User login
FDA boxed warning leads to drop off in use of ESAs
The Food and Drug Administration’s 2007 “boxed warning” about serious adverse events associated with the use of erythropoietin-stimulating agents (ESAs) was followed by a substantial reduction in their use among patients recovering from colorectal, breast, or lung cancer, according to a new report.
Boxed warnings are considered one of the strongest mechanisms with which the FDA can communicate concerns about drug safety to the public. However, some critics have questioned the effectiveness of these warnings, and the available evidence “remains inconclusive, largely because almost all of [the data] were drawn from observational studies using pre-post designs without control groups,” said John Bian, PhD, of the University of South Carolina College of Pharmacy and Hollings Cancer Center, Columbia, and his associates.
The investigators analyzed data in the SEER cancer registry for the period immediately before and immediately after the 2007 boxed warning was issued. Their sample comprised 45,319 patients aged 66 years and older who were treated either in the “pre” warning period (January 2004-September 2006) or the “post” period (April 2007-September 2009). This included a control group of 3,375 patients with myelodysplastic syndromes. Use of ESAs in these patients was off-label and was not targeted by the boxed warning (J Clin Oncol. 2017 Apr 25. doi: 10.1200/JCO.2017.72.6273).The use of ESAs declined sharply after the boxed warning was issued, except in the control group. The proportion of breast cancer patients receiving ESAs dropped from 49%-55% before 2007 to 30% in 2007, 16% in 2008, and 9% in 2009.
Similarly, the proportion of colorectal cancer patients receiving ESAs declined from about 35%-40% before 2007 to 18% in 2007, 11% in 2008, and 9% in 2009. The proportion of lung cancer patients receiving ESAs decreased from 56%-58% before 2007 to 40% in 2007, 29% in 2008, and 24% in 2009. In contrast, the proportion of patients with myelodysplastic syndromes receiving ESAs – the control group – remained relatively stable at 39%-42% before 2007, 35% in 2007, and 32% in 2008 and 2009.
This represents a reduction of approximately 40% overall in the use of ESAs among targeted patients after the warning was issued. However, this decrease appeared to have little effect on the incidence of hospitalization for venous thromboembolism in this patient population, Dr. Bian and his associates noted.
The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
The Food and Drug Administration’s 2007 “boxed warning” about serious adverse events associated with the use of erythropoietin-stimulating agents (ESAs) was followed by a substantial reduction in their use among patients recovering from colorectal, breast, or lung cancer, according to a new report.
Boxed warnings are considered one of the strongest mechanisms with which the FDA can communicate concerns about drug safety to the public. However, some critics have questioned the effectiveness of these warnings, and the available evidence “remains inconclusive, largely because almost all of [the data] were drawn from observational studies using pre-post designs without control groups,” said John Bian, PhD, of the University of South Carolina College of Pharmacy and Hollings Cancer Center, Columbia, and his associates.
The investigators analyzed data in the SEER cancer registry for the period immediately before and immediately after the 2007 boxed warning was issued. Their sample comprised 45,319 patients aged 66 years and older who were treated either in the “pre” warning period (January 2004-September 2006) or the “post” period (April 2007-September 2009). This included a control group of 3,375 patients with myelodysplastic syndromes. Use of ESAs in these patients was off-label and was not targeted by the boxed warning (J Clin Oncol. 2017 Apr 25. doi: 10.1200/JCO.2017.72.6273).The use of ESAs declined sharply after the boxed warning was issued, except in the control group. The proportion of breast cancer patients receiving ESAs dropped from 49%-55% before 2007 to 30% in 2007, 16% in 2008, and 9% in 2009.
Similarly, the proportion of colorectal cancer patients receiving ESAs declined from about 35%-40% before 2007 to 18% in 2007, 11% in 2008, and 9% in 2009. The proportion of lung cancer patients receiving ESAs decreased from 56%-58% before 2007 to 40% in 2007, 29% in 2008, and 24% in 2009. In contrast, the proportion of patients with myelodysplastic syndromes receiving ESAs – the control group – remained relatively stable at 39%-42% before 2007, 35% in 2007, and 32% in 2008 and 2009.
This represents a reduction of approximately 40% overall in the use of ESAs among targeted patients after the warning was issued. However, this decrease appeared to have little effect on the incidence of hospitalization for venous thromboembolism in this patient population, Dr. Bian and his associates noted.
The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
The Food and Drug Administration’s 2007 “boxed warning” about serious adverse events associated with the use of erythropoietin-stimulating agents (ESAs) was followed by a substantial reduction in their use among patients recovering from colorectal, breast, or lung cancer, according to a new report.
Boxed warnings are considered one of the strongest mechanisms with which the FDA can communicate concerns about drug safety to the public. However, some critics have questioned the effectiveness of these warnings, and the available evidence “remains inconclusive, largely because almost all of [the data] were drawn from observational studies using pre-post designs without control groups,” said John Bian, PhD, of the University of South Carolina College of Pharmacy and Hollings Cancer Center, Columbia, and his associates.
The investigators analyzed data in the SEER cancer registry for the period immediately before and immediately after the 2007 boxed warning was issued. Their sample comprised 45,319 patients aged 66 years and older who were treated either in the “pre” warning period (January 2004-September 2006) or the “post” period (April 2007-September 2009). This included a control group of 3,375 patients with myelodysplastic syndromes. Use of ESAs in these patients was off-label and was not targeted by the boxed warning (J Clin Oncol. 2017 Apr 25. doi: 10.1200/JCO.2017.72.6273).The use of ESAs declined sharply after the boxed warning was issued, except in the control group. The proportion of breast cancer patients receiving ESAs dropped from 49%-55% before 2007 to 30% in 2007, 16% in 2008, and 9% in 2009.
Similarly, the proportion of colorectal cancer patients receiving ESAs declined from about 35%-40% before 2007 to 18% in 2007, 11% in 2008, and 9% in 2009. The proportion of lung cancer patients receiving ESAs decreased from 56%-58% before 2007 to 40% in 2007, 29% in 2008, and 24% in 2009. In contrast, the proportion of patients with myelodysplastic syndromes receiving ESAs – the control group – remained relatively stable at 39%-42% before 2007, 35% in 2007, and 32% in 2008 and 2009.
This represents a reduction of approximately 40% overall in the use of ESAs among targeted patients after the warning was issued. However, this decrease appeared to have little effect on the incidence of hospitalization for venous thromboembolism in this patient population, Dr. Bian and his associates noted.
The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
Key clinical point:
Major finding: The use of ESAs among cancer patients that were targeted by the boxed warning dropped by about 40% after the warning was issued.
Data source: A retrospective cohort study involving 45,319 cancer patients enrolled in the SEER data registry during 2004-2009.
Disclosures: The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
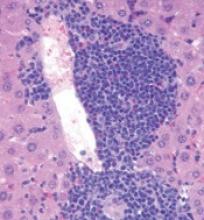
Role of TET2 in hematologic malignancies
New research appears to explain how TET2 mutations increase the risk of hematologic malignancies.
In studying mouse models and patient samples, researchers found evidence to suggest that loss of TET2 opens the door for mutations that drive lymphoid and myeloid malignancies.
The researchers said loss of TET2 leads to hypermutagenicity in hematopoietic stem and progenitor cells (HSPCs), and although TET2-deficient HSPCs are likely not malignant, the higher mutation rates in these cells may result in additional driver mutations in TET2 target genes over time.
“If you lose TET2, it’s not a malignant state per se,” said Mingjiang Xu, MD, PhD, of the University of Miami Miller School of Medicine in Florida.
“But it’s creating a situation for other mutations to happen, leading to all types of blood cancer.”
Dr Xu and his colleagues reported these findings in Nature Communications.
The researchers found that Tet2-knockout mice developed spontaneous, lethal hematologic malignancies. Most (92%) developed myeloid malignancies, but 3.5% developed T-cell malignancies, and 4.5% developed B-cell malignancies.
In sequencing tumor and non-tumor cells from the Tet2-knockout mice, the researchers observed that loss of Tet2 leads to hypermutagenicity in HSPCs.
The team identified 190 genes with recurrent single-nucleotide variants. This included genes that are recurrently altered in human hematologic malignancies—Apc, Nf1, Flt3, Cbl, Notch1, and Mll2.
The researchers also analyzed samples from patients with acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.
The team found that patients with TET2 mutations had “significantly more mutational events than patients with wild-type TET2.” And TET2 mutations were associated with subclonal events in APC, NF1, ASXL1, CBL, and ZRSR2, among other genes.
These findings suggest that targeting TET2 could potentially prevent the development of hematologic malignancies.
The researchers noted that TET2 mutations occur in healthy elderly individuals with clonal hematopoiesis, and these individuals would be ideal candidates for a preventive therapy targeting TET2.
“We are developing a method to target TET2,” Dr Xu said. “If we target that population [with TET2 mutations] for early therapy, we could potentially prevent those downstream mutations from happening.” ![]()
New research appears to explain how TET2 mutations increase the risk of hematologic malignancies.
In studying mouse models and patient samples, researchers found evidence to suggest that loss of TET2 opens the door for mutations that drive lymphoid and myeloid malignancies.
The researchers said loss of TET2 leads to hypermutagenicity in hematopoietic stem and progenitor cells (HSPCs), and although TET2-deficient HSPCs are likely not malignant, the higher mutation rates in these cells may result in additional driver mutations in TET2 target genes over time.
“If you lose TET2, it’s not a malignant state per se,” said Mingjiang Xu, MD, PhD, of the University of Miami Miller School of Medicine in Florida.
“But it’s creating a situation for other mutations to happen, leading to all types of blood cancer.”
Dr Xu and his colleagues reported these findings in Nature Communications.
The researchers found that Tet2-knockout mice developed spontaneous, lethal hematologic malignancies. Most (92%) developed myeloid malignancies, but 3.5% developed T-cell malignancies, and 4.5% developed B-cell malignancies.
In sequencing tumor and non-tumor cells from the Tet2-knockout mice, the researchers observed that loss of Tet2 leads to hypermutagenicity in HSPCs.
The team identified 190 genes with recurrent single-nucleotide variants. This included genes that are recurrently altered in human hematologic malignancies—Apc, Nf1, Flt3, Cbl, Notch1, and Mll2.
The researchers also analyzed samples from patients with acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.
The team found that patients with TET2 mutations had “significantly more mutational events than patients with wild-type TET2.” And TET2 mutations were associated with subclonal events in APC, NF1, ASXL1, CBL, and ZRSR2, among other genes.
These findings suggest that targeting TET2 could potentially prevent the development of hematologic malignancies.
The researchers noted that TET2 mutations occur in healthy elderly individuals with clonal hematopoiesis, and these individuals would be ideal candidates for a preventive therapy targeting TET2.
“We are developing a method to target TET2,” Dr Xu said. “If we target that population [with TET2 mutations] for early therapy, we could potentially prevent those downstream mutations from happening.” ![]()
New research appears to explain how TET2 mutations increase the risk of hematologic malignancies.
In studying mouse models and patient samples, researchers found evidence to suggest that loss of TET2 opens the door for mutations that drive lymphoid and myeloid malignancies.
The researchers said loss of TET2 leads to hypermutagenicity in hematopoietic stem and progenitor cells (HSPCs), and although TET2-deficient HSPCs are likely not malignant, the higher mutation rates in these cells may result in additional driver mutations in TET2 target genes over time.
“If you lose TET2, it’s not a malignant state per se,” said Mingjiang Xu, MD, PhD, of the University of Miami Miller School of Medicine in Florida.
“But it’s creating a situation for other mutations to happen, leading to all types of blood cancer.”
Dr Xu and his colleagues reported these findings in Nature Communications.
The researchers found that Tet2-knockout mice developed spontaneous, lethal hematologic malignancies. Most (92%) developed myeloid malignancies, but 3.5% developed T-cell malignancies, and 4.5% developed B-cell malignancies.
In sequencing tumor and non-tumor cells from the Tet2-knockout mice, the researchers observed that loss of Tet2 leads to hypermutagenicity in HSPCs.
The team identified 190 genes with recurrent single-nucleotide variants. This included genes that are recurrently altered in human hematologic malignancies—Apc, Nf1, Flt3, Cbl, Notch1, and Mll2.
The researchers also analyzed samples from patients with acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.
The team found that patients with TET2 mutations had “significantly more mutational events than patients with wild-type TET2.” And TET2 mutations were associated with subclonal events in APC, NF1, ASXL1, CBL, and ZRSR2, among other genes.
These findings suggest that targeting TET2 could potentially prevent the development of hematologic malignancies.
The researchers noted that TET2 mutations occur in healthy elderly individuals with clonal hematopoiesis, and these individuals would be ideal candidates for a preventive therapy targeting TET2.
“We are developing a method to target TET2,” Dr Xu said. “If we target that population [with TET2 mutations] for early therapy, we could potentially prevent those downstream mutations from happening.” ![]()
Eltrombopag improves frequency, speed, robustness of hematologic recovery
Adding the thrombopoietin-receptor agonist eltrombopag to standard immunosuppressive therapy markedly improved the frequency, speed, and robustness of hematologic recovery in a phase I-II trial of patients with severe aplastic anemia, according to a report published online April 20 in the New England Journal of Medicine.
Previously, researchers found that eltrombopag was effective against aplastic anemia that was refractory to immunosuppression, inducing higher platelet counts, hemoglobin levels, and neutrophil numbers when used as a single agent. They then examined whether adding the drug to immunosuppression in treatment-naive patients would improve the response in this nonrandomized prospective trial, said Danielle M. Townsley, MD, of the hematology branch, National Heart, Lung, and Blood Institute, and her associates.
Ninety-two consecutive patients aged 3-82 years were divided into three cohorts with different timing and duration of treatment. Cohort 1 (30 patients) received eltrombopag from day 14 to 6 months; cohort 2 (31 patients) received it from day 14 to 3 months; and cohort 3 (31 patients) received it from day 1 to 6 months.
The primary efficacy endpoint – the rate of complete hematologic response at 6 months – was highest, at 58%, in cohort 3, which had the earliest initiation and the longest duration of eltrombopag treatment. It was lowest, at 26%, in cohort 2, which had the shortest duration of eltrombopag treatment. The rate of complete hematologic response was intermediate in cohort 1, at 33% (N Engl J Med. 2017 Apr 20. doi:10.1056/NEJMoa1613878).
These complete response rates all exceeded those reported historically, which range from 10% to 20%. Moreover, the rate of partial or complete hematologic response was 87% across all three cohorts, compared with the 66% partial or complete response rate that would be expected with standard immunosuppression alone. Absolute neutrophil counts and platelet counts in all three cohorts also were higher at both 3 and 6 months than were those that have been reported historically.
The average time to independence from transfusions in the entire study population was 1 month, and clinically meaningful improvements in neutrophil levels were seen within a few weeks of initiating eltrombopag.
Two cases of severe cutaneous eruptions led to discontinuation of the study drug. Liver abnormalities were frequent but transient and did not limit the use of eltrombopag.
If these results are validated in future studies, and if no unexpected late complications are identified, adding eltrombopag to standard immunosuppressive therapy “may help patients optimize the timing of allogeneic stem-cell transplantation or avoid it. Of particular interest would be use of eltrombopag in combination with less toxic immunosuppressive regimens that omit antithymocyte globulin, particularly in older patients, in patients with coexisting conditions, and in developing countries where aplastic anemia is prevalent and conventional therapies [are] extremely costly,” Dr. Townsley and her associates noted.
One such confirmatory study, a large randomized placebo-controlled trial (NCT02099747), is now underway in Europe, they wrote.
This trial was supported by the National Heart, Lung, and Blood Institute. Dr. Townsley and her associates reported ties to GlaxoSmithKline and Novartis, makers of eltrombopag.
Adding the thrombopoietin-receptor agonist eltrombopag to standard immunosuppressive therapy markedly improved the frequency, speed, and robustness of hematologic recovery in a phase I-II trial of patients with severe aplastic anemia, according to a report published online April 20 in the New England Journal of Medicine.
Previously, researchers found that eltrombopag was effective against aplastic anemia that was refractory to immunosuppression, inducing higher platelet counts, hemoglobin levels, and neutrophil numbers when used as a single agent. They then examined whether adding the drug to immunosuppression in treatment-naive patients would improve the response in this nonrandomized prospective trial, said Danielle M. Townsley, MD, of the hematology branch, National Heart, Lung, and Blood Institute, and her associates.
Ninety-two consecutive patients aged 3-82 years were divided into three cohorts with different timing and duration of treatment. Cohort 1 (30 patients) received eltrombopag from day 14 to 6 months; cohort 2 (31 patients) received it from day 14 to 3 months; and cohort 3 (31 patients) received it from day 1 to 6 months.
The primary efficacy endpoint – the rate of complete hematologic response at 6 months – was highest, at 58%, in cohort 3, which had the earliest initiation and the longest duration of eltrombopag treatment. It was lowest, at 26%, in cohort 2, which had the shortest duration of eltrombopag treatment. The rate of complete hematologic response was intermediate in cohort 1, at 33% (N Engl J Med. 2017 Apr 20. doi:10.1056/NEJMoa1613878).
These complete response rates all exceeded those reported historically, which range from 10% to 20%. Moreover, the rate of partial or complete hematologic response was 87% across all three cohorts, compared with the 66% partial or complete response rate that would be expected with standard immunosuppression alone. Absolute neutrophil counts and platelet counts in all three cohorts also were higher at both 3 and 6 months than were those that have been reported historically.
The average time to independence from transfusions in the entire study population was 1 month, and clinically meaningful improvements in neutrophil levels were seen within a few weeks of initiating eltrombopag.
Two cases of severe cutaneous eruptions led to discontinuation of the study drug. Liver abnormalities were frequent but transient and did not limit the use of eltrombopag.
If these results are validated in future studies, and if no unexpected late complications are identified, adding eltrombopag to standard immunosuppressive therapy “may help patients optimize the timing of allogeneic stem-cell transplantation or avoid it. Of particular interest would be use of eltrombopag in combination with less toxic immunosuppressive regimens that omit antithymocyte globulin, particularly in older patients, in patients with coexisting conditions, and in developing countries where aplastic anemia is prevalent and conventional therapies [are] extremely costly,” Dr. Townsley and her associates noted.
One such confirmatory study, a large randomized placebo-controlled trial (NCT02099747), is now underway in Europe, they wrote.
This trial was supported by the National Heart, Lung, and Blood Institute. Dr. Townsley and her associates reported ties to GlaxoSmithKline and Novartis, makers of eltrombopag.
Adding the thrombopoietin-receptor agonist eltrombopag to standard immunosuppressive therapy markedly improved the frequency, speed, and robustness of hematologic recovery in a phase I-II trial of patients with severe aplastic anemia, according to a report published online April 20 in the New England Journal of Medicine.
Previously, researchers found that eltrombopag was effective against aplastic anemia that was refractory to immunosuppression, inducing higher platelet counts, hemoglobin levels, and neutrophil numbers when used as a single agent. They then examined whether adding the drug to immunosuppression in treatment-naive patients would improve the response in this nonrandomized prospective trial, said Danielle M. Townsley, MD, of the hematology branch, National Heart, Lung, and Blood Institute, and her associates.
Ninety-two consecutive patients aged 3-82 years were divided into three cohorts with different timing and duration of treatment. Cohort 1 (30 patients) received eltrombopag from day 14 to 6 months; cohort 2 (31 patients) received it from day 14 to 3 months; and cohort 3 (31 patients) received it from day 1 to 6 months.
The primary efficacy endpoint – the rate of complete hematologic response at 6 months – was highest, at 58%, in cohort 3, which had the earliest initiation and the longest duration of eltrombopag treatment. It was lowest, at 26%, in cohort 2, which had the shortest duration of eltrombopag treatment. The rate of complete hematologic response was intermediate in cohort 1, at 33% (N Engl J Med. 2017 Apr 20. doi:10.1056/NEJMoa1613878).
These complete response rates all exceeded those reported historically, which range from 10% to 20%. Moreover, the rate of partial or complete hematologic response was 87% across all three cohorts, compared with the 66% partial or complete response rate that would be expected with standard immunosuppression alone. Absolute neutrophil counts and platelet counts in all three cohorts also were higher at both 3 and 6 months than were those that have been reported historically.
The average time to independence from transfusions in the entire study population was 1 month, and clinically meaningful improvements in neutrophil levels were seen within a few weeks of initiating eltrombopag.
Two cases of severe cutaneous eruptions led to discontinuation of the study drug. Liver abnormalities were frequent but transient and did not limit the use of eltrombopag.
If these results are validated in future studies, and if no unexpected late complications are identified, adding eltrombopag to standard immunosuppressive therapy “may help patients optimize the timing of allogeneic stem-cell transplantation or avoid it. Of particular interest would be use of eltrombopag in combination with less toxic immunosuppressive regimens that omit antithymocyte globulin, particularly in older patients, in patients with coexisting conditions, and in developing countries where aplastic anemia is prevalent and conventional therapies [are] extremely costly,” Dr. Townsley and her associates noted.
One such confirmatory study, a large randomized placebo-controlled trial (NCT02099747), is now underway in Europe, they wrote.
This trial was supported by the National Heart, Lung, and Blood Institute. Dr. Townsley and her associates reported ties to GlaxoSmithKline and Novartis, makers of eltrombopag.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding eltrombopag to standard immunosuppression markedly improves the frequency, speed, and robustness of hematologic recovery in patients with severe aplastic anemia.
Major finding: The rate of partial or complete hematologic response was 87% across all 3 cohorts, compared with the 66% partial or complete response rate that would be expected with standard immunosuppression alone.
Data source: A prospective phase I-II study involving 92 patients aged 3-82 years who were followed for a median of 2 years.
Disclosures: This trial was supported by the National Heart, Lung, and Blood Institute. Dr. Townsley and her associates reported ties to GlaxoSmithKline and Novartis, makers of eltrombopag.
Combo improves response rates in treatment-naïve SAA
Adding eltrombopag to immunosuppressive therapy (IST) can produce high rates of response in treatment-naïve severe aplastic anemia (SAA), according to research published in NEJM.
In patients who received eltrombopag for 6 months, the overall response rate (ORR) was 94%, and the complete response (CR) rate was 58%.
Researchers noted that these rates are “markedly higher” than response rates observed in historical controls who received IST alone.
The team also said the safety profile of eltrombopag in this study was consistent with the known safety profile of the drug.
“[E]ltrombopag plus standard immunosuppressive therapy appeared to increase the overall response rate and substantially increase the frequency, speed, and robustness of hematologic recovery in patients with SAA compared to historical controls,” said study author Danielle Townsley, MD, of the National Heart, Lung and Blood Institute (NHLBI).
She and her colleagues at NHLBI conducted this research through a Cooperative Research and Development Agreement with Novartis, the company that markets eltrombopag as Promacta/Revolade.
Patients and treatment
This phase 1/2 trial included 92 patients with treatment-naïve SAA. The patients’ median age was 32 (range, 3-82), and 54% of them were male.
At baseline, the patients’ median neutrophil count was 310/mm3 (range, 0-1810), their median reticulocyte count was 19,950/mm3 (range, 1600-60,400), and their median platelet count was 9000/mm3 (range, 0-37,000). Their median thrombopoietin level was 3163 pg/ml (range, 1806-4955).
All patients received horse antithymocyte globulin on days 1 to 4 and cyclosporine from day 1 to 6 months. The patients also received eltrombopag at an age-dependent dose.
They received eltrombopag at 150 mg daily if they were 12 years or older, 75 mg daily if they were 6 to 11, and 2.5 mg/kg/day if they were 2 to 5 years of age.
Patients were also split into 3 cohorts according to treatment duration:
- Cohort 1 received eltrombopag from day 14 to the 6-month mark.
- Cohort 2 received eltrombopag from day 14 to the 3-month mark.
- Cohort 3 received eltrombopag from day 1 to 6 months.
Response
The study’s primary efficacy endpoint was hematologic CR at 6 months. CR was defined as an absolute neutrophil count of at least 1000/mm3, a hemoglobin level of at least 10 g/dL, and a platelet count of at least 100,000/mm3.
Secondary endpoints included partial response (PR) and ORR, among other endpoints. ORR was the rate of CR plus PR. Patients had a PR if they had blood counts that no longer met the criteria for SAA but they did not meet criteria for CR.
For all cohorts, at 6 months, the ORR was 87%, and the CR rate was 39%.
In cohort 1, the ORR was 80%, and the CR rate was 33%.
In cohort 2, the ORR was 87%, and the CR rate was 26%.
In cohort 3, the ORR was 94%, and the CR rate was 58%.
The researchers noted that ORRs were higher across all cohorts than the ORR observed in a historical cohort (66%). The cohort consisted of 102 patients who had received standard IST while serving as controls in 1 of 2 recent NHLBI clinical trials.
Relapse
Thirty-two percent of responding patients (25/78) relapsed after 6 months.
After the study protocol was amended to allow the continuation of low-dose cyclosporine from 6 months to 2 years, the frequency of relapse decreased.
Fourteen percent of responders receiving low-dose cyclosporine beyond 6 months relapsed (6/43), compared to 54% of patients who stopped cyclosporine at 6 months (19/35).
Restarting full-dose cyclosporine reversed relapse and increased blood counts in 13 of 25 patients. Adding eltrombopag reversed relapse in an additional 10 patients.
Survival
The 2-year overall survival rate was 97% for the entire study population and 99% when data were censored for transplant.
Twelve patients under went transplant after eltrombopag. Six of them had not responded or were still transfusion-dependent, 3 relapsed, and 3 had clonal evolution.
Three patients died—1 while on study and 2 after transplant.
Clonal evolution and PNH
The researchers said the frequency of clonal evolution in this study was similar to that observed in the historical cohort of patients who received standard IST. The rate of clonal evolution at 2 years was 8% in both groups.
In the current study, 7 patients had clonal evolution at 2 years. Five patients had loss of chromosome 7, which was associated with dysplastic bone marrow changes in 3 patients.
One patient with a complex karyotype progressed to acute myeloid leukemia.
Two patients developed hemolytic paroxysmal nocturnal hemoglobinuria (PNH).
Safety
Two severe adverse events (AEs) were attributed to eltrombopag. Both were cutaneous eruptions—a grade 2 and a grade 3 event. Both AEs led to discontinuation of the drug.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks of treatment due to transient elevations in liver enzymes.
AEs not attributed to eltrombopag were neutropenic infections and AEs known to be associated with IST.
The single patient who died on study was a non-responder who died 3 months after starting treatment. The death was due to paraneoplastic encephalopathy, which was attributed to thymoma that predated study entry. ![]()
Adding eltrombopag to immunosuppressive therapy (IST) can produce high rates of response in treatment-naïve severe aplastic anemia (SAA), according to research published in NEJM.
In patients who received eltrombopag for 6 months, the overall response rate (ORR) was 94%, and the complete response (CR) rate was 58%.
Researchers noted that these rates are “markedly higher” than response rates observed in historical controls who received IST alone.
The team also said the safety profile of eltrombopag in this study was consistent with the known safety profile of the drug.
“[E]ltrombopag plus standard immunosuppressive therapy appeared to increase the overall response rate and substantially increase the frequency, speed, and robustness of hematologic recovery in patients with SAA compared to historical controls,” said study author Danielle Townsley, MD, of the National Heart, Lung and Blood Institute (NHLBI).
She and her colleagues at NHLBI conducted this research through a Cooperative Research and Development Agreement with Novartis, the company that markets eltrombopag as Promacta/Revolade.
Patients and treatment
This phase 1/2 trial included 92 patients with treatment-naïve SAA. The patients’ median age was 32 (range, 3-82), and 54% of them were male.
At baseline, the patients’ median neutrophil count was 310/mm3 (range, 0-1810), their median reticulocyte count was 19,950/mm3 (range, 1600-60,400), and their median platelet count was 9000/mm3 (range, 0-37,000). Their median thrombopoietin level was 3163 pg/ml (range, 1806-4955).
All patients received horse antithymocyte globulin on days 1 to 4 and cyclosporine from day 1 to 6 months. The patients also received eltrombopag at an age-dependent dose.
They received eltrombopag at 150 mg daily if they were 12 years or older, 75 mg daily if they were 6 to 11, and 2.5 mg/kg/day if they were 2 to 5 years of age.
Patients were also split into 3 cohorts according to treatment duration:
- Cohort 1 received eltrombopag from day 14 to the 6-month mark.
- Cohort 2 received eltrombopag from day 14 to the 3-month mark.
- Cohort 3 received eltrombopag from day 1 to 6 months.
Response
The study’s primary efficacy endpoint was hematologic CR at 6 months. CR was defined as an absolute neutrophil count of at least 1000/mm3, a hemoglobin level of at least 10 g/dL, and a platelet count of at least 100,000/mm3.
Secondary endpoints included partial response (PR) and ORR, among other endpoints. ORR was the rate of CR plus PR. Patients had a PR if they had blood counts that no longer met the criteria for SAA but they did not meet criteria for CR.
For all cohorts, at 6 months, the ORR was 87%, and the CR rate was 39%.
In cohort 1, the ORR was 80%, and the CR rate was 33%.
In cohort 2, the ORR was 87%, and the CR rate was 26%.
In cohort 3, the ORR was 94%, and the CR rate was 58%.
The researchers noted that ORRs were higher across all cohorts than the ORR observed in a historical cohort (66%). The cohort consisted of 102 patients who had received standard IST while serving as controls in 1 of 2 recent NHLBI clinical trials.
Relapse
Thirty-two percent of responding patients (25/78) relapsed after 6 months.
After the study protocol was amended to allow the continuation of low-dose cyclosporine from 6 months to 2 years, the frequency of relapse decreased.
Fourteen percent of responders receiving low-dose cyclosporine beyond 6 months relapsed (6/43), compared to 54% of patients who stopped cyclosporine at 6 months (19/35).
Restarting full-dose cyclosporine reversed relapse and increased blood counts in 13 of 25 patients. Adding eltrombopag reversed relapse in an additional 10 patients.
Survival
The 2-year overall survival rate was 97% for the entire study population and 99% when data were censored for transplant.
Twelve patients under went transplant after eltrombopag. Six of them had not responded or were still transfusion-dependent, 3 relapsed, and 3 had clonal evolution.
Three patients died—1 while on study and 2 after transplant.
Clonal evolution and PNH
The researchers said the frequency of clonal evolution in this study was similar to that observed in the historical cohort of patients who received standard IST. The rate of clonal evolution at 2 years was 8% in both groups.
In the current study, 7 patients had clonal evolution at 2 years. Five patients had loss of chromosome 7, which was associated with dysplastic bone marrow changes in 3 patients.
One patient with a complex karyotype progressed to acute myeloid leukemia.
Two patients developed hemolytic paroxysmal nocturnal hemoglobinuria (PNH).
Safety
Two severe adverse events (AEs) were attributed to eltrombopag. Both were cutaneous eruptions—a grade 2 and a grade 3 event. Both AEs led to discontinuation of the drug.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks of treatment due to transient elevations in liver enzymes.
AEs not attributed to eltrombopag were neutropenic infections and AEs known to be associated with IST.
The single patient who died on study was a non-responder who died 3 months after starting treatment. The death was due to paraneoplastic encephalopathy, which was attributed to thymoma that predated study entry. ![]()
Adding eltrombopag to immunosuppressive therapy (IST) can produce high rates of response in treatment-naïve severe aplastic anemia (SAA), according to research published in NEJM.
In patients who received eltrombopag for 6 months, the overall response rate (ORR) was 94%, and the complete response (CR) rate was 58%.
Researchers noted that these rates are “markedly higher” than response rates observed in historical controls who received IST alone.
The team also said the safety profile of eltrombopag in this study was consistent with the known safety profile of the drug.
“[E]ltrombopag plus standard immunosuppressive therapy appeared to increase the overall response rate and substantially increase the frequency, speed, and robustness of hematologic recovery in patients with SAA compared to historical controls,” said study author Danielle Townsley, MD, of the National Heart, Lung and Blood Institute (NHLBI).
She and her colleagues at NHLBI conducted this research through a Cooperative Research and Development Agreement with Novartis, the company that markets eltrombopag as Promacta/Revolade.
Patients and treatment
This phase 1/2 trial included 92 patients with treatment-naïve SAA. The patients’ median age was 32 (range, 3-82), and 54% of them were male.
At baseline, the patients’ median neutrophil count was 310/mm3 (range, 0-1810), their median reticulocyte count was 19,950/mm3 (range, 1600-60,400), and their median platelet count was 9000/mm3 (range, 0-37,000). Their median thrombopoietin level was 3163 pg/ml (range, 1806-4955).
All patients received horse antithymocyte globulin on days 1 to 4 and cyclosporine from day 1 to 6 months. The patients also received eltrombopag at an age-dependent dose.
They received eltrombopag at 150 mg daily if they were 12 years or older, 75 mg daily if they were 6 to 11, and 2.5 mg/kg/day if they were 2 to 5 years of age.
Patients were also split into 3 cohorts according to treatment duration:
- Cohort 1 received eltrombopag from day 14 to the 6-month mark.
- Cohort 2 received eltrombopag from day 14 to the 3-month mark.
- Cohort 3 received eltrombopag from day 1 to 6 months.
Response
The study’s primary efficacy endpoint was hematologic CR at 6 months. CR was defined as an absolute neutrophil count of at least 1000/mm3, a hemoglobin level of at least 10 g/dL, and a platelet count of at least 100,000/mm3.
Secondary endpoints included partial response (PR) and ORR, among other endpoints. ORR was the rate of CR plus PR. Patients had a PR if they had blood counts that no longer met the criteria for SAA but they did not meet criteria for CR.
For all cohorts, at 6 months, the ORR was 87%, and the CR rate was 39%.
In cohort 1, the ORR was 80%, and the CR rate was 33%.
In cohort 2, the ORR was 87%, and the CR rate was 26%.
In cohort 3, the ORR was 94%, and the CR rate was 58%.
The researchers noted that ORRs were higher across all cohorts than the ORR observed in a historical cohort (66%). The cohort consisted of 102 patients who had received standard IST while serving as controls in 1 of 2 recent NHLBI clinical trials.
Relapse
Thirty-two percent of responding patients (25/78) relapsed after 6 months.
After the study protocol was amended to allow the continuation of low-dose cyclosporine from 6 months to 2 years, the frequency of relapse decreased.
Fourteen percent of responders receiving low-dose cyclosporine beyond 6 months relapsed (6/43), compared to 54% of patients who stopped cyclosporine at 6 months (19/35).
Restarting full-dose cyclosporine reversed relapse and increased blood counts in 13 of 25 patients. Adding eltrombopag reversed relapse in an additional 10 patients.
Survival
The 2-year overall survival rate was 97% for the entire study population and 99% when data were censored for transplant.
Twelve patients under went transplant after eltrombopag. Six of them had not responded or were still transfusion-dependent, 3 relapsed, and 3 had clonal evolution.
Three patients died—1 while on study and 2 after transplant.
Clonal evolution and PNH
The researchers said the frequency of clonal evolution in this study was similar to that observed in the historical cohort of patients who received standard IST. The rate of clonal evolution at 2 years was 8% in both groups.
In the current study, 7 patients had clonal evolution at 2 years. Five patients had loss of chromosome 7, which was associated with dysplastic bone marrow changes in 3 patients.
One patient with a complex karyotype progressed to acute myeloid leukemia.
Two patients developed hemolytic paroxysmal nocturnal hemoglobinuria (PNH).
Safety
Two severe adverse events (AEs) were attributed to eltrombopag. Both were cutaneous eruptions—a grade 2 and a grade 3 event. Both AEs led to discontinuation of the drug.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks of treatment due to transient elevations in liver enzymes.
AEs not attributed to eltrombopag were neutropenic infections and AEs known to be associated with IST.
The single patient who died on study was a non-responder who died 3 months after starting treatment. The death was due to paraneoplastic encephalopathy, which was attributed to thymoma that predated study entry. ![]()
FDA: REMS no longer necessary for epoetin, darbepoetin
The Food and Drug Administration no longer requires certification of doctors and hospitals to prescribe epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) for chemotherapy anemia.
A Risk Evaluation and Mitigation Strategy (REMS) program was put in place in 2011 to make sure that the benefits of erythropoiesis-stimulating agents (ESAs) outweighed the risks when prescribed. Under the program, providers were required to become certified in the ESA REMS and to demonstrate that each patient had received counseling on the benefits and risks of the therapies prior to use.
Amgen’s prescriber surveys demonstrated “acceptable knowledge” of the need to counsel patients about the risks. Utilization data indicated “appropriate prescribing” as an alternative to transfusion.
In addition, in an evaluation of the impact of multiple regulatory actions, the FDA determined that full implementation of the ESA REMS in 2011 had minimal impact on trends in ESA utilization metrics, the FDA wrote.
The FDA concluded that regulatory actions and label changes – and the cut in payments for nonrenal indications from the Center for Medicare & Medicaid Services – were enough to reduce overuse in chemotherapy.
However, while the REMS is no longer necessary, the FDA says serious risks of shortened overall survival and/or increased risk of tumor progression or recurrence associated with these drugs remain and health care providers should continue to discuss the risks and benefits of using ESAs with each patient before initiating use.
The Food and Drug Administration no longer requires certification of doctors and hospitals to prescribe epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) for chemotherapy anemia.
A Risk Evaluation and Mitigation Strategy (REMS) program was put in place in 2011 to make sure that the benefits of erythropoiesis-stimulating agents (ESAs) outweighed the risks when prescribed. Under the program, providers were required to become certified in the ESA REMS and to demonstrate that each patient had received counseling on the benefits and risks of the therapies prior to use.
Amgen’s prescriber surveys demonstrated “acceptable knowledge” of the need to counsel patients about the risks. Utilization data indicated “appropriate prescribing” as an alternative to transfusion.
In addition, in an evaluation of the impact of multiple regulatory actions, the FDA determined that full implementation of the ESA REMS in 2011 had minimal impact on trends in ESA utilization metrics, the FDA wrote.
The FDA concluded that regulatory actions and label changes – and the cut in payments for nonrenal indications from the Center for Medicare & Medicaid Services – were enough to reduce overuse in chemotherapy.
However, while the REMS is no longer necessary, the FDA says serious risks of shortened overall survival and/or increased risk of tumor progression or recurrence associated with these drugs remain and health care providers should continue to discuss the risks and benefits of using ESAs with each patient before initiating use.
The Food and Drug Administration no longer requires certification of doctors and hospitals to prescribe epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) for chemotherapy anemia.
A Risk Evaluation and Mitigation Strategy (REMS) program was put in place in 2011 to make sure that the benefits of erythropoiesis-stimulating agents (ESAs) outweighed the risks when prescribed. Under the program, providers were required to become certified in the ESA REMS and to demonstrate that each patient had received counseling on the benefits and risks of the therapies prior to use.
Amgen’s prescriber surveys demonstrated “acceptable knowledge” of the need to counsel patients about the risks. Utilization data indicated “appropriate prescribing” as an alternative to transfusion.
In addition, in an evaluation of the impact of multiple regulatory actions, the FDA determined that full implementation of the ESA REMS in 2011 had minimal impact on trends in ESA utilization metrics, the FDA wrote.
The FDA concluded that regulatory actions and label changes – and the cut in payments for nonrenal indications from the Center for Medicare & Medicaid Services – were enough to reduce overuse in chemotherapy.
However, while the REMS is no longer necessary, the FDA says serious risks of shortened overall survival and/or increased risk of tumor progression or recurrence associated with these drugs remain and health care providers should continue to discuss the risks and benefits of using ESAs with each patient before initiating use.
FDA: REMS for ESAs no longer needed, though risks persist
The US Food and Drug Administration (FDA) has determined that the risk evaluation and mitigation strategy (REMS) for erythropoiesis-stimulating agents (ESAs) is no longer necessary.
The REMS was limited to the use of epoetin alfa (marketed as Epogen and Procrit) and darbepoetin alfa (marketed as Aranesp) to treat patients with anemia due to myelosuppressive chemotherapy.
The FDA said the REMS is no longer necessary to ensure that the benefits of Epogen/Procrit and Aranesp outweigh the risks these drugs pose, which include shortened overall survival and an increased risk of tumor progression or recurrence in patients with cancer.
The FDA has released the REMS requirements for these ESAs and said the risks the drugs pose can be communicated by the current product prescribing information.
The FDA decided the REMS is no longer needed based on its own analyses and an evaluation of the REMS assessment submitted by Amgen, Inc., the company that markets Epogen/Procrit and Aranesp.
Details on the analyses and evaluation are available from the following page on the FDA website: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp). ![]()
The US Food and Drug Administration (FDA) has determined that the risk evaluation and mitigation strategy (REMS) for erythropoiesis-stimulating agents (ESAs) is no longer necessary.
The REMS was limited to the use of epoetin alfa (marketed as Epogen and Procrit) and darbepoetin alfa (marketed as Aranesp) to treat patients with anemia due to myelosuppressive chemotherapy.
The FDA said the REMS is no longer necessary to ensure that the benefits of Epogen/Procrit and Aranesp outweigh the risks these drugs pose, which include shortened overall survival and an increased risk of tumor progression or recurrence in patients with cancer.
The FDA has released the REMS requirements for these ESAs and said the risks the drugs pose can be communicated by the current product prescribing information.
The FDA decided the REMS is no longer needed based on its own analyses and an evaluation of the REMS assessment submitted by Amgen, Inc., the company that markets Epogen/Procrit and Aranesp.
Details on the analyses and evaluation are available from the following page on the FDA website: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp). ![]()
The US Food and Drug Administration (FDA) has determined that the risk evaluation and mitigation strategy (REMS) for erythropoiesis-stimulating agents (ESAs) is no longer necessary.
The REMS was limited to the use of epoetin alfa (marketed as Epogen and Procrit) and darbepoetin alfa (marketed as Aranesp) to treat patients with anemia due to myelosuppressive chemotherapy.
The FDA said the REMS is no longer necessary to ensure that the benefits of Epogen/Procrit and Aranesp outweigh the risks these drugs pose, which include shortened overall survival and an increased risk of tumor progression or recurrence in patients with cancer.
The FDA has released the REMS requirements for these ESAs and said the risks the drugs pose can be communicated by the current product prescribing information.
The FDA decided the REMS is no longer needed based on its own analyses and an evaluation of the REMS assessment submitted by Amgen, Inc., the company that markets Epogen/Procrit and Aranesp.
Details on the analyses and evaluation are available from the following page on the FDA website: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp). ![]()
Study supports use of tPA in stroke patients with SCD
A new study suggests that having sickle cell disease (SCD) should not prevent patients from receiving tissue plasminogen activator (tPA) to treat ischemic stroke if they otherwise qualify for the treatment.
Researchers compared outcomes of tPA treatment in stroke patients with and without SCD and found no significant differences between the groups with regard to serious complications, length of hospital stay, or in-hospital mortality.
“Having sickle cell disease did not adversely affect any of the indicators we measured,” said Robert J. Adams, MD, of the Medical University of South Carolina in Charleston.
The SCD patients did have a higher rate of intracranial hemorrhage (ICH) than patients without SCD, although the rate was not significantly higher. Still, the researchers said further study is needed to look more closely at this outcome.
Dr Adams and his colleagues reported their findings in the journal Stroke.
The team noted that use of tPA has never been contraindicated in SCD, but guidelines recommend acute exchange transfusion for stroke in SCD, rather than tPA.
To gain more insight into the effects of tPA in patients with SCD, the researchers analyzed in-hospital data compiled by the quality improvement program Get With The Guidelines – Stroke.
The data included 2,016,652 stroke patients seen at 1952 participating US hospitals between January 2008 and March 2015. From these patients, the researchers identified 832 with SCD and 3328 age-, sex-, and race-matched controls.
There was no significant difference between the 2 cohorts in the rate of tPA use—8.2% for SCD patients and 9.4% for controls (P=0.3024).
Likewise, there was no significant difference in the timeliness of tPA administration. The median door-to-needle time was 73 minutes for SCD patients and 79 minutes for controls (P=0.3891).
Among patients who received tPA, there was no significant difference in the overall rate of serious complications, which occurred in 6.6% of the SCD patients and 6.0% of controls (P=0.7732).
Serious complications included symptomatic ICH, which occurred in 4.9% of the SCD patients and 3.2% of controls who received tPA (P= 0.4502).
Although this difference was not significant, the researchers said additional studies are needed to track the ICH rate in SCD patients receiving tPA.
The researchers also calculated the odds ratios (ORs) for various outcomes in tPA-treated SCD patients compared to controls.
In an analysis adjusted for multiple covariates, the OR for in-hospital mortality was 1.21 for SCD patients (P=0.4150), the OR for being discharged home was 0.90 (P=0.2686), and the OR for having a hospital stay lasting beyond 4 days was 1.15 (P=0.1151).
“People with sickle cell disease and an acute stroke who would otherwise qualify for tPA did not have worse outcomes than stroke patients who did not have sickle cell disease,” Dr Adams noted.
He and his colleagues said these findings suggest tPA is safe for patients with SCD and could potentially be used as a complementary therapy to red blood cell exchange, the current guideline-recommended frontline therapy for ischemic stroke in patients with SCD.
“These findings suggest that a future randomized trial that compares using red blood cell exchange alone versus combination therapy with tPA and red blood cell exchange should be undertaken to evaluate the outcomes of [ischemic stroke] in patients with sickle cell disease,” said study author Julie Kanter, MD, of the Medical University of South Carolina in Charleston. ![]()
A new study suggests that having sickle cell disease (SCD) should not prevent patients from receiving tissue plasminogen activator (tPA) to treat ischemic stroke if they otherwise qualify for the treatment.
Researchers compared outcomes of tPA treatment in stroke patients with and without SCD and found no significant differences between the groups with regard to serious complications, length of hospital stay, or in-hospital mortality.
“Having sickle cell disease did not adversely affect any of the indicators we measured,” said Robert J. Adams, MD, of the Medical University of South Carolina in Charleston.
The SCD patients did have a higher rate of intracranial hemorrhage (ICH) than patients without SCD, although the rate was not significantly higher. Still, the researchers said further study is needed to look more closely at this outcome.
Dr Adams and his colleagues reported their findings in the journal Stroke.
The team noted that use of tPA has never been contraindicated in SCD, but guidelines recommend acute exchange transfusion for stroke in SCD, rather than tPA.
To gain more insight into the effects of tPA in patients with SCD, the researchers analyzed in-hospital data compiled by the quality improvement program Get With The Guidelines – Stroke.
The data included 2,016,652 stroke patients seen at 1952 participating US hospitals between January 2008 and March 2015. From these patients, the researchers identified 832 with SCD and 3328 age-, sex-, and race-matched controls.
There was no significant difference between the 2 cohorts in the rate of tPA use—8.2% for SCD patients and 9.4% for controls (P=0.3024).
Likewise, there was no significant difference in the timeliness of tPA administration. The median door-to-needle time was 73 minutes for SCD patients and 79 minutes for controls (P=0.3891).
Among patients who received tPA, there was no significant difference in the overall rate of serious complications, which occurred in 6.6% of the SCD patients and 6.0% of controls (P=0.7732).
Serious complications included symptomatic ICH, which occurred in 4.9% of the SCD patients and 3.2% of controls who received tPA (P= 0.4502).
Although this difference was not significant, the researchers said additional studies are needed to track the ICH rate in SCD patients receiving tPA.
The researchers also calculated the odds ratios (ORs) for various outcomes in tPA-treated SCD patients compared to controls.
In an analysis adjusted for multiple covariates, the OR for in-hospital mortality was 1.21 for SCD patients (P=0.4150), the OR for being discharged home was 0.90 (P=0.2686), and the OR for having a hospital stay lasting beyond 4 days was 1.15 (P=0.1151).
“People with sickle cell disease and an acute stroke who would otherwise qualify for tPA did not have worse outcomes than stroke patients who did not have sickle cell disease,” Dr Adams noted.
He and his colleagues said these findings suggest tPA is safe for patients with SCD and could potentially be used as a complementary therapy to red blood cell exchange, the current guideline-recommended frontline therapy for ischemic stroke in patients with SCD.
“These findings suggest that a future randomized trial that compares using red blood cell exchange alone versus combination therapy with tPA and red blood cell exchange should be undertaken to evaluate the outcomes of [ischemic stroke] in patients with sickle cell disease,” said study author Julie Kanter, MD, of the Medical University of South Carolina in Charleston. ![]()
A new study suggests that having sickle cell disease (SCD) should not prevent patients from receiving tissue plasminogen activator (tPA) to treat ischemic stroke if they otherwise qualify for the treatment.
Researchers compared outcomes of tPA treatment in stroke patients with and without SCD and found no significant differences between the groups with regard to serious complications, length of hospital stay, or in-hospital mortality.
“Having sickle cell disease did not adversely affect any of the indicators we measured,” said Robert J. Adams, MD, of the Medical University of South Carolina in Charleston.
The SCD patients did have a higher rate of intracranial hemorrhage (ICH) than patients without SCD, although the rate was not significantly higher. Still, the researchers said further study is needed to look more closely at this outcome.
Dr Adams and his colleagues reported their findings in the journal Stroke.
The team noted that use of tPA has never been contraindicated in SCD, but guidelines recommend acute exchange transfusion for stroke in SCD, rather than tPA.
To gain more insight into the effects of tPA in patients with SCD, the researchers analyzed in-hospital data compiled by the quality improvement program Get With The Guidelines – Stroke.
The data included 2,016,652 stroke patients seen at 1952 participating US hospitals between January 2008 and March 2015. From these patients, the researchers identified 832 with SCD and 3328 age-, sex-, and race-matched controls.
There was no significant difference between the 2 cohorts in the rate of tPA use—8.2% for SCD patients and 9.4% for controls (P=0.3024).
Likewise, there was no significant difference in the timeliness of tPA administration. The median door-to-needle time was 73 minutes for SCD patients and 79 minutes for controls (P=0.3891).
Among patients who received tPA, there was no significant difference in the overall rate of serious complications, which occurred in 6.6% of the SCD patients and 6.0% of controls (P=0.7732).
Serious complications included symptomatic ICH, which occurred in 4.9% of the SCD patients and 3.2% of controls who received tPA (P= 0.4502).
Although this difference was not significant, the researchers said additional studies are needed to track the ICH rate in SCD patients receiving tPA.
The researchers also calculated the odds ratios (ORs) for various outcomes in tPA-treated SCD patients compared to controls.
In an analysis adjusted for multiple covariates, the OR for in-hospital mortality was 1.21 for SCD patients (P=0.4150), the OR for being discharged home was 0.90 (P=0.2686), and the OR for having a hospital stay lasting beyond 4 days was 1.15 (P=0.1151).
“People with sickle cell disease and an acute stroke who would otherwise qualify for tPA did not have worse outcomes than stroke patients who did not have sickle cell disease,” Dr Adams noted.
He and his colleagues said these findings suggest tPA is safe for patients with SCD and could potentially be used as a complementary therapy to red blood cell exchange, the current guideline-recommended frontline therapy for ischemic stroke in patients with SCD.
“These findings suggest that a future randomized trial that compares using red blood cell exchange alone versus combination therapy with tPA and red blood cell exchange should be undertaken to evaluate the outcomes of [ischemic stroke] in patients with sickle cell disease,” said study author Julie Kanter, MD, of the Medical University of South Carolina in Charleston. ![]()
FDA clears direct-to-consumer marketing of genetic risk tests
The US Food and Drug Administration (FDA) has authorized marketing of 23andMe Personal Genome Service (PGS) Genetic Health Risk (GHR) tests for 10 medical conditions.
These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual’s genetic predisposition to certain conditions, including factor XI deficiency, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hereditary hemochromatosis, hereditary thrombophilia, and other conditions.
The GHR tests work by isolating DNA from a saliva sample, which is then tested for more than 500,000 genetic variants.
Consumers receive reports of the results, which tell them if they have an increased risk of developing any of the following 10 conditions.
Factor XI deficiency
The 23andMe PGS Genetic Health Risk Report for Factor XI Deficiency is indicated for reporting of the F283L, E117X, and IVS14+1G>A variants in the F11 gene.
This report describes if a person has a variant associated with factor XI deficiency and the potential for a higher risk of excessive bleeding following trauma or surgery, but it does not describe a person’s overall risk for excessive bleeding. This report is most relevant for people of Ashkenazi Jewish descent.
G6PD deficiency
The 23andMe PGS Genetic Health Risk Report for Glucose-6-Phosphate-Dehydrogenase Deficiency is indicated for reporting of the Val68Met variant in the G6PD gene.
This report describes if a person has a variant associated with G6PD deficiency and a higher risk for episodes of anemia, but it does not describe a person’s overall risk of developing anemia. This report is most relevant for people of African descent.
Hereditary hemochromatosis
The 23andMe PGS Genetic Health Risk Report for Hereditary Hemochromatosis is indicated for reporting of the C282Y and H63D variants in the HFE gene.
This report describes if a person has variants associated with hereditary hemochromatosis and a higher risk for iron overload, but it does not describe a person’s overall risk of developing iron overload. This report is most relevant for people of European descent.
Hereditary thrombophilia
The 23andMe PGS Genetic Health Risk Report for Hereditary Thrombophilia is indicated for reporting of the factor V Leiden variant in the F5 gene, as well as the prothrombin G20210A variant in the F2 gene.
This report describes if a person has variants associated with a higher risk of thrombosis, but it does not describe a person’s overall risk of developing thrombosis. This report is most relevant for people of European descent.
Alpha-1 antitrypsin deficiency (AATD)
The 23andMe PGS Genetic Health Risk Report for Alpha-I Antitrypsin Deficiency is indicated for reporting of the PI*Z and PI*S variants in the SERPINA1 gene.
This report describes if a person has variants associated with AATD and a higher risk for lung or liver disease, but it does not describe a person’s overall risk of developing lung or liver disease. This report is most relevant for people of European descent.
Celiac disease
The 23andMe PGS Genetic Health Risk Report for Celiac Disease is indicated for reporting of a variant in the HLA-DQ2.5 haplotype.
The report describes if a person has a haplotype associated with an increased risk of developing celiac disease, but it does not describe a person’s overall risk for developing celiac disease. This report is most relevant for people of European descent.
Early onset primary dystonia (DYT1/TOR1A-related)
The 23andMe PGS Genetic Health Risk Report for Early-Onset Primary Dystonia (DYT1/TOR1A-Related) is indicated for reporting of the deltaE302/303 variant in the DYT1 gene.
This report describes if a person has variants associated with a higher risk for early-onset primary dystonia, but it does not describe a person’s overall risk of developing dystonia. This report is most relevant for people of Ashkenazi Jewish descent.
Gaucher disease
The 23andMe PGS Genetic Health Risk Report for Gaucher Disease Type 1 is indicated for reporting of the N370S, 84GG, and V394L variants in the GBA gene.
This report describes if a person has variants associated with an increased risk for developing carrier status for Gaucher disease type 1 in adults. This report also describes if a result is associated with personal risk for developing symptoms of Gaucher disease type 1, but it does not describe a person’s overall risk of developing Gaucher disease type 1.
This test is most relevant for people of Ashkenazi Jewish descent.
Late-onset Alzheimer’s disease
The 23andMe PGS Genetic Health Risk Report for Late-onset Alzheimer’s Disease is indicated for reporting of the ε4 variant in the APOE gene.
The report describes if a person’s genetic result is associated with an increased risk of developing late-onset Alzheimer’s disease, but it does not describe a person’s overall risk of developing Alzheimer’s disease.
The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Parkinson’s disease
The 23andMe PGS Genetic Health Risk Report for Parkinson’s Disease is indicated for reporting of the G2019S variant in the LRRK2 gene and the N370S variant in the GBA gene.
The report describes if a person’s genetic result is associated with an increased risk of developing Parkinson’s disease, but it does not describe a person’s overall risk of developing Parkinson’s disease. The test is most relevant for people of European, Ashkenazi Jewish, and North African Berber descent.
Access to testing
23andMe, Inc. said it will release its first set of GHR tests—for hereditary thrombophilia, late-onset Alzheimer’s disease, Parkinson’s disease, alpha-1 antitrypsin deficiency, and Gaucher disease—this month. The remaining tests will follow.
New 23andMe Health + Ancestry Service customers in the US will have access to these tests. Current 23andMe customers will be notified directly regarding their eligibility.
About the marketing authorization
The FDA reviewed data for the 23andMe GHR tests through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the tests’ accuracy, reliability, and clinical relevance. These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for these and similar GHR tests.
The FDA intends to exempt additional 23andMe GHR tests from premarket review, and GHR tests from other makers may be exempt after submitting their first premarket notification. A proposed exemption of this kind would allow other, similar tests to enter the market as quickly as possible after a one-time FDA review.
Excluded from the current marketing authorization and any future, related exemption are GHR tests that function as diagnostic tests. ![]()
The US Food and Drug Administration (FDA) has authorized marketing of 23andMe Personal Genome Service (PGS) Genetic Health Risk (GHR) tests for 10 medical conditions.
These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual’s genetic predisposition to certain conditions, including factor XI deficiency, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hereditary hemochromatosis, hereditary thrombophilia, and other conditions.
The GHR tests work by isolating DNA from a saliva sample, which is then tested for more than 500,000 genetic variants.
Consumers receive reports of the results, which tell them if they have an increased risk of developing any of the following 10 conditions.
Factor XI deficiency
The 23andMe PGS Genetic Health Risk Report for Factor XI Deficiency is indicated for reporting of the F283L, E117X, and IVS14+1G>A variants in the F11 gene.
This report describes if a person has a variant associated with factor XI deficiency and the potential for a higher risk of excessive bleeding following trauma or surgery, but it does not describe a person’s overall risk for excessive bleeding. This report is most relevant for people of Ashkenazi Jewish descent.
G6PD deficiency
The 23andMe PGS Genetic Health Risk Report for Glucose-6-Phosphate-Dehydrogenase Deficiency is indicated for reporting of the Val68Met variant in the G6PD gene.
This report describes if a person has a variant associated with G6PD deficiency and a higher risk for episodes of anemia, but it does not describe a person’s overall risk of developing anemia. This report is most relevant for people of African descent.
Hereditary hemochromatosis
The 23andMe PGS Genetic Health Risk Report for Hereditary Hemochromatosis is indicated for reporting of the C282Y and H63D variants in the HFE gene.
This report describes if a person has variants associated with hereditary hemochromatosis and a higher risk for iron overload, but it does not describe a person’s overall risk of developing iron overload. This report is most relevant for people of European descent.
Hereditary thrombophilia
The 23andMe PGS Genetic Health Risk Report for Hereditary Thrombophilia is indicated for reporting of the factor V Leiden variant in the F5 gene, as well as the prothrombin G20210A variant in the F2 gene.
This report describes if a person has variants associated with a higher risk of thrombosis, but it does not describe a person’s overall risk of developing thrombosis. This report is most relevant for people of European descent.
Alpha-1 antitrypsin deficiency (AATD)
The 23andMe PGS Genetic Health Risk Report for Alpha-I Antitrypsin Deficiency is indicated for reporting of the PI*Z and PI*S variants in the SERPINA1 gene.
This report describes if a person has variants associated with AATD and a higher risk for lung or liver disease, but it does not describe a person’s overall risk of developing lung or liver disease. This report is most relevant for people of European descent.
Celiac disease
The 23andMe PGS Genetic Health Risk Report for Celiac Disease is indicated for reporting of a variant in the HLA-DQ2.5 haplotype.
The report describes if a person has a haplotype associated with an increased risk of developing celiac disease, but it does not describe a person’s overall risk for developing celiac disease. This report is most relevant for people of European descent.
Early onset primary dystonia (DYT1/TOR1A-related)
The 23andMe PGS Genetic Health Risk Report for Early-Onset Primary Dystonia (DYT1/TOR1A-Related) is indicated for reporting of the deltaE302/303 variant in the DYT1 gene.
This report describes if a person has variants associated with a higher risk for early-onset primary dystonia, but it does not describe a person’s overall risk of developing dystonia. This report is most relevant for people of Ashkenazi Jewish descent.
Gaucher disease
The 23andMe PGS Genetic Health Risk Report for Gaucher Disease Type 1 is indicated for reporting of the N370S, 84GG, and V394L variants in the GBA gene.
This report describes if a person has variants associated with an increased risk for developing carrier status for Gaucher disease type 1 in adults. This report also describes if a result is associated with personal risk for developing symptoms of Gaucher disease type 1, but it does not describe a person’s overall risk of developing Gaucher disease type 1.
This test is most relevant for people of Ashkenazi Jewish descent.
Late-onset Alzheimer’s disease
The 23andMe PGS Genetic Health Risk Report for Late-onset Alzheimer’s Disease is indicated for reporting of the ε4 variant in the APOE gene.
The report describes if a person’s genetic result is associated with an increased risk of developing late-onset Alzheimer’s disease, but it does not describe a person’s overall risk of developing Alzheimer’s disease.
The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Parkinson’s disease
The 23andMe PGS Genetic Health Risk Report for Parkinson’s Disease is indicated for reporting of the G2019S variant in the LRRK2 gene and the N370S variant in the GBA gene.
The report describes if a person’s genetic result is associated with an increased risk of developing Parkinson’s disease, but it does not describe a person’s overall risk of developing Parkinson’s disease. The test is most relevant for people of European, Ashkenazi Jewish, and North African Berber descent.
Access to testing
23andMe, Inc. said it will release its first set of GHR tests—for hereditary thrombophilia, late-onset Alzheimer’s disease, Parkinson’s disease, alpha-1 antitrypsin deficiency, and Gaucher disease—this month. The remaining tests will follow.
New 23andMe Health + Ancestry Service customers in the US will have access to these tests. Current 23andMe customers will be notified directly regarding their eligibility.
About the marketing authorization
The FDA reviewed data for the 23andMe GHR tests through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the tests’ accuracy, reliability, and clinical relevance. These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for these and similar GHR tests.
The FDA intends to exempt additional 23andMe GHR tests from premarket review, and GHR tests from other makers may be exempt after submitting their first premarket notification. A proposed exemption of this kind would allow other, similar tests to enter the market as quickly as possible after a one-time FDA review.
Excluded from the current marketing authorization and any future, related exemption are GHR tests that function as diagnostic tests. ![]()
The US Food and Drug Administration (FDA) has authorized marketing of 23andMe Personal Genome Service (PGS) Genetic Health Risk (GHR) tests for 10 medical conditions.
These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual’s genetic predisposition to certain conditions, including factor XI deficiency, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hereditary hemochromatosis, hereditary thrombophilia, and other conditions.
The GHR tests work by isolating DNA from a saliva sample, which is then tested for more than 500,000 genetic variants.
Consumers receive reports of the results, which tell them if they have an increased risk of developing any of the following 10 conditions.
Factor XI deficiency
The 23andMe PGS Genetic Health Risk Report for Factor XI Deficiency is indicated for reporting of the F283L, E117X, and IVS14+1G>A variants in the F11 gene.
This report describes if a person has a variant associated with factor XI deficiency and the potential for a higher risk of excessive bleeding following trauma or surgery, but it does not describe a person’s overall risk for excessive bleeding. This report is most relevant for people of Ashkenazi Jewish descent.
G6PD deficiency
The 23andMe PGS Genetic Health Risk Report for Glucose-6-Phosphate-Dehydrogenase Deficiency is indicated for reporting of the Val68Met variant in the G6PD gene.
This report describes if a person has a variant associated with G6PD deficiency and a higher risk for episodes of anemia, but it does not describe a person’s overall risk of developing anemia. This report is most relevant for people of African descent.
Hereditary hemochromatosis
The 23andMe PGS Genetic Health Risk Report for Hereditary Hemochromatosis is indicated for reporting of the C282Y and H63D variants in the HFE gene.
This report describes if a person has variants associated with hereditary hemochromatosis and a higher risk for iron overload, but it does not describe a person’s overall risk of developing iron overload. This report is most relevant for people of European descent.
Hereditary thrombophilia
The 23andMe PGS Genetic Health Risk Report for Hereditary Thrombophilia is indicated for reporting of the factor V Leiden variant in the F5 gene, as well as the prothrombin G20210A variant in the F2 gene.
This report describes if a person has variants associated with a higher risk of thrombosis, but it does not describe a person’s overall risk of developing thrombosis. This report is most relevant for people of European descent.
Alpha-1 antitrypsin deficiency (AATD)
The 23andMe PGS Genetic Health Risk Report for Alpha-I Antitrypsin Deficiency is indicated for reporting of the PI*Z and PI*S variants in the SERPINA1 gene.
This report describes if a person has variants associated with AATD and a higher risk for lung or liver disease, but it does not describe a person’s overall risk of developing lung or liver disease. This report is most relevant for people of European descent.
Celiac disease
The 23andMe PGS Genetic Health Risk Report for Celiac Disease is indicated for reporting of a variant in the HLA-DQ2.5 haplotype.
The report describes if a person has a haplotype associated with an increased risk of developing celiac disease, but it does not describe a person’s overall risk for developing celiac disease. This report is most relevant for people of European descent.
Early onset primary dystonia (DYT1/TOR1A-related)
The 23andMe PGS Genetic Health Risk Report for Early-Onset Primary Dystonia (DYT1/TOR1A-Related) is indicated for reporting of the deltaE302/303 variant in the DYT1 gene.
This report describes if a person has variants associated with a higher risk for early-onset primary dystonia, but it does not describe a person’s overall risk of developing dystonia. This report is most relevant for people of Ashkenazi Jewish descent.
Gaucher disease
The 23andMe PGS Genetic Health Risk Report for Gaucher Disease Type 1 is indicated for reporting of the N370S, 84GG, and V394L variants in the GBA gene.
This report describes if a person has variants associated with an increased risk for developing carrier status for Gaucher disease type 1 in adults. This report also describes if a result is associated with personal risk for developing symptoms of Gaucher disease type 1, but it does not describe a person’s overall risk of developing Gaucher disease type 1.
This test is most relevant for people of Ashkenazi Jewish descent.
Late-onset Alzheimer’s disease
The 23andMe PGS Genetic Health Risk Report for Late-onset Alzheimer’s Disease is indicated for reporting of the ε4 variant in the APOE gene.
The report describes if a person’s genetic result is associated with an increased risk of developing late-onset Alzheimer’s disease, but it does not describe a person’s overall risk of developing Alzheimer’s disease.
The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Parkinson’s disease
The 23andMe PGS Genetic Health Risk Report for Parkinson’s Disease is indicated for reporting of the G2019S variant in the LRRK2 gene and the N370S variant in the GBA gene.
The report describes if a person’s genetic result is associated with an increased risk of developing Parkinson’s disease, but it does not describe a person’s overall risk of developing Parkinson’s disease. The test is most relevant for people of European, Ashkenazi Jewish, and North African Berber descent.
Access to testing
23andMe, Inc. said it will release its first set of GHR tests—for hereditary thrombophilia, late-onset Alzheimer’s disease, Parkinson’s disease, alpha-1 antitrypsin deficiency, and Gaucher disease—this month. The remaining tests will follow.
New 23andMe Health + Ancestry Service customers in the US will have access to these tests. Current 23andMe customers will be notified directly regarding their eligibility.
About the marketing authorization
The FDA reviewed data for the 23andMe GHR tests through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the tests’ accuracy, reliability, and clinical relevance. These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for these and similar GHR tests.
The FDA intends to exempt additional 23andMe GHR tests from premarket review, and GHR tests from other makers may be exempt after submitting their first premarket notification. A proposed exemption of this kind would allow other, similar tests to enter the market as quickly as possible after a one-time FDA review.
Excluded from the current marketing authorization and any future, related exemption are GHR tests that function as diagnostic tests. ![]()
Drug granted fast track designation for PNH
The US Food and Drug Administration (FDA) has granted fast track designation to Coversin™ for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients who have polymorphisms conferring eculizumab resistance.
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9, and also independently inhibits LTB4 activity.
Coversin is being developed by Akari Therapeutics.
Akari is evaluating Coversin in a pair of phase 2 trials.
In the first trial, researchers are evaluating Coversin in patients with PNH who have never received a complement-blocking therapy. Interim results from this ongoing trial are scheduled to be presented at Akari’s Research and Development Day on April 24 in New York, New York.
In the second phase 2 trial, researchers are evaluating Coversin in patients with PNH who have C5 polymorphisms that confer resistance to eculizumab.
One patient has been enrolled in this trial and has received Coversin for over a year. The treatment has resulted in significant lactate dehydrogenase reduction and complete complement blockade.
About fast track designation
The FDA created the fast track program to facilitate the development and expedite the review of drugs that show promise for treating serious or life-threatening diseases and address unmet medical needs.
Companies developing drugs that receive fast track designation benefit from more frequent communications and meetings with the FDA to review their drug’s development plan, including the design of proposed clinical trials, use of biomarkers, and the extent of data needed for approval.
Drugs with fast track designation may qualify for priority review as well, if relevant criteria are met. Priority review shortens the FDA review process from 10 months to 6 months.
Fast track designation also allows for a rolling review process, whereby completed sections of the investigational new drug application can be submitted for FDA review as they become available, instead of waiting for all sections to be completed. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to Coversin™ for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients who have polymorphisms conferring eculizumab resistance.
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9, and also independently inhibits LTB4 activity.
Coversin is being developed by Akari Therapeutics.
Akari is evaluating Coversin in a pair of phase 2 trials.
In the first trial, researchers are evaluating Coversin in patients with PNH who have never received a complement-blocking therapy. Interim results from this ongoing trial are scheduled to be presented at Akari’s Research and Development Day on April 24 in New York, New York.
In the second phase 2 trial, researchers are evaluating Coversin in patients with PNH who have C5 polymorphisms that confer resistance to eculizumab.
One patient has been enrolled in this trial and has received Coversin for over a year. The treatment has resulted in significant lactate dehydrogenase reduction and complete complement blockade.
About fast track designation
The FDA created the fast track program to facilitate the development and expedite the review of drugs that show promise for treating serious or life-threatening diseases and address unmet medical needs.
Companies developing drugs that receive fast track designation benefit from more frequent communications and meetings with the FDA to review their drug’s development plan, including the design of proposed clinical trials, use of biomarkers, and the extent of data needed for approval.
Drugs with fast track designation may qualify for priority review as well, if relevant criteria are met. Priority review shortens the FDA review process from 10 months to 6 months.
Fast track designation also allows for a rolling review process, whereby completed sections of the investigational new drug application can be submitted for FDA review as they become available, instead of waiting for all sections to be completed. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to Coversin™ for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients who have polymorphisms conferring eculizumab resistance.
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9, and also independently inhibits LTB4 activity.
Coversin is being developed by Akari Therapeutics.
Akari is evaluating Coversin in a pair of phase 2 trials.
In the first trial, researchers are evaluating Coversin in patients with PNH who have never received a complement-blocking therapy. Interim results from this ongoing trial are scheduled to be presented at Akari’s Research and Development Day on April 24 in New York, New York.
In the second phase 2 trial, researchers are evaluating Coversin in patients with PNH who have C5 polymorphisms that confer resistance to eculizumab.
One patient has been enrolled in this trial and has received Coversin for over a year. The treatment has resulted in significant lactate dehydrogenase reduction and complete complement blockade.
About fast track designation
The FDA created the fast track program to facilitate the development and expedite the review of drugs that show promise for treating serious or life-threatening diseases and address unmet medical needs.
Companies developing drugs that receive fast track designation benefit from more frequent communications and meetings with the FDA to review their drug’s development plan, including the design of proposed clinical trials, use of biomarkers, and the extent of data needed for approval.
Drugs with fast track designation may qualify for priority review as well, if relevant criteria are met. Priority review shortens the FDA review process from 10 months to 6 months.
Fast track designation also allows for a rolling review process, whereby completed sections of the investigational new drug application can be submitted for FDA review as they become available, instead of waiting for all sections to be completed. ![]()
Drug induces remission in patient with severe TA-TMA
MARSEILLE—An investigational drug has successfully treated a severe case of transplant-associated thrombotic microangiopathy (TA-TMA), according to a presentation at the 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation.
The drug is OMS721, a monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system.
The patient received OMS721, which is being developed by Omeros Corporation, under a compassionate-use protocol.
Marco Zecca, MD, of the Fondazione IRCCS Policlinico San Matteo in Italy, and his colleagues provided details on this patient in a poster presented at the meeting (Physician Poster Abstracts-Day 1, abstract A437).
The female patient had undergone a hematopoietic stem cell transplant to treat Diamond‐Blackfan anemia. At age 14, she received a transplant from an HLA-compatible, unrelated donor.
Seven months later, she was diagnosed with TA-TMA. The patient was initially treated with eculizumab but had to stop taking the drug after she developed acute pulmonary edema.
She was then treated with plasma exchange but experienced a TA-TMA relapse at 11 months. The patient was again treated with eculizumab and again had to discontinue the drug after developing acute pulmonary edema.
The patient’s condition continued to worsen, and she soon required hemodialysis 3 times a week as well as daily platelet transfusions.
Dr Zecca requested OMS721 as compassionate-use treatment for the patient, and Omeros complied.
Two months after starting OMS721, the patient was able to discontinue hemodialysis and decrease her platelet transfusion requirements. She did not experience any adverse events related to OMS721.
Recently, the patient’s dose was tapered, but she developed a viral infection that reactivated her TA-TMA.
A return to the original dose of OMS721 was successful. Now, the patient no longer requires dialysis or transfusions.
“This patient had severe TMA that I believe would have caused her death,” Dr Zecca said. “Her positive response to OMS721 treatment, both initially and following her virus-induced relapse during tapering, was impressive.”
“The results of OMS721 treatment in this challenge-rechallenge scenario underscore the important effects of the drug. Since the poster was produced, her TMA has remained in remission, and we have been able to discontinue her platelet transfusions. Her rapid response has been heartening, and we all are grateful for this remarkable outcome.” ![]()
MARSEILLE—An investigational drug has successfully treated a severe case of transplant-associated thrombotic microangiopathy (TA-TMA), according to a presentation at the 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation.
The drug is OMS721, a monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system.
The patient received OMS721, which is being developed by Omeros Corporation, under a compassionate-use protocol.
Marco Zecca, MD, of the Fondazione IRCCS Policlinico San Matteo in Italy, and his colleagues provided details on this patient in a poster presented at the meeting (Physician Poster Abstracts-Day 1, abstract A437).
The female patient had undergone a hematopoietic stem cell transplant to treat Diamond‐Blackfan anemia. At age 14, she received a transplant from an HLA-compatible, unrelated donor.
Seven months later, she was diagnosed with TA-TMA. The patient was initially treated with eculizumab but had to stop taking the drug after she developed acute pulmonary edema.
She was then treated with plasma exchange but experienced a TA-TMA relapse at 11 months. The patient was again treated with eculizumab and again had to discontinue the drug after developing acute pulmonary edema.
The patient’s condition continued to worsen, and she soon required hemodialysis 3 times a week as well as daily platelet transfusions.
Dr Zecca requested OMS721 as compassionate-use treatment for the patient, and Omeros complied.
Two months after starting OMS721, the patient was able to discontinue hemodialysis and decrease her platelet transfusion requirements. She did not experience any adverse events related to OMS721.
Recently, the patient’s dose was tapered, but she developed a viral infection that reactivated her TA-TMA.
A return to the original dose of OMS721 was successful. Now, the patient no longer requires dialysis or transfusions.
“This patient had severe TMA that I believe would have caused her death,” Dr Zecca said. “Her positive response to OMS721 treatment, both initially and following her virus-induced relapse during tapering, was impressive.”
“The results of OMS721 treatment in this challenge-rechallenge scenario underscore the important effects of the drug. Since the poster was produced, her TMA has remained in remission, and we have been able to discontinue her platelet transfusions. Her rapid response has been heartening, and we all are grateful for this remarkable outcome.” ![]()
MARSEILLE—An investigational drug has successfully treated a severe case of transplant-associated thrombotic microangiopathy (TA-TMA), according to a presentation at the 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation.
The drug is OMS721, a monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system.
The patient received OMS721, which is being developed by Omeros Corporation, under a compassionate-use protocol.
Marco Zecca, MD, of the Fondazione IRCCS Policlinico San Matteo in Italy, and his colleagues provided details on this patient in a poster presented at the meeting (Physician Poster Abstracts-Day 1, abstract A437).
The female patient had undergone a hematopoietic stem cell transplant to treat Diamond‐Blackfan anemia. At age 14, she received a transplant from an HLA-compatible, unrelated donor.
Seven months later, she was diagnosed with TA-TMA. The patient was initially treated with eculizumab but had to stop taking the drug after she developed acute pulmonary edema.
She was then treated with plasma exchange but experienced a TA-TMA relapse at 11 months. The patient was again treated with eculizumab and again had to discontinue the drug after developing acute pulmonary edema.
The patient’s condition continued to worsen, and she soon required hemodialysis 3 times a week as well as daily platelet transfusions.
Dr Zecca requested OMS721 as compassionate-use treatment for the patient, and Omeros complied.
Two months after starting OMS721, the patient was able to discontinue hemodialysis and decrease her platelet transfusion requirements. She did not experience any adverse events related to OMS721.
Recently, the patient’s dose was tapered, but she developed a viral infection that reactivated her TA-TMA.
A return to the original dose of OMS721 was successful. Now, the patient no longer requires dialysis or transfusions.
“This patient had severe TMA that I believe would have caused her death,” Dr Zecca said. “Her positive response to OMS721 treatment, both initially and following her virus-induced relapse during tapering, was impressive.”
“The results of OMS721 treatment in this challenge-rechallenge scenario underscore the important effects of the drug. Since the poster was produced, her TMA has remained in remission, and we have been able to discontinue her platelet transfusions. Her rapid response has been heartening, and we all are grateful for this remarkable outcome.”