User login
CAR T coverage: Drugmakers say no to patient reported outcomes
Drugmakers and physician groups expressed concern about connecting patient reported outcomes (PRO) to coverage decisions for chimeric antigen receptor T-cell therapy (CAR T) at a recent Medicare meeting.
Despite these objections, members of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) generally expressed confidence in the use of PROs in a series of votes about PRO instruments’ use in cancer care trials.
MEDCAC opened a national coverage analysis in May 2018 at the request of UnitedHealthcare. The insurer had asked the Centers for Medicare & Medicaid Services to clarify the circumstances for coverage of the new CAR T therapies.
In presentations at an Aug. 22 MEDCAC meeting, representatives from the two manufacturers that have approved CAR T treatments on the market cautioned against using PROs in the context of coverage decisions, even though both companies used PRO data in their clinical trials and are collecting it in the postmarketing period.
“While Kite recognizes the importance of PROs in ... clinical trials, the PRO CAR T science, where these instruments are most appropriate for CAR T, remains a still to be determined and still is evolving and still quite early in the developments,” said William Go, MD, PhD, vice president of clinical development at Kite Pharmaceuticals, a Gilead company.
Despite PROs playing an active role in the ongoing development and clinical trials that Kite is running, in the context of CAR T, “we feel PROs are not quite ready for real-world coverage decisions at this time,” Dr. Go said.
A representative from Novartis agreed. “It is important to clearly define research objectives when considering whether to collect patient reported outcomes data, particularly given the burden associated with advanced disease experienced by the patients that are likely to receive this therapy,” Ilia Ferrusi, PhD, associate director, HEOR (CAR T Therapy), U.S. Oncology, Novartis, told the panel.
She noted that Novartis experienced challenges collecting PRO data, which resulted in risks to data quality and interpretation. “This could very well be amplified in larger trials or in real-world practice.”
The presenters’ comments were echoed by written comments submitted to the CMS prior to the MEDCAC meeting.
The American Society for Blood and Marrow Transplantation (ASBMT), despite supporting the collection of patient-reported outcomes, particularly in oncology, “strongly objects to any mechanism that would tie patient access or provider reimbursement to the reporting of PROs, especially in the case of CAR T therapy,” the organization said in a letter to the CMS.
Optimal PRO instruments and time points “are currently unknown” as the PROs used today were developed around chemotherapy use.
“Mandating the use of a current instrument or set of outcomes through NCD [national coverage determination] or CED [coverage with evidence development] will set a course of collecting data that is very likely to be inaccurate and inadequate,” they said.
The ASBMT also noted there is significant heterogeneity in the CAR T constructs and associated disease indications. “Products will utilize different scientific constructs and the time frames for clinical response and/or potential onset of toxicities may differ for each construct, which will challenge the establishment of set time points for data collection.”
And while presentations and comments focused on PROs in the context of CAR T, conversation and voting on the use of PROs was in the broader context of oncology clinical trials, not specific to CAR T, changing the nature of the debate and calling into question the point of the meeting and how it will inform the national coverage determination on CAR T specifically.
The June 15 meeting announcement published in the Federal Register stated that the advisory committee “will specifically focus on appraisal of evidence-based PRO assessments to provide information that impacts patients, their providers, and caregivers after a CAR T-cell therapy intervention for the patient’s cancer.”
However, the committee was asked to evaluate evidence on a number of potential PRO instruments in the broader context of oncology clinical trials without a cancer-specific or treatment-specific context, leading to a debate over how different types of cancer will have different kinds of PRO assessments related to them.
The CMS is expected to make a national coverage decision on CAR T-cell therapy by Feb. 16, 2019.
Drugmakers and physician groups expressed concern about connecting patient reported outcomes (PRO) to coverage decisions for chimeric antigen receptor T-cell therapy (CAR T) at a recent Medicare meeting.
Despite these objections, members of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) generally expressed confidence in the use of PROs in a series of votes about PRO instruments’ use in cancer care trials.
MEDCAC opened a national coverage analysis in May 2018 at the request of UnitedHealthcare. The insurer had asked the Centers for Medicare & Medicaid Services to clarify the circumstances for coverage of the new CAR T therapies.
In presentations at an Aug. 22 MEDCAC meeting, representatives from the two manufacturers that have approved CAR T treatments on the market cautioned against using PROs in the context of coverage decisions, even though both companies used PRO data in their clinical trials and are collecting it in the postmarketing period.
“While Kite recognizes the importance of PROs in ... clinical trials, the PRO CAR T science, where these instruments are most appropriate for CAR T, remains a still to be determined and still is evolving and still quite early in the developments,” said William Go, MD, PhD, vice president of clinical development at Kite Pharmaceuticals, a Gilead company.
Despite PROs playing an active role in the ongoing development and clinical trials that Kite is running, in the context of CAR T, “we feel PROs are not quite ready for real-world coverage decisions at this time,” Dr. Go said.
A representative from Novartis agreed. “It is important to clearly define research objectives when considering whether to collect patient reported outcomes data, particularly given the burden associated with advanced disease experienced by the patients that are likely to receive this therapy,” Ilia Ferrusi, PhD, associate director, HEOR (CAR T Therapy), U.S. Oncology, Novartis, told the panel.
She noted that Novartis experienced challenges collecting PRO data, which resulted in risks to data quality and interpretation. “This could very well be amplified in larger trials or in real-world practice.”
The presenters’ comments were echoed by written comments submitted to the CMS prior to the MEDCAC meeting.
The American Society for Blood and Marrow Transplantation (ASBMT), despite supporting the collection of patient-reported outcomes, particularly in oncology, “strongly objects to any mechanism that would tie patient access or provider reimbursement to the reporting of PROs, especially in the case of CAR T therapy,” the organization said in a letter to the CMS.
Optimal PRO instruments and time points “are currently unknown” as the PROs used today were developed around chemotherapy use.
“Mandating the use of a current instrument or set of outcomes through NCD [national coverage determination] or CED [coverage with evidence development] will set a course of collecting data that is very likely to be inaccurate and inadequate,” they said.
The ASBMT also noted there is significant heterogeneity in the CAR T constructs and associated disease indications. “Products will utilize different scientific constructs and the time frames for clinical response and/or potential onset of toxicities may differ for each construct, which will challenge the establishment of set time points for data collection.”
And while presentations and comments focused on PROs in the context of CAR T, conversation and voting on the use of PROs was in the broader context of oncology clinical trials, not specific to CAR T, changing the nature of the debate and calling into question the point of the meeting and how it will inform the national coverage determination on CAR T specifically.
The June 15 meeting announcement published in the Federal Register stated that the advisory committee “will specifically focus on appraisal of evidence-based PRO assessments to provide information that impacts patients, their providers, and caregivers after a CAR T-cell therapy intervention for the patient’s cancer.”
However, the committee was asked to evaluate evidence on a number of potential PRO instruments in the broader context of oncology clinical trials without a cancer-specific or treatment-specific context, leading to a debate over how different types of cancer will have different kinds of PRO assessments related to them.
The CMS is expected to make a national coverage decision on CAR T-cell therapy by Feb. 16, 2019.
Drugmakers and physician groups expressed concern about connecting patient reported outcomes (PRO) to coverage decisions for chimeric antigen receptor T-cell therapy (CAR T) at a recent Medicare meeting.
Despite these objections, members of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) generally expressed confidence in the use of PROs in a series of votes about PRO instruments’ use in cancer care trials.
MEDCAC opened a national coverage analysis in May 2018 at the request of UnitedHealthcare. The insurer had asked the Centers for Medicare & Medicaid Services to clarify the circumstances for coverage of the new CAR T therapies.
In presentations at an Aug. 22 MEDCAC meeting, representatives from the two manufacturers that have approved CAR T treatments on the market cautioned against using PROs in the context of coverage decisions, even though both companies used PRO data in their clinical trials and are collecting it in the postmarketing period.
“While Kite recognizes the importance of PROs in ... clinical trials, the PRO CAR T science, where these instruments are most appropriate for CAR T, remains a still to be determined and still is evolving and still quite early in the developments,” said William Go, MD, PhD, vice president of clinical development at Kite Pharmaceuticals, a Gilead company.
Despite PROs playing an active role in the ongoing development and clinical trials that Kite is running, in the context of CAR T, “we feel PROs are not quite ready for real-world coverage decisions at this time,” Dr. Go said.
A representative from Novartis agreed. “It is important to clearly define research objectives when considering whether to collect patient reported outcomes data, particularly given the burden associated with advanced disease experienced by the patients that are likely to receive this therapy,” Ilia Ferrusi, PhD, associate director, HEOR (CAR T Therapy), U.S. Oncology, Novartis, told the panel.
She noted that Novartis experienced challenges collecting PRO data, which resulted in risks to data quality and interpretation. “This could very well be amplified in larger trials or in real-world practice.”
The presenters’ comments were echoed by written comments submitted to the CMS prior to the MEDCAC meeting.
The American Society for Blood and Marrow Transplantation (ASBMT), despite supporting the collection of patient-reported outcomes, particularly in oncology, “strongly objects to any mechanism that would tie patient access or provider reimbursement to the reporting of PROs, especially in the case of CAR T therapy,” the organization said in a letter to the CMS.
Optimal PRO instruments and time points “are currently unknown” as the PROs used today were developed around chemotherapy use.
“Mandating the use of a current instrument or set of outcomes through NCD [national coverage determination] or CED [coverage with evidence development] will set a course of collecting data that is very likely to be inaccurate and inadequate,” they said.
The ASBMT also noted there is significant heterogeneity in the CAR T constructs and associated disease indications. “Products will utilize different scientific constructs and the time frames for clinical response and/or potential onset of toxicities may differ for each construct, which will challenge the establishment of set time points for data collection.”
And while presentations and comments focused on PROs in the context of CAR T, conversation and voting on the use of PROs was in the broader context of oncology clinical trials, not specific to CAR T, changing the nature of the debate and calling into question the point of the meeting and how it will inform the national coverage determination on CAR T specifically.
The June 15 meeting announcement published in the Federal Register stated that the advisory committee “will specifically focus on appraisal of evidence-based PRO assessments to provide information that impacts patients, their providers, and caregivers after a CAR T-cell therapy intervention for the patient’s cancer.”
However, the committee was asked to evaluate evidence on a number of potential PRO instruments in the broader context of oncology clinical trials without a cancer-specific or treatment-specific context, leading to a debate over how different types of cancer will have different kinds of PRO assessments related to them.
The CMS is expected to make a national coverage decision on CAR T-cell therapy by Feb. 16, 2019.
Signal strength may limit potency of CAR T-cell therapy
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
FROM SCIENCE SIGNALING
Key clinical point:
Major finding: T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in a mouse model of lymphoma, while the 4-1BB CAR signal led to T cells that better retained their function in vivo and had a longer median survival in the model.
Study details: Analysis of CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells using mass spectrometry, plus analysis of efficacy in a mouse model of lymphoma.
Disclosures: Study authors reported disclosures related to Juno therapeutics and a patent application related to use of mutant CD28 CARs for cellular therapy.
Source: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
CMS finalizes CAR T-cell therapy inpatient payments
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
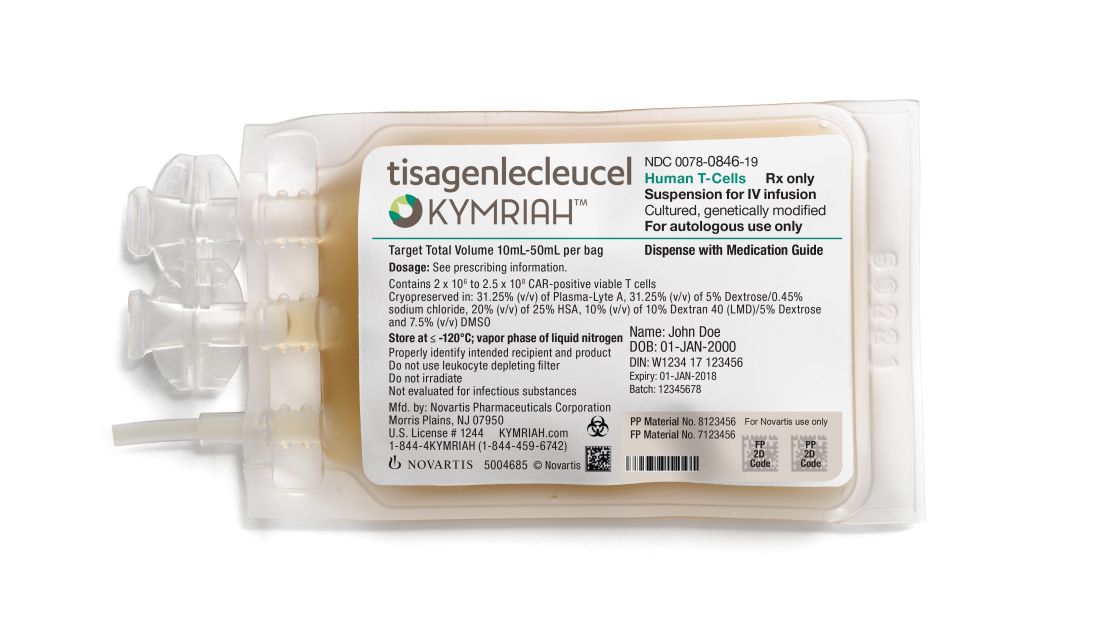
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Feds aim to streamline gene therapy oversight
“As the NIH, the FDA, and research entities have moved to strengthen their individual oversight efforts, some overlaps have occurred,” Dr. Collins and Dr. Gottlieb wrote Aug. 15 in the New England Journal of Medicine. “Substantial duplication has arisen in the submission of initial protocols, annual reports, amendments, and reports of serious adverse events. Originally, these overlaps – which affect no other field in biomedical research – were viewed as harmonized reporting that enabled FDA to conduct regulatory oversight while maintaining confidentiality with sponsors and allowed NIH to provide transparency with regard to the research.”
With three approved treatments – two CAR T therapies and a treatment for a genetic retinal dystrophy – and more than 700 active investigational new drug applications in front of the FDA, “it seems reasonable to envision a day when gene therapy will be a mainstay of treatment for many diseases,” according to the editorial.
To remedy the situation, NIH officials Aug. 16 posted a proposed update to NIH guidance that seeks “to reduce the duplicative oversight burden by further limiting the role of NIH and RAC [Recombinant DNA Advisory Committee] in assessing gene therapy protocols and reviewing their safety information,” Dr. Collins and Dr. Gottlieb wrote. “Specifically, these proposals will eliminate RAC review and reporting requirements to the NIH for human gene therapy protocols. They will also revise the responsibility of institutional Biosafety Committees, which have local oversight for this research, making their review of human gene therapy protocols consistent with review of other research subject to the NIH Guidelines. Such streamlining will also appropriately place the focus of the NIH Guidelines squarely back on laboratory biosafety.”
RAC was originally established in 1974 to advise the NIH director on research involving manipulation of nucleic acids, but was later expanded to review and discuss protocols for gene therapy in humans.
The proposed structure provides an opportunity “to return the RAC to the spirit in which it was founded. ... The NIH envisions using the RAC as an advisory board on today’s emerging technologies, such as gene editing, synthetic biology, and neurotechnology, while harnessing the attributes that have long ensured its transparency,” they wrote.
This latest proposal follows a suite of FDA draft guidances on gene therapies issued in July that proposed new guidance on manufacturing issues, long-term follow-up, and pathways for clinical development in certain areas.
The guidance is scheduled for publication in the Federal Register on Aug. 17.
SOURCE: Collins FS and Gottlieb S. N Engl J Med. doi: 10.1056/NEJMp1810628.
“As the NIH, the FDA, and research entities have moved to strengthen their individual oversight efforts, some overlaps have occurred,” Dr. Collins and Dr. Gottlieb wrote Aug. 15 in the New England Journal of Medicine. “Substantial duplication has arisen in the submission of initial protocols, annual reports, amendments, and reports of serious adverse events. Originally, these overlaps – which affect no other field in biomedical research – were viewed as harmonized reporting that enabled FDA to conduct regulatory oversight while maintaining confidentiality with sponsors and allowed NIH to provide transparency with regard to the research.”
With three approved treatments – two CAR T therapies and a treatment for a genetic retinal dystrophy – and more than 700 active investigational new drug applications in front of the FDA, “it seems reasonable to envision a day when gene therapy will be a mainstay of treatment for many diseases,” according to the editorial.
To remedy the situation, NIH officials Aug. 16 posted a proposed update to NIH guidance that seeks “to reduce the duplicative oversight burden by further limiting the role of NIH and RAC [Recombinant DNA Advisory Committee] in assessing gene therapy protocols and reviewing their safety information,” Dr. Collins and Dr. Gottlieb wrote. “Specifically, these proposals will eliminate RAC review and reporting requirements to the NIH for human gene therapy protocols. They will also revise the responsibility of institutional Biosafety Committees, which have local oversight for this research, making their review of human gene therapy protocols consistent with review of other research subject to the NIH Guidelines. Such streamlining will also appropriately place the focus of the NIH Guidelines squarely back on laboratory biosafety.”
RAC was originally established in 1974 to advise the NIH director on research involving manipulation of nucleic acids, but was later expanded to review and discuss protocols for gene therapy in humans.
The proposed structure provides an opportunity “to return the RAC to the spirit in which it was founded. ... The NIH envisions using the RAC as an advisory board on today’s emerging technologies, such as gene editing, synthetic biology, and neurotechnology, while harnessing the attributes that have long ensured its transparency,” they wrote.
This latest proposal follows a suite of FDA draft guidances on gene therapies issued in July that proposed new guidance on manufacturing issues, long-term follow-up, and pathways for clinical development in certain areas.
The guidance is scheduled for publication in the Federal Register on Aug. 17.
SOURCE: Collins FS and Gottlieb S. N Engl J Med. doi: 10.1056/NEJMp1810628.
“As the NIH, the FDA, and research entities have moved to strengthen their individual oversight efforts, some overlaps have occurred,” Dr. Collins and Dr. Gottlieb wrote Aug. 15 in the New England Journal of Medicine. “Substantial duplication has arisen in the submission of initial protocols, annual reports, amendments, and reports of serious adverse events. Originally, these overlaps – which affect no other field in biomedical research – were viewed as harmonized reporting that enabled FDA to conduct regulatory oversight while maintaining confidentiality with sponsors and allowed NIH to provide transparency with regard to the research.”
With three approved treatments – two CAR T therapies and a treatment for a genetic retinal dystrophy – and more than 700 active investigational new drug applications in front of the FDA, “it seems reasonable to envision a day when gene therapy will be a mainstay of treatment for many diseases,” according to the editorial.
To remedy the situation, NIH officials Aug. 16 posted a proposed update to NIH guidance that seeks “to reduce the duplicative oversight burden by further limiting the role of NIH and RAC [Recombinant DNA Advisory Committee] in assessing gene therapy protocols and reviewing their safety information,” Dr. Collins and Dr. Gottlieb wrote. “Specifically, these proposals will eliminate RAC review and reporting requirements to the NIH for human gene therapy protocols. They will also revise the responsibility of institutional Biosafety Committees, which have local oversight for this research, making their review of human gene therapy protocols consistent with review of other research subject to the NIH Guidelines. Such streamlining will also appropriately place the focus of the NIH Guidelines squarely back on laboratory biosafety.”
RAC was originally established in 1974 to advise the NIH director on research involving manipulation of nucleic acids, but was later expanded to review and discuss protocols for gene therapy in humans.
The proposed structure provides an opportunity “to return the RAC to the spirit in which it was founded. ... The NIH envisions using the RAC as an advisory board on today’s emerging technologies, such as gene editing, synthetic biology, and neurotechnology, while harnessing the attributes that have long ensured its transparency,” they wrote.
This latest proposal follows a suite of FDA draft guidances on gene therapies issued in July that proposed new guidance on manufacturing issues, long-term follow-up, and pathways for clinical development in certain areas.
The guidance is scheduled for publication in the Federal Register on Aug. 17.
SOURCE: Collins FS and Gottlieb S. N Engl J Med. doi: 10.1056/NEJMp1810628.
Key clinical point: NIH and FDA are looking to streamline gene therapy development oversight.
Major finding: The proposal would return the function of the Recombinant DNA Advisory Committee (RAC) to a function more in line with its original mission.
Study details: Full proposal details are to be published in the Federal Register on Aug. 17.
Disclosures: The agency leaders reported no relevant disclosures in the production of the proposal.
Source: Collins FS and Gottlieb S. N Engl J Med. doi: 10.1056/NEJMp1810628.
CAR T Therapy: From Bench to Bedside and Back
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
CMS proposes inpatient payment model for CAR T therapies
Physicians may finally have some clarity on payment for inpatient administration of two chimeric antigen receptor–T-cell therapies if a proposed rule from the Centers of Medicare & Medicaid Services becomes final.
The agency is seeking to assign ICD-10-PCS codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019. The agency is also considering the creation of a new Medicare Severity–Diagnosis Related Group (MS-DRG) code for procedures involving the use of chimeric antigen receptor (CAR) T-cell therapy drugs. The proposal was published in May in the Federal Register.
The proposal demonstrates that CMS is listening to physicians’ concerns about CAR T payments and working to provide a more reasonable framework, said Stephanie Farnia, director of health policy and strategic relations for the American Society for Blood and Marrow Transplantation.
“The primary point of significance is that CAR-T care episodes should be assigned to a specific MS-DRG in FY2019, which will give physicians a clearer sense of inpatient reimbursement in advance,” she said in an interview.
Uncertainty about inpatient payment for administration of the two approved CAR-T therapies have been a lingering concern of specialists using, or interested in using, the therapies. In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians noted that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay per CMS’ 3-day payment window rule.
In outlining the most recent payment proposal, CMS stated that its clinical advisers believe that patients receiving treatment with CAR T-cell therapy would have similar clinical characteristics and comorbidities as patients treated with autologous bone marrow transplant therapy, who are currently assigned to MS-DRG 016 “Autologous Bone Marrow Transplant with CC/MCC”. Therefore, CMS officials said they are suggesting ICD-10-PCS procedure codes XW033C3 and XW043C3 to pre-MDC MS-DRG 016. Additionally, the agency is proposing to revise the title of MS-DRG 016 from “Autologous Bone Marrow Transplant with CC/MCC” to “Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy.”
However, the agency emphasized that it invites public comment on alternative payment approaches for CAR T-cell therapies in the context of the pending, new technology add-on payment applications by the CAR-T drugmakers Novartis Pharmaceuticals and Kite Pharma. If approved in the final rule, the technology add-on payments would provide an additional and separate payment equivalent to up to 50% of the product cost plus the MS-DRG payment received for the episode of care.
Other items in the proposed rule include approximate base payments for hematopoietic stem cell transplantation for fiscal 2019, which starts Oct. 1, 2018. The approximate per unit payment is $5,498, which translates to a total base payment of $64,790 for allogeneic bone marrow transplant (MS-DRG 014) for instance. In an American Society for Blood and Marrow Transplantation blog, Ms. Farnia outlined the approximate base weights and estimated reimbursements calculated from the rule.
CMS did not make any changes to the payment model for allogeneic hematopoietic cell transplantation despite continued requests by physicians for donor acquisition charges. Ms. Farnia encourages doctors to express to CMS the importance of such reimbursement during the comment period.
Public comments on the inpatient payment proposal are due by June 25, 2018, and can be submitted at https://www.regulations.gov. Additionally, CMS will convene a meeting of the Medicare Evidence Development & Coverage Advisory Committee on August 22, 2018, to consider whether to issue a national coverage policy for CAR T-cell therapies that would set consistent parameters for patient access.
Physicians may finally have some clarity on payment for inpatient administration of two chimeric antigen receptor–T-cell therapies if a proposed rule from the Centers of Medicare & Medicaid Services becomes final.
The agency is seeking to assign ICD-10-PCS codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019. The agency is also considering the creation of a new Medicare Severity–Diagnosis Related Group (MS-DRG) code for procedures involving the use of chimeric antigen receptor (CAR) T-cell therapy drugs. The proposal was published in May in the Federal Register.
The proposal demonstrates that CMS is listening to physicians’ concerns about CAR T payments and working to provide a more reasonable framework, said Stephanie Farnia, director of health policy and strategic relations for the American Society for Blood and Marrow Transplantation.
“The primary point of significance is that CAR-T care episodes should be assigned to a specific MS-DRG in FY2019, which will give physicians a clearer sense of inpatient reimbursement in advance,” she said in an interview.
Uncertainty about inpatient payment for administration of the two approved CAR-T therapies have been a lingering concern of specialists using, or interested in using, the therapies. In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians noted that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay per CMS’ 3-day payment window rule.
In outlining the most recent payment proposal, CMS stated that its clinical advisers believe that patients receiving treatment with CAR T-cell therapy would have similar clinical characteristics and comorbidities as patients treated with autologous bone marrow transplant therapy, who are currently assigned to MS-DRG 016 “Autologous Bone Marrow Transplant with CC/MCC”. Therefore, CMS officials said they are suggesting ICD-10-PCS procedure codes XW033C3 and XW043C3 to pre-MDC MS-DRG 016. Additionally, the agency is proposing to revise the title of MS-DRG 016 from “Autologous Bone Marrow Transplant with CC/MCC” to “Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy.”
However, the agency emphasized that it invites public comment on alternative payment approaches for CAR T-cell therapies in the context of the pending, new technology add-on payment applications by the CAR-T drugmakers Novartis Pharmaceuticals and Kite Pharma. If approved in the final rule, the technology add-on payments would provide an additional and separate payment equivalent to up to 50% of the product cost plus the MS-DRG payment received for the episode of care.
Other items in the proposed rule include approximate base payments for hematopoietic stem cell transplantation for fiscal 2019, which starts Oct. 1, 2018. The approximate per unit payment is $5,498, which translates to a total base payment of $64,790 for allogeneic bone marrow transplant (MS-DRG 014) for instance. In an American Society for Blood and Marrow Transplantation blog, Ms. Farnia outlined the approximate base weights and estimated reimbursements calculated from the rule.
CMS did not make any changes to the payment model for allogeneic hematopoietic cell transplantation despite continued requests by physicians for donor acquisition charges. Ms. Farnia encourages doctors to express to CMS the importance of such reimbursement during the comment period.
Public comments on the inpatient payment proposal are due by June 25, 2018, and can be submitted at https://www.regulations.gov. Additionally, CMS will convene a meeting of the Medicare Evidence Development & Coverage Advisory Committee on August 22, 2018, to consider whether to issue a national coverage policy for CAR T-cell therapies that would set consistent parameters for patient access.
Physicians may finally have some clarity on payment for inpatient administration of two chimeric antigen receptor–T-cell therapies if a proposed rule from the Centers of Medicare & Medicaid Services becomes final.
The agency is seeking to assign ICD-10-PCS codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019. The agency is also considering the creation of a new Medicare Severity–Diagnosis Related Group (MS-DRG) code for procedures involving the use of chimeric antigen receptor (CAR) T-cell therapy drugs. The proposal was published in May in the Federal Register.
The proposal demonstrates that CMS is listening to physicians’ concerns about CAR T payments and working to provide a more reasonable framework, said Stephanie Farnia, director of health policy and strategic relations for the American Society for Blood and Marrow Transplantation.
“The primary point of significance is that CAR-T care episodes should be assigned to a specific MS-DRG in FY2019, which will give physicians a clearer sense of inpatient reimbursement in advance,” she said in an interview.
Uncertainty about inpatient payment for administration of the two approved CAR-T therapies have been a lingering concern of specialists using, or interested in using, the therapies. In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians noted that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay per CMS’ 3-day payment window rule.
In outlining the most recent payment proposal, CMS stated that its clinical advisers believe that patients receiving treatment with CAR T-cell therapy would have similar clinical characteristics and comorbidities as patients treated with autologous bone marrow transplant therapy, who are currently assigned to MS-DRG 016 “Autologous Bone Marrow Transplant with CC/MCC”. Therefore, CMS officials said they are suggesting ICD-10-PCS procedure codes XW033C3 and XW043C3 to pre-MDC MS-DRG 016. Additionally, the agency is proposing to revise the title of MS-DRG 016 from “Autologous Bone Marrow Transplant with CC/MCC” to “Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy.”
However, the agency emphasized that it invites public comment on alternative payment approaches for CAR T-cell therapies in the context of the pending, new technology add-on payment applications by the CAR-T drugmakers Novartis Pharmaceuticals and Kite Pharma. If approved in the final rule, the technology add-on payments would provide an additional and separate payment equivalent to up to 50% of the product cost plus the MS-DRG payment received for the episode of care.
Other items in the proposed rule include approximate base payments for hematopoietic stem cell transplantation for fiscal 2019, which starts Oct. 1, 2018. The approximate per unit payment is $5,498, which translates to a total base payment of $64,790 for allogeneic bone marrow transplant (MS-DRG 014) for instance. In an American Society for Blood and Marrow Transplantation blog, Ms. Farnia outlined the approximate base weights and estimated reimbursements calculated from the rule.
CMS did not make any changes to the payment model for allogeneic hematopoietic cell transplantation despite continued requests by physicians for donor acquisition charges. Ms. Farnia encourages doctors to express to CMS the importance of such reimbursement during the comment period.
Public comments on the inpatient payment proposal are due by June 25, 2018, and can be submitted at https://www.regulations.gov. Additionally, CMS will convene a meeting of the Medicare Evidence Development & Coverage Advisory Committee on August 22, 2018, to consider whether to issue a national coverage policy for CAR T-cell therapies that would set consistent parameters for patient access.
Simplified HCT-CI better predicts outcomes in young patients
NEWPORT BEACH, CALIF. – A revised hematopoietic stem cell transplantation comorbidity index developed for adolescents and young adults is useful for predicting nonrelapse mortality in this specific population, according to Brian Friend, MD.
In a retrospective study of 241 patients aged 15-39 years who underwent a first allogeneic hematopoietic stem cell transplant (HCT) between 2005 and 2015 at the University of California, Los Angeles, nonrelapse mortality incidence was particularly high, with rates of 26%, 28%, and 30% at 1, 2, and 3 years, respectively, Dr. Friend, a clinical research fellow at the David Geffen School of Medicine, Los Angeles, reported in a poster at the Acute Leukemia Forum of Hemedicus.
Rather, a history of pulmonary disease – found in 44% of the patients – was the most common comorbidity, and although this was based on pulmonary function tests alone and not necessarily on patient symptoms, it was a surprising finding, he said. It was associated with lower overall survival and with nonrelapse mortality, he added.
A psychosocial component, which took into account factors such as stressors, social support, financial issues, and substance abuse, was also fairly frequent in the patients, but was not necessarily associated with worse outcomes, he noted.
“In multivariable analysis, only a history of prior malignancy (hazard ratio, 2.04) and moderate and severe pulmonary disease (hazard ratios, 1.39 and 1.84, respectively) were associated with a higher incidence of nonrelapse mortality,” he reported.
The existing HCT-CI was developed in adults to help risk-stratify patients undergoing transplant, but adolescents and young adults undergoing HCT tend to have fewer comorbidities compared with older adults, though they still having a significant nonrelapse mortality rate, Dr. Friend said.
“We sought to develop a modified HCT-CI that would be more practical and efficient in predicting outcomes of adolescent and young adult patients,” he wrote.
Data were collected on 15 comorbidities included in the original HCT-CI study, as well as the psychosocial risk factors. The relationship between multiple variables and the incidence of nonrelapse mortality was investigated via the Fine and Gray competing risk model with adjustments for patient- and transplant-specific factors.
A few things were “looked at differently,” he said, explaining, for example, that multiple cardiovascular risk factors were combined into one since they are rare in younger patients.
The study demonstrated that an index including only a few comorbidities important in adolescents and young adults is more predictive in these younger patients vs. adults, suggesting that a simpler model is more practical and useful, Dr. Friend said.
A larger study is planned in conjunction with the Center for International Blood and Marrow Transplant Research (CIBMTR). The researchers for that study will take an in-depth look at this younger population in an effort to develop a novel risk score for them. Other future efforts will focus on developing interventions to target high risk patients – and in particular, modifiable risk factors – with a focus on preventive measures, he said.
Dr. Friend reported having no financial disclosures.
NEWPORT BEACH, CALIF. – A revised hematopoietic stem cell transplantation comorbidity index developed for adolescents and young adults is useful for predicting nonrelapse mortality in this specific population, according to Brian Friend, MD.
In a retrospective study of 241 patients aged 15-39 years who underwent a first allogeneic hematopoietic stem cell transplant (HCT) between 2005 and 2015 at the University of California, Los Angeles, nonrelapse mortality incidence was particularly high, with rates of 26%, 28%, and 30% at 1, 2, and 3 years, respectively, Dr. Friend, a clinical research fellow at the David Geffen School of Medicine, Los Angeles, reported in a poster at the Acute Leukemia Forum of Hemedicus.
Rather, a history of pulmonary disease – found in 44% of the patients – was the most common comorbidity, and although this was based on pulmonary function tests alone and not necessarily on patient symptoms, it was a surprising finding, he said. It was associated with lower overall survival and with nonrelapse mortality, he added.
A psychosocial component, which took into account factors such as stressors, social support, financial issues, and substance abuse, was also fairly frequent in the patients, but was not necessarily associated with worse outcomes, he noted.
“In multivariable analysis, only a history of prior malignancy (hazard ratio, 2.04) and moderate and severe pulmonary disease (hazard ratios, 1.39 and 1.84, respectively) were associated with a higher incidence of nonrelapse mortality,” he reported.
The existing HCT-CI was developed in adults to help risk-stratify patients undergoing transplant, but adolescents and young adults undergoing HCT tend to have fewer comorbidities compared with older adults, though they still having a significant nonrelapse mortality rate, Dr. Friend said.
“We sought to develop a modified HCT-CI that would be more practical and efficient in predicting outcomes of adolescent and young adult patients,” he wrote.
Data were collected on 15 comorbidities included in the original HCT-CI study, as well as the psychosocial risk factors. The relationship between multiple variables and the incidence of nonrelapse mortality was investigated via the Fine and Gray competing risk model with adjustments for patient- and transplant-specific factors.
A few things were “looked at differently,” he said, explaining, for example, that multiple cardiovascular risk factors were combined into one since they are rare in younger patients.
The study demonstrated that an index including only a few comorbidities important in adolescents and young adults is more predictive in these younger patients vs. adults, suggesting that a simpler model is more practical and useful, Dr. Friend said.
A larger study is planned in conjunction with the Center for International Blood and Marrow Transplant Research (CIBMTR). The researchers for that study will take an in-depth look at this younger population in an effort to develop a novel risk score for them. Other future efforts will focus on developing interventions to target high risk patients – and in particular, modifiable risk factors – with a focus on preventive measures, he said.
Dr. Friend reported having no financial disclosures.
NEWPORT BEACH, CALIF. – A revised hematopoietic stem cell transplantation comorbidity index developed for adolescents and young adults is useful for predicting nonrelapse mortality in this specific population, according to Brian Friend, MD.
In a retrospective study of 241 patients aged 15-39 years who underwent a first allogeneic hematopoietic stem cell transplant (HCT) between 2005 and 2015 at the University of California, Los Angeles, nonrelapse mortality incidence was particularly high, with rates of 26%, 28%, and 30% at 1, 2, and 3 years, respectively, Dr. Friend, a clinical research fellow at the David Geffen School of Medicine, Los Angeles, reported in a poster at the Acute Leukemia Forum of Hemedicus.
Rather, a history of pulmonary disease – found in 44% of the patients – was the most common comorbidity, and although this was based on pulmonary function tests alone and not necessarily on patient symptoms, it was a surprising finding, he said. It was associated with lower overall survival and with nonrelapse mortality, he added.
A psychosocial component, which took into account factors such as stressors, social support, financial issues, and substance abuse, was also fairly frequent in the patients, but was not necessarily associated with worse outcomes, he noted.
“In multivariable analysis, only a history of prior malignancy (hazard ratio, 2.04) and moderate and severe pulmonary disease (hazard ratios, 1.39 and 1.84, respectively) were associated with a higher incidence of nonrelapse mortality,” he reported.
The existing HCT-CI was developed in adults to help risk-stratify patients undergoing transplant, but adolescents and young adults undergoing HCT tend to have fewer comorbidities compared with older adults, though they still having a significant nonrelapse mortality rate, Dr. Friend said.
“We sought to develop a modified HCT-CI that would be more practical and efficient in predicting outcomes of adolescent and young adult patients,” he wrote.
Data were collected on 15 comorbidities included in the original HCT-CI study, as well as the psychosocial risk factors. The relationship between multiple variables and the incidence of nonrelapse mortality was investigated via the Fine and Gray competing risk model with adjustments for patient- and transplant-specific factors.
A few things were “looked at differently,” he said, explaining, for example, that multiple cardiovascular risk factors were combined into one since they are rare in younger patients.
The study demonstrated that an index including only a few comorbidities important in adolescents and young adults is more predictive in these younger patients vs. adults, suggesting that a simpler model is more practical and useful, Dr. Friend said.
A larger study is planned in conjunction with the Center for International Blood and Marrow Transplant Research (CIBMTR). The researchers for that study will take an in-depth look at this younger population in an effort to develop a novel risk score for them. Other future efforts will focus on developing interventions to target high risk patients – and in particular, modifiable risk factors – with a focus on preventive measures, he said.
Dr. Friend reported having no financial disclosures.
REPORTING FROM ALF 2018
Key clinical point:
Major finding: As many as 60% of the comorbidities included in the HCT-CI had no significant prevalence in young patients.
Study details: A retrospective study of 241 adolescents and young adults.
Disclosures: Dr. Friend reported having no financial disclosures.
Source: Friend B et al. ALF 2018, Poster Session.
Genetic markers may help predict allogeneic SCT outcomes
SALT LAKE CITY – The presence of p2X7 receptor single nucleotide polymorphisms (SNPs) associated with gain and loss of function may help predict outcomes after allogeneic stem cell transplantation, according to findings from a clinical correlate analysis of recipient and donor DNA samples.
The findings require validation in future studies, but suggest that the presence of the SNPs and p2X7 haplotypes 2 and 4 could be incorporated into disease risk models to improve transplant decision making, David Stuart Ritchie, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The analysis, which specifically looked for the presence of 16 previously identified SNPs and the haplotypes 2 and 4 in p2x7, was performed on pretransplant DNA samples from 333 allogeneic stem cell transplant recipients and 228 donors at a single center between 2002 and 2013. The findings were correlated with patient outcomes.
Five SNPs were excluded from correlation because of low frequency, and of the 11 remaining SNPs, 3 were found to be significantly associated with reduced incidence of acute and/or chronic graft-versus-host disease (GVHD), and 2 were significantly associated with increased relapse or transplant-related mortality, said Dr. Ritchie of the University of Melbourne, Parkville, Australia.
The loss-of-function SNPs rs28360457 and rs3751133 – each linked with decreased inflammation – were significantly associated with a reduced incidence of acute GVHD when comparing grade 0 with grades 1-4 GVHD (P = .0234 and P = .0411, respectively), but not when comparing grades 0-1 and grades 2-4 GVHD.
SNP rs3751133 was also significantly associated with reduced incidence of chronic GVHD when comparing grade 0 with grades 0-4 GVHD (P = .01), but not when comparing grades 0-1 and grades 2-4 GVHD, he said.
The loss-of-function SNP rs1653624 – which is linked with decreased phagocytosis – was associated with an increased incidence of acute GVHD when comparing grade 0 vs. grade 1-4 GVHD (P = .01), but not when comparing grades 0-1 and grades 2-4 GVHD (P = NS).
SNP rs7958311, which had increased surface expression, was associated with a trend toward increased relapse risk (P = .053), and the loss-of-function SNP rs1653624 was associated with an excess of early transplant-related mortality (P = .0471).
“Individual SNPs are interesting, but perhaps more interesting are the haplotypes,” Dr. Ritchie said.
Haplotype 4, which was found in 8 recipients, involves rs1718119 and rs7958311. Those SNPs were previously shown to have 300% and 195% increased expression, respectively, with or without rs2230912, which has been shown to be decreased by 72%, and with or without loss of function rs1653624. Haplotype 4 is associated with a net increase in p2X7 activity, he explained.
In the current study, haplotype 4 was only found to involve rs1718119 co-inherited with rs2230912, and was associated with substantially decreased relapse-free survival overall (hazard ratio, 0.6946), when compared with haplotype 2 (HR, 0.2078), he said.
The differences between haplotype 4 and haplotype 2, and between haplotype 4 and patients with neither haplotype, were highly statistically significant.
Relapse-free survival did not differ significantly between those with haplotype 2 and those with neither haplotype (HR, 0.7717). Similarly, overall survival was significantly poorer among those with haplotype 4 versus haplotype 2 or no haplotype (HR, 0.2812 and 0.2882, respectively), but no difference was seen in overall survival between those with haplotype 2 and those with no haplotype (HR, 1.003), he said.
P2X7 is a purinergic signaling receptor located at chromosome 12q24–a region associated with inflammatory disorders. It plays an important role in immunogenic cell death. It is expressed in all leukocytes, with the highest level of expression seen in monocyte lineage. Binding of its ligand – extracellular adenosine 5’-triphosphate – leads to activation of dendritic cells and release of IL1b leading to T cell recruitment and the production of memory T cells.
Both gain- and loss-of-function SNPs in p2X7 have been reported and implicated in GVHD and other inflammatory disorders, Dr. Ritchie said, explaining the rationale for studying their correlations with outcomes after allogeneic stem cell transplantation.
While such transplants are highly effective for treating hematologic malignancies, outcomes can be adversely affected by infection, acute organ dysfunction, and GVHD. Pretransplant conditioning regimens are associated with high levels of immunogenic cell death and the release of extracellular adenosine 5’-triphosphate, therefore signaling through the p2X7 receptor may lead to activation of downstream effectors that influence transplant outcome, he noted.
“We hypothesized that germline gain or loss of function polymorphisms in this receptor in recipients of allogeneic transplantation would result in an adverse outcome,” he said.
The mean age of the recipients whose samples were analyzed was 46 years, and about half were women. Most (83.8%) had a peripheral blood graft source and 64% of transplants were from related donors. The nonrelapse mortality at 24 months was 12.98%, Dr. Ritchie said, noting that their indications for transplantation were “fairly representative of the adult transplant population, dominated by acute leukemia with a range of other acute conditions.”
The findings – particularly those with respect to haplotype 4, which had the most substantial impact – could play a role in patient risk assessment.
“Potentially, although it is a relatively uncommon haplotype, pretransplant identification of haplotype 4 may well have implications for transplant decision making, given the fact that the majority of our patients with this haplotype did not survive posttransplant,” he concluded, noting that an effort to validate the findings is ongoing in an additional 300 patients.
Dr. Ritchie reported having no financial disclosures.
SOURCE: Koldej R et al. The 2018 BMT Tandem Meetings, Abstract 22.
SALT LAKE CITY – The presence of p2X7 receptor single nucleotide polymorphisms (SNPs) associated with gain and loss of function may help predict outcomes after allogeneic stem cell transplantation, according to findings from a clinical correlate analysis of recipient and donor DNA samples.
The findings require validation in future studies, but suggest that the presence of the SNPs and p2X7 haplotypes 2 and 4 could be incorporated into disease risk models to improve transplant decision making, David Stuart Ritchie, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The analysis, which specifically looked for the presence of 16 previously identified SNPs and the haplotypes 2 and 4 in p2x7, was performed on pretransplant DNA samples from 333 allogeneic stem cell transplant recipients and 228 donors at a single center between 2002 and 2013. The findings were correlated with patient outcomes.
Five SNPs were excluded from correlation because of low frequency, and of the 11 remaining SNPs, 3 were found to be significantly associated with reduced incidence of acute and/or chronic graft-versus-host disease (GVHD), and 2 were significantly associated with increased relapse or transplant-related mortality, said Dr. Ritchie of the University of Melbourne, Parkville, Australia.
The loss-of-function SNPs rs28360457 and rs3751133 – each linked with decreased inflammation – were significantly associated with a reduced incidence of acute GVHD when comparing grade 0 with grades 1-4 GVHD (P = .0234 and P = .0411, respectively), but not when comparing grades 0-1 and grades 2-4 GVHD.
SNP rs3751133 was also significantly associated with reduced incidence of chronic GVHD when comparing grade 0 with grades 0-4 GVHD (P = .01), but not when comparing grades 0-1 and grades 2-4 GVHD, he said.
The loss-of-function SNP rs1653624 – which is linked with decreased phagocytosis – was associated with an increased incidence of acute GVHD when comparing grade 0 vs. grade 1-4 GVHD (P = .01), but not when comparing grades 0-1 and grades 2-4 GVHD (P = NS).
SNP rs7958311, which had increased surface expression, was associated with a trend toward increased relapse risk (P = .053), and the loss-of-function SNP rs1653624 was associated with an excess of early transplant-related mortality (P = .0471).
“Individual SNPs are interesting, but perhaps more interesting are the haplotypes,” Dr. Ritchie said.
Haplotype 4, which was found in 8 recipients, involves rs1718119 and rs7958311. Those SNPs were previously shown to have 300% and 195% increased expression, respectively, with or without rs2230912, which has been shown to be decreased by 72%, and with or without loss of function rs1653624. Haplotype 4 is associated with a net increase in p2X7 activity, he explained.
In the current study, haplotype 4 was only found to involve rs1718119 co-inherited with rs2230912, and was associated with substantially decreased relapse-free survival overall (hazard ratio, 0.6946), when compared with haplotype 2 (HR, 0.2078), he said.
The differences between haplotype 4 and haplotype 2, and between haplotype 4 and patients with neither haplotype, were highly statistically significant.
Relapse-free survival did not differ significantly between those with haplotype 2 and those with neither haplotype (HR, 0.7717). Similarly, overall survival was significantly poorer among those with haplotype 4 versus haplotype 2 or no haplotype (HR, 0.2812 and 0.2882, respectively), but no difference was seen in overall survival between those with haplotype 2 and those with no haplotype (HR, 1.003), he said.
P2X7 is a purinergic signaling receptor located at chromosome 12q24–a region associated with inflammatory disorders. It plays an important role in immunogenic cell death. It is expressed in all leukocytes, with the highest level of expression seen in monocyte lineage. Binding of its ligand – extracellular adenosine 5’-triphosphate – leads to activation of dendritic cells and release of IL1b leading to T cell recruitment and the production of memory T cells.
Both gain- and loss-of-function SNPs in p2X7 have been reported and implicated in GVHD and other inflammatory disorders, Dr. Ritchie said, explaining the rationale for studying their correlations with outcomes after allogeneic stem cell transplantation.
While such transplants are highly effective for treating hematologic malignancies, outcomes can be adversely affected by infection, acute organ dysfunction, and GVHD. Pretransplant conditioning regimens are associated with high levels of immunogenic cell death and the release of extracellular adenosine 5’-triphosphate, therefore signaling through the p2X7 receptor may lead to activation of downstream effectors that influence transplant outcome, he noted.
“We hypothesized that germline gain or loss of function polymorphisms in this receptor in recipients of allogeneic transplantation would result in an adverse outcome,” he said.
The mean age of the recipients whose samples were analyzed was 46 years, and about half were women. Most (83.8%) had a peripheral blood graft source and 64% of transplants were from related donors. The nonrelapse mortality at 24 months was 12.98%, Dr. Ritchie said, noting that their indications for transplantation were “fairly representative of the adult transplant population, dominated by acute leukemia with a range of other acute conditions.”
The findings – particularly those with respect to haplotype 4, which had the most substantial impact – could play a role in patient risk assessment.
“Potentially, although it is a relatively uncommon haplotype, pretransplant identification of haplotype 4 may well have implications for transplant decision making, given the fact that the majority of our patients with this haplotype did not survive posttransplant,” he concluded, noting that an effort to validate the findings is ongoing in an additional 300 patients.
Dr. Ritchie reported having no financial disclosures.
SOURCE: Koldej R et al. The 2018 BMT Tandem Meetings, Abstract 22.
SALT LAKE CITY – The presence of p2X7 receptor single nucleotide polymorphisms (SNPs) associated with gain and loss of function may help predict outcomes after allogeneic stem cell transplantation, according to findings from a clinical correlate analysis of recipient and donor DNA samples.
The findings require validation in future studies, but suggest that the presence of the SNPs and p2X7 haplotypes 2 and 4 could be incorporated into disease risk models to improve transplant decision making, David Stuart Ritchie, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The analysis, which specifically looked for the presence of 16 previously identified SNPs and the haplotypes 2 and 4 in p2x7, was performed on pretransplant DNA samples from 333 allogeneic stem cell transplant recipients and 228 donors at a single center between 2002 and 2013. The findings were correlated with patient outcomes.
Five SNPs were excluded from correlation because of low frequency, and of the 11 remaining SNPs, 3 were found to be significantly associated with reduced incidence of acute and/or chronic graft-versus-host disease (GVHD), and 2 were significantly associated with increased relapse or transplant-related mortality, said Dr. Ritchie of the University of Melbourne, Parkville, Australia.
The loss-of-function SNPs rs28360457 and rs3751133 – each linked with decreased inflammation – were significantly associated with a reduced incidence of acute GVHD when comparing grade 0 with grades 1-4 GVHD (P = .0234 and P = .0411, respectively), but not when comparing grades 0-1 and grades 2-4 GVHD.
SNP rs3751133 was also significantly associated with reduced incidence of chronic GVHD when comparing grade 0 with grades 0-4 GVHD (P = .01), but not when comparing grades 0-1 and grades 2-4 GVHD, he said.
The loss-of-function SNP rs1653624 – which is linked with decreased phagocytosis – was associated with an increased incidence of acute GVHD when comparing grade 0 vs. grade 1-4 GVHD (P = .01), but not when comparing grades 0-1 and grades 2-4 GVHD (P = NS).
SNP rs7958311, which had increased surface expression, was associated with a trend toward increased relapse risk (P = .053), and the loss-of-function SNP rs1653624 was associated with an excess of early transplant-related mortality (P = .0471).
“Individual SNPs are interesting, but perhaps more interesting are the haplotypes,” Dr. Ritchie said.
Haplotype 4, which was found in 8 recipients, involves rs1718119 and rs7958311. Those SNPs were previously shown to have 300% and 195% increased expression, respectively, with or without rs2230912, which has been shown to be decreased by 72%, and with or without loss of function rs1653624. Haplotype 4 is associated with a net increase in p2X7 activity, he explained.
In the current study, haplotype 4 was only found to involve rs1718119 co-inherited with rs2230912, and was associated with substantially decreased relapse-free survival overall (hazard ratio, 0.6946), when compared with haplotype 2 (HR, 0.2078), he said.
The differences between haplotype 4 and haplotype 2, and between haplotype 4 and patients with neither haplotype, were highly statistically significant.
Relapse-free survival did not differ significantly between those with haplotype 2 and those with neither haplotype (HR, 0.7717). Similarly, overall survival was significantly poorer among those with haplotype 4 versus haplotype 2 or no haplotype (HR, 0.2812 and 0.2882, respectively), but no difference was seen in overall survival between those with haplotype 2 and those with no haplotype (HR, 1.003), he said.
P2X7 is a purinergic signaling receptor located at chromosome 12q24–a region associated with inflammatory disorders. It plays an important role in immunogenic cell death. It is expressed in all leukocytes, with the highest level of expression seen in monocyte lineage. Binding of its ligand – extracellular adenosine 5’-triphosphate – leads to activation of dendritic cells and release of IL1b leading to T cell recruitment and the production of memory T cells.
Both gain- and loss-of-function SNPs in p2X7 have been reported and implicated in GVHD and other inflammatory disorders, Dr. Ritchie said, explaining the rationale for studying their correlations with outcomes after allogeneic stem cell transplantation.
While such transplants are highly effective for treating hematologic malignancies, outcomes can be adversely affected by infection, acute organ dysfunction, and GVHD. Pretransplant conditioning regimens are associated with high levels of immunogenic cell death and the release of extracellular adenosine 5’-triphosphate, therefore signaling through the p2X7 receptor may lead to activation of downstream effectors that influence transplant outcome, he noted.
“We hypothesized that germline gain or loss of function polymorphisms in this receptor in recipients of allogeneic transplantation would result in an adverse outcome,” he said.
The mean age of the recipients whose samples were analyzed was 46 years, and about half were women. Most (83.8%) had a peripheral blood graft source and 64% of transplants were from related donors. The nonrelapse mortality at 24 months was 12.98%, Dr. Ritchie said, noting that their indications for transplantation were “fairly representative of the adult transplant population, dominated by acute leukemia with a range of other acute conditions.”
The findings – particularly those with respect to haplotype 4, which had the most substantial impact – could play a role in patient risk assessment.
“Potentially, although it is a relatively uncommon haplotype, pretransplant identification of haplotype 4 may well have implications for transplant decision making, given the fact that the majority of our patients with this haplotype did not survive posttransplant,” he concluded, noting that an effort to validate the findings is ongoing in an additional 300 patients.
Dr. Ritchie reported having no financial disclosures.
SOURCE: Koldej R et al. The 2018 BMT Tandem Meetings, Abstract 22.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Haplotype 4 was associated with substantially decreased relapse-free survival overall (hazard ratio, 0.6946) versus haplotype 2 (HR, 0.2078).
Study details: A clinical correlate analysis of 561 DNA samples.
Disclosures: Dr. Ritchie reported having no financial disclosures.
Source: Koldej R et al. The 2018 BMT Tandem Meetings, Abstract 22.
Study shows value of pretransplant assessment of function, endurance
SALT LAKE CITY – Comprehensive assessment of functional status and endurance prior to allogeneic hematopoietic cell transplantation (HCT) provides important insights into posttransplant outcomes, and when used in combination with other measures may improve the patient selection process, a chart review suggests.
In 349 patients, results of the prospective assessment of physical performance and endurance, along with HCT Comorbidity Index (HCT-CI) score and Karnofsky Performance Scale score (KPS), were compared with day 100-plus nonrelapse mortality and overall survival. The measures were also compared with the novel measures of hospital length of stay, and death during HCT admission, Shabnam Rehman, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
However, heart rate recovery in less than 3 minutes after performing 25 step-ups on each side was associated with shorter length of stay with 89% of those patients, compared with 11% of patients who were not able to recover their heart rate in less than 3 minutes, being discharged within 30 days, she said.
“Similarly, patients who are able to perform at least 11 sit-to-stands in 30 seconds are more likely to be discharged earlier (63% vs. 14% discharged within 30 days),” she said. “The converse is also true.”
That is, only 16% of those not able to recover their heart rate within 3 minutes had a 30-day or shorter stay, while 31% had at least a 60-day stay. In addition, just 13% of those with limited endurance had a 30-day stay or shorter, while 24% had at least a 60-day stay, she explained.
Further, patients with limited endurance, and those unable to perform 10 or more sit-to-stands in 30 seconds were more likely to die during their first transplant admission. Of those with limited endurance, 31% died during admission and 13% survived, and of those with good endurance 69% died during admission and 87% survived. Among patients who were unable to perform more than 10 sit-to-stands, 42% died during admission and 20% survived, and of those able to perform 11 or more, 38% died during admission, and 53% survived.
Overall survival was associated with age, KPS, HCT-CI, and age-adjusted HCT-CI, she noted.
“Patients who were over age 40 and more, and patients with a KPS of 60 or 70, belong to the high- to intermediate-risk group more likely to have decreased overall survival as has been shown in previous studies,” she said. “In addition to validating these findings, we also found that the semiquantitative measures, including pain and endurance, were also associated with overall survival.”
Those with pain present or limited endurance had significantly poorer overall survival (P = .007 and P = .01, respectively), and this finding was reflected in the quantitative measures of sit-to-stands (P = .01) and step-ups (P = .001), even when stratified by age-adjusted HCT-CI, she said.
In addition, a number of risk factors present at the pretreatment assessment were found to be significantly associated with requirement of an assistive device at discharge. These included pain, weakness in the lower extremities, use of an assistive device, inability to perform 25 step-ups and more than 10 sit-to-stands in 30 seconds, and limited endurance (P values ranging from .02 to less than .0001). Requirement of a device was associated with poorer overall survival (P = .03), she said.
Study participants were adults aged 18 years and older (median, 58 years) undergoing a first allogeneic HCT at a single center between 2010 and 2016. Most (83%) were older than age 40 years and 58% were men. About half (51%) had acute myeloid leukemia, and 64% overall had a KPS score of 60-70.
Physical therapists assessed physical performance of all patients within 4 weeks pre-HCT; testing included 25 7-inch step-ups on each side, unassisted sit-to-stands from an 18-inch chair in 30 seconds, weight-bearing ability, need for assistance with ambulation, motor strength in four extremities, sensory or coordination impairment, self-reported pain, and time to recovery of heart rate and oxygen saturation to pre-exercise levels.
“The HCT-CI is a validated tool that predicts nonrelapse mortality and overall survival, but comorbidity alone as a single domain is not a surrogate of overall health or reflection on the true biological age of our patients,” Dr. Rehman said, noting that studies have shown that functional impairment is associated with shorter overall survival, and that patient-reported physical functioning is predictive of overall survival. “The assessment of functional impairment becomes more critical given the aging U.S. population and older patients receiving transplant.”
Traditionally, functional status has been assessed via the KPS, which is a subjective measure and lacks precision, and the HCT-CI has not been studied in the context of the novel outcome measures addressed in the current study, she noted.
The current findings highlight the prognostic value of a more quantitative pretransplant assessment, which can help improve the patient selection process.
“We are in the process of analyzing some more outcomes of these pretransplant assessments, and developing a score that can, in conjunction with other predictive tools, help us improve pretransplant risk stratification and devise interventions that can improve the endurance and overall survival of the patients,” she concluded.
Dr. Rehman reported having no financial disclosures.
SOURCE: Rehman S et al., The 2018 BTM Tandem Meetings, Abstract 19.
SALT LAKE CITY – Comprehensive assessment of functional status and endurance prior to allogeneic hematopoietic cell transplantation (HCT) provides important insights into posttransplant outcomes, and when used in combination with other measures may improve the patient selection process, a chart review suggests.
In 349 patients, results of the prospective assessment of physical performance and endurance, along with HCT Comorbidity Index (HCT-CI) score and Karnofsky Performance Scale score (KPS), were compared with day 100-plus nonrelapse mortality and overall survival. The measures were also compared with the novel measures of hospital length of stay, and death during HCT admission, Shabnam Rehman, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
However, heart rate recovery in less than 3 minutes after performing 25 step-ups on each side was associated with shorter length of stay with 89% of those patients, compared with 11% of patients who were not able to recover their heart rate in less than 3 minutes, being discharged within 30 days, she said.
“Similarly, patients who are able to perform at least 11 sit-to-stands in 30 seconds are more likely to be discharged earlier (63% vs. 14% discharged within 30 days),” she said. “The converse is also true.”
That is, only 16% of those not able to recover their heart rate within 3 minutes had a 30-day or shorter stay, while 31% had at least a 60-day stay. In addition, just 13% of those with limited endurance had a 30-day stay or shorter, while 24% had at least a 60-day stay, she explained.
Further, patients with limited endurance, and those unable to perform 10 or more sit-to-stands in 30 seconds were more likely to die during their first transplant admission. Of those with limited endurance, 31% died during admission and 13% survived, and of those with good endurance 69% died during admission and 87% survived. Among patients who were unable to perform more than 10 sit-to-stands, 42% died during admission and 20% survived, and of those able to perform 11 or more, 38% died during admission, and 53% survived.
Overall survival was associated with age, KPS, HCT-CI, and age-adjusted HCT-CI, she noted.
“Patients who were over age 40 and more, and patients with a KPS of 60 or 70, belong to the high- to intermediate-risk group more likely to have decreased overall survival as has been shown in previous studies,” she said. “In addition to validating these findings, we also found that the semiquantitative measures, including pain and endurance, were also associated with overall survival.”
Those with pain present or limited endurance had significantly poorer overall survival (P = .007 and P = .01, respectively), and this finding was reflected in the quantitative measures of sit-to-stands (P = .01) and step-ups (P = .001), even when stratified by age-adjusted HCT-CI, she said.
In addition, a number of risk factors present at the pretreatment assessment were found to be significantly associated with requirement of an assistive device at discharge. These included pain, weakness in the lower extremities, use of an assistive device, inability to perform 25 step-ups and more than 10 sit-to-stands in 30 seconds, and limited endurance (P values ranging from .02 to less than .0001). Requirement of a device was associated with poorer overall survival (P = .03), she said.
Study participants were adults aged 18 years and older (median, 58 years) undergoing a first allogeneic HCT at a single center between 2010 and 2016. Most (83%) were older than age 40 years and 58% were men. About half (51%) had acute myeloid leukemia, and 64% overall had a KPS score of 60-70.
Physical therapists assessed physical performance of all patients within 4 weeks pre-HCT; testing included 25 7-inch step-ups on each side, unassisted sit-to-stands from an 18-inch chair in 30 seconds, weight-bearing ability, need for assistance with ambulation, motor strength in four extremities, sensory or coordination impairment, self-reported pain, and time to recovery of heart rate and oxygen saturation to pre-exercise levels.
“The HCT-CI is a validated tool that predicts nonrelapse mortality and overall survival, but comorbidity alone as a single domain is not a surrogate of overall health or reflection on the true biological age of our patients,” Dr. Rehman said, noting that studies have shown that functional impairment is associated with shorter overall survival, and that patient-reported physical functioning is predictive of overall survival. “The assessment of functional impairment becomes more critical given the aging U.S. population and older patients receiving transplant.”
Traditionally, functional status has been assessed via the KPS, which is a subjective measure and lacks precision, and the HCT-CI has not been studied in the context of the novel outcome measures addressed in the current study, she noted.
The current findings highlight the prognostic value of a more quantitative pretransplant assessment, which can help improve the patient selection process.
“We are in the process of analyzing some more outcomes of these pretransplant assessments, and developing a score that can, in conjunction with other predictive tools, help us improve pretransplant risk stratification and devise interventions that can improve the endurance and overall survival of the patients,” she concluded.
Dr. Rehman reported having no financial disclosures.
SOURCE: Rehman S et al., The 2018 BTM Tandem Meetings, Abstract 19.
SALT LAKE CITY – Comprehensive assessment of functional status and endurance prior to allogeneic hematopoietic cell transplantation (HCT) provides important insights into posttransplant outcomes, and when used in combination with other measures may improve the patient selection process, a chart review suggests.
In 349 patients, results of the prospective assessment of physical performance and endurance, along with HCT Comorbidity Index (HCT-CI) score and Karnofsky Performance Scale score (KPS), were compared with day 100-plus nonrelapse mortality and overall survival. The measures were also compared with the novel measures of hospital length of stay, and death during HCT admission, Shabnam Rehman, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
However, heart rate recovery in less than 3 minutes after performing 25 step-ups on each side was associated with shorter length of stay with 89% of those patients, compared with 11% of patients who were not able to recover their heart rate in less than 3 minutes, being discharged within 30 days, she said.
“Similarly, patients who are able to perform at least 11 sit-to-stands in 30 seconds are more likely to be discharged earlier (63% vs. 14% discharged within 30 days),” she said. “The converse is also true.”
That is, only 16% of those not able to recover their heart rate within 3 minutes had a 30-day or shorter stay, while 31% had at least a 60-day stay. In addition, just 13% of those with limited endurance had a 30-day stay or shorter, while 24% had at least a 60-day stay, she explained.
Further, patients with limited endurance, and those unable to perform 10 or more sit-to-stands in 30 seconds were more likely to die during their first transplant admission. Of those with limited endurance, 31% died during admission and 13% survived, and of those with good endurance 69% died during admission and 87% survived. Among patients who were unable to perform more than 10 sit-to-stands, 42% died during admission and 20% survived, and of those able to perform 11 or more, 38% died during admission, and 53% survived.
Overall survival was associated with age, KPS, HCT-CI, and age-adjusted HCT-CI, she noted.
“Patients who were over age 40 and more, and patients with a KPS of 60 or 70, belong to the high- to intermediate-risk group more likely to have decreased overall survival as has been shown in previous studies,” she said. “In addition to validating these findings, we also found that the semiquantitative measures, including pain and endurance, were also associated with overall survival.”
Those with pain present or limited endurance had significantly poorer overall survival (P = .007 and P = .01, respectively), and this finding was reflected in the quantitative measures of sit-to-stands (P = .01) and step-ups (P = .001), even when stratified by age-adjusted HCT-CI, she said.
In addition, a number of risk factors present at the pretreatment assessment were found to be significantly associated with requirement of an assistive device at discharge. These included pain, weakness in the lower extremities, use of an assistive device, inability to perform 25 step-ups and more than 10 sit-to-stands in 30 seconds, and limited endurance (P values ranging from .02 to less than .0001). Requirement of a device was associated with poorer overall survival (P = .03), she said.
Study participants were adults aged 18 years and older (median, 58 years) undergoing a first allogeneic HCT at a single center between 2010 and 2016. Most (83%) were older than age 40 years and 58% were men. About half (51%) had acute myeloid leukemia, and 64% overall had a KPS score of 60-70.
Physical therapists assessed physical performance of all patients within 4 weeks pre-HCT; testing included 25 7-inch step-ups on each side, unassisted sit-to-stands from an 18-inch chair in 30 seconds, weight-bearing ability, need for assistance with ambulation, motor strength in four extremities, sensory or coordination impairment, self-reported pain, and time to recovery of heart rate and oxygen saturation to pre-exercise levels.
“The HCT-CI is a validated tool that predicts nonrelapse mortality and overall survival, but comorbidity alone as a single domain is not a surrogate of overall health or reflection on the true biological age of our patients,” Dr. Rehman said, noting that studies have shown that functional impairment is associated with shorter overall survival, and that patient-reported physical functioning is predictive of overall survival. “The assessment of functional impairment becomes more critical given the aging U.S. population and older patients receiving transplant.”
Traditionally, functional status has been assessed via the KPS, which is a subjective measure and lacks precision, and the HCT-CI has not been studied in the context of the novel outcome measures addressed in the current study, she noted.
The current findings highlight the prognostic value of a more quantitative pretransplant assessment, which can help improve the patient selection process.
“We are in the process of analyzing some more outcomes of these pretransplant assessments, and developing a score that can, in conjunction with other predictive tools, help us improve pretransplant risk stratification and devise interventions that can improve the endurance and overall survival of the patients,” she concluded.
Dr. Rehman reported having no financial disclosures.
SOURCE: Rehman S et al., The 2018 BTM Tandem Meetings, Abstract 19.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: The 30-day discharge rates were 89% versus 11% in those with and without good heart rate recovery, respectively.
Study details: A retrospective review of prospectively collected data for 349 patients.
Disclosures: Dr. Rehman reported having no financial disclosures.
Source: Rehman S et al. The 2018 BMT Tandem Meetings, Abstract 19.
Reduced intensity conditioning doesn’t protect fertility
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Of 21 males receiving reduced intensity conditioning or myeloablative conditioning, all but one had azoospermia or oligoteratospermia.
Study details: Observational cohort study of 41 males and 25 females receiving pediatric HSCT.
Disclosures: Dr. Oquendo del Toro reported having no conflicts of interest.
Source: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.