found on CCTA to have coronary disease also have good outcomes in the 20 months' follow-up, Dr. Hulten and his associates pointed out.
They also noted that many of the authors of studies included in the meta-analysis “reported prognosis based on a relatively simple classification of CAD luminal stenosis, specifically, no CAD, nonobstructive CAD, or potentially obstructive CAD (greater than 50% stenosis).” With studies defining “normal” CCTA in slightly different ways, it was not possible to have stratification details that would be clinically informative, the authors wrote. They also noted that their research included data from “different generations of CT scanning technology” the newer of which are known to have improved image quality and accuracy.
As in many other many studies of noninvasive coronary risk stratification tests, the investigators wrote, a limitation of their study is verification bias: “The observed increase in MACE is driven in part by coronary revascularization, demonstrating evidence of a work-up or verification bias. That is, patients with CCTA evidence of a greater than 50% stenosis are more likely to undergo catheterization and subsequent revascularization.”
For symptomatic patients who undergo cardiac computed tomography angiography to evaluate suspected coronary artery disease, a finding of no CAD conveys an excellent prognosis, according to a meta-analysis of 18 studies of diagnostic cardiac computed tomography angiography (CCTA).
The meta-analysis clearly showed that the low (0.16%) annualized event rate that follows such negative test results is comparable to the background event rate among healthy, low-risk individuals in the general population. It is also comparable to the event rates observed after risk-stratification modalities such as stress echocardiography and myocardial perfusion scanning, said Dr. Edward A. Hulten of the cardiology service at Walter Reed Army Medical Center, Washington, and his associates.
The diagnostic accuracy of CCTA has been reported in more than 50 studies, but the technology's prognostic value has been less well established, the investigators observed. This meta-analysis shows that “the concept that CCTA offers anatomic but not prognostic value compared with widely used functional stress testing is no longer accurate,” they wrote.
Dr. Hulten and his colleagues performed a meta-analysis of prospective and retrospective observational studies in which 9,592 patients suspected of having CAD were evaluated using CCTA and followed for a median of 20 months. Seventeen of these studies were rated as good quality.
For the patients whose CCTA results indicated no CAD, the annualized rate of major adverse cardiovascular events (MACE) was 0.16%. There were no coronary revascularizations, myocardial infarctions, or admissions for unstable angina; the only events were from all-cause mortality.
“Considered in concert with the wealth of data regarding the high anatomic accuracy for CCTA, these results are convincing for CCTA to effectively diagnose CAD and convey risk strata for future adverse cardiovascular events,” the researchers said (J. Am. Coll. Cardiol. 2010;57 [doi:10.1016/jacc.2010.10.011]).
Positive results on CCTA correlated with major adverse cardiovascular events. The average annualized MACE rate for a finding of CAD was 8.8% per year (vs. 0.17% per year for negative findings). Revascularization procedures accounted for most of these events; the annualized rate of death or MI was 3.2% vs. 0.15 for positive vs. negative scans. Moreover, the rate of adverse events increased as the severity of coronary artery disease on CCTA exam increased.
Although a negative CCTA scan can be considered strongly predictive of an excellent outcome, the reverse is not true: A positive CCTA scan cannot be considered strongly predictive of future adverse events. With the annualized MACE rate at only 8.8%, the great majority of patients found on CCTA to have coronary disease also have good outcomes in the 20 months' follow-up, Dr. Hulten and his associates pointed out.
They also noted that many of the authors of studies included in the meta-analysis “reported prognosis based on a relatively simple classification of CAD luminal stenosis, specifically, no CAD, nonobstructive CAD, or potentially obstructive CAD (greater than 50% stenosis).” With studies defining “normal” CCTA in slightly different ways, it was not possible to have stratification details that would be clinically informative, the authors wrote. They also noted that their research included data from “different generations of CT scanning technology” the newer of which are known to have improved image quality and accuracy.
As in many other many studies of noninvasive coronary risk stratification tests, the investigators wrote, a limitation of their study is verification bias: “The observed increase in MACE is driven in part by coronary revascularization, demonstrating evidence of a work-up or verification bias. That is, patients with CCTA evidence of a greater than 50% stenosis are more likely to undergo catheterization and subsequent revascularization.”
For symptomatic patients who undergo cardiac computed tomography angiography to evaluate suspected coronary artery disease, a finding of no CAD conveys an excellent prognosis, according to a meta-analysis of 18 studies of diagnostic cardiac computed tomography angiography (CCTA).
The meta-analysis clearly showed that the low (0.16%) annualized event rate that follows such negative test results is comparable to the background event rate among healthy, low-risk individuals in the general population. It is also comparable to the event rates observed after risk-stratification modalities such as stress echocardiography and myocardial perfusion scanning, said Dr. Edward A. Hulten of the cardiology service at Walter Reed Army Medical Center, Washington, and his associates.
The diagnostic accuracy of CCTA has been reported in more than 50 studies, but the technology's prognostic value has been less well established, the investigators observed. This meta-analysis shows that “the concept that CCTA offers anatomic but not prognostic value compared with widely used functional stress testing is no longer accurate,” they wrote.
Dr. Hulten and his colleagues performed a meta-analysis of prospective and retrospective observational studies in which 9,592 patients suspected of having CAD were evaluated using CCTA and followed for a median of 20 months. Seventeen of these studies were rated as good quality.
For the patients whose CCTA results indicated no CAD, the annualized rate of major adverse cardiovascular events (MACE) was 0.16%. There were no coronary revascularizations, myocardial infarctions, or admissions for unstable angina; the only events were from all-cause mortality.
“Considered in concert with the wealth of data regarding the high anatomic accuracy for CCTA, these results are convincing for CCTA to effectively diagnose CAD and convey risk strata for future adverse cardiovascular events,” the researchers said (J. Am. Coll. Cardiol. 2010;57 [doi:10.1016/jacc.2010.10.011]).
Positive results on CCTA correlated with major adverse cardiovascular events. The average annualized MACE rate for a finding of CAD was 8.8% per year (vs. 0.17% per year for negative findings). Revascularization procedures accounted for most of these events; the annualized rate of death or MI was 3.2% vs. 0.15 for positive vs. negative scans. Moreover, the rate of adverse events increased as the severity of coronary artery disease on CCTA exam increased.
Although a negative CCTA scan can be considered strongly predictive of an excellent outcome, the reverse is not true: A positive CCTA scan cannot be considered strongly predictive of future adverse events. With the annualized MACE rate at only 8.8%, the great majority of patients found on CCTA to have coronary disease also have good outcomes in the 20 months' follow-up, Dr. Hulten and his associates pointed out.
They also noted that many of the authors of studies included in the meta-analysis “reported prognosis based on a relatively simple classification of CAD luminal stenosis, specifically, no CAD, nonobstructive CAD, or potentially obstructive CAD (greater than 50% stenosis).” With studies defining “normal” CCTA in slightly different ways, it was not possible to have stratification details that would be clinically informative, the authors wrote. They also noted that their research included data from “different generations of CT scanning technology” the newer of which are known to have improved image quality and accuracy.
As in many other many studies of noninvasive coronary risk stratification tests, the investigators wrote, a limitation of their study is verification bias: “The observed increase in MACE is driven in part by coronary revascularization, demonstrating evidence of a work-up or verification bias. That is, patients with CCTA evidence of a greater than 50% stenosis are more likely to undergo catheterization and subsequent revascularization.”