User login
ASCO details how to manage ongoing cancer drug shortage
As of November 30, the US Food and Drug Administration lists 16 commonly used oncology drugs currently in shortage, including methotrexate, capecitabine, vinblastine, carboplatin, and cisplatin, along with another 13 discontinued agents.
The ASCO guidance, which is updated regularly on ASCO’s drug shortage website, covers dozens of clinical situations involving breast, gastrointestinal, genitourinary, gynecologic, thoracic, and head & neck cancers, as well as Hodgkin lymphoma.
The recommendations, published earlier in JCO Oncology Practice, represent the work of a Drug Shortages Advisory Group with over 40 oncologists, ethicists, and patient advocates brought together by ASCO in collaboration with the Society for Gynecologic Oncology.
In the guidance, the advisory group also provides some context about why these shortage issues have persisted, including a paucity of generic options, quality control issues, and reluctance among manufacturers to produce older drugs with slim profit margins.
And “while ASCO continues to work to address the root causes of the shortages, this guidance document aims to support clinicians, as they navigate the complexities of treatment planning amid the drug shortage, and patients with cancer who are already enduring physical and emotional hardships,” the advisory group writes.
The overall message in the guidance: conserve oncology drugs in limited supply to use when needed most.
The recommendations highlight alternative regimens, when available, and what to do in situations when there are no alternatives, advice that has become particularly relevant for the oncology workhorses cisplatin and carboplatin.
More generally, when ranges of acceptable doses and dose frequencies exist for drugs in short supply, clinicians should opt for the lowest dose at the longest interval. Dose rounding and multi-use vials should also be used to eliminate waste, and alternatives should be used whenever possible. If an alternative agent with similar efficacy and safety is available, the agent in limited supply should not be ordered.
In certain settings where no reasonable alternatives to platinum regimens exist, the advisory group recommends patients travel to where platinum agents are available. The group noted this strategy specifically for patients with non–small cell lung cancer or testicular germ cell cancers, but also acknowledged that this option “may cause additional financial toxicity, hardship, and distress.”
Other, more granular advice includes holding carboplatin in reserve for patients with early-stage triple-negative breast cancer on neoadjuvant therapy who don’t respond well to upfront doxorubicin, cyclophosphamide, and pembrolizumab.
In addition to providing strategies to manage the ongoing cancer drug shortages, ASCO advises counseling for patients and clinicians struggling with the “psychological or moral distress” from the ongoing shortages.
“Unfortunately, drug shortages place the patient and the provider in a challenging situation, possibly resulting in inferior outcomes, delayed or denied care, and increased adverse events,” the advisory group writes. “ASCO will continue to respond to the oncology drug shortage crisis through policy and advocacy efforts, provide ethical guidance for allocation and prioritization decisions, and maintain shortage-specific clinical guidance as long as necessary.”
A version of this article appeared on Medscape.com.
As of November 30, the US Food and Drug Administration lists 16 commonly used oncology drugs currently in shortage, including methotrexate, capecitabine, vinblastine, carboplatin, and cisplatin, along with another 13 discontinued agents.
The ASCO guidance, which is updated regularly on ASCO’s drug shortage website, covers dozens of clinical situations involving breast, gastrointestinal, genitourinary, gynecologic, thoracic, and head & neck cancers, as well as Hodgkin lymphoma.
The recommendations, published earlier in JCO Oncology Practice, represent the work of a Drug Shortages Advisory Group with over 40 oncologists, ethicists, and patient advocates brought together by ASCO in collaboration with the Society for Gynecologic Oncology.
In the guidance, the advisory group also provides some context about why these shortage issues have persisted, including a paucity of generic options, quality control issues, and reluctance among manufacturers to produce older drugs with slim profit margins.
And “while ASCO continues to work to address the root causes of the shortages, this guidance document aims to support clinicians, as they navigate the complexities of treatment planning amid the drug shortage, and patients with cancer who are already enduring physical and emotional hardships,” the advisory group writes.
The overall message in the guidance: conserve oncology drugs in limited supply to use when needed most.
The recommendations highlight alternative regimens, when available, and what to do in situations when there are no alternatives, advice that has become particularly relevant for the oncology workhorses cisplatin and carboplatin.
More generally, when ranges of acceptable doses and dose frequencies exist for drugs in short supply, clinicians should opt for the lowest dose at the longest interval. Dose rounding and multi-use vials should also be used to eliminate waste, and alternatives should be used whenever possible. If an alternative agent with similar efficacy and safety is available, the agent in limited supply should not be ordered.
In certain settings where no reasonable alternatives to platinum regimens exist, the advisory group recommends patients travel to where platinum agents are available. The group noted this strategy specifically for patients with non–small cell lung cancer or testicular germ cell cancers, but also acknowledged that this option “may cause additional financial toxicity, hardship, and distress.”
Other, more granular advice includes holding carboplatin in reserve for patients with early-stage triple-negative breast cancer on neoadjuvant therapy who don’t respond well to upfront doxorubicin, cyclophosphamide, and pembrolizumab.
In addition to providing strategies to manage the ongoing cancer drug shortages, ASCO advises counseling for patients and clinicians struggling with the “psychological or moral distress” from the ongoing shortages.
“Unfortunately, drug shortages place the patient and the provider in a challenging situation, possibly resulting in inferior outcomes, delayed or denied care, and increased adverse events,” the advisory group writes. “ASCO will continue to respond to the oncology drug shortage crisis through policy and advocacy efforts, provide ethical guidance for allocation and prioritization decisions, and maintain shortage-specific clinical guidance as long as necessary.”
A version of this article appeared on Medscape.com.
As of November 30, the US Food and Drug Administration lists 16 commonly used oncology drugs currently in shortage, including methotrexate, capecitabine, vinblastine, carboplatin, and cisplatin, along with another 13 discontinued agents.
The ASCO guidance, which is updated regularly on ASCO’s drug shortage website, covers dozens of clinical situations involving breast, gastrointestinal, genitourinary, gynecologic, thoracic, and head & neck cancers, as well as Hodgkin lymphoma.
The recommendations, published earlier in JCO Oncology Practice, represent the work of a Drug Shortages Advisory Group with over 40 oncologists, ethicists, and patient advocates brought together by ASCO in collaboration with the Society for Gynecologic Oncology.
In the guidance, the advisory group also provides some context about why these shortage issues have persisted, including a paucity of generic options, quality control issues, and reluctance among manufacturers to produce older drugs with slim profit margins.
And “while ASCO continues to work to address the root causes of the shortages, this guidance document aims to support clinicians, as they navigate the complexities of treatment planning amid the drug shortage, and patients with cancer who are already enduring physical and emotional hardships,” the advisory group writes.
The overall message in the guidance: conserve oncology drugs in limited supply to use when needed most.
The recommendations highlight alternative regimens, when available, and what to do in situations when there are no alternatives, advice that has become particularly relevant for the oncology workhorses cisplatin and carboplatin.
More generally, when ranges of acceptable doses and dose frequencies exist for drugs in short supply, clinicians should opt for the lowest dose at the longest interval. Dose rounding and multi-use vials should also be used to eliminate waste, and alternatives should be used whenever possible. If an alternative agent with similar efficacy and safety is available, the agent in limited supply should not be ordered.
In certain settings where no reasonable alternatives to platinum regimens exist, the advisory group recommends patients travel to where platinum agents are available. The group noted this strategy specifically for patients with non–small cell lung cancer or testicular germ cell cancers, but also acknowledged that this option “may cause additional financial toxicity, hardship, and distress.”
Other, more granular advice includes holding carboplatin in reserve for patients with early-stage triple-negative breast cancer on neoadjuvant therapy who don’t respond well to upfront doxorubicin, cyclophosphamide, and pembrolizumab.
In addition to providing strategies to manage the ongoing cancer drug shortages, ASCO advises counseling for patients and clinicians struggling with the “psychological or moral distress” from the ongoing shortages.
“Unfortunately, drug shortages place the patient and the provider in a challenging situation, possibly resulting in inferior outcomes, delayed or denied care, and increased adverse events,” the advisory group writes. “ASCO will continue to respond to the oncology drug shortage crisis through policy and advocacy efforts, provide ethical guidance for allocation and prioritization decisions, and maintain shortage-specific clinical guidance as long as necessary.”
A version of this article appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
Avoid anti-HER2 cancer therapies during pregnancy
TOPLINE:
, according to a recent analysis.
METHODOLOGY:
- Current guidelines do not recommend treating pregnant women with trastuzumab, given documented safety concerns. Other anti-HER2 agents are also discouraged in this setting because of a lack of safety data. However, when considering the efficacy of these drugs in HER2-positive breast cancer, having a better understanding of the potential toxicities in pregnant patients is important.
- In the current case-control analysis, the team explored the risk for adverse effects among pregnant women exposed to anti-HER2 agents vs other anticancer drugs.
- The researchers leveraged the World Health Organization’s pharmacovigilance database, VigiBase, to identify reports with at least one pregnancy-related complication and one suspected anticancer drug.
- The researchers classified exposure to the drugs as occurring before pregnancy, during pregnancy, or via breast milk, semen, or skin. The team then examined 30 maternal and fetal or neonatal adverse outcomes and grouped them into seven categories: abortions, stillbirths, congenital malformations, pregnancy complications, preterm birth, neonatal complications, and delivery complications.
- The most used anti-HER2 agent was trastuzumab (n = 302), followed by pertuzumab (n = 55), trastuzumab-emtansine (n = 20), and lapatinib (n = 18).
TAKEAWAY:
- Among 3,558 reports included in the analysis, 328 patients were exposed to anti-HER2 drugs compared with 3,230 patients who received other anticancer agents.
- Pregnancy, fetal, or newborn adverse outcomes were reported in 61.3% of women treated with anti-HER2 agents and 56.3% of those receiving other anticancer drugs.
- The five most frequently reported complications in the anti-HER2 group were oligohydramnios (23.8%), preterm birth (17.4%), intrauterine growth restriction (9.8%), neonatal respiratory disorder (7.3%), and spontaneous abortion (7.3%).
- Adverse outcomes overreported in women who received anti-HER2 agents included oligohydramnios (reporting odds ratio [ROR], 17.68), congenital tract disorders (ROR, 9.98), and neonatal kidney failure (ROR, 9.15). Cardiovascular malformations were also overreported among women receiving trastuzumab-emtansine (ROR, 4.46), as were intrauterine growth restrictions for those treated with lapatinib (ROR, 7.68).
IN PRACTICE:
Exposure to anti-HER2 agents was associated with “severe specific adverse pregnancy and fetal or newborn outcomes compared with exposure to other anticancer treatments,” with a “strong, highly significant overreporting of congenital respiratory tract disorders and neonatal kidney failure,” which can lead to oligohydramnios, the authors wrote. The authors also noted that when delaying anti-HER2 therapy is not possible, it’s imperative to monitor patients closely for oligohydramnios.
SOURCE:
The study, led by Paul Gougis, MD, Institut Curie Centre de Recherche, Paris, , was published online in JAMA Network Open.
LIMITATIONS:
Potential inconsistencies in the collection of pharmacovigilance data could limit the generalizability of the results in the general population. The group of women exposed to other anticancer therapies may also constitute a different patient population from that given anti-HER2 therapies.
DISCLOSURES:
Coauthor Jean-Philippe Spano, MD, PhD, declared relationships Gilead, AstraZeneca, Lilly, Pfizer, Novartis, Daiichi Sankyo, and GSK.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent analysis.
METHODOLOGY:
- Current guidelines do not recommend treating pregnant women with trastuzumab, given documented safety concerns. Other anti-HER2 agents are also discouraged in this setting because of a lack of safety data. However, when considering the efficacy of these drugs in HER2-positive breast cancer, having a better understanding of the potential toxicities in pregnant patients is important.
- In the current case-control analysis, the team explored the risk for adverse effects among pregnant women exposed to anti-HER2 agents vs other anticancer drugs.
- The researchers leveraged the World Health Organization’s pharmacovigilance database, VigiBase, to identify reports with at least one pregnancy-related complication and one suspected anticancer drug.
- The researchers classified exposure to the drugs as occurring before pregnancy, during pregnancy, or via breast milk, semen, or skin. The team then examined 30 maternal and fetal or neonatal adverse outcomes and grouped them into seven categories: abortions, stillbirths, congenital malformations, pregnancy complications, preterm birth, neonatal complications, and delivery complications.
- The most used anti-HER2 agent was trastuzumab (n = 302), followed by pertuzumab (n = 55), trastuzumab-emtansine (n = 20), and lapatinib (n = 18).
TAKEAWAY:
- Among 3,558 reports included in the analysis, 328 patients were exposed to anti-HER2 drugs compared with 3,230 patients who received other anticancer agents.
- Pregnancy, fetal, or newborn adverse outcomes were reported in 61.3% of women treated with anti-HER2 agents and 56.3% of those receiving other anticancer drugs.
- The five most frequently reported complications in the anti-HER2 group were oligohydramnios (23.8%), preterm birth (17.4%), intrauterine growth restriction (9.8%), neonatal respiratory disorder (7.3%), and spontaneous abortion (7.3%).
- Adverse outcomes overreported in women who received anti-HER2 agents included oligohydramnios (reporting odds ratio [ROR], 17.68), congenital tract disorders (ROR, 9.98), and neonatal kidney failure (ROR, 9.15). Cardiovascular malformations were also overreported among women receiving trastuzumab-emtansine (ROR, 4.46), as were intrauterine growth restrictions for those treated with lapatinib (ROR, 7.68).
IN PRACTICE:
Exposure to anti-HER2 agents was associated with “severe specific adverse pregnancy and fetal or newborn outcomes compared with exposure to other anticancer treatments,” with a “strong, highly significant overreporting of congenital respiratory tract disorders and neonatal kidney failure,” which can lead to oligohydramnios, the authors wrote. The authors also noted that when delaying anti-HER2 therapy is not possible, it’s imperative to monitor patients closely for oligohydramnios.
SOURCE:
The study, led by Paul Gougis, MD, Institut Curie Centre de Recherche, Paris, , was published online in JAMA Network Open.
LIMITATIONS:
Potential inconsistencies in the collection of pharmacovigilance data could limit the generalizability of the results in the general population. The group of women exposed to other anticancer therapies may also constitute a different patient population from that given anti-HER2 therapies.
DISCLOSURES:
Coauthor Jean-Philippe Spano, MD, PhD, declared relationships Gilead, AstraZeneca, Lilly, Pfizer, Novartis, Daiichi Sankyo, and GSK.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent analysis.
METHODOLOGY:
- Current guidelines do not recommend treating pregnant women with trastuzumab, given documented safety concerns. Other anti-HER2 agents are also discouraged in this setting because of a lack of safety data. However, when considering the efficacy of these drugs in HER2-positive breast cancer, having a better understanding of the potential toxicities in pregnant patients is important.
- In the current case-control analysis, the team explored the risk for adverse effects among pregnant women exposed to anti-HER2 agents vs other anticancer drugs.
- The researchers leveraged the World Health Organization’s pharmacovigilance database, VigiBase, to identify reports with at least one pregnancy-related complication and one suspected anticancer drug.
- The researchers classified exposure to the drugs as occurring before pregnancy, during pregnancy, or via breast milk, semen, or skin. The team then examined 30 maternal and fetal or neonatal adverse outcomes and grouped them into seven categories: abortions, stillbirths, congenital malformations, pregnancy complications, preterm birth, neonatal complications, and delivery complications.
- The most used anti-HER2 agent was trastuzumab (n = 302), followed by pertuzumab (n = 55), trastuzumab-emtansine (n = 20), and lapatinib (n = 18).
TAKEAWAY:
- Among 3,558 reports included in the analysis, 328 patients were exposed to anti-HER2 drugs compared with 3,230 patients who received other anticancer agents.
- Pregnancy, fetal, or newborn adverse outcomes were reported in 61.3% of women treated with anti-HER2 agents and 56.3% of those receiving other anticancer drugs.
- The five most frequently reported complications in the anti-HER2 group were oligohydramnios (23.8%), preterm birth (17.4%), intrauterine growth restriction (9.8%), neonatal respiratory disorder (7.3%), and spontaneous abortion (7.3%).
- Adverse outcomes overreported in women who received anti-HER2 agents included oligohydramnios (reporting odds ratio [ROR], 17.68), congenital tract disorders (ROR, 9.98), and neonatal kidney failure (ROR, 9.15). Cardiovascular malformations were also overreported among women receiving trastuzumab-emtansine (ROR, 4.46), as were intrauterine growth restrictions for those treated with lapatinib (ROR, 7.68).
IN PRACTICE:
Exposure to anti-HER2 agents was associated with “severe specific adverse pregnancy and fetal or newborn outcomes compared with exposure to other anticancer treatments,” with a “strong, highly significant overreporting of congenital respiratory tract disorders and neonatal kidney failure,” which can lead to oligohydramnios, the authors wrote. The authors also noted that when delaying anti-HER2 therapy is not possible, it’s imperative to monitor patients closely for oligohydramnios.
SOURCE:
The study, led by Paul Gougis, MD, Institut Curie Centre de Recherche, Paris, , was published online in JAMA Network Open.
LIMITATIONS:
Potential inconsistencies in the collection of pharmacovigilance data could limit the generalizability of the results in the general population. The group of women exposed to other anticancer therapies may also constitute a different patient population from that given anti-HER2 therapies.
DISCLOSURES:
Coauthor Jean-Philippe Spano, MD, PhD, declared relationships Gilead, AstraZeneca, Lilly, Pfizer, Novartis, Daiichi Sankyo, and GSK.
A version of this article appeared on Medscape.com.
Commentary: Vaginal Estrogen Therapy, ILC, And Oral Estrogen Receptor Degraders In Breast Cancer, December 2023
Prior studies show inconsistent outcomes in patients with invasive lobular carcinoma (ILC) and data in premenopausal women is limited. The retrospective cohort study by Yoon and colleagues analyzed the data from three databases and included 225,938 premenopausal women with stage I-III ILC or invasive ductal carcinoma (IDC) in their study to evaluate survival trends in young women with ILC. In the Surveillance, Epidemiology, and End Results (SEER) database, patients with ILC vs IDC showed superior breast cancer severity score (BCSS) outcomes during the first 10 years after diagnosis (HR 0.73; P < .001); similar results were seen in the Asan Medical Center Research (AMCR) database (HR 0.50; 95% CI 0.29-0.86; P = .01). After 10 years, the trend reversed, and BCSS outcomes worsened by 80% in patients with ILC in the SEER database (HR 1.80; P < .001). This was also seen in both the Korean Breast Cancer Registry (HR 2.79; 95% CI 1.32-5.88; P = .007) and AMCR database (HR 2.23; 95% CI 1.04-4.79; P = .04). These findings remained consistent after adjusting for tumor characteristics including age, stage, tumor grade, hormone receptor status, and after controlling for treatment with chemotherapy and radiation. In addition, in the SEER database, the histologic type exerted a statistically significant time-dependent association with BCSS, with ILC showing decreasing BCSS over time (time interaction HR 1.93; 95% CI 1.78-2.10; P < .001). Furthermore, on annual hazard function analysis, the ILC annual peak event of BCSS occurred 5 years after diagnosis, whereas the IDC recurrence events peaked at 5 years before diagnosis, suggesting a higher late recurrence rate for ILC. These findings may have implications on the duration of endocrine therapy used in these patients given concern for worse long-term outcomes in premenopausal patients with ILC.
Oral selective estrogen receptor degraders (SERD) have recently emerged as a new therapeutic mechanism for patients with hormone receptor–positive breast cancer who have developed resistance to other endocrine therapies. Two of these agents, elacestrant and camizestrant, have demonstrated statistically significant progression-free survival benefit in these populations, particularly in tumors with ESR1 mutations. The efficacy of these agents in tumors with ESR1 wild-type subgroup remains uncertain. A meta-analysis by Wong and colleagues of individual patient data from four randomized clinical trials (ACELERA, AMEERA-3, EMERALD, and SERENA-2) included 1290 patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer who received oral SERD or endocrine therapies (ET) of the physician's choice. In the overall cohort, oral SERD showed improved progression-free survival (PFS) outcomes compared with ET of the physician's choice (HR 0.783; 95% CI 0.681-0.900; P < .001). This was also noted in the subgroup of patients with ESR1 mutations (HR 0.557; 95% CI 0.440-0.705; P < .001); although no significant PFS benefit was observed with oral SERD in the ESR1 wild-type subgroup (HR 0.944; 95% CI 0.783-1.138; P = .543). These results suggest that the PFS benefit observed with oral SERD is mainly seen in patients with ESR1-mutated tumors, and, therefore, these drugs should be prescribed accordingly.
Additional Reference
- Cold S, Cold F, Jensen M-B, et al. Systemic or vaginal hormone therapy after early breast cancer: A Danish observational cohort study. J Natl Cancer Inst. 2022;114:1347–1354. doi: 10.1093/jnci/djac112
Prior studies show inconsistent outcomes in patients with invasive lobular carcinoma (ILC) and data in premenopausal women is limited. The retrospective cohort study by Yoon and colleagues analyzed the data from three databases and included 225,938 premenopausal women with stage I-III ILC or invasive ductal carcinoma (IDC) in their study to evaluate survival trends in young women with ILC. In the Surveillance, Epidemiology, and End Results (SEER) database, patients with ILC vs IDC showed superior breast cancer severity score (BCSS) outcomes during the first 10 years after diagnosis (HR 0.73; P < .001); similar results were seen in the Asan Medical Center Research (AMCR) database (HR 0.50; 95% CI 0.29-0.86; P = .01). After 10 years, the trend reversed, and BCSS outcomes worsened by 80% in patients with ILC in the SEER database (HR 1.80; P < .001). This was also seen in both the Korean Breast Cancer Registry (HR 2.79; 95% CI 1.32-5.88; P = .007) and AMCR database (HR 2.23; 95% CI 1.04-4.79; P = .04). These findings remained consistent after adjusting for tumor characteristics including age, stage, tumor grade, hormone receptor status, and after controlling for treatment with chemotherapy and radiation. In addition, in the SEER database, the histologic type exerted a statistically significant time-dependent association with BCSS, with ILC showing decreasing BCSS over time (time interaction HR 1.93; 95% CI 1.78-2.10; P < .001). Furthermore, on annual hazard function analysis, the ILC annual peak event of BCSS occurred 5 years after diagnosis, whereas the IDC recurrence events peaked at 5 years before diagnosis, suggesting a higher late recurrence rate for ILC. These findings may have implications on the duration of endocrine therapy used in these patients given concern for worse long-term outcomes in premenopausal patients with ILC.
Oral selective estrogen receptor degraders (SERD) have recently emerged as a new therapeutic mechanism for patients with hormone receptor–positive breast cancer who have developed resistance to other endocrine therapies. Two of these agents, elacestrant and camizestrant, have demonstrated statistically significant progression-free survival benefit in these populations, particularly in tumors with ESR1 mutations. The efficacy of these agents in tumors with ESR1 wild-type subgroup remains uncertain. A meta-analysis by Wong and colleagues of individual patient data from four randomized clinical trials (ACELERA, AMEERA-3, EMERALD, and SERENA-2) included 1290 patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer who received oral SERD or endocrine therapies (ET) of the physician's choice. In the overall cohort, oral SERD showed improved progression-free survival (PFS) outcomes compared with ET of the physician's choice (HR 0.783; 95% CI 0.681-0.900; P < .001). This was also noted in the subgroup of patients with ESR1 mutations (HR 0.557; 95% CI 0.440-0.705; P < .001); although no significant PFS benefit was observed with oral SERD in the ESR1 wild-type subgroup (HR 0.944; 95% CI 0.783-1.138; P = .543). These results suggest that the PFS benefit observed with oral SERD is mainly seen in patients with ESR1-mutated tumors, and, therefore, these drugs should be prescribed accordingly.
Additional Reference
- Cold S, Cold F, Jensen M-B, et al. Systemic or vaginal hormone therapy after early breast cancer: A Danish observational cohort study. J Natl Cancer Inst. 2022;114:1347–1354. doi: 10.1093/jnci/djac112
Prior studies show inconsistent outcomes in patients with invasive lobular carcinoma (ILC) and data in premenopausal women is limited. The retrospective cohort study by Yoon and colleagues analyzed the data from three databases and included 225,938 premenopausal women with stage I-III ILC or invasive ductal carcinoma (IDC) in their study to evaluate survival trends in young women with ILC. In the Surveillance, Epidemiology, and End Results (SEER) database, patients with ILC vs IDC showed superior breast cancer severity score (BCSS) outcomes during the first 10 years after diagnosis (HR 0.73; P < .001); similar results were seen in the Asan Medical Center Research (AMCR) database (HR 0.50; 95% CI 0.29-0.86; P = .01). After 10 years, the trend reversed, and BCSS outcomes worsened by 80% in patients with ILC in the SEER database (HR 1.80; P < .001). This was also seen in both the Korean Breast Cancer Registry (HR 2.79; 95% CI 1.32-5.88; P = .007) and AMCR database (HR 2.23; 95% CI 1.04-4.79; P = .04). These findings remained consistent after adjusting for tumor characteristics including age, stage, tumor grade, hormone receptor status, and after controlling for treatment with chemotherapy and radiation. In addition, in the SEER database, the histologic type exerted a statistically significant time-dependent association with BCSS, with ILC showing decreasing BCSS over time (time interaction HR 1.93; 95% CI 1.78-2.10; P < .001). Furthermore, on annual hazard function analysis, the ILC annual peak event of BCSS occurred 5 years after diagnosis, whereas the IDC recurrence events peaked at 5 years before diagnosis, suggesting a higher late recurrence rate for ILC. These findings may have implications on the duration of endocrine therapy used in these patients given concern for worse long-term outcomes in premenopausal patients with ILC.
Oral selective estrogen receptor degraders (SERD) have recently emerged as a new therapeutic mechanism for patients with hormone receptor–positive breast cancer who have developed resistance to other endocrine therapies. Two of these agents, elacestrant and camizestrant, have demonstrated statistically significant progression-free survival benefit in these populations, particularly in tumors with ESR1 mutations. The efficacy of these agents in tumors with ESR1 wild-type subgroup remains uncertain. A meta-analysis by Wong and colleagues of individual patient data from four randomized clinical trials (ACELERA, AMEERA-3, EMERALD, and SERENA-2) included 1290 patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer who received oral SERD or endocrine therapies (ET) of the physician's choice. In the overall cohort, oral SERD showed improved progression-free survival (PFS) outcomes compared with ET of the physician's choice (HR 0.783; 95% CI 0.681-0.900; P < .001). This was also noted in the subgroup of patients with ESR1 mutations (HR 0.557; 95% CI 0.440-0.705; P < .001); although no significant PFS benefit was observed with oral SERD in the ESR1 wild-type subgroup (HR 0.944; 95% CI 0.783-1.138; P = .543). These results suggest that the PFS benefit observed with oral SERD is mainly seen in patients with ESR1-mutated tumors, and, therefore, these drugs should be prescribed accordingly.
Additional Reference
- Cold S, Cold F, Jensen M-B, et al. Systemic or vaginal hormone therapy after early breast cancer: A Danish observational cohort study. J Natl Cancer Inst. 2022;114:1347–1354. doi: 10.1093/jnci/djac112
FDA OKs new agent to block chemotherapy-induced neutropenia
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
2023 USPSTF mammography age to start screening in average-risk patients: What’s new is old again
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.
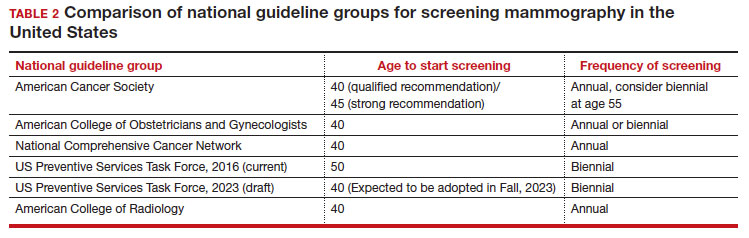
In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
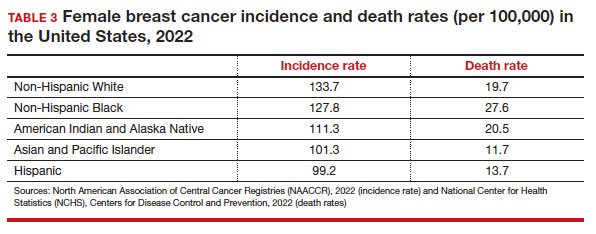
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14
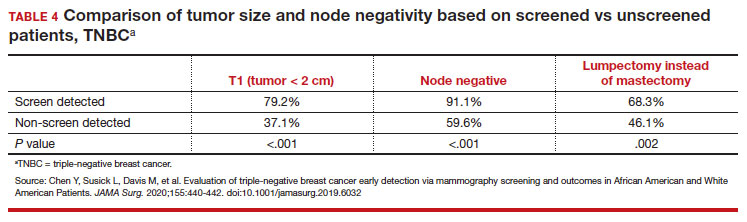
While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.
Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
Commentary: Obesity, Pregnancy, and Adjuvant Chemotherapy in BC, December 2023
Breast cancer in young women presents a unique set of challenges owing to life-stage at the time of diagnosis and treatment. Oncofertility, family planning, and pregnancy are essential issues to address at the time of initial consultation and throughout the survivorship setting. Various studies have provided supportive evidence regarding the safety of pregnancy after breast cancer diagnosis and treatment.3 HR+ breast cancer is associated with its own distinctive considerations related to pregnancy and its timing, including the use of endocrine therapy for 5-10 years, the role of female hormones during pregnancy, and late patterns of recurrence that characterize this subtype. A meta-analysis including eight eligible studies and 3805 women with HR+ early breast cancer investigated the prognostic impact of future pregnancy among these patients (Arecco et al). A total of 1285 women had a pregnancy after breast cancer diagnosis and treatment; there was no difference in disease-free survival (hazard ratio 0.96; 95% CI 0.75-1.24; P = .781) and better overall survival (OS; hazard ratio 0.46; 95% CI 0.27-0.77; P < .005) in those with vs those without subsequent pregnancy. Added to this body of data is the prospective POSITIVE trial, which showed that a temporary pause of endocrine therapy for an attempt at conceiving appears to be safe in young women with early HR+ breast cancer with short-term follow-up.4 Future research efforts investigating outcomes after assisted reproductive technologies in this population, those with germline mutations, and extended follow-up of studies, such as POSITIVE, will continue to inform guidance for and management of young women with breast cancer.
Guidelines favor the use of adjuvant chemotherapy for small, node-negative, triple-negative breast cancer (TNBC), specifically T1b and T1c tumors.5 However, high-quality data to inform this decision-making are sparse, and it is valuable to consider the magnitude of benefit weighed against possible risks and side effects of treatment, as well as patient comorbidities. A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database including 11,510 patients (3388 with T1b and 8122 with T1c TNBC) evaluated the impact of adjuvant chemotherapy on OS and breast cancer–specific survival (BCSS) (Carbajal-Ochoa et al). The use of adjuvant chemotherapy was associated with improved OS (hazard ratio 0.54; 95% CI 0.47-0.62; P < .001) and BCSS (hazard ratio 0.79; 95% CI 0.63-0.99; P = .043) among T1c TNBC. For those with T1b tumors, adjuvant chemotherapy improved OS (hazard ratio 0.52; 95% CI 0.41-0.68; P < .001) but did not improve BCSS (hazard ratio 0.70; 95% CI 0.45-1.07; P = .10). A better understanding of the molecular drivers implicated in this heterogeneous subtype, and predictors of response and resistance, will aid in identifying those patients who have greater benefit and those who can potentially be spared chemotherapy-related toxicities.
Additional References
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. doi: 10.1186/s12885-021-08343-0>
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi: 10.1056/NEJMoa2212856
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. doi: 10.1093/annonc/mdx308
Breast cancer in young women presents a unique set of challenges owing to life-stage at the time of diagnosis and treatment. Oncofertility, family planning, and pregnancy are essential issues to address at the time of initial consultation and throughout the survivorship setting. Various studies have provided supportive evidence regarding the safety of pregnancy after breast cancer diagnosis and treatment.3 HR+ breast cancer is associated with its own distinctive considerations related to pregnancy and its timing, including the use of endocrine therapy for 5-10 years, the role of female hormones during pregnancy, and late patterns of recurrence that characterize this subtype. A meta-analysis including eight eligible studies and 3805 women with HR+ early breast cancer investigated the prognostic impact of future pregnancy among these patients (Arecco et al). A total of 1285 women had a pregnancy after breast cancer diagnosis and treatment; there was no difference in disease-free survival (hazard ratio 0.96; 95% CI 0.75-1.24; P = .781) and better overall survival (OS; hazard ratio 0.46; 95% CI 0.27-0.77; P < .005) in those with vs those without subsequent pregnancy. Added to this body of data is the prospective POSITIVE trial, which showed that a temporary pause of endocrine therapy for an attempt at conceiving appears to be safe in young women with early HR+ breast cancer with short-term follow-up.4 Future research efforts investigating outcomes after assisted reproductive technologies in this population, those with germline mutations, and extended follow-up of studies, such as POSITIVE, will continue to inform guidance for and management of young women with breast cancer.
Guidelines favor the use of adjuvant chemotherapy for small, node-negative, triple-negative breast cancer (TNBC), specifically T1b and T1c tumors.5 However, high-quality data to inform this decision-making are sparse, and it is valuable to consider the magnitude of benefit weighed against possible risks and side effects of treatment, as well as patient comorbidities. A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database including 11,510 patients (3388 with T1b and 8122 with T1c TNBC) evaluated the impact of adjuvant chemotherapy on OS and breast cancer–specific survival (BCSS) (Carbajal-Ochoa et al). The use of adjuvant chemotherapy was associated with improved OS (hazard ratio 0.54; 95% CI 0.47-0.62; P < .001) and BCSS (hazard ratio 0.79; 95% CI 0.63-0.99; P = .043) among T1c TNBC. For those with T1b tumors, adjuvant chemotherapy improved OS (hazard ratio 0.52; 95% CI 0.41-0.68; P < .001) but did not improve BCSS (hazard ratio 0.70; 95% CI 0.45-1.07; P = .10). A better understanding of the molecular drivers implicated in this heterogeneous subtype, and predictors of response and resistance, will aid in identifying those patients who have greater benefit and those who can potentially be spared chemotherapy-related toxicities.
Additional References
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. doi: 10.1186/s12885-021-08343-0>
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi: 10.1056/NEJMoa2212856
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. doi: 10.1093/annonc/mdx308
Breast cancer in young women presents a unique set of challenges owing to life-stage at the time of diagnosis and treatment. Oncofertility, family planning, and pregnancy are essential issues to address at the time of initial consultation and throughout the survivorship setting. Various studies have provided supportive evidence regarding the safety of pregnancy after breast cancer diagnosis and treatment.3 HR+ breast cancer is associated with its own distinctive considerations related to pregnancy and its timing, including the use of endocrine therapy for 5-10 years, the role of female hormones during pregnancy, and late patterns of recurrence that characterize this subtype. A meta-analysis including eight eligible studies and 3805 women with HR+ early breast cancer investigated the prognostic impact of future pregnancy among these patients (Arecco et al). A total of 1285 women had a pregnancy after breast cancer diagnosis and treatment; there was no difference in disease-free survival (hazard ratio 0.96; 95% CI 0.75-1.24; P = .781) and better overall survival (OS; hazard ratio 0.46; 95% CI 0.27-0.77; P < .005) in those with vs those without subsequent pregnancy. Added to this body of data is the prospective POSITIVE trial, which showed that a temporary pause of endocrine therapy for an attempt at conceiving appears to be safe in young women with early HR+ breast cancer with short-term follow-up.4 Future research efforts investigating outcomes after assisted reproductive technologies in this population, those with germline mutations, and extended follow-up of studies, such as POSITIVE, will continue to inform guidance for and management of young women with breast cancer.
Guidelines favor the use of adjuvant chemotherapy for small, node-negative, triple-negative breast cancer (TNBC), specifically T1b and T1c tumors.5 However, high-quality data to inform this decision-making are sparse, and it is valuable to consider the magnitude of benefit weighed against possible risks and side effects of treatment, as well as patient comorbidities. A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database including 11,510 patients (3388 with T1b and 8122 with T1c TNBC) evaluated the impact of adjuvant chemotherapy on OS and breast cancer–specific survival (BCSS) (Carbajal-Ochoa et al). The use of adjuvant chemotherapy was associated with improved OS (hazard ratio 0.54; 95% CI 0.47-0.62; P < .001) and BCSS (hazard ratio 0.79; 95% CI 0.63-0.99; P = .043) among T1c TNBC. For those with T1b tumors, adjuvant chemotherapy improved OS (hazard ratio 0.52; 95% CI 0.41-0.68; P < .001) but did not improve BCSS (hazard ratio 0.70; 95% CI 0.45-1.07; P = .10). A better understanding of the molecular drivers implicated in this heterogeneous subtype, and predictors of response and resistance, will aid in identifying those patients who have greater benefit and those who can potentially be spared chemotherapy-related toxicities.
Additional References
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. doi: 10.1186/s12885-021-08343-0>
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi: 10.1056/NEJMoa2212856
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. doi: 10.1093/annonc/mdx308
Commentary: Obesity, Pregnancy, and Adjuvant Chemotherapy in BC, December 2023
Breast cancer in young women presents a unique set of challenges owing to life-stage at the time of diagnosis and treatment. Oncofertility, family planning, and pregnancy are essential issues to address at the time of initial consultation and throughout the survivorship setting. Various studies have provided supportive evidence regarding the safety of pregnancy after breast cancer diagnosis and treatment.3 HR+ breast cancer is associated with its own distinctive considerations related to pregnancy and its timing, including the use of endocrine therapy for 5-10 years, the role of female hormones during pregnancy, and late patterns of recurrence that characterize this subtype. A meta-analysis including eight eligible studies and 3805 women with HR+ early breast cancer investigated the prognostic impact of future pregnancy among these patients (Arecco et al). A total of 1285 women had a pregnancy after breast cancer diagnosis and treatment; there was no difference in disease-free survival (hazard ratio 0.96; 95% CI 0.75-1.24; P = .781) and better overall survival (OS; hazard ratio 0.46; 95% CI 0.27-0.77; P < .005) in those with vs those without subsequent pregnancy. Added to this body of data is the prospective POSITIVE trial, which showed that a temporary pause of endocrine therapy for an attempt at conceiving appears to be safe in young women with early HR+ breast cancer with short-term follow-up.4 Future research efforts investigating outcomes after assisted reproductive technologies in this population, those with germline mutations, and extended follow-up of studies, such as POSITIVE, will continue to inform guidance for and management of young women with breast cancer.
Guidelines favor the use of adjuvant chemotherapy for small, node-negative, triple-negative breast cancer (TNBC), specifically T1b and T1c tumors.5 However, high-quality data to inform this decision-making are sparse, and it is valuable to consider the magnitude of benefit weighed against possible risks and side effects of treatment, as well as patient comorbidities. A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database including 11,510 patients (3388 with T1b and 8122 with T1c TNBC) evaluated the impact of adjuvant chemotherapy on OS and breast cancer–specific survival (BCSS) (Carbajal-Ochoa et al). The use of adjuvant chemotherapy was associated with improved OS (hazard ratio 0.54; 95% CI 0.47-0.62; P < .001) and BCSS (hazard ratio 0.79; 95% CI 0.63-0.99; P = .043) among T1c TNBC. For those with T1b tumors, adjuvant chemotherapy improved OS (hazard ratio 0.52; 95% CI 0.41-0.68; P < .001) but did not improve BCSS (hazard ratio 0.70; 95% CI 0.45-1.07; P = .10). A better understanding of the molecular drivers implicated in this heterogeneous subtype, and predictors of response and resistance, will aid in identifying those patients who have greater benefit and those who can potentially be spared chemotherapy-related toxicities.
Additional References
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. doi: 10.1186/s12885-021-08343-0
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi:10.1056/NEJMoa2212856
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. doi:10.1093/annonc/mdx308
Breast cancer in young women presents a unique set of challenges owing to life-stage at the time of diagnosis and treatment. Oncofertility, family planning, and pregnancy are essential issues to address at the time of initial consultation and throughout the survivorship setting. Various studies have provided supportive evidence regarding the safety of pregnancy after breast cancer diagnosis and treatment.3 HR+ breast cancer is associated with its own distinctive considerations related to pregnancy and its timing, including the use of endocrine therapy for 5-10 years, the role of female hormones during pregnancy, and late patterns of recurrence that characterize this subtype. A meta-analysis including eight eligible studies and 3805 women with HR+ early breast cancer investigated the prognostic impact of future pregnancy among these patients (Arecco et al). A total of 1285 women had a pregnancy after breast cancer diagnosis and treatment; there was no difference in disease-free survival (hazard ratio 0.96; 95% CI 0.75-1.24; P = .781) and better overall survival (OS; hazard ratio 0.46; 95% CI 0.27-0.77; P < .005) in those with vs those without subsequent pregnancy. Added to this body of data is the prospective POSITIVE trial, which showed that a temporary pause of endocrine therapy for an attempt at conceiving appears to be safe in young women with early HR+ breast cancer with short-term follow-up.4 Future research efforts investigating outcomes after assisted reproductive technologies in this population, those with germline mutations, and extended follow-up of studies, such as POSITIVE, will continue to inform guidance for and management of young women with breast cancer.
Guidelines favor the use of adjuvant chemotherapy for small, node-negative, triple-negative breast cancer (TNBC), specifically T1b and T1c tumors.5 However, high-quality data to inform this decision-making are sparse, and it is valuable to consider the magnitude of benefit weighed against possible risks and side effects of treatment, as well as patient comorbidities. A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database including 11,510 patients (3388 with T1b and 8122 with T1c TNBC) evaluated the impact of adjuvant chemotherapy on OS and breast cancer–specific survival (BCSS) (Carbajal-Ochoa et al). The use of adjuvant chemotherapy was associated with improved OS (hazard ratio 0.54; 95% CI 0.47-0.62; P < .001) and BCSS (hazard ratio 0.79; 95% CI 0.63-0.99; P = .043) among T1c TNBC. For those with T1b tumors, adjuvant chemotherapy improved OS (hazard ratio 0.52; 95% CI 0.41-0.68; P < .001) but did not improve BCSS (hazard ratio 0.70; 95% CI 0.45-1.07; P = .10). A better understanding of the molecular drivers implicated in this heterogeneous subtype, and predictors of response and resistance, will aid in identifying those patients who have greater benefit and those who can potentially be spared chemotherapy-related toxicities.
Additional References
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. doi: 10.1186/s12885-021-08343-0
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi:10.1056/NEJMoa2212856
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. doi:10.1093/annonc/mdx308
Breast cancer in young women presents a unique set of challenges owing to life-stage at the time of diagnosis and treatment. Oncofertility, family planning, and pregnancy are essential issues to address at the time of initial consultation and throughout the survivorship setting. Various studies have provided supportive evidence regarding the safety of pregnancy after breast cancer diagnosis and treatment.3 HR+ breast cancer is associated with its own distinctive considerations related to pregnancy and its timing, including the use of endocrine therapy for 5-10 years, the role of female hormones during pregnancy, and late patterns of recurrence that characterize this subtype. A meta-analysis including eight eligible studies and 3805 women with HR+ early breast cancer investigated the prognostic impact of future pregnancy among these patients (Arecco et al). A total of 1285 women had a pregnancy after breast cancer diagnosis and treatment; there was no difference in disease-free survival (hazard ratio 0.96; 95% CI 0.75-1.24; P = .781) and better overall survival (OS; hazard ratio 0.46; 95% CI 0.27-0.77; P < .005) in those with vs those without subsequent pregnancy. Added to this body of data is the prospective POSITIVE trial, which showed that a temporary pause of endocrine therapy for an attempt at conceiving appears to be safe in young women with early HR+ breast cancer with short-term follow-up.4 Future research efforts investigating outcomes after assisted reproductive technologies in this population, those with germline mutations, and extended follow-up of studies, such as POSITIVE, will continue to inform guidance for and management of young women with breast cancer.
Guidelines favor the use of adjuvant chemotherapy for small, node-negative, triple-negative breast cancer (TNBC), specifically T1b and T1c tumors.5 However, high-quality data to inform this decision-making are sparse, and it is valuable to consider the magnitude of benefit weighed against possible risks and side effects of treatment, as well as patient comorbidities. A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database including 11,510 patients (3388 with T1b and 8122 with T1c TNBC) evaluated the impact of adjuvant chemotherapy on OS and breast cancer–specific survival (BCSS) (Carbajal-Ochoa et al). The use of adjuvant chemotherapy was associated with improved OS (hazard ratio 0.54; 95% CI 0.47-0.62; P < .001) and BCSS (hazard ratio 0.79; 95% CI 0.63-0.99; P = .043) among T1c TNBC. For those with T1b tumors, adjuvant chemotherapy improved OS (hazard ratio 0.52; 95% CI 0.41-0.68; P < .001) but did not improve BCSS (hazard ratio 0.70; 95% CI 0.45-1.07; P = .10). A better understanding of the molecular drivers implicated in this heterogeneous subtype, and predictors of response and resistance, will aid in identifying those patients who have greater benefit and those who can potentially be spared chemotherapy-related toxicities.
Additional References
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. doi: 10.1186/s12885-021-08343-0
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi:10.1056/NEJMoa2212856
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. doi:10.1093/annonc/mdx308
Breast milk liquid biopsy under study for early-stage breast cancer detection
Breast cancer has a worse prognosis when diagnosed during pregnancy or postpartum. Methods for early detection are needed, as evidenced every day in the multidisciplinary unit for treating pregnancy-associated breast cancer, which operates within the breast unit at the Vall d’Hebron University Hospital in Barcelona.
The team working in this field is led by Cristina Saura, PhD, who is also head of the Breast Cancer Group at the Vall d’Hebron Institute of Oncology (VHIO). The results of a study recently published in Cancer Discovery show, for the first time, that breast milk from breast cancer patients contains circulating tumor DNA that can be detected by a liquid biopsy of the milk.
Dr. Saura explained in an interview why they began to pursue this research, which, in one sense, fell into their laps. “In this case, it arose from the concerns of a breast cancer patient who was diagnosed while pregnant with her third daughter. She was actually the one who came up with the idea for the project. She was worried that she had transmitted the tumor through her breast milk to her second daughter while breastfeeding. She had been breastfeeding for a long time and had stretched it out until shortly before she was diagnosed with breast cancer. So she brought us a sample of breast milk that she had stored in her freezer.
“So, thanks to her, that’s where our project started. Though we knew that breast cancer is not transmitted through breast milk, we decided to test the sample and look for markers that could help our research. In the end, when we analyzed the patient’s breast milk, we found DNA with the same mutation that was present in her tumor,” explained Dr. Saura. She noted that the breast milk they analyzed had been frozen for more than a year before the patient’s cancer diagnosis.
In terms of methodology, Ana Vivancos, PhD, head of the VHIO cancer genomics group and also one of the authors of the study, explained that they used two techniques to analyze the breast milk and blood samples: next-generation sequencing and droplet digital polymerase chain reaction. These methods confirmed the presence of ctDNA in the breast milk.
High-sensitivity genomic panel
“We were able to detect tumor mutations in milk samples from 13 of the 15 patients with breast cancer who were tested, while circulating tumor DNA was detected in only one of all the blood samples that were collected at the same time,” said Dr. Vivancos. “The samples from the two patients for whom no mutation was detected were discovered to be colostrum that had been collected during the first few hours of lactation.”
As a next step to make this finding practically useful, the research team designed a genomic panel using next-generation sequencing as a potential method for early detection of breast cancer. “We’ve developed a panel that uses hybrid capture chemistry and unique molecular identifiers that ensure better sensitivity during next-generation sequencing. The panel has been calibrated, based on the existing literature, to detect the genes that are most frequently mutated in breast cancer in young women under 45 years old.”
According to Dr. Vivancos, the sensitivity of this panel exceeds 70%. This means that for all the patient samples analyzed using this panel, 7 out of 10 cases are detected with 100% specificity.
“In practice, the panel design allows us to detect mutations in more than 95% of breast cancer cases in women under 45 years old. noted Dr. Vivancos.
As for this unresolved need, Dr. Saura explained that there is currently no system or tool available to allow early suspicion of breast tumors in pregnant women prior to diagnosis. “That’s exactly the goal of this research: to screen for breast cancer in women who have just given birth. Now, it needs to be validated in a larger group of women in a clinical trial.”
More direct contact with tumor cells
In Dr. Saura’s opinion, in Spain, just like taking a small blood sample from newborns in a heel-prick test to rule out metabolic diseases, milk samples could be taken from women who give birth to rule out or diagnose breast cancer.
As to the potential advantages that breast milk liquid biopsy could have over similar techniques like blood liquid biopsy, Dr. Vivancos pointed to the results of her study: “We have seen that breast milk liquid biopsy was positive for the presence of circulating tumor DNA in 87% of cases, whereas blood only revealed the presence of this marker in 8% of cases. This difference indicates that breast milk is a biofluid that is in more direct contact with tumor cells and therefore will be more informative in earlier stages.”
Dr. Saura explained that the data does not lie when it comes to these tumors in pregnant or postpartum women. “In general, they tend to have a worse prognosis because, in most cases, they are diagnosed in advanced stages. Furthermore, it is typically assumed that the physiological changes in the breasts during gestation and lactation, which are considered to be normal, may hide a developing tumor. The fact is that postpartum breast cancer, understood to be the 10 years after delivery, accounts for 40%-45% of breast cancer cases diagnosed before age 45.”
The researchers plan to continue this project. “Our next step to confirm the usefulness of breast milk as a new tool for liquid biopsy for early detection of breast cancer during the postpartum period is to perform this noninvasive test in thousands of women,” said Dr. Saura.
Goal: Standardize the test as a screening method
“Based on the results we’ve published, we’re starting a study aimed at collecting breast milk samples from 5,000 healthy women around the world who became pregnant at age 40 or older, or who got pregnant at any age and carry mutations that increase their risk of breast cancer,” Dr. Saura added.
When asked when they expect to have preliminary results from this new study, Dr. Saura stated that it’s not yet possible to say exactly when. “We’re still waiting for funding to continue this project, but we continue performing analyses on a case-by-case basis. Of course, if we detect any abnormalities in these women, we will follow the established protocol to confirm diagnosis and start treatment if necessary.”
When asked whether it is reasonable to expect breast milk liquid biopsy to become normalized as a screening method for women of childbearing age who have a history or risk factors for developing breast cancer, Dr. Vivancos said, “That’s the scenario we see in the future and what we wish to contribute toward by providing scientific evidence to make it a reality.”
“For now, our goal is to validate whether circulating tumor DNA can be detected by breast milk liquid biopsy even before breast cancer can be diagnosed using conventional imaging techniques. If we can validate these preliminary results, we will be able to detect breast cancer early using a noninvasive test like breast milk liquid biopsy,” explained Saura.
Lastly, and in view of the issues that are still unresolved when it comes to the detection and treatment of breast cancer during pregnancy, Dr. Saura highlighted the emotional impact that a diagnosis of pregnancy-related cancer has on women and on those close to them. “But the first thing they need to know is that diagnosis is not necessarily synonymous with termination of the pregnancy. On the contrary, this tumor can be treated during pregnancy, since surgery can be performed at any time, and chemotherapy can be started in the second trimester. Proof of this is the 72 children who have been born under these circumstances in the past 20 years at the Vall d’Hebrón University Hospital. This hospital is a pioneer in Spain thanks to its multidisciplinary program for education and specific follow-up with women who have been diagnosed with a breast tumor during pregnancy.”
Dr. Saura and Dr. Vivancos reported no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Breast cancer has a worse prognosis when diagnosed during pregnancy or postpartum. Methods for early detection are needed, as evidenced every day in the multidisciplinary unit for treating pregnancy-associated breast cancer, which operates within the breast unit at the Vall d’Hebron University Hospital in Barcelona.
The team working in this field is led by Cristina Saura, PhD, who is also head of the Breast Cancer Group at the Vall d’Hebron Institute of Oncology (VHIO). The results of a study recently published in Cancer Discovery show, for the first time, that breast milk from breast cancer patients contains circulating tumor DNA that can be detected by a liquid biopsy of the milk.
Dr. Saura explained in an interview why they began to pursue this research, which, in one sense, fell into their laps. “In this case, it arose from the concerns of a breast cancer patient who was diagnosed while pregnant with her third daughter. She was actually the one who came up with the idea for the project. She was worried that she had transmitted the tumor through her breast milk to her second daughter while breastfeeding. She had been breastfeeding for a long time and had stretched it out until shortly before she was diagnosed with breast cancer. So she brought us a sample of breast milk that she had stored in her freezer.
“So, thanks to her, that’s where our project started. Though we knew that breast cancer is not transmitted through breast milk, we decided to test the sample and look for markers that could help our research. In the end, when we analyzed the patient’s breast milk, we found DNA with the same mutation that was present in her tumor,” explained Dr. Saura. She noted that the breast milk they analyzed had been frozen for more than a year before the patient’s cancer diagnosis.
In terms of methodology, Ana Vivancos, PhD, head of the VHIO cancer genomics group and also one of the authors of the study, explained that they used two techniques to analyze the breast milk and blood samples: next-generation sequencing and droplet digital polymerase chain reaction. These methods confirmed the presence of ctDNA in the breast milk.
High-sensitivity genomic panel
“We were able to detect tumor mutations in milk samples from 13 of the 15 patients with breast cancer who were tested, while circulating tumor DNA was detected in only one of all the blood samples that were collected at the same time,” said Dr. Vivancos. “The samples from the two patients for whom no mutation was detected were discovered to be colostrum that had been collected during the first few hours of lactation.”
As a next step to make this finding practically useful, the research team designed a genomic panel using next-generation sequencing as a potential method for early detection of breast cancer. “We’ve developed a panel that uses hybrid capture chemistry and unique molecular identifiers that ensure better sensitivity during next-generation sequencing. The panel has been calibrated, based on the existing literature, to detect the genes that are most frequently mutated in breast cancer in young women under 45 years old.”
According to Dr. Vivancos, the sensitivity of this panel exceeds 70%. This means that for all the patient samples analyzed using this panel, 7 out of 10 cases are detected with 100% specificity.
“In practice, the panel design allows us to detect mutations in more than 95% of breast cancer cases in women under 45 years old. noted Dr. Vivancos.
As for this unresolved need, Dr. Saura explained that there is currently no system or tool available to allow early suspicion of breast tumors in pregnant women prior to diagnosis. “That’s exactly the goal of this research: to screen for breast cancer in women who have just given birth. Now, it needs to be validated in a larger group of women in a clinical trial.”
More direct contact with tumor cells
In Dr. Saura’s opinion, in Spain, just like taking a small blood sample from newborns in a heel-prick test to rule out metabolic diseases, milk samples could be taken from women who give birth to rule out or diagnose breast cancer.
As to the potential advantages that breast milk liquid biopsy could have over similar techniques like blood liquid biopsy, Dr. Vivancos pointed to the results of her study: “We have seen that breast milk liquid biopsy was positive for the presence of circulating tumor DNA in 87% of cases, whereas blood only revealed the presence of this marker in 8% of cases. This difference indicates that breast milk is a biofluid that is in more direct contact with tumor cells and therefore will be more informative in earlier stages.”
Dr. Saura explained that the data does not lie when it comes to these tumors in pregnant or postpartum women. “In general, they tend to have a worse prognosis because, in most cases, they are diagnosed in advanced stages. Furthermore, it is typically assumed that the physiological changes in the breasts during gestation and lactation, which are considered to be normal, may hide a developing tumor. The fact is that postpartum breast cancer, understood to be the 10 years after delivery, accounts for 40%-45% of breast cancer cases diagnosed before age 45.”
The researchers plan to continue this project. “Our next step to confirm the usefulness of breast milk as a new tool for liquid biopsy for early detection of breast cancer during the postpartum period is to perform this noninvasive test in thousands of women,” said Dr. Saura.
Goal: Standardize the test as a screening method
“Based on the results we’ve published, we’re starting a study aimed at collecting breast milk samples from 5,000 healthy women around the world who became pregnant at age 40 or older, or who got pregnant at any age and carry mutations that increase their risk of breast cancer,” Dr. Saura added.
When asked when they expect to have preliminary results from this new study, Dr. Saura stated that it’s not yet possible to say exactly when. “We’re still waiting for funding to continue this project, but we continue performing analyses on a case-by-case basis. Of course, if we detect any abnormalities in these women, we will follow the established protocol to confirm diagnosis and start treatment if necessary.”
When asked whether it is reasonable to expect breast milk liquid biopsy to become normalized as a screening method for women of childbearing age who have a history or risk factors for developing breast cancer, Dr. Vivancos said, “That’s the scenario we see in the future and what we wish to contribute toward by providing scientific evidence to make it a reality.”
“For now, our goal is to validate whether circulating tumor DNA can be detected by breast milk liquid biopsy even before breast cancer can be diagnosed using conventional imaging techniques. If we can validate these preliminary results, we will be able to detect breast cancer early using a noninvasive test like breast milk liquid biopsy,” explained Saura.
Lastly, and in view of the issues that are still unresolved when it comes to the detection and treatment of breast cancer during pregnancy, Dr. Saura highlighted the emotional impact that a diagnosis of pregnancy-related cancer has on women and on those close to them. “But the first thing they need to know is that diagnosis is not necessarily synonymous with termination of the pregnancy. On the contrary, this tumor can be treated during pregnancy, since surgery can be performed at any time, and chemotherapy can be started in the second trimester. Proof of this is the 72 children who have been born under these circumstances in the past 20 years at the Vall d’Hebrón University Hospital. This hospital is a pioneer in Spain thanks to its multidisciplinary program for education and specific follow-up with women who have been diagnosed with a breast tumor during pregnancy.”
Dr. Saura and Dr. Vivancos reported no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Breast cancer has a worse prognosis when diagnosed during pregnancy or postpartum. Methods for early detection are needed, as evidenced every day in the multidisciplinary unit for treating pregnancy-associated breast cancer, which operates within the breast unit at the Vall d’Hebron University Hospital in Barcelona.
The team working in this field is led by Cristina Saura, PhD, who is also head of the Breast Cancer Group at the Vall d’Hebron Institute of Oncology (VHIO). The results of a study recently published in Cancer Discovery show, for the first time, that breast milk from breast cancer patients contains circulating tumor DNA that can be detected by a liquid biopsy of the milk.
Dr. Saura explained in an interview why they began to pursue this research, which, in one sense, fell into their laps. “In this case, it arose from the concerns of a breast cancer patient who was diagnosed while pregnant with her third daughter. She was actually the one who came up with the idea for the project. She was worried that she had transmitted the tumor through her breast milk to her second daughter while breastfeeding. She had been breastfeeding for a long time and had stretched it out until shortly before she was diagnosed with breast cancer. So she brought us a sample of breast milk that she had stored in her freezer.
“So, thanks to her, that’s where our project started. Though we knew that breast cancer is not transmitted through breast milk, we decided to test the sample and look for markers that could help our research. In the end, when we analyzed the patient’s breast milk, we found DNA with the same mutation that was present in her tumor,” explained Dr. Saura. She noted that the breast milk they analyzed had been frozen for more than a year before the patient’s cancer diagnosis.
In terms of methodology, Ana Vivancos, PhD, head of the VHIO cancer genomics group and also one of the authors of the study, explained that they used two techniques to analyze the breast milk and blood samples: next-generation sequencing and droplet digital polymerase chain reaction. These methods confirmed the presence of ctDNA in the breast milk.
High-sensitivity genomic panel
“We were able to detect tumor mutations in milk samples from 13 of the 15 patients with breast cancer who were tested, while circulating tumor DNA was detected in only one of all the blood samples that were collected at the same time,” said Dr. Vivancos. “The samples from the two patients for whom no mutation was detected were discovered to be colostrum that had been collected during the first few hours of lactation.”
As a next step to make this finding practically useful, the research team designed a genomic panel using next-generation sequencing as a potential method for early detection of breast cancer. “We’ve developed a panel that uses hybrid capture chemistry and unique molecular identifiers that ensure better sensitivity during next-generation sequencing. The panel has been calibrated, based on the existing literature, to detect the genes that are most frequently mutated in breast cancer in young women under 45 years old.”
According to Dr. Vivancos, the sensitivity of this panel exceeds 70%. This means that for all the patient samples analyzed using this panel, 7 out of 10 cases are detected with 100% specificity.
“In practice, the panel design allows us to detect mutations in more than 95% of breast cancer cases in women under 45 years old. noted Dr. Vivancos.
As for this unresolved need, Dr. Saura explained that there is currently no system or tool available to allow early suspicion of breast tumors in pregnant women prior to diagnosis. “That’s exactly the goal of this research: to screen for breast cancer in women who have just given birth. Now, it needs to be validated in a larger group of women in a clinical trial.”
More direct contact with tumor cells
In Dr. Saura’s opinion, in Spain, just like taking a small blood sample from newborns in a heel-prick test to rule out metabolic diseases, milk samples could be taken from women who give birth to rule out or diagnose breast cancer.
As to the potential advantages that breast milk liquid biopsy could have over similar techniques like blood liquid biopsy, Dr. Vivancos pointed to the results of her study: “We have seen that breast milk liquid biopsy was positive for the presence of circulating tumor DNA in 87% of cases, whereas blood only revealed the presence of this marker in 8% of cases. This difference indicates that breast milk is a biofluid that is in more direct contact with tumor cells and therefore will be more informative in earlier stages.”
Dr. Saura explained that the data does not lie when it comes to these tumors in pregnant or postpartum women. “In general, they tend to have a worse prognosis because, in most cases, they are diagnosed in advanced stages. Furthermore, it is typically assumed that the physiological changes in the breasts during gestation and lactation, which are considered to be normal, may hide a developing tumor. The fact is that postpartum breast cancer, understood to be the 10 years after delivery, accounts for 40%-45% of breast cancer cases diagnosed before age 45.”
The researchers plan to continue this project. “Our next step to confirm the usefulness of breast milk as a new tool for liquid biopsy for early detection of breast cancer during the postpartum period is to perform this noninvasive test in thousands of women,” said Dr. Saura.
Goal: Standardize the test as a screening method
“Based on the results we’ve published, we’re starting a study aimed at collecting breast milk samples from 5,000 healthy women around the world who became pregnant at age 40 or older, or who got pregnant at any age and carry mutations that increase their risk of breast cancer,” Dr. Saura added.
When asked when they expect to have preliminary results from this new study, Dr. Saura stated that it’s not yet possible to say exactly when. “We’re still waiting for funding to continue this project, but we continue performing analyses on a case-by-case basis. Of course, if we detect any abnormalities in these women, we will follow the established protocol to confirm diagnosis and start treatment if necessary.”
When asked whether it is reasonable to expect breast milk liquid biopsy to become normalized as a screening method for women of childbearing age who have a history or risk factors for developing breast cancer, Dr. Vivancos said, “That’s the scenario we see in the future and what we wish to contribute toward by providing scientific evidence to make it a reality.”
“For now, our goal is to validate whether circulating tumor DNA can be detected by breast milk liquid biopsy even before breast cancer can be diagnosed using conventional imaging techniques. If we can validate these preliminary results, we will be able to detect breast cancer early using a noninvasive test like breast milk liquid biopsy,” explained Saura.
Lastly, and in view of the issues that are still unresolved when it comes to the detection and treatment of breast cancer during pregnancy, Dr. Saura highlighted the emotional impact that a diagnosis of pregnancy-related cancer has on women and on those close to them. “But the first thing they need to know is that diagnosis is not necessarily synonymous with termination of the pregnancy. On the contrary, this tumor can be treated during pregnancy, since surgery can be performed at any time, and chemotherapy can be started in the second trimester. Proof of this is the 72 children who have been born under these circumstances in the past 20 years at the Vall d’Hebrón University Hospital. This hospital is a pioneer in Spain thanks to its multidisciplinary program for education and specific follow-up with women who have been diagnosed with a breast tumor during pregnancy.”
Dr. Saura and Dr. Vivancos reported no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
FROM CANCER DISCOVERY
Can a Mediterranean diet reduce breast cancer recurrence?
TOPLINE:
However, women at high risk for recurrence who made the greatest improvements in their diet quality demonstrated a 41% lower risk for recurrence, compared with peers who made the fewest improvements.
METHODOLOGY:
- A growing body of evidence suggests that a better dietary quality may improve survival among patients with breast cancer, but whether diet impacts breast cancer–specific mortality remains controversial.
- To better understand the relationship between diet and breast cancer outcomes, investigators recruited 1,542 women with breast cancer who had undergone surgical resection in the past 5 years and were considered high risk for recurrence.
- All women received general recommendations for cancer prevention, while the intervention group received active support to adhere to a macro–Mediterranean-style diet, which encourages mainly consuming whole grains, legumes, and high-fiber vegetables and discourages eating foods high in saturated and trans fats, processed meats, and foods and beverages high in sugar.
- Diet was assessed at baseline, 1 year, and every few months in subsequent years via food frequency diaries. Compliance with dietary recommendations for the whole cohort was assessed using a dietary index developed for the trial.
- In addition to diet, women in the diet intervention group were encouraged to maintain moderate to intense physical activity – 30 minutes, on average, each day – and received pedometers to track steps, aiming for 10,000 per day.
TAKEAWAY:
- Over 5 years of follow-up, the rate of breast cancer recurrence did not differ between women in the diet intervention group and those in the control group. Overall, 95 of 769 women in the intervention group and 98 of 773 in the control group had a breast cancer recurrence (hazard ratio, 0.99).
- When evaluating outcomes in the entire cohort, looking at everyone’s level of compliance with dietary recommendations, women who adhered the most to the dietary guidelines had a 41% lower recurrence risk compared with women who adhered the least (HR, 0.59).
- The greatest protective effect among women who demonstrated high compliance occurred in those with ER-positive cancers (HR, 0.42) and those with ER-positive cancers who received tamoxifen (HR, 0.30).
IN PRACTICE:
This intervention trial “did not confirm the hypothesis that a comprehensive dietary modification reduces breast cancer recurrence and metastases,” but when looking at compliance to the Mediterranean diet overall, the analysis did find “a significantly better prognosis” for women with the best adherence.
SOURCE:
The study, with first author Franco Berrino, MD, PhD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, was published online in Clinical Cancer Research.
LIMITATIONS:
The study relied on self-reported dietary data. No dietary instrument was used to estimate nutrient intake and the dietary index developed for the trial remains unvalidated.
DISCLOSURES:
The study was supported by the Italian Department of Health, the Associazione Italiana per la Ricerca sul Cancro, and the Vita e Salute Foundation. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
However, women at high risk for recurrence who made the greatest improvements in their diet quality demonstrated a 41% lower risk for recurrence, compared with peers who made the fewest improvements.
METHODOLOGY:
- A growing body of evidence suggests that a better dietary quality may improve survival among patients with breast cancer, but whether diet impacts breast cancer–specific mortality remains controversial.
- To better understand the relationship between diet and breast cancer outcomes, investigators recruited 1,542 women with breast cancer who had undergone surgical resection in the past 5 years and were considered high risk for recurrence.
- All women received general recommendations for cancer prevention, while the intervention group received active support to adhere to a macro–Mediterranean-style diet, which encourages mainly consuming whole grains, legumes, and high-fiber vegetables and discourages eating foods high in saturated and trans fats, processed meats, and foods and beverages high in sugar.
- Diet was assessed at baseline, 1 year, and every few months in subsequent years via food frequency diaries. Compliance with dietary recommendations for the whole cohort was assessed using a dietary index developed for the trial.
- In addition to diet, women in the diet intervention group were encouraged to maintain moderate to intense physical activity – 30 minutes, on average, each day – and received pedometers to track steps, aiming for 10,000 per day.
TAKEAWAY:
- Over 5 years of follow-up, the rate of breast cancer recurrence did not differ between women in the diet intervention group and those in the control group. Overall, 95 of 769 women in the intervention group and 98 of 773 in the control group had a breast cancer recurrence (hazard ratio, 0.99).
- When evaluating outcomes in the entire cohort, looking at everyone’s level of compliance with dietary recommendations, women who adhered the most to the dietary guidelines had a 41% lower recurrence risk compared with women who adhered the least (HR, 0.59).
- The greatest protective effect among women who demonstrated high compliance occurred in those with ER-positive cancers (HR, 0.42) and those with ER-positive cancers who received tamoxifen (HR, 0.30).
IN PRACTICE:
This intervention trial “did not confirm the hypothesis that a comprehensive dietary modification reduces breast cancer recurrence and metastases,” but when looking at compliance to the Mediterranean diet overall, the analysis did find “a significantly better prognosis” for women with the best adherence.
SOURCE:
The study, with first author Franco Berrino, MD, PhD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, was published online in Clinical Cancer Research.
LIMITATIONS:
The study relied on self-reported dietary data. No dietary instrument was used to estimate nutrient intake and the dietary index developed for the trial remains unvalidated.
DISCLOSURES:
The study was supported by the Italian Department of Health, the Associazione Italiana per la Ricerca sul Cancro, and the Vita e Salute Foundation. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
However, women at high risk for recurrence who made the greatest improvements in their diet quality demonstrated a 41% lower risk for recurrence, compared with peers who made the fewest improvements.
METHODOLOGY:
- A growing body of evidence suggests that a better dietary quality may improve survival among patients with breast cancer, but whether diet impacts breast cancer–specific mortality remains controversial.
- To better understand the relationship between diet and breast cancer outcomes, investigators recruited 1,542 women with breast cancer who had undergone surgical resection in the past 5 years and were considered high risk for recurrence.
- All women received general recommendations for cancer prevention, while the intervention group received active support to adhere to a macro–Mediterranean-style diet, which encourages mainly consuming whole grains, legumes, and high-fiber vegetables and discourages eating foods high in saturated and trans fats, processed meats, and foods and beverages high in sugar.
- Diet was assessed at baseline, 1 year, and every few months in subsequent years via food frequency diaries. Compliance with dietary recommendations for the whole cohort was assessed using a dietary index developed for the trial.
- In addition to diet, women in the diet intervention group were encouraged to maintain moderate to intense physical activity – 30 minutes, on average, each day – and received pedometers to track steps, aiming for 10,000 per day.
TAKEAWAY:
- Over 5 years of follow-up, the rate of breast cancer recurrence did not differ between women in the diet intervention group and those in the control group. Overall, 95 of 769 women in the intervention group and 98 of 773 in the control group had a breast cancer recurrence (hazard ratio, 0.99).
- When evaluating outcomes in the entire cohort, looking at everyone’s level of compliance with dietary recommendations, women who adhered the most to the dietary guidelines had a 41% lower recurrence risk compared with women who adhered the least (HR, 0.59).
- The greatest protective effect among women who demonstrated high compliance occurred in those with ER-positive cancers (HR, 0.42) and those with ER-positive cancers who received tamoxifen (HR, 0.30).
IN PRACTICE:
This intervention trial “did not confirm the hypothesis that a comprehensive dietary modification reduces breast cancer recurrence and metastases,” but when looking at compliance to the Mediterranean diet overall, the analysis did find “a significantly better prognosis” for women with the best adherence.
SOURCE:
The study, with first author Franco Berrino, MD, PhD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, was published online in Clinical Cancer Research.
LIMITATIONS:
The study relied on self-reported dietary data. No dietary instrument was used to estimate nutrient intake and the dietary index developed for the trial remains unvalidated.
DISCLOSURES:
The study was supported by the Italian Department of Health, the Associazione Italiana per la Ricerca sul Cancro, and the Vita e Salute Foundation. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA OKs capivasertib for certain advanced breast cancers
Specifically, the first-in-class AKT kinase inhibitor approval is for patients with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, whose metastatic disease progressed on at least one endocrine-based regimen or who experienced recurrence on or within 12 months of completing adjuvant therapy, according to the FDA approval announcement.
The FDA also approved a companion diagnostic device, the FoundationOne CDx assay, to identify patients who are eligible for treatment with capivasertib.
Approval of capivasertib was based on findings from the randomized, placebo-controlled, phase 3 CAPItello-291 trial, which involved 708 patients with locally advanced or metastatic HR-positive, HER2-negative breast cancer, including 289 whose tumors had PIK3CA/AKT1/PTEN alterations. All had progressed on aromatase inhibitor–based treatment and may have received up to two prior lines of endocrine therapy and up to one line of chemotherapy.
Patients were randomized to either 400 mg of oral capivasertib or placebo twice daily for 4 days, followed by 3 days off treatment each week over a 28-day treatment cycle. Patients in both arms received 500 mg intramuscular fulvestrant on cycle 1 days 1 and 15, and then every 28 days thereafter. Treatment continued until disease progression or unacceptable toxicity.
In the 289 patients with PIK3CA/AKT1/PTEN–altered tumors, median progression-free survival (PFS) in the capivasertib arm was 7.3 months versus 3.1 months in the placebo group (hazard ratio, 0.50).
An exploratory analysis of PFS in the 313 (44%) patients whose tumors did not have a PIK3CA/AKT1/PTEN alteration demonstrated a less notable benefit to the combination (HR, 0.79; 95% confidence interval, 0.61-1.02), indicating that “the difference in the overall population was primarily attributed to the results seen in the population of patients whose tumors have PIK3CA/AKT1/PTEN alteration,” the FDA explained.
Adverse reactions occurring in at least 20% of patients included decreased lymphocytes, leukocytes, hemoglobin, and neutrophils; increased fasting glucose, creatinine, and triglycerides; and diarrhea, nausea, fatigue, vomiting, and stomatitis.
The recommended capivasertib dose is 400 mg orally twice daily, given about 12 hours apart with or without food, for 4 days followed by 3 off days until disease progression or unacceptable toxicity, according to the prescribing information.
A version of this article first appeared on Medscape.com.
Specifically, the first-in-class AKT kinase inhibitor approval is for patients with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, whose metastatic disease progressed on at least one endocrine-based regimen or who experienced recurrence on or within 12 months of completing adjuvant therapy, according to the FDA approval announcement.
The FDA also approved a companion diagnostic device, the FoundationOne CDx assay, to identify patients who are eligible for treatment with capivasertib.
Approval of capivasertib was based on findings from the randomized, placebo-controlled, phase 3 CAPItello-291 trial, which involved 708 patients with locally advanced or metastatic HR-positive, HER2-negative breast cancer, including 289 whose tumors had PIK3CA/AKT1/PTEN alterations. All had progressed on aromatase inhibitor–based treatment and may have received up to two prior lines of endocrine therapy and up to one line of chemotherapy.
Patients were randomized to either 400 mg of oral capivasertib or placebo twice daily for 4 days, followed by 3 days off treatment each week over a 28-day treatment cycle. Patients in both arms received 500 mg intramuscular fulvestrant on cycle 1 days 1 and 15, and then every 28 days thereafter. Treatment continued until disease progression or unacceptable toxicity.
In the 289 patients with PIK3CA/AKT1/PTEN–altered tumors, median progression-free survival (PFS) in the capivasertib arm was 7.3 months versus 3.1 months in the placebo group (hazard ratio, 0.50).
An exploratory analysis of PFS in the 313 (44%) patients whose tumors did not have a PIK3CA/AKT1/PTEN alteration demonstrated a less notable benefit to the combination (HR, 0.79; 95% confidence interval, 0.61-1.02), indicating that “the difference in the overall population was primarily attributed to the results seen in the population of patients whose tumors have PIK3CA/AKT1/PTEN alteration,” the FDA explained.
Adverse reactions occurring in at least 20% of patients included decreased lymphocytes, leukocytes, hemoglobin, and neutrophils; increased fasting glucose, creatinine, and triglycerides; and diarrhea, nausea, fatigue, vomiting, and stomatitis.
The recommended capivasertib dose is 400 mg orally twice daily, given about 12 hours apart with or without food, for 4 days followed by 3 off days until disease progression or unacceptable toxicity, according to the prescribing information.
A version of this article first appeared on Medscape.com.
Specifically, the first-in-class AKT kinase inhibitor approval is for patients with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, whose metastatic disease progressed on at least one endocrine-based regimen or who experienced recurrence on or within 12 months of completing adjuvant therapy, according to the FDA approval announcement.
The FDA also approved a companion diagnostic device, the FoundationOne CDx assay, to identify patients who are eligible for treatment with capivasertib.
Approval of capivasertib was based on findings from the randomized, placebo-controlled, phase 3 CAPItello-291 trial, which involved 708 patients with locally advanced or metastatic HR-positive, HER2-negative breast cancer, including 289 whose tumors had PIK3CA/AKT1/PTEN alterations. All had progressed on aromatase inhibitor–based treatment and may have received up to two prior lines of endocrine therapy and up to one line of chemotherapy.
Patients were randomized to either 400 mg of oral capivasertib or placebo twice daily for 4 days, followed by 3 days off treatment each week over a 28-day treatment cycle. Patients in both arms received 500 mg intramuscular fulvestrant on cycle 1 days 1 and 15, and then every 28 days thereafter. Treatment continued until disease progression or unacceptable toxicity.
In the 289 patients with PIK3CA/AKT1/PTEN–altered tumors, median progression-free survival (PFS) in the capivasertib arm was 7.3 months versus 3.1 months in the placebo group (hazard ratio, 0.50).
An exploratory analysis of PFS in the 313 (44%) patients whose tumors did not have a PIK3CA/AKT1/PTEN alteration demonstrated a less notable benefit to the combination (HR, 0.79; 95% confidence interval, 0.61-1.02), indicating that “the difference in the overall population was primarily attributed to the results seen in the population of patients whose tumors have PIK3CA/AKT1/PTEN alteration,” the FDA explained.
Adverse reactions occurring in at least 20% of patients included decreased lymphocytes, leukocytes, hemoglobin, and neutrophils; increased fasting glucose, creatinine, and triglycerides; and diarrhea, nausea, fatigue, vomiting, and stomatitis.
The recommended capivasertib dose is 400 mg orally twice daily, given about 12 hours apart with or without food, for 4 days followed by 3 off days until disease progression or unacceptable toxicity, according to the prescribing information.
A version of this article first appeared on Medscape.com.




