User login
A global snapshot of leukemia incidence
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.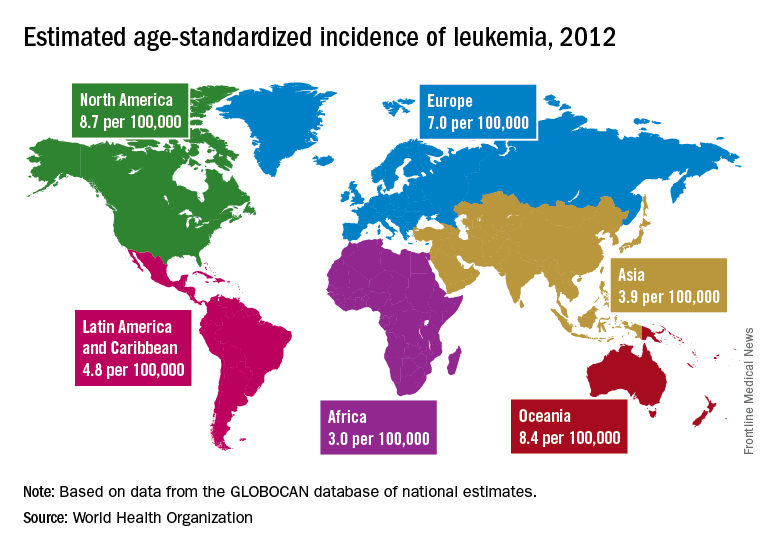
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
FROM THE LANCET HAEMATOLOGY
Consider steroid-induced hypertension when treating pediatric ALL
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Method may predict relapse at BCP-ALL diagnosis
Researchers say they have developed a technique that can help them determine, at diagnosis, whether children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) will relapse after treatment.
The method involves examining individual leukemia cells using mass cytometry.
In looking at the cells’ stage of development and signaling behavior, the researchers were able to identify a subset of malignant cells that predispose a patient to relapse.
The team described this method, which they termed “developmentally dependent predictor of relapse (DDPR),” in Nature Medicine.
Prior research suggested relapse may be driven by treatment-resistant cells that are present from the beginning of disease development.
“We wondered, can we identify those cells at the time the patient first presents to the clinic, and can we treat patients with a specific therapy to target them?” said study author Kara Davis, DO, of Stanford University in California.
Dr Davis and her colleagues used mass cytometry to analyze diagnostic bone marrow samples from 60 patients with BCP-ALL.
To pinpoint the problematic cells among the millions of cells in each patient’s sample, the researchers had to figure out how to organize the data.
“Every patient has vastly different features to their cancer,” Dr Davis said, “and we had to ask, ‘Is there any common thread between them?’”
The solution, the researchers found, was to match BCP-ALL cells and healthy B cells according to their developmental states, comparing the leukemic cells to the healthy cells.
The comparison revealed 6 features of leukemic cell populations that were associated with relapse.
Broadly, the features suggested that pro-BII cells with activated mTOR signaling were associated with relapse, as were pre-BI cells with activated and unresponsive pre-B-cell receptor signaling.
“We do not understand the mechanisms by which malignant cells from the pro-BII and pre-BI stages of development resist treatment,” Dr Davis noted.
However, she and her colleagues were able to show the leukemic cell features identified by DDPR could predict relapse in the BCP-ALL patients.
Of the 60 patients analyzed, there were 54 with at least 3 years of follow-up. The researchers divided these patients into a training cohort (n=44) and a validation cohort (n=10).
The team used an integrated cumulative/dynamic area under the curve (iAUC) and a C-statistic to assess DDPR performance in both cohorts.
In the training cohort, DDPR had an iAUC value of 0.92 and a C-statistic of 0.87. In the validation cohort, DDPR had an iAUC value of 0.85 and a C-statistic of 0.87.
The researchers also said DDPR “performed well” in predicting relapse-free survival in a retrospective analysis of both cohorts (P = 2.8 × 10−7).
Now, the researchers plan to validate DDPR in a larger number of patients and evaluate whether the same general approach could predict relapse in other cancers.
Researchers say they have developed a technique that can help them determine, at diagnosis, whether children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) will relapse after treatment.
The method involves examining individual leukemia cells using mass cytometry.
In looking at the cells’ stage of development and signaling behavior, the researchers were able to identify a subset of malignant cells that predispose a patient to relapse.
The team described this method, which they termed “developmentally dependent predictor of relapse (DDPR),” in Nature Medicine.
Prior research suggested relapse may be driven by treatment-resistant cells that are present from the beginning of disease development.
“We wondered, can we identify those cells at the time the patient first presents to the clinic, and can we treat patients with a specific therapy to target them?” said study author Kara Davis, DO, of Stanford University in California.
Dr Davis and her colleagues used mass cytometry to analyze diagnostic bone marrow samples from 60 patients with BCP-ALL.
To pinpoint the problematic cells among the millions of cells in each patient’s sample, the researchers had to figure out how to organize the data.
“Every patient has vastly different features to their cancer,” Dr Davis said, “and we had to ask, ‘Is there any common thread between them?’”
The solution, the researchers found, was to match BCP-ALL cells and healthy B cells according to their developmental states, comparing the leukemic cells to the healthy cells.
The comparison revealed 6 features of leukemic cell populations that were associated with relapse.
Broadly, the features suggested that pro-BII cells with activated mTOR signaling were associated with relapse, as were pre-BI cells with activated and unresponsive pre-B-cell receptor signaling.
“We do not understand the mechanisms by which malignant cells from the pro-BII and pre-BI stages of development resist treatment,” Dr Davis noted.
However, she and her colleagues were able to show the leukemic cell features identified by DDPR could predict relapse in the BCP-ALL patients.
Of the 60 patients analyzed, there were 54 with at least 3 years of follow-up. The researchers divided these patients into a training cohort (n=44) and a validation cohort (n=10).
The team used an integrated cumulative/dynamic area under the curve (iAUC) and a C-statistic to assess DDPR performance in both cohorts.
In the training cohort, DDPR had an iAUC value of 0.92 and a C-statistic of 0.87. In the validation cohort, DDPR had an iAUC value of 0.85 and a C-statistic of 0.87.
The researchers also said DDPR “performed well” in predicting relapse-free survival in a retrospective analysis of both cohorts (P = 2.8 × 10−7).
Now, the researchers plan to validate DDPR in a larger number of patients and evaluate whether the same general approach could predict relapse in other cancers.
Researchers say they have developed a technique that can help them determine, at diagnosis, whether children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) will relapse after treatment.
The method involves examining individual leukemia cells using mass cytometry.
In looking at the cells’ stage of development and signaling behavior, the researchers were able to identify a subset of malignant cells that predispose a patient to relapse.
The team described this method, which they termed “developmentally dependent predictor of relapse (DDPR),” in Nature Medicine.
Prior research suggested relapse may be driven by treatment-resistant cells that are present from the beginning of disease development.
“We wondered, can we identify those cells at the time the patient first presents to the clinic, and can we treat patients with a specific therapy to target them?” said study author Kara Davis, DO, of Stanford University in California.
Dr Davis and her colleagues used mass cytometry to analyze diagnostic bone marrow samples from 60 patients with BCP-ALL.
To pinpoint the problematic cells among the millions of cells in each patient’s sample, the researchers had to figure out how to organize the data.
“Every patient has vastly different features to their cancer,” Dr Davis said, “and we had to ask, ‘Is there any common thread between them?’”
The solution, the researchers found, was to match BCP-ALL cells and healthy B cells according to their developmental states, comparing the leukemic cells to the healthy cells.
The comparison revealed 6 features of leukemic cell populations that were associated with relapse.
Broadly, the features suggested that pro-BII cells with activated mTOR signaling were associated with relapse, as were pre-BI cells with activated and unresponsive pre-B-cell receptor signaling.
“We do not understand the mechanisms by which malignant cells from the pro-BII and pre-BI stages of development resist treatment,” Dr Davis noted.
However, she and her colleagues were able to show the leukemic cell features identified by DDPR could predict relapse in the BCP-ALL patients.
Of the 60 patients analyzed, there were 54 with at least 3 years of follow-up. The researchers divided these patients into a training cohort (n=44) and a validation cohort (n=10).
The team used an integrated cumulative/dynamic area under the curve (iAUC) and a C-statistic to assess DDPR performance in both cohorts.
In the training cohort, DDPR had an iAUC value of 0.92 and a C-statistic of 0.87. In the validation cohort, DDPR had an iAUC value of 0.85 and a C-statistic of 0.87.
The researchers also said DDPR “performed well” in predicting relapse-free survival in a retrospective analysis of both cohorts (P = 2.8 × 10−7).
Now, the researchers plan to validate DDPR in a larger number of patients and evaluate whether the same general approach could predict relapse in other cancers.
Phase 2 study tests low-dose maintenance therapy for ALL
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
FROM CLINICALTRIALS.GOV
Expanded UCB product can stand alone
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
Disease burden impacts survival, toxicity after CAR T-cell therapy
Adults with relapsed or refractory acute lymphoblastic leukemia (ALL) have better outcomes if they have a low disease burden when receiving chimeric antigen receptor (CAR) T-cell therapy, according to research published in NEJM.
The final analysis of a phase 1 trial showed that patients with a low disease burden at baseline had superior event-free survival (EFS) and overall survival (OS) after therapy, compared to patients with a high disease burden.
Patients with a low disease burden also had a lower rate of cytokine release syndrome (CRS) and neurotoxic events.
“This is the longest follow-up study of people with ALL treated with CAR therapy,” said study author Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“With the long follow-up, we were able to demonstrate, for the first time, that patients with a lower disease burden benefited the most from CAR therapy, with significantly improved survival and reduced toxicity.”
The study included 53 adults with ALL who had a median age of 44 (range, 23-74).
They were heavily pretreated, with 68% receiving CAR T-cell therapy as a third or later salvage treatment. Thirty-six percent of patients had received an allogeneic transplant, and 23% had primary refractory disease.
In this trial, patients received a single infusion of 19-28z CAR T cells after conditioning chemotherapy. The maximum follow-up time was 5.5 years, with a median follow-up of 29 months.
In all, 83% of patients achieved a complete response. The median EFS was 6.1 months, and the median OS was 12.9 months.
The median EFS was significantly longer for patients with a low disease burden (<5% bone marrow blasts) compared to a high disease burden (≥5% bone marrow blasts or extramedullary disease)—10.6 months and 5.6 months, respectively (P=0.01).
The same was true for the median OS, which was 20.1 months in patients with a low disease burden and 12.4 months in those with a high disease burden (P=0.02).
For the entire study population, the rate of CRS was 85%, and 26% of patients had severe CRS. One patient died of severe CRS and multi-organ failure before the researchers began modifying the dose of CAR T cells according to the pretreatment disease burden.
Severe CRS occurred in 41% of patients with a high disease burden and 5% of those with a low disease burden.
In the entire study population, 2% of patients had grade 2 neurotoxic effects, 36% had grade 3, 6% had grade 4, and none had grade 5. The rate of severe neurotoxicity was 42%.
Neurotoxic effects occurred in 59% of patients with a high disease burden and 14% of those with a low disease burden.
“Among all of the clinical and disease factors we examined, pretreatment disease burden was the strongest predictor of long-term outcome after CAR therapy,” Dr Park said. “Our data supports the incorporation of CAR therapy in an earlier treatment setting in ALL, when the disease volume is small, so as to achieve the greatest long-term efficacy and lowest toxicity.”
This work was supported by Juno Therapeutics, the National Institutes of Health, the Carson Family Charitable Trust, the Emerald Foundation, the Mr. and Mrs. Goodwyn Commonwealth Fund, the Terry Fox Run for Cancer Research organized by the Canadian Association of New York, Kate’s Team, William Laurence and Blanche Hughes Foundation, the Center for Experimental Therapeutics at Memorial Sloan Kettering, and the Lake Road Foundation. ![]()
Adults with relapsed or refractory acute lymphoblastic leukemia (ALL) have better outcomes if they have a low disease burden when receiving chimeric antigen receptor (CAR) T-cell therapy, according to research published in NEJM.
The final analysis of a phase 1 trial showed that patients with a low disease burden at baseline had superior event-free survival (EFS) and overall survival (OS) after therapy, compared to patients with a high disease burden.
Patients with a low disease burden also had a lower rate of cytokine release syndrome (CRS) and neurotoxic events.
“This is the longest follow-up study of people with ALL treated with CAR therapy,” said study author Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“With the long follow-up, we were able to demonstrate, for the first time, that patients with a lower disease burden benefited the most from CAR therapy, with significantly improved survival and reduced toxicity.”
The study included 53 adults with ALL who had a median age of 44 (range, 23-74).
They were heavily pretreated, with 68% receiving CAR T-cell therapy as a third or later salvage treatment. Thirty-six percent of patients had received an allogeneic transplant, and 23% had primary refractory disease.
In this trial, patients received a single infusion of 19-28z CAR T cells after conditioning chemotherapy. The maximum follow-up time was 5.5 years, with a median follow-up of 29 months.
In all, 83% of patients achieved a complete response. The median EFS was 6.1 months, and the median OS was 12.9 months.
The median EFS was significantly longer for patients with a low disease burden (<5% bone marrow blasts) compared to a high disease burden (≥5% bone marrow blasts or extramedullary disease)—10.6 months and 5.6 months, respectively (P=0.01).
The same was true for the median OS, which was 20.1 months in patients with a low disease burden and 12.4 months in those with a high disease burden (P=0.02).
For the entire study population, the rate of CRS was 85%, and 26% of patients had severe CRS. One patient died of severe CRS and multi-organ failure before the researchers began modifying the dose of CAR T cells according to the pretreatment disease burden.
Severe CRS occurred in 41% of patients with a high disease burden and 5% of those with a low disease burden.
In the entire study population, 2% of patients had grade 2 neurotoxic effects, 36% had grade 3, 6% had grade 4, and none had grade 5. The rate of severe neurotoxicity was 42%.
Neurotoxic effects occurred in 59% of patients with a high disease burden and 14% of those with a low disease burden.
“Among all of the clinical and disease factors we examined, pretreatment disease burden was the strongest predictor of long-term outcome after CAR therapy,” Dr Park said. “Our data supports the incorporation of CAR therapy in an earlier treatment setting in ALL, when the disease volume is small, so as to achieve the greatest long-term efficacy and lowest toxicity.”
This work was supported by Juno Therapeutics, the National Institutes of Health, the Carson Family Charitable Trust, the Emerald Foundation, the Mr. and Mrs. Goodwyn Commonwealth Fund, the Terry Fox Run for Cancer Research organized by the Canadian Association of New York, Kate’s Team, William Laurence and Blanche Hughes Foundation, the Center for Experimental Therapeutics at Memorial Sloan Kettering, and the Lake Road Foundation. ![]()
Adults with relapsed or refractory acute lymphoblastic leukemia (ALL) have better outcomes if they have a low disease burden when receiving chimeric antigen receptor (CAR) T-cell therapy, according to research published in NEJM.
The final analysis of a phase 1 trial showed that patients with a low disease burden at baseline had superior event-free survival (EFS) and overall survival (OS) after therapy, compared to patients with a high disease burden.
Patients with a low disease burden also had a lower rate of cytokine release syndrome (CRS) and neurotoxic events.
“This is the longest follow-up study of people with ALL treated with CAR therapy,” said study author Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“With the long follow-up, we were able to demonstrate, for the first time, that patients with a lower disease burden benefited the most from CAR therapy, with significantly improved survival and reduced toxicity.”
The study included 53 adults with ALL who had a median age of 44 (range, 23-74).
They were heavily pretreated, with 68% receiving CAR T-cell therapy as a third or later salvage treatment. Thirty-six percent of patients had received an allogeneic transplant, and 23% had primary refractory disease.
In this trial, patients received a single infusion of 19-28z CAR T cells after conditioning chemotherapy. The maximum follow-up time was 5.5 years, with a median follow-up of 29 months.
In all, 83% of patients achieved a complete response. The median EFS was 6.1 months, and the median OS was 12.9 months.
The median EFS was significantly longer for patients with a low disease burden (<5% bone marrow blasts) compared to a high disease burden (≥5% bone marrow blasts or extramedullary disease)—10.6 months and 5.6 months, respectively (P=0.01).
The same was true for the median OS, which was 20.1 months in patients with a low disease burden and 12.4 months in those with a high disease burden (P=0.02).
For the entire study population, the rate of CRS was 85%, and 26% of patients had severe CRS. One patient died of severe CRS and multi-organ failure before the researchers began modifying the dose of CAR T cells according to the pretreatment disease burden.
Severe CRS occurred in 41% of patients with a high disease burden and 5% of those with a low disease burden.
In the entire study population, 2% of patients had grade 2 neurotoxic effects, 36% had grade 3, 6% had grade 4, and none had grade 5. The rate of severe neurotoxicity was 42%.
Neurotoxic effects occurred in 59% of patients with a high disease burden and 14% of those with a low disease burden.
“Among all of the clinical and disease factors we examined, pretreatment disease burden was the strongest predictor of long-term outcome after CAR therapy,” Dr Park said. “Our data supports the incorporation of CAR therapy in an earlier treatment setting in ALL, when the disease volume is small, so as to achieve the greatest long-term efficacy and lowest toxicity.”
This work was supported by Juno Therapeutics, the National Institutes of Health, the Carson Family Charitable Trust, the Emerald Foundation, the Mr. and Mrs. Goodwyn Commonwealth Fund, the Terry Fox Run for Cancer Research organized by the Canadian Association of New York, Kate’s Team, William Laurence and Blanche Hughes Foundation, the Center for Experimental Therapeutics at Memorial Sloan Kettering, and the Lake Road Foundation. ![]()
Socioeconomic deprivation tied to survival in ALL
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
CAR T-cell therapy produces durable CRs in ALL
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Tisagenlecleucel looks effective in phase 2 study of young ALL patients
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Key clinical point:
Major finding: The overall remission rate was 81% at 3 months, and 73% of patients experienced grade 3 or 4 adverse events.
Study details: A multicenter, phase 2 study of 75 patients.
Disclosures: Novartis designed and sponsored this research. Dr. Maude reported receiving fees from Novartis and grant funding from St. Baldrick’s Foundation.
Source: Maude SL et al. N Engl J Med. 2018;378:439-48.
CAR T cells produce longest survival in low disease burden ALL patients
Among patients with B-cell acute lymphoblastic leukemia (ALL) who received an infusion of 19-28z CAR T cells, patients with low disease burden had better survival outcomes and fewer toxic effects than did patients with a high disease burden, according to long-term follow-up results of a phase 1 study.
Median overall survival for B-cell ALL patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02), and 12.9 months for the entire cohort, according to results published in the New England Journal of Medicine.
The 12.9-month overall survival for the full study cohort “compares favorably” to results from another recently reported clinical trial showing overall survival of 7.7 months for adult B-cell ALL patients treated with blinatumomab, an anti–CD19/CD3 bispecific T-cell engager, wrote Jae H. Park, MD, of the Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The CAR T-cell and blinatumomab results cannot be directly compared owing to the differences in study design, patient characteristics, and posttreatment consolidation, but “the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies,” Dr. Park and his colleagues wrote in their report.
The phase 1 trial by Dr. Park and his colleagues included 53 adults with relapsed B-cell ALL who received a single infusion of 19-28z CAR T-cell therapy manufactured at Memorial Sloan Kettering Cancer Center.
After the infusion, 41% of patients with high disease burden (at least 5% bone marrow blasts or extramedullary disease) experienced severe cytokine release syndrome, compared with 5% of those with low disease burden, according to the report.
Likewise, neurotoxic effects were seen in 59% of high disease burden B-ALL patients, compared with 14% of those with low disease burden, the investigators reported.
Low disease burden was associated with a higher rate of complete remission, but this finding did not reach statistical significance. However, low disease burden patients not only had improved overall survival, as noted, but also had a significantly longer event-free survival versus high disease burden patients (10.6 and 5.3 months, respectively; P = .01).
Robust expansion of CAR T cells in vivo was a good predictor of short-term response and toxic effects but did not correlate with longer-term efficacy, according to the researchers. Instead, the ratio of peak CAR T-cell expansion to tumor burden correlated significantly with event-free and overall survival.
That finding “raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden,” the investigators wrote.
The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
SOURCE: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
Among patients with B-cell acute lymphoblastic leukemia (ALL) who received an infusion of 19-28z CAR T cells, patients with low disease burden had better survival outcomes and fewer toxic effects than did patients with a high disease burden, according to long-term follow-up results of a phase 1 study.
Median overall survival for B-cell ALL patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02), and 12.9 months for the entire cohort, according to results published in the New England Journal of Medicine.
The 12.9-month overall survival for the full study cohort “compares favorably” to results from another recently reported clinical trial showing overall survival of 7.7 months for adult B-cell ALL patients treated with blinatumomab, an anti–CD19/CD3 bispecific T-cell engager, wrote Jae H. Park, MD, of the Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The CAR T-cell and blinatumomab results cannot be directly compared owing to the differences in study design, patient characteristics, and posttreatment consolidation, but “the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies,” Dr. Park and his colleagues wrote in their report.
The phase 1 trial by Dr. Park and his colleagues included 53 adults with relapsed B-cell ALL who received a single infusion of 19-28z CAR T-cell therapy manufactured at Memorial Sloan Kettering Cancer Center.
After the infusion, 41% of patients with high disease burden (at least 5% bone marrow blasts or extramedullary disease) experienced severe cytokine release syndrome, compared with 5% of those with low disease burden, according to the report.
Likewise, neurotoxic effects were seen in 59% of high disease burden B-ALL patients, compared with 14% of those with low disease burden, the investigators reported.
Low disease burden was associated with a higher rate of complete remission, but this finding did not reach statistical significance. However, low disease burden patients not only had improved overall survival, as noted, but also had a significantly longer event-free survival versus high disease burden patients (10.6 and 5.3 months, respectively; P = .01).
Robust expansion of CAR T cells in vivo was a good predictor of short-term response and toxic effects but did not correlate with longer-term efficacy, according to the researchers. Instead, the ratio of peak CAR T-cell expansion to tumor burden correlated significantly with event-free and overall survival.
That finding “raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden,” the investigators wrote.
The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
SOURCE: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
Among patients with B-cell acute lymphoblastic leukemia (ALL) who received an infusion of 19-28z CAR T cells, patients with low disease burden had better survival outcomes and fewer toxic effects than did patients with a high disease burden, according to long-term follow-up results of a phase 1 study.
Median overall survival for B-cell ALL patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02), and 12.9 months for the entire cohort, according to results published in the New England Journal of Medicine.
The 12.9-month overall survival for the full study cohort “compares favorably” to results from another recently reported clinical trial showing overall survival of 7.7 months for adult B-cell ALL patients treated with blinatumomab, an anti–CD19/CD3 bispecific T-cell engager, wrote Jae H. Park, MD, of the Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The CAR T-cell and blinatumomab results cannot be directly compared owing to the differences in study design, patient characteristics, and posttreatment consolidation, but “the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies,” Dr. Park and his colleagues wrote in their report.
The phase 1 trial by Dr. Park and his colleagues included 53 adults with relapsed B-cell ALL who received a single infusion of 19-28z CAR T-cell therapy manufactured at Memorial Sloan Kettering Cancer Center.
After the infusion, 41% of patients with high disease burden (at least 5% bone marrow blasts or extramedullary disease) experienced severe cytokine release syndrome, compared with 5% of those with low disease burden, according to the report.
Likewise, neurotoxic effects were seen in 59% of high disease burden B-ALL patients, compared with 14% of those with low disease burden, the investigators reported.
Low disease burden was associated with a higher rate of complete remission, but this finding did not reach statistical significance. However, low disease burden patients not only had improved overall survival, as noted, but also had a significantly longer event-free survival versus high disease burden patients (10.6 and 5.3 months, respectively; P = .01).
Robust expansion of CAR T cells in vivo was a good predictor of short-term response and toxic effects but did not correlate with longer-term efficacy, according to the researchers. Instead, the ratio of peak CAR T-cell expansion to tumor burden correlated significantly with event-free and overall survival.
That finding “raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden,” the investigators wrote.
The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
SOURCE: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Median overall survival for patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02).
Study details: A long-term follow-up of a phase 1 trial including 53 adults with relapsed B-cell ALL.
Disclosures: The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
Source: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.











