User login
Crizanlizumab appears effective across subgroups
Crizanlizumab can reduce vaso-occlusive crises (VOCs) across subgroups of patients with sickle cell disease (SCD), according to a post-hoc analysis of the phase 2 SUSTAIN trial.
Researchers found crizanlizumab was more effective than placebo at delaying time to first VOC and eliminating crises in patients who had numerous previous crises, exhibited the HbSS genotype, or were taking concomitant hydroxyurea.
Abdullah Kutlar, MD, of the Medical College of Georgia in Augusta, and his colleagues reported these findings in the American Journal of Hematology.
The phase 2 SUSTAIN trial previously showed that crizanlizumab—a humanized, anti–P-selectin monoclonal antibody—reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months.
Additionally, a subgroup analysis showed there was a lower frequency of VOCs with crizanlizumab at 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post-hoc analysis took a deeper look at these observations across the same subgroups. Specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events in 132 patients.
Crizanlizumab eliminated VOCs about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (5 to 10 VOCs in the year prior)—28.0% and 4.2%, respectively.
Crizanlizumab eliminated VOCs about twice as often as placebo in patients with the HbSS genotype—31.9% and 17.0%, respectively—and in patients who were using concomitant hydroxyurea—33.3% and 17.5%, respectively.
Further analysis showed that crizanlizumab delayed time to first VOC across all subgroups.
In patients with the HbSS genotype, the time to first VOC was 4.07 months with crizanlizumab and 1.12 months with placebo.
In patients with a higher frequency of previous VOCs, the time to first on-study VOC was 2.43 months with crizanlizumab and 1.03 months with placebo.
In patients taking hydroxyurea, the time to first VOC was 2.43 months with crizanlizumab and 1.45 months with placebo.
Safety was comparable across subgroups.
This study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Crizanlizumab can reduce vaso-occlusive crises (VOCs) across subgroups of patients with sickle cell disease (SCD), according to a post-hoc analysis of the phase 2 SUSTAIN trial.
Researchers found crizanlizumab was more effective than placebo at delaying time to first VOC and eliminating crises in patients who had numerous previous crises, exhibited the HbSS genotype, or were taking concomitant hydroxyurea.
Abdullah Kutlar, MD, of the Medical College of Georgia in Augusta, and his colleagues reported these findings in the American Journal of Hematology.
The phase 2 SUSTAIN trial previously showed that crizanlizumab—a humanized, anti–P-selectin monoclonal antibody—reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months.
Additionally, a subgroup analysis showed there was a lower frequency of VOCs with crizanlizumab at 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post-hoc analysis took a deeper look at these observations across the same subgroups. Specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events in 132 patients.
Crizanlizumab eliminated VOCs about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (5 to 10 VOCs in the year prior)—28.0% and 4.2%, respectively.
Crizanlizumab eliminated VOCs about twice as often as placebo in patients with the HbSS genotype—31.9% and 17.0%, respectively—and in patients who were using concomitant hydroxyurea—33.3% and 17.5%, respectively.
Further analysis showed that crizanlizumab delayed time to first VOC across all subgroups.
In patients with the HbSS genotype, the time to first VOC was 4.07 months with crizanlizumab and 1.12 months with placebo.
In patients with a higher frequency of previous VOCs, the time to first on-study VOC was 2.43 months with crizanlizumab and 1.03 months with placebo.
In patients taking hydroxyurea, the time to first VOC was 2.43 months with crizanlizumab and 1.45 months with placebo.
Safety was comparable across subgroups.
This study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Crizanlizumab can reduce vaso-occlusive crises (VOCs) across subgroups of patients with sickle cell disease (SCD), according to a post-hoc analysis of the phase 2 SUSTAIN trial.
Researchers found crizanlizumab was more effective than placebo at delaying time to first VOC and eliminating crises in patients who had numerous previous crises, exhibited the HbSS genotype, or were taking concomitant hydroxyurea.
Abdullah Kutlar, MD, of the Medical College of Georgia in Augusta, and his colleagues reported these findings in the American Journal of Hematology.
The phase 2 SUSTAIN trial previously showed that crizanlizumab—a humanized, anti–P-selectin monoclonal antibody—reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months.
Additionally, a subgroup analysis showed there was a lower frequency of VOCs with crizanlizumab at 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post-hoc analysis took a deeper look at these observations across the same subgroups. Specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events in 132 patients.
Crizanlizumab eliminated VOCs about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (5 to 10 VOCs in the year prior)—28.0% and 4.2%, respectively.
Crizanlizumab eliminated VOCs about twice as often as placebo in patients with the HbSS genotype—31.9% and 17.0%, respectively—and in patients who were using concomitant hydroxyurea—33.3% and 17.5%, respectively.
Further analysis showed that crizanlizumab delayed time to first VOC across all subgroups.
In patients with the HbSS genotype, the time to first VOC was 4.07 months with crizanlizumab and 1.12 months with placebo.
In patients with a higher frequency of previous VOCs, the time to first on-study VOC was 2.43 months with crizanlizumab and 1.03 months with placebo.
In patients taking hydroxyurea, the time to first VOC was 2.43 months with crizanlizumab and 1.45 months with placebo.
Safety was comparable across subgroups.
This study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Crizanlizumab relieves sickle cell crises across subgroups
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
FROM THE AMERICAN JOURNAL OF HEMATOLOGY
Key clinical point:
Major finding: Crizanlizumab eliminated vaso-occlusive crises about seven times more frequently than did placebo in patients with numerous crises (28.0% vs. 4.2%).
Study details: A post hoc analysis of 132 patients from the phase 2 SUSTAIN trial.
Disclosures: The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Source: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Variant not associated with CLL, AIHA, or ITP in certain patients
DUBROVNIK, CROATIA—New research suggests there is no association between the PTPN22 R620W polymorphism and chronic lymphocytic leukemia (CLL) or autoimmune hematologic disorders in patients from the Republic of Macedonia.
Past studies have shown an association between the PTPN22 R620W variant and both CLL1 and autoimmune diseases2 in patients from Northwest Europe.
However, a study of Macedonian patients suggests there is no association between the variant and CLL, autoimmune hemolytic anemia (AIHA), or idiopathic thrombocytopenic purpura (ITP) for patients from Southeast Europe.
Irina Panovska-Stavridis, PhD, of Ss. Cyril and Methodius University in Skopje, Republic of Macedonia, and her colleagues presented this finding at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
“A lot of data from the literature suggests [the PTPN22 R620W variant ] has a role in developing multiple immune diseases, but it is validated just in patients from Northwest Europe,” Dr. Panovska-Stavridis noted.
Therefore, she and her colleagues decided to assess the frequency of the PTPN22 R620W variant (C1858T, rs2476601) in individuals from Southeast Europe, particularly the Republic of Macedonia.
The researchers evaluated 320 patients—168 with CLL, 66 with AIHA, and 86 with ITP—and 182 age- and sex-matched control subjects with no history of malignant or autoimmune disease.
The team found a similar frequency of the minor T allele and genotype distribution in control subjects and patients.
| CLL | AIHA | ITP | Controls | |
| Minor T allele | 0.107 | 0.067 | 0.036 | 0.05 |
| CC genotype | 0.809 | 0.166 | 0.023 | 0.901 |
| CT genotype | 0.9 | 0.067 | 0.033 | 0.099 |
| TT genotype | 0.928 | 0.072 | 0 | 0 |
Dr. Panovska-Stavridis said these results suggest the PTPN22 R620W variant is not a risk factor for the development of CLL, AIHA, or ITP in patients from Southeast Europe.
She also said the results suggest the influence of the variant on lymphocytic homeostasis is affected by certain genetic and environmental factors, and the development of CLL and autoimmune diseases is influenced by race/ethnicity-based variations in the germline composition of the IGHV locus in correlation with environmental factors.
Dr. Panovska-Stavridis did not declare any conflicts of interest.
1. Hebbring S et al. Blood. 2013 121:237-238; doi: https://doi.org/10.1182/blood-2012-08-450221
2. Burb GL et al. FEBS Lett. 2011 Dec 1;585(23):3689-98. doi: 10.1016/j.febslet.2011.04.032
DUBROVNIK, CROATIA—New research suggests there is no association between the PTPN22 R620W polymorphism and chronic lymphocytic leukemia (CLL) or autoimmune hematologic disorders in patients from the Republic of Macedonia.
Past studies have shown an association between the PTPN22 R620W variant and both CLL1 and autoimmune diseases2 in patients from Northwest Europe.
However, a study of Macedonian patients suggests there is no association between the variant and CLL, autoimmune hemolytic anemia (AIHA), or idiopathic thrombocytopenic purpura (ITP) for patients from Southeast Europe.
Irina Panovska-Stavridis, PhD, of Ss. Cyril and Methodius University in Skopje, Republic of Macedonia, and her colleagues presented this finding at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
“A lot of data from the literature suggests [the PTPN22 R620W variant ] has a role in developing multiple immune diseases, but it is validated just in patients from Northwest Europe,” Dr. Panovska-Stavridis noted.
Therefore, she and her colleagues decided to assess the frequency of the PTPN22 R620W variant (C1858T, rs2476601) in individuals from Southeast Europe, particularly the Republic of Macedonia.
The researchers evaluated 320 patients—168 with CLL, 66 with AIHA, and 86 with ITP—and 182 age- and sex-matched control subjects with no history of malignant or autoimmune disease.
The team found a similar frequency of the minor T allele and genotype distribution in control subjects and patients.
| CLL | AIHA | ITP | Controls | |
| Minor T allele | 0.107 | 0.067 | 0.036 | 0.05 |
| CC genotype | 0.809 | 0.166 | 0.023 | 0.901 |
| CT genotype | 0.9 | 0.067 | 0.033 | 0.099 |
| TT genotype | 0.928 | 0.072 | 0 | 0 |
Dr. Panovska-Stavridis said these results suggest the PTPN22 R620W variant is not a risk factor for the development of CLL, AIHA, or ITP in patients from Southeast Europe.
She also said the results suggest the influence of the variant on lymphocytic homeostasis is affected by certain genetic and environmental factors, and the development of CLL and autoimmune diseases is influenced by race/ethnicity-based variations in the germline composition of the IGHV locus in correlation with environmental factors.
Dr. Panovska-Stavridis did not declare any conflicts of interest.
1. Hebbring S et al. Blood. 2013 121:237-238; doi: https://doi.org/10.1182/blood-2012-08-450221
2. Burb GL et al. FEBS Lett. 2011 Dec 1;585(23):3689-98. doi: 10.1016/j.febslet.2011.04.032
DUBROVNIK, CROATIA—New research suggests there is no association between the PTPN22 R620W polymorphism and chronic lymphocytic leukemia (CLL) or autoimmune hematologic disorders in patients from the Republic of Macedonia.
Past studies have shown an association between the PTPN22 R620W variant and both CLL1 and autoimmune diseases2 in patients from Northwest Europe.
However, a study of Macedonian patients suggests there is no association between the variant and CLL, autoimmune hemolytic anemia (AIHA), or idiopathic thrombocytopenic purpura (ITP) for patients from Southeast Europe.
Irina Panovska-Stavridis, PhD, of Ss. Cyril and Methodius University in Skopje, Republic of Macedonia, and her colleagues presented this finding at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
“A lot of data from the literature suggests [the PTPN22 R620W variant ] has a role in developing multiple immune diseases, but it is validated just in patients from Northwest Europe,” Dr. Panovska-Stavridis noted.
Therefore, she and her colleagues decided to assess the frequency of the PTPN22 R620W variant (C1858T, rs2476601) in individuals from Southeast Europe, particularly the Republic of Macedonia.
The researchers evaluated 320 patients—168 with CLL, 66 with AIHA, and 86 with ITP—and 182 age- and sex-matched control subjects with no history of malignant or autoimmune disease.
The team found a similar frequency of the minor T allele and genotype distribution in control subjects and patients.
| CLL | AIHA | ITP | Controls | |
| Minor T allele | 0.107 | 0.067 | 0.036 | 0.05 |
| CC genotype | 0.809 | 0.166 | 0.023 | 0.901 |
| CT genotype | 0.9 | 0.067 | 0.033 | 0.099 |
| TT genotype | 0.928 | 0.072 | 0 | 0 |
Dr. Panovska-Stavridis said these results suggest the PTPN22 R620W variant is not a risk factor for the development of CLL, AIHA, or ITP in patients from Southeast Europe.
She also said the results suggest the influence of the variant on lymphocytic homeostasis is affected by certain genetic and environmental factors, and the development of CLL and autoimmune diseases is influenced by race/ethnicity-based variations in the germline composition of the IGHV locus in correlation with environmental factors.
Dr. Panovska-Stavridis did not declare any conflicts of interest.
1. Hebbring S et al. Blood. 2013 121:237-238; doi: https://doi.org/10.1182/blood-2012-08-450221
2. Burb GL et al. FEBS Lett. 2011 Dec 1;585(23):3689-98. doi: 10.1016/j.febslet.2011.04.032
Researchers develop genetics-based prognostic tool for MDS
Researchers have developed a new risk model for primary myelodysplastic syndromes (MDS) that integrates genetic and clinical information.
The research team considered the current standard for prognostication—the revised International Prognostic Scoring System (IPSS-R)—to be too complex, limited to newly diagnosed cases, and missing information on mutations and age.
So they devised a “simpler and more contemporary” prognostic system, the Mayo Alliance Prognostic Model for MDS.
The team, from the Mayo Clinic in Rochester, Minnesota, and the National Taiwan University Hospital (NTUH), described the new model in Mayo Clinic Proceedings.
Lead author Ayalew Tefferi, MD, of the Mayo Clinic, said the new model “is not an enhancement of the international prognostic scoring system tool, it's a complete makeover."
The team analyzed mutation information from 357 patients with primary MDS or leukemic transformation treated at the Mayo Clinic from the end of December 1994 through mid-December 2017.
The patients were a median age of 74 and 70% were males.
They compared the Mayo patients to 328 NTUH patients, who were a median age of 66 and 65% were males.
Multivariate analysis of the Mayo cohort identified the following as predictors of inferior overall survival:
- Monosomal karyotype (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6)
- Non-monosomal karyotype abnormalities other than single/double del(5q) (HR, 1.8; 95% CI, 1.3-2.6)
- RUNX1 (HR, 2.0; 95% CI, 1.2-3.1)
- ASXL1 (HR, 1.7; 95% CI, 1.2-2.3) mutations
- Absence of SF3B1 mutations (HR, 1.6; 95% CI, 1.1-2.4)
- Age greater than 70 years (HR, 2.2; 95% CI, 1.6-3.1)
- Hemoglobin level less than 8 g/dL in women or less than 9 g/dL in men (HR, 2.3; 95% CI, 1.7-3.1)
- Platelet count less than 75 x 109/L (HR, 1.5; 95% CI, 1.1-2.1)
- 10% or more bone marrow blasts (HR, 1.7; 95% CI, 1.1-2.8)
They then provided values to reflect the prognostic contribution of each of the above predictors and devised the new 4-tiered Mayo prognostic model.
Median 5-year overall survival rates in the 4 categories in the Mayo model were 73% (low risk), 34% (intermediate-1), 7% (intermediate-2), and 0% (high risk; 9-month median survival).
The team then validated the Mayo alliance model by using the NTUH cohort and compared it to the IPSS-R.
The investigators were able to confirm superior predictive accuracy of their model and a substantial discordance between the the Mayo model and the IPSS-R in terms of the pattern of risk distribution.
Examples of discordance included:
- More than 25% of patients belonging to the high-risk category according to the Mayo alliance model were classified as IPSS-R low or intermediate risk
- Almost 50% of patients with intermediate-2 risk category according to the Mayo alliance model were classified as IPSS-R very low or low risk
- Almost 50% of patients with IPSS-R very low risk were classified as intermediate-2 or intermediate-1 risk according to the Mayo alliance model
The authors wrote that this “suggests a fundamental and not incremental advantage for the new Mayo alliance model.”
Researchers have developed a new risk model for primary myelodysplastic syndromes (MDS) that integrates genetic and clinical information.
The research team considered the current standard for prognostication—the revised International Prognostic Scoring System (IPSS-R)—to be too complex, limited to newly diagnosed cases, and missing information on mutations and age.
So they devised a “simpler and more contemporary” prognostic system, the Mayo Alliance Prognostic Model for MDS.
The team, from the Mayo Clinic in Rochester, Minnesota, and the National Taiwan University Hospital (NTUH), described the new model in Mayo Clinic Proceedings.
Lead author Ayalew Tefferi, MD, of the Mayo Clinic, said the new model “is not an enhancement of the international prognostic scoring system tool, it's a complete makeover."
The team analyzed mutation information from 357 patients with primary MDS or leukemic transformation treated at the Mayo Clinic from the end of December 1994 through mid-December 2017.
The patients were a median age of 74 and 70% were males.
They compared the Mayo patients to 328 NTUH patients, who were a median age of 66 and 65% were males.
Multivariate analysis of the Mayo cohort identified the following as predictors of inferior overall survival:
- Monosomal karyotype (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6)
- Non-monosomal karyotype abnormalities other than single/double del(5q) (HR, 1.8; 95% CI, 1.3-2.6)
- RUNX1 (HR, 2.0; 95% CI, 1.2-3.1)
- ASXL1 (HR, 1.7; 95% CI, 1.2-2.3) mutations
- Absence of SF3B1 mutations (HR, 1.6; 95% CI, 1.1-2.4)
- Age greater than 70 years (HR, 2.2; 95% CI, 1.6-3.1)
- Hemoglobin level less than 8 g/dL in women or less than 9 g/dL in men (HR, 2.3; 95% CI, 1.7-3.1)
- Platelet count less than 75 x 109/L (HR, 1.5; 95% CI, 1.1-2.1)
- 10% or more bone marrow blasts (HR, 1.7; 95% CI, 1.1-2.8)
They then provided values to reflect the prognostic contribution of each of the above predictors and devised the new 4-tiered Mayo prognostic model.
Median 5-year overall survival rates in the 4 categories in the Mayo model were 73% (low risk), 34% (intermediate-1), 7% (intermediate-2), and 0% (high risk; 9-month median survival).
The team then validated the Mayo alliance model by using the NTUH cohort and compared it to the IPSS-R.
The investigators were able to confirm superior predictive accuracy of their model and a substantial discordance between the the Mayo model and the IPSS-R in terms of the pattern of risk distribution.
Examples of discordance included:
- More than 25% of patients belonging to the high-risk category according to the Mayo alliance model were classified as IPSS-R low or intermediate risk
- Almost 50% of patients with intermediate-2 risk category according to the Mayo alliance model were classified as IPSS-R very low or low risk
- Almost 50% of patients with IPSS-R very low risk were classified as intermediate-2 or intermediate-1 risk according to the Mayo alliance model
The authors wrote that this “suggests a fundamental and not incremental advantage for the new Mayo alliance model.”
Researchers have developed a new risk model for primary myelodysplastic syndromes (MDS) that integrates genetic and clinical information.
The research team considered the current standard for prognostication—the revised International Prognostic Scoring System (IPSS-R)—to be too complex, limited to newly diagnosed cases, and missing information on mutations and age.
So they devised a “simpler and more contemporary” prognostic system, the Mayo Alliance Prognostic Model for MDS.
The team, from the Mayo Clinic in Rochester, Minnesota, and the National Taiwan University Hospital (NTUH), described the new model in Mayo Clinic Proceedings.
Lead author Ayalew Tefferi, MD, of the Mayo Clinic, said the new model “is not an enhancement of the international prognostic scoring system tool, it's a complete makeover."
The team analyzed mutation information from 357 patients with primary MDS or leukemic transformation treated at the Mayo Clinic from the end of December 1994 through mid-December 2017.
The patients were a median age of 74 and 70% were males.
They compared the Mayo patients to 328 NTUH patients, who were a median age of 66 and 65% were males.
Multivariate analysis of the Mayo cohort identified the following as predictors of inferior overall survival:
- Monosomal karyotype (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6)
- Non-monosomal karyotype abnormalities other than single/double del(5q) (HR, 1.8; 95% CI, 1.3-2.6)
- RUNX1 (HR, 2.0; 95% CI, 1.2-3.1)
- ASXL1 (HR, 1.7; 95% CI, 1.2-2.3) mutations
- Absence of SF3B1 mutations (HR, 1.6; 95% CI, 1.1-2.4)
- Age greater than 70 years (HR, 2.2; 95% CI, 1.6-3.1)
- Hemoglobin level less than 8 g/dL in women or less than 9 g/dL in men (HR, 2.3; 95% CI, 1.7-3.1)
- Platelet count less than 75 x 109/L (HR, 1.5; 95% CI, 1.1-2.1)
- 10% or more bone marrow blasts (HR, 1.7; 95% CI, 1.1-2.8)
They then provided values to reflect the prognostic contribution of each of the above predictors and devised the new 4-tiered Mayo prognostic model.
Median 5-year overall survival rates in the 4 categories in the Mayo model were 73% (low risk), 34% (intermediate-1), 7% (intermediate-2), and 0% (high risk; 9-month median survival).
The team then validated the Mayo alliance model by using the NTUH cohort and compared it to the IPSS-R.
The investigators were able to confirm superior predictive accuracy of their model and a substantial discordance between the the Mayo model and the IPSS-R in terms of the pattern of risk distribution.
Examples of discordance included:
- More than 25% of patients belonging to the high-risk category according to the Mayo alliance model were classified as IPSS-R low or intermediate risk
- Almost 50% of patients with intermediate-2 risk category according to the Mayo alliance model were classified as IPSS-R very low or low risk
- Almost 50% of patients with IPSS-R very low risk were classified as intermediate-2 or intermediate-1 risk according to the Mayo alliance model
The authors wrote that this “suggests a fundamental and not incremental advantage for the new Mayo alliance model.”
NHLBI commits to a sickle cell cure
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
CHMP backs proposed biosimilars of pegfilgrastim
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
Sequencing informs prognosis after HSCT in MDS
Gene sequencing early after transplant may provide important prognostic information in patients with myelodysplastic syndromes (MDS), according to a new study.
Patients who had disease-associated mutations in the bone marrow 30 days after hematopoietic stem cell transplant (HSCT) were significantly more likely to experience disease progression and have lower rates of progression-free survival (PFS) at 1 year.
“Using our sequencing method, we’re identifying residual tumor cells before a pathologist could see them under the microscope and before a patient develops symptoms,” said Matthew J. Walter, MD, of Washington University in St. Louis, Mo.
“At that moment, there may be time to intervene in ways that could delay the cancer from coming back or potentially prevent it completely.”
Dr. Walter and his colleagues described results with their sequencing method in The New England Journal of Medicine.
The researchers sequenced bone marrow and skin (control) samples from 90 adults with MDS who underwent allogeneic HSCT.
The team used enhanced exome sequencing to detect mutations before HSCT and evaluated mutation clearance using error-corrected sequencing to genotype mutations in bone marrow samples collected 30 days after HSCT.
The researchers detected at least one validated somatic mutation in the pre-HSCT samples from 86 of 90 patients.
Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after HSCT. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the researchers explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not progress (P<0.001).
Progression occurred in 53.1% of patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days, whereas progression occurred in 13% of patients who did not have such mutations. After adjusting for conditioning regimen, the hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P<0.001).
The 1-year PFS rate was 31.3% in patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days and 59.3% in patients who did not have the mutations. After adjusting for conditioning, the HR for progression or death was 2.22 (P=0.005).
The researchers noted that PFS was lower in patients who had received reduced-intensity conditioning and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 (P≤0.001), when compared to other combinations of conditioning regimen and mutation status.
In multivariable analyses, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than four-fold risk of progression (HR, 4.48; P<0.001) and a more than two-fold risk of progression or death (HR, 2.39; P=0.002).
“Now that we have detected mutations early and shown that it predicts a higher risk of recurrence, we want to determine the best course of action for those high-risk patients,” Dr. Walter said.
He and his colleagues acknowledged that the high-coverage exome sequencing technique used for this study is not routinely available in the clinic. Therefore, the researchers also analyzed samples using a subset of genes that are usually included in gene sequencing panels for MDS and acute myeloid leukemia.
The researchers noted that this 40-gene panel revealed fewer patients (n=68; 79%) with mutations, but “the prognostic value of detection of measurable residual disease was still highly clinically significant.”
With this approach, the presence of at least one mutation with a variant allele frequency of at least 0.5% 30 days after HSCT was associated with a higher risk of disease progression at 1 year (HR, 3.39; P=0.001) and a higher risk of progression or death at 1 year (HR, 2.09; P=0.02).
This study was supported by grants from the Leukemia and Lymphoma Society and other groups.
Gene sequencing early after transplant may provide important prognostic information in patients with myelodysplastic syndromes (MDS), according to a new study.
Patients who had disease-associated mutations in the bone marrow 30 days after hematopoietic stem cell transplant (HSCT) were significantly more likely to experience disease progression and have lower rates of progression-free survival (PFS) at 1 year.
“Using our sequencing method, we’re identifying residual tumor cells before a pathologist could see them under the microscope and before a patient develops symptoms,” said Matthew J. Walter, MD, of Washington University in St. Louis, Mo.
“At that moment, there may be time to intervene in ways that could delay the cancer from coming back or potentially prevent it completely.”
Dr. Walter and his colleagues described results with their sequencing method in The New England Journal of Medicine.
The researchers sequenced bone marrow and skin (control) samples from 90 adults with MDS who underwent allogeneic HSCT.
The team used enhanced exome sequencing to detect mutations before HSCT and evaluated mutation clearance using error-corrected sequencing to genotype mutations in bone marrow samples collected 30 days after HSCT.
The researchers detected at least one validated somatic mutation in the pre-HSCT samples from 86 of 90 patients.
Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after HSCT. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the researchers explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not progress (P<0.001).
Progression occurred in 53.1% of patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days, whereas progression occurred in 13% of patients who did not have such mutations. After adjusting for conditioning regimen, the hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P<0.001).
The 1-year PFS rate was 31.3% in patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days and 59.3% in patients who did not have the mutations. After adjusting for conditioning, the HR for progression or death was 2.22 (P=0.005).
The researchers noted that PFS was lower in patients who had received reduced-intensity conditioning and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 (P≤0.001), when compared to other combinations of conditioning regimen and mutation status.
In multivariable analyses, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than four-fold risk of progression (HR, 4.48; P<0.001) and a more than two-fold risk of progression or death (HR, 2.39; P=0.002).
“Now that we have detected mutations early and shown that it predicts a higher risk of recurrence, we want to determine the best course of action for those high-risk patients,” Dr. Walter said.
He and his colleagues acknowledged that the high-coverage exome sequencing technique used for this study is not routinely available in the clinic. Therefore, the researchers also analyzed samples using a subset of genes that are usually included in gene sequencing panels for MDS and acute myeloid leukemia.
The researchers noted that this 40-gene panel revealed fewer patients (n=68; 79%) with mutations, but “the prognostic value of detection of measurable residual disease was still highly clinically significant.”
With this approach, the presence of at least one mutation with a variant allele frequency of at least 0.5% 30 days after HSCT was associated with a higher risk of disease progression at 1 year (HR, 3.39; P=0.001) and a higher risk of progression or death at 1 year (HR, 2.09; P=0.02).
This study was supported by grants from the Leukemia and Lymphoma Society and other groups.
Gene sequencing early after transplant may provide important prognostic information in patients with myelodysplastic syndromes (MDS), according to a new study.
Patients who had disease-associated mutations in the bone marrow 30 days after hematopoietic stem cell transplant (HSCT) were significantly more likely to experience disease progression and have lower rates of progression-free survival (PFS) at 1 year.
“Using our sequencing method, we’re identifying residual tumor cells before a pathologist could see them under the microscope and before a patient develops symptoms,” said Matthew J. Walter, MD, of Washington University in St. Louis, Mo.
“At that moment, there may be time to intervene in ways that could delay the cancer from coming back or potentially prevent it completely.”
Dr. Walter and his colleagues described results with their sequencing method in The New England Journal of Medicine.
The researchers sequenced bone marrow and skin (control) samples from 90 adults with MDS who underwent allogeneic HSCT.
The team used enhanced exome sequencing to detect mutations before HSCT and evaluated mutation clearance using error-corrected sequencing to genotype mutations in bone marrow samples collected 30 days after HSCT.
The researchers detected at least one validated somatic mutation in the pre-HSCT samples from 86 of 90 patients.
Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after HSCT. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the researchers explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not progress (P<0.001).
Progression occurred in 53.1% of patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days, whereas progression occurred in 13% of patients who did not have such mutations. After adjusting for conditioning regimen, the hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P<0.001).
The 1-year PFS rate was 31.3% in patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days and 59.3% in patients who did not have the mutations. After adjusting for conditioning, the HR for progression or death was 2.22 (P=0.005).
The researchers noted that PFS was lower in patients who had received reduced-intensity conditioning and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 (P≤0.001), when compared to other combinations of conditioning regimen and mutation status.
In multivariable analyses, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than four-fold risk of progression (HR, 4.48; P<0.001) and a more than two-fold risk of progression or death (HR, 2.39; P=0.002).
“Now that we have detected mutations early and shown that it predicts a higher risk of recurrence, we want to determine the best course of action for those high-risk patients,” Dr. Walter said.
He and his colleagues acknowledged that the high-coverage exome sequencing technique used for this study is not routinely available in the clinic. Therefore, the researchers also analyzed samples using a subset of genes that are usually included in gene sequencing panels for MDS and acute myeloid leukemia.
The researchers noted that this 40-gene panel revealed fewer patients (n=68; 79%) with mutations, but “the prognostic value of detection of measurable residual disease was still highly clinically significant.”
With this approach, the presence of at least one mutation with a variant allele frequency of at least 0.5% 30 days after HSCT was associated with a higher risk of disease progression at 1 year (HR, 3.39; P=0.001) and a higher risk of progression or death at 1 year (HR, 2.09; P=0.02).
This study was supported by grants from the Leukemia and Lymphoma Society and other groups.
HIF1A could be therapeutic target for MDS
The transcription factor HIF1A could be a therapeutic target for “a broad spectrum” of patients with myelodysplastic syndromes (MDS), according to researchers.
Preclinical experiments indicated that HIF1A fuels the biological processes that cause different types of MDS.
Researchers also found that inhibiting HIF1A reversed MDS symptoms and prolonged survival in mouse models of MDS.
Gang Huang, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio, and his colleagues reported these findings in Cancer Discovery.
The researchers identified HIF1A’s role in MDS by first analyzing cells from healthy donors and MDS patients, including patients with refractory anemia, refractory anemia with ring sideroblasts, and refractory anemia with excess blasts type 1 and 2.
The researchers observed increased gene expression of HIF1A-induced genes in the cells from MDS patients. The team also found a high frequency of HIF1A-expressing cells in the MDS cohort, regardless of the patients’ IPSS-R risk.
The researchers conducted experiments in mouse models to study the onset of MDS and its genetic and molecular drivers. The results suggested that dysregulation of HIF1A has a central role in the onset of MDS, including different manifestations and symptoms found in patients.
“We know the genomes of MDS patients have recurrent mutations in different transcriptional, epigenetic, and metabolic regulators, but the incidence of these mutations does not directly correspond to the disease when it occurs,” Dr. Huang noted.
“Our study shows that malfunctions in the signaling of HIF1A could be generating the diverse medical problems doctors see in MDS patients.”
Specifically, the researchers found that MDS-associated mutations—DNMT3A, TET2, ASXL1, RUNX1, and MLL1—induced HIF1A signaling. And activation of HIF1A signaling in hematopoietic cells induced MDS phenotypes in mice.
The team said this suggests dysregulation of HIF1A signaling could generate diverse MDS phenotypes by “functioning as a signaling funnel” for MDS driver mutations.
The researchers also showed that inhibition of HIF1A could reverse MDS phenotypes. They said HIF1A deletion rescued dysplasia formation, partially rescued thrombocytopenia, and abrogated MDS development in mouse models.
Treatment with echinomycin, an inhibitor of HIF1A-mediated target gene activation, prolonged survival in mouse models of MDS and decreased MDSL cell numbers in the bone marrow and spleen.
This research was supported by the Kyoto University Foundation, the MDS Foundation, the Cincinnati Children’s Hospital Research Foundation, the Leukemia Research Foundation, and others.
The transcription factor HIF1A could be a therapeutic target for “a broad spectrum” of patients with myelodysplastic syndromes (MDS), according to researchers.
Preclinical experiments indicated that HIF1A fuels the biological processes that cause different types of MDS.
Researchers also found that inhibiting HIF1A reversed MDS symptoms and prolonged survival in mouse models of MDS.
Gang Huang, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio, and his colleagues reported these findings in Cancer Discovery.
The researchers identified HIF1A’s role in MDS by first analyzing cells from healthy donors and MDS patients, including patients with refractory anemia, refractory anemia with ring sideroblasts, and refractory anemia with excess blasts type 1 and 2.
The researchers observed increased gene expression of HIF1A-induced genes in the cells from MDS patients. The team also found a high frequency of HIF1A-expressing cells in the MDS cohort, regardless of the patients’ IPSS-R risk.
The researchers conducted experiments in mouse models to study the onset of MDS and its genetic and molecular drivers. The results suggested that dysregulation of HIF1A has a central role in the onset of MDS, including different manifestations and symptoms found in patients.
“We know the genomes of MDS patients have recurrent mutations in different transcriptional, epigenetic, and metabolic regulators, but the incidence of these mutations does not directly correspond to the disease when it occurs,” Dr. Huang noted.
“Our study shows that malfunctions in the signaling of HIF1A could be generating the diverse medical problems doctors see in MDS patients.”
Specifically, the researchers found that MDS-associated mutations—DNMT3A, TET2, ASXL1, RUNX1, and MLL1—induced HIF1A signaling. And activation of HIF1A signaling in hematopoietic cells induced MDS phenotypes in mice.
The team said this suggests dysregulation of HIF1A signaling could generate diverse MDS phenotypes by “functioning as a signaling funnel” for MDS driver mutations.
The researchers also showed that inhibition of HIF1A could reverse MDS phenotypes. They said HIF1A deletion rescued dysplasia formation, partially rescued thrombocytopenia, and abrogated MDS development in mouse models.
Treatment with echinomycin, an inhibitor of HIF1A-mediated target gene activation, prolonged survival in mouse models of MDS and decreased MDSL cell numbers in the bone marrow and spleen.
This research was supported by the Kyoto University Foundation, the MDS Foundation, the Cincinnati Children’s Hospital Research Foundation, the Leukemia Research Foundation, and others.
The transcription factor HIF1A could be a therapeutic target for “a broad spectrum” of patients with myelodysplastic syndromes (MDS), according to researchers.
Preclinical experiments indicated that HIF1A fuels the biological processes that cause different types of MDS.
Researchers also found that inhibiting HIF1A reversed MDS symptoms and prolonged survival in mouse models of MDS.
Gang Huang, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio, and his colleagues reported these findings in Cancer Discovery.
The researchers identified HIF1A’s role in MDS by first analyzing cells from healthy donors and MDS patients, including patients with refractory anemia, refractory anemia with ring sideroblasts, and refractory anemia with excess blasts type 1 and 2.
The researchers observed increased gene expression of HIF1A-induced genes in the cells from MDS patients. The team also found a high frequency of HIF1A-expressing cells in the MDS cohort, regardless of the patients’ IPSS-R risk.
The researchers conducted experiments in mouse models to study the onset of MDS and its genetic and molecular drivers. The results suggested that dysregulation of HIF1A has a central role in the onset of MDS, including different manifestations and symptoms found in patients.
“We know the genomes of MDS patients have recurrent mutations in different transcriptional, epigenetic, and metabolic regulators, but the incidence of these mutations does not directly correspond to the disease when it occurs,” Dr. Huang noted.
“Our study shows that malfunctions in the signaling of HIF1A could be generating the diverse medical problems doctors see in MDS patients.”
Specifically, the researchers found that MDS-associated mutations—DNMT3A, TET2, ASXL1, RUNX1, and MLL1—induced HIF1A signaling. And activation of HIF1A signaling in hematopoietic cells induced MDS phenotypes in mice.
The team said this suggests dysregulation of HIF1A signaling could generate diverse MDS phenotypes by “functioning as a signaling funnel” for MDS driver mutations.
The researchers also showed that inhibition of HIF1A could reverse MDS phenotypes. They said HIF1A deletion rescued dysplasia formation, partially rescued thrombocytopenia, and abrogated MDS development in mouse models.
Treatment with echinomycin, an inhibitor of HIF1A-mediated target gene activation, prolonged survival in mouse models of MDS and decreased MDSL cell numbers in the bone marrow and spleen.
This research was supported by the Kyoto University Foundation, the MDS Foundation, the Cincinnati Children’s Hospital Research Foundation, the Leukemia Research Foundation, and others.
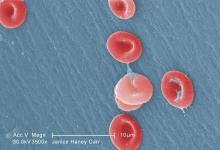
Research may help explain how VOCs occur
Researchers say they have gained new insight that may help explain how vaso-occlusive crises (VOCs) occur in patients with sickle cell disease (SCD).
The team assessed how red blood cell (RBC) adhesion and polymerization of deoxygenated sickle hemoglobin affect the mechanisms underlying VOCs.
Experiments showed that hypoxia enhances sickle RBC adherence, and hemoglobin S polymerization enhances adherence for sickle reticulocytes and mature erythrocytes.
However, sickle reticulocytes have “unique adhesion dynamics” and therefore appear more likely to cause VOCs.
The researchers described these discoveries in an article set to be published this week in the Proceedings of the National Academy of Sciences.
To investigate how RBCs interact with blood vessels to set off a VOC, the researchers built a microfluidic system that mimics post-capillary vessels. These vessels, which carry deoxygenated blood away from the capillaries, are where vaso-occlusions are most likely to occur.
The microfluidic system is designed to allow the researchers to control the oxygen level. The team used the system to test blood from eight SCD patients.
The researchers found that, under hypoxic conditions, sickle RBCs are two to four times more likely to adhere to the blood vessel walls than they are when oxygen levels are normal.
The team also found that hemoglobin S polymerization enhances the adherence of sickle reticulocytes and sickle mature erythrocytes. The hemoglobin S forms stiff fibers that grow and push the cell membrane outward, and these fibers help the cells adhere more firmly to the lining of the blood vessel.
“There has been little understanding of why, under hypoxia, there is much more adhesion,” said study author Subra Suresh, DSc, of Nanyang Technological University in Singapore.
“The experiments of this study provide some key insights into the processes and mechanisms responsible for increased adhesion.”
The researchers also found that, in SCD patients, reticulocytes are more likely than mature erythrocytes to adhere to blood vessels.
“We observed the growth of sickle hemoglobin fibers stretching reticulocytes within minutes,” said study author Dimitrios Papageorgiou, PhD, of the Massachusetts Institute of Technology in Cambridge.
“It looks like they’re trying to grab more of the surface and adhere more strongly.”
The researchers said these and other findings suggest polymerization and adhesion stimulate each other.
The team now hopes to devise a more complete model of vaso-occlusion that combines their new findings with previous work. The previous work involved measuring how long it takes SCD patients’ blood cells to stiffen, making them more likely to block blood flow in tiny blood vessels.
The researchers also hope their findings might help them devise a way to predict VOCs in individual SCD patients.
Researchers say they have gained new insight that may help explain how vaso-occlusive crises (VOCs) occur in patients with sickle cell disease (SCD).
The team assessed how red blood cell (RBC) adhesion and polymerization of deoxygenated sickle hemoglobin affect the mechanisms underlying VOCs.
Experiments showed that hypoxia enhances sickle RBC adherence, and hemoglobin S polymerization enhances adherence for sickle reticulocytes and mature erythrocytes.
However, sickle reticulocytes have “unique adhesion dynamics” and therefore appear more likely to cause VOCs.
The researchers described these discoveries in an article set to be published this week in the Proceedings of the National Academy of Sciences.
To investigate how RBCs interact with blood vessels to set off a VOC, the researchers built a microfluidic system that mimics post-capillary vessels. These vessels, which carry deoxygenated blood away from the capillaries, are where vaso-occlusions are most likely to occur.
The microfluidic system is designed to allow the researchers to control the oxygen level. The team used the system to test blood from eight SCD patients.
The researchers found that, under hypoxic conditions, sickle RBCs are two to four times more likely to adhere to the blood vessel walls than they are when oxygen levels are normal.
The team also found that hemoglobin S polymerization enhances the adherence of sickle reticulocytes and sickle mature erythrocytes. The hemoglobin S forms stiff fibers that grow and push the cell membrane outward, and these fibers help the cells adhere more firmly to the lining of the blood vessel.
“There has been little understanding of why, under hypoxia, there is much more adhesion,” said study author Subra Suresh, DSc, of Nanyang Technological University in Singapore.
“The experiments of this study provide some key insights into the processes and mechanisms responsible for increased adhesion.”
The researchers also found that, in SCD patients, reticulocytes are more likely than mature erythrocytes to adhere to blood vessels.
“We observed the growth of sickle hemoglobin fibers stretching reticulocytes within minutes,” said study author Dimitrios Papageorgiou, PhD, of the Massachusetts Institute of Technology in Cambridge.
“It looks like they’re trying to grab more of the surface and adhere more strongly.”
The researchers said these and other findings suggest polymerization and adhesion stimulate each other.
The team now hopes to devise a more complete model of vaso-occlusion that combines their new findings with previous work. The previous work involved measuring how long it takes SCD patients’ blood cells to stiffen, making them more likely to block blood flow in tiny blood vessels.
The researchers also hope their findings might help them devise a way to predict VOCs in individual SCD patients.
Researchers say they have gained new insight that may help explain how vaso-occlusive crises (VOCs) occur in patients with sickle cell disease (SCD).
The team assessed how red blood cell (RBC) adhesion and polymerization of deoxygenated sickle hemoglobin affect the mechanisms underlying VOCs.
Experiments showed that hypoxia enhances sickle RBC adherence, and hemoglobin S polymerization enhances adherence for sickle reticulocytes and mature erythrocytes.
However, sickle reticulocytes have “unique adhesion dynamics” and therefore appear more likely to cause VOCs.
The researchers described these discoveries in an article set to be published this week in the Proceedings of the National Academy of Sciences.
To investigate how RBCs interact with blood vessels to set off a VOC, the researchers built a microfluidic system that mimics post-capillary vessels. These vessels, which carry deoxygenated blood away from the capillaries, are where vaso-occlusions are most likely to occur.
The microfluidic system is designed to allow the researchers to control the oxygen level. The team used the system to test blood from eight SCD patients.
The researchers found that, under hypoxic conditions, sickle RBCs are two to four times more likely to adhere to the blood vessel walls than they are when oxygen levels are normal.
The team also found that hemoglobin S polymerization enhances the adherence of sickle reticulocytes and sickle mature erythrocytes. The hemoglobin S forms stiff fibers that grow and push the cell membrane outward, and these fibers help the cells adhere more firmly to the lining of the blood vessel.
“There has been little understanding of why, under hypoxia, there is much more adhesion,” said study author Subra Suresh, DSc, of Nanyang Technological University in Singapore.
“The experiments of this study provide some key insights into the processes and mechanisms responsible for increased adhesion.”
The researchers also found that, in SCD patients, reticulocytes are more likely than mature erythrocytes to adhere to blood vessels.
“We observed the growth of sickle hemoglobin fibers stretching reticulocytes within minutes,” said study author Dimitrios Papageorgiou, PhD, of the Massachusetts Institute of Technology in Cambridge.
“It looks like they’re trying to grab more of the surface and adhere more strongly.”
The researchers said these and other findings suggest polymerization and adhesion stimulate each other.
The team now hopes to devise a more complete model of vaso-occlusion that combines their new findings with previous work. The previous work involved measuring how long it takes SCD patients’ blood cells to stiffen, making them more likely to block blood flow in tiny blood vessels.
The researchers also hope their findings might help them devise a way to predict VOCs in individual SCD patients.
AMP publishes report on DNA variants in CMNs
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.