User login
SPRINT MIND published: Extension trial to add 2 years’ follow-up
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
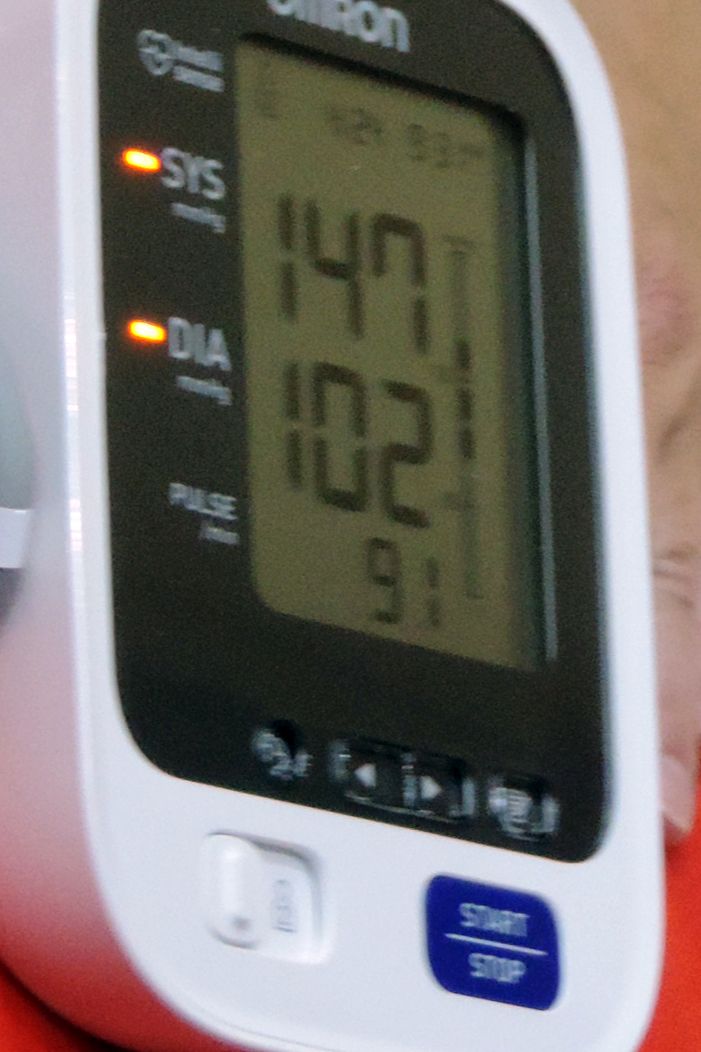
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
FROM JAMA
Key clinical point: Keeping systolic blood pressure lower than 120 mm Hg did not significantly reduce the risk of all-cause dementia in patients with hypertension, but it did lower the risk of mild cognitive impairment and probable dementia.
Major finding: The intensively treated group had a nonsignificant 17% lower risk of dementia, and significant reductions in the risk of MCI (19%) and probable dementia (15%).
Study details: SPRINT MIND was a substudy of the SPRINT antihypertension trial.
Source: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
Frailty may affect the expression of dementia
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
FROM LANCET NEUROLOGY
Key clinical point: Frailty modifies the association between Alzheimer’s disease pathology and Alzheimer dementia.
Major finding: Frailty index score (odds ratio, 1.76) is independently associated with dementia status.
Study details: A cross-sectional analysis of 456 deceased participants in the Rush Memory and Aging Project.
Disclosures: The study had no outside funding.
Source: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
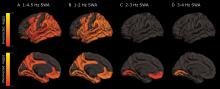
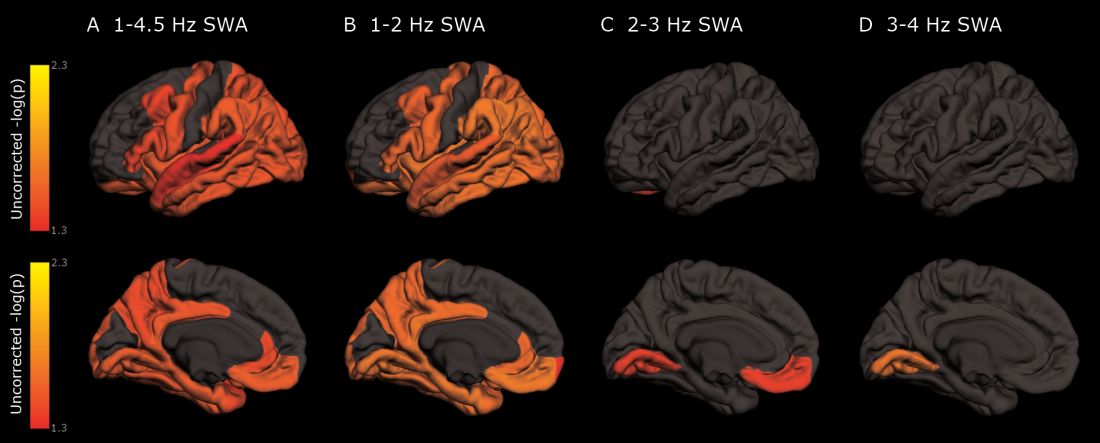
As deep sleep decreases, Alzheimer’s pathology – particularly tau – increases
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Cognitively normal subjects with tau deposition experience altered sleep patterns.
Major finding: Decreased time in non-REM deep sleep was associated with increased tau pathology in Alzheimer’s-affected brain regions and in cerebrospinal fluid.
Study details: The prospective longitudinal study comprised 119 subjects.
Disclosures: The authors reported no relevant financial disclosures.
Source: Lucey BP et al. Sci Transl Med. 2019 Jan 9;11:eaau6550.
Treating OSA with positive airway pressure decreased amyloid levels in CSF
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
FROM ANNALS OF NEUROLOGY
Key clinical point:
Major finding: After treatment, a correlational analysis found decreases in amyloid-beta 40 and 42.
Study details: The prospective, interventional study comprised 18 subjects.
Disclosures: The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
Source: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Researchers exploring ways to mitigate aging’s impact on diabetes
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2018
Nuedexta mainly prescribed for dementia, Parkinson’s
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Only 8.4% of patients had a diagnosis of multiple sclerosis and only 6.8% had amyotrophic lateral sclerosis, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis.
Study details: Population-based cohort study of 12,858 patients prescribed dextromethorphan-quinidine between 2010 and 2017.
Disclosures: Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
Source: Fralick M et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112.
Cerebral small vessel disease progression linked to MCI in hypertensive patients
Patients with hypertension who show substantial progression of cerebral small vessel disease over time have sixfold higher odds of developing mild cognitive impairment than do those without signs of progression on brain MRI, new research has found.
The results, published online Jan. 4 in Hypertension, come from a longitudinal, population-based study of 976 patients with hypertension but with no history of dementia or clinical stroke. Participants underwent a vascular risk assessment, brain MRI, cognitive evaluation, and blood sampling at baseline, and 345 patients were also retested after a mean of nearly 4 years.
Researchers saw significant sixfold higher odds of developing incident mild cognitive impairment (MCI) among individuals who showed marked progression of periventricular white matter hyperintensities – an imaging hallmark of cerebral small vessel disease – compared with individuals who did not show any progression (odds ratio = 6.184; 95% confidence interval, 1.506-25.370; P = .011).
Patients with greater progression of periventricular white matter hyperintensities also showed significantly greater decreases in global cognition scores – both in total DRS-2 Z-score and executive function Z-score – when compared against individuals without white matter hyperintensity progression.
“As MCI is one of the most important risk factors in the development of dementia, future research should investigate the mechanisms by which PVH [periventricular white matter hyperintensities] trigger cognitive impairment and the clinical utility of its assessment,” wrote Joan Jiménez-Balado of Vall d’Hebron Research Institute, Barcelona, and his associates.
However, deep white matter hyperintensity progression – as opposed to periventricular – was not linked to cognitive changes, except in the case of bilateral occipital deep white matter hyperintensity changes, which were linked to a significant worsening in the attention Z-score.
The authors noted that the different impacts of periventricular versus deep white matter hyperintensities may relate to a number of factors. The first was that deep white matter hyperintensities disrupt cortico-cortical connections but periventricular ones are more likely to affect long cortico-subcortical association fibers, which “would be an important variable to determine the impaired networks involved in cognition.”
They also suggested that periventricular and deep white matter hyperintensities may affect different neuromodulator systems; the periventricular white matter could be closer to ascending cholinergic bundles that may play a role in vascular cognitive impairment.
Periventricular white matter hyperintensities may also accelerate the deposition of amyloid because of their association with venous collagenosis, which is linked to ischemia and disruptions of the interstitial fluid circulation.
“On the other hand, [deep white matter hyperintensity] may be more related to hypoperfusion, as deep areas are particularly vulnerable to low [blood pressure],” the authors wrote, while stressing that the pathophysiology of white matter hyperintensities is not fully understood, so further research is needed.
Overall, the 345 patients with follow-up data had a median age of 65 years at baseline and mean blood pressure of 143/78.2 mm Hg at baseline and 146.5/75 mm Hg at follow-up. White matter hyperintensity changes occurred periventricularly in 22% and in deep white matter in 48%. The researchers saw new infarcts in 6.1% of patients, and 5.5% had incident cerebral microbleeds. While incident cerebral microbleeds were significantly associated with declines in the attention Z-score, they did not affect other cognitive functions, and incidental infarcts were also not associated with cognitive changes.
Baseline blood pressure and average blood pressure during follow-up were not associated with changes in cardiac small vessel disease lesions. However, diastolic – but not systolic – blood pressure at baseline and follow-up was positively correlated with total, attention, and executive function DRS-2 Z-scores at follow-up.
Three-quarters of patients showed cognitive changes associated with normal aging both at baseline and follow-up, 9.1% had stable MCI, and 9.1% of patients had incident MCI. However, 6.6% of subjects reverted back to normal aging after having MCI at baseline.
The authors noted that they did not examine markers of neurodegeneration, such as tau or amyloid-beta, which could also be linked to hypertension and cerebral small vessel disease lesions.
The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
SOURCE: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090
Patients with hypertension who show substantial progression of cerebral small vessel disease over time have sixfold higher odds of developing mild cognitive impairment than do those without signs of progression on brain MRI, new research has found.
The results, published online Jan. 4 in Hypertension, come from a longitudinal, population-based study of 976 patients with hypertension but with no history of dementia or clinical stroke. Participants underwent a vascular risk assessment, brain MRI, cognitive evaluation, and blood sampling at baseline, and 345 patients were also retested after a mean of nearly 4 years.
Researchers saw significant sixfold higher odds of developing incident mild cognitive impairment (MCI) among individuals who showed marked progression of periventricular white matter hyperintensities – an imaging hallmark of cerebral small vessel disease – compared with individuals who did not show any progression (odds ratio = 6.184; 95% confidence interval, 1.506-25.370; P = .011).
Patients with greater progression of periventricular white matter hyperintensities also showed significantly greater decreases in global cognition scores – both in total DRS-2 Z-score and executive function Z-score – when compared against individuals without white matter hyperintensity progression.
“As MCI is one of the most important risk factors in the development of dementia, future research should investigate the mechanisms by which PVH [periventricular white matter hyperintensities] trigger cognitive impairment and the clinical utility of its assessment,” wrote Joan Jiménez-Balado of Vall d’Hebron Research Institute, Barcelona, and his associates.
However, deep white matter hyperintensity progression – as opposed to periventricular – was not linked to cognitive changes, except in the case of bilateral occipital deep white matter hyperintensity changes, which were linked to a significant worsening in the attention Z-score.
The authors noted that the different impacts of periventricular versus deep white matter hyperintensities may relate to a number of factors. The first was that deep white matter hyperintensities disrupt cortico-cortical connections but periventricular ones are more likely to affect long cortico-subcortical association fibers, which “would be an important variable to determine the impaired networks involved in cognition.”
They also suggested that periventricular and deep white matter hyperintensities may affect different neuromodulator systems; the periventricular white matter could be closer to ascending cholinergic bundles that may play a role in vascular cognitive impairment.
Periventricular white matter hyperintensities may also accelerate the deposition of amyloid because of their association with venous collagenosis, which is linked to ischemia and disruptions of the interstitial fluid circulation.
“On the other hand, [deep white matter hyperintensity] may be more related to hypoperfusion, as deep areas are particularly vulnerable to low [blood pressure],” the authors wrote, while stressing that the pathophysiology of white matter hyperintensities is not fully understood, so further research is needed.
Overall, the 345 patients with follow-up data had a median age of 65 years at baseline and mean blood pressure of 143/78.2 mm Hg at baseline and 146.5/75 mm Hg at follow-up. White matter hyperintensity changes occurred periventricularly in 22% and in deep white matter in 48%. The researchers saw new infarcts in 6.1% of patients, and 5.5% had incident cerebral microbleeds. While incident cerebral microbleeds were significantly associated with declines in the attention Z-score, they did not affect other cognitive functions, and incidental infarcts were also not associated with cognitive changes.
Baseline blood pressure and average blood pressure during follow-up were not associated with changes in cardiac small vessel disease lesions. However, diastolic – but not systolic – blood pressure at baseline and follow-up was positively correlated with total, attention, and executive function DRS-2 Z-scores at follow-up.
Three-quarters of patients showed cognitive changes associated with normal aging both at baseline and follow-up, 9.1% had stable MCI, and 9.1% of patients had incident MCI. However, 6.6% of subjects reverted back to normal aging after having MCI at baseline.
The authors noted that they did not examine markers of neurodegeneration, such as tau or amyloid-beta, which could also be linked to hypertension and cerebral small vessel disease lesions.
The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
SOURCE: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090
Patients with hypertension who show substantial progression of cerebral small vessel disease over time have sixfold higher odds of developing mild cognitive impairment than do those without signs of progression on brain MRI, new research has found.
The results, published online Jan. 4 in Hypertension, come from a longitudinal, population-based study of 976 patients with hypertension but with no history of dementia or clinical stroke. Participants underwent a vascular risk assessment, brain MRI, cognitive evaluation, and blood sampling at baseline, and 345 patients were also retested after a mean of nearly 4 years.
Researchers saw significant sixfold higher odds of developing incident mild cognitive impairment (MCI) among individuals who showed marked progression of periventricular white matter hyperintensities – an imaging hallmark of cerebral small vessel disease – compared with individuals who did not show any progression (odds ratio = 6.184; 95% confidence interval, 1.506-25.370; P = .011).
Patients with greater progression of periventricular white matter hyperintensities also showed significantly greater decreases in global cognition scores – both in total DRS-2 Z-score and executive function Z-score – when compared against individuals without white matter hyperintensity progression.
“As MCI is one of the most important risk factors in the development of dementia, future research should investigate the mechanisms by which PVH [periventricular white matter hyperintensities] trigger cognitive impairment and the clinical utility of its assessment,” wrote Joan Jiménez-Balado of Vall d’Hebron Research Institute, Barcelona, and his associates.
However, deep white matter hyperintensity progression – as opposed to periventricular – was not linked to cognitive changes, except in the case of bilateral occipital deep white matter hyperintensity changes, which were linked to a significant worsening in the attention Z-score.
The authors noted that the different impacts of periventricular versus deep white matter hyperintensities may relate to a number of factors. The first was that deep white matter hyperintensities disrupt cortico-cortical connections but periventricular ones are more likely to affect long cortico-subcortical association fibers, which “would be an important variable to determine the impaired networks involved in cognition.”
They also suggested that periventricular and deep white matter hyperintensities may affect different neuromodulator systems; the periventricular white matter could be closer to ascending cholinergic bundles that may play a role in vascular cognitive impairment.
Periventricular white matter hyperintensities may also accelerate the deposition of amyloid because of their association with venous collagenosis, which is linked to ischemia and disruptions of the interstitial fluid circulation.
“On the other hand, [deep white matter hyperintensity] may be more related to hypoperfusion, as deep areas are particularly vulnerable to low [blood pressure],” the authors wrote, while stressing that the pathophysiology of white matter hyperintensities is not fully understood, so further research is needed.
Overall, the 345 patients with follow-up data had a median age of 65 years at baseline and mean blood pressure of 143/78.2 mm Hg at baseline and 146.5/75 mm Hg at follow-up. White matter hyperintensity changes occurred periventricularly in 22% and in deep white matter in 48%. The researchers saw new infarcts in 6.1% of patients, and 5.5% had incident cerebral microbleeds. While incident cerebral microbleeds were significantly associated with declines in the attention Z-score, they did not affect other cognitive functions, and incidental infarcts were also not associated with cognitive changes.
Baseline blood pressure and average blood pressure during follow-up were not associated with changes in cardiac small vessel disease lesions. However, diastolic – but not systolic – blood pressure at baseline and follow-up was positively correlated with total, attention, and executive function DRS-2 Z-scores at follow-up.
Three-quarters of patients showed cognitive changes associated with normal aging both at baseline and follow-up, 9.1% had stable MCI, and 9.1% of patients had incident MCI. However, 6.6% of subjects reverted back to normal aging after having MCI at baseline.
The authors noted that they did not examine markers of neurodegeneration, such as tau or amyloid-beta, which could also be linked to hypertension and cerebral small vessel disease lesions.
The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
SOURCE: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090
FROM HYPERTENSION
Key clinical point: Cerebral small vessel disease changes are associated with the development of mild cognitive impairment in hypertensive patients.
Major finding: Periventricular white matter hyperintensities in patients with hypertension were associated with sixfold higher odds of mild cognitive impairment.
Study details: A longitudinal, population-based study of 345 patients with hypertension.
Disclosures: The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
Source: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090.
Can lifestyle modifications delay or prevent Alzheimer’s disease?
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4

Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4

Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Can higher MAP post cardiac arrest improve neurologic outcomes?
CHICAGO – A European clinical trial that targeted a mean arterial blood pressure after cardiac arrest higher than what the existing guidelines recommend found that the approach was safe, improved blood flow and oxygen to the brain, helped patients recover quicker, and reduced the number of adverse cardiac events, although it did not reduce the extent of anoxic brain damage or improve functional outcomes, the lead investigator reported at the American Heart Association scientific sessions.
The Neuroprotect trial randomly assigned 112 adult survivors of an out-of-hospital cardiac arrest who were unconscious upon admission to two study groups: early goal-directed hemodynamic optimization (EGDHO), in which researchers used a targeted mean arterial pressure (MAP) of 85-100 mm Hg and mixed venous oxygen saturation between 65% and 75% during the first 36 hours after ICU admission; and the standard care group, in which they used the guideline-recommended MAP target of 65 mm Hg, said Koen Ameloot, MD, of East Limburg Hospital in Genk, Belgium.
“EGDHO clearly improved cerebral perfusion and oxygenation, thereby for the first time providing the proof of concept for this new hemodynamic target,” Dr. Ameloot said. “However, this did not result in the reduction of the extent of anoxic brain hemorrhage or effusion rate on MRI or an improvement in functional outcome at 180 days.”
He noted the trial was predicated on improving upon the so-called “two-hit” model of cardiac arrest sequelae: the first hit being the no-flow and low-flow period before achieving restoration of spontaneous circulation; the second hit being hypoperfusion and reperfusion injury during ICU stay.
Dr. Ameloot referenced a study in which he and other coauthors reported that patients with a MAP target of 65 mm Hg “experience a profound drop of cerebral oxygen saturation during the first 12 hours of ICU stay that may cause additional brain damage” (Resuscitation. 2018;123:92-7).
The researchers explored the question of what is the optimal MAP if a target of 65 mm Hg is too low, Dr. Ameloot said. “We showed that maximal brain oxygenation is achieved with a MAP of 100 mm Hg, while lower MAPs were associated with submaximal brain perfusion and higher MAPs with excessive after-load, a reduction in stroke volume, and suboptimal cerebral oxygenation.”
During the 36-hour intervention period, the EGDHO patients received higher doses of norepinephrine, Dr. Ameloot said. “This resulted in significant improvement of cerebral oxygenation during the first 12 hours and was paralleled by significantly higher cerebral perfusion in the subset of patients in whom Doppler measurements were performed,” he said. “While patients allocated to the MAP 65 mm Hg target experienced a profound drop of cerebral oxygenation during the critical first 6-12 hours of ICU stay, cerebral oxygenation was maintained at 67% in patients assigned to EGDHO.”
However, the rate of anoxic brain damage, measured as the percentage of irreversibly damaged anoxic voxels on diffusion-weighted MRI – the primary endpoint of the study – was actually higher in the EGDHO group, 16% vs. 12%, Dr. Ameloot said. “The percentage of anoxic voxels was only a poor predictor of favorable neurological outcome at 180 days, questioning the validity of the primary endpoint,” he said. He also noted that 23% of the trial participants did not have an MRI scan because of higher than expected 5-day rates of death.
“The percentage of patients with favorable neurological outcome tended to be somewhat higher in the intervention arm, although this did not reach statistical significance at ICU discharge and at 180 days,” Dr. Ameloot said. He noted that 42% of the intervention group and 33% of controls in the full-analysis set (P = .30) and 43% and 27%, respectively, in the per-protocol set (P = .15) had a favorable neurological outcome, as calculated using the Glasgow-Pittsburgh Cerebral Performance Category scores of 1 or 2, at 180 days.
The study did not reveal any noteworthy differences in ICU stay (7 vs. 8 days, P = .13) or days on mechanical ventilation (5 vs. 7, P = .31), although fewer patients in the EGDHO group required a tracheostomy (4% vs. 18%, P = .02). The intervention group also had lower rates of cardiac events, including recurrent cardiac arrest, limb ischemia, new atrial fibrillation, and pulmonary edema (13% vs. 33%; P = .02), Dr. Ameloot said.
Future post-hoc analyses of the data will explore the hypothesis that higher blood pressure leads to improved coronary perfusion and reduced infarct size, thus improving prognosis, he added.
“Should this trial therefore be the definite end to the promising hypothesis that improving brain oxygenation might reduce the second hit in post–cardiac arrest patients? I don’t think so,” Dr. Ameloot said. He noted a few limits to the study: that the perfusion rate on MRI was a poor predictor of 180-day outcome; that more patients than expected entered the trial without receiving basic life support and with nonshockable rhythms; and that there was possibly less extensive brain damage among controls at baseline. “Only an adequately powered clinical trial can provide an answer about the effects of EGDHO in post–cardiac arrest patients,” Dr. Ameloot said.
Dr. Ameloot had no financial relationships to disclose.
SOURCE: Ameloot K et al. AHA 2018, Abstract 18620
CHICAGO – A European clinical trial that targeted a mean arterial blood pressure after cardiac arrest higher than what the existing guidelines recommend found that the approach was safe, improved blood flow and oxygen to the brain, helped patients recover quicker, and reduced the number of adverse cardiac events, although it did not reduce the extent of anoxic brain damage or improve functional outcomes, the lead investigator reported at the American Heart Association scientific sessions.
The Neuroprotect trial randomly assigned 112 adult survivors of an out-of-hospital cardiac arrest who were unconscious upon admission to two study groups: early goal-directed hemodynamic optimization (EGDHO), in which researchers used a targeted mean arterial pressure (MAP) of 85-100 mm Hg and mixed venous oxygen saturation between 65% and 75% during the first 36 hours after ICU admission; and the standard care group, in which they used the guideline-recommended MAP target of 65 mm Hg, said Koen Ameloot, MD, of East Limburg Hospital in Genk, Belgium.
“EGDHO clearly improved cerebral perfusion and oxygenation, thereby for the first time providing the proof of concept for this new hemodynamic target,” Dr. Ameloot said. “However, this did not result in the reduction of the extent of anoxic brain hemorrhage or effusion rate on MRI or an improvement in functional outcome at 180 days.”
He noted the trial was predicated on improving upon the so-called “two-hit” model of cardiac arrest sequelae: the first hit being the no-flow and low-flow period before achieving restoration of spontaneous circulation; the second hit being hypoperfusion and reperfusion injury during ICU stay.
Dr. Ameloot referenced a study in which he and other coauthors reported that patients with a MAP target of 65 mm Hg “experience a profound drop of cerebral oxygen saturation during the first 12 hours of ICU stay that may cause additional brain damage” (Resuscitation. 2018;123:92-7).
The researchers explored the question of what is the optimal MAP if a target of 65 mm Hg is too low, Dr. Ameloot said. “We showed that maximal brain oxygenation is achieved with a MAP of 100 mm Hg, while lower MAPs were associated with submaximal brain perfusion and higher MAPs with excessive after-load, a reduction in stroke volume, and suboptimal cerebral oxygenation.”
During the 36-hour intervention period, the EGDHO patients received higher doses of norepinephrine, Dr. Ameloot said. “This resulted in significant improvement of cerebral oxygenation during the first 12 hours and was paralleled by significantly higher cerebral perfusion in the subset of patients in whom Doppler measurements were performed,” he said. “While patients allocated to the MAP 65 mm Hg target experienced a profound drop of cerebral oxygenation during the critical first 6-12 hours of ICU stay, cerebral oxygenation was maintained at 67% in patients assigned to EGDHO.”
However, the rate of anoxic brain damage, measured as the percentage of irreversibly damaged anoxic voxels on diffusion-weighted MRI – the primary endpoint of the study – was actually higher in the EGDHO group, 16% vs. 12%, Dr. Ameloot said. “The percentage of anoxic voxels was only a poor predictor of favorable neurological outcome at 180 days, questioning the validity of the primary endpoint,” he said. He also noted that 23% of the trial participants did not have an MRI scan because of higher than expected 5-day rates of death.
“The percentage of patients with favorable neurological outcome tended to be somewhat higher in the intervention arm, although this did not reach statistical significance at ICU discharge and at 180 days,” Dr. Ameloot said. He noted that 42% of the intervention group and 33% of controls in the full-analysis set (P = .30) and 43% and 27%, respectively, in the per-protocol set (P = .15) had a favorable neurological outcome, as calculated using the Glasgow-Pittsburgh Cerebral Performance Category scores of 1 or 2, at 180 days.
The study did not reveal any noteworthy differences in ICU stay (7 vs. 8 days, P = .13) or days on mechanical ventilation (5 vs. 7, P = .31), although fewer patients in the EGDHO group required a tracheostomy (4% vs. 18%, P = .02). The intervention group also had lower rates of cardiac events, including recurrent cardiac arrest, limb ischemia, new atrial fibrillation, and pulmonary edema (13% vs. 33%; P = .02), Dr. Ameloot said.
Future post-hoc analyses of the data will explore the hypothesis that higher blood pressure leads to improved coronary perfusion and reduced infarct size, thus improving prognosis, he added.
“Should this trial therefore be the definite end to the promising hypothesis that improving brain oxygenation might reduce the second hit in post–cardiac arrest patients? I don’t think so,” Dr. Ameloot said. He noted a few limits to the study: that the perfusion rate on MRI was a poor predictor of 180-day outcome; that more patients than expected entered the trial without receiving basic life support and with nonshockable rhythms; and that there was possibly less extensive brain damage among controls at baseline. “Only an adequately powered clinical trial can provide an answer about the effects of EGDHO in post–cardiac arrest patients,” Dr. Ameloot said.
Dr. Ameloot had no financial relationships to disclose.
SOURCE: Ameloot K et al. AHA 2018, Abstract 18620
CHICAGO – A European clinical trial that targeted a mean arterial blood pressure after cardiac arrest higher than what the existing guidelines recommend found that the approach was safe, improved blood flow and oxygen to the brain, helped patients recover quicker, and reduced the number of adverse cardiac events, although it did not reduce the extent of anoxic brain damage or improve functional outcomes, the lead investigator reported at the American Heart Association scientific sessions.
The Neuroprotect trial randomly assigned 112 adult survivors of an out-of-hospital cardiac arrest who were unconscious upon admission to two study groups: early goal-directed hemodynamic optimization (EGDHO), in which researchers used a targeted mean arterial pressure (MAP) of 85-100 mm Hg and mixed venous oxygen saturation between 65% and 75% during the first 36 hours after ICU admission; and the standard care group, in which they used the guideline-recommended MAP target of 65 mm Hg, said Koen Ameloot, MD, of East Limburg Hospital in Genk, Belgium.
“EGDHO clearly improved cerebral perfusion and oxygenation, thereby for the first time providing the proof of concept for this new hemodynamic target,” Dr. Ameloot said. “However, this did not result in the reduction of the extent of anoxic brain hemorrhage or effusion rate on MRI or an improvement in functional outcome at 180 days.”
He noted the trial was predicated on improving upon the so-called “two-hit” model of cardiac arrest sequelae: the first hit being the no-flow and low-flow period before achieving restoration of spontaneous circulation; the second hit being hypoperfusion and reperfusion injury during ICU stay.
Dr. Ameloot referenced a study in which he and other coauthors reported that patients with a MAP target of 65 mm Hg “experience a profound drop of cerebral oxygen saturation during the first 12 hours of ICU stay that may cause additional brain damage” (Resuscitation. 2018;123:92-7).
The researchers explored the question of what is the optimal MAP if a target of 65 mm Hg is too low, Dr. Ameloot said. “We showed that maximal brain oxygenation is achieved with a MAP of 100 mm Hg, while lower MAPs were associated with submaximal brain perfusion and higher MAPs with excessive after-load, a reduction in stroke volume, and suboptimal cerebral oxygenation.”
During the 36-hour intervention period, the EGDHO patients received higher doses of norepinephrine, Dr. Ameloot said. “This resulted in significant improvement of cerebral oxygenation during the first 12 hours and was paralleled by significantly higher cerebral perfusion in the subset of patients in whom Doppler measurements were performed,” he said. “While patients allocated to the MAP 65 mm Hg target experienced a profound drop of cerebral oxygenation during the critical first 6-12 hours of ICU stay, cerebral oxygenation was maintained at 67% in patients assigned to EGDHO.”
However, the rate of anoxic brain damage, measured as the percentage of irreversibly damaged anoxic voxels on diffusion-weighted MRI – the primary endpoint of the study – was actually higher in the EGDHO group, 16% vs. 12%, Dr. Ameloot said. “The percentage of anoxic voxels was only a poor predictor of favorable neurological outcome at 180 days, questioning the validity of the primary endpoint,” he said. He also noted that 23% of the trial participants did not have an MRI scan because of higher than expected 5-day rates of death.
“The percentage of patients with favorable neurological outcome tended to be somewhat higher in the intervention arm, although this did not reach statistical significance at ICU discharge and at 180 days,” Dr. Ameloot said. He noted that 42% of the intervention group and 33% of controls in the full-analysis set (P = .30) and 43% and 27%, respectively, in the per-protocol set (P = .15) had a favorable neurological outcome, as calculated using the Glasgow-Pittsburgh Cerebral Performance Category scores of 1 or 2, at 180 days.
The study did not reveal any noteworthy differences in ICU stay (7 vs. 8 days, P = .13) or days on mechanical ventilation (5 vs. 7, P = .31), although fewer patients in the EGDHO group required a tracheostomy (4% vs. 18%, P = .02). The intervention group also had lower rates of cardiac events, including recurrent cardiac arrest, limb ischemia, new atrial fibrillation, and pulmonary edema (13% vs. 33%; P = .02), Dr. Ameloot said.
Future post-hoc analyses of the data will explore the hypothesis that higher blood pressure leads to improved coronary perfusion and reduced infarct size, thus improving prognosis, he added.
“Should this trial therefore be the definite end to the promising hypothesis that improving brain oxygenation might reduce the second hit in post–cardiac arrest patients? I don’t think so,” Dr. Ameloot said. He noted a few limits to the study: that the perfusion rate on MRI was a poor predictor of 180-day outcome; that more patients than expected entered the trial without receiving basic life support and with nonshockable rhythms; and that there was possibly less extensive brain damage among controls at baseline. “Only an adequately powered clinical trial can provide an answer about the effects of EGDHO in post–cardiac arrest patients,” Dr. Ameloot said.
Dr. Ameloot had no financial relationships to disclose.
SOURCE: Ameloot K et al. AHA 2018, Abstract 18620
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Forty-three percent of patients in the intervention group had a favorable neurological outcome vs. 27% of controls (P = .15).
Study details: The Neuroprotect trial was a multicenter, randomized, open-label, assessor-blinded trial of 112 post–cardiac arrest patients.
Disclosures: Dr. Ameloot had no financial relationships to disclose.
Source: Ameloot K et al. AHA 2018, Abstract 18620
Neurologic Disease Eventually Affects Half of Women and One-Third of Men
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].