User login
Ulipristal reduces bleeding with contraceptive implant
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Treatment with ulipristal was associated with 5 fewer bleeding days per month, compared with placebo (P = .002).
Study details: The double-blind, placebo-controlled trial involved 65 women with etonogestrel implants who reported more than one bleeding episode in a 24-day time frame.
Disclosures: The study was funded by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences (NCATS). The authors reported financial disclosures related to Bayer and Merck.
Source: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
2018 Update on contraception
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.
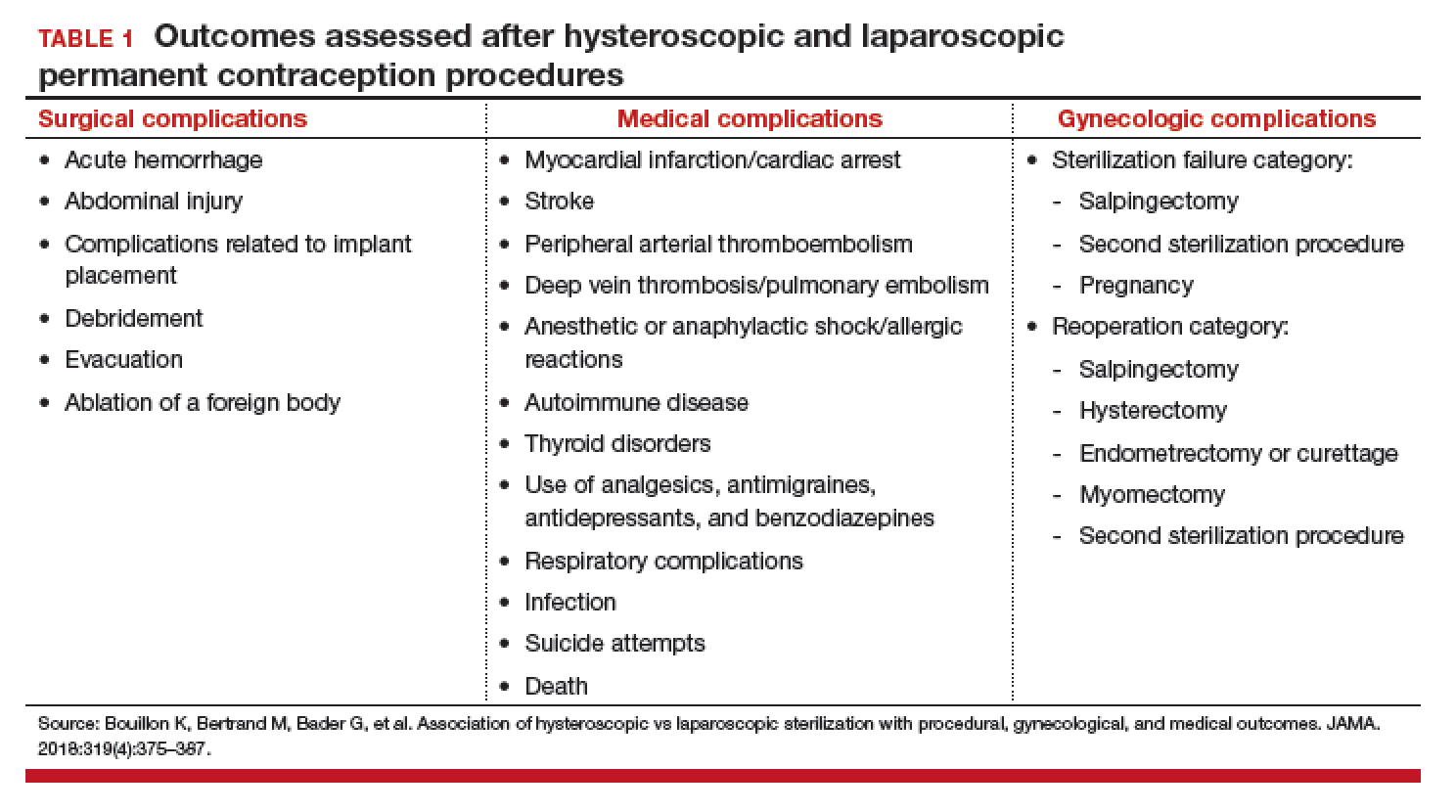
Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).
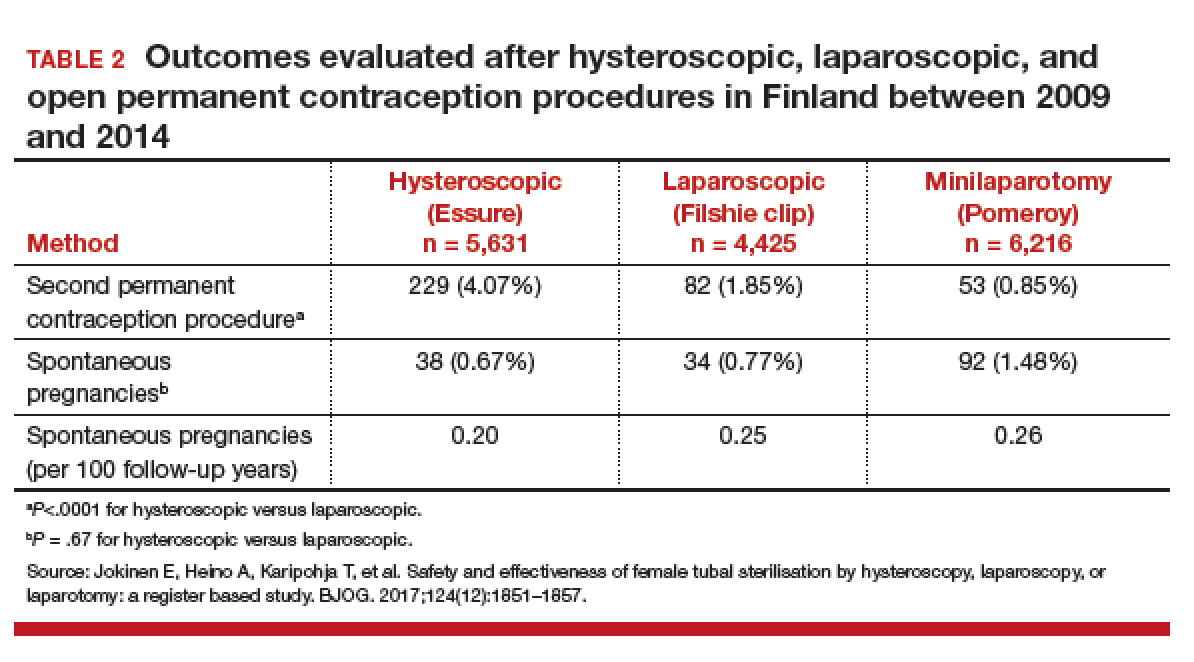
At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
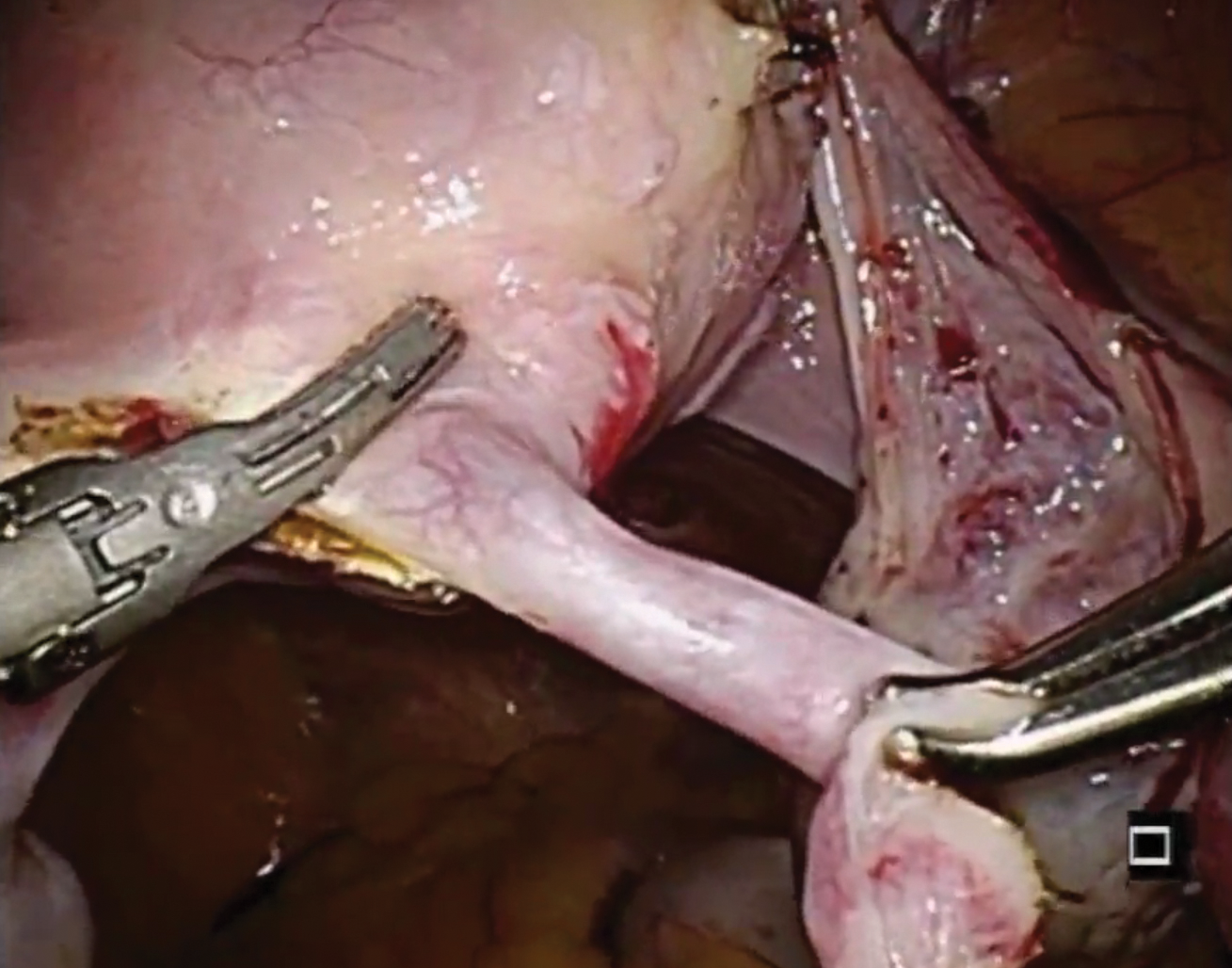
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.
Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.

Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).

At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.

Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).

At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Is the most effective emergency contraception easily obtained at US pharmacies?
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.
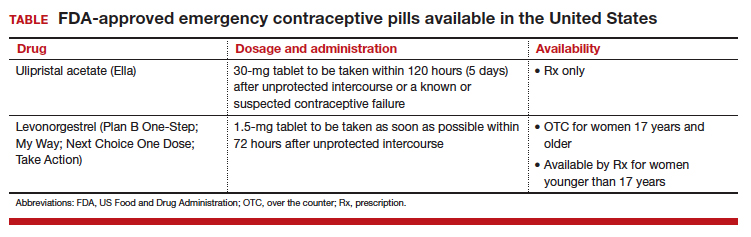
According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
FDA approves vaginal ring contraceptive Annovera
The first vaginal ring contraceptive that can be used for 1 year has been approved by the Food and Drug Administration.
The FDA granted approval of Annovera (segesterone acetate and ethinyl estradiol vaginal system), a reusable donut-shaped ring, to the Population Council. The nonbiodegradable, flexible vaginal system is placed in the vagina for 3 weeks followed by 1 week out of the vagina, at which time women may experience a menstrual period. This schedule is repeated every 4 weeks for 13 28-day menstrual cycles.
The contraceptive ring is washed and stored in a compact case for the 7 days when it is not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30° C (86° F).
“Today’s approval builds on available birth control options,” said Victor Crentsil, MD, acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
The efficacy and safety of Annovera were studied in three, open-label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about 2%-4% of women may get pregnant during the first year they use Annovera.
Annovera carries a boxed warning regarding cigarette smoking and serious cardiovascular events. Women over age 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated for women with a high risk of arterial or venous thrombotic diseases; a history of breast cancer or another estrogen- or progestin-sensitive cancer; liver tumors, acute hepatitis, or severe (decompensated) cirrhosis; undiagnosed abnormal uterine bleeding; hypersensitivity to any of the components of Annovera; and use of hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, according to an FDA press release.
The most common side effects of Annovera are similar to those of other combined hormonal contraceptive products and include headache, nausea and vomiting, yeast infections, abdominal pain, dysmenorrhea, breast tenderness, irregular bleeding, diarrhea, and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism and the effects of CYP3A-modulating drugs and tampon use on the pharmacokinetics of Annovera.
It is very exciting to see ongoing research and development for new contraceptives and approval of these new methods! We know that each person’s contraceptive needs are unique and having more options from which to choose will help us as providers connect our patients to methods that meet their goals and individual needs. These two methods fill important gaps in our current contraceptive portfolio: a patient-controlled, long-acting method and a facilitated non-hormonal, non-prescription method (the Natural Cycles app). I am looking forward to hearing feedback from patients about their thoughts and experiences with these new methods.
Melissa Kottke, MD, MPH, MBA, is an associate professor of obstetrics and gynecology and director of the Jane Fonda Center for Adolescent Reproductive Health, Emory University, Atlanta.
It is very exciting to see ongoing research and development for new contraceptives and approval of these new methods! We know that each person’s contraceptive needs are unique and having more options from which to choose will help us as providers connect our patients to methods that meet their goals and individual needs. These two methods fill important gaps in our current contraceptive portfolio: a patient-controlled, long-acting method and a facilitated non-hormonal, non-prescription method (the Natural Cycles app). I am looking forward to hearing feedback from patients about their thoughts and experiences with these new methods.
Melissa Kottke, MD, MPH, MBA, is an associate professor of obstetrics and gynecology and director of the Jane Fonda Center for Adolescent Reproductive Health, Emory University, Atlanta.
It is very exciting to see ongoing research and development for new contraceptives and approval of these new methods! We know that each person’s contraceptive needs are unique and having more options from which to choose will help us as providers connect our patients to methods that meet their goals and individual needs. These two methods fill important gaps in our current contraceptive portfolio: a patient-controlled, long-acting method and a facilitated non-hormonal, non-prescription method (the Natural Cycles app). I am looking forward to hearing feedback from patients about their thoughts and experiences with these new methods.
Melissa Kottke, MD, MPH, MBA, is an associate professor of obstetrics and gynecology and director of the Jane Fonda Center for Adolescent Reproductive Health, Emory University, Atlanta.
The first vaginal ring contraceptive that can be used for 1 year has been approved by the Food and Drug Administration.
The FDA granted approval of Annovera (segesterone acetate and ethinyl estradiol vaginal system), a reusable donut-shaped ring, to the Population Council. The nonbiodegradable, flexible vaginal system is placed in the vagina for 3 weeks followed by 1 week out of the vagina, at which time women may experience a menstrual period. This schedule is repeated every 4 weeks for 13 28-day menstrual cycles.
The contraceptive ring is washed and stored in a compact case for the 7 days when it is not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30° C (86° F).
“Today’s approval builds on available birth control options,” said Victor Crentsil, MD, acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
The efficacy and safety of Annovera were studied in three, open-label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about 2%-4% of women may get pregnant during the first year they use Annovera.
Annovera carries a boxed warning regarding cigarette smoking and serious cardiovascular events. Women over age 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated for women with a high risk of arterial or venous thrombotic diseases; a history of breast cancer or another estrogen- or progestin-sensitive cancer; liver tumors, acute hepatitis, or severe (decompensated) cirrhosis; undiagnosed abnormal uterine bleeding; hypersensitivity to any of the components of Annovera; and use of hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, according to an FDA press release.
The most common side effects of Annovera are similar to those of other combined hormonal contraceptive products and include headache, nausea and vomiting, yeast infections, abdominal pain, dysmenorrhea, breast tenderness, irregular bleeding, diarrhea, and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism and the effects of CYP3A-modulating drugs and tampon use on the pharmacokinetics of Annovera.
The first vaginal ring contraceptive that can be used for 1 year has been approved by the Food and Drug Administration.
The FDA granted approval of Annovera (segesterone acetate and ethinyl estradiol vaginal system), a reusable donut-shaped ring, to the Population Council. The nonbiodegradable, flexible vaginal system is placed in the vagina for 3 weeks followed by 1 week out of the vagina, at which time women may experience a menstrual period. This schedule is repeated every 4 weeks for 13 28-day menstrual cycles.
The contraceptive ring is washed and stored in a compact case for the 7 days when it is not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30° C (86° F).
“Today’s approval builds on available birth control options,” said Victor Crentsil, MD, acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
The efficacy and safety of Annovera were studied in three, open-label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about 2%-4% of women may get pregnant during the first year they use Annovera.
Annovera carries a boxed warning regarding cigarette smoking and serious cardiovascular events. Women over age 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated for women with a high risk of arterial or venous thrombotic diseases; a history of breast cancer or another estrogen- or progestin-sensitive cancer; liver tumors, acute hepatitis, or severe (decompensated) cirrhosis; undiagnosed abnormal uterine bleeding; hypersensitivity to any of the components of Annovera; and use of hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, according to an FDA press release.
The most common side effects of Annovera are similar to those of other combined hormonal contraceptive products and include headache, nausea and vomiting, yeast infections, abdominal pain, dysmenorrhea, breast tenderness, irregular bleeding, diarrhea, and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism and the effects of CYP3A-modulating drugs and tampon use on the pharmacokinetics of Annovera.
Continuation, complication rates similar for implants, IUDs
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
FROM CONTRACEPTION
Key clinical point:
Major finding: For implants and IUDS, respectively, 1-year continuation rates were similar (adjusted 81% vs. 77%, P = .01), as were complication rates (unadjusted 8% for implants, 7% for IUDs).
Study details: Retrospective analysis of 3,305 Medicaid-covered women treated with the two types of contraceptives during 2012-2015 in the District of Columbia and Maryland.
Disclosures: MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen discloses serving on the Diclegis speakers bureau.
Source: Romano MJ et al. Contraception. 2018 Aug;98:125-9.
New IUD expelled less often after C-section than older device
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
FROM CONTRACEPTION
Key clinical point: A newly developed IUD appears to be expelled less frequently than an older device after insertion following cesarean section births.
Major finding: 61 of 70 IUDs (88%) remained in place in women who received the frameless copper-releasing Gyn-CS device, while 54 of 70 (79%) did so in those who received the TCu380A device (P = .30).
Study details: Randomized trial of 140 women who underwent cesarean section followed by insertion of one of the two IUD types (n = 70 for each).
Disclosures: No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
Source: Unal C et al. Contraception. 2018 Aug;98:135-40.
FDA approves first mobile app for contraceptive use
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
Contraceptive considerations for women with headache and migraine
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6
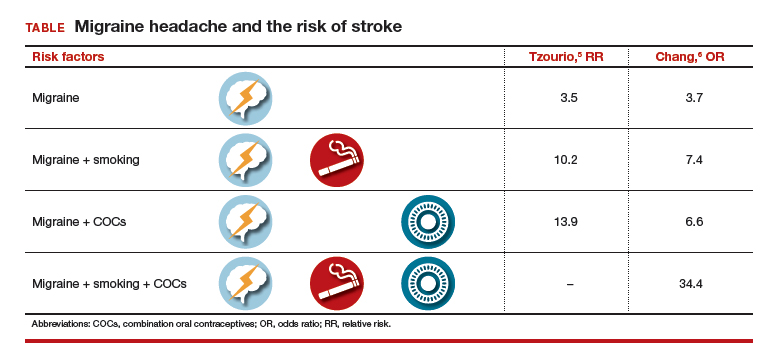
Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6

Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6

Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
CDC apps specific for ObGyns
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.
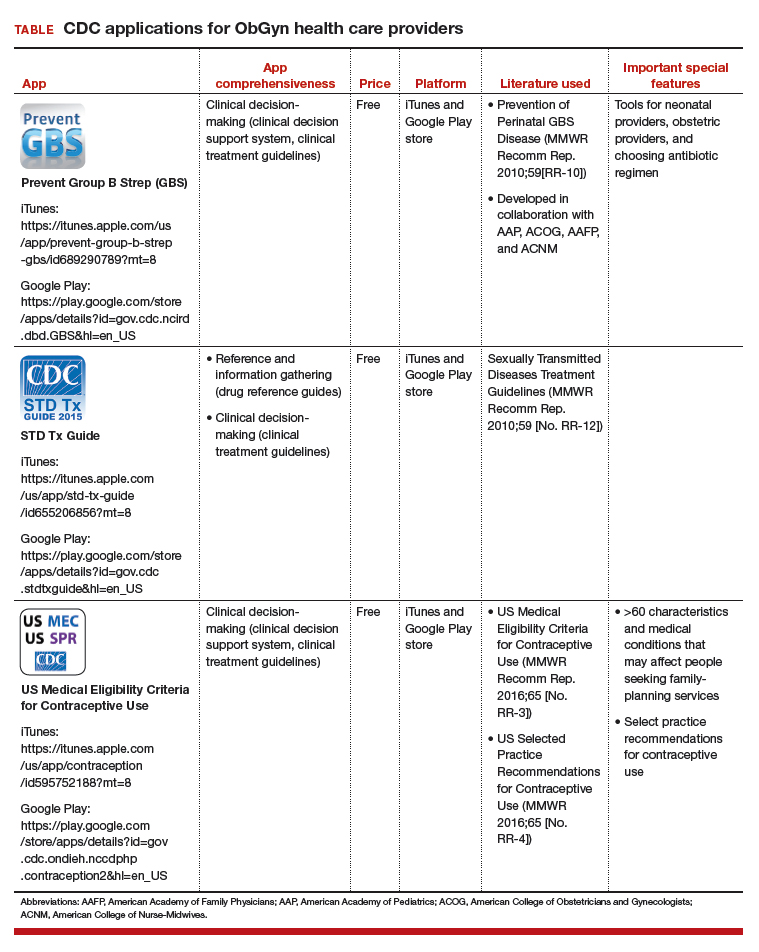
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
IN THIS ARTICLE
- Details on recommended apps