User login
Treating bipolar mania in the outpatient setting: Risk vs reward
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
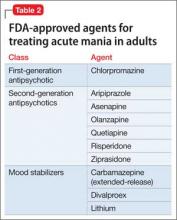
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Suicide assessment and management self-test: How do you score?
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
Pediatric Surgical Comanagement
According to the 2012 Society of Hospital Medicine (SHM) survey, 94% of adult hospitalists and 74% of pediatric hospitalists provide inpatient care to surgical patients.[1] Many of these programs involve comanagement, which the SHM Comanagement Advisory Panel has described as a system of care featuring shared responsibility, authority, and accountability for hospitalized patients with medical and surgical needs.[2] Collaboration between medical and surgical teams for these patients has occurred commonly at some community institutions for decades, but may only be emerging at some tertiary care hospitals. The trend of comanagement appears to be increasing in popularity in adult medicine.[3] As in adult patients, comanagement for children undergoing surgical procedures, particularly those children with special healthcare needs (CSHCNs), has been proposed as a strategy for improving quality and costs. In this review, we will describe structural, quality, and financial implications of pediatric hospitalist comanagement programs, each of which include both potential benefits and drawbacks, as well as discuss a future research agenda for these programs.
ORGANIZATIONAL NEEDS AND STRUCTURE OF COMANAGEMENT PROGRAMS
Patterns of comanagement likely depend on hospital size and structure, both in adult patients[3] and in pediatrics. Children hospitalized for surgical procedures generally fall into 1 of 2 groups: those who are typically healthy and at low risk for complications, and those who are medically complex and at high risk. Healthy children often undergo high‐prevalence, low‐complexity surgical procedures such as tonsillectomy and hernia repairs;[4] these patients are commonly cared for at community hospitals by adult and pediatric surgeons. Whereas medically complex children also undergo these common procedures, they are more likely to be cared for at tertiary care centers and are also more likely to undergo higher‐complexity surgeries such as spinal fusions, hip osteotomies, and ventriculoperitoneal shunt placements.[4] Hospitalist comanagement programs at community hospitals and tertiary care centers may therefore have evolved differently in response to different needs of patients, providers, and organizations,[5] though some institutions may not fall neatly into 1 of these 2 categories.
Comanagement in Community Hospitals
A significant number of pediatric patients are hospitalized each year in community hospitals.[6] As noted above, children undergoing surgery in these settings are generally healthy, and may be cared for by surgeons with varying amounts of pediatric expertise. In this model, the surgeon may frequently be offsite when not in the operating room, necessitating some type of onsite postoperative coverage. In pediatrics, following adult models, this coverage need may be relatively straightforward: surgeons perform the procedure, followed by a medical team assuming postoperative care with surgical consultation. Because of general surgeons' varying experience with children, the American Academy of Pediatrics suggests that patients younger than 14 years or weighing less than 40 kg cared for by providers without routine pediatric experience should have a pediatric‐trained provider involved in their care,[7] though this suggestion does not mandate comanagement.
For children cared for by adult providers who have little experience with areas of pediatric‐specific care such as medication dosing and assessment of deterioration, we believe that involvement of a pediatric provider may impact care in a number of ways. A pediatric hospitalist's availability on the inpatient unit may allow him or her to better manage routine issues such as pain control and intravenous fluids without surgeon input, improving the efficiency of care. A pediatric hospitalist's general pediatric training may allow him or her to more quickly recognize when a child is medically deteriorating, or when transfer to a tertiary care center may be necessary, making care safer. However, no studies have specifically examined these variables. Future research should measure outcomes such as transfers to higher levels of care, medication errors, length of stay (LOS), and complication rates, especially in community hospital settings.
Comanagement in Tertiary Care Referral Centers
At tertiary care referral centers, surgeries in children are most often performed by pediatric surgeons. In these settings, providing routine hospitalist comanagement to all patients may be neither cost‐effective nor feasible. Adult studies have suggested that population‐targeted models can significantly improve several clinical outcomes. For example, in several studies of patients 65 years and older hospitalized with hip fractures, comanagement with a geriatric hospitalist was associated with improved clinical outcomes and shortened LOS.[8, 9, 10, 11, 12]
An analogous group of pediatric patients to the geriatric population may be CSHCNs or children who are medically complex. Several frameworks have been proposed to identify these patients.[13] Many institutions classify medically complex patients as those with complex chronic medical conditions (CCCs).[13, 14, 15] One framework to identify CSHCNs suggested including not only children with CCCs, but also those with (1) substantial service needs and/or family burden; (2) severe functional limitations; and/or (3) high rates of healthcare system utilization, often requiring the care of several subspecialty providers.[16] As the needs of these patients may be quite diverse, pediatric hospitalists may be involved in many aspects of their care, from preoperative evaluation,[17] to establishing protocols for best practices, to communicating with primary care providers, and even seeing patients in postoperative follow‐up clinics. These patients are known to be at high risk for surgical complications, readmissions,[14] medical errors,[18] lapses in communication, and high care costs. In 1 study, comanagement for children with neuromuscular scoliosis hospitalized for spinal fusion surgery has been associated with shorter LOS and less variability in LOS.[19] However, drawbacks of comanagement programs involving CSHCNs may include difficulty with consistent identification of the population who will most benefit from comanagement and higher initial costs of care.[18]
Models of Comanagement and Comanagement Agreements
A comanagement agreement should address 5 major questions: (1) Who is the primary service? (2) Who is the consulting/comanaging service? (3) Are consults as‐needed or automatic? (4) Who writes orders for the patient? (5) Which staffing model will be used for patient care?[20] Although each question above may be answered differently in different systems, the correct comanagement program is a program that aligns most closely with the patient population and care setting.[11, 20]
Several different models exist for hospitalistsurgeon comanagement programs[20, 21] (Table 1). Under the consultation model (model I), hospitalists become involved in the care of surgical patients only when requested to do so by the surgical team. Criteria for requesting this kind of consultation and the extent of responsibility afforded to the medical team are often not clearly defined, and may differ from hospital to hospital or even surgeon to surgeon.[22] Hospitalist involvement with adult patients with postoperative medical complications, which presumably employed this as‐needed model, has been associated with lower mortality and LOS[23]; whether this involvement provides similar benefits in children with postoperative complications has not been explicitly studied.
| Model | Attending Service | Consulting Service | Automatic Consultation | Who Writes Orders? | Notes |
|---|---|---|---|---|---|
| |||||
| I | Surgery | Pediatrics | No | Surgery | Similar to traditional consultation |
| II | Surgery | Pediatrics | Yes | Usually surgery | Basic comanagement, consultant may sign off |
| III | Pediatrics | Surgery | Yes | Usually pediatrics | Basic comanagement, consultant may sign off |
| IV | Combined | N/A | N/A | Each service writes own | True comanagement, no sign‐off from either service permitted |
The remaining models involve compulsory participation by both surgical and medical services. In models II and III, patients may be evaluated preoperatively; those felt to meet specific criteria for high medical complexity are either admitted to a medical service with automatic surgical consultation or admitted to a surgical service with automatic medical consultation. In both cases, writing of orders is handled in the same manner as any consultation model; depending on the service agreement, consulting services may sign off or may be required to be involved until discharge. In model IV, care is fully comanaged by medical and surgical services, with each service having ownership over orders pertaining to their discipline. Ethical concerns about such agreements outlined by the American Medical Association include whether all patients cared for under agreements II to IV will truly benefit from the cost of multispecialty care, and whether informed consent from patients themselves should be required given the cost implications.[24]
Comanagement models also vary with respect to frontline provider staffing. Models may incorporate nurse practitioners, hospitalists, physician assistants, or a combination thereof. These providers may assume a variety of roles, including preoperative patient evaluation, direct care of patients while hospitalized, and/or coordination of inpatient and outpatient postoperative care. Staffing requirements for hospitalists and/or mid‐level providers will differ significantly at different institutions based on surgical volume, patient complexity, and other local factors.
COMANAGEMENT AND QUALITY
Comanagement as a Family‐Centered Initiative
Development of a family‐centered culture of care, including care coordination, lies at the core of pediatric hospital medicine, particularly for CSHCNs.[25, 26] In the outpatient setting, family‐centered care has been associated with improved quality of care for CSHCNs.[27, 28] For families of hospitalized children, issues such as involvement in care and timely information transfer have been identified as high priorities.[29] An important tool for addressing these needs is family‐centered rounds (FCRs), which represent multidisciplinary rounds at the bedside involving families and patients as active shared decision makers in conjunction with the medical team.[30, 31] Although FCRs have not been studied in comanagement arrangements specifically, evidence suggests that this tool improves family centeredness and patient safety in nonsurgical patients,[32] and FCRs can likely have a similar impact on postoperative care.
A pediatric hospitalist comanagement program may impact quality and safety of care in a number of other ways. Hospitalists may offer improved access to clinical information for nurses and families, making care safer. One study of comanagement in adult neurosurgical patients found that access to hospitalists led to improved quality and safety of care as perceived by nurses and other members of the care team.[33] A study in pediatric patients found that nurses overwhelmingly supported having hospitalist involvement in complex children undergoing surgery; the same study found that pediatric hospitalists were particularly noted for their communication skills.[34]
Assessing Clinical Outcomes in Pediatric Hospitalist Comanagement Programs
Most studies evaluating the impact of surgical comanagement programs have focused on global metrics such as LOS, overall complication rates, and resource utilization. In adults, results of these studies have been mixed, suggesting that patient selection may be an important factor.[35] In pediatrics, 2 US studies have assessed these metrics at single centers. Simon et al. found that involvement of a pediatric hospitalist in comanagement of patients undergoing spinal fusion surgery significantly decreased LOS.[19] Rappaport et al. found that patients comanaged by hospitalists had lower utilization of laboratory tests and parenteral nutrition, though initial program costs significantly increased.[36] Studies outside the United States, including a study from Sweden,[37] have suggested that a multidisciplinary approach to children's surgical care, including the presence of pediatric specialists, reduced infection rate and other complications. These studies provide general support for the role of hospitalists in comanagement, although determining which aspects of care are most impacted may be difficult.
Comanagement programs might impact safety and quality negatively as well. Care may be fragmented, leading to provider and family dissatisfaction. Poor communication and multiple handoffs among multidisciplinary team members might interfere with the central role of the nurse in patient care.[38] Comanagement programs might lead to provider disengagement if providers feel that others will assume roles with which they may be unfamiliar or poorly trained.[35] This lack of knowledge may also affect communication with families, leading to conflicting messages among the care team and family frustration. In addition, the impact of comanagement programs on trainees such as residents, both surgical and pediatric, has received limited study.[39] Assessing pediatric comanagement programs' impact on communication, family‐centeredness, and trainees deserves further study.
FINANCIAL IMPLICATIONS OF PEDIATRIC COMANAGEMENT PROGRAMS
Children undergoing surgery require significant financial resources for their care. A study of 38 major US children's hospitals found that 3 of the top 10 conditions with the highest annual expenditures were surgical procedures.[4] The most costly procedure was spinal fusion for scoliosis, accounting for an average of $45,000 per admission and $610 million annually. Although a significant portion of these costs represented surgical devices and operating room time, these totals also included the cost of hospital services and in‐hospital complications. CSHCNs more often undergo high‐complexity procedures such as spinal fusions[36] and face greater risk for costly postoperative complications. The financial benefits that come from reductions in outcomes such as LOS and readmissions in this population are potentially large, but may depend on the payment model as described below.
Billing Models in Comanagement Programs
Several billing constructs exist in comanagement models. At many institutions, comanagement billing may resemble that for traditional consultation: the pediatric hospitalist bills for his/her services using standard initial and subsequent consultation billing coding for the child's medical conditions, and may sign off when the hospitalist feels recommendations are complete. Other models may also exist. Model IV comanagement may involve a prearranged financial agreement, in which billing modifiers are used to differentiate surgical care only (modifier 54) and postoperative medical care only (modifier 55). These modifiers, typically used for Medicare patients, indicate a split in a global surgical fee.[40]
The SHM has outlined financial considerations that should be addressed at the time of program inception and updated periodically, including identifying how each party will bill, who bills for which service, and monitoring collection rates and rejected claims.[2] Regardless of billing model, the main focus of comanagement must be quality of care, not financial considerations; situations in which the latter are emphasized at the expense of patient care may be unethical or illegal.[24] Regardless, surgical comanagement programs should seek maximal reimbursement in order to remain viable.
Value of Comanagement for a Healthcare Organization Under Fee‐for‐Service Payment
The value of any comanagement program is highly dependent on both the institution's payor mix and the healthcare organization's overarching goals. From a business perspective, a multidisciplinary approach may be perceived as resource intensive, but formal cost‐effectiveness analyses over time are limited. Theoretically, a traditional payment model involving hospitalist comanagement would be financially beneficial for a healthcare institution by allowing surgeons and surgical trainees more time to operate. However, these savings are difficult to quantify. Despite the fact that most hospitalist programs have no direct financial benefit to the institution, many hospital leaders seem willing to subsidize hospitalist programs based on measures such as patient and referring physician satisfaction with hospitalist care.[41] Postoperative complications, although unfortunate, may be a source of revenue under this model if paid by insurance companies in the usual manner, leading to a misalignment of quality and financial goals. Regardless, whether these programs are considered worthy investments to healthcare organizations will ultimately depend on evolving billing and reimbursement structures; to date, no formal survey of how comanagement programs bill and are reimbursed has been performed.
Value for an Organization in an Accountable Care Organization Model
Although a detailed discussion of accountable care organizations (ACOs) is beyond the scope of this article, stronger incentives in these structures for reducing resource utilization and complication rates may make hospitalist comanagement attractive in an ACO model.[42] ACO program evaluation is expected to be based on such data as patient surveys, documentation of care coordination, and several disease‐specific metrics.[43, 44] Several children's hospital‐based systems and mixed health systems have dedicated significant resources to establishing networks of providers that bridge inpatient and outpatient episodes of surgical care.[27, 28] One adult study has suggested lower costs associated with hospitalist comanagement for geriatric patients with hip fractures.[12] Comanagement programs may help meet quality and value goals, including enhancing care coordination between inpatient and outpatient care, and therefore may prove to be a beneficial investment for institutions. As the healthcare landscape evolves, formal study of the costs and benefits of pediatric comanagement models in ACO‐type care structures will be important.
SETTING A RESEARCH AGENDA
Pediatric hospitalist comanagement programs require vigorous study to evaluate their impact. Potential research targets include not only clinical data such as LOS, perioperative complication rates, readmission rates, and resource utilization, but also data regarding surgeon, nursing, and family satisfaction. These programs should also be evaluated in terms of how they impact trainees, both surgical and pediatric. Because of comanagement programs' complexities, we anticipate that they will impart both positive and negative effects on some of these factors. These programs will also require evaluation over time as they require significant education on the part of staff and families.[36]
In addition to affecting global metrics, pediatric hospitalists may also have a positive impact on surgical care by demonstrating leadership to improve systems of care relevant to surgical patients, including the use of guidelines. The American College of Surgeons' National Surgical Quality Improvement Program has identified 2 priorities that hospitalists may impact: surgical site infection (SSI) and pulmonary complications, which combined comprise greater than half of all 30‐day postoperative complications.[45] Regarding SSI prevention, hospitalist researchers are making valuable contributions to literature surrounding adherence to Centers for Disease Control and Prevention and Pediatric Orthopedic Society of North America guidelines.[46, 47, 48] Research is ongoing regarding how human and systems factors may impact the effectiveness of these guidelines, but also how reliably these guidelines are implemented.[49] In the area of pulmonary complications, pediatric hospitalists have followed the example of successful initiatives in adult surgery patients by developing and implementing postoperative protocols to prevent pulmonary complications such as postoperative pneumonia.[50] At 1 center, pediatric hospitalists have led efforts to implement a standardized respiratory care pathway for high‐risk orthopedic patients.[51] Evaluation of the effectiveness of such programs is currently ongoing, but early data show similar benefits to those demonstrated in adults.
CONCLUSIONS
Pediatric hospitalist comanagement programs for surgical patients have largely followed the path of adult programs. Limited data suggest that certain clinical outcomes may be improved under comanagement, but patient selection may be important. Although there is significant variety between programs, there exist several common themes, including the importance of clear delineation of roles and a central goal of improved care coordination. Ongoing research will hopefully shed more light on the impact of these programs, especially with regard to patient safety, hospitalist‐led quality‐improvement programs, and financial implications, particularly in different structures of care and reimbursement models.
Disclosure: Nothing to report.
- 2012 State of Hospital Medicine Report, Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/survey. Accessed on September 1, 2014.
- Society of Hospital Medicine Co‐Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co‐management. SHM website. Available at: http://tools.hospitalmedicine.org/Implementation/Co‐ManagementWhitePaper‐final_5‐10‐10.pdf. Accessed on September 25, 2014.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , . Pediatric hospitalist comanagement of surgical patients: challenges and opportunities. Clin Pediatr (Phila). 2008;47(2):114–121.
- , . Utilization of pediatric hospitals in New York State. Pediatrics. 2003;111(5 pt 1):1068–1071.
- ; Committee on Hospital Care. Physicians' roles in coordinating care of hospitalized children. Pediatrics. 2003;111(3):707–709.
- , , , et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219–225.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , . Geriatric co‐management of proximal femur fractures: total quality management and protocol‐driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356.
- , , , , , . Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , , . Pediatric hospital medicine and children with medical complexity: past, present, and future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):113–119.
- , , , et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140.
- , , , , . Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684–688.
- , , , , . Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , . Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189.
- . How best to design surgical comanagement services for pediatric surgical patients? Hosp Pediatr. 2013;3(3):242–243.
- , , , , , . Effects of provider characteristics on care coordination under comanagement. J Hosp Med. 2010;5(9):508–513.
- , , , . Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17(9):e333–e339.
- American Medical Association. 2008 report of the Council on Medical Service, policy sunset report for 1998 AMA socioeconomic policies. CMS Report 4, A‐08. Available at: http://www.ama‐assn.org/resources/doc/cms/a‐08cms4.pdf. Accessed on September 1, 2014.
- Committee on Hospital Care and Institute for Patient‐and Family‐Centered Care. Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129(2):394–404.
- Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460.
- , , , , , . Care coordination for CSHCN: associations with family‐provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428–S434.
- , , , et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.
- , , . Parents' priorities and satisfaction with acute pediatric care. Arch Pediatr Adolesc Med. 2005;159(2):127–131.
- . Family‐centered rounds. Pediatr Clin North Am. 2014;61(4):663–670.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- , , , , ; PIPS Group. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15(suppl 1):17–25.
- , , , et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004–2010.
- , , . Nurses' assessment of pediatric physicians: are hospitalists different? J Healthc Manag Am Coll Healthc Exec. 2008;53(1):14–24; discussion 24–25.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- , , , et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233–241.
- , , . Outcome of major spinal deformity surgery in high‐risk patients: comparison between two departments. Evid Based Spine Care J. 2010;1(3):11–18.
- , , , , , . Meeting the complex needs of the health care team: identification of nurse‐team communication practices perceived to enhance patient outcomes. Qual Health Res. 2010;20(1):15–28.
- , , , . A pediatric residency experience with surgical comanagement. Hosp Pediatr. 2013;3(2):144–148.
- U.S. Department of Health and Human Services. Centers for Medicaid and Medicare Services. MLN matters. Available at: http://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/Downloads/MM7872.pdf. Accessed on September 1,2014.
- , , ; Research Advisory Committee of the American Board of Pediatrics. Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- . Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364(16):e32.
- American Academy of Pediatrics. Accountable Care Organizations (ACOs) and Pediatricians: Evaluation and Engagement. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/Pages/Accountable‐Care‐Organizations‐and‐Pediatricians‐Evaluation‐and‐Engagement.aspx. Accessed on September 25, 2014.
- , , , , . Delivery system characteristics and their association with quality and costs of care: implications for accountable care organizations [published online ahead of print February 21, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000014.
- , , , et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121.
- , , , et al. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–278. Available at: http://www.cdc.gov/hicpac/pdf/guidelines/SSI_1999.pdf. Accessed on September 1, 2014.
- , , , et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high‐risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–478.
- , , , et al. Perioperative antibiotic use for spinal surgery procedures in US children's hospitals. Spine. 2013;38(7):609–616. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , , , , , . A human factors intervention to improve post‐operative antibiotic timing and prevent surgical site infection. Paper presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- , , , , . I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , . Early postoperative respiratory care improves outcomes, adds value for hospitalized pediatric orthopedic patients. Poster presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
According to the 2012 Society of Hospital Medicine (SHM) survey, 94% of adult hospitalists and 74% of pediatric hospitalists provide inpatient care to surgical patients.[1] Many of these programs involve comanagement, which the SHM Comanagement Advisory Panel has described as a system of care featuring shared responsibility, authority, and accountability for hospitalized patients with medical and surgical needs.[2] Collaboration between medical and surgical teams for these patients has occurred commonly at some community institutions for decades, but may only be emerging at some tertiary care hospitals. The trend of comanagement appears to be increasing in popularity in adult medicine.[3] As in adult patients, comanagement for children undergoing surgical procedures, particularly those children with special healthcare needs (CSHCNs), has been proposed as a strategy for improving quality and costs. In this review, we will describe structural, quality, and financial implications of pediatric hospitalist comanagement programs, each of which include both potential benefits and drawbacks, as well as discuss a future research agenda for these programs.
ORGANIZATIONAL NEEDS AND STRUCTURE OF COMANAGEMENT PROGRAMS
Patterns of comanagement likely depend on hospital size and structure, both in adult patients[3] and in pediatrics. Children hospitalized for surgical procedures generally fall into 1 of 2 groups: those who are typically healthy and at low risk for complications, and those who are medically complex and at high risk. Healthy children often undergo high‐prevalence, low‐complexity surgical procedures such as tonsillectomy and hernia repairs;[4] these patients are commonly cared for at community hospitals by adult and pediatric surgeons. Whereas medically complex children also undergo these common procedures, they are more likely to be cared for at tertiary care centers and are also more likely to undergo higher‐complexity surgeries such as spinal fusions, hip osteotomies, and ventriculoperitoneal shunt placements.[4] Hospitalist comanagement programs at community hospitals and tertiary care centers may therefore have evolved differently in response to different needs of patients, providers, and organizations,[5] though some institutions may not fall neatly into 1 of these 2 categories.
Comanagement in Community Hospitals
A significant number of pediatric patients are hospitalized each year in community hospitals.[6] As noted above, children undergoing surgery in these settings are generally healthy, and may be cared for by surgeons with varying amounts of pediatric expertise. In this model, the surgeon may frequently be offsite when not in the operating room, necessitating some type of onsite postoperative coverage. In pediatrics, following adult models, this coverage need may be relatively straightforward: surgeons perform the procedure, followed by a medical team assuming postoperative care with surgical consultation. Because of general surgeons' varying experience with children, the American Academy of Pediatrics suggests that patients younger than 14 years or weighing less than 40 kg cared for by providers without routine pediatric experience should have a pediatric‐trained provider involved in their care,[7] though this suggestion does not mandate comanagement.
For children cared for by adult providers who have little experience with areas of pediatric‐specific care such as medication dosing and assessment of deterioration, we believe that involvement of a pediatric provider may impact care in a number of ways. A pediatric hospitalist's availability on the inpatient unit may allow him or her to better manage routine issues such as pain control and intravenous fluids without surgeon input, improving the efficiency of care. A pediatric hospitalist's general pediatric training may allow him or her to more quickly recognize when a child is medically deteriorating, or when transfer to a tertiary care center may be necessary, making care safer. However, no studies have specifically examined these variables. Future research should measure outcomes such as transfers to higher levels of care, medication errors, length of stay (LOS), and complication rates, especially in community hospital settings.
Comanagement in Tertiary Care Referral Centers
At tertiary care referral centers, surgeries in children are most often performed by pediatric surgeons. In these settings, providing routine hospitalist comanagement to all patients may be neither cost‐effective nor feasible. Adult studies have suggested that population‐targeted models can significantly improve several clinical outcomes. For example, in several studies of patients 65 years and older hospitalized with hip fractures, comanagement with a geriatric hospitalist was associated with improved clinical outcomes and shortened LOS.[8, 9, 10, 11, 12]
An analogous group of pediatric patients to the geriatric population may be CSHCNs or children who are medically complex. Several frameworks have been proposed to identify these patients.[13] Many institutions classify medically complex patients as those with complex chronic medical conditions (CCCs).[13, 14, 15] One framework to identify CSHCNs suggested including not only children with CCCs, but also those with (1) substantial service needs and/or family burden; (2) severe functional limitations; and/or (3) high rates of healthcare system utilization, often requiring the care of several subspecialty providers.[16] As the needs of these patients may be quite diverse, pediatric hospitalists may be involved in many aspects of their care, from preoperative evaluation,[17] to establishing protocols for best practices, to communicating with primary care providers, and even seeing patients in postoperative follow‐up clinics. These patients are known to be at high risk for surgical complications, readmissions,[14] medical errors,[18] lapses in communication, and high care costs. In 1 study, comanagement for children with neuromuscular scoliosis hospitalized for spinal fusion surgery has been associated with shorter LOS and less variability in LOS.[19] However, drawbacks of comanagement programs involving CSHCNs may include difficulty with consistent identification of the population who will most benefit from comanagement and higher initial costs of care.[18]
Models of Comanagement and Comanagement Agreements
A comanagement agreement should address 5 major questions: (1) Who is the primary service? (2) Who is the consulting/comanaging service? (3) Are consults as‐needed or automatic? (4) Who writes orders for the patient? (5) Which staffing model will be used for patient care?[20] Although each question above may be answered differently in different systems, the correct comanagement program is a program that aligns most closely with the patient population and care setting.[11, 20]
Several different models exist for hospitalistsurgeon comanagement programs[20, 21] (Table 1). Under the consultation model (model I), hospitalists become involved in the care of surgical patients only when requested to do so by the surgical team. Criteria for requesting this kind of consultation and the extent of responsibility afforded to the medical team are often not clearly defined, and may differ from hospital to hospital or even surgeon to surgeon.[22] Hospitalist involvement with adult patients with postoperative medical complications, which presumably employed this as‐needed model, has been associated with lower mortality and LOS[23]; whether this involvement provides similar benefits in children with postoperative complications has not been explicitly studied.
| Model | Attending Service | Consulting Service | Automatic Consultation | Who Writes Orders? | Notes |
|---|---|---|---|---|---|
| |||||
| I | Surgery | Pediatrics | No | Surgery | Similar to traditional consultation |
| II | Surgery | Pediatrics | Yes | Usually surgery | Basic comanagement, consultant may sign off |
| III | Pediatrics | Surgery | Yes | Usually pediatrics | Basic comanagement, consultant may sign off |
| IV | Combined | N/A | N/A | Each service writes own | True comanagement, no sign‐off from either service permitted |
The remaining models involve compulsory participation by both surgical and medical services. In models II and III, patients may be evaluated preoperatively; those felt to meet specific criteria for high medical complexity are either admitted to a medical service with automatic surgical consultation or admitted to a surgical service with automatic medical consultation. In both cases, writing of orders is handled in the same manner as any consultation model; depending on the service agreement, consulting services may sign off or may be required to be involved until discharge. In model IV, care is fully comanaged by medical and surgical services, with each service having ownership over orders pertaining to their discipline. Ethical concerns about such agreements outlined by the American Medical Association include whether all patients cared for under agreements II to IV will truly benefit from the cost of multispecialty care, and whether informed consent from patients themselves should be required given the cost implications.[24]
Comanagement models also vary with respect to frontline provider staffing. Models may incorporate nurse practitioners, hospitalists, physician assistants, or a combination thereof. These providers may assume a variety of roles, including preoperative patient evaluation, direct care of patients while hospitalized, and/or coordination of inpatient and outpatient postoperative care. Staffing requirements for hospitalists and/or mid‐level providers will differ significantly at different institutions based on surgical volume, patient complexity, and other local factors.
COMANAGEMENT AND QUALITY
Comanagement as a Family‐Centered Initiative
Development of a family‐centered culture of care, including care coordination, lies at the core of pediatric hospital medicine, particularly for CSHCNs.[25, 26] In the outpatient setting, family‐centered care has been associated with improved quality of care for CSHCNs.[27, 28] For families of hospitalized children, issues such as involvement in care and timely information transfer have been identified as high priorities.[29] An important tool for addressing these needs is family‐centered rounds (FCRs), which represent multidisciplinary rounds at the bedside involving families and patients as active shared decision makers in conjunction with the medical team.[30, 31] Although FCRs have not been studied in comanagement arrangements specifically, evidence suggests that this tool improves family centeredness and patient safety in nonsurgical patients,[32] and FCRs can likely have a similar impact on postoperative care.
A pediatric hospitalist comanagement program may impact quality and safety of care in a number of other ways. Hospitalists may offer improved access to clinical information for nurses and families, making care safer. One study of comanagement in adult neurosurgical patients found that access to hospitalists led to improved quality and safety of care as perceived by nurses and other members of the care team.[33] A study in pediatric patients found that nurses overwhelmingly supported having hospitalist involvement in complex children undergoing surgery; the same study found that pediatric hospitalists were particularly noted for their communication skills.[34]
Assessing Clinical Outcomes in Pediatric Hospitalist Comanagement Programs
Most studies evaluating the impact of surgical comanagement programs have focused on global metrics such as LOS, overall complication rates, and resource utilization. In adults, results of these studies have been mixed, suggesting that patient selection may be an important factor.[35] In pediatrics, 2 US studies have assessed these metrics at single centers. Simon et al. found that involvement of a pediatric hospitalist in comanagement of patients undergoing spinal fusion surgery significantly decreased LOS.[19] Rappaport et al. found that patients comanaged by hospitalists had lower utilization of laboratory tests and parenteral nutrition, though initial program costs significantly increased.[36] Studies outside the United States, including a study from Sweden,[37] have suggested that a multidisciplinary approach to children's surgical care, including the presence of pediatric specialists, reduced infection rate and other complications. These studies provide general support for the role of hospitalists in comanagement, although determining which aspects of care are most impacted may be difficult.
Comanagement programs might impact safety and quality negatively as well. Care may be fragmented, leading to provider and family dissatisfaction. Poor communication and multiple handoffs among multidisciplinary team members might interfere with the central role of the nurse in patient care.[38] Comanagement programs might lead to provider disengagement if providers feel that others will assume roles with which they may be unfamiliar or poorly trained.[35] This lack of knowledge may also affect communication with families, leading to conflicting messages among the care team and family frustration. In addition, the impact of comanagement programs on trainees such as residents, both surgical and pediatric, has received limited study.[39] Assessing pediatric comanagement programs' impact on communication, family‐centeredness, and trainees deserves further study.
FINANCIAL IMPLICATIONS OF PEDIATRIC COMANAGEMENT PROGRAMS
Children undergoing surgery require significant financial resources for their care. A study of 38 major US children's hospitals found that 3 of the top 10 conditions with the highest annual expenditures were surgical procedures.[4] The most costly procedure was spinal fusion for scoliosis, accounting for an average of $45,000 per admission and $610 million annually. Although a significant portion of these costs represented surgical devices and operating room time, these totals also included the cost of hospital services and in‐hospital complications. CSHCNs more often undergo high‐complexity procedures such as spinal fusions[36] and face greater risk for costly postoperative complications. The financial benefits that come from reductions in outcomes such as LOS and readmissions in this population are potentially large, but may depend on the payment model as described below.
Billing Models in Comanagement Programs
Several billing constructs exist in comanagement models. At many institutions, comanagement billing may resemble that for traditional consultation: the pediatric hospitalist bills for his/her services using standard initial and subsequent consultation billing coding for the child's medical conditions, and may sign off when the hospitalist feels recommendations are complete. Other models may also exist. Model IV comanagement may involve a prearranged financial agreement, in which billing modifiers are used to differentiate surgical care only (modifier 54) and postoperative medical care only (modifier 55). These modifiers, typically used for Medicare patients, indicate a split in a global surgical fee.[40]
The SHM has outlined financial considerations that should be addressed at the time of program inception and updated periodically, including identifying how each party will bill, who bills for which service, and monitoring collection rates and rejected claims.[2] Regardless of billing model, the main focus of comanagement must be quality of care, not financial considerations; situations in which the latter are emphasized at the expense of patient care may be unethical or illegal.[24] Regardless, surgical comanagement programs should seek maximal reimbursement in order to remain viable.
Value of Comanagement for a Healthcare Organization Under Fee‐for‐Service Payment
The value of any comanagement program is highly dependent on both the institution's payor mix and the healthcare organization's overarching goals. From a business perspective, a multidisciplinary approach may be perceived as resource intensive, but formal cost‐effectiveness analyses over time are limited. Theoretically, a traditional payment model involving hospitalist comanagement would be financially beneficial for a healthcare institution by allowing surgeons and surgical trainees more time to operate. However, these savings are difficult to quantify. Despite the fact that most hospitalist programs have no direct financial benefit to the institution, many hospital leaders seem willing to subsidize hospitalist programs based on measures such as patient and referring physician satisfaction with hospitalist care.[41] Postoperative complications, although unfortunate, may be a source of revenue under this model if paid by insurance companies in the usual manner, leading to a misalignment of quality and financial goals. Regardless, whether these programs are considered worthy investments to healthcare organizations will ultimately depend on evolving billing and reimbursement structures; to date, no formal survey of how comanagement programs bill and are reimbursed has been performed.
Value for an Organization in an Accountable Care Organization Model
Although a detailed discussion of accountable care organizations (ACOs) is beyond the scope of this article, stronger incentives in these structures for reducing resource utilization and complication rates may make hospitalist comanagement attractive in an ACO model.[42] ACO program evaluation is expected to be based on such data as patient surveys, documentation of care coordination, and several disease‐specific metrics.[43, 44] Several children's hospital‐based systems and mixed health systems have dedicated significant resources to establishing networks of providers that bridge inpatient and outpatient episodes of surgical care.[27, 28] One adult study has suggested lower costs associated with hospitalist comanagement for geriatric patients with hip fractures.[12] Comanagement programs may help meet quality and value goals, including enhancing care coordination between inpatient and outpatient care, and therefore may prove to be a beneficial investment for institutions. As the healthcare landscape evolves, formal study of the costs and benefits of pediatric comanagement models in ACO‐type care structures will be important.
SETTING A RESEARCH AGENDA
Pediatric hospitalist comanagement programs require vigorous study to evaluate their impact. Potential research targets include not only clinical data such as LOS, perioperative complication rates, readmission rates, and resource utilization, but also data regarding surgeon, nursing, and family satisfaction. These programs should also be evaluated in terms of how they impact trainees, both surgical and pediatric. Because of comanagement programs' complexities, we anticipate that they will impart both positive and negative effects on some of these factors. These programs will also require evaluation over time as they require significant education on the part of staff and families.[36]
In addition to affecting global metrics, pediatric hospitalists may also have a positive impact on surgical care by demonstrating leadership to improve systems of care relevant to surgical patients, including the use of guidelines. The American College of Surgeons' National Surgical Quality Improvement Program has identified 2 priorities that hospitalists may impact: surgical site infection (SSI) and pulmonary complications, which combined comprise greater than half of all 30‐day postoperative complications.[45] Regarding SSI prevention, hospitalist researchers are making valuable contributions to literature surrounding adherence to Centers for Disease Control and Prevention and Pediatric Orthopedic Society of North America guidelines.[46, 47, 48] Research is ongoing regarding how human and systems factors may impact the effectiveness of these guidelines, but also how reliably these guidelines are implemented.[49] In the area of pulmonary complications, pediatric hospitalists have followed the example of successful initiatives in adult surgery patients by developing and implementing postoperative protocols to prevent pulmonary complications such as postoperative pneumonia.[50] At 1 center, pediatric hospitalists have led efforts to implement a standardized respiratory care pathway for high‐risk orthopedic patients.[51] Evaluation of the effectiveness of such programs is currently ongoing, but early data show similar benefits to those demonstrated in adults.
CONCLUSIONS
Pediatric hospitalist comanagement programs for surgical patients have largely followed the path of adult programs. Limited data suggest that certain clinical outcomes may be improved under comanagement, but patient selection may be important. Although there is significant variety between programs, there exist several common themes, including the importance of clear delineation of roles and a central goal of improved care coordination. Ongoing research will hopefully shed more light on the impact of these programs, especially with regard to patient safety, hospitalist‐led quality‐improvement programs, and financial implications, particularly in different structures of care and reimbursement models.
Disclosure: Nothing to report.
According to the 2012 Society of Hospital Medicine (SHM) survey, 94% of adult hospitalists and 74% of pediatric hospitalists provide inpatient care to surgical patients.[1] Many of these programs involve comanagement, which the SHM Comanagement Advisory Panel has described as a system of care featuring shared responsibility, authority, and accountability for hospitalized patients with medical and surgical needs.[2] Collaboration between medical and surgical teams for these patients has occurred commonly at some community institutions for decades, but may only be emerging at some tertiary care hospitals. The trend of comanagement appears to be increasing in popularity in adult medicine.[3] As in adult patients, comanagement for children undergoing surgical procedures, particularly those children with special healthcare needs (CSHCNs), has been proposed as a strategy for improving quality and costs. In this review, we will describe structural, quality, and financial implications of pediatric hospitalist comanagement programs, each of which include both potential benefits and drawbacks, as well as discuss a future research agenda for these programs.
ORGANIZATIONAL NEEDS AND STRUCTURE OF COMANAGEMENT PROGRAMS
Patterns of comanagement likely depend on hospital size and structure, both in adult patients[3] and in pediatrics. Children hospitalized for surgical procedures generally fall into 1 of 2 groups: those who are typically healthy and at low risk for complications, and those who are medically complex and at high risk. Healthy children often undergo high‐prevalence, low‐complexity surgical procedures such as tonsillectomy and hernia repairs;[4] these patients are commonly cared for at community hospitals by adult and pediatric surgeons. Whereas medically complex children also undergo these common procedures, they are more likely to be cared for at tertiary care centers and are also more likely to undergo higher‐complexity surgeries such as spinal fusions, hip osteotomies, and ventriculoperitoneal shunt placements.[4] Hospitalist comanagement programs at community hospitals and tertiary care centers may therefore have evolved differently in response to different needs of patients, providers, and organizations,[5] though some institutions may not fall neatly into 1 of these 2 categories.
Comanagement in Community Hospitals
A significant number of pediatric patients are hospitalized each year in community hospitals.[6] As noted above, children undergoing surgery in these settings are generally healthy, and may be cared for by surgeons with varying amounts of pediatric expertise. In this model, the surgeon may frequently be offsite when not in the operating room, necessitating some type of onsite postoperative coverage. In pediatrics, following adult models, this coverage need may be relatively straightforward: surgeons perform the procedure, followed by a medical team assuming postoperative care with surgical consultation. Because of general surgeons' varying experience with children, the American Academy of Pediatrics suggests that patients younger than 14 years or weighing less than 40 kg cared for by providers without routine pediatric experience should have a pediatric‐trained provider involved in their care,[7] though this suggestion does not mandate comanagement.
For children cared for by adult providers who have little experience with areas of pediatric‐specific care such as medication dosing and assessment of deterioration, we believe that involvement of a pediatric provider may impact care in a number of ways. A pediatric hospitalist's availability on the inpatient unit may allow him or her to better manage routine issues such as pain control and intravenous fluids without surgeon input, improving the efficiency of care. A pediatric hospitalist's general pediatric training may allow him or her to more quickly recognize when a child is medically deteriorating, or when transfer to a tertiary care center may be necessary, making care safer. However, no studies have specifically examined these variables. Future research should measure outcomes such as transfers to higher levels of care, medication errors, length of stay (LOS), and complication rates, especially in community hospital settings.
Comanagement in Tertiary Care Referral Centers
At tertiary care referral centers, surgeries in children are most often performed by pediatric surgeons. In these settings, providing routine hospitalist comanagement to all patients may be neither cost‐effective nor feasible. Adult studies have suggested that population‐targeted models can significantly improve several clinical outcomes. For example, in several studies of patients 65 years and older hospitalized with hip fractures, comanagement with a geriatric hospitalist was associated with improved clinical outcomes and shortened LOS.[8, 9, 10, 11, 12]
An analogous group of pediatric patients to the geriatric population may be CSHCNs or children who are medically complex. Several frameworks have been proposed to identify these patients.[13] Many institutions classify medically complex patients as those with complex chronic medical conditions (CCCs).[13, 14, 15] One framework to identify CSHCNs suggested including not only children with CCCs, but also those with (1) substantial service needs and/or family burden; (2) severe functional limitations; and/or (3) high rates of healthcare system utilization, often requiring the care of several subspecialty providers.[16] As the needs of these patients may be quite diverse, pediatric hospitalists may be involved in many aspects of their care, from preoperative evaluation,[17] to establishing protocols for best practices, to communicating with primary care providers, and even seeing patients in postoperative follow‐up clinics. These patients are known to be at high risk for surgical complications, readmissions,[14] medical errors,[18] lapses in communication, and high care costs. In 1 study, comanagement for children with neuromuscular scoliosis hospitalized for spinal fusion surgery has been associated with shorter LOS and less variability in LOS.[19] However, drawbacks of comanagement programs involving CSHCNs may include difficulty with consistent identification of the population who will most benefit from comanagement and higher initial costs of care.[18]
Models of Comanagement and Comanagement Agreements
A comanagement agreement should address 5 major questions: (1) Who is the primary service? (2) Who is the consulting/comanaging service? (3) Are consults as‐needed or automatic? (4) Who writes orders for the patient? (5) Which staffing model will be used for patient care?[20] Although each question above may be answered differently in different systems, the correct comanagement program is a program that aligns most closely with the patient population and care setting.[11, 20]
Several different models exist for hospitalistsurgeon comanagement programs[20, 21] (Table 1). Under the consultation model (model I), hospitalists become involved in the care of surgical patients only when requested to do so by the surgical team. Criteria for requesting this kind of consultation and the extent of responsibility afforded to the medical team are often not clearly defined, and may differ from hospital to hospital or even surgeon to surgeon.[22] Hospitalist involvement with adult patients with postoperative medical complications, which presumably employed this as‐needed model, has been associated with lower mortality and LOS[23]; whether this involvement provides similar benefits in children with postoperative complications has not been explicitly studied.
| Model | Attending Service | Consulting Service | Automatic Consultation | Who Writes Orders? | Notes |
|---|---|---|---|---|---|
| |||||
| I | Surgery | Pediatrics | No | Surgery | Similar to traditional consultation |
| II | Surgery | Pediatrics | Yes | Usually surgery | Basic comanagement, consultant may sign off |
| III | Pediatrics | Surgery | Yes | Usually pediatrics | Basic comanagement, consultant may sign off |
| IV | Combined | N/A | N/A | Each service writes own | True comanagement, no sign‐off from either service permitted |
The remaining models involve compulsory participation by both surgical and medical services. In models II and III, patients may be evaluated preoperatively; those felt to meet specific criteria for high medical complexity are either admitted to a medical service with automatic surgical consultation or admitted to a surgical service with automatic medical consultation. In both cases, writing of orders is handled in the same manner as any consultation model; depending on the service agreement, consulting services may sign off or may be required to be involved until discharge. In model IV, care is fully comanaged by medical and surgical services, with each service having ownership over orders pertaining to their discipline. Ethical concerns about such agreements outlined by the American Medical Association include whether all patients cared for under agreements II to IV will truly benefit from the cost of multispecialty care, and whether informed consent from patients themselves should be required given the cost implications.[24]
Comanagement models also vary with respect to frontline provider staffing. Models may incorporate nurse practitioners, hospitalists, physician assistants, or a combination thereof. These providers may assume a variety of roles, including preoperative patient evaluation, direct care of patients while hospitalized, and/or coordination of inpatient and outpatient postoperative care. Staffing requirements for hospitalists and/or mid‐level providers will differ significantly at different institutions based on surgical volume, patient complexity, and other local factors.
COMANAGEMENT AND QUALITY
Comanagement as a Family‐Centered Initiative
Development of a family‐centered culture of care, including care coordination, lies at the core of pediatric hospital medicine, particularly for CSHCNs.[25, 26] In the outpatient setting, family‐centered care has been associated with improved quality of care for CSHCNs.[27, 28] For families of hospitalized children, issues such as involvement in care and timely information transfer have been identified as high priorities.[29] An important tool for addressing these needs is family‐centered rounds (FCRs), which represent multidisciplinary rounds at the bedside involving families and patients as active shared decision makers in conjunction with the medical team.[30, 31] Although FCRs have not been studied in comanagement arrangements specifically, evidence suggests that this tool improves family centeredness and patient safety in nonsurgical patients,[32] and FCRs can likely have a similar impact on postoperative care.
A pediatric hospitalist comanagement program may impact quality and safety of care in a number of other ways. Hospitalists may offer improved access to clinical information for nurses and families, making care safer. One study of comanagement in adult neurosurgical patients found that access to hospitalists led to improved quality and safety of care as perceived by nurses and other members of the care team.[33] A study in pediatric patients found that nurses overwhelmingly supported having hospitalist involvement in complex children undergoing surgery; the same study found that pediatric hospitalists were particularly noted for their communication skills.[34]
Assessing Clinical Outcomes in Pediatric Hospitalist Comanagement Programs
Most studies evaluating the impact of surgical comanagement programs have focused on global metrics such as LOS, overall complication rates, and resource utilization. In adults, results of these studies have been mixed, suggesting that patient selection may be an important factor.[35] In pediatrics, 2 US studies have assessed these metrics at single centers. Simon et al. found that involvement of a pediatric hospitalist in comanagement of patients undergoing spinal fusion surgery significantly decreased LOS.[19] Rappaport et al. found that patients comanaged by hospitalists had lower utilization of laboratory tests and parenteral nutrition, though initial program costs significantly increased.[36] Studies outside the United States, including a study from Sweden,[37] have suggested that a multidisciplinary approach to children's surgical care, including the presence of pediatric specialists, reduced infection rate and other complications. These studies provide general support for the role of hospitalists in comanagement, although determining which aspects of care are most impacted may be difficult.
Comanagement programs might impact safety and quality negatively as well. Care may be fragmented, leading to provider and family dissatisfaction. Poor communication and multiple handoffs among multidisciplinary team members might interfere with the central role of the nurse in patient care.[38] Comanagement programs might lead to provider disengagement if providers feel that others will assume roles with which they may be unfamiliar or poorly trained.[35] This lack of knowledge may also affect communication with families, leading to conflicting messages among the care team and family frustration. In addition, the impact of comanagement programs on trainees such as residents, both surgical and pediatric, has received limited study.[39] Assessing pediatric comanagement programs' impact on communication, family‐centeredness, and trainees deserves further study.
FINANCIAL IMPLICATIONS OF PEDIATRIC COMANAGEMENT PROGRAMS
Children undergoing surgery require significant financial resources for their care. A study of 38 major US children's hospitals found that 3 of the top 10 conditions with the highest annual expenditures were surgical procedures.[4] The most costly procedure was spinal fusion for scoliosis, accounting for an average of $45,000 per admission and $610 million annually. Although a significant portion of these costs represented surgical devices and operating room time, these totals also included the cost of hospital services and in‐hospital complications. CSHCNs more often undergo high‐complexity procedures such as spinal fusions[36] and face greater risk for costly postoperative complications. The financial benefits that come from reductions in outcomes such as LOS and readmissions in this population are potentially large, but may depend on the payment model as described below.
Billing Models in Comanagement Programs
Several billing constructs exist in comanagement models. At many institutions, comanagement billing may resemble that for traditional consultation: the pediatric hospitalist bills for his/her services using standard initial and subsequent consultation billing coding for the child's medical conditions, and may sign off when the hospitalist feels recommendations are complete. Other models may also exist. Model IV comanagement may involve a prearranged financial agreement, in which billing modifiers are used to differentiate surgical care only (modifier 54) and postoperative medical care only (modifier 55). These modifiers, typically used for Medicare patients, indicate a split in a global surgical fee.[40]
The SHM has outlined financial considerations that should be addressed at the time of program inception and updated periodically, including identifying how each party will bill, who bills for which service, and monitoring collection rates and rejected claims.[2] Regardless of billing model, the main focus of comanagement must be quality of care, not financial considerations; situations in which the latter are emphasized at the expense of patient care may be unethical or illegal.[24] Regardless, surgical comanagement programs should seek maximal reimbursement in order to remain viable.
Value of Comanagement for a Healthcare Organization Under Fee‐for‐Service Payment
The value of any comanagement program is highly dependent on both the institution's payor mix and the healthcare organization's overarching goals. From a business perspective, a multidisciplinary approach may be perceived as resource intensive, but formal cost‐effectiveness analyses over time are limited. Theoretically, a traditional payment model involving hospitalist comanagement would be financially beneficial for a healthcare institution by allowing surgeons and surgical trainees more time to operate. However, these savings are difficult to quantify. Despite the fact that most hospitalist programs have no direct financial benefit to the institution, many hospital leaders seem willing to subsidize hospitalist programs based on measures such as patient and referring physician satisfaction with hospitalist care.[41] Postoperative complications, although unfortunate, may be a source of revenue under this model if paid by insurance companies in the usual manner, leading to a misalignment of quality and financial goals. Regardless, whether these programs are considered worthy investments to healthcare organizations will ultimately depend on evolving billing and reimbursement structures; to date, no formal survey of how comanagement programs bill and are reimbursed has been performed.
Value for an Organization in an Accountable Care Organization Model
Although a detailed discussion of accountable care organizations (ACOs) is beyond the scope of this article, stronger incentives in these structures for reducing resource utilization and complication rates may make hospitalist comanagement attractive in an ACO model.[42] ACO program evaluation is expected to be based on such data as patient surveys, documentation of care coordination, and several disease‐specific metrics.[43, 44] Several children's hospital‐based systems and mixed health systems have dedicated significant resources to establishing networks of providers that bridge inpatient and outpatient episodes of surgical care.[27, 28] One adult study has suggested lower costs associated with hospitalist comanagement for geriatric patients with hip fractures.[12] Comanagement programs may help meet quality and value goals, including enhancing care coordination between inpatient and outpatient care, and therefore may prove to be a beneficial investment for institutions. As the healthcare landscape evolves, formal study of the costs and benefits of pediatric comanagement models in ACO‐type care structures will be important.
SETTING A RESEARCH AGENDA
Pediatric hospitalist comanagement programs require vigorous study to evaluate their impact. Potential research targets include not only clinical data such as LOS, perioperative complication rates, readmission rates, and resource utilization, but also data regarding surgeon, nursing, and family satisfaction. These programs should also be evaluated in terms of how they impact trainees, both surgical and pediatric. Because of comanagement programs' complexities, we anticipate that they will impart both positive and negative effects on some of these factors. These programs will also require evaluation over time as they require significant education on the part of staff and families.[36]
In addition to affecting global metrics, pediatric hospitalists may also have a positive impact on surgical care by demonstrating leadership to improve systems of care relevant to surgical patients, including the use of guidelines. The American College of Surgeons' National Surgical Quality Improvement Program has identified 2 priorities that hospitalists may impact: surgical site infection (SSI) and pulmonary complications, which combined comprise greater than half of all 30‐day postoperative complications.[45] Regarding SSI prevention, hospitalist researchers are making valuable contributions to literature surrounding adherence to Centers for Disease Control and Prevention and Pediatric Orthopedic Society of North America guidelines.[46, 47, 48] Research is ongoing regarding how human and systems factors may impact the effectiveness of these guidelines, but also how reliably these guidelines are implemented.[49] In the area of pulmonary complications, pediatric hospitalists have followed the example of successful initiatives in adult surgery patients by developing and implementing postoperative protocols to prevent pulmonary complications such as postoperative pneumonia.[50] At 1 center, pediatric hospitalists have led efforts to implement a standardized respiratory care pathway for high‐risk orthopedic patients.[51] Evaluation of the effectiveness of such programs is currently ongoing, but early data show similar benefits to those demonstrated in adults.
CONCLUSIONS
Pediatric hospitalist comanagement programs for surgical patients have largely followed the path of adult programs. Limited data suggest that certain clinical outcomes may be improved under comanagement, but patient selection may be important. Although there is significant variety between programs, there exist several common themes, including the importance of clear delineation of roles and a central goal of improved care coordination. Ongoing research will hopefully shed more light on the impact of these programs, especially with regard to patient safety, hospitalist‐led quality‐improvement programs, and financial implications, particularly in different structures of care and reimbursement models.
Disclosure: Nothing to report.
- 2012 State of Hospital Medicine Report, Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/survey. Accessed on September 1, 2014.
- Society of Hospital Medicine Co‐Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co‐management. SHM website. Available at: http://tools.hospitalmedicine.org/Implementation/Co‐ManagementWhitePaper‐final_5‐10‐10.pdf. Accessed on September 25, 2014.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , . Pediatric hospitalist comanagement of surgical patients: challenges and opportunities. Clin Pediatr (Phila). 2008;47(2):114–121.
- , . Utilization of pediatric hospitals in New York State. Pediatrics. 2003;111(5 pt 1):1068–1071.
- ; Committee on Hospital Care. Physicians' roles in coordinating care of hospitalized children. Pediatrics. 2003;111(3):707–709.
- , , , et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219–225.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , . Geriatric co‐management of proximal femur fractures: total quality management and protocol‐driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356.
- , , , , , . Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , , . Pediatric hospital medicine and children with medical complexity: past, present, and future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):113–119.
- , , , et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140.
- , , , , . Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684–688.
- , , , , . Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , . Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189.
- . How best to design surgical comanagement services for pediatric surgical patients? Hosp Pediatr. 2013;3(3):242–243.
- , , , , , . Effects of provider characteristics on care coordination under comanagement. J Hosp Med. 2010;5(9):508–513.
- , , , . Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17(9):e333–e339.
- American Medical Association. 2008 report of the Council on Medical Service, policy sunset report for 1998 AMA socioeconomic policies. CMS Report 4, A‐08. Available at: http://www.ama‐assn.org/resources/doc/cms/a‐08cms4.pdf. Accessed on September 1, 2014.
- Committee on Hospital Care and Institute for Patient‐and Family‐Centered Care. Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129(2):394–404.
- Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460.
- , , , , , . Care coordination for CSHCN: associations with family‐provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428–S434.
- , , , et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.
- , , . Parents' priorities and satisfaction with acute pediatric care. Arch Pediatr Adolesc Med. 2005;159(2):127–131.
- . Family‐centered rounds. Pediatr Clin North Am. 2014;61(4):663–670.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- , , , , ; PIPS Group. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15(suppl 1):17–25.
- , , , et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004–2010.
- , , . Nurses' assessment of pediatric physicians: are hospitalists different? J Healthc Manag Am Coll Healthc Exec. 2008;53(1):14–24; discussion 24–25.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- , , , et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233–241.
- , , . Outcome of major spinal deformity surgery in high‐risk patients: comparison between two departments. Evid Based Spine Care J. 2010;1(3):11–18.
- , , , , , . Meeting the complex needs of the health care team: identification of nurse‐team communication practices perceived to enhance patient outcomes. Qual Health Res. 2010;20(1):15–28.
- , , , . A pediatric residency experience with surgical comanagement. Hosp Pediatr. 2013;3(2):144–148.
- U.S. Department of Health and Human Services. Centers for Medicaid and Medicare Services. MLN matters. Available at: http://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/Downloads/MM7872.pdf. Accessed on September 1,2014.
- , , ; Research Advisory Committee of the American Board of Pediatrics. Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- . Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364(16):e32.
- American Academy of Pediatrics. Accountable Care Organizations (ACOs) and Pediatricians: Evaluation and Engagement. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/Pages/Accountable‐Care‐Organizations‐and‐Pediatricians‐Evaluation‐and‐Engagement.aspx. Accessed on September 25, 2014.
- , , , , . Delivery system characteristics and their association with quality and costs of care: implications for accountable care organizations [published online ahead of print February 21, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000014.
- , , , et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121.
- , , , et al. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–278. Available at: http://www.cdc.gov/hicpac/pdf/guidelines/SSI_1999.pdf. Accessed on September 1, 2014.
- , , , et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high‐risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–478.
- , , , et al. Perioperative antibiotic use for spinal surgery procedures in US children's hospitals. Spine. 2013;38(7):609–616. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , , , , , . A human factors intervention to improve post‐operative antibiotic timing and prevent surgical site infection. Paper presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- , , , , . I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , . Early postoperative respiratory care improves outcomes, adds value for hospitalized pediatric orthopedic patients. Poster presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- 2012 State of Hospital Medicine Report, Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/survey. Accessed on September 1, 2014.
- Society of Hospital Medicine Co‐Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co‐management. SHM website. Available at: http://tools.hospitalmedicine.org/Implementation/Co‐ManagementWhitePaper‐final_5‐10‐10.pdf. Accessed on September 25, 2014.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , . Pediatric hospitalist comanagement of surgical patients: challenges and opportunities. Clin Pediatr (Phila). 2008;47(2):114–121.
- , . Utilization of pediatric hospitals in New York State. Pediatrics. 2003;111(5 pt 1):1068–1071.
- ; Committee on Hospital Care. Physicians' roles in coordinating care of hospitalized children. Pediatrics. 2003;111(3):707–709.
- , , , et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219–225.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , . Geriatric co‐management of proximal femur fractures: total quality management and protocol‐driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356.
- , , , , , . Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , , . Pediatric hospital medicine and children with medical complexity: past, present, and future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):113–119.
- , , , et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140.
- , , , , . Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684–688.
- , , , , . Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , . Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189.
- . How best to design surgical comanagement services for pediatric surgical patients? Hosp Pediatr. 2013;3(3):242–243.
- , , , , , . Effects of provider characteristics on care coordination under comanagement. J Hosp Med. 2010;5(9):508–513.
- , , , . Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17(9):e333–e339.
- American Medical Association. 2008 report of the Council on Medical Service, policy sunset report for 1998 AMA socioeconomic policies. CMS Report 4, A‐08. Available at: http://www.ama‐assn.org/resources/doc/cms/a‐08cms4.pdf. Accessed on September 1, 2014.
- Committee on Hospital Care and Institute for Patient‐and Family‐Centered Care. Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129(2):394–404.
- Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460.
- , , , , , . Care coordination for CSHCN: associations with family‐provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428–S434.
- , , , et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.
- , , . Parents' priorities and satisfaction with acute pediatric care. Arch Pediatr Adolesc Med. 2005;159(2):127–131.
- . Family‐centered rounds. Pediatr Clin North Am. 2014;61(4):663–670.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- , , , , ; PIPS Group. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15(suppl 1):17–25.
- , , , et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004–2010.
- , , . Nurses' assessment of pediatric physicians: are hospitalists different? J Healthc Manag Am Coll Healthc Exec. 2008;53(1):14–24; discussion 24–25.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- , , , et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233–241.
- , , . Outcome of major spinal deformity surgery in high‐risk patients: comparison between two departments. Evid Based Spine Care J. 2010;1(3):11–18.
- , , , , , . Meeting the complex needs of the health care team: identification of nurse‐team communication practices perceived to enhance patient outcomes. Qual Health Res. 2010;20(1):15–28.
- , , , . A pediatric residency experience with surgical comanagement. Hosp Pediatr. 2013;3(2):144–148.
- U.S. Department of Health and Human Services. Centers for Medicaid and Medicare Services. MLN matters. Available at: http://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/Downloads/MM7872.pdf. Accessed on September 1,2014.
- , , ; Research Advisory Committee of the American Board of Pediatrics. Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- . Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364(16):e32.
- American Academy of Pediatrics. Accountable Care Organizations (ACOs) and Pediatricians: Evaluation and Engagement. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/Pages/Accountable‐Care‐Organizations‐and‐Pediatricians‐Evaluation‐and‐Engagement.aspx. Accessed on September 25, 2014.
- , , , , . Delivery system characteristics and their association with quality and costs of care: implications for accountable care organizations [published online ahead of print February 21, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000014.
- , , , et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121.
- , , , et al. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–278. Available at: http://www.cdc.gov/hicpac/pdf/guidelines/SSI_1999.pdf. Accessed on September 1, 2014.
- , , , et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high‐risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–478.
- , , , et al. Perioperative antibiotic use for spinal surgery procedures in US children's hospitals. Spine. 2013;38(7):609–616. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , , , , , . A human factors intervention to improve post‐operative antibiotic timing and prevent surgical site infection. Paper presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- , , , , . I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , . Early postoperative respiratory care improves outcomes, adds value for hospitalized pediatric orthopedic patients. Poster presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
Uterine rupture, child stillborn: $3.8M net award
Uterine rupture, child stillborn: $3.8M net award
At 35 weeks' gestation, a woman went to the emergency department (ED) with abdominal pain, fast heartbeat, and irregular contractions. Her history included three cesarean deliveries, including one with a vertical incision. She was admitted, and a cesarean delivery was planned for the next day. After 8 hours, during which the patient’s condition worsened, an emergency cesarean delivery was undertaken. A full rupture of the uterus was found; the baby’s body had extruded into the mother’s abdomen. The child was stillborn.
PARENTS’ CLAIM The stillbirth could have been avoided if the nurses had communicated the mother’s worsening condition to the physicians.
DEFENDANTS’ DEFENSE After the hospital and physicians settled prior to trial, the case continued against the nurse in charge of the mother’s care and the nurse-staffing group. Negligence was denied; all protocols were followed.
VERDICT A $2.9 million Illinois verdict was returned. With a $900,000 settlement from the hospital and physicians, the net award was $3.8 million.
_______________
Where did rare strep A infection come from?
A 36-year-old woman reported heavy vaginal bleeding to her ObGyn. She underwent endometrial ablation in her physician’s office.
The next day, the woman called the office to report abdominal pain. She was told to stop the medication she was taking, and if the pain continued to the next day, to go to an ED. The next day, the patient went to the ED and was found to be in septic shock. During emergency laparotomy, 50 mL of purulent fluid were drained and an emergency hysterectomy was performed. Three days later, the patient died from pulmonary arrest caused by toxic shock syndrome. An autopsy revealed that the patient’s sepsis was caused by group A streptococci (GAS) infection.
ESTATE’S CLAIM The patient was not a proper candidate for endometrial ablation because of her history of chronic cervical infection. The ObGyn perforated the cervix during the procedure and tried to conceal it. At autopsy, bone wax was found in the rectal lumen that had been used to cover up damage to the cervix. The ObGyn introduced GAS bacteria into the patient’s system. The ObGyn’s staff failed to ask the proper questions when she called the day after the procedure. She should have been told to go directly to the ED.
DEFENDANTS’ DEFENSE The ObGyn did not perforate the cervix or uterus during the procedure. GAS infection is so rare that it would have been difficult to foresee or diagnose. Potentially, the patient had a chronic
cervical infection before ablation.
VERDICT A Texas defense verdict was returned.
_______________
DURING INSERTION, IUD PERFORATES UTERINE WALL; LATER FOUND BELOW LIVER
On July 21, a 46-year-old woman went to an ObGyn for placement of an intrauterine device (IUD). Shortly after the ObGyn inserted the levonorgestrel-releasing intrauterine system (Mirena, Bayer HealthCare), the patient reported severe pelvic and abdominal pain. On July 26, the patient underwent surgical removal of the IUD.
She was discharged on July 29 but continued to report pain. She was readmitted to the hospital the next day and treated for pain. She was bed ridden for 3 weeks after IUD-removal surgery, and had a 3-month recovery before feeling pain free.
PATIENT’S CLAIM The ObGyn was negligent in perforating the patient’s uterine wall during IUD insertion, causing the device to ultimately migrate under the patient’s liver.
DEFENDANTS’ DEFENSE Uterine perforation is a known complication of IUD insertion. The IUD escaped from the patient’s uterus at a later time and not during the insertion procedure.
VERDICT A Florida verdict of $208,839 was returned; the amount was reduced to $161,058 because the medical expenses were written off by the health-care providers.
_______________
Was travel appropriate for this pregnant woman?
A woman with a history of two premature deliveries and one miscarriage became pregnant again. She received prenatal care at an Army hospital. She traveled to Spain, where the baby was born at 31 weeks’ gestation. The baby required treatment in a neonatal intensive care unit (NICU) for 17 days. The child has cerebral palsy, with tetraplegia of all four extremities. She cannot walk without assistance and suffers severe cognitive and vision impairment.
PARENTS’ CLAIM The ObGyn at the Army hospital should not have approved the mother’s request for travel; he did so, despite knowing that the mother was at high risk for premature birth. The military medical hospital to which she was assigned in Spain could not manage a high-risk pregnancy, didn’t have a NICU, and didn’t have specialists to treat premature infants.
DEFENDANTS’ DEFENSE The ObGyn argued that he did not have access to the medical records showing the mother’s history. The patient countered that the ObGyn did indeed have the patient’s records, as he had discussed them with her.
VERDICT A $10,409,700 California verdict was returned against the ObGyn and the government facility.
_______________
Triple-negative BrCa not diagnosed until metastasized: $5.2M
After finding lumps in both breasts, a woman in her 30s saw a nurse practitioner (NP) at an Army hospital. A radiologist reported no mass in the right breast and multiple benign-appearing anechoic lesions in the left breast after bilateral mammography and ultrasonography (US) in July 2008. The Chief of Mammography Services recommended referral to a breast surgeon, but the patient never received the letter. It was placed in her mammography file, not in the treatment file.
In November 2008, the patient returned to the clinic. Bilateral diagnostic mammography and US were ordered, but for unknown reasons, cancelled. US of the left breast was interpreted as benign in January 2009.
After imaging in March 2010, followed by a needle biopsy of the right breast, a radiologist reported finding intermediate-grade infiltrating ductal carcinoma.
The patient sought care outside the military medical system at a large university hospital. In April 2010, stage 3 triple-negative invasive ductal carcinoma (IDC) was identified. The patient underwent chemotherapy, a double mastectomy, removal of 21 lymph nodes, and breast reconstruction. She was given a 60% chance of recurrence in 5–7 years.
PATIENT’S CLAIM It was negligent to not inform her of imaging results. Biopsy should have been performed in 2008, when the IDC was likely at stage 1; treatment would have been far less aggressive. Electronic medical records showed that the 2008 mammography and US results had been “signed off” by an NP at the clinic.
DEFENDANTS’ DEFENSE While unable to concede liability, the government agency did not contest the point.
VERDICT A $5.2 million Tennessee federal court bench verdict was returned, citing failures in communication, poor and improper record keeping and retention, failure to follow-up, and an unexplained cancellation of a medical order.
_______________
Woman dies from cervical cancer: $2.3M
In 2001, a 41-year-old woman had abnormal Pap smear results but her gynecologist did not order more testing. The patient was told to return in 3 months, but she did not return until 2007—reporting abnormal bleeding, vaginal discharge, and pain. Her Pap results were normal, however, and the gynecologist did not order further testing. In 2009, the patient was found to have advanced cervical cancer. She died 2 years later.
ESTATE’S CLAIM Further testing should have been ordered in 2001, which would have likely revealed dysplasia, which can lead to cancer. The laboratory incorrectly interpreted the 2007 Pap test; if the results had been properly reported, additional testing could have been ordered.
DEFENDANTS’ DEFENSE The laboratory and patient’s estate settled for a confidential amount before trial. The gynecologist denied negligence.
VERDICT A New Jersey jury found the gynecologist 40% at fault for his actions in 2007. The jury found the laboratory 50% at fault, and the patient 10% at fault. A gross verdict of $2.33 million was returned.
_______________
Bowel injury after cesarean delivery; mother dies of sepsis
At 40 4/7 weeks' gestation, a 37-year-old woman gave birth to a healthy child by cesarean delivery. The next day, the patient had an elevated white blood cell (WBC) count with a left shift, her abdomen was tympanic but soft, and she was passing flatus and belching. The ObGyn ordered a Fleet enema; only flatus was released. A covering ObGyn ordered an abdominal radiograph, which the radiologist reported as showing postoperative ileus and mild constipation. The patient was given a second Fleet enema the next day, resulting in watery stool. She vomited 300 mL of dark green fluid.
After a rectal tube was placed 2 days later, one hard brown stool and several brown, pasty, loose, and liquid stools were returned. She vomited several times that day, and was found to have hypoactive bowel sounds with continued tympanic quality in the upper quadrants. Laboratory testing revealed continued elevated WBC count with left shift. The next day, she had hypoactive bowel sounds with brown liquid stools. Later that morning, she was able to tolerate clear liquids. The ObGyn decided to discharge her home with instructions to continue on a clear liquid diet for 2 more days before advancing her diet.
The day after discharge, she was found unresponsive at home. She was taken to the hospital, but resuscitation attempts failed. She died. An autopsy revealed that the cause of death was sepsis.
ESTATE’S CLAIM The ObGyn was negligent in failing to diagnose and treat a postoperative intra-abdominal infection caused by bowel perforation. A surgical consult should have been obtained. The woman was prematurely discharged. The radiologist failed to report the presence of free air on the abdominal x-ray.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million Maryland settlement was reached.
_______________
Right ureter injury detected and repaired
During laparoscopic-assisted vaginal hysterectomy, the ObGyn detected and repaired an injury to the right ureter. The patient’s recovery was delayed by the injury.
PATIENT’S CLAIM The ObGyn was negligent in using a Kleppinger bipolar cauterizing instrument to cauterize the vaginal cuff. Thermal overspray from the instrument or the instrument itself damaged the ureter. The ObGyn was also negligent in not performing diagnostic cystoscopy to confirm patency of the ureter after the repair was made.
PHYSICIAN’S DEFENSE Ureter injury is a known risk of the procedure. All procedures were performed according to protocol.
VERDICT A Florida defense verdict was returned.
_______________
Failure to detect inflammatory BrCa; woman dies
A 42-year-old woman underwent mammography in February 2002 after reporting pain, discoloration, inflammation, and swelling in her left breast. The radiologist who interpreted the mammography suggested a biopsy for a differential diagnosis of mastitis or inflammatory carcinoma. The biopsy results were negative.
The patient’s symptoms persisted, and she underwent US in late May 2002. Another radiologist interpreted the US, noting that the patient could not tolerate compression, which led to less than optimal evaluation. The radiologist suggested that mastitis was the likely cause of the patient’s symptoms.
The patient then consulted a surgeon, who ordered mammography and magnetic resonance imaging (MRI) followed by biopsy, which indicated cancer. The patient underwent a mastectomy but metastasis had already occurred. She died at age 50 prior to the trial.
ESTATE’S CLAIM If the cancer had been diagnosed earlier, the outcome would have been better. Both radiologists misinterpreted the mammographies.
DEFENDANTS’ DEFENSE The mammographies had been properly interpreted. Any missed diagnosis would not have impacted the outcome due to the type of cancer. The scans had been released to the patient, but were subsequently lost; an adverse interference instruction was given to the jury.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine rupture, child stillborn: $3.8M net award
At 35 weeks' gestation, a woman went to the emergency department (ED) with abdominal pain, fast heartbeat, and irregular contractions. Her history included three cesarean deliveries, including one with a vertical incision. She was admitted, and a cesarean delivery was planned for the next day. After 8 hours, during which the patient’s condition worsened, an emergency cesarean delivery was undertaken. A full rupture of the uterus was found; the baby’s body had extruded into the mother’s abdomen. The child was stillborn.
PARENTS’ CLAIM The stillbirth could have been avoided if the nurses had communicated the mother’s worsening condition to the physicians.
DEFENDANTS’ DEFENSE After the hospital and physicians settled prior to trial, the case continued against the nurse in charge of the mother’s care and the nurse-staffing group. Negligence was denied; all protocols were followed.
VERDICT A $2.9 million Illinois verdict was returned. With a $900,000 settlement from the hospital and physicians, the net award was $3.8 million.
_______________
Where did rare strep A infection come from?
A 36-year-old woman reported heavy vaginal bleeding to her ObGyn. She underwent endometrial ablation in her physician’s office.
The next day, the woman called the office to report abdominal pain. She was told to stop the medication she was taking, and if the pain continued to the next day, to go to an ED. The next day, the patient went to the ED and was found to be in septic shock. During emergency laparotomy, 50 mL of purulent fluid were drained and an emergency hysterectomy was performed. Three days later, the patient died from pulmonary arrest caused by toxic shock syndrome. An autopsy revealed that the patient’s sepsis was caused by group A streptococci (GAS) infection.
ESTATE’S CLAIM The patient was not a proper candidate for endometrial ablation because of her history of chronic cervical infection. The ObGyn perforated the cervix during the procedure and tried to conceal it. At autopsy, bone wax was found in the rectal lumen that had been used to cover up damage to the cervix. The ObGyn introduced GAS bacteria into the patient’s system. The ObGyn’s staff failed to ask the proper questions when she called the day after the procedure. She should have been told to go directly to the ED.
DEFENDANTS’ DEFENSE The ObGyn did not perforate the cervix or uterus during the procedure. GAS infection is so rare that it would have been difficult to foresee or diagnose. Potentially, the patient had a chronic
cervical infection before ablation.
VERDICT A Texas defense verdict was returned.
_______________
DURING INSERTION, IUD PERFORATES UTERINE WALL; LATER FOUND BELOW LIVER
On July 21, a 46-year-old woman went to an ObGyn for placement of an intrauterine device (IUD). Shortly after the ObGyn inserted the levonorgestrel-releasing intrauterine system (Mirena, Bayer HealthCare), the patient reported severe pelvic and abdominal pain. On July 26, the patient underwent surgical removal of the IUD.
She was discharged on July 29 but continued to report pain. She was readmitted to the hospital the next day and treated for pain. She was bed ridden for 3 weeks after IUD-removal surgery, and had a 3-month recovery before feeling pain free.
PATIENT’S CLAIM The ObGyn was negligent in perforating the patient’s uterine wall during IUD insertion, causing the device to ultimately migrate under the patient’s liver.
DEFENDANTS’ DEFENSE Uterine perforation is a known complication of IUD insertion. The IUD escaped from the patient’s uterus at a later time and not during the insertion procedure.
VERDICT A Florida verdict of $208,839 was returned; the amount was reduced to $161,058 because the medical expenses were written off by the health-care providers.
_______________
Was travel appropriate for this pregnant woman?
A woman with a history of two premature deliveries and one miscarriage became pregnant again. She received prenatal care at an Army hospital. She traveled to Spain, where the baby was born at 31 weeks’ gestation. The baby required treatment in a neonatal intensive care unit (NICU) for 17 days. The child has cerebral palsy, with tetraplegia of all four extremities. She cannot walk without assistance and suffers severe cognitive and vision impairment.
PARENTS’ CLAIM The ObGyn at the Army hospital should not have approved the mother’s request for travel; he did so, despite knowing that the mother was at high risk for premature birth. The military medical hospital to which she was assigned in Spain could not manage a high-risk pregnancy, didn’t have a NICU, and didn’t have specialists to treat premature infants.
DEFENDANTS’ DEFENSE The ObGyn argued that he did not have access to the medical records showing the mother’s history. The patient countered that the ObGyn did indeed have the patient’s records, as he had discussed them with her.
VERDICT A $10,409,700 California verdict was returned against the ObGyn and the government facility.
_______________
Triple-negative BrCa not diagnosed until metastasized: $5.2M
After finding lumps in both breasts, a woman in her 30s saw a nurse practitioner (NP) at an Army hospital. A radiologist reported no mass in the right breast and multiple benign-appearing anechoic lesions in the left breast after bilateral mammography and ultrasonography (US) in July 2008. The Chief of Mammography Services recommended referral to a breast surgeon, but the patient never received the letter. It was placed in her mammography file, not in the treatment file.
In November 2008, the patient returned to the clinic. Bilateral diagnostic mammography and US were ordered, but for unknown reasons, cancelled. US of the left breast was interpreted as benign in January 2009.
After imaging in March 2010, followed by a needle biopsy of the right breast, a radiologist reported finding intermediate-grade infiltrating ductal carcinoma.
The patient sought care outside the military medical system at a large university hospital. In April 2010, stage 3 triple-negative invasive ductal carcinoma (IDC) was identified. The patient underwent chemotherapy, a double mastectomy, removal of 21 lymph nodes, and breast reconstruction. She was given a 60% chance of recurrence in 5–7 years.
PATIENT’S CLAIM It was negligent to not inform her of imaging results. Biopsy should have been performed in 2008, when the IDC was likely at stage 1; treatment would have been far less aggressive. Electronic medical records showed that the 2008 mammography and US results had been “signed off” by an NP at the clinic.
DEFENDANTS’ DEFENSE While unable to concede liability, the government agency did not contest the point.
VERDICT A $5.2 million Tennessee federal court bench verdict was returned, citing failures in communication, poor and improper record keeping and retention, failure to follow-up, and an unexplained cancellation of a medical order.
_______________
Woman dies from cervical cancer: $2.3M
In 2001, a 41-year-old woman had abnormal Pap smear results but her gynecologist did not order more testing. The patient was told to return in 3 months, but she did not return until 2007—reporting abnormal bleeding, vaginal discharge, and pain. Her Pap results were normal, however, and the gynecologist did not order further testing. In 2009, the patient was found to have advanced cervical cancer. She died 2 years later.
ESTATE’S CLAIM Further testing should have been ordered in 2001, which would have likely revealed dysplasia, which can lead to cancer. The laboratory incorrectly interpreted the 2007 Pap test; if the results had been properly reported, additional testing could have been ordered.
DEFENDANTS’ DEFENSE The laboratory and patient’s estate settled for a confidential amount before trial. The gynecologist denied negligence.
VERDICT A New Jersey jury found the gynecologist 40% at fault for his actions in 2007. The jury found the laboratory 50% at fault, and the patient 10% at fault. A gross verdict of $2.33 million was returned.
_______________
Bowel injury after cesarean delivery; mother dies of sepsis
At 40 4/7 weeks' gestation, a 37-year-old woman gave birth to a healthy child by cesarean delivery. The next day, the patient had an elevated white blood cell (WBC) count with a left shift, her abdomen was tympanic but soft, and she was passing flatus and belching. The ObGyn ordered a Fleet enema; only flatus was released. A covering ObGyn ordered an abdominal radiograph, which the radiologist reported as showing postoperative ileus and mild constipation. The patient was given a second Fleet enema the next day, resulting in watery stool. She vomited 300 mL of dark green fluid.
After a rectal tube was placed 2 days later, one hard brown stool and several brown, pasty, loose, and liquid stools were returned. She vomited several times that day, and was found to have hypoactive bowel sounds with continued tympanic quality in the upper quadrants. Laboratory testing revealed continued elevated WBC count with left shift. The next day, she had hypoactive bowel sounds with brown liquid stools. Later that morning, she was able to tolerate clear liquids. The ObGyn decided to discharge her home with instructions to continue on a clear liquid diet for 2 more days before advancing her diet.
The day after discharge, she was found unresponsive at home. She was taken to the hospital, but resuscitation attempts failed. She died. An autopsy revealed that the cause of death was sepsis.
ESTATE’S CLAIM The ObGyn was negligent in failing to diagnose and treat a postoperative intra-abdominal infection caused by bowel perforation. A surgical consult should have been obtained. The woman was prematurely discharged. The radiologist failed to report the presence of free air on the abdominal x-ray.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million Maryland settlement was reached.
_______________
Right ureter injury detected and repaired
During laparoscopic-assisted vaginal hysterectomy, the ObGyn detected and repaired an injury to the right ureter. The patient’s recovery was delayed by the injury.
PATIENT’S CLAIM The ObGyn was negligent in using a Kleppinger bipolar cauterizing instrument to cauterize the vaginal cuff. Thermal overspray from the instrument or the instrument itself damaged the ureter. The ObGyn was also negligent in not performing diagnostic cystoscopy to confirm patency of the ureter after the repair was made.
PHYSICIAN’S DEFENSE Ureter injury is a known risk of the procedure. All procedures were performed according to protocol.
VERDICT A Florida defense verdict was returned.
_______________
Failure to detect inflammatory BrCa; woman dies
A 42-year-old woman underwent mammography in February 2002 after reporting pain, discoloration, inflammation, and swelling in her left breast. The radiologist who interpreted the mammography suggested a biopsy for a differential diagnosis of mastitis or inflammatory carcinoma. The biopsy results were negative.
The patient’s symptoms persisted, and she underwent US in late May 2002. Another radiologist interpreted the US, noting that the patient could not tolerate compression, which led to less than optimal evaluation. The radiologist suggested that mastitis was the likely cause of the patient’s symptoms.
The patient then consulted a surgeon, who ordered mammography and magnetic resonance imaging (MRI) followed by biopsy, which indicated cancer. The patient underwent a mastectomy but metastasis had already occurred. She died at age 50 prior to the trial.
ESTATE’S CLAIM If the cancer had been diagnosed earlier, the outcome would have been better. Both radiologists misinterpreted the mammographies.
DEFENDANTS’ DEFENSE The mammographies had been properly interpreted. Any missed diagnosis would not have impacted the outcome due to the type of cancer. The scans had been released to the patient, but were subsequently lost; an adverse interference instruction was given to the jury.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine rupture, child stillborn: $3.8M net award
At 35 weeks' gestation, a woman went to the emergency department (ED) with abdominal pain, fast heartbeat, and irregular contractions. Her history included three cesarean deliveries, including one with a vertical incision. She was admitted, and a cesarean delivery was planned for the next day. After 8 hours, during which the patient’s condition worsened, an emergency cesarean delivery was undertaken. A full rupture of the uterus was found; the baby’s body had extruded into the mother’s abdomen. The child was stillborn.
PARENTS’ CLAIM The stillbirth could have been avoided if the nurses had communicated the mother’s worsening condition to the physicians.
DEFENDANTS’ DEFENSE After the hospital and physicians settled prior to trial, the case continued against the nurse in charge of the mother’s care and the nurse-staffing group. Negligence was denied; all protocols were followed.
VERDICT A $2.9 million Illinois verdict was returned. With a $900,000 settlement from the hospital and physicians, the net award was $3.8 million.
_______________
Where did rare strep A infection come from?
A 36-year-old woman reported heavy vaginal bleeding to her ObGyn. She underwent endometrial ablation in her physician’s office.
The next day, the woman called the office to report abdominal pain. She was told to stop the medication she was taking, and if the pain continued to the next day, to go to an ED. The next day, the patient went to the ED and was found to be in septic shock. During emergency laparotomy, 50 mL of purulent fluid were drained and an emergency hysterectomy was performed. Three days later, the patient died from pulmonary arrest caused by toxic shock syndrome. An autopsy revealed that the patient’s sepsis was caused by group A streptococci (GAS) infection.
ESTATE’S CLAIM The patient was not a proper candidate for endometrial ablation because of her history of chronic cervical infection. The ObGyn perforated the cervix during the procedure and tried to conceal it. At autopsy, bone wax was found in the rectal lumen that had been used to cover up damage to the cervix. The ObGyn introduced GAS bacteria into the patient’s system. The ObGyn’s staff failed to ask the proper questions when she called the day after the procedure. She should have been told to go directly to the ED.
DEFENDANTS’ DEFENSE The ObGyn did not perforate the cervix or uterus during the procedure. GAS infection is so rare that it would have been difficult to foresee or diagnose. Potentially, the patient had a chronic
cervical infection before ablation.
VERDICT A Texas defense verdict was returned.
_______________
DURING INSERTION, IUD PERFORATES UTERINE WALL; LATER FOUND BELOW LIVER
On July 21, a 46-year-old woman went to an ObGyn for placement of an intrauterine device (IUD). Shortly after the ObGyn inserted the levonorgestrel-releasing intrauterine system (Mirena, Bayer HealthCare), the patient reported severe pelvic and abdominal pain. On July 26, the patient underwent surgical removal of the IUD.
She was discharged on July 29 but continued to report pain. She was readmitted to the hospital the next day and treated for pain. She was bed ridden for 3 weeks after IUD-removal surgery, and had a 3-month recovery before feeling pain free.
PATIENT’S CLAIM The ObGyn was negligent in perforating the patient’s uterine wall during IUD insertion, causing the device to ultimately migrate under the patient’s liver.
DEFENDANTS’ DEFENSE Uterine perforation is a known complication of IUD insertion. The IUD escaped from the patient’s uterus at a later time and not during the insertion procedure.
VERDICT A Florida verdict of $208,839 was returned; the amount was reduced to $161,058 because the medical expenses were written off by the health-care providers.
_______________
Was travel appropriate for this pregnant woman?
A woman with a history of two premature deliveries and one miscarriage became pregnant again. She received prenatal care at an Army hospital. She traveled to Spain, where the baby was born at 31 weeks’ gestation. The baby required treatment in a neonatal intensive care unit (NICU) for 17 days. The child has cerebral palsy, with tetraplegia of all four extremities. She cannot walk without assistance and suffers severe cognitive and vision impairment.
PARENTS’ CLAIM The ObGyn at the Army hospital should not have approved the mother’s request for travel; he did so, despite knowing that the mother was at high risk for premature birth. The military medical hospital to which she was assigned in Spain could not manage a high-risk pregnancy, didn’t have a NICU, and didn’t have specialists to treat premature infants.
DEFENDANTS’ DEFENSE The ObGyn argued that he did not have access to the medical records showing the mother’s history. The patient countered that the ObGyn did indeed have the patient’s records, as he had discussed them with her.
VERDICT A $10,409,700 California verdict was returned against the ObGyn and the government facility.
_______________
Triple-negative BrCa not diagnosed until metastasized: $5.2M
After finding lumps in both breasts, a woman in her 30s saw a nurse practitioner (NP) at an Army hospital. A radiologist reported no mass in the right breast and multiple benign-appearing anechoic lesions in the left breast after bilateral mammography and ultrasonography (US) in July 2008. The Chief of Mammography Services recommended referral to a breast surgeon, but the patient never received the letter. It was placed in her mammography file, not in the treatment file.
In November 2008, the patient returned to the clinic. Bilateral diagnostic mammography and US were ordered, but for unknown reasons, cancelled. US of the left breast was interpreted as benign in January 2009.
After imaging in March 2010, followed by a needle biopsy of the right breast, a radiologist reported finding intermediate-grade infiltrating ductal carcinoma.
The patient sought care outside the military medical system at a large university hospital. In April 2010, stage 3 triple-negative invasive ductal carcinoma (IDC) was identified. The patient underwent chemotherapy, a double mastectomy, removal of 21 lymph nodes, and breast reconstruction. She was given a 60% chance of recurrence in 5–7 years.
PATIENT’S CLAIM It was negligent to not inform her of imaging results. Biopsy should have been performed in 2008, when the IDC was likely at stage 1; treatment would have been far less aggressive. Electronic medical records showed that the 2008 mammography and US results had been “signed off” by an NP at the clinic.
DEFENDANTS’ DEFENSE While unable to concede liability, the government agency did not contest the point.
VERDICT A $5.2 million Tennessee federal court bench verdict was returned, citing failures in communication, poor and improper record keeping and retention, failure to follow-up, and an unexplained cancellation of a medical order.
_______________
Woman dies from cervical cancer: $2.3M
In 2001, a 41-year-old woman had abnormal Pap smear results but her gynecologist did not order more testing. The patient was told to return in 3 months, but she did not return until 2007—reporting abnormal bleeding, vaginal discharge, and pain. Her Pap results were normal, however, and the gynecologist did not order further testing. In 2009, the patient was found to have advanced cervical cancer. She died 2 years later.
ESTATE’S CLAIM Further testing should have been ordered in 2001, which would have likely revealed dysplasia, which can lead to cancer. The laboratory incorrectly interpreted the 2007 Pap test; if the results had been properly reported, additional testing could have been ordered.
DEFENDANTS’ DEFENSE The laboratory and patient’s estate settled for a confidential amount before trial. The gynecologist denied negligence.
VERDICT A New Jersey jury found the gynecologist 40% at fault for his actions in 2007. The jury found the laboratory 50% at fault, and the patient 10% at fault. A gross verdict of $2.33 million was returned.
_______________
Bowel injury after cesarean delivery; mother dies of sepsis
At 40 4/7 weeks' gestation, a 37-year-old woman gave birth to a healthy child by cesarean delivery. The next day, the patient had an elevated white blood cell (WBC) count with a left shift, her abdomen was tympanic but soft, and she was passing flatus and belching. The ObGyn ordered a Fleet enema; only flatus was released. A covering ObGyn ordered an abdominal radiograph, which the radiologist reported as showing postoperative ileus and mild constipation. The patient was given a second Fleet enema the next day, resulting in watery stool. She vomited 300 mL of dark green fluid.
After a rectal tube was placed 2 days later, one hard brown stool and several brown, pasty, loose, and liquid stools were returned. She vomited several times that day, and was found to have hypoactive bowel sounds with continued tympanic quality in the upper quadrants. Laboratory testing revealed continued elevated WBC count with left shift. The next day, she had hypoactive bowel sounds with brown liquid stools. Later that morning, she was able to tolerate clear liquids. The ObGyn decided to discharge her home with instructions to continue on a clear liquid diet for 2 more days before advancing her diet.
The day after discharge, she was found unresponsive at home. She was taken to the hospital, but resuscitation attempts failed. She died. An autopsy revealed that the cause of death was sepsis.
ESTATE’S CLAIM The ObGyn was negligent in failing to diagnose and treat a postoperative intra-abdominal infection caused by bowel perforation. A surgical consult should have been obtained. The woman was prematurely discharged. The radiologist failed to report the presence of free air on the abdominal x-ray.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million Maryland settlement was reached.
_______________
Right ureter injury detected and repaired
During laparoscopic-assisted vaginal hysterectomy, the ObGyn detected and repaired an injury to the right ureter. The patient’s recovery was delayed by the injury.
PATIENT’S CLAIM The ObGyn was negligent in using a Kleppinger bipolar cauterizing instrument to cauterize the vaginal cuff. Thermal overspray from the instrument or the instrument itself damaged the ureter. The ObGyn was also negligent in not performing diagnostic cystoscopy to confirm patency of the ureter after the repair was made.
PHYSICIAN’S DEFENSE Ureter injury is a known risk of the procedure. All procedures were performed according to protocol.
VERDICT A Florida defense verdict was returned.
_______________
Failure to detect inflammatory BrCa; woman dies
A 42-year-old woman underwent mammography in February 2002 after reporting pain, discoloration, inflammation, and swelling in her left breast. The radiologist who interpreted the mammography suggested a biopsy for a differential diagnosis of mastitis or inflammatory carcinoma. The biopsy results were negative.
The patient’s symptoms persisted, and she underwent US in late May 2002. Another radiologist interpreted the US, noting that the patient could not tolerate compression, which led to less than optimal evaluation. The radiologist suggested that mastitis was the likely cause of the patient’s symptoms.
The patient then consulted a surgeon, who ordered mammography and magnetic resonance imaging (MRI) followed by biopsy, which indicated cancer. The patient underwent a mastectomy but metastasis had already occurred. She died at age 50 prior to the trial.
ESTATE’S CLAIM If the cancer had been diagnosed earlier, the outcome would have been better. Both radiologists misinterpreted the mammographies.
DEFENDANTS’ DEFENSE The mammographies had been properly interpreted. Any missed diagnosis would not have impacted the outcome due to the type of cancer. The scans had been released to the patient, but were subsequently lost; an adverse interference instruction was given to the jury.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
More inclusions:
- Where did rare strep A infection come from?
- During insertion, IUD perforates uterine wall; Later found below liver
- Was travel appropriate for this pregnant woman?
- Triple-negative BrCa not diagnosed until metastasized: $5.2M
- Woman dies from cervical cancer: $2.3M
- Bowel injury after cesarean delivery; mother dies of sepsis
- Right ureter injury detected and repaired
- Failure to detect inflammatory BrCa; woman dies
The Affordable Care Act: What’s the latest?
mmmm
When I last wrote about the Affordable Care Act (ACA), in May 2014, I focused on the contraception issue. Since then, the US Supreme Court ruled, in Burwell v. Hobby Lobby, that closely held, for-profit companies with religious objections to covering birth control can opt out of the requirement to provide contraceptive coverage to their employees.
In this article, I explore that decision and what it means for women’s health. I also present data on the uninsured rate in the United States, which has dropped significantly since enactment of the ACA, and I discuss one increasingly common barrier to access to care—the use of narrow networks by insurers.
A corporation now can hold a religious belief
The Supreme Court’s majority 5-4 ruling recognized, for the first time, that a for-profit corporation can hold a religious belief, but the Court limited this claim to closely held corporations. The Court also decided that the ACA placed a substantial burden on the corporations’ religious beliefs and concluded that there are less burdensome ways to accomplish the law’s intent, rendering the contraceptive coverage provision in the ACA in violation of the Religious Freedom Restoration Act (RFRA). The Court limited its ruling to the contraceptive coverage requirement, essentially turning the requirement into an option for many employers.
Are contraceptives abortifacients?
The religious belief at the center of Burwell v. Hobby Lobby was that life begins at conception, which the Green family—the owners of Hobby Lobby—equate to fertilization. Hobby Lobby’s attorneys also asserted that four contraceptives approved by the US Food and Drug Administration and included in the ACA mandate may prevent implantation of a fertilized egg, thereby constituting abortion.
Although there is no scientific answer as to when life begins, ACOG and the medical community agree that pregnancy begins at implantation. In its amicus brief to the US Supreme Court, ACOG asserted the medical community’s consensus that the four contraceptives prevent pregnancy rather than end it, and are not abortifacients:
- emergency contraceptive pills: levonorgestrel (Plan B) and its generic equivalents and ulipristal acetate (ella)
- the copper IUD (ParaGard)
- levonorgestrel-releasing intrauterine systems (Mirena, Skyla).
What is a closely held corporation?
In general, according to the Pew Research Center, a closely held corporation is a private company (not publicly traded) with a limited number of shareholders. The Internal Revenue Service (IRS), an important source, defines a closely held corporation as one in which more than half of the stock is owned (directly or indirectly) by five or fewer individuals at any time in the second half of the year.
“S” corporations are also considered closely held. These are corporations with 100 or fewer shareholders, with all members of the same family counted as one shareholder. “S” corporations don’t pay income tax; their shareholders pay tax on their personal returns, based on the corporations’ profits and losses.
Hobby Lobby is organized as an “S” corporation. According to the IRS, in 2011, there were 4,158,572 “S” corporations, 99.4% of them with 10 or fewer shareholders.1
The US Census Bureau estimates that, in 2012, about 2.9 million “S” corporations employed more than 29 million people. Many closely held corporations are quite large.2 According to the Pew Research Center, family-owned Cargill employs 140,000 people and had $136.7 billion in revenue in fiscal 2013. Hobby Lobby has estimated revenues of $3.3 billion and 23,000 employees.2
What’s next?
ACOG helped secure coverage of contraceptives in the ACA and is working with the US Congress and our women’s health partners to restore this important care. Days after the Supreme Court decision, Senator Patty Murray (D-WA) introduced the Protect Women’s Health from Corporate Interference Act, S. 2578, with 46 cosponsors as of this writing. ACOG fully supports this bill, also known as the “Not My Boss’ Business Bill,” which would reestablish the contraceptive coverage mandate as well as other care required by federal law. This bill still maintains the exemption from contraceptive coverage for houses of worship and the accommodation for religious nonprofits.
In introducing her bill, Senator Murray pointed out that “the contraceptive coverage requirement has already made a tremendous difference in women’s lives—24 million more prescriptions for oral contraceptives were filled with no copay in 2013 than in 2012, and women have saved $483 million in out-of-pocket costs for oral contraceptives.”3
Uninsured rate is declining
The Commonwealth Fund shows that, from July–September 2013 to April–June 2014, the nation’s uninsured rate fell from 20% to 15%, resulting in 9.5 million fewer uninsured adults.4 The biggest drop occurred among young adults, with the uninsured rate falling from 28% to 18%, and in states that adopted the Medicaid expansion, where uninsured rates fell from 28% to 17%.4
States that didn’t expand their Medicaid program didn’t show any noticeable change, with the uninsured rate declining only two points, from 38% to 36%.4
Coverage resulted in access to care for the majority of the newly covered. Sixty percent of people with new coverage visited a provider or hospital or paid for a prescription. Sixty-two percent of these individuals said they wouldn’t have been able to access this care before getting this coverage. Eighty-one percent of people with new coverage said they were better off now than before.4
ACA works better in some states than others
The Kaiser Family Foundation looked at four successful states—Colorado, Connecticut, Kentucky, and Washington state—to see what lessons can be learned. Important commonalities include the fact that the states run their own marketplace, adopted the Medi-caid expansion, and conducted extensive outreach and public education, including engaging providers in patient outreach and enrollment.5
Other tools of success were developing good marketing and branding, providing consumer-friendly assistance, and attention to systems and operations.5
How the Hobby Lobby decision affects individual states
Because the Supreme Court’s decision concerned interpretation of a federal law—the Religious Freedom Restoration Act (RFRA)—it does not supersede state laws that mandate coverage of contraceptives.
Twenty-eight states have laws or rulings requiring insurers to cover contraceptives, most of them dating from the 1990s and providing some exemption for religious insurers or plans. Only Illinois allows an exemption for secular bodies.
Although these state laws remain in effect, state officials may opt to stop enforcing them with regard to certain companies. For example, after the Hobby Lobby decision, Wisconsin officials announced that they no longer will enforce contraceptive coverage when a company has a religious objection.
For companies that self-fund or self-insure worker health coverage, the state coverage laws don’t apply—only federal law does. These companies do not have to adhere to state insurance mandates.
Some states have their own version of the RFRA. See the chart at right for details on a state-by-state basis.
The Supreme Court ruling also has no effect on state laws that guarantee access to emergency contraception in hospital emergency departments and that require pharmacists to dispense contraceptives.
| State | Contraceptive equity law? | Employer/insurer exemption to equity law? | Religious freedom law? |
| Alabama | ✔ | ||
| Alaska | |||
| Arizona | ✔ | ✔ | ✔ |
| Arkansas | ✔ | ✔ | |
| California | ✔ | ✔ | |
| Colorado | ✔ | ||
| Connecticut | ✔ | ✔ | ✔ |
| Delaware | ✔ | ✔ | |
| Florida | ✔ | ||
| Georgia | ✔ | ||
| Hawaii | ✔ | ✔ | |
| Idaho | ✔ | ||
| Illinois | ✔ | ✔ | ✔ |
| Indiana | |||
| Iowa | ✔ | ||
| Kansas | |||
| Kentucky | ✔ | ||
| Louisiana | ✔ | ||
| Maine | ✔ | ✔ | |
| Maryland | ✔ | ✔ | |
| Massachusetts | ✔ | ✔ | |
| Michigan | ✔ | ✔ | |
| Minnesota | |||
| Mississippi | ✔ | ||
| Missouri | ✔ | ✔ | ✔ |
| Montana | ✔ | ||
| Nebraska | |||
| Nevada | ✔ | ✔ | |
| New Hampshire | ✔ | ||
| New Jersey | ✔ | ✔ | |
| New Mexico | ✔ | ✔ | ✔ |
| New York | ✔ | ✔ | |
| North Carolina | ✔ | ✔ | |
| North Dakota | |||
| Ohio | |||
| Oklahoma | ✔ | ||
| Oregon | ✔ | ✔ | |
| Pennsylvania | ✔ | ||
| Rhode Island | ✔ | ✔ | ✔ |
| South Carolina | ✔ | ||
| South Dakota | |||
| Tennessee | ✔ | ||
| Texas | ✔ | ||
| Utah | |||
| Vermont | ✔ | ||
| Virginia | ✔ | ||
| Washington | ✔ | ||
| West Virginia | ✔ | ✔ | |
| Wisconsin | ✔ | ||
| Wyoming | |||
| TOTAL | 28 | 20 | 18 |
Narrow networks limit access to care
Huge concerns abound regarding implementation and real-life experiences related to the ACA. A number of them—high deductibles, low payment rates, limited access to physicians, long drive and wait times—can be related to “narrow networks.” Insurers exclude certain providers and offer all providers lower payment rates (which leads some physicians to drop out of the plan); they also create tiers (charging consumers lower copays and deductibles for using inner-tier preferred providers and high out-of-pocket costs for using other providers, even though they may be in the network).
Narrow networks work for insurers as an effective tool for lowering provider payment rates to keep premiums low and gain market share. The narrower the network, the lower are physician payments and premiums.
The ACA promises expanded access to high-quality, affordable health care for millions of Americans—a promise being compromised in many areas of the country through narrow networks. In these instances, insurers offering new plans in a health-care marketplace limit patient access to the numbers, types, and locations of physicians and hospitals covered under certain plans. Insurers typically offer patients low premiums, offer selected providers a high volume of patients at low payment levels, and exclude other providers whom the insurer deems to be high-cost.
Narrow networks aren’t new
As with so many elements of the ACA, narrow networks aren’t a new phenomenon. Many of us remember the public relations price that HMOs paid in the 1980s and 1990s for exceedingly limiting patients’ access to care while charging low premiums. The consumer outcry led the National Association of Insurance Commissioners to urge states to require managed-care plans to maintain adequate networks, the approach adopted by the federal government in the ACA.6
The pendulum swung in the next decade to broader networks in which consumers had much greater access, but premiums increased by an average of 11% per year.6 Employers then pushed insurers to reduce premium costs, leading back to narrow networks in the years just before the ACA. Narrow network plans accounted for 23% of all employer-sponsored plans in 2012, up from 15% in 2007.6
Increasing consolidation contributes to narrow networks
The trend toward narrower networks is also linked to increasing consolidation in health care. As health systems grow and individual or small group practices disappear, insurers rely on being able to credibly threaten to exclude systems and big groups from their networks as leverage in payment negotiations. By restricting the choice of providers in a plan, the insurer can promise more customers for the doctors and hospitals that are included, and negotiate lower payments to those providers.
The downside for physicians is clear:
- low payment rates
- exclusion from networks and coverage
- inability to refer patients to providers the physician determines to be best for that patient’s needs.
- The downside for patients:
- If they have to go out of network to get needed care, they may end up paying high out-of-pocket costs
- If they delay or forego care, their health may suffer significantly.
The insurance industry’s position is that patients have choices. Plans with access to more hospitals and specialists are available but usually at a higher price.
Narrow networks are one way to achieve low premiums
In the months leading up to ACA enactment, insurers got to work developing plans designed to be sold on the exchanges that would attract consumers through low-cost premiums and still maximize profits, especially now that insurers, under the ACA, are barred from excluding sick enrollees or increasing premiums for women, in addition to other important protections.
In previous articles, we’ve explored these landmark protections. Insurers in the individual market used to be able to keep premiums relatively low, and profits up, through use of preexisting coverage exclusions, benefit exclusions including noncoverage for maternity care or prescription drugs, and high cost sharing. Not anymore.
Since enactment of the ACA, narrow networks seem to be the preferred, and most effective, payment negotiation tool of many insurers offering plans through the exchanges, reflecting the trend we’re already seeing in the private health insurance marketplace.
NPR spotlights the difficulty of finding a specialist
The consumer and provider problems of narrow networks have been gaining attention in the media. In July, the National Public Radio (NPR) Web site carried an article entitled, “Patients with low-cost insurance struggle to find specialists,” with a key subtitle: “So you found an exchange plan. But can you find a provider?”7
In the NPR article, author Carrie Feibel reported on the situation in a majority-immigrant area of southwest Houston.
There, many patients at the local clinic have health insurance coverage for the first time, an important step toward healthier lives for themselves and their families. But many people in need of a specialist are learning that their insurance card doesn’t guarantee them access to a needed surgeon or hospital. They’ve purchased a narrow-network insurance plan, with a low premium but few specialists who accept that insurance.7
The two largest hospital chains in Houston—Houston Methodist and Memorial Hermann—as well as Houston’s MD Anderson Cancer Center, don’t participate in the Blue Cross Blue Shield HMO Silver plan, a plan popular with low-income consumers because of its low premium.7
Will the government take action?
The ACA actually guards against overly narrow networks and established the first national standard for network adequacy—a standard that needs fuller development, for sure. Plans sold on the exchanges are required to establish networks that include, among other providers, essential community providers, who typically care for mostly low-income and medically underserved populations. Networks also must include sufficient numbers and types of providers, including “providers that specialize in mental health and substance abuse services, to assure that all services will be accessible without unreasonable delay.”8
Insurers also must provide people who are considering purchasing their products with an accurate directory—both online and a hard copy—identifying providers not accepting new patients in the network. And plans are prohibited from charging out-of-network cost-sharing for emergency services.
Much of the oversight and many of the details—how much is adequate? what is unreasonable?—are left to the states, many of which have years of experience grappling with the downsides and delicate balance of networks.
The Urban Institute points out that Vermont and Delaware set standards for maximum geographic distance and drive times for primary care services. In California, plans must make it easy for consumers to reach urban providers on public transportation.6
Professional societies are taking note
Today, the misuse of narrow networks by exchange plans also has gotten the attention of the American Medical Association, ACOG, and many other national medical specialty societies, in addition to the states and federal government.
The trick, many health-care policy experts agree, is to find the right balance. How broad can the network be before premiums soar? Most agree that consumers must be able to choose between plans with confidence, without any cost or access surprises, meaning much better transparency. And many agree that provider quality, in addition to cost, has to find its way into the equation.
This year, the Center for Consumer Information and Insurance Oversight, a part of the federal Department of Health and Human Services created by the ACA to investigate these kinds of issues, is investigating access to hospital systems, mental health services, oncology, and primary care providers and is developing time, distance, and other standards that insurers will have to adhere to.
Employer groups oppose strong standards or limits on narrow networks. Recently, representatives of the US Chamber of Commerce, the National Retail Federation, and others warned Congress to stay out of this fight. They understand that more generous networks mean higher premiums. These employer representative groups prefer to strengthen consumer protections like directories and keep low the cost of health insurance that they provide for their employees.
Acknowledgment
The author acknowledges the work and expertise of ACOG's state government affairs team for the state analysis—Kathryn Moore, Director, and Kate Vlach, Senior Manager—as well as advocacy partners.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Internal Revenue Service. SOI Tax States, Table 1, Returns of Active Corporations, Form 1120S. http://www.irs.gov/uac/SOI-Tax-Stats-Table-1-Returns-of-Active-Corporations,-Form-1120S. Updated June 27, 2014. Accessed September 4, 2014.
2. DeSilver D. What is a ‘closely held corporation,’ anyway, and how many are there? Pew Research Center: Fact Tank. http://www.pewresearch.org/fact-tank/2014/07/07/what-is-a-closely-held-corporation-anyway-and-how-many-are-there/. Published July 7, 2014. Accessed September 4, 2014.
3. Murray P. Protect Women’s Health From Corporate Interference Act: Summary. http://www.murray.senate.gov/public/_cache/files/30554052-0f84-485a-babc-ccc04af85bb6/protect-women-s-health-from-corporate-interference-act---one-page-summary---final.pdf. Accessed September 4, 2014.
4. The Commonwealth Fund. New Survey: After First ACA Enrollment Period, Uninsured Rate Dropped from 20% to 15%; Largest Declines Among Young Adults, Latinos, and Low-Income People. http://www.commonwealthfund.org/publications/press-releases/2014/jul/after-first-aca -enrollment-period. Published July 10,2014. Accessed September 4, 2014.
5. Artiga S, Stephens J, Rudowitz R, Perry M. What Worked and What’s Next? Strategies in Four States Leading ACA Enrollment Efforts. Kaiser Family Foundation. http://kff.org/health-reform/issue-brief/what-worked-and-whats-next-strategies-in-four-states-leading-aca-enrollment-efforts/. Published July 16, 2014. Accessed September 4, 2014.
6. Corlette S, Volk J. Narrow Provider Networks in New Health Plans: Balancing Affordability with Access to Quality Care. Urban Institute: Georgetown University Center on Health Insurance Reforms. http://www.urban.org/UploadedPDF/413135-New-Provider-Networks-in-New-Health-Plans.pdf. Published May 2014. Accessed September 4, 2014.
7. Feibel C. Patients With Low-Cost Insurance Struggle to Find Specialists. National Public Radio. http://www.npr.org/blogs/health/2014/07/16/331419293/patients-with-low-cost-insurance-struggle-to-find-specialists. Published July 16, 2014. Accessed September 4, 2014.
8. Patient Protection and Affordable Care Act: Preexisting Condition Exclusions, Lifetime and Annual Limits, Rescissions, and Patient Protections. US Department of Health and Human Services. http://www.regulations.go/#!documentDetail;D=HHS-OS-2010-0014-0001. Published June 28, 2010. Accessed September 9, 2014.
mmmm
When I last wrote about the Affordable Care Act (ACA), in May 2014, I focused on the contraception issue. Since then, the US Supreme Court ruled, in Burwell v. Hobby Lobby, that closely held, for-profit companies with religious objections to covering birth control can opt out of the requirement to provide contraceptive coverage to their employees.
In this article, I explore that decision and what it means for women’s health. I also present data on the uninsured rate in the United States, which has dropped significantly since enactment of the ACA, and I discuss one increasingly common barrier to access to care—the use of narrow networks by insurers.
A corporation now can hold a religious belief
The Supreme Court’s majority 5-4 ruling recognized, for the first time, that a for-profit corporation can hold a religious belief, but the Court limited this claim to closely held corporations. The Court also decided that the ACA placed a substantial burden on the corporations’ religious beliefs and concluded that there are less burdensome ways to accomplish the law’s intent, rendering the contraceptive coverage provision in the ACA in violation of the Religious Freedom Restoration Act (RFRA). The Court limited its ruling to the contraceptive coverage requirement, essentially turning the requirement into an option for many employers.
Are contraceptives abortifacients?
The religious belief at the center of Burwell v. Hobby Lobby was that life begins at conception, which the Green family—the owners of Hobby Lobby—equate to fertilization. Hobby Lobby’s attorneys also asserted that four contraceptives approved by the US Food and Drug Administration and included in the ACA mandate may prevent implantation of a fertilized egg, thereby constituting abortion.
Although there is no scientific answer as to when life begins, ACOG and the medical community agree that pregnancy begins at implantation. In its amicus brief to the US Supreme Court, ACOG asserted the medical community’s consensus that the four contraceptives prevent pregnancy rather than end it, and are not abortifacients:
- emergency contraceptive pills: levonorgestrel (Plan B) and its generic equivalents and ulipristal acetate (ella)
- the copper IUD (ParaGard)
- levonorgestrel-releasing intrauterine systems (Mirena, Skyla).
What is a closely held corporation?
In general, according to the Pew Research Center, a closely held corporation is a private company (not publicly traded) with a limited number of shareholders. The Internal Revenue Service (IRS), an important source, defines a closely held corporation as one in which more than half of the stock is owned (directly or indirectly) by five or fewer individuals at any time in the second half of the year.
“S” corporations are also considered closely held. These are corporations with 100 or fewer shareholders, with all members of the same family counted as one shareholder. “S” corporations don’t pay income tax; their shareholders pay tax on their personal returns, based on the corporations’ profits and losses.
Hobby Lobby is organized as an “S” corporation. According to the IRS, in 2011, there were 4,158,572 “S” corporations, 99.4% of them with 10 or fewer shareholders.1
The US Census Bureau estimates that, in 2012, about 2.9 million “S” corporations employed more than 29 million people. Many closely held corporations are quite large.2 According to the Pew Research Center, family-owned Cargill employs 140,000 people and had $136.7 billion in revenue in fiscal 2013. Hobby Lobby has estimated revenues of $3.3 billion and 23,000 employees.2
What’s next?
ACOG helped secure coverage of contraceptives in the ACA and is working with the US Congress and our women’s health partners to restore this important care. Days after the Supreme Court decision, Senator Patty Murray (D-WA) introduced the Protect Women’s Health from Corporate Interference Act, S. 2578, with 46 cosponsors as of this writing. ACOG fully supports this bill, also known as the “Not My Boss’ Business Bill,” which would reestablish the contraceptive coverage mandate as well as other care required by federal law. This bill still maintains the exemption from contraceptive coverage for houses of worship and the accommodation for religious nonprofits.
In introducing her bill, Senator Murray pointed out that “the contraceptive coverage requirement has already made a tremendous difference in women’s lives—24 million more prescriptions for oral contraceptives were filled with no copay in 2013 than in 2012, and women have saved $483 million in out-of-pocket costs for oral contraceptives.”3
Uninsured rate is declining
The Commonwealth Fund shows that, from July–September 2013 to April–June 2014, the nation’s uninsured rate fell from 20% to 15%, resulting in 9.5 million fewer uninsured adults.4 The biggest drop occurred among young adults, with the uninsured rate falling from 28% to 18%, and in states that adopted the Medicaid expansion, where uninsured rates fell from 28% to 17%.4
States that didn’t expand their Medicaid program didn’t show any noticeable change, with the uninsured rate declining only two points, from 38% to 36%.4
Coverage resulted in access to care for the majority of the newly covered. Sixty percent of people with new coverage visited a provider or hospital or paid for a prescription. Sixty-two percent of these individuals said they wouldn’t have been able to access this care before getting this coverage. Eighty-one percent of people with new coverage said they were better off now than before.4
ACA works better in some states than others
The Kaiser Family Foundation looked at four successful states—Colorado, Connecticut, Kentucky, and Washington state—to see what lessons can be learned. Important commonalities include the fact that the states run their own marketplace, adopted the Medi-caid expansion, and conducted extensive outreach and public education, including engaging providers in patient outreach and enrollment.5
Other tools of success were developing good marketing and branding, providing consumer-friendly assistance, and attention to systems and operations.5
How the Hobby Lobby decision affects individual states
Because the Supreme Court’s decision concerned interpretation of a federal law—the Religious Freedom Restoration Act (RFRA)—it does not supersede state laws that mandate coverage of contraceptives.
Twenty-eight states have laws or rulings requiring insurers to cover contraceptives, most of them dating from the 1990s and providing some exemption for religious insurers or plans. Only Illinois allows an exemption for secular bodies.
Although these state laws remain in effect, state officials may opt to stop enforcing them with regard to certain companies. For example, after the Hobby Lobby decision, Wisconsin officials announced that they no longer will enforce contraceptive coverage when a company has a religious objection.
For companies that self-fund or self-insure worker health coverage, the state coverage laws don’t apply—only federal law does. These companies do not have to adhere to state insurance mandates.
Some states have their own version of the RFRA. See the chart at right for details on a state-by-state basis.
The Supreme Court ruling also has no effect on state laws that guarantee access to emergency contraception in hospital emergency departments and that require pharmacists to dispense contraceptives.
| State | Contraceptive equity law? | Employer/insurer exemption to equity law? | Religious freedom law? |
| Alabama | ✔ | ||
| Alaska | |||
| Arizona | ✔ | ✔ | ✔ |
| Arkansas | ✔ | ✔ | |
| California | ✔ | ✔ | |
| Colorado | ✔ | ||
| Connecticut | ✔ | ✔ | ✔ |
| Delaware | ✔ | ✔ | |
| Florida | ✔ | ||
| Georgia | ✔ | ||
| Hawaii | ✔ | ✔ | |
| Idaho | ✔ | ||
| Illinois | ✔ | ✔ | ✔ |
| Indiana | |||
| Iowa | ✔ | ||
| Kansas | |||
| Kentucky | ✔ | ||
| Louisiana | ✔ | ||
| Maine | ✔ | ✔ | |
| Maryland | ✔ | ✔ | |
| Massachusetts | ✔ | ✔ | |
| Michigan | ✔ | ✔ | |
| Minnesota | |||
| Mississippi | ✔ | ||
| Missouri | ✔ | ✔ | ✔ |
| Montana | ✔ | ||
| Nebraska | |||
| Nevada | ✔ | ✔ | |
| New Hampshire | ✔ | ||
| New Jersey | ✔ | ✔ | |
| New Mexico | ✔ | ✔ | ✔ |
| New York | ✔ | ✔ | |
| North Carolina | ✔ | ✔ | |
| North Dakota | |||
| Ohio | |||
| Oklahoma | ✔ | ||
| Oregon | ✔ | ✔ | |
| Pennsylvania | ✔ | ||
| Rhode Island | ✔ | ✔ | ✔ |
| South Carolina | ✔ | ||
| South Dakota | |||
| Tennessee | ✔ | ||
| Texas | ✔ | ||
| Utah | |||
| Vermont | ✔ | ||
| Virginia | ✔ | ||
| Washington | ✔ | ||
| West Virginia | ✔ | ✔ | |
| Wisconsin | ✔ | ||
| Wyoming | |||
| TOTAL | 28 | 20 | 18 |
Narrow networks limit access to care
Huge concerns abound regarding implementation and real-life experiences related to the ACA. A number of them—high deductibles, low payment rates, limited access to physicians, long drive and wait times—can be related to “narrow networks.” Insurers exclude certain providers and offer all providers lower payment rates (which leads some physicians to drop out of the plan); they also create tiers (charging consumers lower copays and deductibles for using inner-tier preferred providers and high out-of-pocket costs for using other providers, even though they may be in the network).
Narrow networks work for insurers as an effective tool for lowering provider payment rates to keep premiums low and gain market share. The narrower the network, the lower are physician payments and premiums.
The ACA promises expanded access to high-quality, affordable health care for millions of Americans—a promise being compromised in many areas of the country through narrow networks. In these instances, insurers offering new plans in a health-care marketplace limit patient access to the numbers, types, and locations of physicians and hospitals covered under certain plans. Insurers typically offer patients low premiums, offer selected providers a high volume of patients at low payment levels, and exclude other providers whom the insurer deems to be high-cost.
Narrow networks aren’t new
As with so many elements of the ACA, narrow networks aren’t a new phenomenon. Many of us remember the public relations price that HMOs paid in the 1980s and 1990s for exceedingly limiting patients’ access to care while charging low premiums. The consumer outcry led the National Association of Insurance Commissioners to urge states to require managed-care plans to maintain adequate networks, the approach adopted by the federal government in the ACA.6
The pendulum swung in the next decade to broader networks in which consumers had much greater access, but premiums increased by an average of 11% per year.6 Employers then pushed insurers to reduce premium costs, leading back to narrow networks in the years just before the ACA. Narrow network plans accounted for 23% of all employer-sponsored plans in 2012, up from 15% in 2007.6
Increasing consolidation contributes to narrow networks
The trend toward narrower networks is also linked to increasing consolidation in health care. As health systems grow and individual or small group practices disappear, insurers rely on being able to credibly threaten to exclude systems and big groups from their networks as leverage in payment negotiations. By restricting the choice of providers in a plan, the insurer can promise more customers for the doctors and hospitals that are included, and negotiate lower payments to those providers.
The downside for physicians is clear:
- low payment rates
- exclusion from networks and coverage
- inability to refer patients to providers the physician determines to be best for that patient’s needs.
- The downside for patients:
- If they have to go out of network to get needed care, they may end up paying high out-of-pocket costs
- If they delay or forego care, their health may suffer significantly.
The insurance industry’s position is that patients have choices. Plans with access to more hospitals and specialists are available but usually at a higher price.
Narrow networks are one way to achieve low premiums
In the months leading up to ACA enactment, insurers got to work developing plans designed to be sold on the exchanges that would attract consumers through low-cost premiums and still maximize profits, especially now that insurers, under the ACA, are barred from excluding sick enrollees or increasing premiums for women, in addition to other important protections.
In previous articles, we’ve explored these landmark protections. Insurers in the individual market used to be able to keep premiums relatively low, and profits up, through use of preexisting coverage exclusions, benefit exclusions including noncoverage for maternity care or prescription drugs, and high cost sharing. Not anymore.
Since enactment of the ACA, narrow networks seem to be the preferred, and most effective, payment negotiation tool of many insurers offering plans through the exchanges, reflecting the trend we’re already seeing in the private health insurance marketplace.
NPR spotlights the difficulty of finding a specialist
The consumer and provider problems of narrow networks have been gaining attention in the media. In July, the National Public Radio (NPR) Web site carried an article entitled, “Patients with low-cost insurance struggle to find specialists,” with a key subtitle: “So you found an exchange plan. But can you find a provider?”7
In the NPR article, author Carrie Feibel reported on the situation in a majority-immigrant area of southwest Houston.
There, many patients at the local clinic have health insurance coverage for the first time, an important step toward healthier lives for themselves and their families. But many people in need of a specialist are learning that their insurance card doesn’t guarantee them access to a needed surgeon or hospital. They’ve purchased a narrow-network insurance plan, with a low premium but few specialists who accept that insurance.7
The two largest hospital chains in Houston—Houston Methodist and Memorial Hermann—as well as Houston’s MD Anderson Cancer Center, don’t participate in the Blue Cross Blue Shield HMO Silver plan, a plan popular with low-income consumers because of its low premium.7
Will the government take action?
The ACA actually guards against overly narrow networks and established the first national standard for network adequacy—a standard that needs fuller development, for sure. Plans sold on the exchanges are required to establish networks that include, among other providers, essential community providers, who typically care for mostly low-income and medically underserved populations. Networks also must include sufficient numbers and types of providers, including “providers that specialize in mental health and substance abuse services, to assure that all services will be accessible without unreasonable delay.”8
Insurers also must provide people who are considering purchasing their products with an accurate directory—both online and a hard copy—identifying providers not accepting new patients in the network. And plans are prohibited from charging out-of-network cost-sharing for emergency services.
Much of the oversight and many of the details—how much is adequate? what is unreasonable?—are left to the states, many of which have years of experience grappling with the downsides and delicate balance of networks.
The Urban Institute points out that Vermont and Delaware set standards for maximum geographic distance and drive times for primary care services. In California, plans must make it easy for consumers to reach urban providers on public transportation.6
Professional societies are taking note
Today, the misuse of narrow networks by exchange plans also has gotten the attention of the American Medical Association, ACOG, and many other national medical specialty societies, in addition to the states and federal government.
The trick, many health-care policy experts agree, is to find the right balance. How broad can the network be before premiums soar? Most agree that consumers must be able to choose between plans with confidence, without any cost or access surprises, meaning much better transparency. And many agree that provider quality, in addition to cost, has to find its way into the equation.
This year, the Center for Consumer Information and Insurance Oversight, a part of the federal Department of Health and Human Services created by the ACA to investigate these kinds of issues, is investigating access to hospital systems, mental health services, oncology, and primary care providers and is developing time, distance, and other standards that insurers will have to adhere to.
Employer groups oppose strong standards or limits on narrow networks. Recently, representatives of the US Chamber of Commerce, the National Retail Federation, and others warned Congress to stay out of this fight. They understand that more generous networks mean higher premiums. These employer representative groups prefer to strengthen consumer protections like directories and keep low the cost of health insurance that they provide for their employees.
Acknowledgment
The author acknowledges the work and expertise of ACOG's state government affairs team for the state analysis—Kathryn Moore, Director, and Kate Vlach, Senior Manager—as well as advocacy partners.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
mmmm
When I last wrote about the Affordable Care Act (ACA), in May 2014, I focused on the contraception issue. Since then, the US Supreme Court ruled, in Burwell v. Hobby Lobby, that closely held, for-profit companies with religious objections to covering birth control can opt out of the requirement to provide contraceptive coverage to their employees.
In this article, I explore that decision and what it means for women’s health. I also present data on the uninsured rate in the United States, which has dropped significantly since enactment of the ACA, and I discuss one increasingly common barrier to access to care—the use of narrow networks by insurers.
A corporation now can hold a religious belief
The Supreme Court’s majority 5-4 ruling recognized, for the first time, that a for-profit corporation can hold a religious belief, but the Court limited this claim to closely held corporations. The Court also decided that the ACA placed a substantial burden on the corporations’ religious beliefs and concluded that there are less burdensome ways to accomplish the law’s intent, rendering the contraceptive coverage provision in the ACA in violation of the Religious Freedom Restoration Act (RFRA). The Court limited its ruling to the contraceptive coverage requirement, essentially turning the requirement into an option for many employers.
Are contraceptives abortifacients?
The religious belief at the center of Burwell v. Hobby Lobby was that life begins at conception, which the Green family—the owners of Hobby Lobby—equate to fertilization. Hobby Lobby’s attorneys also asserted that four contraceptives approved by the US Food and Drug Administration and included in the ACA mandate may prevent implantation of a fertilized egg, thereby constituting abortion.
Although there is no scientific answer as to when life begins, ACOG and the medical community agree that pregnancy begins at implantation. In its amicus brief to the US Supreme Court, ACOG asserted the medical community’s consensus that the four contraceptives prevent pregnancy rather than end it, and are not abortifacients:
- emergency contraceptive pills: levonorgestrel (Plan B) and its generic equivalents and ulipristal acetate (ella)
- the copper IUD (ParaGard)
- levonorgestrel-releasing intrauterine systems (Mirena, Skyla).
What is a closely held corporation?
In general, according to the Pew Research Center, a closely held corporation is a private company (not publicly traded) with a limited number of shareholders. The Internal Revenue Service (IRS), an important source, defines a closely held corporation as one in which more than half of the stock is owned (directly or indirectly) by five or fewer individuals at any time in the second half of the year.
“S” corporations are also considered closely held. These are corporations with 100 or fewer shareholders, with all members of the same family counted as one shareholder. “S” corporations don’t pay income tax; their shareholders pay tax on their personal returns, based on the corporations’ profits and losses.
Hobby Lobby is organized as an “S” corporation. According to the IRS, in 2011, there were 4,158,572 “S” corporations, 99.4% of them with 10 or fewer shareholders.1
The US Census Bureau estimates that, in 2012, about 2.9 million “S” corporations employed more than 29 million people. Many closely held corporations are quite large.2 According to the Pew Research Center, family-owned Cargill employs 140,000 people and had $136.7 billion in revenue in fiscal 2013. Hobby Lobby has estimated revenues of $3.3 billion and 23,000 employees.2
What’s next?
ACOG helped secure coverage of contraceptives in the ACA and is working with the US Congress and our women’s health partners to restore this important care. Days after the Supreme Court decision, Senator Patty Murray (D-WA) introduced the Protect Women’s Health from Corporate Interference Act, S. 2578, with 46 cosponsors as of this writing. ACOG fully supports this bill, also known as the “Not My Boss’ Business Bill,” which would reestablish the contraceptive coverage mandate as well as other care required by federal law. This bill still maintains the exemption from contraceptive coverage for houses of worship and the accommodation for religious nonprofits.
In introducing her bill, Senator Murray pointed out that “the contraceptive coverage requirement has already made a tremendous difference in women’s lives—24 million more prescriptions for oral contraceptives were filled with no copay in 2013 than in 2012, and women have saved $483 million in out-of-pocket costs for oral contraceptives.”3
Uninsured rate is declining
The Commonwealth Fund shows that, from July–September 2013 to April–June 2014, the nation’s uninsured rate fell from 20% to 15%, resulting in 9.5 million fewer uninsured adults.4 The biggest drop occurred among young adults, with the uninsured rate falling from 28% to 18%, and in states that adopted the Medicaid expansion, where uninsured rates fell from 28% to 17%.4
States that didn’t expand their Medicaid program didn’t show any noticeable change, with the uninsured rate declining only two points, from 38% to 36%.4
Coverage resulted in access to care for the majority of the newly covered. Sixty percent of people with new coverage visited a provider or hospital or paid for a prescription. Sixty-two percent of these individuals said they wouldn’t have been able to access this care before getting this coverage. Eighty-one percent of people with new coverage said they were better off now than before.4
ACA works better in some states than others
The Kaiser Family Foundation looked at four successful states—Colorado, Connecticut, Kentucky, and Washington state—to see what lessons can be learned. Important commonalities include the fact that the states run their own marketplace, adopted the Medi-caid expansion, and conducted extensive outreach and public education, including engaging providers in patient outreach and enrollment.5
Other tools of success were developing good marketing and branding, providing consumer-friendly assistance, and attention to systems and operations.5
How the Hobby Lobby decision affects individual states
Because the Supreme Court’s decision concerned interpretation of a federal law—the Religious Freedom Restoration Act (RFRA)—it does not supersede state laws that mandate coverage of contraceptives.
Twenty-eight states have laws or rulings requiring insurers to cover contraceptives, most of them dating from the 1990s and providing some exemption for religious insurers or plans. Only Illinois allows an exemption for secular bodies.
Although these state laws remain in effect, state officials may opt to stop enforcing them with regard to certain companies. For example, after the Hobby Lobby decision, Wisconsin officials announced that they no longer will enforce contraceptive coverage when a company has a religious objection.
For companies that self-fund or self-insure worker health coverage, the state coverage laws don’t apply—only federal law does. These companies do not have to adhere to state insurance mandates.
Some states have their own version of the RFRA. See the chart at right for details on a state-by-state basis.
The Supreme Court ruling also has no effect on state laws that guarantee access to emergency contraception in hospital emergency departments and that require pharmacists to dispense contraceptives.
| State | Contraceptive equity law? | Employer/insurer exemption to equity law? | Religious freedom law? |
| Alabama | ✔ | ||
| Alaska | |||
| Arizona | ✔ | ✔ | ✔ |
| Arkansas | ✔ | ✔ | |
| California | ✔ | ✔ | |
| Colorado | ✔ | ||
| Connecticut | ✔ | ✔ | ✔ |
| Delaware | ✔ | ✔ | |
| Florida | ✔ | ||
| Georgia | ✔ | ||
| Hawaii | ✔ | ✔ | |
| Idaho | ✔ | ||
| Illinois | ✔ | ✔ | ✔ |
| Indiana | |||
| Iowa | ✔ | ||
| Kansas | |||
| Kentucky | ✔ | ||
| Louisiana | ✔ | ||
| Maine | ✔ | ✔ | |
| Maryland | ✔ | ✔ | |
| Massachusetts | ✔ | ✔ | |
| Michigan | ✔ | ✔ | |
| Minnesota | |||
| Mississippi | ✔ | ||
| Missouri | ✔ | ✔ | ✔ |
| Montana | ✔ | ||
| Nebraska | |||
| Nevada | ✔ | ✔ | |
| New Hampshire | ✔ | ||
| New Jersey | ✔ | ✔ | |
| New Mexico | ✔ | ✔ | ✔ |
| New York | ✔ | ✔ | |
| North Carolina | ✔ | ✔ | |
| North Dakota | |||
| Ohio | |||
| Oklahoma | ✔ | ||
| Oregon | ✔ | ✔ | |
| Pennsylvania | ✔ | ||
| Rhode Island | ✔ | ✔ | ✔ |
| South Carolina | ✔ | ||
| South Dakota | |||
| Tennessee | ✔ | ||
| Texas | ✔ | ||
| Utah | |||
| Vermont | ✔ | ||
| Virginia | ✔ | ||
| Washington | ✔ | ||
| West Virginia | ✔ | ✔ | |
| Wisconsin | ✔ | ||
| Wyoming | |||
| TOTAL | 28 | 20 | 18 |
Narrow networks limit access to care
Huge concerns abound regarding implementation and real-life experiences related to the ACA. A number of them—high deductibles, low payment rates, limited access to physicians, long drive and wait times—can be related to “narrow networks.” Insurers exclude certain providers and offer all providers lower payment rates (which leads some physicians to drop out of the plan); they also create tiers (charging consumers lower copays and deductibles for using inner-tier preferred providers and high out-of-pocket costs for using other providers, even though they may be in the network).
Narrow networks work for insurers as an effective tool for lowering provider payment rates to keep premiums low and gain market share. The narrower the network, the lower are physician payments and premiums.
The ACA promises expanded access to high-quality, affordable health care for millions of Americans—a promise being compromised in many areas of the country through narrow networks. In these instances, insurers offering new plans in a health-care marketplace limit patient access to the numbers, types, and locations of physicians and hospitals covered under certain plans. Insurers typically offer patients low premiums, offer selected providers a high volume of patients at low payment levels, and exclude other providers whom the insurer deems to be high-cost.
Narrow networks aren’t new
As with so many elements of the ACA, narrow networks aren’t a new phenomenon. Many of us remember the public relations price that HMOs paid in the 1980s and 1990s for exceedingly limiting patients’ access to care while charging low premiums. The consumer outcry led the National Association of Insurance Commissioners to urge states to require managed-care plans to maintain adequate networks, the approach adopted by the federal government in the ACA.6
The pendulum swung in the next decade to broader networks in which consumers had much greater access, but premiums increased by an average of 11% per year.6 Employers then pushed insurers to reduce premium costs, leading back to narrow networks in the years just before the ACA. Narrow network plans accounted for 23% of all employer-sponsored plans in 2012, up from 15% in 2007.6
Increasing consolidation contributes to narrow networks
The trend toward narrower networks is also linked to increasing consolidation in health care. As health systems grow and individual or small group practices disappear, insurers rely on being able to credibly threaten to exclude systems and big groups from their networks as leverage in payment negotiations. By restricting the choice of providers in a plan, the insurer can promise more customers for the doctors and hospitals that are included, and negotiate lower payments to those providers.
The downside for physicians is clear:
- low payment rates
- exclusion from networks and coverage
- inability to refer patients to providers the physician determines to be best for that patient’s needs.
- The downside for patients:
- If they have to go out of network to get needed care, they may end up paying high out-of-pocket costs
- If they delay or forego care, their health may suffer significantly.
The insurance industry’s position is that patients have choices. Plans with access to more hospitals and specialists are available but usually at a higher price.
Narrow networks are one way to achieve low premiums
In the months leading up to ACA enactment, insurers got to work developing plans designed to be sold on the exchanges that would attract consumers through low-cost premiums and still maximize profits, especially now that insurers, under the ACA, are barred from excluding sick enrollees or increasing premiums for women, in addition to other important protections.
In previous articles, we’ve explored these landmark protections. Insurers in the individual market used to be able to keep premiums relatively low, and profits up, through use of preexisting coverage exclusions, benefit exclusions including noncoverage for maternity care or prescription drugs, and high cost sharing. Not anymore.
Since enactment of the ACA, narrow networks seem to be the preferred, and most effective, payment negotiation tool of many insurers offering plans through the exchanges, reflecting the trend we’re already seeing in the private health insurance marketplace.
NPR spotlights the difficulty of finding a specialist
The consumer and provider problems of narrow networks have been gaining attention in the media. In July, the National Public Radio (NPR) Web site carried an article entitled, “Patients with low-cost insurance struggle to find specialists,” with a key subtitle: “So you found an exchange plan. But can you find a provider?”7
In the NPR article, author Carrie Feibel reported on the situation in a majority-immigrant area of southwest Houston.
There, many patients at the local clinic have health insurance coverage for the first time, an important step toward healthier lives for themselves and their families. But many people in need of a specialist are learning that their insurance card doesn’t guarantee them access to a needed surgeon or hospital. They’ve purchased a narrow-network insurance plan, with a low premium but few specialists who accept that insurance.7
The two largest hospital chains in Houston—Houston Methodist and Memorial Hermann—as well as Houston’s MD Anderson Cancer Center, don’t participate in the Blue Cross Blue Shield HMO Silver plan, a plan popular with low-income consumers because of its low premium.7
Will the government take action?
The ACA actually guards against overly narrow networks and established the first national standard for network adequacy—a standard that needs fuller development, for sure. Plans sold on the exchanges are required to establish networks that include, among other providers, essential community providers, who typically care for mostly low-income and medically underserved populations. Networks also must include sufficient numbers and types of providers, including “providers that specialize in mental health and substance abuse services, to assure that all services will be accessible without unreasonable delay.”8
Insurers also must provide people who are considering purchasing their products with an accurate directory—both online and a hard copy—identifying providers not accepting new patients in the network. And plans are prohibited from charging out-of-network cost-sharing for emergency services.
Much of the oversight and many of the details—how much is adequate? what is unreasonable?—are left to the states, many of which have years of experience grappling with the downsides and delicate balance of networks.
The Urban Institute points out that Vermont and Delaware set standards for maximum geographic distance and drive times for primary care services. In California, plans must make it easy for consumers to reach urban providers on public transportation.6
Professional societies are taking note
Today, the misuse of narrow networks by exchange plans also has gotten the attention of the American Medical Association, ACOG, and many other national medical specialty societies, in addition to the states and federal government.
The trick, many health-care policy experts agree, is to find the right balance. How broad can the network be before premiums soar? Most agree that consumers must be able to choose between plans with confidence, without any cost or access surprises, meaning much better transparency. And many agree that provider quality, in addition to cost, has to find its way into the equation.
This year, the Center for Consumer Information and Insurance Oversight, a part of the federal Department of Health and Human Services created by the ACA to investigate these kinds of issues, is investigating access to hospital systems, mental health services, oncology, and primary care providers and is developing time, distance, and other standards that insurers will have to adhere to.
Employer groups oppose strong standards or limits on narrow networks. Recently, representatives of the US Chamber of Commerce, the National Retail Federation, and others warned Congress to stay out of this fight. They understand that more generous networks mean higher premiums. These employer representative groups prefer to strengthen consumer protections like directories and keep low the cost of health insurance that they provide for their employees.
Acknowledgment
The author acknowledges the work and expertise of ACOG's state government affairs team for the state analysis—Kathryn Moore, Director, and Kate Vlach, Senior Manager—as well as advocacy partners.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Internal Revenue Service. SOI Tax States, Table 1, Returns of Active Corporations, Form 1120S. http://www.irs.gov/uac/SOI-Tax-Stats-Table-1-Returns-of-Active-Corporations,-Form-1120S. Updated June 27, 2014. Accessed September 4, 2014.
2. DeSilver D. What is a ‘closely held corporation,’ anyway, and how many are there? Pew Research Center: Fact Tank. http://www.pewresearch.org/fact-tank/2014/07/07/what-is-a-closely-held-corporation-anyway-and-how-many-are-there/. Published July 7, 2014. Accessed September 4, 2014.
3. Murray P. Protect Women’s Health From Corporate Interference Act: Summary. http://www.murray.senate.gov/public/_cache/files/30554052-0f84-485a-babc-ccc04af85bb6/protect-women-s-health-from-corporate-interference-act---one-page-summary---final.pdf. Accessed September 4, 2014.
4. The Commonwealth Fund. New Survey: After First ACA Enrollment Period, Uninsured Rate Dropped from 20% to 15%; Largest Declines Among Young Adults, Latinos, and Low-Income People. http://www.commonwealthfund.org/publications/press-releases/2014/jul/after-first-aca -enrollment-period. Published July 10,2014. Accessed September 4, 2014.
5. Artiga S, Stephens J, Rudowitz R, Perry M. What Worked and What’s Next? Strategies in Four States Leading ACA Enrollment Efforts. Kaiser Family Foundation. http://kff.org/health-reform/issue-brief/what-worked-and-whats-next-strategies-in-four-states-leading-aca-enrollment-efforts/. Published July 16, 2014. Accessed September 4, 2014.
6. Corlette S, Volk J. Narrow Provider Networks in New Health Plans: Balancing Affordability with Access to Quality Care. Urban Institute: Georgetown University Center on Health Insurance Reforms. http://www.urban.org/UploadedPDF/413135-New-Provider-Networks-in-New-Health-Plans.pdf. Published May 2014. Accessed September 4, 2014.
7. Feibel C. Patients With Low-Cost Insurance Struggle to Find Specialists. National Public Radio. http://www.npr.org/blogs/health/2014/07/16/331419293/patients-with-low-cost-insurance-struggle-to-find-specialists. Published July 16, 2014. Accessed September 4, 2014.
8. Patient Protection and Affordable Care Act: Preexisting Condition Exclusions, Lifetime and Annual Limits, Rescissions, and Patient Protections. US Department of Health and Human Services. http://www.regulations.go/#!documentDetail;D=HHS-OS-2010-0014-0001. Published June 28, 2010. Accessed September 9, 2014.
1. Internal Revenue Service. SOI Tax States, Table 1, Returns of Active Corporations, Form 1120S. http://www.irs.gov/uac/SOI-Tax-Stats-Table-1-Returns-of-Active-Corporations,-Form-1120S. Updated June 27, 2014. Accessed September 4, 2014.
2. DeSilver D. What is a ‘closely held corporation,’ anyway, and how many are there? Pew Research Center: Fact Tank. http://www.pewresearch.org/fact-tank/2014/07/07/what-is-a-closely-held-corporation-anyway-and-how-many-are-there/. Published July 7, 2014. Accessed September 4, 2014.
3. Murray P. Protect Women’s Health From Corporate Interference Act: Summary. http://www.murray.senate.gov/public/_cache/files/30554052-0f84-485a-babc-ccc04af85bb6/protect-women-s-health-from-corporate-interference-act---one-page-summary---final.pdf. Accessed September 4, 2014.
4. The Commonwealth Fund. New Survey: After First ACA Enrollment Period, Uninsured Rate Dropped from 20% to 15%; Largest Declines Among Young Adults, Latinos, and Low-Income People. http://www.commonwealthfund.org/publications/press-releases/2014/jul/after-first-aca -enrollment-period. Published July 10,2014. Accessed September 4, 2014.
5. Artiga S, Stephens J, Rudowitz R, Perry M. What Worked and What’s Next? Strategies in Four States Leading ACA Enrollment Efforts. Kaiser Family Foundation. http://kff.org/health-reform/issue-brief/what-worked-and-whats-next-strategies-in-four-states-leading-aca-enrollment-efforts/. Published July 16, 2014. Accessed September 4, 2014.
6. Corlette S, Volk J. Narrow Provider Networks in New Health Plans: Balancing Affordability with Access to Quality Care. Urban Institute: Georgetown University Center on Health Insurance Reforms. http://www.urban.org/UploadedPDF/413135-New-Provider-Networks-in-New-Health-Plans.pdf. Published May 2014. Accessed September 4, 2014.
7. Feibel C. Patients With Low-Cost Insurance Struggle to Find Specialists. National Public Radio. http://www.npr.org/blogs/health/2014/07/16/331419293/patients-with-low-cost-insurance-struggle-to-find-specialists. Published July 16, 2014. Accessed September 4, 2014.
8. Patient Protection and Affordable Care Act: Preexisting Condition Exclusions, Lifetime and Annual Limits, Rescissions, and Patient Protections. US Department of Health and Human Services. http://www.regulations.go/#!documentDetail;D=HHS-OS-2010-0014-0001. Published June 28, 2010. Accessed September 9, 2014.
Inside the article:
Are contraceptives abortifacients?
How the Hobby Lobby decision affects individual states
Narrow networks limit access to care
Metastatic Brain Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Managing snoring: When to consider surgery
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
KEY POINTS
- The treatment of snoring begins with a thorough history and physical examination.
- Polysomnography is almost always necessary to rule out other sleep disorders, such as obstructive sleep apnea. This is particularly important if an elective surgical intervention is planned.
- Surgical procedures for snoring include septoplasty with or without radiofrequency ablation of the upper airway, injection snoreplasty, Pillar implants, and laser-assisted uvulopalatoplasty.
- Although studies indicate that these procedures are effective, no well-controlled study has compared one procedure against another. The choice of procedure is often determined by the expertise of the surgeon, and the outcome is highly dependent on the skill of the surgeon.
Diabetes therapy and cancer risk: Where do we stand when treating patients?
In the last quarter century, many new drugs have become available for treating type 2 diabetes mellitus. The American Association of Clinical Endocrinologists incorporated these new agents in its updated glycemic control algorithm in 2013.1 Because diabetes affects 25.8 million Americans and can lead to blindness, renal failure, cardiovascular disease, and amputation, agents that help us treat it more effectively are valuable.2
One of the barriers to effective treatment is the side effects of the agents. Because some of these drugs have been in use for only a short time, concerns of potential adverse effects have arisen. Cancer is one such concern, especially since type 2 diabetes mellitus by itself increases the risk of cancer by 20% to 50% compared with no diabetes.3
Type 2 diabetes has been linked to risk of cancers of the pancreas,4 colorectum,5,6 liver,7 kidney,8,9 breast,10 bladder,11 and endometri-um,12 as well as to hematologic malignancies such as non-Hodgkin lymphoma.13 The risk of bladder cancer appears to depend on how long the patient has had type 2 diabetes. Newton et al,14 in a prospective cohort study, found that those who had diabetes for more than 15 years and used insulin had the highest risk of bladder cancer. On the other hand, the risk of prostate cancer is actually lower in people with diabetes,15 particularly in those who have had diabetes for longer than 4 years.16
Cancer and type 2 diabetes share many risk factors and underlying pathophysiologic mechanisms. Nonmodifiable risk factors for both diseases include advanced age, male sex, ethnicity (African American men appear to be most vulnerable to both cancer and diabetes),17,18 and family history. Modifiable risk factors include lower socioeconomic status, obesity, and alcohol consumption. These common risk factors lead to hyperinsulinemia and insulin resistance, changes in mitochondrial function, low-grade inflammation, and oxidative stress,3 which promote both diabetes and cancer. Diabetes therapy may influence several of these processes.
Several classes of diabetes drugs, including exogenous insulin,19–22 insulin secretagogues,23–25 and incretin-based therapies,26–28 have been under scrutiny because of their potential influences on cancer development in a population already at risk (Table 1).
INSULIN ANALOGUES: MIXED EVIDENCE
Insulin promotes cell division by binding to insulin receptor isoform A and insulin-like growth factor 1 receptors.29 Because endogenous hyperinsulinemia has been linked to cancer risk, growth, and proliferation, some speculate that exogenous insulin may also increase cancer risk.
In 2009, a retrospective study by Hemkens et al linked the long-acting insulin analogue glargine to risk of cancer.19 This finding set off a tumult of controversy within the medical community and concern among patients. Several limitations of the study were brought to light, including a short duration of follow-up, and several other studies have refuted the study’s findings.20,21
More recently, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial22 found no higher cancer risk with glargine use than with placebo. This study enrolled 12,537 participants from 573 sites in 40 countries. Specifically, risks with glargine use were as follows:
- Any cancer—hazard ratio 1.00, 95% confidence interval (CI) 0.88–1.13, P = .97
- Cancer death—hazard ratio 0.94, 95% CI 0.77–1.15, P = .52.
However, the study was designed to assess cardiovascular outcomes, not cancer risk. Furthermore, the participants were not typical of patients seen in clinical practice: their insulin doses were lower (the median insulin dose was 0.4 units/kg/day by year 6, whereas in clinical practice, those with type 2 diabetes mellitus often use more than 1 unit/kg/day, depending on duration of diabetes, diet, and exercise regimen), and their baseline median hemoglobin A1c level was only 6.4%. And one may argue that the median follow-up of 6.2 years was too short for cancer to develop.22
In vitro studies indicate that long-acting analogue insulin therapy may promote cancer cell growth more than endogenous insulin,30 but epidemiologic data have not unequivocally substantiated this.20–22 There is no clear evidence that analogue insulin therapy raises cancer risk above that of human recombinant insulin, and starting insulin therapy should not be delayed because of concerns about cancer risk, particularly in uncontrolled diabetes.
INSULIN SECRETAGOGUES
Sulfonylureas: Higher risk
Before 1995, only two classes of diabetes drugs were approved by the US Food and Drug Administration (FDA)—insulin and sulfonylureas.
Sulfonylureas lower blood sugar levels by binding to sulfonylurea receptors and inhibiting adenosine triphosphate-dependent potassium channels. The resulting change in resting potential causes an influx of calcium, ultimately leading to insulin secretion.
Sulfonylureas are effective, and because of their low cost, physicians often pick them as a second-line agent after metformin.
The main disadvantage of sulfonylureas is the risk of hypoglycemia, particularly in patients with renal failure, the elderly, and diabetic patients who are unaware of when they are hypoglycemic. Other potential drawbacks are that they impair cardiac ischemic preconditioning31 and possibly increase cancer risk.21,32 (Ischemic preconditioning is the process in which transient episodes of ischemia “condition” the myocardium so that it better withstands future episodes with minimal anginal pain and tissue injury.33) Of the sulfonylureas, glyburide has been most implicated in cardiovascular risk.32
In a retrospective cohort study of 62,809 patients from a general-practice database in the United Kingdom, Currie et al21 found that sulfonylurea monotherapy was associated with a 36% higher risk of cancer (95% CI 1.19–1.54, P < .001) than metformin monotherapy. Prescribing bias may have influenced the results: practitioners are more likely to prescribe sulfonylureas to leaner patients, who have a greater likelihood of occult cancer. However, other studies also found that the cancer death rate is higher in those who take a sulfonylurea alone than in those who use metformin alone.23,24
Some evidence indicates that long-acting sulfonylurea formulations (eg, glyburide) likely hold the most danger, certainly in regard to hypoglycemia, but it is less clear if this translates to cancer concerns.31
Meglitinides: Limited evidence
Meglitinides, the other class of insulin secretagogues, are less commonly used but are similar to sulfonylureas in the way they increase endogenous insulin levels. The data are limited regarding cancer risk and meglitinide therapy, but the magnitude of the association is similar to that with sulfonylurea therapy.25
INSULIN SENSITIZERS
There are currently two classes of insulin sensitizers: biguanides and thiazolidinediones (TZDs, also known as glitazones). These drugs show less risk of both cancer incidence and cancer death than insulin secretagogues such as sulfonylureas.21,23,24 In fact, they may decrease cancer potential by alteration of signaling via the AKT/mTOR (v-akt murine thymoma viral oncogene homolog 1/mammalian target of rapamycin) pathway.34
Metformin, a biguanide, is the oral drug of choice
Metformin is the only biguanide currently available in the United States. It was approved by the FDA in 1995, although it had been in clinical use since the 1950s. Inexpensive and familiar, it is the oral antihyperglycemic of choice if there are no contraindications to it, such as renal dysfunction (creatinine ≥ 1.4 mg/dL in women and ≥ 1.5 mg/dL in men), acute decompensated heart failure, or pulmonary or hepatic insufficiency, all of which may lead to an increased risk of lactic acidosis.1
Metformin lowers blood sugar levels primarily by inhibiting hepatic glucose production (gluconeogenesis) and by improving peripheral insulin sensitivity. It directly activates AMP-activated protein kinase (AMPK), which affects insulin signaling and glucose and fat metabolism.35 It may exert further beneficial effects by acutely increasing glucagon-like peptide-1 (GLP-1) levels and inducing islet incretin-receptor gene expression.36 Although the exact mechanisms have not been fully elucidated, metformin’s insulin-sensitizing properties are likely from favorable effects on insulin receptor expression, tyrosine kinase activity, and influences on the incretin pathway.36,37 These effects also mitigate carcinogenesis, both directly (via AMPK and liver kinase B1, a tumor-suppressor gene) and indirectly (via reduction of hyperinsulinemia).35
Overall, biguanide therapy is associated with a lower cancer incidence or, at worst, no effect on cancer incidence. In vitro studies demonstrate that metformin both suppresses cancer cell growth and induces apoptosis, resulting in fewer live cancer cells.34 Several retrospective studies found lower cancer risk in metformin users than in patients receiving antidiabetes drugs other than insulin-sensitizing agents,21,23,25,38–40 while others have shown no effect.41 Use of metformin was specifically associated with lower risk of cancers of the liver, colon and rectum, and lung.42 Further, metformin users have a lower cancer mortality rate than nonusers.24,43
Thiazolidinediones
TZDs, such as pioglitazone, work by binding to peroxisome proliferator-activated gamma receptors in the cell nucleus, altering gene transcription.44 They reduce insulin resistance and levels of endogenous insulin levels and free fatty acids.44
Concern over bladder cancer risk with TZD use, particularly with pioglitazone, has increased in the last few years, as various cohort studies found a statistically significant increased risk with this agent.44 The risk appears to rise with cumulative dose.45,46
Randomized controlled trials also found an increased risk of bladder cancer with TZD therapy, although the difference was not statistically significant.47–49 In a mean follow-up of 8.7 years, the Prospective Pioglitazone Clinical Trial in Macrovascular Events reported 23 cases of bladder cancer in the pioglitazone group vs 22 cases in the placebo group, for rates of 0.9% vs 0.8% (relative risk [RR] 1.06, 95% CI 0.59–1.89).49
On the other hand, the risk of cancer of the breast, colon, and lung has been found to be lower with TZD use.47 In vitro studies support the clinical data, showing that TZDs inhibit growth of human cancer cells derived from cancers of the lung, colon, breast, stomach, ovary, and prostate.50–53
Home et al54 compared rosiglitazone against a sulfonylurea in patients already taking metformin in the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial. Malignancies developed in 6.7% of the sulfonylurea group compared with 5.1% of the rosiglitazone group, for a hazard ratio of 1.33 (95% CI 0.94–1.88).
Both ADOPT (A Diabetes Outcome Progression Trial) and the RECORD trial found rosiglitazone comparable to metformin in terms of cancer risk.54
Colmers et al47 pooled data from four randomized controlled trials, seven cohort studies, and nine case-control studies to assess the risk of cancer with TZD use in type 2 diabetes. Both the randomized and observational data showed neutral overall cancer risk with TZDs. However, pooled data from observational studies showed significantly lower risk with TZD use in terms of:
- Colorectal cancer RR 0.93, 95% CI 0.87–1.00
- Lung cancer RR 0.91, 95% CI 0.84–0.98
- Breast cancer RR 0.89, 95% CI 0.81–0.98.
INCRETIN-BASED THERAPIES
Incretins are hormones released from the gut in response to food ingestion, triggering release of insulin before blood glucose levels rise. Their action explains why insulin secretion increases more after an oral glucose load than after an intravenous glucose load, a phenomenon called the incretin effect.55
There are two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). They have short a half-life because they are rapidly degraded by dipeptidyl peptidase-IV (DPP-IV).55 Available incretin-based therapies are GLP-1 receptor agonists and DPP-IV inhibitors.
When used as monotherapy, incretin-based therapies do not cause hypoglycemia because their effect is glucose-dependent.55 GLP-1 receptor antagonists have the added benefit of inducing weight loss, but DPP-IV inhibitors are considered to be weight-neutral.
GLP-1 receptor agonists
Exenatide, the first of the GLP-1 receptor agonists, was approved in 2005. The original formulation (Byetta) is taken by injection twice daily, and timing in conjunction with food intake is important: it should be taken within 60 minutes before the morning and evening meals. Extended-release exenatide (Bydureon) is a once-weekly formulation taken without regard to timing of food intake. Exenatide (either twice-daily Byetta or once-weekly Bydureon) should not be used in those with creatinine clearance less than 30 mL/min or end-stage renal disease and should be used with caution in patients with renal transplantation.
Liraglutide (Victoza), a once-daily formulation, can be injected irrespective of food intake. The dose does not have to be adjusted for renal function, although it should be used with caution in those with renal impairment, including end-stage renal disease. Approval for a 3-mg formulation is pending with the FDA as a weight-loss drug on the basis of promising results in a randomized phase 3 trial.56
Albiglutide (Tanzeum), a once-weekly GLP-1 receptor antagonist, was recently approved by the FDA.
DPP-IV inhibitors
Whereas GLP-1 receptor agonists are injected, the DPP-IV inhibitors have the advantage of being oral agents.
Sitagliptin (Januvia), the first DPP-IV inhibitor, became available in the United States in 2006. Since then, three more have become available: saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).
Concerns about thyroid cancer with incretin drugs
Concerns of increased risk of cancer, particularly of the thyroid and pancreas, have been raised since GLP-1 receptor agonists and DPP-IV inhibitors became available.
Studies in rodents have shown C-cell hyperplasia, sometimes resulting in increased incidence of thyroid carcinoma, and dose-dependent rises in serum calcitonin, particularly with liraglutide.26 This has raised concern about an increased risk of medullary thyroid carcinoma in humans. However, the density of C cells in rodents is up to 45 times greater than in humans, and C cells also express functional GLP-1 receptors.26
Gier et al27 assessed the expression of calcitonin and human GLP-1 receptors in normal C cells, C cell hyperplasia, and medullary cancer. In this study, calcitonin and GLP-1 receptor were co-expressed in medullary thyroid cancer (10 of 12 cases) and C-cell hyperplasia (9 of 9 cases) more commonly than in normal C cells (5 of 15 cases). Further, GLP-1 receptor was expressed in 3 of 17 cases of papillary thyroid cancer.
Calcitonin, a polypeptide hormone produced by thyroid C cells and used as a medullary thyroid cancer biomarker, was increased in a slightly higher percentage of patients treated with liraglutide than in controls, without an increase above the normal range.57
A meta-analysis by Alves et al58 of 25 studies found that neither exenatide (no cases reported) nor liraglutide (odds ratio 1.54, 95% CI 0.40–6.02) was associated with increased thyroid cancer risk.
MacConell et al59 pooled the results of 19 placebo-controlled trials of twice-daily exenatide and found a thyroid cancer incidence rate of 0.3 per 100 patient-years (< 0.1%) vs 0 per 100 patient-years in pooled comparators.
Concerns about pancreatic cancer with incretin drugs
Increased risk of acute pancreatitis is a potential side effect of both DPP-IV inhibitors and GLP-1 receptor agonists and has led to speculation that this translates to an increased risk of pancreatic cancer.
In a point-counterpoint debate, Butler et al28 argued that incretin-based medications have questionable safety, with increased rates of pancreatitis possibly leading to pancreatic cancer. In counterpoint, Nauck60 argued that the risk of pancreatitis or cancer is extremely low, and clinical cases are unsubstantiated.
Bailey61 outlined the complexities and difficulties in drawing firm conclusions from individual clinical trials regarding possible adverse effects of diabetes drugs. The trials are typically designed to assess hemoglobin A1c reduction at varying doses and are typically restricted in patient selection, patient numbers, and drug-exposure duration, which may introduce allocation and ascertainment biases. The attempt to draw firm conclusions from such trials can be problematic and can lead to increased alarm, warranted or not.
Type 2 diabetes mellitus itself is associated with an increased incidence of pancreatic cancer, and whether incretin therapy enhances this risk is still controversial. Whether more episodes of acute pancreatitis without chronic pancreatitis can be extrapolated to an increased incidence of pancreatic cancer is doubtful. A normal pancreatic duct cell may take up to 12 years to become a tumor cell from which pancreatic carcinoma develops, another 7 years to develop metastatic capacity, and another 3 years before a diagnosis is made from clinical symptoms (which are usually accompanied by metastases).62
The risks and benefits of incretin therapies remain a contentious issue, and there are no clear prospective data at this time on increased pancreatic cancer incidence. Long-term prospective studies designed to analyze these specific outcomes (pancreatitis, pancreatic cancer, and medullary thyroid cancer) need to be undertaken.63
OTHER DIABETES THERAPIES
Alpha glucosidase inhibitors
Oral glucosidase inhibitors ameliorate hyperglycemia by inhibiting alpha glucosidase enzymes in the brush border of the small intestines, preventing conversion of polysaccharides to monosaccharides.64 This slows digestion of carbohydrates and glucose release into the bloodstream and blunts the postprandial hyperglycemic excursion.
The two alpha glucosidase inhibitors currently available in the United States are acarbose and miglitol, and although data are limited, they do not appear to increase the risk of cancer.65,66
Sodium-glucose-linked cotransporter 2 inhibitors
The newest class of oral diabetes agents to be approved are the sodium-glucose-linked cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga).
SGLT2 is a protein in the S1 segment of the proximal renal tubules responsible for over 90% of renal glucose reabsorption. SGLT2 inhibitors lower serum glucose levels by promoting glycosuria and have also been shown to have favorable effects on blood pressure and weight.67,68
Canagliflozin was the first of its class to gain FDA approval in the United States. It has not been found to be associated with increased cancer risk.68
Dapagliflozin, originally approved in Europe, was approved in the United States on January 8, 2014. Because of a possible increased incidence of breast and bladder malignancies, the FDA advisory committee initially recommended against approval and required further data. In those who were treated, nine cases of bladder cancer and nine cases of breast cancer were reported, compared with one case of bladder cancer and no cases of breast cancer in the control group; however, the difference was not statistically significant.68
Since SGLT2 inhibitors are still new, data on long-term outcomes are lacking. Early clinical data do not show a significant increase in cancer risk.
WHAT THIS MEANS IN PRACTICE
Many studies have found associations between diabetes, obesity, hyperinsulinemia, and cancer risk. In the last decade, concerns implicating antihyperglycemic agents in cancer development have arisen but have not been well substantiated. At this time, there are no definitive prospective data indicating that the currently available type 2 diabetes therapies increase the incidence of cancer beyond the inherent increased risk in this population. What, then, is one to do?
Educate. Lifestyle modification, including weight management, should continue to be emphasized in diabetes education, as no therapy is completely effective without adjunct modifications in diet and physical activity. Epidemiologic studies have shown the benefits of lifestyle modifications, which ameliorate many of the adverse metabolic conditions that coexist in type 2 diabetes and cancer.
Screen for cancer. Given the associations between diabetes and malignancy, cancer screening is especially important in this high-risk population.
Customize therapy to individual patients. Those with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid sitagliptin until definitive clinical data become available.
Moreover, patients with a personal or family history of medullary thyroid cancer should not receive GLP-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma. Until we have further elucidating data, it is not possible to say whether a family history of any of the other types of cancer should represent a contraindication to the use of any of these agents.
Discuss. The multitude of diabetes therapies warrants physician-patient discussions that carefully weigh the risks and benefits of additional agents to optimize glycemic control and metabolic factors in individual patients.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Centers for Disease Control and Prevention (CDC). Diabetes data and trends. www.cdc.gov/diabetes/statistics/. Accessed April 8, 2014.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687.
- Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006; 101:1872–1879.
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380.
- Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112.
- Washio M, Mori M, Khan M, et al; JACC Study Group. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 2007; 14:393–397.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823.
- Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007; 50:1365–1374.
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008; 31:2391–2397.
- Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 2013; 132:2186–2191.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:2056–2062.
- Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005; 161:147–152.
- Centers for Disease Control and Prevention. Diabetes public health resource. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pubs/estimates14.htm. Accessed August 12, 2014.
- Centers for Disease Control and Prevention. Cancer prevention and control cancer rates by race and ethnicity. www.cdc.gov/cancer/dcpc/data/race.htm. Accessed August 12, 2014.
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; 52:1732–1744.
- Colhoun HMSDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; 52:1755–1765.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Baur DM, Klotsche J, Hamnvik OP, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011; 60:1363–1371.
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29:254–258.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137:482–488.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013; 36:2118–2125.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011; 18:R125–R147.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab 2003; 88:528–530.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012; 107:620–626.
- Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrmann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990; 82:2044–2051.
- Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med 2011; 15:825–836.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122:253–270.
- Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011; 54:339–349.
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323–1332.
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011; 123:200–204.
- Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011; 20:337–344.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7:e33411.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
- Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344:e3645.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2012; 38:475–484.
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32:187–202.
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A, Perez A. Pioglitazone and bladder malignancy during observational follow-up of PROactive: 6-year update. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA.
- Akinyeke TO, Stewart LV. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol Ther 2011; 11:1046–1058.
- Ban JO, Oh JH, Son SM, et al. Troglitazone, a PPAR agonist, inhibits human prostate cancer cell growth through inactivation of NFKB via suppression of GSK-3B expression. Cancer Biol Ther 2011; 12:288–296.
- Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog 2010; 49:235–246.
- Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2010; 10:390.
- Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti GADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845.
- Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 41:299–307.
- Wadden TA, Hollander P, Klein S, et al; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37:1443–1451.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5,594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5:29–41.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care 2013; 36:2098–2106.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467:1114–1117.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 1995; 18:303–311.
- Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009; 46:279–284.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011; 54:2009–2015.
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022.
- Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5:313–527.
In the last quarter century, many new drugs have become available for treating type 2 diabetes mellitus. The American Association of Clinical Endocrinologists incorporated these new agents in its updated glycemic control algorithm in 2013.1 Because diabetes affects 25.8 million Americans and can lead to blindness, renal failure, cardiovascular disease, and amputation, agents that help us treat it more effectively are valuable.2
One of the barriers to effective treatment is the side effects of the agents. Because some of these drugs have been in use for only a short time, concerns of potential adverse effects have arisen. Cancer is one such concern, especially since type 2 diabetes mellitus by itself increases the risk of cancer by 20% to 50% compared with no diabetes.3
Type 2 diabetes has been linked to risk of cancers of the pancreas,4 colorectum,5,6 liver,7 kidney,8,9 breast,10 bladder,11 and endometri-um,12 as well as to hematologic malignancies such as non-Hodgkin lymphoma.13 The risk of bladder cancer appears to depend on how long the patient has had type 2 diabetes. Newton et al,14 in a prospective cohort study, found that those who had diabetes for more than 15 years and used insulin had the highest risk of bladder cancer. On the other hand, the risk of prostate cancer is actually lower in people with diabetes,15 particularly in those who have had diabetes for longer than 4 years.16
Cancer and type 2 diabetes share many risk factors and underlying pathophysiologic mechanisms. Nonmodifiable risk factors for both diseases include advanced age, male sex, ethnicity (African American men appear to be most vulnerable to both cancer and diabetes),17,18 and family history. Modifiable risk factors include lower socioeconomic status, obesity, and alcohol consumption. These common risk factors lead to hyperinsulinemia and insulin resistance, changes in mitochondrial function, low-grade inflammation, and oxidative stress,3 which promote both diabetes and cancer. Diabetes therapy may influence several of these processes.
Several classes of diabetes drugs, including exogenous insulin,19–22 insulin secretagogues,23–25 and incretin-based therapies,26–28 have been under scrutiny because of their potential influences on cancer development in a population already at risk (Table 1).
INSULIN ANALOGUES: MIXED EVIDENCE
Insulin promotes cell division by binding to insulin receptor isoform A and insulin-like growth factor 1 receptors.29 Because endogenous hyperinsulinemia has been linked to cancer risk, growth, and proliferation, some speculate that exogenous insulin may also increase cancer risk.
In 2009, a retrospective study by Hemkens et al linked the long-acting insulin analogue glargine to risk of cancer.19 This finding set off a tumult of controversy within the medical community and concern among patients. Several limitations of the study were brought to light, including a short duration of follow-up, and several other studies have refuted the study’s findings.20,21
More recently, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial22 found no higher cancer risk with glargine use than with placebo. This study enrolled 12,537 participants from 573 sites in 40 countries. Specifically, risks with glargine use were as follows:
- Any cancer—hazard ratio 1.00, 95% confidence interval (CI) 0.88–1.13, P = .97
- Cancer death—hazard ratio 0.94, 95% CI 0.77–1.15, P = .52.
However, the study was designed to assess cardiovascular outcomes, not cancer risk. Furthermore, the participants were not typical of patients seen in clinical practice: their insulin doses were lower (the median insulin dose was 0.4 units/kg/day by year 6, whereas in clinical practice, those with type 2 diabetes mellitus often use more than 1 unit/kg/day, depending on duration of diabetes, diet, and exercise regimen), and their baseline median hemoglobin A1c level was only 6.4%. And one may argue that the median follow-up of 6.2 years was too short for cancer to develop.22
In vitro studies indicate that long-acting analogue insulin therapy may promote cancer cell growth more than endogenous insulin,30 but epidemiologic data have not unequivocally substantiated this.20–22 There is no clear evidence that analogue insulin therapy raises cancer risk above that of human recombinant insulin, and starting insulin therapy should not be delayed because of concerns about cancer risk, particularly in uncontrolled diabetes.
INSULIN SECRETAGOGUES
Sulfonylureas: Higher risk
Before 1995, only two classes of diabetes drugs were approved by the US Food and Drug Administration (FDA)—insulin and sulfonylureas.
Sulfonylureas lower blood sugar levels by binding to sulfonylurea receptors and inhibiting adenosine triphosphate-dependent potassium channels. The resulting change in resting potential causes an influx of calcium, ultimately leading to insulin secretion.
Sulfonylureas are effective, and because of their low cost, physicians often pick them as a second-line agent after metformin.
The main disadvantage of sulfonylureas is the risk of hypoglycemia, particularly in patients with renal failure, the elderly, and diabetic patients who are unaware of when they are hypoglycemic. Other potential drawbacks are that they impair cardiac ischemic preconditioning31 and possibly increase cancer risk.21,32 (Ischemic preconditioning is the process in which transient episodes of ischemia “condition” the myocardium so that it better withstands future episodes with minimal anginal pain and tissue injury.33) Of the sulfonylureas, glyburide has been most implicated in cardiovascular risk.32
In a retrospective cohort study of 62,809 patients from a general-practice database in the United Kingdom, Currie et al21 found that sulfonylurea monotherapy was associated with a 36% higher risk of cancer (95% CI 1.19–1.54, P < .001) than metformin monotherapy. Prescribing bias may have influenced the results: practitioners are more likely to prescribe sulfonylureas to leaner patients, who have a greater likelihood of occult cancer. However, other studies also found that the cancer death rate is higher in those who take a sulfonylurea alone than in those who use metformin alone.23,24
Some evidence indicates that long-acting sulfonylurea formulations (eg, glyburide) likely hold the most danger, certainly in regard to hypoglycemia, but it is less clear if this translates to cancer concerns.31
Meglitinides: Limited evidence
Meglitinides, the other class of insulin secretagogues, are less commonly used but are similar to sulfonylureas in the way they increase endogenous insulin levels. The data are limited regarding cancer risk and meglitinide therapy, but the magnitude of the association is similar to that with sulfonylurea therapy.25
INSULIN SENSITIZERS
There are currently two classes of insulin sensitizers: biguanides and thiazolidinediones (TZDs, also known as glitazones). These drugs show less risk of both cancer incidence and cancer death than insulin secretagogues such as sulfonylureas.21,23,24 In fact, they may decrease cancer potential by alteration of signaling via the AKT/mTOR (v-akt murine thymoma viral oncogene homolog 1/mammalian target of rapamycin) pathway.34
Metformin, a biguanide, is the oral drug of choice
Metformin is the only biguanide currently available in the United States. It was approved by the FDA in 1995, although it had been in clinical use since the 1950s. Inexpensive and familiar, it is the oral antihyperglycemic of choice if there are no contraindications to it, such as renal dysfunction (creatinine ≥ 1.4 mg/dL in women and ≥ 1.5 mg/dL in men), acute decompensated heart failure, or pulmonary or hepatic insufficiency, all of which may lead to an increased risk of lactic acidosis.1
Metformin lowers blood sugar levels primarily by inhibiting hepatic glucose production (gluconeogenesis) and by improving peripheral insulin sensitivity. It directly activates AMP-activated protein kinase (AMPK), which affects insulin signaling and glucose and fat metabolism.35 It may exert further beneficial effects by acutely increasing glucagon-like peptide-1 (GLP-1) levels and inducing islet incretin-receptor gene expression.36 Although the exact mechanisms have not been fully elucidated, metformin’s insulin-sensitizing properties are likely from favorable effects on insulin receptor expression, tyrosine kinase activity, and influences on the incretin pathway.36,37 These effects also mitigate carcinogenesis, both directly (via AMPK and liver kinase B1, a tumor-suppressor gene) and indirectly (via reduction of hyperinsulinemia).35
Overall, biguanide therapy is associated with a lower cancer incidence or, at worst, no effect on cancer incidence. In vitro studies demonstrate that metformin both suppresses cancer cell growth and induces apoptosis, resulting in fewer live cancer cells.34 Several retrospective studies found lower cancer risk in metformin users than in patients receiving antidiabetes drugs other than insulin-sensitizing agents,21,23,25,38–40 while others have shown no effect.41 Use of metformin was specifically associated with lower risk of cancers of the liver, colon and rectum, and lung.42 Further, metformin users have a lower cancer mortality rate than nonusers.24,43
Thiazolidinediones
TZDs, such as pioglitazone, work by binding to peroxisome proliferator-activated gamma receptors in the cell nucleus, altering gene transcription.44 They reduce insulin resistance and levels of endogenous insulin levels and free fatty acids.44
Concern over bladder cancer risk with TZD use, particularly with pioglitazone, has increased in the last few years, as various cohort studies found a statistically significant increased risk with this agent.44 The risk appears to rise with cumulative dose.45,46
Randomized controlled trials also found an increased risk of bladder cancer with TZD therapy, although the difference was not statistically significant.47–49 In a mean follow-up of 8.7 years, the Prospective Pioglitazone Clinical Trial in Macrovascular Events reported 23 cases of bladder cancer in the pioglitazone group vs 22 cases in the placebo group, for rates of 0.9% vs 0.8% (relative risk [RR] 1.06, 95% CI 0.59–1.89).49
On the other hand, the risk of cancer of the breast, colon, and lung has been found to be lower with TZD use.47 In vitro studies support the clinical data, showing that TZDs inhibit growth of human cancer cells derived from cancers of the lung, colon, breast, stomach, ovary, and prostate.50–53
Home et al54 compared rosiglitazone against a sulfonylurea in patients already taking metformin in the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial. Malignancies developed in 6.7% of the sulfonylurea group compared with 5.1% of the rosiglitazone group, for a hazard ratio of 1.33 (95% CI 0.94–1.88).
Both ADOPT (A Diabetes Outcome Progression Trial) and the RECORD trial found rosiglitazone comparable to metformin in terms of cancer risk.54
Colmers et al47 pooled data from four randomized controlled trials, seven cohort studies, and nine case-control studies to assess the risk of cancer with TZD use in type 2 diabetes. Both the randomized and observational data showed neutral overall cancer risk with TZDs. However, pooled data from observational studies showed significantly lower risk with TZD use in terms of:
- Colorectal cancer RR 0.93, 95% CI 0.87–1.00
- Lung cancer RR 0.91, 95% CI 0.84–0.98
- Breast cancer RR 0.89, 95% CI 0.81–0.98.
INCRETIN-BASED THERAPIES
Incretins are hormones released from the gut in response to food ingestion, triggering release of insulin before blood glucose levels rise. Their action explains why insulin secretion increases more after an oral glucose load than after an intravenous glucose load, a phenomenon called the incretin effect.55
There are two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). They have short a half-life because they are rapidly degraded by dipeptidyl peptidase-IV (DPP-IV).55 Available incretin-based therapies are GLP-1 receptor agonists and DPP-IV inhibitors.
When used as monotherapy, incretin-based therapies do not cause hypoglycemia because their effect is glucose-dependent.55 GLP-1 receptor antagonists have the added benefit of inducing weight loss, but DPP-IV inhibitors are considered to be weight-neutral.
GLP-1 receptor agonists
Exenatide, the first of the GLP-1 receptor agonists, was approved in 2005. The original formulation (Byetta) is taken by injection twice daily, and timing in conjunction with food intake is important: it should be taken within 60 minutes before the morning and evening meals. Extended-release exenatide (Bydureon) is a once-weekly formulation taken without regard to timing of food intake. Exenatide (either twice-daily Byetta or once-weekly Bydureon) should not be used in those with creatinine clearance less than 30 mL/min or end-stage renal disease and should be used with caution in patients with renal transplantation.
Liraglutide (Victoza), a once-daily formulation, can be injected irrespective of food intake. The dose does not have to be adjusted for renal function, although it should be used with caution in those with renal impairment, including end-stage renal disease. Approval for a 3-mg formulation is pending with the FDA as a weight-loss drug on the basis of promising results in a randomized phase 3 trial.56
Albiglutide (Tanzeum), a once-weekly GLP-1 receptor antagonist, was recently approved by the FDA.
DPP-IV inhibitors
Whereas GLP-1 receptor agonists are injected, the DPP-IV inhibitors have the advantage of being oral agents.
Sitagliptin (Januvia), the first DPP-IV inhibitor, became available in the United States in 2006. Since then, three more have become available: saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).
Concerns about thyroid cancer with incretin drugs
Concerns of increased risk of cancer, particularly of the thyroid and pancreas, have been raised since GLP-1 receptor agonists and DPP-IV inhibitors became available.
Studies in rodents have shown C-cell hyperplasia, sometimes resulting in increased incidence of thyroid carcinoma, and dose-dependent rises in serum calcitonin, particularly with liraglutide.26 This has raised concern about an increased risk of medullary thyroid carcinoma in humans. However, the density of C cells in rodents is up to 45 times greater than in humans, and C cells also express functional GLP-1 receptors.26
Gier et al27 assessed the expression of calcitonin and human GLP-1 receptors in normal C cells, C cell hyperplasia, and medullary cancer. In this study, calcitonin and GLP-1 receptor were co-expressed in medullary thyroid cancer (10 of 12 cases) and C-cell hyperplasia (9 of 9 cases) more commonly than in normal C cells (5 of 15 cases). Further, GLP-1 receptor was expressed in 3 of 17 cases of papillary thyroid cancer.
Calcitonin, a polypeptide hormone produced by thyroid C cells and used as a medullary thyroid cancer biomarker, was increased in a slightly higher percentage of patients treated with liraglutide than in controls, without an increase above the normal range.57
A meta-analysis by Alves et al58 of 25 studies found that neither exenatide (no cases reported) nor liraglutide (odds ratio 1.54, 95% CI 0.40–6.02) was associated with increased thyroid cancer risk.
MacConell et al59 pooled the results of 19 placebo-controlled trials of twice-daily exenatide and found a thyroid cancer incidence rate of 0.3 per 100 patient-years (< 0.1%) vs 0 per 100 patient-years in pooled comparators.
Concerns about pancreatic cancer with incretin drugs
Increased risk of acute pancreatitis is a potential side effect of both DPP-IV inhibitors and GLP-1 receptor agonists and has led to speculation that this translates to an increased risk of pancreatic cancer.
In a point-counterpoint debate, Butler et al28 argued that incretin-based medications have questionable safety, with increased rates of pancreatitis possibly leading to pancreatic cancer. In counterpoint, Nauck60 argued that the risk of pancreatitis or cancer is extremely low, and clinical cases are unsubstantiated.
Bailey61 outlined the complexities and difficulties in drawing firm conclusions from individual clinical trials regarding possible adverse effects of diabetes drugs. The trials are typically designed to assess hemoglobin A1c reduction at varying doses and are typically restricted in patient selection, patient numbers, and drug-exposure duration, which may introduce allocation and ascertainment biases. The attempt to draw firm conclusions from such trials can be problematic and can lead to increased alarm, warranted or not.
Type 2 diabetes mellitus itself is associated with an increased incidence of pancreatic cancer, and whether incretin therapy enhances this risk is still controversial. Whether more episodes of acute pancreatitis without chronic pancreatitis can be extrapolated to an increased incidence of pancreatic cancer is doubtful. A normal pancreatic duct cell may take up to 12 years to become a tumor cell from which pancreatic carcinoma develops, another 7 years to develop metastatic capacity, and another 3 years before a diagnosis is made from clinical symptoms (which are usually accompanied by metastases).62
The risks and benefits of incretin therapies remain a contentious issue, and there are no clear prospective data at this time on increased pancreatic cancer incidence. Long-term prospective studies designed to analyze these specific outcomes (pancreatitis, pancreatic cancer, and medullary thyroid cancer) need to be undertaken.63
OTHER DIABETES THERAPIES
Alpha glucosidase inhibitors
Oral glucosidase inhibitors ameliorate hyperglycemia by inhibiting alpha glucosidase enzymes in the brush border of the small intestines, preventing conversion of polysaccharides to monosaccharides.64 This slows digestion of carbohydrates and glucose release into the bloodstream and blunts the postprandial hyperglycemic excursion.
The two alpha glucosidase inhibitors currently available in the United States are acarbose and miglitol, and although data are limited, they do not appear to increase the risk of cancer.65,66
Sodium-glucose-linked cotransporter 2 inhibitors
The newest class of oral diabetes agents to be approved are the sodium-glucose-linked cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga).
SGLT2 is a protein in the S1 segment of the proximal renal tubules responsible for over 90% of renal glucose reabsorption. SGLT2 inhibitors lower serum glucose levels by promoting glycosuria and have also been shown to have favorable effects on blood pressure and weight.67,68
Canagliflozin was the first of its class to gain FDA approval in the United States. It has not been found to be associated with increased cancer risk.68
Dapagliflozin, originally approved in Europe, was approved in the United States on January 8, 2014. Because of a possible increased incidence of breast and bladder malignancies, the FDA advisory committee initially recommended against approval and required further data. In those who were treated, nine cases of bladder cancer and nine cases of breast cancer were reported, compared with one case of bladder cancer and no cases of breast cancer in the control group; however, the difference was not statistically significant.68
Since SGLT2 inhibitors are still new, data on long-term outcomes are lacking. Early clinical data do not show a significant increase in cancer risk.
WHAT THIS MEANS IN PRACTICE
Many studies have found associations between diabetes, obesity, hyperinsulinemia, and cancer risk. In the last decade, concerns implicating antihyperglycemic agents in cancer development have arisen but have not been well substantiated. At this time, there are no definitive prospective data indicating that the currently available type 2 diabetes therapies increase the incidence of cancer beyond the inherent increased risk in this population. What, then, is one to do?
Educate. Lifestyle modification, including weight management, should continue to be emphasized in diabetes education, as no therapy is completely effective without adjunct modifications in diet and physical activity. Epidemiologic studies have shown the benefits of lifestyle modifications, which ameliorate many of the adverse metabolic conditions that coexist in type 2 diabetes and cancer.
Screen for cancer. Given the associations between diabetes and malignancy, cancer screening is especially important in this high-risk population.
Customize therapy to individual patients. Those with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid sitagliptin until definitive clinical data become available.
Moreover, patients with a personal or family history of medullary thyroid cancer should not receive GLP-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma. Until we have further elucidating data, it is not possible to say whether a family history of any of the other types of cancer should represent a contraindication to the use of any of these agents.
Discuss. The multitude of diabetes therapies warrants physician-patient discussions that carefully weigh the risks and benefits of additional agents to optimize glycemic control and metabolic factors in individual patients.
In the last quarter century, many new drugs have become available for treating type 2 diabetes mellitus. The American Association of Clinical Endocrinologists incorporated these new agents in its updated glycemic control algorithm in 2013.1 Because diabetes affects 25.8 million Americans and can lead to blindness, renal failure, cardiovascular disease, and amputation, agents that help us treat it more effectively are valuable.2
One of the barriers to effective treatment is the side effects of the agents. Because some of these drugs have been in use for only a short time, concerns of potential adverse effects have arisen. Cancer is one such concern, especially since type 2 diabetes mellitus by itself increases the risk of cancer by 20% to 50% compared with no diabetes.3
Type 2 diabetes has been linked to risk of cancers of the pancreas,4 colorectum,5,6 liver,7 kidney,8,9 breast,10 bladder,11 and endometri-um,12 as well as to hematologic malignancies such as non-Hodgkin lymphoma.13 The risk of bladder cancer appears to depend on how long the patient has had type 2 diabetes. Newton et al,14 in a prospective cohort study, found that those who had diabetes for more than 15 years and used insulin had the highest risk of bladder cancer. On the other hand, the risk of prostate cancer is actually lower in people with diabetes,15 particularly in those who have had diabetes for longer than 4 years.16
Cancer and type 2 diabetes share many risk factors and underlying pathophysiologic mechanisms. Nonmodifiable risk factors for both diseases include advanced age, male sex, ethnicity (African American men appear to be most vulnerable to both cancer and diabetes),17,18 and family history. Modifiable risk factors include lower socioeconomic status, obesity, and alcohol consumption. These common risk factors lead to hyperinsulinemia and insulin resistance, changes in mitochondrial function, low-grade inflammation, and oxidative stress,3 which promote both diabetes and cancer. Diabetes therapy may influence several of these processes.
Several classes of diabetes drugs, including exogenous insulin,19–22 insulin secretagogues,23–25 and incretin-based therapies,26–28 have been under scrutiny because of their potential influences on cancer development in a population already at risk (Table 1).
INSULIN ANALOGUES: MIXED EVIDENCE
Insulin promotes cell division by binding to insulin receptor isoform A and insulin-like growth factor 1 receptors.29 Because endogenous hyperinsulinemia has been linked to cancer risk, growth, and proliferation, some speculate that exogenous insulin may also increase cancer risk.
In 2009, a retrospective study by Hemkens et al linked the long-acting insulin analogue glargine to risk of cancer.19 This finding set off a tumult of controversy within the medical community and concern among patients. Several limitations of the study were brought to light, including a short duration of follow-up, and several other studies have refuted the study’s findings.20,21
More recently, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial22 found no higher cancer risk with glargine use than with placebo. This study enrolled 12,537 participants from 573 sites in 40 countries. Specifically, risks with glargine use were as follows:
- Any cancer—hazard ratio 1.00, 95% confidence interval (CI) 0.88–1.13, P = .97
- Cancer death—hazard ratio 0.94, 95% CI 0.77–1.15, P = .52.
However, the study was designed to assess cardiovascular outcomes, not cancer risk. Furthermore, the participants were not typical of patients seen in clinical practice: their insulin doses were lower (the median insulin dose was 0.4 units/kg/day by year 6, whereas in clinical practice, those with type 2 diabetes mellitus often use more than 1 unit/kg/day, depending on duration of diabetes, diet, and exercise regimen), and their baseline median hemoglobin A1c level was only 6.4%. And one may argue that the median follow-up of 6.2 years was too short for cancer to develop.22
In vitro studies indicate that long-acting analogue insulin therapy may promote cancer cell growth more than endogenous insulin,30 but epidemiologic data have not unequivocally substantiated this.20–22 There is no clear evidence that analogue insulin therapy raises cancer risk above that of human recombinant insulin, and starting insulin therapy should not be delayed because of concerns about cancer risk, particularly in uncontrolled diabetes.
INSULIN SECRETAGOGUES
Sulfonylureas: Higher risk
Before 1995, only two classes of diabetes drugs were approved by the US Food and Drug Administration (FDA)—insulin and sulfonylureas.
Sulfonylureas lower blood sugar levels by binding to sulfonylurea receptors and inhibiting adenosine triphosphate-dependent potassium channels. The resulting change in resting potential causes an influx of calcium, ultimately leading to insulin secretion.
Sulfonylureas are effective, and because of their low cost, physicians often pick them as a second-line agent after metformin.
The main disadvantage of sulfonylureas is the risk of hypoglycemia, particularly in patients with renal failure, the elderly, and diabetic patients who are unaware of when they are hypoglycemic. Other potential drawbacks are that they impair cardiac ischemic preconditioning31 and possibly increase cancer risk.21,32 (Ischemic preconditioning is the process in which transient episodes of ischemia “condition” the myocardium so that it better withstands future episodes with minimal anginal pain and tissue injury.33) Of the sulfonylureas, glyburide has been most implicated in cardiovascular risk.32
In a retrospective cohort study of 62,809 patients from a general-practice database in the United Kingdom, Currie et al21 found that sulfonylurea monotherapy was associated with a 36% higher risk of cancer (95% CI 1.19–1.54, P < .001) than metformin monotherapy. Prescribing bias may have influenced the results: practitioners are more likely to prescribe sulfonylureas to leaner patients, who have a greater likelihood of occult cancer. However, other studies also found that the cancer death rate is higher in those who take a sulfonylurea alone than in those who use metformin alone.23,24
Some evidence indicates that long-acting sulfonylurea formulations (eg, glyburide) likely hold the most danger, certainly in regard to hypoglycemia, but it is less clear if this translates to cancer concerns.31
Meglitinides: Limited evidence
Meglitinides, the other class of insulin secretagogues, are less commonly used but are similar to sulfonylureas in the way they increase endogenous insulin levels. The data are limited regarding cancer risk and meglitinide therapy, but the magnitude of the association is similar to that with sulfonylurea therapy.25
INSULIN SENSITIZERS
There are currently two classes of insulin sensitizers: biguanides and thiazolidinediones (TZDs, also known as glitazones). These drugs show less risk of both cancer incidence and cancer death than insulin secretagogues such as sulfonylureas.21,23,24 In fact, they may decrease cancer potential by alteration of signaling via the AKT/mTOR (v-akt murine thymoma viral oncogene homolog 1/mammalian target of rapamycin) pathway.34
Metformin, a biguanide, is the oral drug of choice
Metformin is the only biguanide currently available in the United States. It was approved by the FDA in 1995, although it had been in clinical use since the 1950s. Inexpensive and familiar, it is the oral antihyperglycemic of choice if there are no contraindications to it, such as renal dysfunction (creatinine ≥ 1.4 mg/dL in women and ≥ 1.5 mg/dL in men), acute decompensated heart failure, or pulmonary or hepatic insufficiency, all of which may lead to an increased risk of lactic acidosis.1
Metformin lowers blood sugar levels primarily by inhibiting hepatic glucose production (gluconeogenesis) and by improving peripheral insulin sensitivity. It directly activates AMP-activated protein kinase (AMPK), which affects insulin signaling and glucose and fat metabolism.35 It may exert further beneficial effects by acutely increasing glucagon-like peptide-1 (GLP-1) levels and inducing islet incretin-receptor gene expression.36 Although the exact mechanisms have not been fully elucidated, metformin’s insulin-sensitizing properties are likely from favorable effects on insulin receptor expression, tyrosine kinase activity, and influences on the incretin pathway.36,37 These effects also mitigate carcinogenesis, both directly (via AMPK and liver kinase B1, a tumor-suppressor gene) and indirectly (via reduction of hyperinsulinemia).35
Overall, biguanide therapy is associated with a lower cancer incidence or, at worst, no effect on cancer incidence. In vitro studies demonstrate that metformin both suppresses cancer cell growth and induces apoptosis, resulting in fewer live cancer cells.34 Several retrospective studies found lower cancer risk in metformin users than in patients receiving antidiabetes drugs other than insulin-sensitizing agents,21,23,25,38–40 while others have shown no effect.41 Use of metformin was specifically associated with lower risk of cancers of the liver, colon and rectum, and lung.42 Further, metformin users have a lower cancer mortality rate than nonusers.24,43
Thiazolidinediones
TZDs, such as pioglitazone, work by binding to peroxisome proliferator-activated gamma receptors in the cell nucleus, altering gene transcription.44 They reduce insulin resistance and levels of endogenous insulin levels and free fatty acids.44
Concern over bladder cancer risk with TZD use, particularly with pioglitazone, has increased in the last few years, as various cohort studies found a statistically significant increased risk with this agent.44 The risk appears to rise with cumulative dose.45,46
Randomized controlled trials also found an increased risk of bladder cancer with TZD therapy, although the difference was not statistically significant.47–49 In a mean follow-up of 8.7 years, the Prospective Pioglitazone Clinical Trial in Macrovascular Events reported 23 cases of bladder cancer in the pioglitazone group vs 22 cases in the placebo group, for rates of 0.9% vs 0.8% (relative risk [RR] 1.06, 95% CI 0.59–1.89).49
On the other hand, the risk of cancer of the breast, colon, and lung has been found to be lower with TZD use.47 In vitro studies support the clinical data, showing that TZDs inhibit growth of human cancer cells derived from cancers of the lung, colon, breast, stomach, ovary, and prostate.50–53
Home et al54 compared rosiglitazone against a sulfonylurea in patients already taking metformin in the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial. Malignancies developed in 6.7% of the sulfonylurea group compared with 5.1% of the rosiglitazone group, for a hazard ratio of 1.33 (95% CI 0.94–1.88).
Both ADOPT (A Diabetes Outcome Progression Trial) and the RECORD trial found rosiglitazone comparable to metformin in terms of cancer risk.54
Colmers et al47 pooled data from four randomized controlled trials, seven cohort studies, and nine case-control studies to assess the risk of cancer with TZD use in type 2 diabetes. Both the randomized and observational data showed neutral overall cancer risk with TZDs. However, pooled data from observational studies showed significantly lower risk with TZD use in terms of:
- Colorectal cancer RR 0.93, 95% CI 0.87–1.00
- Lung cancer RR 0.91, 95% CI 0.84–0.98
- Breast cancer RR 0.89, 95% CI 0.81–0.98.
INCRETIN-BASED THERAPIES
Incretins are hormones released from the gut in response to food ingestion, triggering release of insulin before blood glucose levels rise. Their action explains why insulin secretion increases more after an oral glucose load than after an intravenous glucose load, a phenomenon called the incretin effect.55
There are two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). They have short a half-life because they are rapidly degraded by dipeptidyl peptidase-IV (DPP-IV).55 Available incretin-based therapies are GLP-1 receptor agonists and DPP-IV inhibitors.
When used as monotherapy, incretin-based therapies do not cause hypoglycemia because their effect is glucose-dependent.55 GLP-1 receptor antagonists have the added benefit of inducing weight loss, but DPP-IV inhibitors are considered to be weight-neutral.
GLP-1 receptor agonists
Exenatide, the first of the GLP-1 receptor agonists, was approved in 2005. The original formulation (Byetta) is taken by injection twice daily, and timing in conjunction with food intake is important: it should be taken within 60 minutes before the morning and evening meals. Extended-release exenatide (Bydureon) is a once-weekly formulation taken without regard to timing of food intake. Exenatide (either twice-daily Byetta or once-weekly Bydureon) should not be used in those with creatinine clearance less than 30 mL/min or end-stage renal disease and should be used with caution in patients with renal transplantation.
Liraglutide (Victoza), a once-daily formulation, can be injected irrespective of food intake. The dose does not have to be adjusted for renal function, although it should be used with caution in those with renal impairment, including end-stage renal disease. Approval for a 3-mg formulation is pending with the FDA as a weight-loss drug on the basis of promising results in a randomized phase 3 trial.56
Albiglutide (Tanzeum), a once-weekly GLP-1 receptor antagonist, was recently approved by the FDA.
DPP-IV inhibitors
Whereas GLP-1 receptor agonists are injected, the DPP-IV inhibitors have the advantage of being oral agents.
Sitagliptin (Januvia), the first DPP-IV inhibitor, became available in the United States in 2006. Since then, three more have become available: saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).
Concerns about thyroid cancer with incretin drugs
Concerns of increased risk of cancer, particularly of the thyroid and pancreas, have been raised since GLP-1 receptor agonists and DPP-IV inhibitors became available.
Studies in rodents have shown C-cell hyperplasia, sometimes resulting in increased incidence of thyroid carcinoma, and dose-dependent rises in serum calcitonin, particularly with liraglutide.26 This has raised concern about an increased risk of medullary thyroid carcinoma in humans. However, the density of C cells in rodents is up to 45 times greater than in humans, and C cells also express functional GLP-1 receptors.26
Gier et al27 assessed the expression of calcitonin and human GLP-1 receptors in normal C cells, C cell hyperplasia, and medullary cancer. In this study, calcitonin and GLP-1 receptor were co-expressed in medullary thyroid cancer (10 of 12 cases) and C-cell hyperplasia (9 of 9 cases) more commonly than in normal C cells (5 of 15 cases). Further, GLP-1 receptor was expressed in 3 of 17 cases of papillary thyroid cancer.
Calcitonin, a polypeptide hormone produced by thyroid C cells and used as a medullary thyroid cancer biomarker, was increased in a slightly higher percentage of patients treated with liraglutide than in controls, without an increase above the normal range.57
A meta-analysis by Alves et al58 of 25 studies found that neither exenatide (no cases reported) nor liraglutide (odds ratio 1.54, 95% CI 0.40–6.02) was associated with increased thyroid cancer risk.
MacConell et al59 pooled the results of 19 placebo-controlled trials of twice-daily exenatide and found a thyroid cancer incidence rate of 0.3 per 100 patient-years (< 0.1%) vs 0 per 100 patient-years in pooled comparators.
Concerns about pancreatic cancer with incretin drugs
Increased risk of acute pancreatitis is a potential side effect of both DPP-IV inhibitors and GLP-1 receptor agonists and has led to speculation that this translates to an increased risk of pancreatic cancer.
In a point-counterpoint debate, Butler et al28 argued that incretin-based medications have questionable safety, with increased rates of pancreatitis possibly leading to pancreatic cancer. In counterpoint, Nauck60 argued that the risk of pancreatitis or cancer is extremely low, and clinical cases are unsubstantiated.
Bailey61 outlined the complexities and difficulties in drawing firm conclusions from individual clinical trials regarding possible adverse effects of diabetes drugs. The trials are typically designed to assess hemoglobin A1c reduction at varying doses and are typically restricted in patient selection, patient numbers, and drug-exposure duration, which may introduce allocation and ascertainment biases. The attempt to draw firm conclusions from such trials can be problematic and can lead to increased alarm, warranted or not.
Type 2 diabetes mellitus itself is associated with an increased incidence of pancreatic cancer, and whether incretin therapy enhances this risk is still controversial. Whether more episodes of acute pancreatitis without chronic pancreatitis can be extrapolated to an increased incidence of pancreatic cancer is doubtful. A normal pancreatic duct cell may take up to 12 years to become a tumor cell from which pancreatic carcinoma develops, another 7 years to develop metastatic capacity, and another 3 years before a diagnosis is made from clinical symptoms (which are usually accompanied by metastases).62
The risks and benefits of incretin therapies remain a contentious issue, and there are no clear prospective data at this time on increased pancreatic cancer incidence. Long-term prospective studies designed to analyze these specific outcomes (pancreatitis, pancreatic cancer, and medullary thyroid cancer) need to be undertaken.63
OTHER DIABETES THERAPIES
Alpha glucosidase inhibitors
Oral glucosidase inhibitors ameliorate hyperglycemia by inhibiting alpha glucosidase enzymes in the brush border of the small intestines, preventing conversion of polysaccharides to monosaccharides.64 This slows digestion of carbohydrates and glucose release into the bloodstream and blunts the postprandial hyperglycemic excursion.
The two alpha glucosidase inhibitors currently available in the United States are acarbose and miglitol, and although data are limited, they do not appear to increase the risk of cancer.65,66
Sodium-glucose-linked cotransporter 2 inhibitors
The newest class of oral diabetes agents to be approved are the sodium-glucose-linked cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga).
SGLT2 is a protein in the S1 segment of the proximal renal tubules responsible for over 90% of renal glucose reabsorption. SGLT2 inhibitors lower serum glucose levels by promoting glycosuria and have also been shown to have favorable effects on blood pressure and weight.67,68
Canagliflozin was the first of its class to gain FDA approval in the United States. It has not been found to be associated with increased cancer risk.68
Dapagliflozin, originally approved in Europe, was approved in the United States on January 8, 2014. Because of a possible increased incidence of breast and bladder malignancies, the FDA advisory committee initially recommended against approval and required further data. In those who were treated, nine cases of bladder cancer and nine cases of breast cancer were reported, compared with one case of bladder cancer and no cases of breast cancer in the control group; however, the difference was not statistically significant.68
Since SGLT2 inhibitors are still new, data on long-term outcomes are lacking. Early clinical data do not show a significant increase in cancer risk.
WHAT THIS MEANS IN PRACTICE
Many studies have found associations between diabetes, obesity, hyperinsulinemia, and cancer risk. In the last decade, concerns implicating antihyperglycemic agents in cancer development have arisen but have not been well substantiated. At this time, there are no definitive prospective data indicating that the currently available type 2 diabetes therapies increase the incidence of cancer beyond the inherent increased risk in this population. What, then, is one to do?
Educate. Lifestyle modification, including weight management, should continue to be emphasized in diabetes education, as no therapy is completely effective without adjunct modifications in diet and physical activity. Epidemiologic studies have shown the benefits of lifestyle modifications, which ameliorate many of the adverse metabolic conditions that coexist in type 2 diabetes and cancer.
Screen for cancer. Given the associations between diabetes and malignancy, cancer screening is especially important in this high-risk population.
Customize therapy to individual patients. Those with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid sitagliptin until definitive clinical data become available.
Moreover, patients with a personal or family history of medullary thyroid cancer should not receive GLP-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma. Until we have further elucidating data, it is not possible to say whether a family history of any of the other types of cancer should represent a contraindication to the use of any of these agents.
Discuss. The multitude of diabetes therapies warrants physician-patient discussions that carefully weigh the risks and benefits of additional agents to optimize glycemic control and metabolic factors in individual patients.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Centers for Disease Control and Prevention (CDC). Diabetes data and trends. www.cdc.gov/diabetes/statistics/. Accessed April 8, 2014.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687.
- Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006; 101:1872–1879.
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380.
- Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112.
- Washio M, Mori M, Khan M, et al; JACC Study Group. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 2007; 14:393–397.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823.
- Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007; 50:1365–1374.
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008; 31:2391–2397.
- Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 2013; 132:2186–2191.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:2056–2062.
- Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005; 161:147–152.
- Centers for Disease Control and Prevention. Diabetes public health resource. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pubs/estimates14.htm. Accessed August 12, 2014.
- Centers for Disease Control and Prevention. Cancer prevention and control cancer rates by race and ethnicity. www.cdc.gov/cancer/dcpc/data/race.htm. Accessed August 12, 2014.
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; 52:1732–1744.
- Colhoun HMSDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; 52:1755–1765.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Baur DM, Klotsche J, Hamnvik OP, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011; 60:1363–1371.
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29:254–258.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137:482–488.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013; 36:2118–2125.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011; 18:R125–R147.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab 2003; 88:528–530.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012; 107:620–626.
- Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrmann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990; 82:2044–2051.
- Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med 2011; 15:825–836.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122:253–270.
- Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011; 54:339–349.
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323–1332.
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011; 123:200–204.
- Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011; 20:337–344.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7:e33411.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
- Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344:e3645.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2012; 38:475–484.
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32:187–202.
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A, Perez A. Pioglitazone and bladder malignancy during observational follow-up of PROactive: 6-year update. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA.
- Akinyeke TO, Stewart LV. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol Ther 2011; 11:1046–1058.
- Ban JO, Oh JH, Son SM, et al. Troglitazone, a PPAR agonist, inhibits human prostate cancer cell growth through inactivation of NFKB via suppression of GSK-3B expression. Cancer Biol Ther 2011; 12:288–296.
- Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog 2010; 49:235–246.
- Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2010; 10:390.
- Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti GADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845.
- Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 41:299–307.
- Wadden TA, Hollander P, Klein S, et al; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37:1443–1451.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5,594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5:29–41.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care 2013; 36:2098–2106.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467:1114–1117.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 1995; 18:303–311.
- Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009; 46:279–284.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011; 54:2009–2015.
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022.
- Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5:313–527.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Centers for Disease Control and Prevention (CDC). Diabetes data and trends. www.cdc.gov/diabetes/statistics/. Accessed April 8, 2014.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687.
- Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006; 101:1872–1879.
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380.
- Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112.
- Washio M, Mori M, Khan M, et al; JACC Study Group. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 2007; 14:393–397.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823.
- Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007; 50:1365–1374.
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008; 31:2391–2397.
- Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 2013; 132:2186–2191.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:2056–2062.
- Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005; 161:147–152.
- Centers for Disease Control and Prevention. Diabetes public health resource. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pubs/estimates14.htm. Accessed August 12, 2014.
- Centers for Disease Control and Prevention. Cancer prevention and control cancer rates by race and ethnicity. www.cdc.gov/cancer/dcpc/data/race.htm. Accessed August 12, 2014.
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; 52:1732–1744.
- Colhoun HMSDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; 52:1755–1765.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Baur DM, Klotsche J, Hamnvik OP, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011; 60:1363–1371.
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29:254–258.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137:482–488.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013; 36:2118–2125.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011; 18:R125–R147.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab 2003; 88:528–530.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012; 107:620–626.
- Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrmann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990; 82:2044–2051.
- Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med 2011; 15:825–836.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122:253–270.
- Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011; 54:339–349.
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323–1332.
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011; 123:200–204.
- Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011; 20:337–344.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7:e33411.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
- Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344:e3645.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2012; 38:475–484.
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32:187–202.
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A, Perez A. Pioglitazone and bladder malignancy during observational follow-up of PROactive: 6-year update. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA.
- Akinyeke TO, Stewart LV. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol Ther 2011; 11:1046–1058.
- Ban JO, Oh JH, Son SM, et al. Troglitazone, a PPAR agonist, inhibits human prostate cancer cell growth through inactivation of NFKB via suppression of GSK-3B expression. Cancer Biol Ther 2011; 12:288–296.
- Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog 2010; 49:235–246.
- Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2010; 10:390.
- Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti GADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845.
- Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 41:299–307.
- Wadden TA, Hollander P, Klein S, et al; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37:1443–1451.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5,594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5:29–41.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care 2013; 36:2098–2106.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467:1114–1117.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 1995; 18:303–311.
- Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009; 46:279–284.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011; 54:2009–2015.
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022.
- Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5:313–527.
KEY POINTS
- Exogenous insulin, insulin secretagogues, and incretin-based therapies are under scrutiny because of their potential influences on cancer development in a population already at risk.
- At present, we lack adequate prospective data on the cancer risk from diabetes drugs.
- Patients with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid incretin therapies until definitive clinical data become available.
- Patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 should not receive glucagon-like peptide-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma.
- Given the associations between diabetes and malignancy, cancer screening is especially important.
When patients on target-specific oral anticoagulants need surgery
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
KEY POINTS
- How long before surgery to stop a TSOAC depends on the bleeding risk of the procedure and the patient’s renal function.
- Perioperative bridging is generally unnecessary for patients on TSOACs.
- Routine coagulation assays such as the prothrombin time and activated partial thromboplastin time do not reliably reflect the degree of anticoagulation with TSOACs.
- There are no specific antidotes or standardized reversal strategies for TSOACs.
- TSOACs have a rapid onset of action and should only be restarted postoperatively once hemostasis has been confirmed.
Be prepared to adjust dosing of psychotropics after bariatric surgery
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.