User login
Will finding the depression−inflammation link lead to tailored treatments for MDD?
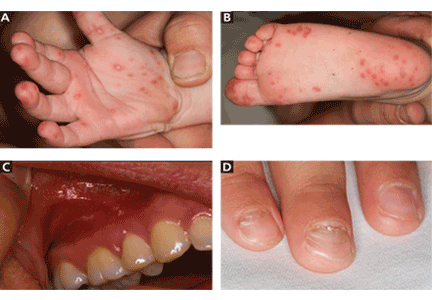
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
Sedative-hypnotics for sleepless geriatric patients
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
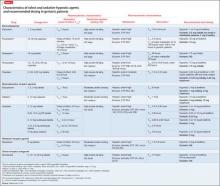
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
How to document SUICIDE risk
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
How do you score on this self-assessment of suicide risk management?: First of 2 parts
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
Management of Gastroenteropancreatic Neuroendocrine Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Radon and lung cancer: Assessing and mitigating the risk
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
KEY POINTS
- Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232.
- Radon 222 decays to polonium 218 and then, after several intermediate steps, to polonium 214, both of which emit alpha particles, which are highly effective in damaging tissues.
- Radon exposure is associated with increased lung cancer incidence in underground miners. In the general population, it is estimated to be the second most common cause of lung cancer, after cigarette smoking.
- There is no evidence yet of a benefit of lung cancer screening based on radon exposure.
The protein-sparing modified fast for obese patients with type 2 diabetes: What to expect
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
KEY POINTS
- The PSMF is indicated in patients who have a body mass index (BMI) of 30 kg/m2 or more, or a BMI of 27 kg/m2 or more with one or more comorbidities such as type 2 diabetes.
- The PSMF provides less than 800 kcal/day during an initial intensive phase of about 6 months, with gradual reintroduction of calories during a refeeding phase lasting 6 to 8 weeks.
- Patients on the PSMF under medical supervision rapidly lose fat while maintaining lean body mass.
- Unfortunately, many patients tend to regain weight after completing a PSMF program. Additional strategies are needed to maintain weight loss.
Hand, foot, and mouth disease: Identifying and managing an acute viral syndrome
Hand, foot, and mouth disease (HFMD) is typically a benign childhood infection—except when it isn’t so benign or when it occurs in adults.
The usual presentation is in a child with fever, oral ulcerations, and papules on the palms of the hands and the soles of the feet.1 However, severe complications can occur, including central nervous system involvement and cardiopulmonary failure, and can lead to significant morbidity and even death.2 Fortunately, these complications are rare.
Less common in North America than in other regions, HFMD has recurrently broken out in many areas of Southern Asia and the surrounding Pacific region. However, several North American outbreaks have been documented in recent years and have affected unexpected numbers of immunocompetent adults, demonstrating that this disease is of worldwide importance in adults as well as children.3
Because HFMD has the potential to reach epidemic levels in the United States, early recognition is paramount, and primary care physicians need to be familiar with its common signs and symptoms.
USUALLY A SUMMER DISEASE
HFMD occurs all around the world, exhibiting seasonal variation in temperate climates. In these locations, individual cases and regional outbreaks usually occur in the spring, summer, and fall. No sexual predisposition has been documented. Most symptomatic cases are in children under the age of 10.
OUTBREAKS AROUND THE WORLD
The disease was first described more than 40 years ago, with several large outbreaks in the last 16 years.
1998—An outbreak in Taiwan affected more than 1.5 million people, mostly children. Severe cases numbered just over 400, and 78 children died.4
2008—China,5 Singapore,6 Vietnam,7 Mongolia,8 and Brunei9 were stricken with an outbreak that affected 30,000 people and led to more than 50 deaths.
2009—An outbreak in the Henan and Shandong provinces of eastern China killed 35 people.10
2010—In several southern Chinese regions, more than 70,000 people were infected, with almost 600 deaths.11
2011 to the present. The United States has had several outbreaks in the last 3 years. Although HFMD is not one of the diseases that must be reported to public health authorities in the United States, from November 2011 to February 2012 the US Centers for Disease Control and Prevention (CDC) received reports of 63 possible cases: 38 in Alabama, 17 in Nevada, 7 in California, and 1 in Connecticut.1 Fifteen of the patients were adults, and more than half had contacts who were sick.
The most recent US outbreak, in Alabama,12 was atypical because it occurred in the winter.
CAUSED BY ENTEROVIRUSES
HFMD is caused by infection with a variety of viruses in the genus Enterovirus, a large group that in turn is part of the larger Picornaviridae family.13 The taxonomy of this genus is complicated and subject to revision; species include coxsackieviruses, polioviruses, enteroviruses, and echoviruses. They are all small, nonenveloped, single-stranded RNA viruses.
The most common strains that cause HFMD are coxsackievirus A16 and enterovirus 71. In addition, coxsackievirus A6 may be emerging, and many other coxsackievirus strains have been directly implicated, including A5, A7, A9, A10, B2, and B5.
Coxsackievirus A16 is the leading cause of HFMD.
Enterovirus 71 is the second most common cause of HFMD and has also caused outbreaks. It usually results in benign disease. However, among the causes of HFMD, it is associated with more prominent central nervous system involvement14 and is the most common cause of viral meningoencephalitis in children.
Coxsackievirus A6. In December 2011, the California Department of Public Health isolated a strain of coxsackievirus A6 that caused extensive rash and nail shedding.15 Among the 63 possible cases of HFMD reported to the CDC from November 2011 to February 2012, specimens for clinical testing were obtained in 34, and 25 of those demonstrated coxsackievirus A6 infection.3
FEVER, ORAL ULCERS, RASH ON HANDS AND FEET
The typical clinical manifestations of HFMD are fever, stomatitis with oral ulcers, and an exanthem affecting the palms, soles, and other parts of the body. These last less than 7 to 10 days, usually occur during the spring to fall months, and have a benign course.
The incubation period is 3 to 5 days, with a prodrome that may include fever, malaise, abdominal pain, and myalgia before the onset of oral and cutaneous findings. Painful oral ulcers may precede the exanthem and can result in dehydration.16
The cutaneous manifestation of HFMD is typically a papulovesicular rash affecting the palms, soles, and buttocks (Figure 1). Other sites may include the knees, elbows, and the dorsal surfaces of the hands and feet. The lesions may be maculopapular and can be either asymptomatic or tender and painful. Desquamation can follow the exanthem, and lesions usually resolve without scarring or secondary infection.16,17
Table 1 and Table 2 compare HFMD with other common illnesses that can cause similar skin and mucosal findings. In particular, herpangina has the identical clinical presentation as HFMD except that it does not cause skin lesions. It is caused by many of the same enteroviruses linked to HFMD.
Different viruses, different signs?
The numerous viruses that cause HFMD usually cause similar signs and symptoms during bouts of typical, self-limited disease. However, neurologic and cardiopulmonary involvement, which are fortunately rare, are more often associated with enterovirus 71 infection.
Nail manifestations are common in HFMD. Nail separation from the nail matrix (onychomadesis) was associated with coxsackievirus A6 infection during a 2010 outbreak of HFMD in Taiwan and in a 2009 outbreak in Finland.18 Moreover, this virus was cultured from a nail specimen in one patient, suggesting viral infiltration as the cause of nail-matrix arrest.19
Perioral skin eruptions, desquamation, and Beau lines have also been associated with coxsackievirus A6.18 Beau lines are transverse depressions of the nail, most evident in the central nail plate; when seen on multiple nails, they imply a systemic illness causing disruption of nail matrix growth.20
Atypical HFMD and coxsackievirus A6
Atypical HFMD has recently been described in connection with coxsackievirus A6. Lott et al21 reported five cases of coxsackievirus A6-associated HFMD in 2013. Atypically, three of the affected patients presented in winter months, two were adults, and two had widespread skin involvement.21
Mathes et al22 reported a series of 80 cases of enteroviral infections in which the lesions had a predilection for the antecubital and popliteal fossae and were similar in severity and distribution to those seen in eczema herpeticum or Kaposi varicelliform eruption in patients with and patients without a history of atopic dermatitis. They named this find-clinical finding of pronounced coxsackievirus-associated skin disease at sites previously affected by atopic dermatitis.
Additional cutaneous findings of coxsackievirus A6 infection may include onychomadesis, Beau lines, and vesiculobullous lesions. Patients with atypical, coxsackievirus A6-associated HFMD may not have oral lesions.23
In the five cases reported by Lott et al,21 significant systemic symptoms (fever, chills, diarrhea, and myalgias) led all but one of the patients to seek care in an emergency department. However, atypical HFMD has not been associated with life-threatening illness.
Atypical HFMD associated with coxsackievirus A6 is an emerging entity in the United States, and the acuity of both cutaneous and systemic symptoms poses a diagnostic dilemma. Furthermore, infection has been documented in immunocompetent adults.23 Familiarity with the clinical findings may expedite appropriate care, prevent spread to contacts, and avoid unnecessary testing.
Neurologic and cardiopulmonary manifestations
Enteroviruses are the most common causes of viral meningoencephalitis in the United States. They mainly affect children and cause serious and potentially chronic disease in those with humoral immunodeficiencies.24 Neurologic and cardiopulmonary manifestations of HFMD are varied and extremely rare in the United States but should always be viewed clinically as signs of concern and severe disease.
Signs of potentially fatal disease that have been observed in young children include tachycardia, tachypnea, hypotension, hypertension, gastrointestinal bleeding, neurologic symptoms, leukocytosis, absence of oral lesions, and vomiting.2 Signs of dysautonomia, myoclonus, ataxia, and brainstem involvement may portend fatal disease in which rapid decompensation is the result of cardiogenic shock due to loss of ventricular contractility, causing pulmonary edema and end-organ dysfunction.16
Neurologic manifestations associated with enterovirus 71 infection include aseptic meningitis, a poliomyelitis-like syndrome, brainstem encephalitis, neurogenic pulmonary edema, opsoclonus-myoclonus syndrome, cerebellar ataxia, Guillain-Barré syndrome, and transverse myelitis.
Because some patients who have neurologic disease respond to treatment with high-dose methylprednisolone and intravenous immune globulin, there is reason to suspect that an autoimmune phenomenon triggered by the culprit enterovirus may be the cause of many of the neurologic symptoms.25
A 2012 meta-analysis26 found that an elevated white blood cell count and hyperglycemia could be clinically useful in distinguishing benign from severe HFMD. In patients with benign HFMD, white blood cell counts and blood glucose values were no different from those in healthy controls.26
DIAGNOSIS IS USUALLY CLINICAL
Most enteroviral infections are asymptomatic, but HFMD is a possibility if a patient has mild illness, fever, and a maculopapular or vesicular rash on the palms of the hands and soles of the feet, sometimes associated with oral ulcers (herpangina). Skin lesions can also be found on the legs, face, buttocks, and trunk.
In the United States, HFMD most commonly occurs in children under age 4 and is usually caused by coxsackievirus A16. Adults can also be affected, especially if they were in contact with children in child care, which was the case in approximately half of nonpediatric patients who tested positive for HFMD during an outbreak in several states between November 2011 and February 2012.3
The clinical characteristics of HFMD caused by enterovirus 71 may be somewhat different, with smaller vesicles, diffuse erythema of the trunk and limbs, and higher fever (temperature ≥ 39°C [102.2°F] for more than 3 days).27 However, the rash of coxsackievirus A16 HFMD may be more extensive and severe.
Other clinical manifestations of HFMD include nail dystrophies such as Beau lines and nail shedding, hyperglycemia, dehydration, and more serious and potentially life-threatening complications such as pulmonary edema28 and viral meningoencephalitis.29
Laboratory testing
In mild cases of HFMD, particularly in patients with a high probability of having the disease based on their clinical characteristics and sick contacts, laboratory testing is not necessary. Testing is usually reserved for severe cases and public health investigation of outbreaks.
Viral culture is the gold standard for diagnosing HFMD, but the final results can take nearly a week.
Polymerase chain reaction testing is faster, with a turnaround time of less than 1 day. It identifies viral RNA and is highly sensitive for detecting central nervous system infection.30
Where should samples be collected? Serum viremia precedes invasion of the skin and mucous membranes, so plasma can be tested. Inside the body, enteroviruses initially replicate in the gastrointestinal tract, although collecting a rectal swab or a stool sample is somewhat invasive. Further, in an enterovirus 71 epidemic in Taiwan, 93% of the patients had positive throat swabs, but only 30% tested positive by rectal swabs or analysis of the feces.27 At present, throat and vesicle specimens are considered to be the most useful sources for diagnostic purposes.16
ELISAs. Newly developed IgM-capture enzyme-linked immunosorbent assays (ELISAs) for coxsackievirus A16 and enterovirus 71 appear quite promising for diagnosing HFMD. These tests are inexpensive and detect IgM antibodies early and in a high percentage of patients. In the first week of the disease, the IgM detection rate was found to be 90.2% for enterovirus 71 and 68% for coxsackievirus A16.31
Cross-reactivity between these two viruses was a problem with ELISA testing in the past, causing false-positive results for enterovirus 71 in patients who in fact had coxsackievirus A16. The problem appears to be resolved in new versions that use specific enterovirus 71 proteins, eg, VP1.32
RECOGNITION AND PREVENTION ARE THE BEST MEDICINE
Recognizing HFMD early is crucial, because making the clinical diagnosis can identify patients who have signs of severe disease and can help protect future contacts and decrease the risk of an epidemic.
Infected patients continue to shed the virus for a long time, making hand hygiene and environmental control measures in health care settings and daycare centers of vital importance, to prevent spread of the infection.
Enteroviruses are stable in the environment and therefore capable of fecal-oral and oral-oral transmission. Humans are the only known natural hosts. No chemoprophylaxis or vaccination has been established to prevent HFMD. The recurrence of large-scale epidemics in the developing world is perhaps explained by ineffective sewage treatment and limited access to clean drinking water, especially in light of the fecal-oral spread of the virus. Intrafamilial spread of HFMD has been shown to be an important means of disease transmission, and asymptomatic adult carriers of these viruses may spread it to young children.33
The different viruses that cause HFMD result in a similar clinical presentation in most patients. Therefore, identifying HFMD caused by enterovirus 71, which carries a risk of severe and even fatal disease in young children vs a virus such as coxsackievirus A16, can be very difficult in practice without virologic testing. Thus, when diagnosed with HFMD, patients should be counseled to control all variables that could lead to further spread of the disease.
An analysis of epidemics in Asia suggested that public health awareness may have averted deaths in successive epidemics, highlighting the need to identify HFMD epidemics in communities and to educate patients and families about measures to prevent further spread of the virus in addition to standard supportive care.34
The CDC recommends35:
- Frequent hand-washing after toileting and changing diapers
- Disinfecting frequently used surfaces and objects, including toys
- Avoiding close contact with infected individuals and sharing of personal items such as utensils and cups.
These measures should be recommended to all affected patients.35
NO PROVEN ANTIVIRAL TREATMENT
No proven antiviral treatment exists for HFMD. Thus, the goals of treatment are typically supportive, as for any self-limited viral syndrome.16
Does acyclovir help? Shelley et al36 treated 13 patients (12 children and 1 adult) with acyclovir within 1 to 2 days of the onset of the HFMD rash and reported that it was beneficial, with significant relief of fever and skin lesions within 24 hours of starting therapy. These anecdotal results have not been replicated, and acyclovir is not an established treatment for HFMD.
If acyclovir does help, how does it work? Acyclovir, like other common antiviral medications, inactivates thymidine kinase, an enzyme produced by herpesviruses but not by HFMD-causing viruses like coxsackievirus A16. Shelley et al proposed that acyclovir may enhance the antiviral effect of the patient’s own interferon.36
Intravenous immunoglobulin has been used in severe cases during outbreaks in Asia, with retrospective data showing a potential ability to halt disease progression if used before the development of cardiopulmonary failure. However, this has not been studied prospectively and is not currently recommended.16
Acknowledgment: We would like to thank Dr. Salvador Alvarez of the Mayo Clinic Department of Infectious Disease and Dr. Donald Lookingbill of the Mayo Clinic Department of Dermatology for their collaboration.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease (HFMD). www.cdc.gov/hand-foot-mouth/index.html. Accessed June 10, 2014.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis 2010; 63:229–233.
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213–214.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935.
- BBC News. China virus toll continues rise. May 5, 2008. http://news.bbc.co.uk/2/hi/asia-pacific/7383796.stm. Accessed February 5, 2014.
- Suhaimi ND. HFMD: 1,000 cases a week in Singapore is unusual, says doc. Straits Times April 20, 2008.
- Viet Nam News: HFMD cases prompt tighter health screening at airport. May 15, 2008.
- UBPOST. EV-71 virus continues dramatic rise. May 22, 2008.
- Begawan BS. 1,053 HFMD cases recorded. Brunei Times. November 7, 2008.
- Chinaview. Hand-foot-mouth disease death toll rises to 17 in East China’s Shandong Province. April 9, 2009.
- Chinaview. China reports 537 deaths from hand-foot-mouth disease this year. June 24, 2010.
- Wolfson H. Outbreak of hand, foot and mouth disease severe in Alabama. Birmingham News February 13, 2012.
- Centers for Disease Control and Prevention (CDC). Non-Polio Enterovirus Infections. www.cdc.gov/non-polio-enterovirus/. Accessed June 10, 2014.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9:78–85.
- California Department of Public Health. Coxsackievirus A6 (CVA6). 2011. www.cdph.ca.gov/programs/cder/Pages/CVA6.aspx. Accessed June 10, 2014.
- World Health Organization: Western Pacific Region. A Guide to Clinical management and Public Health Response for Hand, Foot, and Mouth Disease (HFMD).
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol 2010; 22:216–218.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis 2011; 11:346.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis 2009; 15:1485–1488.
- Tosti A, Piraccini BM. Nail Disorders. In:Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology JV. 3rded. Elsevier Limited; 2012:1129–1144.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol 2013; 69:736–741.
- Mathes EF, Oza V, Frieden IJ, et al. ”Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 2013; 132:e149–e157.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol 2013; 5:203–209.
- Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep 2002; 4:309–316.
- Akiyama K, Imazeki R, Yoshii F, Koide T, Muto J. An adult case of hand, foot, and mouth disease caused by enterovirus 71 accompanied by opsoclonus myoclonica. Tokai J Exp Clin Med 2008; 33:143–145.
- Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS One 2012; 7:e29003.
- Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88.
- Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190.
- Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682–1686.
- Mayo Clinic Laboratories. Enterovirus, Molecular Detection, PCR, Plasma. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89893. Accessed June 10, 2014.
- Yu N, Guo M, He SJ, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 2012; 9:12.
- Wang C, You A, Tian X, et al. Analysis and solution of false-positives when testing CVA16 sera using an antibody assay against the EV71 virus. Virus Res 2013; 176:33–36.
- Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6:e25287.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci 2011; 24:214–221.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease: Prevention & Treatment. www.cdc.gov/hand-foot-mouth/about/prevention-treatment.html. Accessed June 10, 2014.
- Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis 1996; 57:232–234.
Hand, foot, and mouth disease (HFMD) is typically a benign childhood infection—except when it isn’t so benign or when it occurs in adults.
The usual presentation is in a child with fever, oral ulcerations, and papules on the palms of the hands and the soles of the feet.1 However, severe complications can occur, including central nervous system involvement and cardiopulmonary failure, and can lead to significant morbidity and even death.2 Fortunately, these complications are rare.
Less common in North America than in other regions, HFMD has recurrently broken out in many areas of Southern Asia and the surrounding Pacific region. However, several North American outbreaks have been documented in recent years and have affected unexpected numbers of immunocompetent adults, demonstrating that this disease is of worldwide importance in adults as well as children.3
Because HFMD has the potential to reach epidemic levels in the United States, early recognition is paramount, and primary care physicians need to be familiar with its common signs and symptoms.
USUALLY A SUMMER DISEASE
HFMD occurs all around the world, exhibiting seasonal variation in temperate climates. In these locations, individual cases and regional outbreaks usually occur in the spring, summer, and fall. No sexual predisposition has been documented. Most symptomatic cases are in children under the age of 10.
OUTBREAKS AROUND THE WORLD
The disease was first described more than 40 years ago, with several large outbreaks in the last 16 years.
1998—An outbreak in Taiwan affected more than 1.5 million people, mostly children. Severe cases numbered just over 400, and 78 children died.4
2008—China,5 Singapore,6 Vietnam,7 Mongolia,8 and Brunei9 were stricken with an outbreak that affected 30,000 people and led to more than 50 deaths.
2009—An outbreak in the Henan and Shandong provinces of eastern China killed 35 people.10
2010—In several southern Chinese regions, more than 70,000 people were infected, with almost 600 deaths.11
2011 to the present. The United States has had several outbreaks in the last 3 years. Although HFMD is not one of the diseases that must be reported to public health authorities in the United States, from November 2011 to February 2012 the US Centers for Disease Control and Prevention (CDC) received reports of 63 possible cases: 38 in Alabama, 17 in Nevada, 7 in California, and 1 in Connecticut.1 Fifteen of the patients were adults, and more than half had contacts who were sick.
The most recent US outbreak, in Alabama,12 was atypical because it occurred in the winter.
CAUSED BY ENTEROVIRUSES
HFMD is caused by infection with a variety of viruses in the genus Enterovirus, a large group that in turn is part of the larger Picornaviridae family.13 The taxonomy of this genus is complicated and subject to revision; species include coxsackieviruses, polioviruses, enteroviruses, and echoviruses. They are all small, nonenveloped, single-stranded RNA viruses.
The most common strains that cause HFMD are coxsackievirus A16 and enterovirus 71. In addition, coxsackievirus A6 may be emerging, and many other coxsackievirus strains have been directly implicated, including A5, A7, A9, A10, B2, and B5.
Coxsackievirus A16 is the leading cause of HFMD.
Enterovirus 71 is the second most common cause of HFMD and has also caused outbreaks. It usually results in benign disease. However, among the causes of HFMD, it is associated with more prominent central nervous system involvement14 and is the most common cause of viral meningoencephalitis in children.
Coxsackievirus A6. In December 2011, the California Department of Public Health isolated a strain of coxsackievirus A6 that caused extensive rash and nail shedding.15 Among the 63 possible cases of HFMD reported to the CDC from November 2011 to February 2012, specimens for clinical testing were obtained in 34, and 25 of those demonstrated coxsackievirus A6 infection.3
FEVER, ORAL ULCERS, RASH ON HANDS AND FEET
The typical clinical manifestations of HFMD are fever, stomatitis with oral ulcers, and an exanthem affecting the palms, soles, and other parts of the body. These last less than 7 to 10 days, usually occur during the spring to fall months, and have a benign course.
The incubation period is 3 to 5 days, with a prodrome that may include fever, malaise, abdominal pain, and myalgia before the onset of oral and cutaneous findings. Painful oral ulcers may precede the exanthem and can result in dehydration.16
The cutaneous manifestation of HFMD is typically a papulovesicular rash affecting the palms, soles, and buttocks (Figure 1). Other sites may include the knees, elbows, and the dorsal surfaces of the hands and feet. The lesions may be maculopapular and can be either asymptomatic or tender and painful. Desquamation can follow the exanthem, and lesions usually resolve without scarring or secondary infection.16,17

Table 1 and Table 2 compare HFMD with other common illnesses that can cause similar skin and mucosal findings. In particular, herpangina has the identical clinical presentation as HFMD except that it does not cause skin lesions. It is caused by many of the same enteroviruses linked to HFMD.
Different viruses, different signs?
The numerous viruses that cause HFMD usually cause similar signs and symptoms during bouts of typical, self-limited disease. However, neurologic and cardiopulmonary involvement, which are fortunately rare, are more often associated with enterovirus 71 infection.
Nail manifestations are common in HFMD. Nail separation from the nail matrix (onychomadesis) was associated with coxsackievirus A6 infection during a 2010 outbreak of HFMD in Taiwan and in a 2009 outbreak in Finland.18 Moreover, this virus was cultured from a nail specimen in one patient, suggesting viral infiltration as the cause of nail-matrix arrest.19
Perioral skin eruptions, desquamation, and Beau lines have also been associated with coxsackievirus A6.18 Beau lines are transverse depressions of the nail, most evident in the central nail plate; when seen on multiple nails, they imply a systemic illness causing disruption of nail matrix growth.20
Atypical HFMD and coxsackievirus A6
Atypical HFMD has recently been described in connection with coxsackievirus A6. Lott et al21 reported five cases of coxsackievirus A6-associated HFMD in 2013. Atypically, three of the affected patients presented in winter months, two were adults, and two had widespread skin involvement.21
Mathes et al22 reported a series of 80 cases of enteroviral infections in which the lesions had a predilection for the antecubital and popliteal fossae and were similar in severity and distribution to those seen in eczema herpeticum or Kaposi varicelliform eruption in patients with and patients without a history of atopic dermatitis. They named this find-clinical finding of pronounced coxsackievirus-associated skin disease at sites previously affected by atopic dermatitis.
Additional cutaneous findings of coxsackievirus A6 infection may include onychomadesis, Beau lines, and vesiculobullous lesions. Patients with atypical, coxsackievirus A6-associated HFMD may not have oral lesions.23
In the five cases reported by Lott et al,21 significant systemic symptoms (fever, chills, diarrhea, and myalgias) led all but one of the patients to seek care in an emergency department. However, atypical HFMD has not been associated with life-threatening illness.
Atypical HFMD associated with coxsackievirus A6 is an emerging entity in the United States, and the acuity of both cutaneous and systemic symptoms poses a diagnostic dilemma. Furthermore, infection has been documented in immunocompetent adults.23 Familiarity with the clinical findings may expedite appropriate care, prevent spread to contacts, and avoid unnecessary testing.
Neurologic and cardiopulmonary manifestations
Enteroviruses are the most common causes of viral meningoencephalitis in the United States. They mainly affect children and cause serious and potentially chronic disease in those with humoral immunodeficiencies.24 Neurologic and cardiopulmonary manifestations of HFMD are varied and extremely rare in the United States but should always be viewed clinically as signs of concern and severe disease.
Signs of potentially fatal disease that have been observed in young children include tachycardia, tachypnea, hypotension, hypertension, gastrointestinal bleeding, neurologic symptoms, leukocytosis, absence of oral lesions, and vomiting.2 Signs of dysautonomia, myoclonus, ataxia, and brainstem involvement may portend fatal disease in which rapid decompensation is the result of cardiogenic shock due to loss of ventricular contractility, causing pulmonary edema and end-organ dysfunction.16
Neurologic manifestations associated with enterovirus 71 infection include aseptic meningitis, a poliomyelitis-like syndrome, brainstem encephalitis, neurogenic pulmonary edema, opsoclonus-myoclonus syndrome, cerebellar ataxia, Guillain-Barré syndrome, and transverse myelitis.
Because some patients who have neurologic disease respond to treatment with high-dose methylprednisolone and intravenous immune globulin, there is reason to suspect that an autoimmune phenomenon triggered by the culprit enterovirus may be the cause of many of the neurologic symptoms.25
A 2012 meta-analysis26 found that an elevated white blood cell count and hyperglycemia could be clinically useful in distinguishing benign from severe HFMD. In patients with benign HFMD, white blood cell counts and blood glucose values were no different from those in healthy controls.26
DIAGNOSIS IS USUALLY CLINICAL
Most enteroviral infections are asymptomatic, but HFMD is a possibility if a patient has mild illness, fever, and a maculopapular or vesicular rash on the palms of the hands and soles of the feet, sometimes associated with oral ulcers (herpangina). Skin lesions can also be found on the legs, face, buttocks, and trunk.
In the United States, HFMD most commonly occurs in children under age 4 and is usually caused by coxsackievirus A16. Adults can also be affected, especially if they were in contact with children in child care, which was the case in approximately half of nonpediatric patients who tested positive for HFMD during an outbreak in several states between November 2011 and February 2012.3
The clinical characteristics of HFMD caused by enterovirus 71 may be somewhat different, with smaller vesicles, diffuse erythema of the trunk and limbs, and higher fever (temperature ≥ 39°C [102.2°F] for more than 3 days).27 However, the rash of coxsackievirus A16 HFMD may be more extensive and severe.
Other clinical manifestations of HFMD include nail dystrophies such as Beau lines and nail shedding, hyperglycemia, dehydration, and more serious and potentially life-threatening complications such as pulmonary edema28 and viral meningoencephalitis.29
Laboratory testing
In mild cases of HFMD, particularly in patients with a high probability of having the disease based on their clinical characteristics and sick contacts, laboratory testing is not necessary. Testing is usually reserved for severe cases and public health investigation of outbreaks.
Viral culture is the gold standard for diagnosing HFMD, but the final results can take nearly a week.
Polymerase chain reaction testing is faster, with a turnaround time of less than 1 day. It identifies viral RNA and is highly sensitive for detecting central nervous system infection.30
Where should samples be collected? Serum viremia precedes invasion of the skin and mucous membranes, so plasma can be tested. Inside the body, enteroviruses initially replicate in the gastrointestinal tract, although collecting a rectal swab or a stool sample is somewhat invasive. Further, in an enterovirus 71 epidemic in Taiwan, 93% of the patients had positive throat swabs, but only 30% tested positive by rectal swabs or analysis of the feces.27 At present, throat and vesicle specimens are considered to be the most useful sources for diagnostic purposes.16
ELISAs. Newly developed IgM-capture enzyme-linked immunosorbent assays (ELISAs) for coxsackievirus A16 and enterovirus 71 appear quite promising for diagnosing HFMD. These tests are inexpensive and detect IgM antibodies early and in a high percentage of patients. In the first week of the disease, the IgM detection rate was found to be 90.2% for enterovirus 71 and 68% for coxsackievirus A16.31
Cross-reactivity between these two viruses was a problem with ELISA testing in the past, causing false-positive results for enterovirus 71 in patients who in fact had coxsackievirus A16. The problem appears to be resolved in new versions that use specific enterovirus 71 proteins, eg, VP1.32
RECOGNITION AND PREVENTION ARE THE BEST MEDICINE
Recognizing HFMD early is crucial, because making the clinical diagnosis can identify patients who have signs of severe disease and can help protect future contacts and decrease the risk of an epidemic.
Infected patients continue to shed the virus for a long time, making hand hygiene and environmental control measures in health care settings and daycare centers of vital importance, to prevent spread of the infection.
Enteroviruses are stable in the environment and therefore capable of fecal-oral and oral-oral transmission. Humans are the only known natural hosts. No chemoprophylaxis or vaccination has been established to prevent HFMD. The recurrence of large-scale epidemics in the developing world is perhaps explained by ineffective sewage treatment and limited access to clean drinking water, especially in light of the fecal-oral spread of the virus. Intrafamilial spread of HFMD has been shown to be an important means of disease transmission, and asymptomatic adult carriers of these viruses may spread it to young children.33
The different viruses that cause HFMD result in a similar clinical presentation in most patients. Therefore, identifying HFMD caused by enterovirus 71, which carries a risk of severe and even fatal disease in young children vs a virus such as coxsackievirus A16, can be very difficult in practice without virologic testing. Thus, when diagnosed with HFMD, patients should be counseled to control all variables that could lead to further spread of the disease.
An analysis of epidemics in Asia suggested that public health awareness may have averted deaths in successive epidemics, highlighting the need to identify HFMD epidemics in communities and to educate patients and families about measures to prevent further spread of the virus in addition to standard supportive care.34
The CDC recommends35:
- Frequent hand-washing after toileting and changing diapers
- Disinfecting frequently used surfaces and objects, including toys
- Avoiding close contact with infected individuals and sharing of personal items such as utensils and cups.
These measures should be recommended to all affected patients.35
NO PROVEN ANTIVIRAL TREATMENT
No proven antiviral treatment exists for HFMD. Thus, the goals of treatment are typically supportive, as for any self-limited viral syndrome.16
Does acyclovir help? Shelley et al36 treated 13 patients (12 children and 1 adult) with acyclovir within 1 to 2 days of the onset of the HFMD rash and reported that it was beneficial, with significant relief of fever and skin lesions within 24 hours of starting therapy. These anecdotal results have not been replicated, and acyclovir is not an established treatment for HFMD.
If acyclovir does help, how does it work? Acyclovir, like other common antiviral medications, inactivates thymidine kinase, an enzyme produced by herpesviruses but not by HFMD-causing viruses like coxsackievirus A16. Shelley et al proposed that acyclovir may enhance the antiviral effect of the patient’s own interferon.36
Intravenous immunoglobulin has been used in severe cases during outbreaks in Asia, with retrospective data showing a potential ability to halt disease progression if used before the development of cardiopulmonary failure. However, this has not been studied prospectively and is not currently recommended.16
Acknowledgment: We would like to thank Dr. Salvador Alvarez of the Mayo Clinic Department of Infectious Disease and Dr. Donald Lookingbill of the Mayo Clinic Department of Dermatology for their collaboration.
Hand, foot, and mouth disease (HFMD) is typically a benign childhood infection—except when it isn’t so benign or when it occurs in adults.
The usual presentation is in a child with fever, oral ulcerations, and papules on the palms of the hands and the soles of the feet.1 However, severe complications can occur, including central nervous system involvement and cardiopulmonary failure, and can lead to significant morbidity and even death.2 Fortunately, these complications are rare.
Less common in North America than in other regions, HFMD has recurrently broken out in many areas of Southern Asia and the surrounding Pacific region. However, several North American outbreaks have been documented in recent years and have affected unexpected numbers of immunocompetent adults, demonstrating that this disease is of worldwide importance in adults as well as children.3
Because HFMD has the potential to reach epidemic levels in the United States, early recognition is paramount, and primary care physicians need to be familiar with its common signs and symptoms.
USUALLY A SUMMER DISEASE
HFMD occurs all around the world, exhibiting seasonal variation in temperate climates. In these locations, individual cases and regional outbreaks usually occur in the spring, summer, and fall. No sexual predisposition has been documented. Most symptomatic cases are in children under the age of 10.
OUTBREAKS AROUND THE WORLD
The disease was first described more than 40 years ago, with several large outbreaks in the last 16 years.
1998—An outbreak in Taiwan affected more than 1.5 million people, mostly children. Severe cases numbered just over 400, and 78 children died.4
2008—China,5 Singapore,6 Vietnam,7 Mongolia,8 and Brunei9 were stricken with an outbreak that affected 30,000 people and led to more than 50 deaths.
2009—An outbreak in the Henan and Shandong provinces of eastern China killed 35 people.10
2010—In several southern Chinese regions, more than 70,000 people were infected, with almost 600 deaths.11
2011 to the present. The United States has had several outbreaks in the last 3 years. Although HFMD is not one of the diseases that must be reported to public health authorities in the United States, from November 2011 to February 2012 the US Centers for Disease Control and Prevention (CDC) received reports of 63 possible cases: 38 in Alabama, 17 in Nevada, 7 in California, and 1 in Connecticut.1 Fifteen of the patients were adults, and more than half had contacts who were sick.
The most recent US outbreak, in Alabama,12 was atypical because it occurred in the winter.
CAUSED BY ENTEROVIRUSES
HFMD is caused by infection with a variety of viruses in the genus Enterovirus, a large group that in turn is part of the larger Picornaviridae family.13 The taxonomy of this genus is complicated and subject to revision; species include coxsackieviruses, polioviruses, enteroviruses, and echoviruses. They are all small, nonenveloped, single-stranded RNA viruses.
The most common strains that cause HFMD are coxsackievirus A16 and enterovirus 71. In addition, coxsackievirus A6 may be emerging, and many other coxsackievirus strains have been directly implicated, including A5, A7, A9, A10, B2, and B5.
Coxsackievirus A16 is the leading cause of HFMD.
Enterovirus 71 is the second most common cause of HFMD and has also caused outbreaks. It usually results in benign disease. However, among the causes of HFMD, it is associated with more prominent central nervous system involvement14 and is the most common cause of viral meningoencephalitis in children.
Coxsackievirus A6. In December 2011, the California Department of Public Health isolated a strain of coxsackievirus A6 that caused extensive rash and nail shedding.15 Among the 63 possible cases of HFMD reported to the CDC from November 2011 to February 2012, specimens for clinical testing were obtained in 34, and 25 of those demonstrated coxsackievirus A6 infection.3
FEVER, ORAL ULCERS, RASH ON HANDS AND FEET
The typical clinical manifestations of HFMD are fever, stomatitis with oral ulcers, and an exanthem affecting the palms, soles, and other parts of the body. These last less than 7 to 10 days, usually occur during the spring to fall months, and have a benign course.
The incubation period is 3 to 5 days, with a prodrome that may include fever, malaise, abdominal pain, and myalgia before the onset of oral and cutaneous findings. Painful oral ulcers may precede the exanthem and can result in dehydration.16
The cutaneous manifestation of HFMD is typically a papulovesicular rash affecting the palms, soles, and buttocks (Figure 1). Other sites may include the knees, elbows, and the dorsal surfaces of the hands and feet. The lesions may be maculopapular and can be either asymptomatic or tender and painful. Desquamation can follow the exanthem, and lesions usually resolve without scarring or secondary infection.16,17

Table 1 and Table 2 compare HFMD with other common illnesses that can cause similar skin and mucosal findings. In particular, herpangina has the identical clinical presentation as HFMD except that it does not cause skin lesions. It is caused by many of the same enteroviruses linked to HFMD.
Different viruses, different signs?
The numerous viruses that cause HFMD usually cause similar signs and symptoms during bouts of typical, self-limited disease. However, neurologic and cardiopulmonary involvement, which are fortunately rare, are more often associated with enterovirus 71 infection.
Nail manifestations are common in HFMD. Nail separation from the nail matrix (onychomadesis) was associated with coxsackievirus A6 infection during a 2010 outbreak of HFMD in Taiwan and in a 2009 outbreak in Finland.18 Moreover, this virus was cultured from a nail specimen in one patient, suggesting viral infiltration as the cause of nail-matrix arrest.19
Perioral skin eruptions, desquamation, and Beau lines have also been associated with coxsackievirus A6.18 Beau lines are transverse depressions of the nail, most evident in the central nail plate; when seen on multiple nails, they imply a systemic illness causing disruption of nail matrix growth.20
Atypical HFMD and coxsackievirus A6
Atypical HFMD has recently been described in connection with coxsackievirus A6. Lott et al21 reported five cases of coxsackievirus A6-associated HFMD in 2013. Atypically, three of the affected patients presented in winter months, two were adults, and two had widespread skin involvement.21
Mathes et al22 reported a series of 80 cases of enteroviral infections in which the lesions had a predilection for the antecubital and popliteal fossae and were similar in severity and distribution to those seen in eczema herpeticum or Kaposi varicelliform eruption in patients with and patients without a history of atopic dermatitis. They named this find-clinical finding of pronounced coxsackievirus-associated skin disease at sites previously affected by atopic dermatitis.
Additional cutaneous findings of coxsackievirus A6 infection may include onychomadesis, Beau lines, and vesiculobullous lesions. Patients with atypical, coxsackievirus A6-associated HFMD may not have oral lesions.23
In the five cases reported by Lott et al,21 significant systemic symptoms (fever, chills, diarrhea, and myalgias) led all but one of the patients to seek care in an emergency department. However, atypical HFMD has not been associated with life-threatening illness.
Atypical HFMD associated with coxsackievirus A6 is an emerging entity in the United States, and the acuity of both cutaneous and systemic symptoms poses a diagnostic dilemma. Furthermore, infection has been documented in immunocompetent adults.23 Familiarity with the clinical findings may expedite appropriate care, prevent spread to contacts, and avoid unnecessary testing.
Neurologic and cardiopulmonary manifestations
Enteroviruses are the most common causes of viral meningoencephalitis in the United States. They mainly affect children and cause serious and potentially chronic disease in those with humoral immunodeficiencies.24 Neurologic and cardiopulmonary manifestations of HFMD are varied and extremely rare in the United States but should always be viewed clinically as signs of concern and severe disease.
Signs of potentially fatal disease that have been observed in young children include tachycardia, tachypnea, hypotension, hypertension, gastrointestinal bleeding, neurologic symptoms, leukocytosis, absence of oral lesions, and vomiting.2 Signs of dysautonomia, myoclonus, ataxia, and brainstem involvement may portend fatal disease in which rapid decompensation is the result of cardiogenic shock due to loss of ventricular contractility, causing pulmonary edema and end-organ dysfunction.16
Neurologic manifestations associated with enterovirus 71 infection include aseptic meningitis, a poliomyelitis-like syndrome, brainstem encephalitis, neurogenic pulmonary edema, opsoclonus-myoclonus syndrome, cerebellar ataxia, Guillain-Barré syndrome, and transverse myelitis.
Because some patients who have neurologic disease respond to treatment with high-dose methylprednisolone and intravenous immune globulin, there is reason to suspect that an autoimmune phenomenon triggered by the culprit enterovirus may be the cause of many of the neurologic symptoms.25
A 2012 meta-analysis26 found that an elevated white blood cell count and hyperglycemia could be clinically useful in distinguishing benign from severe HFMD. In patients with benign HFMD, white blood cell counts and blood glucose values were no different from those in healthy controls.26
DIAGNOSIS IS USUALLY CLINICAL
Most enteroviral infections are asymptomatic, but HFMD is a possibility if a patient has mild illness, fever, and a maculopapular or vesicular rash on the palms of the hands and soles of the feet, sometimes associated with oral ulcers (herpangina). Skin lesions can also be found on the legs, face, buttocks, and trunk.
In the United States, HFMD most commonly occurs in children under age 4 and is usually caused by coxsackievirus A16. Adults can also be affected, especially if they were in contact with children in child care, which was the case in approximately half of nonpediatric patients who tested positive for HFMD during an outbreak in several states between November 2011 and February 2012.3
The clinical characteristics of HFMD caused by enterovirus 71 may be somewhat different, with smaller vesicles, diffuse erythema of the trunk and limbs, and higher fever (temperature ≥ 39°C [102.2°F] for more than 3 days).27 However, the rash of coxsackievirus A16 HFMD may be more extensive and severe.
Other clinical manifestations of HFMD include nail dystrophies such as Beau lines and nail shedding, hyperglycemia, dehydration, and more serious and potentially life-threatening complications such as pulmonary edema28 and viral meningoencephalitis.29
Laboratory testing
In mild cases of HFMD, particularly in patients with a high probability of having the disease based on their clinical characteristics and sick contacts, laboratory testing is not necessary. Testing is usually reserved for severe cases and public health investigation of outbreaks.
Viral culture is the gold standard for diagnosing HFMD, but the final results can take nearly a week.
Polymerase chain reaction testing is faster, with a turnaround time of less than 1 day. It identifies viral RNA and is highly sensitive for detecting central nervous system infection.30
Where should samples be collected? Serum viremia precedes invasion of the skin and mucous membranes, so plasma can be tested. Inside the body, enteroviruses initially replicate in the gastrointestinal tract, although collecting a rectal swab or a stool sample is somewhat invasive. Further, in an enterovirus 71 epidemic in Taiwan, 93% of the patients had positive throat swabs, but only 30% tested positive by rectal swabs or analysis of the feces.27 At present, throat and vesicle specimens are considered to be the most useful sources for diagnostic purposes.16
ELISAs. Newly developed IgM-capture enzyme-linked immunosorbent assays (ELISAs) for coxsackievirus A16 and enterovirus 71 appear quite promising for diagnosing HFMD. These tests are inexpensive and detect IgM antibodies early and in a high percentage of patients. In the first week of the disease, the IgM detection rate was found to be 90.2% for enterovirus 71 and 68% for coxsackievirus A16.31
Cross-reactivity between these two viruses was a problem with ELISA testing in the past, causing false-positive results for enterovirus 71 in patients who in fact had coxsackievirus A16. The problem appears to be resolved in new versions that use specific enterovirus 71 proteins, eg, VP1.32
RECOGNITION AND PREVENTION ARE THE BEST MEDICINE
Recognizing HFMD early is crucial, because making the clinical diagnosis can identify patients who have signs of severe disease and can help protect future contacts and decrease the risk of an epidemic.
Infected patients continue to shed the virus for a long time, making hand hygiene and environmental control measures in health care settings and daycare centers of vital importance, to prevent spread of the infection.
Enteroviruses are stable in the environment and therefore capable of fecal-oral and oral-oral transmission. Humans are the only known natural hosts. No chemoprophylaxis or vaccination has been established to prevent HFMD. The recurrence of large-scale epidemics in the developing world is perhaps explained by ineffective sewage treatment and limited access to clean drinking water, especially in light of the fecal-oral spread of the virus. Intrafamilial spread of HFMD has been shown to be an important means of disease transmission, and asymptomatic adult carriers of these viruses may spread it to young children.33
The different viruses that cause HFMD result in a similar clinical presentation in most patients. Therefore, identifying HFMD caused by enterovirus 71, which carries a risk of severe and even fatal disease in young children vs a virus such as coxsackievirus A16, can be very difficult in practice without virologic testing. Thus, when diagnosed with HFMD, patients should be counseled to control all variables that could lead to further spread of the disease.
An analysis of epidemics in Asia suggested that public health awareness may have averted deaths in successive epidemics, highlighting the need to identify HFMD epidemics in communities and to educate patients and families about measures to prevent further spread of the virus in addition to standard supportive care.34
The CDC recommends35:
- Frequent hand-washing after toileting and changing diapers
- Disinfecting frequently used surfaces and objects, including toys
- Avoiding close contact with infected individuals and sharing of personal items such as utensils and cups.
These measures should be recommended to all affected patients.35
NO PROVEN ANTIVIRAL TREATMENT
No proven antiviral treatment exists for HFMD. Thus, the goals of treatment are typically supportive, as for any self-limited viral syndrome.16
Does acyclovir help? Shelley et al36 treated 13 patients (12 children and 1 adult) with acyclovir within 1 to 2 days of the onset of the HFMD rash and reported that it was beneficial, with significant relief of fever and skin lesions within 24 hours of starting therapy. These anecdotal results have not been replicated, and acyclovir is not an established treatment for HFMD.
If acyclovir does help, how does it work? Acyclovir, like other common antiviral medications, inactivates thymidine kinase, an enzyme produced by herpesviruses but not by HFMD-causing viruses like coxsackievirus A16. Shelley et al proposed that acyclovir may enhance the antiviral effect of the patient’s own interferon.36
Intravenous immunoglobulin has been used in severe cases during outbreaks in Asia, with retrospective data showing a potential ability to halt disease progression if used before the development of cardiopulmonary failure. However, this has not been studied prospectively and is not currently recommended.16
Acknowledgment: We would like to thank Dr. Salvador Alvarez of the Mayo Clinic Department of Infectious Disease and Dr. Donald Lookingbill of the Mayo Clinic Department of Dermatology for their collaboration.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease (HFMD). www.cdc.gov/hand-foot-mouth/index.html. Accessed June 10, 2014.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis 2010; 63:229–233.
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213–214.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935.
- BBC News. China virus toll continues rise. May 5, 2008. http://news.bbc.co.uk/2/hi/asia-pacific/7383796.stm. Accessed February 5, 2014.
- Suhaimi ND. HFMD: 1,000 cases a week in Singapore is unusual, says doc. Straits Times April 20, 2008.
- Viet Nam News: HFMD cases prompt tighter health screening at airport. May 15, 2008.
- UBPOST. EV-71 virus continues dramatic rise. May 22, 2008.
- Begawan BS. 1,053 HFMD cases recorded. Brunei Times. November 7, 2008.
- Chinaview. Hand-foot-mouth disease death toll rises to 17 in East China’s Shandong Province. April 9, 2009.
- Chinaview. China reports 537 deaths from hand-foot-mouth disease this year. June 24, 2010.
- Wolfson H. Outbreak of hand, foot and mouth disease severe in Alabama. Birmingham News February 13, 2012.
- Centers for Disease Control and Prevention (CDC). Non-Polio Enterovirus Infections. www.cdc.gov/non-polio-enterovirus/. Accessed June 10, 2014.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9:78–85.
- California Department of Public Health. Coxsackievirus A6 (CVA6). 2011. www.cdph.ca.gov/programs/cder/Pages/CVA6.aspx. Accessed June 10, 2014.
- World Health Organization: Western Pacific Region. A Guide to Clinical management and Public Health Response for Hand, Foot, and Mouth Disease (HFMD).
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol 2010; 22:216–218.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis 2011; 11:346.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis 2009; 15:1485–1488.
- Tosti A, Piraccini BM. Nail Disorders. In:Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology JV. 3rded. Elsevier Limited; 2012:1129–1144.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol 2013; 69:736–741.
- Mathes EF, Oza V, Frieden IJ, et al. ”Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 2013; 132:e149–e157.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol 2013; 5:203–209.
- Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep 2002; 4:309–316.
- Akiyama K, Imazeki R, Yoshii F, Koide T, Muto J. An adult case of hand, foot, and mouth disease caused by enterovirus 71 accompanied by opsoclonus myoclonica. Tokai J Exp Clin Med 2008; 33:143–145.
- Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS One 2012; 7:e29003.
- Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88.
- Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190.
- Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682–1686.
- Mayo Clinic Laboratories. Enterovirus, Molecular Detection, PCR, Plasma. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89893. Accessed June 10, 2014.
- Yu N, Guo M, He SJ, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 2012; 9:12.
- Wang C, You A, Tian X, et al. Analysis and solution of false-positives when testing CVA16 sera using an antibody assay against the EV71 virus. Virus Res 2013; 176:33–36.
- Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6:e25287.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci 2011; 24:214–221.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease: Prevention & Treatment. www.cdc.gov/hand-foot-mouth/about/prevention-treatment.html. Accessed June 10, 2014.
- Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis 1996; 57:232–234.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease (HFMD). www.cdc.gov/hand-foot-mouth/index.html. Accessed June 10, 2014.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis 2010; 63:229–233.
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213–214.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935.
- BBC News. China virus toll continues rise. May 5, 2008. http://news.bbc.co.uk/2/hi/asia-pacific/7383796.stm. Accessed February 5, 2014.
- Suhaimi ND. HFMD: 1,000 cases a week in Singapore is unusual, says doc. Straits Times April 20, 2008.
- Viet Nam News: HFMD cases prompt tighter health screening at airport. May 15, 2008.
- UBPOST. EV-71 virus continues dramatic rise. May 22, 2008.
- Begawan BS. 1,053 HFMD cases recorded. Brunei Times. November 7, 2008.
- Chinaview. Hand-foot-mouth disease death toll rises to 17 in East China’s Shandong Province. April 9, 2009.
- Chinaview. China reports 537 deaths from hand-foot-mouth disease this year. June 24, 2010.
- Wolfson H. Outbreak of hand, foot and mouth disease severe in Alabama. Birmingham News February 13, 2012.
- Centers for Disease Control and Prevention (CDC). Non-Polio Enterovirus Infections. www.cdc.gov/non-polio-enterovirus/. Accessed June 10, 2014.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9:78–85.
- California Department of Public Health. Coxsackievirus A6 (CVA6). 2011. www.cdph.ca.gov/programs/cder/Pages/CVA6.aspx. Accessed June 10, 2014.
- World Health Organization: Western Pacific Region. A Guide to Clinical management and Public Health Response for Hand, Foot, and Mouth Disease (HFMD).
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol 2010; 22:216–218.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis 2011; 11:346.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis 2009; 15:1485–1488.
- Tosti A, Piraccini BM. Nail Disorders. In:Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology JV. 3rded. Elsevier Limited; 2012:1129–1144.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol 2013; 69:736–741.
- Mathes EF, Oza V, Frieden IJ, et al. ”Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 2013; 132:e149–e157.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol 2013; 5:203–209.
- Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep 2002; 4:309–316.
- Akiyama K, Imazeki R, Yoshii F, Koide T, Muto J. An adult case of hand, foot, and mouth disease caused by enterovirus 71 accompanied by opsoclonus myoclonica. Tokai J Exp Clin Med 2008; 33:143–145.
- Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS One 2012; 7:e29003.
- Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88.
- Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190.
- Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682–1686.
- Mayo Clinic Laboratories. Enterovirus, Molecular Detection, PCR, Plasma. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89893. Accessed June 10, 2014.
- Yu N, Guo M, He SJ, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 2012; 9:12.
- Wang C, You A, Tian X, et al. Analysis and solution of false-positives when testing CVA16 sera using an antibody assay against the EV71 virus. Virus Res 2013; 176:33–36.
- Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6:e25287.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci 2011; 24:214–221.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease: Prevention & Treatment. www.cdc.gov/hand-foot-mouth/about/prevention-treatment.html. Accessed June 10, 2014.
- Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis 1996; 57:232–234.
KEY POINTS
- In Asian and Pacific nations, HFMD has been a significant public health concern since 1997, with recurrent epidemics and, in some cases, severe complications, including central nervous system disease, pulmonary edema, and death.
- Coxsackievirus A16 and enterovirus 71 are the most common agents of HFMD. In addition, coxsackievirus A6 seems to be emerging.
- Neurologic and cardiopulmonary involvement are more often associated with enterovirus 71 infection.
- In March 2012, 63 cases of severe HFMD were reported in Alabama, California, Connecticut, and Nevada. Fifteen of the patients were adults, and more than half had positive sick contacts. Of the 34 patients who underwent serologic testing, 25 were positive for coxsackievirus A6, an unusual pathogen for HFMD in the United States, associated with more severe skin findings.
- Treatment focuses on supportive care and prevention.
Was fetus’ wrist injured during cesarean delivery?
Was fetus’ wrist injured during cesarean delivery?
At 34 weeks’ gestation, a 39-year-old woman went to the hospital in preterm labor. Her history included a prior cesarean delivery. Ultrasonography (US) showed that the fetus was in a double-footling breech position. The ObGyn decided to perform a cesarean delivery when the fetal heart-rate monitor indicated distress.
After making a midline incision through the earlier scar, the ObGyn created a low transverse uterine incision with a scalpel. The mother’s uterus was thick because labor had not progressed. When the ObGyn was unable to deliver the baby through the low transverse incision, she performed a T-extension of the incision using bandage scissors while placing her free hand inside the uterus to shield the fetus from injury. After extensive manipulation, the baby was delivered and immediately handed to a neonatologist. After surgery, the neonatologist told the mother that the baby had sustained two lacerations to the ulnar side of the right wrist. The newborn was airlifted to another hospital for treatment of sepsis. There, an orthopedic hand surgeon examined the child and determined that the lacerations were superficial and only required sutures. The orthopedist saw the infant a month later and believed there was no significant wrist injury.
When the child began preschool, she started to experience cold intolerance and difficulty writing with her right hand. The child was referred to a pediatric neurologist, who found no nerve damage and ordered occupational therapy.
The original orthopedic surgeon examined the child when she was 7 years old and determined that the flexor carpi ulnaris tendon had been completely severed with a partial injury to the ulnar nerve. He recommended a return visit at age 14 for full assessment of the wrist injury.
PARENTS’ CLAIM The ObGyn did not properly shield the fetus when performing the T-extension incision during cesarean delivery. The child’s weakness will increase with age, ruling out some occupations.
PHYSICIAN’S DEFENSE The ObGyn was not negligent; she had provided adequate protection of the fetus during both incisions.
VERDICT An Illinois defense verdict was returned.
Woman dies after tubal ligation
After a 42-year-old woman underwent tubal ligation, her surgeon was concerned about a possible bowel perforation and admitted her to the hospital. The next morning, a computed tomography (CT) scan of the abdomen did not reveal bowel injury.
That afternoon, when the patient reported shortness of breath, the surgeon called the hospitalist with concern for pulmonary embolism (PE). The hospitalist immediately ordered a CT scan of the chest, initiated PE protocol, and wrote “r/o PE” on the chart. A radiologist reminded the hospitalist of the earlier CT scan with concern for kidney damage from another dye study. The hospitalist cancelled the CT scan and PE protocol. After waiting 17 hours to run any further tests, a CT scan revealed massive bilateral PE. The patient was transferred to the ICU, but died the next day.
PATIENT’S CLAIM The 17-hour delay was negligent.
PHYSICIAN’S DEFENSE There was no negligence. The patient died of septic shock, not PE.
VERDICT A $4 million Virginia verdict was returned.
Child born without hand and forearm
During prenatal care, a mother underwent US at 20 and 36 weeks; both studies were reported as normal. The child was born missing his left hand and part of his left forearm due to a congenital amputation. The child will require prosthetics for life.
PATIENT’S CLAIM The condition should have been seen during prenatal US; an abortion was still an option at 20 weeks.
DEFENDANTS’ DEFENSE US was properly performed and evaluated. It can be difficult to differentiate the right from left extremities.
VERDICT A California defense verdict was returned.
After starting Yasmin, woman has stroke with permanent paralysis: $16.5M total award
When a 37-year-old woman reported irregular menstruation, her ObGyn prescribed drospirenone/ethinyl estradiol (Yasmin; Bayer). Thirteen days after starting the drug, the patient had a stroke. She is paralyzed on her left side, has limited ability to speak, cannot use her left arm and leg, and requires 24-hour care.
PATIENT’S CLAIM The ObGyn should have recognized that Yasmin was not appropriate for this patient because of the drug’s clotting risks. The patient’s risk factors included her age (over 35), borderline hypertension, overweight, history of smoking, and high cholesterol. The ObGyn should have offered safer alternatives, such as a progesterone-only pill. The US Food and Drug Administration (FDA) issued a safety warning that all drospirenone-containing drugs may be associated with a higher risk of venous thrombosis during the first 6 months of use.
DEFENDANTS’ DEFENSE According to Bayer, Yasmin is safe, and remains on the market. It was an appropriate drug to treat her irregular bleeding.
VERDICT Claims against the medical center that referred the patient to the ObGyn were settled for $2.5 million before trial. A $14 million Illinois verdict was returned against the ObGyn, for a total award of $16.5 million.
Who is at fault when pelvic mesh erodes?
In January 2011, an ObGyn implanted the Gynecare TVT Obturator System (TVT‑O; Ethicon) during a midurethral sling procedure to treat stress urinary incontinence (SUI) in a woman in her 60s. Shortly thereafter, the ObGyn left practice because of early-onset Alzheimer’s disease, and the patient’s care was taken over by a gynecologist.
At the 2-month postoperative visit, the gynecologist found that the mesh had eroded into the patient’s vagina. The gynecologist simply cut the mesh with a scissor, charted that a small erosion was present, and prescribed estrogen cream.
The patient continued to report pain, discomfort, pressure, difficulty voiding urine, continued incontinence, vaginal discharge, scarring, infection, odor, and bleeding.
PATIENT’S CLAIM The polypropylene mesh used during the midurethral sling procedure has been shown to be incompatible with human tissue. It promotes an immune response, which stimulates degradation of the pelvic tissue and can contribute to the development of severe adverse reactions to the mesh. Ethicon negligently designed, manufactured, marketed, labeled, and packaged the pelvic mesh products.
DEFENDANTS’ DEFENSE Proper warnings were provided about the health risks associated with polypropylene mesh products. The medical device was not properly sized.
VERDICT A Texas jury rejected the patient’s claims that Ethicon did not provide proper warnings about the sling’s health risks and declined to award punitive damages.
However, the jury decided that the mesh implant was defectively designed, and returned a $1.2 million verdict against Ethicon.
Was suspected bowel injury treated properly?
A 40-year-old woman was referred to an ObGyn after reporting abnormal uterine bleeding to her primary care physician. The patient had very light menses every few weeks. The ObGyn performed an ablation procedure, without relief. A month later, the ObGyn performed robot-assisted laparoscopic hysterectomy. The next day, the patient reported abdominal pain. Suspecting a bowel injury, the ObGyn ordered a CT scan; the bowel appeared normal, so the ObGyn referred the patient to a surgeon. During exploratory laparotomy, the surgeon found and repaired a bowel injury. The patient developed significant complications from a necrotizing infection that included respiratory distress and ongoing wound care.
PATIENT’S CLAIM Conservative treatment should have been offered before surgery. The ObGyn should have waited longer after the ablation procedure before doing the hysterectomy. The ObGyn should have checked for a possible bowel injury before closing the hysterectomy.
PHYSICIAN’S DEFENSE The bowel injury is a known complication of the procedure and was recognized and repaired in a timely manner.
VERDICT A Kentucky defense verdict was returned.
Pap smear improperly interpreted: Woman dies from cervical cancer
A 37-year-old woman underwent a pap smear in 2008 that was read by a cytotechnologist as normal. Two years later, the patient was found to have a golf-ball–sized cancerous tumor. She died from cervical cancer in 2011.
ESTATE’S CLAIM The cytotechnologist was negligent in misreading the 2008 Pap smear. If treatment had been started in 2008, the cancer could have been resolved with a simple conization biopsy.
DEFENDANTS’ DEFENSE The Pap smear interpretation was reasonable. The cancer could not have been diagnosed in 2008. The patient was at fault for failing to follow-up Pap smears during the next 2 years.
VERDICT After assigning 75% fault to the cytotechnologist and 25% fault to the patient, a Florida jury returned a $20,870,200 verdict, which was reduced to $15,816,699.
Disastrous off-label use of anticoagulation
When a pelvic abscess was found, a 50-year-old woman was admitted to the hospital for treatment. She was taking warfarin due to a history of venous thromboembolism.
Before the procedure, her physicians attempted to temporarily reverse her anticoagulation by administering Factor IX Complex (Profilnine SD, Grifols Biologicals). The dose ordered for the patient was nearly double the maximum recommended weight-based dose. Almost immediately after receiving the infusion, the patient went into cardiopulmonary arrest and died. An autopsy found the cause of death to be pulmonary emboli (PE).
ESTATE’S CLAIM An excessive dose of Profilnine caused PE. At the time of the incident, Profilnine was not FDA approved for warfarin reversal, although some off-label uses were recognized in emergent situations, such as intracranial bleeds.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $1.25 million Virginia settlement was reached.
Vesicovaginal fistula from ureteral injury
At a women’s health clinic, a patient reported continuous, heavy vaginal bleeding; pain; and shortness of breath when walking. She had a history of endometritis and multiple abdominal surgeries. Examination disclosed a profuse vaginal discharge, a normal cervix, and an enlarged uterus. The patient consented to abdominal hysterectomy and bilateral salpingo-oophorectomy performed by an ObGyn assisted by a resident.
During surgery, the ObGyn found that the patient’s uterus was at 16 to 20 weeks’ gestation size, with multiple serosal uterine fibroids and frank pus and necrosed fibroid tumors within the uterine cavity. The procedure took longer than planned because of extensive adhesions. After surgery, the patient was anemic and was given a beta-blocker for tachycardia. She was discharged 3 days later with 48 hours’ worth of intravenous antibiotics.
A month later, the patient reported urinary incontinence. She saw a urologist, who found a vesicovaginal fistula. The patient underwent nephrostomy-tube placement. Right ureterolysis and a right ureteral reimplant was performed 4 months later.
PATIENT’S CLAIM The ObGyn injured the right ureter during surgery.
DEFENDANTS’ DEFENSE The ureter injury is a known risk of the procedure. The injury was due to an infection or delayed effects of ischemia. The patient had a good recovery with no residual injury.
VERDICT A Michigan defense verdict was returned.
Why did mother die after delivering twins?
After a 35-year-old woman gave birth to twins by cesarean delivery, she died. At autopsy, 4 liters of blood were found in her abdomen.
ESTATE’S CLAIM The ObGyn failed to recognize and treat an arterial or venous bleed during surgery.
DEFENDANTS’ DEFENSE The patient died from amniotic fluid embolism. Autopsy results showed right ventricular heart failure, respiratory failure, and disseminated intravascular coagulation.
VERDICT A Florida defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Was fetus’ wrist injured during cesarean delivery?
At 34 weeks’ gestation, a 39-year-old woman went to the hospital in preterm labor. Her history included a prior cesarean delivery. Ultrasonography (US) showed that the fetus was in a double-footling breech position. The ObGyn decided to perform a cesarean delivery when the fetal heart-rate monitor indicated distress.
After making a midline incision through the earlier scar, the ObGyn created a low transverse uterine incision with a scalpel. The mother’s uterus was thick because labor had not progressed. When the ObGyn was unable to deliver the baby through the low transverse incision, she performed a T-extension of the incision using bandage scissors while placing her free hand inside the uterus to shield the fetus from injury. After extensive manipulation, the baby was delivered and immediately handed to a neonatologist. After surgery, the neonatologist told the mother that the baby had sustained two lacerations to the ulnar side of the right wrist. The newborn was airlifted to another hospital for treatment of sepsis. There, an orthopedic hand surgeon examined the child and determined that the lacerations were superficial and only required sutures. The orthopedist saw the infant a month later and believed there was no significant wrist injury.
When the child began preschool, she started to experience cold intolerance and difficulty writing with her right hand. The child was referred to a pediatric neurologist, who found no nerve damage and ordered occupational therapy.
The original orthopedic surgeon examined the child when she was 7 years old and determined that the flexor carpi ulnaris tendon had been completely severed with a partial injury to the ulnar nerve. He recommended a return visit at age 14 for full assessment of the wrist injury.
PARENTS’ CLAIM The ObGyn did not properly shield the fetus when performing the T-extension incision during cesarean delivery. The child’s weakness will increase with age, ruling out some occupations.
PHYSICIAN’S DEFENSE The ObGyn was not negligent; she had provided adequate protection of the fetus during both incisions.
VERDICT An Illinois defense verdict was returned.
Woman dies after tubal ligation
After a 42-year-old woman underwent tubal ligation, her surgeon was concerned about a possible bowel perforation and admitted her to the hospital. The next morning, a computed tomography (CT) scan of the abdomen did not reveal bowel injury.
That afternoon, when the patient reported shortness of breath, the surgeon called the hospitalist with concern for pulmonary embolism (PE). The hospitalist immediately ordered a CT scan of the chest, initiated PE protocol, and wrote “r/o PE” on the chart. A radiologist reminded the hospitalist of the earlier CT scan with concern for kidney damage from another dye study. The hospitalist cancelled the CT scan and PE protocol. After waiting 17 hours to run any further tests, a CT scan revealed massive bilateral PE. The patient was transferred to the ICU, but died the next day.
PATIENT’S CLAIM The 17-hour delay was negligent.
PHYSICIAN’S DEFENSE There was no negligence. The patient died of septic shock, not PE.
VERDICT A $4 million Virginia verdict was returned.
Child born without hand and forearm
During prenatal care, a mother underwent US at 20 and 36 weeks; both studies were reported as normal. The child was born missing his left hand and part of his left forearm due to a congenital amputation. The child will require prosthetics for life.
PATIENT’S CLAIM The condition should have been seen during prenatal US; an abortion was still an option at 20 weeks.
DEFENDANTS’ DEFENSE US was properly performed and evaluated. It can be difficult to differentiate the right from left extremities.
VERDICT A California defense verdict was returned.
After starting Yasmin, woman has stroke with permanent paralysis: $16.5M total award
When a 37-year-old woman reported irregular menstruation, her ObGyn prescribed drospirenone/ethinyl estradiol (Yasmin; Bayer). Thirteen days after starting the drug, the patient had a stroke. She is paralyzed on her left side, has limited ability to speak, cannot use her left arm and leg, and requires 24-hour care.
PATIENT’S CLAIM The ObGyn should have recognized that Yasmin was not appropriate for this patient because of the drug’s clotting risks. The patient’s risk factors included her age (over 35), borderline hypertension, overweight, history of smoking, and high cholesterol. The ObGyn should have offered safer alternatives, such as a progesterone-only pill. The US Food and Drug Administration (FDA) issued a safety warning that all drospirenone-containing drugs may be associated with a higher risk of venous thrombosis during the first 6 months of use.
DEFENDANTS’ DEFENSE According to Bayer, Yasmin is safe, and remains on the market. It was an appropriate drug to treat her irregular bleeding.
VERDICT Claims against the medical center that referred the patient to the ObGyn were settled for $2.5 million before trial. A $14 million Illinois verdict was returned against the ObGyn, for a total award of $16.5 million.
Who is at fault when pelvic mesh erodes?
In January 2011, an ObGyn implanted the Gynecare TVT Obturator System (TVT‑O; Ethicon) during a midurethral sling procedure to treat stress urinary incontinence (SUI) in a woman in her 60s. Shortly thereafter, the ObGyn left practice because of early-onset Alzheimer’s disease, and the patient’s care was taken over by a gynecologist.
At the 2-month postoperative visit, the gynecologist found that the mesh had eroded into the patient’s vagina. The gynecologist simply cut the mesh with a scissor, charted that a small erosion was present, and prescribed estrogen cream.
The patient continued to report pain, discomfort, pressure, difficulty voiding urine, continued incontinence, vaginal discharge, scarring, infection, odor, and bleeding.
PATIENT’S CLAIM The polypropylene mesh used during the midurethral sling procedure has been shown to be incompatible with human tissue. It promotes an immune response, which stimulates degradation of the pelvic tissue and can contribute to the development of severe adverse reactions to the mesh. Ethicon negligently designed, manufactured, marketed, labeled, and packaged the pelvic mesh products.
DEFENDANTS’ DEFENSE Proper warnings were provided about the health risks associated with polypropylene mesh products. The medical device was not properly sized.
VERDICT A Texas jury rejected the patient’s claims that Ethicon did not provide proper warnings about the sling’s health risks and declined to award punitive damages.
However, the jury decided that the mesh implant was defectively designed, and returned a $1.2 million verdict against Ethicon.
Was suspected bowel injury treated properly?
A 40-year-old woman was referred to an ObGyn after reporting abnormal uterine bleeding to her primary care physician. The patient had very light menses every few weeks. The ObGyn performed an ablation procedure, without relief. A month later, the ObGyn performed robot-assisted laparoscopic hysterectomy. The next day, the patient reported abdominal pain. Suspecting a bowel injury, the ObGyn ordered a CT scan; the bowel appeared normal, so the ObGyn referred the patient to a surgeon. During exploratory laparotomy, the surgeon found and repaired a bowel injury. The patient developed significant complications from a necrotizing infection that included respiratory distress and ongoing wound care.
PATIENT’S CLAIM Conservative treatment should have been offered before surgery. The ObGyn should have waited longer after the ablation procedure before doing the hysterectomy. The ObGyn should have checked for a possible bowel injury before closing the hysterectomy.
PHYSICIAN’S DEFENSE The bowel injury is a known complication of the procedure and was recognized and repaired in a timely manner.
VERDICT A Kentucky defense verdict was returned.
Pap smear improperly interpreted: Woman dies from cervical cancer
A 37-year-old woman underwent a pap smear in 2008 that was read by a cytotechnologist as normal. Two years later, the patient was found to have a golf-ball–sized cancerous tumor. She died from cervical cancer in 2011.
ESTATE’S CLAIM The cytotechnologist was negligent in misreading the 2008 Pap smear. If treatment had been started in 2008, the cancer could have been resolved with a simple conization biopsy.
DEFENDANTS’ DEFENSE The Pap smear interpretation was reasonable. The cancer could not have been diagnosed in 2008. The patient was at fault for failing to follow-up Pap smears during the next 2 years.
VERDICT After assigning 75% fault to the cytotechnologist and 25% fault to the patient, a Florida jury returned a $20,870,200 verdict, which was reduced to $15,816,699.
Disastrous off-label use of anticoagulation
When a pelvic abscess was found, a 50-year-old woman was admitted to the hospital for treatment. She was taking warfarin due to a history of venous thromboembolism.
Before the procedure, her physicians attempted to temporarily reverse her anticoagulation by administering Factor IX Complex (Profilnine SD, Grifols Biologicals). The dose ordered for the patient was nearly double the maximum recommended weight-based dose. Almost immediately after receiving the infusion, the patient went into cardiopulmonary arrest and died. An autopsy found the cause of death to be pulmonary emboli (PE).
ESTATE’S CLAIM An excessive dose of Profilnine caused PE. At the time of the incident, Profilnine was not FDA approved for warfarin reversal, although some off-label uses were recognized in emergent situations, such as intracranial bleeds.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $1.25 million Virginia settlement was reached.
Vesicovaginal fistula from ureteral injury
At a women’s health clinic, a patient reported continuous, heavy vaginal bleeding; pain; and shortness of breath when walking. She had a history of endometritis and multiple abdominal surgeries. Examination disclosed a profuse vaginal discharge, a normal cervix, and an enlarged uterus. The patient consented to abdominal hysterectomy and bilateral salpingo-oophorectomy performed by an ObGyn assisted by a resident.
During surgery, the ObGyn found that the patient’s uterus was at 16 to 20 weeks’ gestation size, with multiple serosal uterine fibroids and frank pus and necrosed fibroid tumors within the uterine cavity. The procedure took longer than planned because of extensive adhesions. After surgery, the patient was anemic and was given a beta-blocker for tachycardia. She was discharged 3 days later with 48 hours’ worth of intravenous antibiotics.
A month later, the patient reported urinary incontinence. She saw a urologist, who found a vesicovaginal fistula. The patient underwent nephrostomy-tube placement. Right ureterolysis and a right ureteral reimplant was performed 4 months later.
PATIENT’S CLAIM The ObGyn injured the right ureter during surgery.
DEFENDANTS’ DEFENSE The ureter injury is a known risk of the procedure. The injury was due to an infection or delayed effects of ischemia. The patient had a good recovery with no residual injury.
VERDICT A Michigan defense verdict was returned.
Why did mother die after delivering twins?
After a 35-year-old woman gave birth to twins by cesarean delivery, she died. At autopsy, 4 liters of blood were found in her abdomen.
ESTATE’S CLAIM The ObGyn failed to recognize and treat an arterial or venous bleed during surgery.
DEFENDANTS’ DEFENSE The patient died from amniotic fluid embolism. Autopsy results showed right ventricular heart failure, respiratory failure, and disseminated intravascular coagulation.
VERDICT A Florida defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Was fetus’ wrist injured during cesarean delivery?
At 34 weeks’ gestation, a 39-year-old woman went to the hospital in preterm labor. Her history included a prior cesarean delivery. Ultrasonography (US) showed that the fetus was in a double-footling breech position. The ObGyn decided to perform a cesarean delivery when the fetal heart-rate monitor indicated distress.
After making a midline incision through the earlier scar, the ObGyn created a low transverse uterine incision with a scalpel. The mother’s uterus was thick because labor had not progressed. When the ObGyn was unable to deliver the baby through the low transverse incision, she performed a T-extension of the incision using bandage scissors while placing her free hand inside the uterus to shield the fetus from injury. After extensive manipulation, the baby was delivered and immediately handed to a neonatologist. After surgery, the neonatologist told the mother that the baby had sustained two lacerations to the ulnar side of the right wrist. The newborn was airlifted to another hospital for treatment of sepsis. There, an orthopedic hand surgeon examined the child and determined that the lacerations were superficial and only required sutures. The orthopedist saw the infant a month later and believed there was no significant wrist injury.
When the child began preschool, she started to experience cold intolerance and difficulty writing with her right hand. The child was referred to a pediatric neurologist, who found no nerve damage and ordered occupational therapy.
The original orthopedic surgeon examined the child when she was 7 years old and determined that the flexor carpi ulnaris tendon had been completely severed with a partial injury to the ulnar nerve. He recommended a return visit at age 14 for full assessment of the wrist injury.
PARENTS’ CLAIM The ObGyn did not properly shield the fetus when performing the T-extension incision during cesarean delivery. The child’s weakness will increase with age, ruling out some occupations.
PHYSICIAN’S DEFENSE The ObGyn was not negligent; she had provided adequate protection of the fetus during both incisions.
VERDICT An Illinois defense verdict was returned.
Woman dies after tubal ligation
After a 42-year-old woman underwent tubal ligation, her surgeon was concerned about a possible bowel perforation and admitted her to the hospital. The next morning, a computed tomography (CT) scan of the abdomen did not reveal bowel injury.
That afternoon, when the patient reported shortness of breath, the surgeon called the hospitalist with concern for pulmonary embolism (PE). The hospitalist immediately ordered a CT scan of the chest, initiated PE protocol, and wrote “r/o PE” on the chart. A radiologist reminded the hospitalist of the earlier CT scan with concern for kidney damage from another dye study. The hospitalist cancelled the CT scan and PE protocol. After waiting 17 hours to run any further tests, a CT scan revealed massive bilateral PE. The patient was transferred to the ICU, but died the next day.
PATIENT’S CLAIM The 17-hour delay was negligent.
PHYSICIAN’S DEFENSE There was no negligence. The patient died of septic shock, not PE.
VERDICT A $4 million Virginia verdict was returned.
Child born without hand and forearm
During prenatal care, a mother underwent US at 20 and 36 weeks; both studies were reported as normal. The child was born missing his left hand and part of his left forearm due to a congenital amputation. The child will require prosthetics for life.
PATIENT’S CLAIM The condition should have been seen during prenatal US; an abortion was still an option at 20 weeks.
DEFENDANTS’ DEFENSE US was properly performed and evaluated. It can be difficult to differentiate the right from left extremities.
VERDICT A California defense verdict was returned.
After starting Yasmin, woman has stroke with permanent paralysis: $16.5M total award
When a 37-year-old woman reported irregular menstruation, her ObGyn prescribed drospirenone/ethinyl estradiol (Yasmin; Bayer). Thirteen days after starting the drug, the patient had a stroke. She is paralyzed on her left side, has limited ability to speak, cannot use her left arm and leg, and requires 24-hour care.
PATIENT’S CLAIM The ObGyn should have recognized that Yasmin was not appropriate for this patient because of the drug’s clotting risks. The patient’s risk factors included her age (over 35), borderline hypertension, overweight, history of smoking, and high cholesterol. The ObGyn should have offered safer alternatives, such as a progesterone-only pill. The US Food and Drug Administration (FDA) issued a safety warning that all drospirenone-containing drugs may be associated with a higher risk of venous thrombosis during the first 6 months of use.
DEFENDANTS’ DEFENSE According to Bayer, Yasmin is safe, and remains on the market. It was an appropriate drug to treat her irregular bleeding.
VERDICT Claims against the medical center that referred the patient to the ObGyn were settled for $2.5 million before trial. A $14 million Illinois verdict was returned against the ObGyn, for a total award of $16.5 million.
Who is at fault when pelvic mesh erodes?
In January 2011, an ObGyn implanted the Gynecare TVT Obturator System (TVT‑O; Ethicon) during a midurethral sling procedure to treat stress urinary incontinence (SUI) in a woman in her 60s. Shortly thereafter, the ObGyn left practice because of early-onset Alzheimer’s disease, and the patient’s care was taken over by a gynecologist.
At the 2-month postoperative visit, the gynecologist found that the mesh had eroded into the patient’s vagina. The gynecologist simply cut the mesh with a scissor, charted that a small erosion was present, and prescribed estrogen cream.
The patient continued to report pain, discomfort, pressure, difficulty voiding urine, continued incontinence, vaginal discharge, scarring, infection, odor, and bleeding.
PATIENT’S CLAIM The polypropylene mesh used during the midurethral sling procedure has been shown to be incompatible with human tissue. It promotes an immune response, which stimulates degradation of the pelvic tissue and can contribute to the development of severe adverse reactions to the mesh. Ethicon negligently designed, manufactured, marketed, labeled, and packaged the pelvic mesh products.
DEFENDANTS’ DEFENSE Proper warnings were provided about the health risks associated with polypropylene mesh products. The medical device was not properly sized.
VERDICT A Texas jury rejected the patient’s claims that Ethicon did not provide proper warnings about the sling’s health risks and declined to award punitive damages.
However, the jury decided that the mesh implant was defectively designed, and returned a $1.2 million verdict against Ethicon.
Was suspected bowel injury treated properly?
A 40-year-old woman was referred to an ObGyn after reporting abnormal uterine bleeding to her primary care physician. The patient had very light menses every few weeks. The ObGyn performed an ablation procedure, without relief. A month later, the ObGyn performed robot-assisted laparoscopic hysterectomy. The next day, the patient reported abdominal pain. Suspecting a bowel injury, the ObGyn ordered a CT scan; the bowel appeared normal, so the ObGyn referred the patient to a surgeon. During exploratory laparotomy, the surgeon found and repaired a bowel injury. The patient developed significant complications from a necrotizing infection that included respiratory distress and ongoing wound care.
PATIENT’S CLAIM Conservative treatment should have been offered before surgery. The ObGyn should have waited longer after the ablation procedure before doing the hysterectomy. The ObGyn should have checked for a possible bowel injury before closing the hysterectomy.
PHYSICIAN’S DEFENSE The bowel injury is a known complication of the procedure and was recognized and repaired in a timely manner.
VERDICT A Kentucky defense verdict was returned.
Pap smear improperly interpreted: Woman dies from cervical cancer
A 37-year-old woman underwent a pap smear in 2008 that was read by a cytotechnologist as normal. Two years later, the patient was found to have a golf-ball–sized cancerous tumor. She died from cervical cancer in 2011.
ESTATE’S CLAIM The cytotechnologist was negligent in misreading the 2008 Pap smear. If treatment had been started in 2008, the cancer could have been resolved with a simple conization biopsy.
DEFENDANTS’ DEFENSE The Pap smear interpretation was reasonable. The cancer could not have been diagnosed in 2008. The patient was at fault for failing to follow-up Pap smears during the next 2 years.
VERDICT After assigning 75% fault to the cytotechnologist and 25% fault to the patient, a Florida jury returned a $20,870,200 verdict, which was reduced to $15,816,699.
Disastrous off-label use of anticoagulation
When a pelvic abscess was found, a 50-year-old woman was admitted to the hospital for treatment. She was taking warfarin due to a history of venous thromboembolism.
Before the procedure, her physicians attempted to temporarily reverse her anticoagulation by administering Factor IX Complex (Profilnine SD, Grifols Biologicals). The dose ordered for the patient was nearly double the maximum recommended weight-based dose. Almost immediately after receiving the infusion, the patient went into cardiopulmonary arrest and died. An autopsy found the cause of death to be pulmonary emboli (PE).
ESTATE’S CLAIM An excessive dose of Profilnine caused PE. At the time of the incident, Profilnine was not FDA approved for warfarin reversal, although some off-label uses were recognized in emergent situations, such as intracranial bleeds.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $1.25 million Virginia settlement was reached.
Vesicovaginal fistula from ureteral injury
At a women’s health clinic, a patient reported continuous, heavy vaginal bleeding; pain; and shortness of breath when walking. She had a history of endometritis and multiple abdominal surgeries. Examination disclosed a profuse vaginal discharge, a normal cervix, and an enlarged uterus. The patient consented to abdominal hysterectomy and bilateral salpingo-oophorectomy performed by an ObGyn assisted by a resident.
During surgery, the ObGyn found that the patient’s uterus was at 16 to 20 weeks’ gestation size, with multiple serosal uterine fibroids and frank pus and necrosed fibroid tumors within the uterine cavity. The procedure took longer than planned because of extensive adhesions. After surgery, the patient was anemic and was given a beta-blocker for tachycardia. She was discharged 3 days later with 48 hours’ worth of intravenous antibiotics.
A month later, the patient reported urinary incontinence. She saw a urologist, who found a vesicovaginal fistula. The patient underwent nephrostomy-tube placement. Right ureterolysis and a right ureteral reimplant was performed 4 months later.
PATIENT’S CLAIM The ObGyn injured the right ureter during surgery.
DEFENDANTS’ DEFENSE The ureter injury is a known risk of the procedure. The injury was due to an infection or delayed effects of ischemia. The patient had a good recovery with no residual injury.
VERDICT A Michigan defense verdict was returned.
Why did mother die after delivering twins?
After a 35-year-old woman gave birth to twins by cesarean delivery, she died. At autopsy, 4 liters of blood were found in her abdomen.
ESTATE’S CLAIM The ObGyn failed to recognize and treat an arterial or venous bleed during surgery.
DEFENDANTS’ DEFENSE The patient died from amniotic fluid embolism. Autopsy results showed right ventricular heart failure, respiratory failure, and disseminated intravascular coagulation.
VERDICT A Florida defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Using the Internet in your practice. Part 3: Maximizing your online reach through SEO and pay-per-click
It’s high time to go beyond relying on YellowPages.com to attract patients to your practice. According to research and analysis company BIA/Kelsey, “nearly all consumers (97%) now use online media to shop locally.”1 Today’s patients are Internet savvy and expect their ObGyn not only to be a good physician who diagnoses and treats their conditions but also to demonstrate his or her electronic technical abilities, through online communication, a Web site, email newsletter outreach, and, yes, a social media presence.
Yet, being the best in your field or having an incredible Web site won’t matter if your existing patients and potential new patients can’t find you on the Internet. The solution? Get your Web site onto the first page of Google’s search results. Why? Google is still by far the dominant search engine in the United States, and it has the overwhelming ability to offer you an endless supply of patients. And can your practice survive without a steady flow of new patients?
Type a keyword into the Google search window and dozens, hundreds, or thousands of sites may become visible. Google lists 10 sites per page, however, and when was the last time you went to the second, third, or fourth results page looking for something? If your Web site does not appear on the first results page, you are essentially invisible. The good news is that you can easily and inexpensively reach the top of a search engine’s results page by knowing and applying best practices for effective search engine optimization (SEO; a free endeavor, although your time investment should considered) and/or utilizing a pay-per-click (PPC) service (for a fee that’s comparable to what you used to pay for your old Yellow Pages listing). In this article, we aim to provide to you the know-how to embark on these endeavors by defining SEO and PPC and relaying effective principles for marketing your practice to potential new patients.
What is SEO?
SEO refers to techniques that help your Web site rank higher in organic (natural) results, which helps your site, and you, become more visible to people who are seeking your services. Search engines specialize in offering Web surfers the best information about their search terms, or keywords. To do this, Google uses more than 200 different algorithms, some of which we know but most of which are not published.
The algorithms are used to determine where your Web site ranks according to a number of factors relevant to the content and set-up of each page of your Web site (on-page SEO) as well as everything you do outside of your Web site (off-page SEO) to enhance your SEO rankings. This relevance is calculated by looking at both on-page and off-page factors, including:
- what you are doing in relation to your competition
- how long your Web site has been active
- search engine submission
- article submission
- directory submission
- linking strategies.
You have to work on both on-page and off-page factors many times a month to convince the search engines that you are a Web site worth visiting. Over time, your site will start to rise in the rankings and gain qualified traffic. Then you can concentrate on converting those Web site visitors to office-based patients.
A warning: If you use unethical tactics to get your Web site on the first page of the search results, Google will catch up to you—and your Web-site rankings will plummet.
Five important steps to increase SEO
There’s a system to reach the top of any search engine’s results page. The most important steps are to:
- Use keywords in your Web site coding, or page description (called meta tags).
- Use keywords in your Web site copy.
- Develop in-bound links.
- Post new keyword-related content regularly—typically accomplished through a blog.
- Integrate your keywords in social media postings.
1 and 2. Keywords are key
Keywords are what an Internet surfer enters into a search function and what the search engine crawlers hunt for. The crawlers then direct the surfer to the Web site that is perceived as the best source of information.
Here are the most popular keywords used by potential patients looking for ObGyns: obstetrician, gynecologist, gynecology, vaginal discharge, vaginal dryness, breast self-exam, breast cancer screening, prolapsed bladder, pelvic pain, and adolescent gynecology. You also should include your city’s name as a keyword.
Use keywords in Web site coding and copy. Unless you are experienced in Web development, you’re better off hiring a professional who knows Internet coding to help you develop HTML meta tags, anchor text, a sitemap, etc. You easily can incorporate keywords in the copy on your Web site, but the keyword density should be no more than approximately 3% to 5% of the copy. (If it is more than 5%, it is considered “stuffing,” and not looked on kindly by Google.)
3. In-bound links: Who’s linking to you?
As the search engine crawlers scan Web pages for indexing, they also look for links from other Web sites. The greater number of quality in-bound links a Web site has, the stronger influence or authority it accrues.
In-bound links are weighted differently: a link from a highly authoritative Web site like NYTimes.com will give a Web site a bigger boost than a link from a small blog site. Links from high-ranking sites, such as city directories, hospitals, and online medical directories, improve your Web-site ranking. You should be submitting your Web site address and keyword description to these appropriate directories for in-bound links on a weekly or monthly basis.
4. Develop new content by blogging
Search engines place a high value on new content, and the easiest way to add new keyword-related content is to blog. Writing a 400-word keyword–relevant blog on a regular basis will provide the search engine crawlers with new content to graze.
As an added bonus, there are many medical ezines—small magazines and newsletters distributed by any electronic method—that regularly need content. Publication of your blog article will provide additional back-links to your Web site and improve your SEO rankings. This is your opportunity to go “viral,” have your material read by thousands, and increase visits to your Web site. When your name appears on multiple sites, you create the perception of demonstrating your expertise in various topics, techniques, and therapeutic options.
5. Search engines love social media
Newer technologies are given greater weight in determining Web page ranking. Start with blogging and then add Facebook, YouTube, and Twitter. Always remember to link from these sites with relevant keywords to the exact page on your site that contains the best information for those keywords.
Advertise your practice using PPC (Google AdWords)
Google, Yahoo, and other Internet portals make their money by selling advertisements on search-results pages. Both paid and organic listings appear on the search results pages, but they are displayed in different locations. On Google, PPC listings are found on the top and right side of each page under the header “Ads” (FIGURE 1). The organic or natural search (no payment required) is on the left below the ads.
On Google, the PPC function is called “AdWords” (http://www.google.com/adwords). On the AdWords page, a listing is found that offers how many times people type in certain words or phrases—keywords. Google AdWords allows the marketplace to bid on keywords; the higher the bid, the closer to the top position on the first page of the Google search results. Depending on monthly search volume, popularity, and competition, you can pay anywhere from pennies to $25 each time a Web surfer clicks on your ad. In FIGURE 2, you can see that a suggested bid for “Gynecologist Miami” is $2.96.
You must constantly monitor and manage your AdWords account. Test different landing pages, adjust your copy, and change offerings to make sure you are converting your paid traffic to patients. Otherwise, you can spend hundreds of dollars each month without achieving the desired outcome.
By doing your own research with Google AdWords’ keyword planner, you will see the variations of keywords that you can use in the copy of your Web site and related content for organic SE
Patient conversion: Your ultimate goal
Google is only one piece of the Internet marketing puzzle. Once you have invested in mastering the SEO rankings (by doing it yourself or by paying for professional help), it’s up to your Web site to convert the visitor to a paying patient. To maximize your return on investment (ROI), implement marketing strategies and a patient conversion system on your Web site. When a prospective patient lands there, you have less than 10 seconds to engage her. In Part 1of this series, we discuss features that will keep your visitor involved while she navigates the site and make it easy for her to make an appointment. Don’t lose her because she can’t find your contact information hidden at the bottom of the page.2
If you don’t want to spend the time and effort to do it yourself, outsourcing is a cost-effective solution, and a trackable and measurable way for you to calculate your ROI.
Bottom line: Be seen on the Internet. We are all connected to the Internet every waking moment. This is where we go for information; this is how we communicate with each other; and this is where we create relationships. If you want to build your practice, you have to be where your patient can find you—on the top of an Internet search results page.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Pacheco E, Udowitz R. Nearly all consumers (97%) now use online media to shop locally, according to BIA/Kelsey and ConStat [press release]. BIA/Kelsey Web site. http://www.biakelsey.com/company/press-releases/100310-nearly-all-consumers-now-use-online-media-to-shop-locally.asp. Published March 10, 2010. Accessed August 12, 2014.
2. Baum NH, Romano R. Using the Internet in your practice. Part 1: why social media are important and how to get started. OBG Manag. 2014;26(2):25–36.
It’s high time to go beyond relying on YellowPages.com to attract patients to your practice. According to research and analysis company BIA/Kelsey, “nearly all consumers (97%) now use online media to shop locally.”1 Today’s patients are Internet savvy and expect their ObGyn not only to be a good physician who diagnoses and treats their conditions but also to demonstrate his or her electronic technical abilities, through online communication, a Web site, email newsletter outreach, and, yes, a social media presence.
Yet, being the best in your field or having an incredible Web site won’t matter if your existing patients and potential new patients can’t find you on the Internet. The solution? Get your Web site onto the first page of Google’s search results. Why? Google is still by far the dominant search engine in the United States, and it has the overwhelming ability to offer you an endless supply of patients. And can your practice survive without a steady flow of new patients?
Type a keyword into the Google search window and dozens, hundreds, or thousands of sites may become visible. Google lists 10 sites per page, however, and when was the last time you went to the second, third, or fourth results page looking for something? If your Web site does not appear on the first results page, you are essentially invisible. The good news is that you can easily and inexpensively reach the top of a search engine’s results page by knowing and applying best practices for effective search engine optimization (SEO; a free endeavor, although your time investment should considered) and/or utilizing a pay-per-click (PPC) service (for a fee that’s comparable to what you used to pay for your old Yellow Pages listing). In this article, we aim to provide to you the know-how to embark on these endeavors by defining SEO and PPC and relaying effective principles for marketing your practice to potential new patients.
What is SEO?
SEO refers to techniques that help your Web site rank higher in organic (natural) results, which helps your site, and you, become more visible to people who are seeking your services. Search engines specialize in offering Web surfers the best information about their search terms, or keywords. To do this, Google uses more than 200 different algorithms, some of which we know but most of which are not published.
The algorithms are used to determine where your Web site ranks according to a number of factors relevant to the content and set-up of each page of your Web site (on-page SEO) as well as everything you do outside of your Web site (off-page SEO) to enhance your SEO rankings. This relevance is calculated by looking at both on-page and off-page factors, including:
- what you are doing in relation to your competition
- how long your Web site has been active
- search engine submission
- article submission
- directory submission
- linking strategies.
You have to work on both on-page and off-page factors many times a month to convince the search engines that you are a Web site worth visiting. Over time, your site will start to rise in the rankings and gain qualified traffic. Then you can concentrate on converting those Web site visitors to office-based patients.
A warning: If you use unethical tactics to get your Web site on the first page of the search results, Google will catch up to you—and your Web-site rankings will plummet.
Five important steps to increase SEO
There’s a system to reach the top of any search engine’s results page. The most important steps are to:
- Use keywords in your Web site coding, or page description (called meta tags).
- Use keywords in your Web site copy.
- Develop in-bound links.
- Post new keyword-related content regularly—typically accomplished through a blog.
- Integrate your keywords in social media postings.
1 and 2. Keywords are key
Keywords are what an Internet surfer enters into a search function and what the search engine crawlers hunt for. The crawlers then direct the surfer to the Web site that is perceived as the best source of information.
Here are the most popular keywords used by potential patients looking for ObGyns: obstetrician, gynecologist, gynecology, vaginal discharge, vaginal dryness, breast self-exam, breast cancer screening, prolapsed bladder, pelvic pain, and adolescent gynecology. You also should include your city’s name as a keyword.
Use keywords in Web site coding and copy. Unless you are experienced in Web development, you’re better off hiring a professional who knows Internet coding to help you develop HTML meta tags, anchor text, a sitemap, etc. You easily can incorporate keywords in the copy on your Web site, but the keyword density should be no more than approximately 3% to 5% of the copy. (If it is more than 5%, it is considered “stuffing,” and not looked on kindly by Google.)
3. In-bound links: Who’s linking to you?
As the search engine crawlers scan Web pages for indexing, they also look for links from other Web sites. The greater number of quality in-bound links a Web site has, the stronger influence or authority it accrues.
In-bound links are weighted differently: a link from a highly authoritative Web site like NYTimes.com will give a Web site a bigger boost than a link from a small blog site. Links from high-ranking sites, such as city directories, hospitals, and online medical directories, improve your Web-site ranking. You should be submitting your Web site address and keyword description to these appropriate directories for in-bound links on a weekly or monthly basis.
4. Develop new content by blogging
Search engines place a high value on new content, and the easiest way to add new keyword-related content is to blog. Writing a 400-word keyword–relevant blog on a regular basis will provide the search engine crawlers with new content to graze.
As an added bonus, there are many medical ezines—small magazines and newsletters distributed by any electronic method—that regularly need content. Publication of your blog article will provide additional back-links to your Web site and improve your SEO rankings. This is your opportunity to go “viral,” have your material read by thousands, and increase visits to your Web site. When your name appears on multiple sites, you create the perception of demonstrating your expertise in various topics, techniques, and therapeutic options.
5. Search engines love social media
Newer technologies are given greater weight in determining Web page ranking. Start with blogging and then add Facebook, YouTube, and Twitter. Always remember to link from these sites with relevant keywords to the exact page on your site that contains the best information for those keywords.
Advertise your practice using PPC (Google AdWords)
Google, Yahoo, and other Internet portals make their money by selling advertisements on search-results pages. Both paid and organic listings appear on the search results pages, but they are displayed in different locations. On Google, PPC listings are found on the top and right side of each page under the header “Ads” (FIGURE 1). The organic or natural search (no payment required) is on the left below the ads.
On Google, the PPC function is called “AdWords” (http://www.google.com/adwords). On the AdWords page, a listing is found that offers how many times people type in certain words or phrases—keywords. Google AdWords allows the marketplace to bid on keywords; the higher the bid, the closer to the top position on the first page of the Google search results. Depending on monthly search volume, popularity, and competition, you can pay anywhere from pennies to $25 each time a Web surfer clicks on your ad. In FIGURE 2, you can see that a suggested bid for “Gynecologist Miami” is $2.96.
You must constantly monitor and manage your AdWords account. Test different landing pages, adjust your copy, and change offerings to make sure you are converting your paid traffic to patients. Otherwise, you can spend hundreds of dollars each month without achieving the desired outcome.
By doing your own research with Google AdWords’ keyword planner, you will see the variations of keywords that you can use in the copy of your Web site and related content for organic SE
Patient conversion: Your ultimate goal
Google is only one piece of the Internet marketing puzzle. Once you have invested in mastering the SEO rankings (by doing it yourself or by paying for professional help), it’s up to your Web site to convert the visitor to a paying patient. To maximize your return on investment (ROI), implement marketing strategies and a patient conversion system on your Web site. When a prospective patient lands there, you have less than 10 seconds to engage her. In Part 1of this series, we discuss features that will keep your visitor involved while she navigates the site and make it easy for her to make an appointment. Don’t lose her because she can’t find your contact information hidden at the bottom of the page.2
If you don’t want to spend the time and effort to do it yourself, outsourcing is a cost-effective solution, and a trackable and measurable way for you to calculate your ROI.
Bottom line: Be seen on the Internet. We are all connected to the Internet every waking moment. This is where we go for information; this is how we communicate with each other; and this is where we create relationships. If you want to build your practice, you have to be where your patient can find you—on the top of an Internet search results page.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It’s high time to go beyond relying on YellowPages.com to attract patients to your practice. According to research and analysis company BIA/Kelsey, “nearly all consumers (97%) now use online media to shop locally.”1 Today’s patients are Internet savvy and expect their ObGyn not only to be a good physician who diagnoses and treats their conditions but also to demonstrate his or her electronic technical abilities, through online communication, a Web site, email newsletter outreach, and, yes, a social media presence.
Yet, being the best in your field or having an incredible Web site won’t matter if your existing patients and potential new patients can’t find you on the Internet. The solution? Get your Web site onto the first page of Google’s search results. Why? Google is still by far the dominant search engine in the United States, and it has the overwhelming ability to offer you an endless supply of patients. And can your practice survive without a steady flow of new patients?
Type a keyword into the Google search window and dozens, hundreds, or thousands of sites may become visible. Google lists 10 sites per page, however, and when was the last time you went to the second, third, or fourth results page looking for something? If your Web site does not appear on the first results page, you are essentially invisible. The good news is that you can easily and inexpensively reach the top of a search engine’s results page by knowing and applying best practices for effective search engine optimization (SEO; a free endeavor, although your time investment should considered) and/or utilizing a pay-per-click (PPC) service (for a fee that’s comparable to what you used to pay for your old Yellow Pages listing). In this article, we aim to provide to you the know-how to embark on these endeavors by defining SEO and PPC and relaying effective principles for marketing your practice to potential new patients.
What is SEO?
SEO refers to techniques that help your Web site rank higher in organic (natural) results, which helps your site, and you, become more visible to people who are seeking your services. Search engines specialize in offering Web surfers the best information about their search terms, or keywords. To do this, Google uses more than 200 different algorithms, some of which we know but most of which are not published.
The algorithms are used to determine where your Web site ranks according to a number of factors relevant to the content and set-up of each page of your Web site (on-page SEO) as well as everything you do outside of your Web site (off-page SEO) to enhance your SEO rankings. This relevance is calculated by looking at both on-page and off-page factors, including:
- what you are doing in relation to your competition
- how long your Web site has been active
- search engine submission
- article submission
- directory submission
- linking strategies.
You have to work on both on-page and off-page factors many times a month to convince the search engines that you are a Web site worth visiting. Over time, your site will start to rise in the rankings and gain qualified traffic. Then you can concentrate on converting those Web site visitors to office-based patients.
A warning: If you use unethical tactics to get your Web site on the first page of the search results, Google will catch up to you—and your Web-site rankings will plummet.
Five important steps to increase SEO
There’s a system to reach the top of any search engine’s results page. The most important steps are to:
- Use keywords in your Web site coding, or page description (called meta tags).
- Use keywords in your Web site copy.
- Develop in-bound links.
- Post new keyword-related content regularly—typically accomplished through a blog.
- Integrate your keywords in social media postings.
1 and 2. Keywords are key
Keywords are what an Internet surfer enters into a search function and what the search engine crawlers hunt for. The crawlers then direct the surfer to the Web site that is perceived as the best source of information.
Here are the most popular keywords used by potential patients looking for ObGyns: obstetrician, gynecologist, gynecology, vaginal discharge, vaginal dryness, breast self-exam, breast cancer screening, prolapsed bladder, pelvic pain, and adolescent gynecology. You also should include your city’s name as a keyword.
Use keywords in Web site coding and copy. Unless you are experienced in Web development, you’re better off hiring a professional who knows Internet coding to help you develop HTML meta tags, anchor text, a sitemap, etc. You easily can incorporate keywords in the copy on your Web site, but the keyword density should be no more than approximately 3% to 5% of the copy. (If it is more than 5%, it is considered “stuffing,” and not looked on kindly by Google.)
3. In-bound links: Who’s linking to you?
As the search engine crawlers scan Web pages for indexing, they also look for links from other Web sites. The greater number of quality in-bound links a Web site has, the stronger influence or authority it accrues.
In-bound links are weighted differently: a link from a highly authoritative Web site like NYTimes.com will give a Web site a bigger boost than a link from a small blog site. Links from high-ranking sites, such as city directories, hospitals, and online medical directories, improve your Web-site ranking. You should be submitting your Web site address and keyword description to these appropriate directories for in-bound links on a weekly or monthly basis.
4. Develop new content by blogging
Search engines place a high value on new content, and the easiest way to add new keyword-related content is to blog. Writing a 400-word keyword–relevant blog on a regular basis will provide the search engine crawlers with new content to graze.
As an added bonus, there are many medical ezines—small magazines and newsletters distributed by any electronic method—that regularly need content. Publication of your blog article will provide additional back-links to your Web site and improve your SEO rankings. This is your opportunity to go “viral,” have your material read by thousands, and increase visits to your Web site. When your name appears on multiple sites, you create the perception of demonstrating your expertise in various topics, techniques, and therapeutic options.
5. Search engines love social media
Newer technologies are given greater weight in determining Web page ranking. Start with blogging and then add Facebook, YouTube, and Twitter. Always remember to link from these sites with relevant keywords to the exact page on your site that contains the best information for those keywords.
Advertise your practice using PPC (Google AdWords)
Google, Yahoo, and other Internet portals make their money by selling advertisements on search-results pages. Both paid and organic listings appear on the search results pages, but they are displayed in different locations. On Google, PPC listings are found on the top and right side of each page under the header “Ads” (FIGURE 1). The organic or natural search (no payment required) is on the left below the ads.
On Google, the PPC function is called “AdWords” (http://www.google.com/adwords). On the AdWords page, a listing is found that offers how many times people type in certain words or phrases—keywords. Google AdWords allows the marketplace to bid on keywords; the higher the bid, the closer to the top position on the first page of the Google search results. Depending on monthly search volume, popularity, and competition, you can pay anywhere from pennies to $25 each time a Web surfer clicks on your ad. In FIGURE 2, you can see that a suggested bid for “Gynecologist Miami” is $2.96.
You must constantly monitor and manage your AdWords account. Test different landing pages, adjust your copy, and change offerings to make sure you are converting your paid traffic to patients. Otherwise, you can spend hundreds of dollars each month without achieving the desired outcome.
By doing your own research with Google AdWords’ keyword planner, you will see the variations of keywords that you can use in the copy of your Web site and related content for organic SE
Patient conversion: Your ultimate goal
Google is only one piece of the Internet marketing puzzle. Once you have invested in mastering the SEO rankings (by doing it yourself or by paying for professional help), it’s up to your Web site to convert the visitor to a paying patient. To maximize your return on investment (ROI), implement marketing strategies and a patient conversion system on your Web site. When a prospective patient lands there, you have less than 10 seconds to engage her. In Part 1of this series, we discuss features that will keep your visitor involved while she navigates the site and make it easy for her to make an appointment. Don’t lose her because she can’t find your contact information hidden at the bottom of the page.2
If you don’t want to spend the time and effort to do it yourself, outsourcing is a cost-effective solution, and a trackable and measurable way for you to calculate your ROI.
Bottom line: Be seen on the Internet. We are all connected to the Internet every waking moment. This is where we go for information; this is how we communicate with each other; and this is where we create relationships. If you want to build your practice, you have to be where your patient can find you—on the top of an Internet search results page.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Pacheco E, Udowitz R. Nearly all consumers (97%) now use online media to shop locally, according to BIA/Kelsey and ConStat [press release]. BIA/Kelsey Web site. http://www.biakelsey.com/company/press-releases/100310-nearly-all-consumers-now-use-online-media-to-shop-locally.asp. Published March 10, 2010. Accessed August 12, 2014.
2. Baum NH, Romano R. Using the Internet in your practice. Part 1: why social media are important and how to get started. OBG Manag. 2014;26(2):25–36.
1. Pacheco E, Udowitz R. Nearly all consumers (97%) now use online media to shop locally, according to BIA/Kelsey and ConStat [press release]. BIA/Kelsey Web site. http://www.biakelsey.com/company/press-releases/100310-nearly-all-consumers-now-use-online-media-to-shop-locally.asp. Published March 10, 2010. Accessed August 12, 2014.
2. Baum NH, Romano R. Using the Internet in your practice. Part 1: why social media are important and how to get started. OBG Manag. 2014;26(2):25–36.