User login
ALL regimens clear disease in kids with MPAL
SAN DIEGO—Pediatric patients with mixed phenotype acute leukemia (MPAL) can achieve minimal residual disease (MRD) negativity with acute lymphoblastic leukemia (ALL)-directed chemotherapy, according to new research.
In a retrospective study, most pediatric MPAL patients who received ALL-directed chemotherapy achieved an MRD-negative complete response (CR).
Ninety-three percent of patients achieved a CR at the end of induction with an ALL regimen, 70% were MRD-negative at the end of induction, and 86% were MRD-negative at the end of induction or consolidation.
Etan Orgel, MD, of the University of Southern California, Los Angeles, presented these findings at the ASH 2018 Annual Meeting (abstract 558*).
The study included 94 patients aged 1-21 years who met World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions.
Most patients had B/Myeloid phenotype (89%, n=84), 10% (n=9) had T/Myeloid, and 1% (n=1) had B/T phenotype.
Eighty-seven patients (93%) received ALL induction, and 83 (89%) continued on ALL therapy after induction.
Ninety-three percent (81/87) of patients treated with an ALL induction regimen had a CR at the end of induction. One patient died during induction, and six had induction failures, defined as either disease progression (n=2) or MRD of 5% or greater (n=4).
The MRD-negative rates, defined as MRD less than 0.01%, were 70% (59/84) at the end of induction and 86% (68/79) at the end of induction or consolidation.
Twelve of 14 patients (86%) who were MRD-positive at the end of induction and continued on ALL therapy achieved MRD negativity at the end of consolidation.
Survival
The researchers assessed 5-year survival in patients who received an ALL regimen but did not go on to transplant.
In these patients, the 5-year event-free survival (EFS) was 75%, and the 5-year overall survival (OS) was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said.
“[T]his is very different from the approach used at many adult centers and many of the adult recommendations,” he added.
The 5-year EFS rate was 80% in patients who were MRD-negative at the end of induction and 52% in patients who were MRD-positive at the end of induction. Five-year OS rates were 91% and 84%, respectively.
The 5-year EFS rate was 77% in patients who were MRD-negative at the end of consolidation and was unavailable in the three patients who were MRD-positive. The 5-year OS rates were 89% and not available, respectively.
In a multivariable analysis, MRD was the strongest predictor of EFS (hazard ratio [HR]=3.5) and OS (HR=4.6).
There was a trend toward earlier failure and worse OS (HR=4.49, P=0.074) for T-lineage-containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” Dr. Orgel said.
In closing, he said this research suggests that ALL therapy without transplant may be sufficient to treat most patients with pediatric MPAL. However, he noted that clinical trials are necessary to prospectively validate MRD thresholds at end of induction and consolidation and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” Dr. Orgel said.
He disclosed no conflicts of interest.
* Data in the presentation differ from the abstract.
SAN DIEGO—Pediatric patients with mixed phenotype acute leukemia (MPAL) can achieve minimal residual disease (MRD) negativity with acute lymphoblastic leukemia (ALL)-directed chemotherapy, according to new research.
In a retrospective study, most pediatric MPAL patients who received ALL-directed chemotherapy achieved an MRD-negative complete response (CR).
Ninety-three percent of patients achieved a CR at the end of induction with an ALL regimen, 70% were MRD-negative at the end of induction, and 86% were MRD-negative at the end of induction or consolidation.
Etan Orgel, MD, of the University of Southern California, Los Angeles, presented these findings at the ASH 2018 Annual Meeting (abstract 558*).
The study included 94 patients aged 1-21 years who met World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions.
Most patients had B/Myeloid phenotype (89%, n=84), 10% (n=9) had T/Myeloid, and 1% (n=1) had B/T phenotype.
Eighty-seven patients (93%) received ALL induction, and 83 (89%) continued on ALL therapy after induction.
Ninety-three percent (81/87) of patients treated with an ALL induction regimen had a CR at the end of induction. One patient died during induction, and six had induction failures, defined as either disease progression (n=2) or MRD of 5% or greater (n=4).
The MRD-negative rates, defined as MRD less than 0.01%, were 70% (59/84) at the end of induction and 86% (68/79) at the end of induction or consolidation.
Twelve of 14 patients (86%) who were MRD-positive at the end of induction and continued on ALL therapy achieved MRD negativity at the end of consolidation.
Survival
The researchers assessed 5-year survival in patients who received an ALL regimen but did not go on to transplant.
In these patients, the 5-year event-free survival (EFS) was 75%, and the 5-year overall survival (OS) was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said.
“[T]his is very different from the approach used at many adult centers and many of the adult recommendations,” he added.
The 5-year EFS rate was 80% in patients who were MRD-negative at the end of induction and 52% in patients who were MRD-positive at the end of induction. Five-year OS rates were 91% and 84%, respectively.
The 5-year EFS rate was 77% in patients who were MRD-negative at the end of consolidation and was unavailable in the three patients who were MRD-positive. The 5-year OS rates were 89% and not available, respectively.
In a multivariable analysis, MRD was the strongest predictor of EFS (hazard ratio [HR]=3.5) and OS (HR=4.6).
There was a trend toward earlier failure and worse OS (HR=4.49, P=0.074) for T-lineage-containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” Dr. Orgel said.
In closing, he said this research suggests that ALL therapy without transplant may be sufficient to treat most patients with pediatric MPAL. However, he noted that clinical trials are necessary to prospectively validate MRD thresholds at end of induction and consolidation and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” Dr. Orgel said.
He disclosed no conflicts of interest.
* Data in the presentation differ from the abstract.
SAN DIEGO—Pediatric patients with mixed phenotype acute leukemia (MPAL) can achieve minimal residual disease (MRD) negativity with acute lymphoblastic leukemia (ALL)-directed chemotherapy, according to new research.
In a retrospective study, most pediatric MPAL patients who received ALL-directed chemotherapy achieved an MRD-negative complete response (CR).
Ninety-three percent of patients achieved a CR at the end of induction with an ALL regimen, 70% were MRD-negative at the end of induction, and 86% were MRD-negative at the end of induction or consolidation.
Etan Orgel, MD, of the University of Southern California, Los Angeles, presented these findings at the ASH 2018 Annual Meeting (abstract 558*).
The study included 94 patients aged 1-21 years who met World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions.
Most patients had B/Myeloid phenotype (89%, n=84), 10% (n=9) had T/Myeloid, and 1% (n=1) had B/T phenotype.
Eighty-seven patients (93%) received ALL induction, and 83 (89%) continued on ALL therapy after induction.
Ninety-three percent (81/87) of patients treated with an ALL induction regimen had a CR at the end of induction. One patient died during induction, and six had induction failures, defined as either disease progression (n=2) or MRD of 5% or greater (n=4).
The MRD-negative rates, defined as MRD less than 0.01%, were 70% (59/84) at the end of induction and 86% (68/79) at the end of induction or consolidation.
Twelve of 14 patients (86%) who were MRD-positive at the end of induction and continued on ALL therapy achieved MRD negativity at the end of consolidation.
Survival
The researchers assessed 5-year survival in patients who received an ALL regimen but did not go on to transplant.
In these patients, the 5-year event-free survival (EFS) was 75%, and the 5-year overall survival (OS) was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said.
“[T]his is very different from the approach used at many adult centers and many of the adult recommendations,” he added.
The 5-year EFS rate was 80% in patients who were MRD-negative at the end of induction and 52% in patients who were MRD-positive at the end of induction. Five-year OS rates were 91% and 84%, respectively.
The 5-year EFS rate was 77% in patients who were MRD-negative at the end of consolidation and was unavailable in the three patients who were MRD-positive. The 5-year OS rates were 89% and not available, respectively.
In a multivariable analysis, MRD was the strongest predictor of EFS (hazard ratio [HR]=3.5) and OS (HR=4.6).
There was a trend toward earlier failure and worse OS (HR=4.49, P=0.074) for T-lineage-containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” Dr. Orgel said.
In closing, he said this research suggests that ALL therapy without transplant may be sufficient to treat most patients with pediatric MPAL. However, he noted that clinical trials are necessary to prospectively validate MRD thresholds at end of induction and consolidation and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” Dr. Orgel said.
He disclosed no conflicts of interest.
* Data in the presentation differ from the abstract.
ELIANA update: Tisagenlecleucel responses durable in r/r B-cell ALL
SAN DIEGO – A single infusion of tisagenlecleucel (Kymriah) continues to demonstrate high efficacy a year and a half after pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia (ALL) were enrolled in the pivotal ELIANA trial, Stephan A. Grupp, MD, PhD, reported at a press conference at the annual meeting of the American Society of Hematology.
Of patients in complete remission after receiving the chimeric antigen receptor (CAR) T-cell therapy, 66% were still in remission at 18 months of follow-up and median survival has yet to be reached.
“We think the efficacy is sustained and excellent,” said Dr. Grupp, of the University of Pennsylvania, Philadelphia. “These are durable complete responses even without further therapy. We haven’t reached median duration of response or overall survival, and we can manage toxicity, so I think this is clearly an opportunity for us to treat our patients both in America and across the world.”
The results of ELIANA were the basis for the approval of tisagenlecleucel (Kymriah) for relapsed/refractory B-cell ALL in the United States, and for subsequent approvals by health authorities in the European Union, Switzerland, and Canada.
The international, single-arm, open-label, phase 2 ELIANA trial included 97 patients aged 3-24 years with relapsed or refractory B-cell ALL, most of whom had previously undergone a hematopoietic stem cell transplant. Of those patients, 79 patients went on to receive a single infusion of the CAR T-cell therapy.
The rate of overall remission, defined as the rate of complete remission or complete remission with incomplete blood count recovery, was 82% (65 patients). Among those patients, 66% were still in remission at 18 months, while overall survival was “excellent” at 70%, Dr. Grupp said, and the median overall survival was not reached.
Adverse events prevalence remained relatively unchanged since the previous analyses, according to Dr. Grupp, who added that appropriately trained personnel were able to effectively manage those adverse events across study sites. Cytokine release syndrome (CRS) occurred in 77% of patients. All cases were reversible; 39% received tocilizumab for treatment of CRS with or without other anticytokine therapies; 48% required ICU-level care for CRS, with a median ICU stay of 7 days.
The other most common grade 3/4 nonhematologic adverse events were neutropenia with a body temperature exceeding 38.3° C (62% within 8 weeks of infusion), hypoxia (20%), and hypotension (20%). Grade 3 neurologic effects occurred in 13% of patients, and none had cerebral edema. Based on laboratory results, 43% had grade 3/4 thrombocytopenia and 54% had neutropenia not resolved by day 28; most of these events resolved to grade 2 or less by 3 months.
There were 25 postinfusion deaths, with 2 occurring within 30 days (1 because of disease progression, 1 because of cerebral hemorrhage). Of the 23 deaths after 30 days (range, 53-859 days), 18 were caused by disease progression. The other deaths were caused by encephalitis, systemic mycosis, vasoocclusive hepatobiliary disorder related to allogeneic SCT, bacterial lung infection, and an unknown reason after study withdrawal.
Follow-up is ongoing in the ELIANA study, which is supported by Novartis, the maker of Kymriah.
Dr. Grupp reported research funding from Novartis, consultancy with Novartis, Jazz Pharmaceuticals, and Adaptimmune Therapeutics, and patents or royalties with the University of Pennsylvania.
SOURCE: Grupp SA et al. ASH 2018, Abstract 895.
SAN DIEGO – A single infusion of tisagenlecleucel (Kymriah) continues to demonstrate high efficacy a year and a half after pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia (ALL) were enrolled in the pivotal ELIANA trial, Stephan A. Grupp, MD, PhD, reported at a press conference at the annual meeting of the American Society of Hematology.
Of patients in complete remission after receiving the chimeric antigen receptor (CAR) T-cell therapy, 66% were still in remission at 18 months of follow-up and median survival has yet to be reached.
“We think the efficacy is sustained and excellent,” said Dr. Grupp, of the University of Pennsylvania, Philadelphia. “These are durable complete responses even without further therapy. We haven’t reached median duration of response or overall survival, and we can manage toxicity, so I think this is clearly an opportunity for us to treat our patients both in America and across the world.”
The results of ELIANA were the basis for the approval of tisagenlecleucel (Kymriah) for relapsed/refractory B-cell ALL in the United States, and for subsequent approvals by health authorities in the European Union, Switzerland, and Canada.
The international, single-arm, open-label, phase 2 ELIANA trial included 97 patients aged 3-24 years with relapsed or refractory B-cell ALL, most of whom had previously undergone a hematopoietic stem cell transplant. Of those patients, 79 patients went on to receive a single infusion of the CAR T-cell therapy.
The rate of overall remission, defined as the rate of complete remission or complete remission with incomplete blood count recovery, was 82% (65 patients). Among those patients, 66% were still in remission at 18 months, while overall survival was “excellent” at 70%, Dr. Grupp said, and the median overall survival was not reached.
Adverse events prevalence remained relatively unchanged since the previous analyses, according to Dr. Grupp, who added that appropriately trained personnel were able to effectively manage those adverse events across study sites. Cytokine release syndrome (CRS) occurred in 77% of patients. All cases were reversible; 39% received tocilizumab for treatment of CRS with or without other anticytokine therapies; 48% required ICU-level care for CRS, with a median ICU stay of 7 days.
The other most common grade 3/4 nonhematologic adverse events were neutropenia with a body temperature exceeding 38.3° C (62% within 8 weeks of infusion), hypoxia (20%), and hypotension (20%). Grade 3 neurologic effects occurred in 13% of patients, and none had cerebral edema. Based on laboratory results, 43% had grade 3/4 thrombocytopenia and 54% had neutropenia not resolved by day 28; most of these events resolved to grade 2 or less by 3 months.
There were 25 postinfusion deaths, with 2 occurring within 30 days (1 because of disease progression, 1 because of cerebral hemorrhage). Of the 23 deaths after 30 days (range, 53-859 days), 18 were caused by disease progression. The other deaths were caused by encephalitis, systemic mycosis, vasoocclusive hepatobiliary disorder related to allogeneic SCT, bacterial lung infection, and an unknown reason after study withdrawal.
Follow-up is ongoing in the ELIANA study, which is supported by Novartis, the maker of Kymriah.
Dr. Grupp reported research funding from Novartis, consultancy with Novartis, Jazz Pharmaceuticals, and Adaptimmune Therapeutics, and patents or royalties with the University of Pennsylvania.
SOURCE: Grupp SA et al. ASH 2018, Abstract 895.
SAN DIEGO – A single infusion of tisagenlecleucel (Kymriah) continues to demonstrate high efficacy a year and a half after pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia (ALL) were enrolled in the pivotal ELIANA trial, Stephan A. Grupp, MD, PhD, reported at a press conference at the annual meeting of the American Society of Hematology.
Of patients in complete remission after receiving the chimeric antigen receptor (CAR) T-cell therapy, 66% were still in remission at 18 months of follow-up and median survival has yet to be reached.
“We think the efficacy is sustained and excellent,” said Dr. Grupp, of the University of Pennsylvania, Philadelphia. “These are durable complete responses even without further therapy. We haven’t reached median duration of response or overall survival, and we can manage toxicity, so I think this is clearly an opportunity for us to treat our patients both in America and across the world.”
The results of ELIANA were the basis for the approval of tisagenlecleucel (Kymriah) for relapsed/refractory B-cell ALL in the United States, and for subsequent approvals by health authorities in the European Union, Switzerland, and Canada.
The international, single-arm, open-label, phase 2 ELIANA trial included 97 patients aged 3-24 years with relapsed or refractory B-cell ALL, most of whom had previously undergone a hematopoietic stem cell transplant. Of those patients, 79 patients went on to receive a single infusion of the CAR T-cell therapy.
The rate of overall remission, defined as the rate of complete remission or complete remission with incomplete blood count recovery, was 82% (65 patients). Among those patients, 66% were still in remission at 18 months, while overall survival was “excellent” at 70%, Dr. Grupp said, and the median overall survival was not reached.
Adverse events prevalence remained relatively unchanged since the previous analyses, according to Dr. Grupp, who added that appropriately trained personnel were able to effectively manage those adverse events across study sites. Cytokine release syndrome (CRS) occurred in 77% of patients. All cases were reversible; 39% received tocilizumab for treatment of CRS with or without other anticytokine therapies; 48% required ICU-level care for CRS, with a median ICU stay of 7 days.
The other most common grade 3/4 nonhematologic adverse events were neutropenia with a body temperature exceeding 38.3° C (62% within 8 weeks of infusion), hypoxia (20%), and hypotension (20%). Grade 3 neurologic effects occurred in 13% of patients, and none had cerebral edema. Based on laboratory results, 43% had grade 3/4 thrombocytopenia and 54% had neutropenia not resolved by day 28; most of these events resolved to grade 2 or less by 3 months.
There were 25 postinfusion deaths, with 2 occurring within 30 days (1 because of disease progression, 1 because of cerebral hemorrhage). Of the 23 deaths after 30 days (range, 53-859 days), 18 were caused by disease progression. The other deaths were caused by encephalitis, systemic mycosis, vasoocclusive hepatobiliary disorder related to allogeneic SCT, bacterial lung infection, and an unknown reason after study withdrawal.
Follow-up is ongoing in the ELIANA study, which is supported by Novartis, the maker of Kymriah.
Dr. Grupp reported research funding from Novartis, consultancy with Novartis, Jazz Pharmaceuticals, and Adaptimmune Therapeutics, and patents or royalties with the University of Pennsylvania.
SOURCE: Grupp SA et al. ASH 2018, Abstract 895.
REPORTING FROM ASH 2018
Key clinical point: A single infusion of tisagenlecleucel continues to demonstrate high efficacy a year and a half later in pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia.
Major finding: Among patients who had complete remission, 66% were still in remission at 18 months, while overall survival was 70% and median overall survival had not yet been reached.
Study details: A follow-up of the pivotal ELIANA trial, an international, single-arm, open-label, phase 2 study including 97 patients aged 3-24 years with relapsed or refractory B-cell acute lymphoblastic leukemia.
Disclosures: The study was supported by Novartis. Dr. Grupp reported disclosures with Novartis, Jazz Pharmaceuticals, Adaptimmune Therapeutics, and the University of Pennsylvania.
Source: Grupp SA et al. ASH 2018, Abstract 895.
Stem cell transplant after CAR T cells may reduce B-ALL relapse risk
SAN DIEGO – A hematopoietic cell transplant following chimeric antigen receptor (CAR) T-cell therapy for B-cell acute lymphocytic leukemia (B-ALL) may reduce late relapse risk in certain patients, a retrospective analysis suggests.
Corinne Summers, MD, of Seattle Children’s Hospital, and her colleagues evaluated the potential benefits of allogeneic hematopoietic cell transplant (HCT) in 50 pediatric and young adult B-ALL patients who had sustained leukemic remission after receiving SCRI-CAR19v1, a CD19-specific CAR T-cell product.
Leukemia-free survival was significantly improved for patients with no history of HCT who received CD19 CAR T-cell therapy followed by consolidative HCT, Dr. Summers reported at the annual meeting of the American Society of Hematology.
However, the benefits of consolidative HCT are unclear for patients with a history of HCT, Dr. Summers said at the meeting, noting that larger studies are needed.
In her video interview at ASH 2018, Dr. Summers talked more about the challenges of late leukemic relapse and the potential role of HCT after CAR T-cell therapy.
Dr. Summers reported no disclosures related to her presentation.
SAN DIEGO – A hematopoietic cell transplant following chimeric antigen receptor (CAR) T-cell therapy for B-cell acute lymphocytic leukemia (B-ALL) may reduce late relapse risk in certain patients, a retrospective analysis suggests.
Corinne Summers, MD, of Seattle Children’s Hospital, and her colleagues evaluated the potential benefits of allogeneic hematopoietic cell transplant (HCT) in 50 pediatric and young adult B-ALL patients who had sustained leukemic remission after receiving SCRI-CAR19v1, a CD19-specific CAR T-cell product.
Leukemia-free survival was significantly improved for patients with no history of HCT who received CD19 CAR T-cell therapy followed by consolidative HCT, Dr. Summers reported at the annual meeting of the American Society of Hematology.
However, the benefits of consolidative HCT are unclear for patients with a history of HCT, Dr. Summers said at the meeting, noting that larger studies are needed.
In her video interview at ASH 2018, Dr. Summers talked more about the challenges of late leukemic relapse and the potential role of HCT after CAR T-cell therapy.
Dr. Summers reported no disclosures related to her presentation.
SAN DIEGO – A hematopoietic cell transplant following chimeric antigen receptor (CAR) T-cell therapy for B-cell acute lymphocytic leukemia (B-ALL) may reduce late relapse risk in certain patients, a retrospective analysis suggests.
Corinne Summers, MD, of Seattle Children’s Hospital, and her colleagues evaluated the potential benefits of allogeneic hematopoietic cell transplant (HCT) in 50 pediatric and young adult B-ALL patients who had sustained leukemic remission after receiving SCRI-CAR19v1, a CD19-specific CAR T-cell product.
Leukemia-free survival was significantly improved for patients with no history of HCT who received CD19 CAR T-cell therapy followed by consolidative HCT, Dr. Summers reported at the annual meeting of the American Society of Hematology.
However, the benefits of consolidative HCT are unclear for patients with a history of HCT, Dr. Summers said at the meeting, noting that larger studies are needed.
In her video interview at ASH 2018, Dr. Summers talked more about the challenges of late leukemic relapse and the potential role of HCT after CAR T-cell therapy.
Dr. Summers reported no disclosures related to her presentation.
REPORTING FROM ASH 2018
CAR T-cell studies to be presented at ASH
Several studies set to be presented at the 2018 ASH Annual Meeting provide new insights regarding chimeric antigen receptor (CAR) T-cell therapies.
One study suggests ibrutinib may enhance CAR T-cell therapy in patients with chronic lymphocytic leukemia (CLL), and another suggests checkpoint inhibitors can augment CAR T-cell therapy in certain patients with B-cell acute lymphoblastic leukemia (ALL).
Two additional studies indicate that responses to tisagenlecleucel are durable in both ALL and diffuse large B-cell lymphoma (DLBCL).
A fifth study suggests hematopoietic stem cell transplant (HSCT) may reduce the risk of relapse after CAR T-cell therapy.
ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland, discussed these studies during a media briefing ahead of the ASH Annual Meeting.
Ibrutinib
In the ibrutinib study (abstract 299), patients received the BTK inhibitor starting 2 weeks prior to leukapheresis and continued until 3 months after treatment with JCAR014.
Data suggest this strategy may improve responses and decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory CLL.
Responses occurred in 88% of patients who received ibrutinib and 56% of those who did not.
Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients (26%) in the no-ibrutinib cohort and 0 of 17 patients in the ibrutinib cohort.
These findings are “early and preliminary but very exciting” Dr. Brodsky said.
Checkpoint inhibitors
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy.
The 14 patients studied had early CAR T-cell loss, partial response, or no response to CAR T-cell therapy. Thirteen patients had B-cell ALL, and one had B lymphoblastic lymphoma.
CD19-directed CAR T-cell therapy consisted of tisagenlecleucel in four patients and CTL119 in 10. Thirteen patients received pembrolizumab, and one received nivolumab.
Three of six patients who had early B-cell recovery re-established B-cell aplasia with the addition of a checkpoint inhibitor. In two patients, B-cell aplasia persists with ongoing pembrolizumab.
Four patients who did not respond to or relapsed after their initial CAR T-cell therapy had a partial (n=2) or complete response (n=2) with the addition of pembrolizumab.
There were additional partial responses in the remaining four patients. However, one of these patients (with CD19-dim/negative disease) progressed.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said.
“[This is a] very small [study with] preliminary data but very exciting that it is safe to give checkpoint inhibitors with CAR T cells, and it may be efficacious at getting the immune response back.”
Tisagenlecleucel follow-up
One of the two tisagenlecleucel updates (abstract 895) consists of data from the ELIANA trial, which includes pediatric and young adult patients with relapsed/refractory ALL.
The overall response rate was 82% (65/79). Of the 65 responders, 29 were still in response at follow-up.
The probability of relapse-free survival was 66% at 12 months and 18 months.
“These are some very fast-growing tumors, and these are refractory, resistant patients, so, as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
The other tisagenlecleucel update (abstract 1684) is from the JULIET trial, which includes adults with relapsed or refractory DLBCL (n=99).
The overall response rate was 54%. The probability of relapse-free survival was 66% at 6 months and 64% at both 12 months and 18 months.
HSCT consolidation
Dr. Brodsky also discussed long-term follow-up from a phase 1/2 trial of SCRI-CAR19v1, a CD19-specific CAR T-cell product, in patients with relapsed/refractory ALL (abstract 967).
Of the 50 evaluable patients, 17 had no history of HSCT prior to CAR T-cell therapy.
Three of the 17 patients did not proceed to HSCT after CAR T-cell therapy, and two of these patients relapsed. Of the 14 patients who did undergo HSCT after CAR T-cell therapy, two relapsed.
There were 33 patients with a prior history of HSCT, and 10 of them had another HSCT after CAR T-cell therapy. Five of them are still alive and in remission.
Of the 23 patients who did not undergo another HSCT, eight are still in remission.
“This study is very small, and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Several studies set to be presented at the 2018 ASH Annual Meeting provide new insights regarding chimeric antigen receptor (CAR) T-cell therapies.
One study suggests ibrutinib may enhance CAR T-cell therapy in patients with chronic lymphocytic leukemia (CLL), and another suggests checkpoint inhibitors can augment CAR T-cell therapy in certain patients with B-cell acute lymphoblastic leukemia (ALL).
Two additional studies indicate that responses to tisagenlecleucel are durable in both ALL and diffuse large B-cell lymphoma (DLBCL).
A fifth study suggests hematopoietic stem cell transplant (HSCT) may reduce the risk of relapse after CAR T-cell therapy.
ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland, discussed these studies during a media briefing ahead of the ASH Annual Meeting.
Ibrutinib
In the ibrutinib study (abstract 299), patients received the BTK inhibitor starting 2 weeks prior to leukapheresis and continued until 3 months after treatment with JCAR014.
Data suggest this strategy may improve responses and decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory CLL.
Responses occurred in 88% of patients who received ibrutinib and 56% of those who did not.
Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients (26%) in the no-ibrutinib cohort and 0 of 17 patients in the ibrutinib cohort.
These findings are “early and preliminary but very exciting” Dr. Brodsky said.
Checkpoint inhibitors
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy.
The 14 patients studied had early CAR T-cell loss, partial response, or no response to CAR T-cell therapy. Thirteen patients had B-cell ALL, and one had B lymphoblastic lymphoma.
CD19-directed CAR T-cell therapy consisted of tisagenlecleucel in four patients and CTL119 in 10. Thirteen patients received pembrolizumab, and one received nivolumab.
Three of six patients who had early B-cell recovery re-established B-cell aplasia with the addition of a checkpoint inhibitor. In two patients, B-cell aplasia persists with ongoing pembrolizumab.
Four patients who did not respond to or relapsed after their initial CAR T-cell therapy had a partial (n=2) or complete response (n=2) with the addition of pembrolizumab.
There were additional partial responses in the remaining four patients. However, one of these patients (with CD19-dim/negative disease) progressed.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said.
“[This is a] very small [study with] preliminary data but very exciting that it is safe to give checkpoint inhibitors with CAR T cells, and it may be efficacious at getting the immune response back.”
Tisagenlecleucel follow-up
One of the two tisagenlecleucel updates (abstract 895) consists of data from the ELIANA trial, which includes pediatric and young adult patients with relapsed/refractory ALL.
The overall response rate was 82% (65/79). Of the 65 responders, 29 were still in response at follow-up.
The probability of relapse-free survival was 66% at 12 months and 18 months.
“These are some very fast-growing tumors, and these are refractory, resistant patients, so, as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
The other tisagenlecleucel update (abstract 1684) is from the JULIET trial, which includes adults with relapsed or refractory DLBCL (n=99).
The overall response rate was 54%. The probability of relapse-free survival was 66% at 6 months and 64% at both 12 months and 18 months.
HSCT consolidation
Dr. Brodsky also discussed long-term follow-up from a phase 1/2 trial of SCRI-CAR19v1, a CD19-specific CAR T-cell product, in patients with relapsed/refractory ALL (abstract 967).
Of the 50 evaluable patients, 17 had no history of HSCT prior to CAR T-cell therapy.
Three of the 17 patients did not proceed to HSCT after CAR T-cell therapy, and two of these patients relapsed. Of the 14 patients who did undergo HSCT after CAR T-cell therapy, two relapsed.
There were 33 patients with a prior history of HSCT, and 10 of them had another HSCT after CAR T-cell therapy. Five of them are still alive and in remission.
Of the 23 patients who did not undergo another HSCT, eight are still in remission.
“This study is very small, and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Several studies set to be presented at the 2018 ASH Annual Meeting provide new insights regarding chimeric antigen receptor (CAR) T-cell therapies.
One study suggests ibrutinib may enhance CAR T-cell therapy in patients with chronic lymphocytic leukemia (CLL), and another suggests checkpoint inhibitors can augment CAR T-cell therapy in certain patients with B-cell acute lymphoblastic leukemia (ALL).
Two additional studies indicate that responses to tisagenlecleucel are durable in both ALL and diffuse large B-cell lymphoma (DLBCL).
A fifth study suggests hematopoietic stem cell transplant (HSCT) may reduce the risk of relapse after CAR T-cell therapy.
ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland, discussed these studies during a media briefing ahead of the ASH Annual Meeting.
Ibrutinib
In the ibrutinib study (abstract 299), patients received the BTK inhibitor starting 2 weeks prior to leukapheresis and continued until 3 months after treatment with JCAR014.
Data suggest this strategy may improve responses and decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory CLL.
Responses occurred in 88% of patients who received ibrutinib and 56% of those who did not.
Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients (26%) in the no-ibrutinib cohort and 0 of 17 patients in the ibrutinib cohort.
These findings are “early and preliminary but very exciting” Dr. Brodsky said.
Checkpoint inhibitors
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy.
The 14 patients studied had early CAR T-cell loss, partial response, or no response to CAR T-cell therapy. Thirteen patients had B-cell ALL, and one had B lymphoblastic lymphoma.
CD19-directed CAR T-cell therapy consisted of tisagenlecleucel in four patients and CTL119 in 10. Thirteen patients received pembrolizumab, and one received nivolumab.
Three of six patients who had early B-cell recovery re-established B-cell aplasia with the addition of a checkpoint inhibitor. In two patients, B-cell aplasia persists with ongoing pembrolizumab.
Four patients who did not respond to or relapsed after their initial CAR T-cell therapy had a partial (n=2) or complete response (n=2) with the addition of pembrolizumab.
There were additional partial responses in the remaining four patients. However, one of these patients (with CD19-dim/negative disease) progressed.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said.
“[This is a] very small [study with] preliminary data but very exciting that it is safe to give checkpoint inhibitors with CAR T cells, and it may be efficacious at getting the immune response back.”
Tisagenlecleucel follow-up
One of the two tisagenlecleucel updates (abstract 895) consists of data from the ELIANA trial, which includes pediatric and young adult patients with relapsed/refractory ALL.
The overall response rate was 82% (65/79). Of the 65 responders, 29 were still in response at follow-up.
The probability of relapse-free survival was 66% at 12 months and 18 months.
“These are some very fast-growing tumors, and these are refractory, resistant patients, so, as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
The other tisagenlecleucel update (abstract 1684) is from the JULIET trial, which includes adults with relapsed or refractory DLBCL (n=99).
The overall response rate was 54%. The probability of relapse-free survival was 66% at 6 months and 64% at both 12 months and 18 months.
HSCT consolidation
Dr. Brodsky also discussed long-term follow-up from a phase 1/2 trial of SCRI-CAR19v1, a CD19-specific CAR T-cell product, in patients with relapsed/refractory ALL (abstract 967).
Of the 50 evaluable patients, 17 had no history of HSCT prior to CAR T-cell therapy.
Three of the 17 patients did not proceed to HSCT after CAR T-cell therapy, and two of these patients relapsed. Of the 14 patients who did undergo HSCT after CAR T-cell therapy, two relapsed.
There were 33 patients with a prior history of HSCT, and 10 of them had another HSCT after CAR T-cell therapy. Five of them are still alive and in remission.
Of the 23 patients who did not undergo another HSCT, eight are still in remission.
“This study is very small, and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
CHMP backs blinatumomab for MRD
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
Diet change can improve survival in obese mice with ALL
Switching to a low-fat diet can improve survival in obese mice with acute lymphoblastic leukemia (ALL), according to new research.
Diet-induced obese (DIO) mice with ALL had a survival rate of 17% if they remained on a high-fat diet while treated with vincristine, but survival rose to 92% for mice that were switched to a low-fat diet before treatment.
However, the dietary switch did not impact the survival of DIO mice treated with dexamethasone or L-asparaginase monotherapy.
Researchers reported these findings in Cancer & Metabolism.
“The most exciting thing, to me, about this study is the fact that this shows that a dietary intervention could potentially help us kill leukemia cells in children with acute lymphoblastic leukemia,” said study author Steven Mittelman, MD, PhD, of the University of Southern California, Los Angeles.
“The current treatments for leukemia are very toxic, so finding a way to use a healthy diet, without increasing the toxicity of therapy to treat people with cancer, would be incredible.”
Building on previous research that showed obesity reduced the effectiveness of chemotherapeutic drugs in children with leukemia, the researchers tested whether a dietary intervention could improve ALL outcomes in obese mice.
Methods
The team used NOD/SCID IL2-receptor gamma chain knockout mice raised on either a 60% or 10% fat-calorie diet. Only male mice were studied because female mice do not become as significantly obese on the high-fat diet.
The researchers implanted GFP+ pre-B-cell ALL transgenic mouse cells into DIO and control mice at about 20 weeks old.
Six to seven days after ALL implantation, the researchers randomized the DIO mice to continue their high-fat diet or switch to the control diet (10% calories from fat).
In some experiments, mice received monotherapy with vincristine, and other experiments used L-asparaginase or dexamethasone.
In additional experiments, the researchers implanted DIO and control mice with patient-derived ALL cells with normal karyotype.
After 17 days of engraftment, the researchers switched half the DIO mice to the control diet. The next day, they treated these mice with vincristine, L-asparaginase, or dexamethasone for 4 weeks.
The team monitored the mice daily for food intake, body weight, and onset of progressive leukemia.
Results
Diet had no impact on DIO or control mice that did not receive chemotherapy. Time to progression was the same in these mice.
When vincristine was started on day 7 after ALL implantation, DIO mice switched to the low-fat diet had the best survival, even better than the control mice.
Overall survival was 92% for the DIO mice that switched diets, 42% for the control group, and 17% for the DIO group maintained on the high-fat diet.
Survival experiments performed using L-asparaginase or dexamethasone monotherapy showed no impact of switching diets. The researchers reported there was “no detectable effect on survival in these experiments.”
The team believes this is the first study to test a diet intervention on treatment outcome in hematologic malignancy.
The researchers plan to study dietary intervention further in both obese and non-obese patients.
This study was supported by the National Cancer Institute and funds from the Saban Research Institute, Children’s Hospital of Los Angeles. The researchers had no competing interests to declare.
Switching to a low-fat diet can improve survival in obese mice with acute lymphoblastic leukemia (ALL), according to new research.
Diet-induced obese (DIO) mice with ALL had a survival rate of 17% if they remained on a high-fat diet while treated with vincristine, but survival rose to 92% for mice that were switched to a low-fat diet before treatment.
However, the dietary switch did not impact the survival of DIO mice treated with dexamethasone or L-asparaginase monotherapy.
Researchers reported these findings in Cancer & Metabolism.
“The most exciting thing, to me, about this study is the fact that this shows that a dietary intervention could potentially help us kill leukemia cells in children with acute lymphoblastic leukemia,” said study author Steven Mittelman, MD, PhD, of the University of Southern California, Los Angeles.
“The current treatments for leukemia are very toxic, so finding a way to use a healthy diet, without increasing the toxicity of therapy to treat people with cancer, would be incredible.”
Building on previous research that showed obesity reduced the effectiveness of chemotherapeutic drugs in children with leukemia, the researchers tested whether a dietary intervention could improve ALL outcomes in obese mice.
Methods
The team used NOD/SCID IL2-receptor gamma chain knockout mice raised on either a 60% or 10% fat-calorie diet. Only male mice were studied because female mice do not become as significantly obese on the high-fat diet.
The researchers implanted GFP+ pre-B-cell ALL transgenic mouse cells into DIO and control mice at about 20 weeks old.
Six to seven days after ALL implantation, the researchers randomized the DIO mice to continue their high-fat diet or switch to the control diet (10% calories from fat).
In some experiments, mice received monotherapy with vincristine, and other experiments used L-asparaginase or dexamethasone.
In additional experiments, the researchers implanted DIO and control mice with patient-derived ALL cells with normal karyotype.
After 17 days of engraftment, the researchers switched half the DIO mice to the control diet. The next day, they treated these mice with vincristine, L-asparaginase, or dexamethasone for 4 weeks.
The team monitored the mice daily for food intake, body weight, and onset of progressive leukemia.
Results
Diet had no impact on DIO or control mice that did not receive chemotherapy. Time to progression was the same in these mice.
When vincristine was started on day 7 after ALL implantation, DIO mice switched to the low-fat diet had the best survival, even better than the control mice.
Overall survival was 92% for the DIO mice that switched diets, 42% for the control group, and 17% for the DIO group maintained on the high-fat diet.
Survival experiments performed using L-asparaginase or dexamethasone monotherapy showed no impact of switching diets. The researchers reported there was “no detectable effect on survival in these experiments.”
The team believes this is the first study to test a diet intervention on treatment outcome in hematologic malignancy.
The researchers plan to study dietary intervention further in both obese and non-obese patients.
This study was supported by the National Cancer Institute and funds from the Saban Research Institute, Children’s Hospital of Los Angeles. The researchers had no competing interests to declare.
Switching to a low-fat diet can improve survival in obese mice with acute lymphoblastic leukemia (ALL), according to new research.
Diet-induced obese (DIO) mice with ALL had a survival rate of 17% if they remained on a high-fat diet while treated with vincristine, but survival rose to 92% for mice that were switched to a low-fat diet before treatment.
However, the dietary switch did not impact the survival of DIO mice treated with dexamethasone or L-asparaginase monotherapy.
Researchers reported these findings in Cancer & Metabolism.
“The most exciting thing, to me, about this study is the fact that this shows that a dietary intervention could potentially help us kill leukemia cells in children with acute lymphoblastic leukemia,” said study author Steven Mittelman, MD, PhD, of the University of Southern California, Los Angeles.
“The current treatments for leukemia are very toxic, so finding a way to use a healthy diet, without increasing the toxicity of therapy to treat people with cancer, would be incredible.”
Building on previous research that showed obesity reduced the effectiveness of chemotherapeutic drugs in children with leukemia, the researchers tested whether a dietary intervention could improve ALL outcomes in obese mice.
Methods
The team used NOD/SCID IL2-receptor gamma chain knockout mice raised on either a 60% or 10% fat-calorie diet. Only male mice were studied because female mice do not become as significantly obese on the high-fat diet.
The researchers implanted GFP+ pre-B-cell ALL transgenic mouse cells into DIO and control mice at about 20 weeks old.
Six to seven days after ALL implantation, the researchers randomized the DIO mice to continue their high-fat diet or switch to the control diet (10% calories from fat).
In some experiments, mice received monotherapy with vincristine, and other experiments used L-asparaginase or dexamethasone.
In additional experiments, the researchers implanted DIO and control mice with patient-derived ALL cells with normal karyotype.
After 17 days of engraftment, the researchers switched half the DIO mice to the control diet. The next day, they treated these mice with vincristine, L-asparaginase, or dexamethasone for 4 weeks.
The team monitored the mice daily for food intake, body weight, and onset of progressive leukemia.
Results
Diet had no impact on DIO or control mice that did not receive chemotherapy. Time to progression was the same in these mice.
When vincristine was started on day 7 after ALL implantation, DIO mice switched to the low-fat diet had the best survival, even better than the control mice.
Overall survival was 92% for the DIO mice that switched diets, 42% for the control group, and 17% for the DIO group maintained on the high-fat diet.
Survival experiments performed using L-asparaginase or dexamethasone monotherapy showed no impact of switching diets. The researchers reported there was “no detectable effect on survival in these experiments.”
The team believes this is the first study to test a diet intervention on treatment outcome in hematologic malignancy.
The researchers plan to study dietary intervention further in both obese and non-obese patients.
This study was supported by the National Cancer Institute and funds from the Saban Research Institute, Children’s Hospital of Los Angeles. The researchers had no competing interests to declare.
Prognostic features could improve ALL outcomes
CHICAGO – New recognition of the prognostic value of cytogenetic factors, minimal residual disease activity, and Philadelphia chromosome–like signature could improve the treatment and outcomes of acute lymphoblastic leukemia (ALL), according to Anjali Advani, MD.
CD20
About 80% of ALL is B-cell ALL and the majority of patients have pre–B-cell ALL, Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“And on the B lymphoblast, many antigens are expressed, including CD19, CD20, and CD52,” she said.
Rituximab, a drug often used for the treatment of lymphoma, is a chimeric monoclonal antibody against the protein CD20, which is expressed in 41% of ALL patients.
“Interestingly, CD20 expression in ALL has been associated with an adverse prognostic impact, which suggests that targeting this may potentially improve outcomes in these patients,” Dr. Advani said.
In fact, a recent randomized study by Sébastien Maury, MD, of the University of Paris-Est, and his colleagues, demonstrated that adding 16-18 doses of rituximab to a Berlin-Frankfurt-Münster (BFM)–based chemotherapy in Philadelphia chromosome (Ph)–negative patients aged 18-59 years with CD20-positive pre–B-cell ALL improved 2-year event-free survival from 52% to 65%. The data are consistent with those from prior studies, including a German study that showed a higher degree of minimal residual disease (MRD) negativity in patients treated with rituximab, she noted (N Engl J Med 2016;375:1044-53).
While the study by Dr. Maury and his colleagues didn’t look at MRD, that may offer an explanation for the improved event-free survival in their study, Dr. Advani suggested.
MRD
Minimal residual disease has become a standard part of practice in ALL, but pediatric ALL led the way in using early MRD measurement for risk-stratifying therapy, and it has taken a bit longer for it to be incorporated in the adult disease realm, Dr. Advani said.
Either flow cytometry or polymerase chain reaction (PCR) amplification can be used to measure MRD, she noted.
In one of the larger studies done in adults, researchers used PCR to look at MRD at two time points and stratified patients into three risk groups, including low, intermediate, and high risk (Blood. 2006 Feb 1;107:1116-23).
Measuring MRD at those two time points “clearly separated the prognosis of patients not only in terms of disease-free survival but [in] overall survival,” she said. “So that’s why, for ALL, this has really become very important.”
In the United States, where flow-based cytometry is used more, it is necessary to find a properly equipped laboratory that can provide reliable results, she added. “For example, at our center we actually send our MRD to Fred Hutchinson [Cancer Center in Seattle].”
Johns Hopkins [Baltimore] also has such a lab, and both can arrange to accept send-outs, she said.
The other “really exciting thing” in regard to MRD in ALL is the recent approval of blinatumomab for MRD-positive ALL, she said.
In a study of 113 evaluable patients who were treated with the monoclonal antibody, 78% achieved complete molecular response (Blood. 2018 Apr 5;131:1522-31).
“And probably most importantly, when they looked at those patients who responded to blinatumomab in terms of MRD, these patients had, again, not only improved relapse-free survival but also increased overall survival,” she said. “I think this really explains why in ALL, we are measuring MRD and how it can really impact these patients.”
One of the remaining questions that will be important to address going forward is whether patients with MRD-positive ALL should continue to be considered for transplant; some of these studies have shown “very, very good outcomes” in patients who have not been transplanted, she noted.
Ph-like signature
Another important new prognostic feature in ALL is the presence of the Ph-like signature, a gene expression signature that was initially described in children with poor-risk ALL, and which looks a lot like Ph-positive disease.
“When they delved in further, they identified that this signature actually correlated with multiple different [kinase] fusions ... and it turns out that 20%-25% of young adults have this signature,” she said.
Since event-free survival in young adults with ALL is usually in the 65%-70% range, most of the remaining 30%-35% likely have this signature, she explained.
The kinase fusions associated with the Ph-like signature retain intact tyrosine kinase domains, and the spectrum of the fusions changes across age groups.
Treatments targeting some of these – for example, dasatinib for patients with a Ph-like dasatinib-sensitive kinase mutation – are being investigated.
Additionally, the Children’s Oncology Group has developed a clinically adaptable screening assay to identify the signature, she noted.
“So I would say that, and there is probably some difference in opinion, it is now becoming fairly standard that at diagnosis in an adult with ALL we’re sending the Ph-like signature,” she said. “And again, usually you’re going to have to send this out, and at our center we send it out to [Nationwide Children’s Hospital] in Columbus [Ohio].”
As important as it is to identify the Ph-like signature, given its association with poor prognosis, a number of questions remain, including whether transplant improves outcomes.
“The hope is it probably does, and that’s something that’s being evaluated in studies,” she said, noting that clinical studies are also specifically targeting these patients.
“So these patients should probably be enrolled on a clinical trial, because their outcome is clearly inferior,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
CHICAGO – New recognition of the prognostic value of cytogenetic factors, minimal residual disease activity, and Philadelphia chromosome–like signature could improve the treatment and outcomes of acute lymphoblastic leukemia (ALL), according to Anjali Advani, MD.
CD20
About 80% of ALL is B-cell ALL and the majority of patients have pre–B-cell ALL, Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“And on the B lymphoblast, many antigens are expressed, including CD19, CD20, and CD52,” she said.
Rituximab, a drug often used for the treatment of lymphoma, is a chimeric monoclonal antibody against the protein CD20, which is expressed in 41% of ALL patients.
“Interestingly, CD20 expression in ALL has been associated with an adverse prognostic impact, which suggests that targeting this may potentially improve outcomes in these patients,” Dr. Advani said.
In fact, a recent randomized study by Sébastien Maury, MD, of the University of Paris-Est, and his colleagues, demonstrated that adding 16-18 doses of rituximab to a Berlin-Frankfurt-Münster (BFM)–based chemotherapy in Philadelphia chromosome (Ph)–negative patients aged 18-59 years with CD20-positive pre–B-cell ALL improved 2-year event-free survival from 52% to 65%. The data are consistent with those from prior studies, including a German study that showed a higher degree of minimal residual disease (MRD) negativity in patients treated with rituximab, she noted (N Engl J Med 2016;375:1044-53).
While the study by Dr. Maury and his colleagues didn’t look at MRD, that may offer an explanation for the improved event-free survival in their study, Dr. Advani suggested.
MRD
Minimal residual disease has become a standard part of practice in ALL, but pediatric ALL led the way in using early MRD measurement for risk-stratifying therapy, and it has taken a bit longer for it to be incorporated in the adult disease realm, Dr. Advani said.
Either flow cytometry or polymerase chain reaction (PCR) amplification can be used to measure MRD, she noted.
In one of the larger studies done in adults, researchers used PCR to look at MRD at two time points and stratified patients into three risk groups, including low, intermediate, and high risk (Blood. 2006 Feb 1;107:1116-23).
Measuring MRD at those two time points “clearly separated the prognosis of patients not only in terms of disease-free survival but [in] overall survival,” she said. “So that’s why, for ALL, this has really become very important.”
In the United States, where flow-based cytometry is used more, it is necessary to find a properly equipped laboratory that can provide reliable results, she added. “For example, at our center we actually send our MRD to Fred Hutchinson [Cancer Center in Seattle].”
Johns Hopkins [Baltimore] also has such a lab, and both can arrange to accept send-outs, she said.
The other “really exciting thing” in regard to MRD in ALL is the recent approval of blinatumomab for MRD-positive ALL, she said.
In a study of 113 evaluable patients who were treated with the monoclonal antibody, 78% achieved complete molecular response (Blood. 2018 Apr 5;131:1522-31).
“And probably most importantly, when they looked at those patients who responded to blinatumomab in terms of MRD, these patients had, again, not only improved relapse-free survival but also increased overall survival,” she said. “I think this really explains why in ALL, we are measuring MRD and how it can really impact these patients.”
One of the remaining questions that will be important to address going forward is whether patients with MRD-positive ALL should continue to be considered for transplant; some of these studies have shown “very, very good outcomes” in patients who have not been transplanted, she noted.
Ph-like signature
Another important new prognostic feature in ALL is the presence of the Ph-like signature, a gene expression signature that was initially described in children with poor-risk ALL, and which looks a lot like Ph-positive disease.
“When they delved in further, they identified that this signature actually correlated with multiple different [kinase] fusions ... and it turns out that 20%-25% of young adults have this signature,” she said.
Since event-free survival in young adults with ALL is usually in the 65%-70% range, most of the remaining 30%-35% likely have this signature, she explained.
The kinase fusions associated with the Ph-like signature retain intact tyrosine kinase domains, and the spectrum of the fusions changes across age groups.
Treatments targeting some of these – for example, dasatinib for patients with a Ph-like dasatinib-sensitive kinase mutation – are being investigated.
Additionally, the Children’s Oncology Group has developed a clinically adaptable screening assay to identify the signature, she noted.
“So I would say that, and there is probably some difference in opinion, it is now becoming fairly standard that at diagnosis in an adult with ALL we’re sending the Ph-like signature,” she said. “And again, usually you’re going to have to send this out, and at our center we send it out to [Nationwide Children’s Hospital] in Columbus [Ohio].”
As important as it is to identify the Ph-like signature, given its association with poor prognosis, a number of questions remain, including whether transplant improves outcomes.
“The hope is it probably does, and that’s something that’s being evaluated in studies,” she said, noting that clinical studies are also specifically targeting these patients.
“So these patients should probably be enrolled on a clinical trial, because their outcome is clearly inferior,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
CHICAGO – New recognition of the prognostic value of cytogenetic factors, minimal residual disease activity, and Philadelphia chromosome–like signature could improve the treatment and outcomes of acute lymphoblastic leukemia (ALL), according to Anjali Advani, MD.
CD20
About 80% of ALL is B-cell ALL and the majority of patients have pre–B-cell ALL, Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“And on the B lymphoblast, many antigens are expressed, including CD19, CD20, and CD52,” she said.
Rituximab, a drug often used for the treatment of lymphoma, is a chimeric monoclonal antibody against the protein CD20, which is expressed in 41% of ALL patients.
“Interestingly, CD20 expression in ALL has been associated with an adverse prognostic impact, which suggests that targeting this may potentially improve outcomes in these patients,” Dr. Advani said.
In fact, a recent randomized study by Sébastien Maury, MD, of the University of Paris-Est, and his colleagues, demonstrated that adding 16-18 doses of rituximab to a Berlin-Frankfurt-Münster (BFM)–based chemotherapy in Philadelphia chromosome (Ph)–negative patients aged 18-59 years with CD20-positive pre–B-cell ALL improved 2-year event-free survival from 52% to 65%. The data are consistent with those from prior studies, including a German study that showed a higher degree of minimal residual disease (MRD) negativity in patients treated with rituximab, she noted (N Engl J Med 2016;375:1044-53).
While the study by Dr. Maury and his colleagues didn’t look at MRD, that may offer an explanation for the improved event-free survival in their study, Dr. Advani suggested.
MRD
Minimal residual disease has become a standard part of practice in ALL, but pediatric ALL led the way in using early MRD measurement for risk-stratifying therapy, and it has taken a bit longer for it to be incorporated in the adult disease realm, Dr. Advani said.
Either flow cytometry or polymerase chain reaction (PCR) amplification can be used to measure MRD, she noted.
In one of the larger studies done in adults, researchers used PCR to look at MRD at two time points and stratified patients into three risk groups, including low, intermediate, and high risk (Blood. 2006 Feb 1;107:1116-23).
Measuring MRD at those two time points “clearly separated the prognosis of patients not only in terms of disease-free survival but [in] overall survival,” she said. “So that’s why, for ALL, this has really become very important.”
In the United States, where flow-based cytometry is used more, it is necessary to find a properly equipped laboratory that can provide reliable results, she added. “For example, at our center we actually send our MRD to Fred Hutchinson [Cancer Center in Seattle].”
Johns Hopkins [Baltimore] also has such a lab, and both can arrange to accept send-outs, she said.
The other “really exciting thing” in regard to MRD in ALL is the recent approval of blinatumomab for MRD-positive ALL, she said.
In a study of 113 evaluable patients who were treated with the monoclonal antibody, 78% achieved complete molecular response (Blood. 2018 Apr 5;131:1522-31).
“And probably most importantly, when they looked at those patients who responded to blinatumomab in terms of MRD, these patients had, again, not only improved relapse-free survival but also increased overall survival,” she said. “I think this really explains why in ALL, we are measuring MRD and how it can really impact these patients.”
One of the remaining questions that will be important to address going forward is whether patients with MRD-positive ALL should continue to be considered for transplant; some of these studies have shown “very, very good outcomes” in patients who have not been transplanted, she noted.
Ph-like signature
Another important new prognostic feature in ALL is the presence of the Ph-like signature, a gene expression signature that was initially described in children with poor-risk ALL, and which looks a lot like Ph-positive disease.
“When they delved in further, they identified that this signature actually correlated with multiple different [kinase] fusions ... and it turns out that 20%-25% of young adults have this signature,” she said.
Since event-free survival in young adults with ALL is usually in the 65%-70% range, most of the remaining 30%-35% likely have this signature, she explained.
The kinase fusions associated with the Ph-like signature retain intact tyrosine kinase domains, and the spectrum of the fusions changes across age groups.
Treatments targeting some of these – for example, dasatinib for patients with a Ph-like dasatinib-sensitive kinase mutation – are being investigated.
Additionally, the Children’s Oncology Group has developed a clinically adaptable screening assay to identify the signature, she noted.
“So I would say that, and there is probably some difference in opinion, it is now becoming fairly standard that at diagnosis in an adult with ALL we’re sending the Ph-like signature,” she said. “And again, usually you’re going to have to send this out, and at our center we send it out to [Nationwide Children’s Hospital] in Columbus [Ohio].”
As important as it is to identify the Ph-like signature, given its association with poor prognosis, a number of questions remain, including whether transplant improves outcomes.
“The hope is it probably does, and that’s something that’s being evaluated in studies,” she said, noting that clinical studies are also specifically targeting these patients.
“So these patients should probably be enrolled on a clinical trial, because their outcome is clearly inferior,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
EXPERT ANALYSIS FROM MHM 2018
Report details financial burden of blood cancers
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).
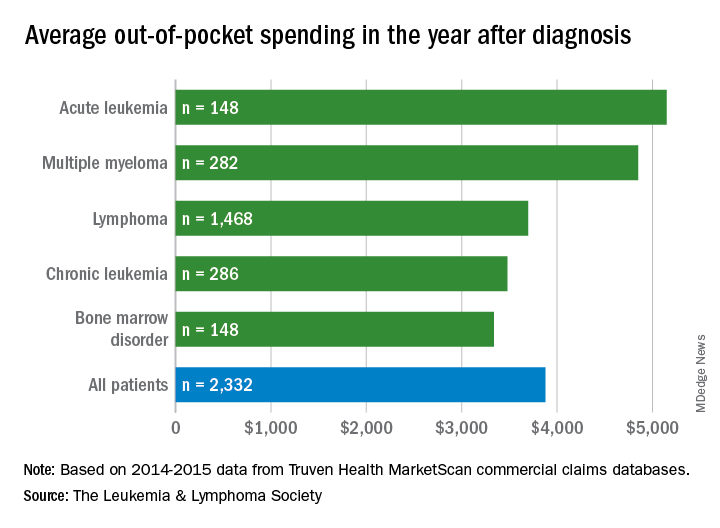
Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
Team tracks changes in height, weight in pediatric ALL
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
Cost-effectiveness of CAR T-cell therapy
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.