User login
Get ready for the Big Easy
CHEST 2019 will be in New Orleans, Louisiana, this year, October 19-23. Here are a few ways to be engaged leading up to the meeting.
Submit Abstracts and Case Reports
Do you have original investigative research to share? There’s still some time to submit your abstracts and case reports for presentation at CHEST 2019 through Friday, March 15. If accepted, all abstracts and case reports will be published as submitted in an online CHEST® journal abstract supplement. No corrections will be made once submission is complete.
View submission details (https://chestmeeting.chestnet.org/abstracts-and-case-reports/)
Call for Moderators
CHEST is currently requesting moderators to facilitate discussions, questions, and answers within assigned sessions on-site at CHEST 2019 in New Orleans. Moderators will be notified June to September of their acceptance as a moderator.
View complete details (https://docs.google.com/forms/d/e/1FAIpQLSdSWFSyKAeIjfyYgGRF6km_95znba63bx6iM9TWl08gpdqzEQ/viewform)
CHEST Challenge 2019
US-based CHEST fellows-in-training - does your fellowship have what it takes to win CHEST Challenge 2019? CHEST Challenge is a fun and exciting competition in which CHEST fellows-in-training compete against programs around the country for honor and prizes! The first round of the competition consists of two parts: social media challenges and online quiz. The aggregate score for both of these components will be used to identify the top three highest scoring teams. These top three teams will then be invited to send three fellows each to the CHEST Challenge Championship, a Jeopardy-style game show that takes place live during the CHEST Annual Meeting.
See the rules and how to participate. (chestchallenge.org)
Apply for CHEST Foundation Grants
The CHEST Foundation has awarded more than $10 million in grant funding to nearly 800 recipients worldwide for clinical research and community service. Each year, the CHEST Foundation offers grants to worthy research candidates, generous community service volunteers, and distinguished scholars in a field of expertise.
The CHEST Foundation is accepting grant applications now through April 8, 2019, in the following areas:
• CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP – Up to $15,000 (multiple recipients selected)*
• The GlaxoSmithKline Distinguished Scholar in Respiratory Health – $150,000*
• CHEST Foundation Research Grant in Asthma – $15,000 – $30,000*
• CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease – $25,000 – $50,000*
• CHEST Foundation Research Grant in Cystic Fibrosis – $15,000 – $30,000*
• CHEST Foundation Research Grant in Lung Cancer – $50,000 – $100,000*
• CHEST Foundation Research Grant in Nontuberculous Mycobacteria Diseases – $30,000 – $60,000*
• CHEST Foundation Research Grant in Pulmonary Arterial Hypertension – $25,000 – $50,000*
• CHEST Foundation Research Grant in Pulmonary Fibrosis – $25,000 – $50,000*
• CHEST Foundation Research Grant in Venous Thromboembolism – $15,000 – $30,000*
• CHEST Foundation Research Grant in Women’s Lung Health – $10,000*
*Amount contingent on funding.
Learn more on how to apply now. (https://foundation.chestnet.org/grants/apply-for-a-grant/)
CHEST 2019 will be in New Orleans, Louisiana, this year, October 19-23. Here are a few ways to be engaged leading up to the meeting.
Submit Abstracts and Case Reports
Do you have original investigative research to share? There’s still some time to submit your abstracts and case reports for presentation at CHEST 2019 through Friday, March 15. If accepted, all abstracts and case reports will be published as submitted in an online CHEST® journal abstract supplement. No corrections will be made once submission is complete.
View submission details (https://chestmeeting.chestnet.org/abstracts-and-case-reports/)
Call for Moderators
CHEST is currently requesting moderators to facilitate discussions, questions, and answers within assigned sessions on-site at CHEST 2019 in New Orleans. Moderators will be notified June to September of their acceptance as a moderator.
View complete details (https://docs.google.com/forms/d/e/1FAIpQLSdSWFSyKAeIjfyYgGRF6km_95znba63bx6iM9TWl08gpdqzEQ/viewform)
CHEST Challenge 2019
US-based CHEST fellows-in-training - does your fellowship have what it takes to win CHEST Challenge 2019? CHEST Challenge is a fun and exciting competition in which CHEST fellows-in-training compete against programs around the country for honor and prizes! The first round of the competition consists of two parts: social media challenges and online quiz. The aggregate score for both of these components will be used to identify the top three highest scoring teams. These top three teams will then be invited to send three fellows each to the CHEST Challenge Championship, a Jeopardy-style game show that takes place live during the CHEST Annual Meeting.
See the rules and how to participate. (chestchallenge.org)
Apply for CHEST Foundation Grants
The CHEST Foundation has awarded more than $10 million in grant funding to nearly 800 recipients worldwide for clinical research and community service. Each year, the CHEST Foundation offers grants to worthy research candidates, generous community service volunteers, and distinguished scholars in a field of expertise.
The CHEST Foundation is accepting grant applications now through April 8, 2019, in the following areas:
• CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP – Up to $15,000 (multiple recipients selected)*
• The GlaxoSmithKline Distinguished Scholar in Respiratory Health – $150,000*
• CHEST Foundation Research Grant in Asthma – $15,000 – $30,000*
• CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease – $25,000 – $50,000*
• CHEST Foundation Research Grant in Cystic Fibrosis – $15,000 – $30,000*
• CHEST Foundation Research Grant in Lung Cancer – $50,000 – $100,000*
• CHEST Foundation Research Grant in Nontuberculous Mycobacteria Diseases – $30,000 – $60,000*
• CHEST Foundation Research Grant in Pulmonary Arterial Hypertension – $25,000 – $50,000*
• CHEST Foundation Research Grant in Pulmonary Fibrosis – $25,000 – $50,000*
• CHEST Foundation Research Grant in Venous Thromboembolism – $15,000 – $30,000*
• CHEST Foundation Research Grant in Women’s Lung Health – $10,000*
*Amount contingent on funding.
Learn more on how to apply now. (https://foundation.chestnet.org/grants/apply-for-a-grant/)
CHEST 2019 will be in New Orleans, Louisiana, this year, October 19-23. Here are a few ways to be engaged leading up to the meeting.
Submit Abstracts and Case Reports
Do you have original investigative research to share? There’s still some time to submit your abstracts and case reports for presentation at CHEST 2019 through Friday, March 15. If accepted, all abstracts and case reports will be published as submitted in an online CHEST® journal abstract supplement. No corrections will be made once submission is complete.
View submission details (https://chestmeeting.chestnet.org/abstracts-and-case-reports/)
Call for Moderators
CHEST is currently requesting moderators to facilitate discussions, questions, and answers within assigned sessions on-site at CHEST 2019 in New Orleans. Moderators will be notified June to September of their acceptance as a moderator.
View complete details (https://docs.google.com/forms/d/e/1FAIpQLSdSWFSyKAeIjfyYgGRF6km_95znba63bx6iM9TWl08gpdqzEQ/viewform)
CHEST Challenge 2019
US-based CHEST fellows-in-training - does your fellowship have what it takes to win CHEST Challenge 2019? CHEST Challenge is a fun and exciting competition in which CHEST fellows-in-training compete against programs around the country for honor and prizes! The first round of the competition consists of two parts: social media challenges and online quiz. The aggregate score for both of these components will be used to identify the top three highest scoring teams. These top three teams will then be invited to send three fellows each to the CHEST Challenge Championship, a Jeopardy-style game show that takes place live during the CHEST Annual Meeting.
See the rules and how to participate. (chestchallenge.org)
Apply for CHEST Foundation Grants
The CHEST Foundation has awarded more than $10 million in grant funding to nearly 800 recipients worldwide for clinical research and community service. Each year, the CHEST Foundation offers grants to worthy research candidates, generous community service volunteers, and distinguished scholars in a field of expertise.
The CHEST Foundation is accepting grant applications now through April 8, 2019, in the following areas:
• CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP – Up to $15,000 (multiple recipients selected)*
• The GlaxoSmithKline Distinguished Scholar in Respiratory Health – $150,000*
• CHEST Foundation Research Grant in Asthma – $15,000 – $30,000*
• CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease – $25,000 – $50,000*
• CHEST Foundation Research Grant in Cystic Fibrosis – $15,000 – $30,000*
• CHEST Foundation Research Grant in Lung Cancer – $50,000 – $100,000*
• CHEST Foundation Research Grant in Nontuberculous Mycobacteria Diseases – $30,000 – $60,000*
• CHEST Foundation Research Grant in Pulmonary Arterial Hypertension – $25,000 – $50,000*
• CHEST Foundation Research Grant in Pulmonary Fibrosis – $25,000 – $50,000*
• CHEST Foundation Research Grant in Venous Thromboembolism – $15,000 – $30,000*
• CHEST Foundation Research Grant in Women’s Lung Health – $10,000*
*Amount contingent on funding.
Learn more on how to apply now. (https://foundation.chestnet.org/grants/apply-for-a-grant/)
CHEST updates guidelines on PAH
The American College of Chest Physicians® (CHEST) has published updates to the evidence-based guidelines on therapy for pulmonary arterial hypertension (PAH). In the latest evidence-based guideline, Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report, experts provide 78 evidence-based recommendations for appropriate use in treating patients with PAH.
“New recommendations and ungraded consensus-based statements were developed in this update based on new studies that were published since the 2014 guidelines. In addition, an evidence-based and consensus-driven treatment algorithm was created to guide the clinician through an organized approach to management,” says CHEST Pulmonary Arterial Hypertension Guidelines Committee Co-Chair, Deborah Jo Levine, MD, FCCP.
As part of the guideline development process, the panel updated the systematic review on the same clinical questions and criteria. Based on the results of the systematic review, the panel developed two new recommendations about pharmacologic therapy for PAH:
•For treatment-naive patients with PAH who are World Health Organization (WHO) functional class II and III, we suggest initial combination therapy with ambrisentan and tadalafil to improve 6-minute walk distance (6MWD).
•For stable or symptomatic patients with PAH on background therapy with ambrisentan, we suggest the addition of tadalafil to improve 6MWD.
The complete guideline article is free to view in the Online First section of the journal CHEST®.
The American College of Chest Physicians® (CHEST) has published updates to the evidence-based guidelines on therapy for pulmonary arterial hypertension (PAH). In the latest evidence-based guideline, Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report, experts provide 78 evidence-based recommendations for appropriate use in treating patients with PAH.
“New recommendations and ungraded consensus-based statements were developed in this update based on new studies that were published since the 2014 guidelines. In addition, an evidence-based and consensus-driven treatment algorithm was created to guide the clinician through an organized approach to management,” says CHEST Pulmonary Arterial Hypertension Guidelines Committee Co-Chair, Deborah Jo Levine, MD, FCCP.
As part of the guideline development process, the panel updated the systematic review on the same clinical questions and criteria. Based on the results of the systematic review, the panel developed two new recommendations about pharmacologic therapy for PAH:
•For treatment-naive patients with PAH who are World Health Organization (WHO) functional class II and III, we suggest initial combination therapy with ambrisentan and tadalafil to improve 6-minute walk distance (6MWD).
•For stable or symptomatic patients with PAH on background therapy with ambrisentan, we suggest the addition of tadalafil to improve 6MWD.
The complete guideline article is free to view in the Online First section of the journal CHEST®.
The American College of Chest Physicians® (CHEST) has published updates to the evidence-based guidelines on therapy for pulmonary arterial hypertension (PAH). In the latest evidence-based guideline, Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report, experts provide 78 evidence-based recommendations for appropriate use in treating patients with PAH.
“New recommendations and ungraded consensus-based statements were developed in this update based on new studies that were published since the 2014 guidelines. In addition, an evidence-based and consensus-driven treatment algorithm was created to guide the clinician through an organized approach to management,” says CHEST Pulmonary Arterial Hypertension Guidelines Committee Co-Chair, Deborah Jo Levine, MD, FCCP.
As part of the guideline development process, the panel updated the systematic review on the same clinical questions and criteria. Based on the results of the systematic review, the panel developed two new recommendations about pharmacologic therapy for PAH:
•For treatment-naive patients with PAH who are World Health Organization (WHO) functional class II and III, we suggest initial combination therapy with ambrisentan and tadalafil to improve 6-minute walk distance (6MWD).
•For stable or symptomatic patients with PAH on background therapy with ambrisentan, we suggest the addition of tadalafil to improve 6MWD.
The complete guideline article is free to view in the Online First section of the journal CHEST®.
CHEST reaccredited by Society for Simulation in Healthcare
The American College of Chest Physicians (CHEST) received reaccreditation from the Society for Simulation in Healthcare (SSH) for the 2018-2023 term in the areas of Teaching/Education, Assessment, and Research. In 2013, CHEST became the first and only medical specialty society to achieve SSH accreditation, a distinction that continues today. Currently, CHEST joins over 125 SSH-accredited programs worldwide, including universities, hospitals, and medical education companies.
The reaccreditation process was the result of months of preparation on behalf of CHEST Simulation Program staff, CHEST Accreditation staff, CHEST Outcomes staff, as well as CHEST’s Live Learning Domain Task Force chairs and other education leadership. This culminated in mid-November at a face-to-face on-site interview with site reviewers representing SSH and CHEST Simulation Program faculty and staff and CHEST leadership. Throughout the process, CHEST was given the opportunity to highlight the unique and innovative ways in which we are utilizing simulation-based education to provide greater clinical insights to enhance patient care.
We recognize that this isn’t only an every-4-year commitment, but it is resultant of the ongoing efforts from a group of dedicated individuals. Thank you to all whose contributions ensured our success!
The American College of Chest Physicians (CHEST) received reaccreditation from the Society for Simulation in Healthcare (SSH) for the 2018-2023 term in the areas of Teaching/Education, Assessment, and Research. In 2013, CHEST became the first and only medical specialty society to achieve SSH accreditation, a distinction that continues today. Currently, CHEST joins over 125 SSH-accredited programs worldwide, including universities, hospitals, and medical education companies.
The reaccreditation process was the result of months of preparation on behalf of CHEST Simulation Program staff, CHEST Accreditation staff, CHEST Outcomes staff, as well as CHEST’s Live Learning Domain Task Force chairs and other education leadership. This culminated in mid-November at a face-to-face on-site interview with site reviewers representing SSH and CHEST Simulation Program faculty and staff and CHEST leadership. Throughout the process, CHEST was given the opportunity to highlight the unique and innovative ways in which we are utilizing simulation-based education to provide greater clinical insights to enhance patient care.
We recognize that this isn’t only an every-4-year commitment, but it is resultant of the ongoing efforts from a group of dedicated individuals. Thank you to all whose contributions ensured our success!
The American College of Chest Physicians (CHEST) received reaccreditation from the Society for Simulation in Healthcare (SSH) for the 2018-2023 term in the areas of Teaching/Education, Assessment, and Research. In 2013, CHEST became the first and only medical specialty society to achieve SSH accreditation, a distinction that continues today. Currently, CHEST joins over 125 SSH-accredited programs worldwide, including universities, hospitals, and medical education companies.
The reaccreditation process was the result of months of preparation on behalf of CHEST Simulation Program staff, CHEST Accreditation staff, CHEST Outcomes staff, as well as CHEST’s Live Learning Domain Task Force chairs and other education leadership. This culminated in mid-November at a face-to-face on-site interview with site reviewers representing SSH and CHEST Simulation Program faculty and staff and CHEST leadership. Throughout the process, CHEST was given the opportunity to highlight the unique and innovative ways in which we are utilizing simulation-based education to provide greater clinical insights to enhance patient care.
We recognize that this isn’t only an every-4-year commitment, but it is resultant of the ongoing efforts from a group of dedicated individuals. Thank you to all whose contributions ensured our success!
In Memoriam
CHEST has been notified of the following deaths.
We extend our sincere condolences.
Faroque A. Khan, MBBS
Venessa Holland, MD, FCCP
CHEST has been notified of the following deaths.
We extend our sincere condolences.
Faroque A. Khan, MBBS
Venessa Holland, MD, FCCP
CHEST has been notified of the following deaths.
We extend our sincere condolences.
Faroque A. Khan, MBBS
Venessa Holland, MD, FCCP
This Month in CHEST: Editor’s picks
Giants in Chest Medicine – Paul D. Stein, MD, Master FCCP’
Rapidly Improving ARDS in Therapeutic Randomized Controlled Trials. By Dr. E. J. Schenck, et al.
The Accuracy of Clinical Staging of Stage I-IIIa Non-Small Cell Lung Cancer: An Analysis
Based on Individual Participant Data. By Dr. N. Navani, et al.
A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian
Subjects: Derivation in 471,446 Chinese Subjects, With Internal Validation and External
Application in 451,199 Korean Subjects. By Dr. Y-G Li, et al.
A Sleep Medicine Curriculum for Pulmonary and Pulmonary/Critical Care Fellowship
Programs: A Multisociety Expert Panel Report. By Dr. D. A. Schulman, et al.
Giants in Chest Medicine – Paul D. Stein, MD, Master FCCP’
Rapidly Improving ARDS in Therapeutic Randomized Controlled Trials. By Dr. E. J. Schenck, et al.
The Accuracy of Clinical Staging of Stage I-IIIa Non-Small Cell Lung Cancer: An Analysis
Based on Individual Participant Data. By Dr. N. Navani, et al.
A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian
Subjects: Derivation in 471,446 Chinese Subjects, With Internal Validation and External
Application in 451,199 Korean Subjects. By Dr. Y-G Li, et al.
A Sleep Medicine Curriculum for Pulmonary and Pulmonary/Critical Care Fellowship
Programs: A Multisociety Expert Panel Report. By Dr. D. A. Schulman, et al.
Giants in Chest Medicine – Paul D. Stein, MD, Master FCCP’
Rapidly Improving ARDS in Therapeutic Randomized Controlled Trials. By Dr. E. J. Schenck, et al.
The Accuracy of Clinical Staging of Stage I-IIIa Non-Small Cell Lung Cancer: An Analysis
Based on Individual Participant Data. By Dr. N. Navani, et al.
A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian
Subjects: Derivation in 471,446 Chinese Subjects, With Internal Validation and External
Application in 451,199 Korean Subjects. By Dr. Y-G Li, et al.
A Sleep Medicine Curriculum for Pulmonary and Pulmonary/Critical Care Fellowship
Programs: A Multisociety Expert Panel Report. By Dr. D. A. Schulman, et al.
Disaster response, practice operations, transplant, women's health
Disaster Response and Global Health
Epigenetics and Disasters
The configuration of the DNA bordering a gene dictates under what conditions a gene is expressed. Random errors or mutations affecting the neighboring DNA or the gene itself can affect how the gene functions. Epigenetics is an emerging field of science looking at environmental and psychosocial factors that do not directly cause mutations but still affect how genes are expressed with implications for the development and inheritance of disease. These external influences are thought to affect why some segments of DNA become accessible for protein production while other segments may not.
Disasters represent stressors with potential for epigenetic impact. Women who were pregnant during the 1998 Quebec ice storm were found to have a correlation between maternal objective stress and a distinctive pattern of DNA methylation in their children 13 years later (Cao-Lei L, et al. PLoS ONE. 2014;9[9] e10765). Methylation is known to affect the activity of a DNA segment and how genes are expressed. Associations have also been found between the severity of hurricanes and the prevalence of autism in the offspring of pregnant women experiencing these disasters (Kinney DK, et al. J Autism Dev Disord. 2008;38:481).
Anthropogenic hazards may also affect the offspring of survivors as suggested by studies of civil war POWs and Dutch Hunger Winter during WW II (Costa, DL, et al. Proc Nat Acad Sci 2018;. 115:44; Heijmans BT et al. Proc Nat Acad Sci. 2008;105[44]: 17046-9).
Epigenetics represents an area for additional research as natural and man-made disasters increase.
Omesh Toolsie, MBBS
Steering Committee Fellow-in-Training
Practice Operations
Medicare Competitive Bidding Process Update
Medicare’s Competitive Bidding Program (CBP), mandated since 2003, asks providers of specific durable medical equipment (including oxygen) to submit competing proposals for services. The best offer is then awarded a 3-year contract. Recently, several reforms to CBP have been proposed. The payment structure has changed to “lead-item pricing,” where a single bid in each category is selected and payment amounts for each product are then calculated based on pricing ratios and fee schedules (CMS DMEPOS Competitive Bidding).
This is in contrast to the prior method of median pricing, which caused financial difficulty and access concerns (Council for Quality Respiratory Care. The Rationale for Reforming Medicare Home Respiratory Therapy Payment Methodology. 2018). Budget neutrality requirements should relax, and oxygen payment structures improve. These proposed changes also include improved coverage of liquid oxygen and addition of home ventilator supplies.
However, effective January 1, 2019, all CBP is suspended through CMS. During the anticipated 2-year gap, any Medicare-enrolled supplier will be able to provide items until new contracts are awarded. Pricing during the gap period is based on a current single price plus consumer price index. These changes will impact CHEST members and their patients moving forward. During the temporary gap period, some areas are seeing decreased accessibility of some DME due to demand. Once reinstated, the changes to the oxygen payment structure should improve access and reduce out-of-pocket costs. The Practice Operations NetWork will continue to provide updates on this topic as they become available.
Timothy Dempsey, MD, MPH
Steering Committee Fellow-in-Training
Megan Sisk, DO
Steering Committee Member
Transplant
Medicare Part D Plans Can Deny Coverage of Select Immunosuppressant Medications in Solid Organ Transplant Recipients
An alarming problem has emerged with some solid organ transplant recipients experiencing immunosuppressant medication claim denials by Medicare Part D plans. Affected patients are those who convert from some other insurance (ie, private insurance or state Medicaid) to Medicare after their transplant and, therefore, rely on Medicare Part D for immunosuppressant drug coverage.
Insurance companies who offer Medicare Part D plans must follow the rules described in the Medicare Prescription Drug Benefit Manual.1 Although the Manual mandates that all immunosuppressant medications are on plan formularies, Part D plans are only required to cover immunosuppressant medications when used for indications approved by the Food and Drug Administration (FDA) or for off-label indications supported by the Centers for Medicare & Medicaid Services (CMS)-approved compendia (Drugdex® and AHFS Drug Information®).
A recent study examining the extent of the problem demonstrated non-renal organ transplant recipients are frequently prescribed and maintained on at least one medication vulnerable to Medicare Part D claim denials at 1 year posttransplant (lung: 71.1%; intestine: 39.7%; pancreas: 36.8%; liver: 19.7%; heart: 18.5%).2 Lung transplant recipients are most vulnerable since no immunosuppressant is FDA-approved for use in lung transplantation, and CMS-approved compendia only support off-label use for tacrolimus and cyclosporine in this population. Therefore, mycophenolate mofetil, mycophenolic acid, azathioprine, everolimus, and sirolimus are vulnerable to denial by Medicare Part D plans when used in lung transplant recipients. Over 95% of lung transplant recipients are maintained on an anti-metabolite, with the majority (88%) maintained on mycophenolate, so this is frequently impacted.2,3 While the transplant community is aware of this issue and has begun work to correct it, it has yet to be solved.2,4 In the meantime, if transplant recipients have been denied for this off-label and off-compendia reason, and appeals of those decisions have also been denied, options for obtaining the denied immunosuppressant medication include discount programs, foundation/grant funding, and industry-sponsored assistance programs.
Jennifer K. McDermott, PharmD
NetWork Member
1. Prescription Drug Benefit Manual. Centers for Medicare & Medicaid Services. Chapter 6: Part D Drugs and Formulary Requirements. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Part-D-Benefits-Manual-Chapter-6.pdf
2. Potter LM et al. Transplant recipients are vulnerable to coverage denial under Medicare Part D. Am J Transplant. 2018;18:1502.
3. Valapour M et al. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant. 2018;18 (Suppl 1): 363.
4. Immunusuppressant Drug Coverage Under Medicare Part D Benefit. American Society of Transplantation. Available at: www.myast.org/public-policy/key-position-statements/immunosuppressant-drug-coverage-under-medicare-part-d-benefit.
Women’s Health
Cannabis Use Affects Women Differently
As we enter an era of legalization, cannabis use is increasingly prevalent. Variances in the risks for women and men have been observed. For most age groups, men have higher rates of use or dependence on illicit drugs than women. However, women are equally likely as men to progress to a substance use disorder. Women may be more susceptible to craving and relapse , which are key phases of the addiction cycle. A study on use among adolescents concluded there was preliminary evidence of a faster transition from initiation of marijuana use to regular use in women, when compared with men (Schepis, et al. J Addict Med. 2011;5[1]:65).
Research studies suggest that marijuana impairs spatial memory in women more so than in men. Studies have suggested that teenage girls who use marijuana may have a higher risk of brain structural abnormalities associated with regular marijuana exposure than teenage boys (Tapert, et al. Addict Biol. 2009;14[4]:457).
A study published in Psychoneuroendocrinology showed that cannabinoid receptor binding site densities exhibit sex differences and can be modulated by estradiol in several limbic brain regions. These findings may account for the sex differences observed with respect to the effects of cannabinoids (Riebe, et al. Psychoneuroendocrinology. 2010;35[8]:1265).
Further research is needed to expand our understanding of the interactions between cannabinoids and sex steroids. Detoxification treatments tailored toward women and men with cannabis addiction show a promising future and necessitate further research.
Anita Rajagopal, MD
Steering Committee Member
Disaster Response and Global Health
Epigenetics and Disasters
The configuration of the DNA bordering a gene dictates under what conditions a gene is expressed. Random errors or mutations affecting the neighboring DNA or the gene itself can affect how the gene functions. Epigenetics is an emerging field of science looking at environmental and psychosocial factors that do not directly cause mutations but still affect how genes are expressed with implications for the development and inheritance of disease. These external influences are thought to affect why some segments of DNA become accessible for protein production while other segments may not.
Disasters represent stressors with potential for epigenetic impact. Women who were pregnant during the 1998 Quebec ice storm were found to have a correlation between maternal objective stress and a distinctive pattern of DNA methylation in their children 13 years later (Cao-Lei L, et al. PLoS ONE. 2014;9[9] e10765). Methylation is known to affect the activity of a DNA segment and how genes are expressed. Associations have also been found between the severity of hurricanes and the prevalence of autism in the offspring of pregnant women experiencing these disasters (Kinney DK, et al. J Autism Dev Disord. 2008;38:481).
Anthropogenic hazards may also affect the offspring of survivors as suggested by studies of civil war POWs and Dutch Hunger Winter during WW II (Costa, DL, et al. Proc Nat Acad Sci 2018;. 115:44; Heijmans BT et al. Proc Nat Acad Sci. 2008;105[44]: 17046-9).
Epigenetics represents an area for additional research as natural and man-made disasters increase.
Omesh Toolsie, MBBS
Steering Committee Fellow-in-Training
Practice Operations
Medicare Competitive Bidding Process Update
Medicare’s Competitive Bidding Program (CBP), mandated since 2003, asks providers of specific durable medical equipment (including oxygen) to submit competing proposals for services. The best offer is then awarded a 3-year contract. Recently, several reforms to CBP have been proposed. The payment structure has changed to “lead-item pricing,” where a single bid in each category is selected and payment amounts for each product are then calculated based on pricing ratios and fee schedules (CMS DMEPOS Competitive Bidding).
This is in contrast to the prior method of median pricing, which caused financial difficulty and access concerns (Council for Quality Respiratory Care. The Rationale for Reforming Medicare Home Respiratory Therapy Payment Methodology. 2018). Budget neutrality requirements should relax, and oxygen payment structures improve. These proposed changes also include improved coverage of liquid oxygen and addition of home ventilator supplies.
However, effective January 1, 2019, all CBP is suspended through CMS. During the anticipated 2-year gap, any Medicare-enrolled supplier will be able to provide items until new contracts are awarded. Pricing during the gap period is based on a current single price plus consumer price index. These changes will impact CHEST members and their patients moving forward. During the temporary gap period, some areas are seeing decreased accessibility of some DME due to demand. Once reinstated, the changes to the oxygen payment structure should improve access and reduce out-of-pocket costs. The Practice Operations NetWork will continue to provide updates on this topic as they become available.
Timothy Dempsey, MD, MPH
Steering Committee Fellow-in-Training
Megan Sisk, DO
Steering Committee Member
Transplant
Medicare Part D Plans Can Deny Coverage of Select Immunosuppressant Medications in Solid Organ Transplant Recipients
An alarming problem has emerged with some solid organ transplant recipients experiencing immunosuppressant medication claim denials by Medicare Part D plans. Affected patients are those who convert from some other insurance (ie, private insurance or state Medicaid) to Medicare after their transplant and, therefore, rely on Medicare Part D for immunosuppressant drug coverage.
Insurance companies who offer Medicare Part D plans must follow the rules described in the Medicare Prescription Drug Benefit Manual.1 Although the Manual mandates that all immunosuppressant medications are on plan formularies, Part D plans are only required to cover immunosuppressant medications when used for indications approved by the Food and Drug Administration (FDA) or for off-label indications supported by the Centers for Medicare & Medicaid Services (CMS)-approved compendia (Drugdex® and AHFS Drug Information®).
A recent study examining the extent of the problem demonstrated non-renal organ transplant recipients are frequently prescribed and maintained on at least one medication vulnerable to Medicare Part D claim denials at 1 year posttransplant (lung: 71.1%; intestine: 39.7%; pancreas: 36.8%; liver: 19.7%; heart: 18.5%).2 Lung transplant recipients are most vulnerable since no immunosuppressant is FDA-approved for use in lung transplantation, and CMS-approved compendia only support off-label use for tacrolimus and cyclosporine in this population. Therefore, mycophenolate mofetil, mycophenolic acid, azathioprine, everolimus, and sirolimus are vulnerable to denial by Medicare Part D plans when used in lung transplant recipients. Over 95% of lung transplant recipients are maintained on an anti-metabolite, with the majority (88%) maintained on mycophenolate, so this is frequently impacted.2,3 While the transplant community is aware of this issue and has begun work to correct it, it has yet to be solved.2,4 In the meantime, if transplant recipients have been denied for this off-label and off-compendia reason, and appeals of those decisions have also been denied, options for obtaining the denied immunosuppressant medication include discount programs, foundation/grant funding, and industry-sponsored assistance programs.
Jennifer K. McDermott, PharmD
NetWork Member
1. Prescription Drug Benefit Manual. Centers for Medicare & Medicaid Services. Chapter 6: Part D Drugs and Formulary Requirements. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Part-D-Benefits-Manual-Chapter-6.pdf
2. Potter LM et al. Transplant recipients are vulnerable to coverage denial under Medicare Part D. Am J Transplant. 2018;18:1502.
3. Valapour M et al. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant. 2018;18 (Suppl 1): 363.
4. Immunusuppressant Drug Coverage Under Medicare Part D Benefit. American Society of Transplantation. Available at: www.myast.org/public-policy/key-position-statements/immunosuppressant-drug-coverage-under-medicare-part-d-benefit.
Women’s Health
Cannabis Use Affects Women Differently
As we enter an era of legalization, cannabis use is increasingly prevalent. Variances in the risks for women and men have been observed. For most age groups, men have higher rates of use or dependence on illicit drugs than women. However, women are equally likely as men to progress to a substance use disorder. Women may be more susceptible to craving and relapse , which are key phases of the addiction cycle. A study on use among adolescents concluded there was preliminary evidence of a faster transition from initiation of marijuana use to regular use in women, when compared with men (Schepis, et al. J Addict Med. 2011;5[1]:65).
Research studies suggest that marijuana impairs spatial memory in women more so than in men. Studies have suggested that teenage girls who use marijuana may have a higher risk of brain structural abnormalities associated with regular marijuana exposure than teenage boys (Tapert, et al. Addict Biol. 2009;14[4]:457).
A study published in Psychoneuroendocrinology showed that cannabinoid receptor binding site densities exhibit sex differences and can be modulated by estradiol in several limbic brain regions. These findings may account for the sex differences observed with respect to the effects of cannabinoids (Riebe, et al. Psychoneuroendocrinology. 2010;35[8]:1265).
Further research is needed to expand our understanding of the interactions between cannabinoids and sex steroids. Detoxification treatments tailored toward women and men with cannabis addiction show a promising future and necessitate further research.
Anita Rajagopal, MD
Steering Committee Member
Disaster Response and Global Health
Epigenetics and Disasters
The configuration of the DNA bordering a gene dictates under what conditions a gene is expressed. Random errors or mutations affecting the neighboring DNA or the gene itself can affect how the gene functions. Epigenetics is an emerging field of science looking at environmental and psychosocial factors that do not directly cause mutations but still affect how genes are expressed with implications for the development and inheritance of disease. These external influences are thought to affect why some segments of DNA become accessible for protein production while other segments may not.
Disasters represent stressors with potential for epigenetic impact. Women who were pregnant during the 1998 Quebec ice storm were found to have a correlation between maternal objective stress and a distinctive pattern of DNA methylation in their children 13 years later (Cao-Lei L, et al. PLoS ONE. 2014;9[9] e10765). Methylation is known to affect the activity of a DNA segment and how genes are expressed. Associations have also been found between the severity of hurricanes and the prevalence of autism in the offspring of pregnant women experiencing these disasters (Kinney DK, et al. J Autism Dev Disord. 2008;38:481).
Anthropogenic hazards may also affect the offspring of survivors as suggested by studies of civil war POWs and Dutch Hunger Winter during WW II (Costa, DL, et al. Proc Nat Acad Sci 2018;. 115:44; Heijmans BT et al. Proc Nat Acad Sci. 2008;105[44]: 17046-9).
Epigenetics represents an area for additional research as natural and man-made disasters increase.
Omesh Toolsie, MBBS
Steering Committee Fellow-in-Training
Practice Operations
Medicare Competitive Bidding Process Update
Medicare’s Competitive Bidding Program (CBP), mandated since 2003, asks providers of specific durable medical equipment (including oxygen) to submit competing proposals for services. The best offer is then awarded a 3-year contract. Recently, several reforms to CBP have been proposed. The payment structure has changed to “lead-item pricing,” where a single bid in each category is selected and payment amounts for each product are then calculated based on pricing ratios and fee schedules (CMS DMEPOS Competitive Bidding).
This is in contrast to the prior method of median pricing, which caused financial difficulty and access concerns (Council for Quality Respiratory Care. The Rationale for Reforming Medicare Home Respiratory Therapy Payment Methodology. 2018). Budget neutrality requirements should relax, and oxygen payment structures improve. These proposed changes also include improved coverage of liquid oxygen and addition of home ventilator supplies.
However, effective January 1, 2019, all CBP is suspended through CMS. During the anticipated 2-year gap, any Medicare-enrolled supplier will be able to provide items until new contracts are awarded. Pricing during the gap period is based on a current single price plus consumer price index. These changes will impact CHEST members and their patients moving forward. During the temporary gap period, some areas are seeing decreased accessibility of some DME due to demand. Once reinstated, the changes to the oxygen payment structure should improve access and reduce out-of-pocket costs. The Practice Operations NetWork will continue to provide updates on this topic as they become available.
Timothy Dempsey, MD, MPH
Steering Committee Fellow-in-Training
Megan Sisk, DO
Steering Committee Member
Transplant
Medicare Part D Plans Can Deny Coverage of Select Immunosuppressant Medications in Solid Organ Transplant Recipients
An alarming problem has emerged with some solid organ transplant recipients experiencing immunosuppressant medication claim denials by Medicare Part D plans. Affected patients are those who convert from some other insurance (ie, private insurance or state Medicaid) to Medicare after their transplant and, therefore, rely on Medicare Part D for immunosuppressant drug coverage.
Insurance companies who offer Medicare Part D plans must follow the rules described in the Medicare Prescription Drug Benefit Manual.1 Although the Manual mandates that all immunosuppressant medications are on plan formularies, Part D plans are only required to cover immunosuppressant medications when used for indications approved by the Food and Drug Administration (FDA) or for off-label indications supported by the Centers for Medicare & Medicaid Services (CMS)-approved compendia (Drugdex® and AHFS Drug Information®).
A recent study examining the extent of the problem demonstrated non-renal organ transplant recipients are frequently prescribed and maintained on at least one medication vulnerable to Medicare Part D claim denials at 1 year posttransplant (lung: 71.1%; intestine: 39.7%; pancreas: 36.8%; liver: 19.7%; heart: 18.5%).2 Lung transplant recipients are most vulnerable since no immunosuppressant is FDA-approved for use in lung transplantation, and CMS-approved compendia only support off-label use for tacrolimus and cyclosporine in this population. Therefore, mycophenolate mofetil, mycophenolic acid, azathioprine, everolimus, and sirolimus are vulnerable to denial by Medicare Part D plans when used in lung transplant recipients. Over 95% of lung transplant recipients are maintained on an anti-metabolite, with the majority (88%) maintained on mycophenolate, so this is frequently impacted.2,3 While the transplant community is aware of this issue and has begun work to correct it, it has yet to be solved.2,4 In the meantime, if transplant recipients have been denied for this off-label and off-compendia reason, and appeals of those decisions have also been denied, options for obtaining the denied immunosuppressant medication include discount programs, foundation/grant funding, and industry-sponsored assistance programs.
Jennifer K. McDermott, PharmD
NetWork Member
1. Prescription Drug Benefit Manual. Centers for Medicare & Medicaid Services. Chapter 6: Part D Drugs and Formulary Requirements. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Part-D-Benefits-Manual-Chapter-6.pdf
2. Potter LM et al. Transplant recipients are vulnerable to coverage denial under Medicare Part D. Am J Transplant. 2018;18:1502.
3. Valapour M et al. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant. 2018;18 (Suppl 1): 363.
4. Immunusuppressant Drug Coverage Under Medicare Part D Benefit. American Society of Transplantation. Available at: www.myast.org/public-policy/key-position-statements/immunosuppressant-drug-coverage-under-medicare-part-d-benefit.
Women’s Health
Cannabis Use Affects Women Differently
As we enter an era of legalization, cannabis use is increasingly prevalent. Variances in the risks for women and men have been observed. For most age groups, men have higher rates of use or dependence on illicit drugs than women. However, women are equally likely as men to progress to a substance use disorder. Women may be more susceptible to craving and relapse , which are key phases of the addiction cycle. A study on use among adolescents concluded there was preliminary evidence of a faster transition from initiation of marijuana use to regular use in women, when compared with men (Schepis, et al. J Addict Med. 2011;5[1]:65).
Research studies suggest that marijuana impairs spatial memory in women more so than in men. Studies have suggested that teenage girls who use marijuana may have a higher risk of brain structural abnormalities associated with regular marijuana exposure than teenage boys (Tapert, et al. Addict Biol. 2009;14[4]:457).
A study published in Psychoneuroendocrinology showed that cannabinoid receptor binding site densities exhibit sex differences and can be modulated by estradiol in several limbic brain regions. These findings may account for the sex differences observed with respect to the effects of cannabinoids (Riebe, et al. Psychoneuroendocrinology. 2010;35[8]:1265).
Further research is needed to expand our understanding of the interactions between cannabinoids and sex steroids. Detoxification treatments tailored toward women and men with cannabis addiction show a promising future and necessitate further research.
Anita Rajagopal, MD
Steering Committee Member
CHEST Foundation’s NetWorks Challenge is just around the corner
The NetWorks Challenge is an annual fundraising competition that encourages NetWork members to contribute to the CHEST Foundation - supporting clinical research grants and community service programs and creating patient education materials - while earning travel grants for their NetWork members to the CHEST Annual Meeting 2019 in New Orleans. Because of your generosity throughout the 2018 NetWorks Challenge, the CHEST Foundation was able to send 59 early career clinicians to CHEST 2018 in San Antonio - marked growth from the 25 clinicians who received the travel grants in 2017.
As we further improve this program based on feedback from NetWorks members, a few elements of the fundraiser are changing in 2019.
Length: This year, the NetWorks Challenge will span 3 months. Contributions made between April 1 and June 30 count toward your NetWork’s fundraising total! Just be sure to list your NetWork when making your contribution on chestfoundation.org/donate. Each month has a unique theme related to CHEST, so be sure to watch our social media profiles to engage with us and each other during the drive.
Additionally, ANY contributions made to the CHEST Foundation during your membership renewal will count toward your NetWorks total amount raised - no matter when your membership is up for renewal. Contributions made in this manner after June 30 will count toward your Network’s 2020 amount raised.
Prizes: This year, every NetWork is eligible to receive travel grants to CHEST 2019 in New Orleans based on the amount raised by the NetWork. Our final winners – the NetWork with the highest amount raised, and the NetWork with the highest percentage of participation from their NetWork, will each receive two additional travel grants to CHEST 2019. Plus, the NetWork with the highest amount raised over the course of the challenge receives an additional prize – a seat in a CHEST Live Learning course of the winner’s choosing, offered at CHEST’s Innovation, Simulation, and Training Center in Glenview, Illinois.
Visit chestfoundation.org/nc for more detailed information.
The NetWorks Challenge is an annual fundraising competition that encourages NetWork members to contribute to the CHEST Foundation - supporting clinical research grants and community service programs and creating patient education materials - while earning travel grants for their NetWork members to the CHEST Annual Meeting 2019 in New Orleans. Because of your generosity throughout the 2018 NetWorks Challenge, the CHEST Foundation was able to send 59 early career clinicians to CHEST 2018 in San Antonio - marked growth from the 25 clinicians who received the travel grants in 2017.
As we further improve this program based on feedback from NetWorks members, a few elements of the fundraiser are changing in 2019.
Length: This year, the NetWorks Challenge will span 3 months. Contributions made between April 1 and June 30 count toward your NetWork’s fundraising total! Just be sure to list your NetWork when making your contribution on chestfoundation.org/donate. Each month has a unique theme related to CHEST, so be sure to watch our social media profiles to engage with us and each other during the drive.
Additionally, ANY contributions made to the CHEST Foundation during your membership renewal will count toward your NetWorks total amount raised - no matter when your membership is up for renewal. Contributions made in this manner after June 30 will count toward your Network’s 2020 amount raised.
Prizes: This year, every NetWork is eligible to receive travel grants to CHEST 2019 in New Orleans based on the amount raised by the NetWork. Our final winners – the NetWork with the highest amount raised, and the NetWork with the highest percentage of participation from their NetWork, will each receive two additional travel grants to CHEST 2019. Plus, the NetWork with the highest amount raised over the course of the challenge receives an additional prize – a seat in a CHEST Live Learning course of the winner’s choosing, offered at CHEST’s Innovation, Simulation, and Training Center in Glenview, Illinois.
Visit chestfoundation.org/nc for more detailed information.
The NetWorks Challenge is an annual fundraising competition that encourages NetWork members to contribute to the CHEST Foundation - supporting clinical research grants and community service programs and creating patient education materials - while earning travel grants for their NetWork members to the CHEST Annual Meeting 2019 in New Orleans. Because of your generosity throughout the 2018 NetWorks Challenge, the CHEST Foundation was able to send 59 early career clinicians to CHEST 2018 in San Antonio - marked growth from the 25 clinicians who received the travel grants in 2017.
As we further improve this program based on feedback from NetWorks members, a few elements of the fundraiser are changing in 2019.
Length: This year, the NetWorks Challenge will span 3 months. Contributions made between April 1 and June 30 count toward your NetWork’s fundraising total! Just be sure to list your NetWork when making your contribution on chestfoundation.org/donate. Each month has a unique theme related to CHEST, so be sure to watch our social media profiles to engage with us and each other during the drive.
Additionally, ANY contributions made to the CHEST Foundation during your membership renewal will count toward your NetWorks total amount raised - no matter when your membership is up for renewal. Contributions made in this manner after June 30 will count toward your Network’s 2020 amount raised.
Prizes: This year, every NetWork is eligible to receive travel grants to CHEST 2019 in New Orleans based on the amount raised by the NetWork. Our final winners – the NetWork with the highest amount raised, and the NetWork with the highest percentage of participation from their NetWork, will each receive two additional travel grants to CHEST 2019. Plus, the NetWork with the highest amount raised over the course of the challenge receives an additional prize – a seat in a CHEST Live Learning course of the winner’s choosing, offered at CHEST’s Innovation, Simulation, and Training Center in Glenview, Illinois.
Visit chestfoundation.org/nc for more detailed information.
Meet the CHEST President-Designate
Steven Q. Simpson, MD, FCCP, is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement. Dr. Simpson acts as a CHEST Regent-at-Large of the Board of Regents, board liaison for the Guidelines Oversight Committee, sits on numerous board task forces and subcommittees and is a member of the CHEST SEEK Critical Care Medicine Editorial Board. He will serve as CHEST President for the 2020-2021 term.
Dr. Simpson is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services. He has conducted research in all areas of severe sepsis, including molecular and cellular mechanisms, translational, quality improvement, and computer modeling studies. He was a founder in 2005 of the Midwest Critical Care Collaborative, a multidisciplinary and interprofessional collaborative effort to improve the quality of critical care services throughout the Midwest. In 2007, he initiated the Kansas Sepsis Project, a statewide program to improve severe sepsis care and outcomes via continuing education both in sepsis and in quality improvement principles and via interprofessional collaborations. Dr. Simpson is an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. He is a member of the board of directors and Chief Medical Officer of Sepsis Alliance, a nationwide patient information and advocacy organization.
During his tenure at the University of New Mexico, he contributed to the discovery of a particular form of sepsis, the hantavirus pulmonary syndrome, and published numerous papers on the clinical description, the hemodynamic description, and the approach to supportive care for patients with the syndrome, including extracorporeal hemodynamic and oxygenation support. Dr. Simpson has authored over 180 scientific articles, book chapters, editorials, abstracts and electronic media publications. He was awarded the 2009 Eli Lilly Distinguished Scholar in Critical Care Medicine Award of the American College of Chest Physicians and the 2013 Roger C. Bone Memorial Lecture in Critical Care Medicine, which recognizes career contributions to the field. He has also been recognized as a Distinguished CHEST Educator in 2017 and 2018.
Steven Q. Simpson, MD, FCCP, is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement. Dr. Simpson acts as a CHEST Regent-at-Large of the Board of Regents, board liaison for the Guidelines Oversight Committee, sits on numerous board task forces and subcommittees and is a member of the CHEST SEEK Critical Care Medicine Editorial Board. He will serve as CHEST President for the 2020-2021 term.
Dr. Simpson is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services. He has conducted research in all areas of severe sepsis, including molecular and cellular mechanisms, translational, quality improvement, and computer modeling studies. He was a founder in 2005 of the Midwest Critical Care Collaborative, a multidisciplinary and interprofessional collaborative effort to improve the quality of critical care services throughout the Midwest. In 2007, he initiated the Kansas Sepsis Project, a statewide program to improve severe sepsis care and outcomes via continuing education both in sepsis and in quality improvement principles and via interprofessional collaborations. Dr. Simpson is an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. He is a member of the board of directors and Chief Medical Officer of Sepsis Alliance, a nationwide patient information and advocacy organization.
During his tenure at the University of New Mexico, he contributed to the discovery of a particular form of sepsis, the hantavirus pulmonary syndrome, and published numerous papers on the clinical description, the hemodynamic description, and the approach to supportive care for patients with the syndrome, including extracorporeal hemodynamic and oxygenation support. Dr. Simpson has authored over 180 scientific articles, book chapters, editorials, abstracts and electronic media publications. He was awarded the 2009 Eli Lilly Distinguished Scholar in Critical Care Medicine Award of the American College of Chest Physicians and the 2013 Roger C. Bone Memorial Lecture in Critical Care Medicine, which recognizes career contributions to the field. He has also been recognized as a Distinguished CHEST Educator in 2017 and 2018.
Steven Q. Simpson, MD, FCCP, is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement. Dr. Simpson acts as a CHEST Regent-at-Large of the Board of Regents, board liaison for the Guidelines Oversight Committee, sits on numerous board task forces and subcommittees and is a member of the CHEST SEEK Critical Care Medicine Editorial Board. He will serve as CHEST President for the 2020-2021 term.
Dr. Simpson is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services. He has conducted research in all areas of severe sepsis, including molecular and cellular mechanisms, translational, quality improvement, and computer modeling studies. He was a founder in 2005 of the Midwest Critical Care Collaborative, a multidisciplinary and interprofessional collaborative effort to improve the quality of critical care services throughout the Midwest. In 2007, he initiated the Kansas Sepsis Project, a statewide program to improve severe sepsis care and outcomes via continuing education both in sepsis and in quality improvement principles and via interprofessional collaborations. Dr. Simpson is an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. He is a member of the board of directors and Chief Medical Officer of Sepsis Alliance, a nationwide patient information and advocacy organization.
During his tenure at the University of New Mexico, he contributed to the discovery of a particular form of sepsis, the hantavirus pulmonary syndrome, and published numerous papers on the clinical description, the hemodynamic description, and the approach to supportive care for patients with the syndrome, including extracorporeal hemodynamic and oxygenation support. Dr. Simpson has authored over 180 scientific articles, book chapters, editorials, abstracts and electronic media publications. He was awarded the 2009 Eli Lilly Distinguished Scholar in Critical Care Medicine Award of the American College of Chest Physicians and the 2013 Roger C. Bone Memorial Lecture in Critical Care Medicine, which recognizes career contributions to the field. He has also been recognized as a Distinguished CHEST Educator in 2017 and 2018.
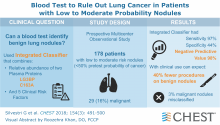
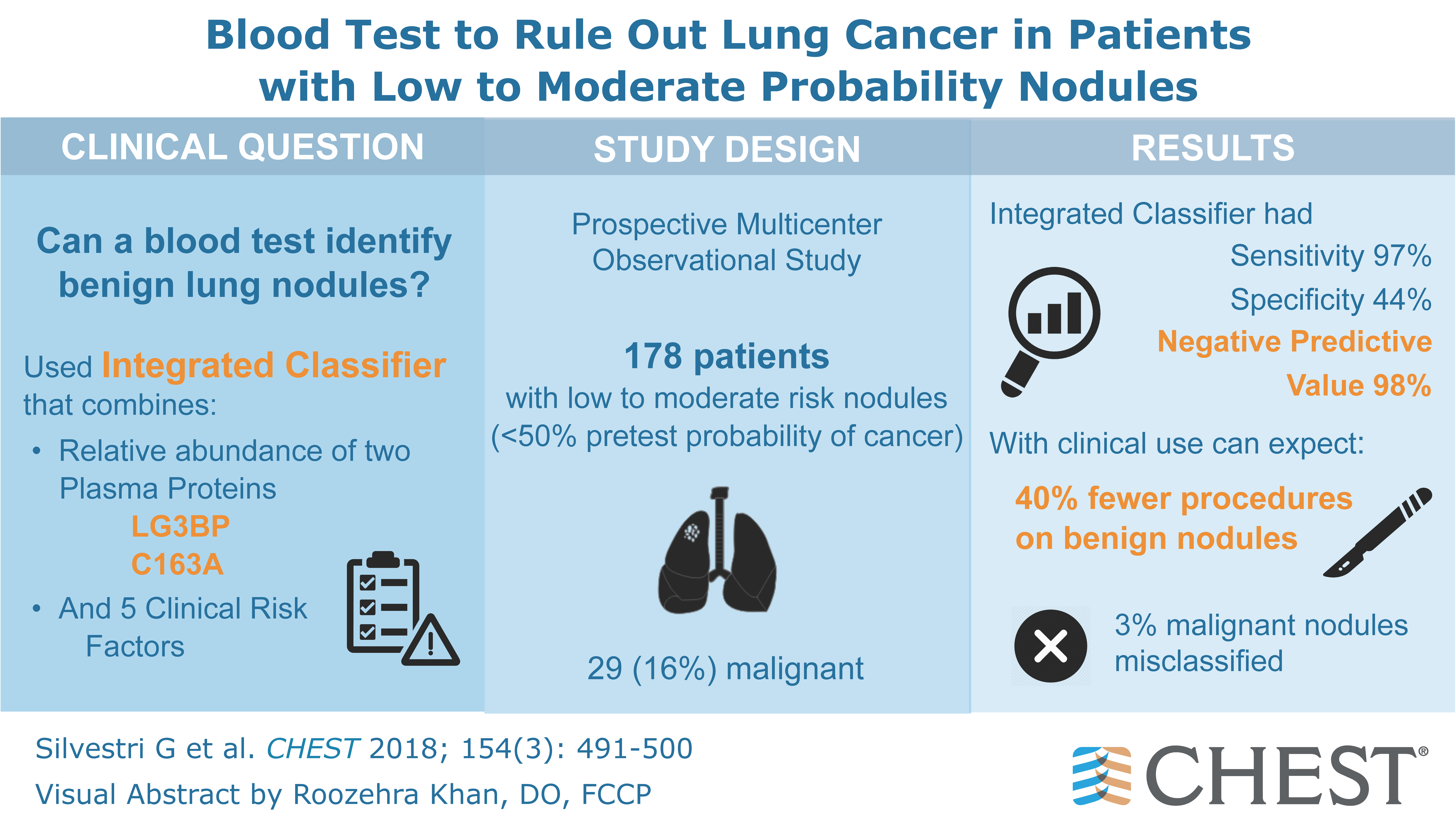
Visual abstracts enhance journal readers’ experience
Physicians’ time is decreasingly their own, and, yet, keeping abreast of clinical literature is increasingly more important. The journal CHEST has introduced a new feature aimed at easing that task and broadening the reach of journal content: visual abstracts.
At the direction of CHEST Editor in Chief Richard Irwin, MD, Master FCCP, Dr. Carroll assembled a team to help carry out an ambitious multimedia strategy (see box). Dr. Irwin charged the Web and Multimedia editorial team with not only extending the reach of journal content but also enhancing readers’ engagement with and understanding of it.
“Our first project was the development of visual abstracts, a type of infographics used to distill the key points of a research abstract into an easily digested graphic form,” says Dr. Carroll, who also is research director of pediatric critical care at Connecticut Children’s Medical Center, Hartford, and a professor of pediatrics at the University of Connecticut School of Medicine, Farmington.
The first visual abstracts were posted to accompany two articles in the July 2018 issue of CHEST. With the exception of August 2018, every issue since has been enhanced with infographics. Insert infographic here (A full gallery of all the visual abstracts so far is available at https://journal.chestnet.org/infographics.) The visual abstracts are available through a number of vehicles: the journal’s website (https://journal.chestnet.org/), the journal’s mobile app (https://journal.chestnet.org/content/mobileaccessinstructions), and social media platforms such as Facebook (https://www.facebook.com/accpchest/) and Twitter (https://twitter.com/accpchest).
“Our goal with the infographics is to promote the exciting research CHEST publishes and to get readers to click through and read the entire article,” says Dr. Carroll. “So far, we’re happy with our results—and we’re looking forward to even greater reach in 2019.”
CHEST Web and Multimedia Section
Editor
Christopher Carroll, MD, MS
Assistant Editors
Yonatan Y. Greenstein, MD, FCCP, Newark, NJ
Roozehra Khan, DO, FCCP, Los Angeles, CA
Dominique J. Pepper, MD, MBChB, MHSc, Bethesda, MD.
###
Physicians’ time is decreasingly their own, and, yet, keeping abreast of clinical literature is increasingly more important. The journal CHEST has introduced a new feature aimed at easing that task and broadening the reach of journal content: visual abstracts.
At the direction of CHEST Editor in Chief Richard Irwin, MD, Master FCCP, Dr. Carroll assembled a team to help carry out an ambitious multimedia strategy (see box). Dr. Irwin charged the Web and Multimedia editorial team with not only extending the reach of journal content but also enhancing readers’ engagement with and understanding of it.
“Our first project was the development of visual abstracts, a type of infographics used to distill the key points of a research abstract into an easily digested graphic form,” says Dr. Carroll, who also is research director of pediatric critical care at Connecticut Children’s Medical Center, Hartford, and a professor of pediatrics at the University of Connecticut School of Medicine, Farmington.
The first visual abstracts were posted to accompany two articles in the July 2018 issue of CHEST. With the exception of August 2018, every issue since has been enhanced with infographics. Insert infographic here (A full gallery of all the visual abstracts so far is available at https://journal.chestnet.org/infographics.) The visual abstracts are available through a number of vehicles: the journal’s website (https://journal.chestnet.org/), the journal’s mobile app (https://journal.chestnet.org/content/mobileaccessinstructions), and social media platforms such as Facebook (https://www.facebook.com/accpchest/) and Twitter (https://twitter.com/accpchest).
“Our goal with the infographics is to promote the exciting research CHEST publishes and to get readers to click through and read the entire article,” says Dr. Carroll. “So far, we’re happy with our results—and we’re looking forward to even greater reach in 2019.”
CHEST Web and Multimedia Section
Editor
Christopher Carroll, MD, MS
Assistant Editors
Yonatan Y. Greenstein, MD, FCCP, Newark, NJ
Roozehra Khan, DO, FCCP, Los Angeles, CA
Dominique J. Pepper, MD, MBChB, MHSc, Bethesda, MD.
###
Physicians’ time is decreasingly their own, and, yet, keeping abreast of clinical literature is increasingly more important. The journal CHEST has introduced a new feature aimed at easing that task and broadening the reach of journal content: visual abstracts.
At the direction of CHEST Editor in Chief Richard Irwin, MD, Master FCCP, Dr. Carroll assembled a team to help carry out an ambitious multimedia strategy (see box). Dr. Irwin charged the Web and Multimedia editorial team with not only extending the reach of journal content but also enhancing readers’ engagement with and understanding of it.
“Our first project was the development of visual abstracts, a type of infographics used to distill the key points of a research abstract into an easily digested graphic form,” says Dr. Carroll, who also is research director of pediatric critical care at Connecticut Children’s Medical Center, Hartford, and a professor of pediatrics at the University of Connecticut School of Medicine, Farmington.
The first visual abstracts were posted to accompany two articles in the July 2018 issue of CHEST. With the exception of August 2018, every issue since has been enhanced with infographics. Insert infographic here (A full gallery of all the visual abstracts so far is available at https://journal.chestnet.org/infographics.) The visual abstracts are available through a number of vehicles: the journal’s website (https://journal.chestnet.org/), the journal’s mobile app (https://journal.chestnet.org/content/mobileaccessinstructions), and social media platforms such as Facebook (https://www.facebook.com/accpchest/) and Twitter (https://twitter.com/accpchest).
“Our goal with the infographics is to promote the exciting research CHEST publishes and to get readers to click through and read the entire article,” says Dr. Carroll. “So far, we’re happy with our results—and we’re looking forward to even greater reach in 2019.”
CHEST Web and Multimedia Section
Editor
Christopher Carroll, MD, MS
Assistant Editors
Yonatan Y. Greenstein, MD, FCCP, Newark, NJ
Roozehra Khan, DO, FCCP, Los Angeles, CA
Dominique J. Pepper, MD, MBChB, MHSc, Bethesda, MD.
###
Five steering committees examine the literature
Airways Disorders
Defining and treating early COPD: Can we make a difference?
There is growing evidence that early COPD—before currently accepted spirometric or symptomatic criteria are present—may be an important clinical entity. The primary pathobiologic mechanisms in early COPD development include both abnormal lung development and accelerated lung aging (Augustí et al. Am J Respir Crit Care Med. 2018 Oct 15;198:8:978).
Martinez and colleagues recently proposed defining early COPD as age <50 with 10+ pack-year smoking history and at least one of the following: (1) early airflow limitation (postbronchodilator FEV1/FVC < lower limit of normal), (2) compatible CT scan abnormalities, (3) rapid decline in FEV1 (≥60 mL/yr) that is accelerated relative to FVC (Martinez et al. Am J Respir Crit Care Med. 2018 Jun 15;197[12]:1540).
A novel multiresolution CT scan imaging protocol described by Koo and coworkers found that substantial loss of small airways— specifically the terminal and transitional bronchioles—occurs in patients with mild-to-moderate COPD even prior to the development of emphysema on CT scan. These findings show that significant destruction of the small airways has occurred prior to the development of mild COPD (Koo et al. Lancet Respir Med. 2018 Aug;6:591).
Pharmacologic treatment for COPD is targeted at the reduction of symptoms and risk of exacerbation, as there remains no conclusive evidence that existing therapies modify long-term decline in lung function. It is unknown if pharmacotherapy for “early COPD” will alter the disease course. While not directly addressing this subset, information may be gleaned from trials on younger, more mild GOLD Stage 1 or Stage 2 patients. The Tie-COPD trial, the largest powered study to date of mild-to-moderate COPD, found that among patients with GOLD stage 1 or 2 COPD treatment with tiotropium compared with placebo for 2 years resulted in significantly higher FEV1 before bronchodilator use (between group difference of 157 mL) and slowed annual decline in FEV1 after bronchodilator use (Zhou et al. N Engl J Med. 2017 Sep 7;377[10]:923).
As our understanding of heterogeneity within COPD increases, striving for improved outcomes from our therapies—an impact on lung function in addition to symptom and exacerbation risk—may need to begin with the study of earlier treatment.
Megan Conroy, MD
Steering Committee Fellow-in-Training
Allen J. Blaivas, DO, FCCP
Steering Committee Vice-Chair
Clinical Pulmonary Medicine
Asthma-COPD overlap: An underappreciated phenotype of obstructive airway disease (OAD)
Asthma-COPD overlap (ACO) is a common yet underappreciated clinical entity within the complex OAD spectrum. Currently, there is no consensus criteria to define ACO; however, a roundtable consensus from an international group (Sin et al. Eur Respir J. 2016 Sep;48:664) suggests using major and minor criteria, with key features being airflow limitation, asthma history, and cigarette or biomass exposure. Several studies have shown that patients with ACO have severe disease, faster lung function decline, greater morbidity and mortality, and lower QoL (Alshabanat et al. PLoS One. 2015 Sep 3;10:e0136065).
There is paucity of data on the pathophysiology, risk factors, and clinical management given exclusion of these patients from clinical trials of asthma and COPD. Indeed, clinicians and researchers now realize that ACO is an umbrella term for multiple subphenotypes, including patients who have predominant asthma with some COPD features and others with predominant COPD with some asthma features. Overall, IgE level, FeNO, sputum, and blood eosinophils are usually higher in ACO than in COPD and relatively similar compared with asthma (Kobayashi et al. Int J Chron Obs Pulmon Dis. 2016 May 26;11:2117).
Most recently, a longitudinal study looked at predictors of ACO among NY firefighters exposed to WTC dust (Singh et al. CHEST. 2018 Dec;154[6]:1301). Pre-exposure low lung function and elevated blood eosinophils and IL4 (T2 inflammatory cytokine) increased risk of developing ACO among those exposed to WTC dust. Further research is required to better understand the interaction of environmental exposure and risk factors in the pathophysiology of ACO. It may be more pragmatic to use the unifying term OAD, as originally proposed in the Dutch hypothesis, and further delineate how several phenotypes of airway disease can be classified by combining traditional approaches with molecular and genomic analysis.
Munish Luthra, MD, FCCP
Steering Committee Member
Samantha D’Annunzio, MD
Steering Committee Member
Critical Care
Mechanical ventilation: One size fits all?
Mechanical ventilation (MV) is a lifesaving intervention in the ICU, but it has been associated with numerous complications ranging from overuse of sedation, atelectasis, and baro or volutrauma.
After 2000, it became well known that using a low tidal volume (VT) strategy (6 mL/kg predicted body weight, PBW) in patients with ARDS produced lower mortality and more ventilator-free days (N Engl J Med. 2000 May 4;342[18]:1301). In addition, a meta-analysis in 2012 demonstrated a lower relative risk of new lung injury, mortality, and pulmonary infections with low VT in non-ARDS patients (Serpa et al. JAMA. 2012 Oct 24/31;308[16]:1651). However, the included studies varied widely in their use of VT (9-12 mL/kg), duration of MV, and in mixed settings (ICU or operating room).
Recently, a large randomized clinical trial compared the effect of low (4-6 mL/kg, PBW) vs intermediate (8-10 mL/kg, PBW) VT ventilation strategy in non-ARDS ICU patients. Interestingly, the study concluded that there is no significant difference in ventilator-free days (21 days in each group), median length ICU and hospital stay, ICU mortality rates, and 28- and 90-day mortality. Also, there was no difference in new-onset ARDS, severe atelectasis, sedation use, and delirium (JAMA. 2018; 320[18]:1872). This study suggests that in non-ARDS patients, MV should be individualized according to each patient’s clinical situation, the nature of the disease, and its effect on lung mechanics, especially in patients who cannot tolerate low tidal volumes.
Margaret A. Disselkamp, MD
Steering Committee Member
Mohammed A. Megri, MD
Fellow-in-Training Steering Committee Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Improving access to sleep medicine care for patients with NMD
Sleep-disordered breathing (SDB) occurs in up to 5% of children, with adverse implications for growth and development. Children with neuromuscular disease are at significantly higher risk than unaffected children (Chiang et al. Children. 2018;5:e78). Respiratory dysfunction that may present as SDB before daytime impairment in gas exchange is evident. Diagnosing and treating SDB (to include OSA, CSA, and hypoventilation syndromes) early can significantly improve morbidity and mortality.
Unfortunately, diagnostic sleep medicine resources are limited. Children may wait up to a year or more for definitive testing with in-laboratory, attended polysomnography (PSG). Among children with neuromuscular disease, fewer than 10% may undergo a sleep clinic evaluation, and, of those that do, they may have only one visit over a 3-year period of care (Rose et al. Pediatr Pulmonol. 2018 Oct;53:1378). Home sleep testing (HST) has been evaluated as an alternative to PSG given lower cost, availability, and advantage of the child sleeping in his/her own bed. Although HST is indicated in adults with a high pretest probability for moderate to severe OSA, it is not indicated in children, given the potential to underestimate disease severity or to miss the diagnosis entirely (Kirk et al. J Clin Sleep Med. 2017 Oct 15;13[10]:1199). HST lacks electroencephalogram (EEG) and capnography. Technical recording mishaps are more common in children, but in-lab PSG has the advantage of on-site troubleshooting by a technologist.
A recently published study by Fishman and colleagues attempted to compare gold standard in-lab PSG to HST with capnography (Fishman et al. J Clin Sleep Med. 2018 Dec 15;14[12]:2013). Despite a well-designed study with a carefully selected population, HST failed to reliably diagnose SDB. HST underestimated disease severity and, in some cases, missed the diagnosis of SDB entirely. The addition of end tidal CO2 monitoring failed to improve diagnostic accuracy, and HST and PSG-ETco2 values were poorly correlated.
Although children with neuromuscular disease face long wait times for sleep evaluations, HST is clearly not the solution for now. It remains to be seen if innovations in HST with extended monitoring (and transcutaneous CO2) become viable. In the meantime, finding ways to improve access to sleep medicine care for children with neuromuscular disease is a must.
Jacob Collen, MD, FCCP
Steering Committee Member
Interstitial and Diffuse Lung Disease
Idiopathic pneumonias that are not all that idiopathic
Despite being defined as an individual entity for research purposes in 2015 (Fisher et al. Eur Respir J. 2015;46:976), interstitial pneumonias with autoimmune features (IPAF) remain a heterogeneous group of interstitial lung diseases that puzzle the clinician. Since the introduction of the IPAF definition, there have been attempts to validate the diagnostic criteria and study their prognostic implications. Some of these studies showed differential prognosis in patients who met the IPAF criteria (Oldham et al. Eur Respir J. 2016;47:1767).
Although the implications of the presence of autoimmune antibodies in idiopathic interstitial pneumonias (IIPs) is not fully understood, the treatment often entails immunosuppression, especially in those with non-UIP patterns of disease and/or clinical features of autoimmune disease. The stakes are high when IIPs are associated with antibodies correlated with rapidly progressive disease, such as MDA-5 antibody or antisynthetase antibodies. Pulmonologists often lack the clinical expertise to detect occult autoimmune disorders, though the role of the rheumatologist in facilitating the diagnosis and treatment of IPAF is not well delineated. Most health-care systems are not equipped with collaborative ILD-rheumatology clinics or even easy access to a rheumatologist. There is a need for real-world pragmatic studies to establish the optimal way to evaluate patients with ILD for autoimmune features and identify patients who would benefit most from an early referral to rheumatology to aid with diagnosis, treatment, and sometimes monitoring for extrapulmonary manifestations of autoimmune disorders.
Avanthika Thanushi Wynn, MD
Steering Committee Fellow-in-Training
Airways Disorders
Defining and treating early COPD: Can we make a difference?
There is growing evidence that early COPD—before currently accepted spirometric or symptomatic criteria are present—may be an important clinical entity. The primary pathobiologic mechanisms in early COPD development include both abnormal lung development and accelerated lung aging (Augustí et al. Am J Respir Crit Care Med. 2018 Oct 15;198:8:978).
Martinez and colleagues recently proposed defining early COPD as age <50 with 10+ pack-year smoking history and at least one of the following: (1) early airflow limitation (postbronchodilator FEV1/FVC < lower limit of normal), (2) compatible CT scan abnormalities, (3) rapid decline in FEV1 (≥60 mL/yr) that is accelerated relative to FVC (Martinez et al. Am J Respir Crit Care Med. 2018 Jun 15;197[12]:1540).
A novel multiresolution CT scan imaging protocol described by Koo and coworkers found that substantial loss of small airways— specifically the terminal and transitional bronchioles—occurs in patients with mild-to-moderate COPD even prior to the development of emphysema on CT scan. These findings show that significant destruction of the small airways has occurred prior to the development of mild COPD (Koo et al. Lancet Respir Med. 2018 Aug;6:591).
Pharmacologic treatment for COPD is targeted at the reduction of symptoms and risk of exacerbation, as there remains no conclusive evidence that existing therapies modify long-term decline in lung function. It is unknown if pharmacotherapy for “early COPD” will alter the disease course. While not directly addressing this subset, information may be gleaned from trials on younger, more mild GOLD Stage 1 or Stage 2 patients. The Tie-COPD trial, the largest powered study to date of mild-to-moderate COPD, found that among patients with GOLD stage 1 or 2 COPD treatment with tiotropium compared with placebo for 2 years resulted in significantly higher FEV1 before bronchodilator use (between group difference of 157 mL) and slowed annual decline in FEV1 after bronchodilator use (Zhou et al. N Engl J Med. 2017 Sep 7;377[10]:923).
As our understanding of heterogeneity within COPD increases, striving for improved outcomes from our therapies—an impact on lung function in addition to symptom and exacerbation risk—may need to begin with the study of earlier treatment.
Megan Conroy, MD
Steering Committee Fellow-in-Training
Allen J. Blaivas, DO, FCCP
Steering Committee Vice-Chair
Clinical Pulmonary Medicine
Asthma-COPD overlap: An underappreciated phenotype of obstructive airway disease (OAD)
Asthma-COPD overlap (ACO) is a common yet underappreciated clinical entity within the complex OAD spectrum. Currently, there is no consensus criteria to define ACO; however, a roundtable consensus from an international group (Sin et al. Eur Respir J. 2016 Sep;48:664) suggests using major and minor criteria, with key features being airflow limitation, asthma history, and cigarette or biomass exposure. Several studies have shown that patients with ACO have severe disease, faster lung function decline, greater morbidity and mortality, and lower QoL (Alshabanat et al. PLoS One. 2015 Sep 3;10:e0136065).
There is paucity of data on the pathophysiology, risk factors, and clinical management given exclusion of these patients from clinical trials of asthma and COPD. Indeed, clinicians and researchers now realize that ACO is an umbrella term for multiple subphenotypes, including patients who have predominant asthma with some COPD features and others with predominant COPD with some asthma features. Overall, IgE level, FeNO, sputum, and blood eosinophils are usually higher in ACO than in COPD and relatively similar compared with asthma (Kobayashi et al. Int J Chron Obs Pulmon Dis. 2016 May 26;11:2117).
Most recently, a longitudinal study looked at predictors of ACO among NY firefighters exposed to WTC dust (Singh et al. CHEST. 2018 Dec;154[6]:1301). Pre-exposure low lung function and elevated blood eosinophils and IL4 (T2 inflammatory cytokine) increased risk of developing ACO among those exposed to WTC dust. Further research is required to better understand the interaction of environmental exposure and risk factors in the pathophysiology of ACO. It may be more pragmatic to use the unifying term OAD, as originally proposed in the Dutch hypothesis, and further delineate how several phenotypes of airway disease can be classified by combining traditional approaches with molecular and genomic analysis.
Munish Luthra, MD, FCCP
Steering Committee Member
Samantha D’Annunzio, MD
Steering Committee Member
Critical Care
Mechanical ventilation: One size fits all?
Mechanical ventilation (MV) is a lifesaving intervention in the ICU, but it has been associated with numerous complications ranging from overuse of sedation, atelectasis, and baro or volutrauma.
After 2000, it became well known that using a low tidal volume (VT) strategy (6 mL/kg predicted body weight, PBW) in patients with ARDS produced lower mortality and more ventilator-free days (N Engl J Med. 2000 May 4;342[18]:1301). In addition, a meta-analysis in 2012 demonstrated a lower relative risk of new lung injury, mortality, and pulmonary infections with low VT in non-ARDS patients (Serpa et al. JAMA. 2012 Oct 24/31;308[16]:1651). However, the included studies varied widely in their use of VT (9-12 mL/kg), duration of MV, and in mixed settings (ICU or operating room).
Recently, a large randomized clinical trial compared the effect of low (4-6 mL/kg, PBW) vs intermediate (8-10 mL/kg, PBW) VT ventilation strategy in non-ARDS ICU patients. Interestingly, the study concluded that there is no significant difference in ventilator-free days (21 days in each group), median length ICU and hospital stay, ICU mortality rates, and 28- and 90-day mortality. Also, there was no difference in new-onset ARDS, severe atelectasis, sedation use, and delirium (JAMA. 2018; 320[18]:1872). This study suggests that in non-ARDS patients, MV should be individualized according to each patient’s clinical situation, the nature of the disease, and its effect on lung mechanics, especially in patients who cannot tolerate low tidal volumes.
Margaret A. Disselkamp, MD
Steering Committee Member
Mohammed A. Megri, MD
Fellow-in-Training Steering Committee Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Improving access to sleep medicine care for patients with NMD
Sleep-disordered breathing (SDB) occurs in up to 5% of children, with adverse implications for growth and development. Children with neuromuscular disease are at significantly higher risk than unaffected children (Chiang et al. Children. 2018;5:e78). Respiratory dysfunction that may present as SDB before daytime impairment in gas exchange is evident. Diagnosing and treating SDB (to include OSA, CSA, and hypoventilation syndromes) early can significantly improve morbidity and mortality.
Unfortunately, diagnostic sleep medicine resources are limited. Children may wait up to a year or more for definitive testing with in-laboratory, attended polysomnography (PSG). Among children with neuromuscular disease, fewer than 10% may undergo a sleep clinic evaluation, and, of those that do, they may have only one visit over a 3-year period of care (Rose et al. Pediatr Pulmonol. 2018 Oct;53:1378). Home sleep testing (HST) has been evaluated as an alternative to PSG given lower cost, availability, and advantage of the child sleeping in his/her own bed. Although HST is indicated in adults with a high pretest probability for moderate to severe OSA, it is not indicated in children, given the potential to underestimate disease severity or to miss the diagnosis entirely (Kirk et al. J Clin Sleep Med. 2017 Oct 15;13[10]:1199). HST lacks electroencephalogram (EEG) and capnography. Technical recording mishaps are more common in children, but in-lab PSG has the advantage of on-site troubleshooting by a technologist.
A recently published study by Fishman and colleagues attempted to compare gold standard in-lab PSG to HST with capnography (Fishman et al. J Clin Sleep Med. 2018 Dec 15;14[12]:2013). Despite a well-designed study with a carefully selected population, HST failed to reliably diagnose SDB. HST underestimated disease severity and, in some cases, missed the diagnosis of SDB entirely. The addition of end tidal CO2 monitoring failed to improve diagnostic accuracy, and HST and PSG-ETco2 values were poorly correlated.
Although children with neuromuscular disease face long wait times for sleep evaluations, HST is clearly not the solution for now. It remains to be seen if innovations in HST with extended monitoring (and transcutaneous CO2) become viable. In the meantime, finding ways to improve access to sleep medicine care for children with neuromuscular disease is a must.
Jacob Collen, MD, FCCP
Steering Committee Member
Interstitial and Diffuse Lung Disease
Idiopathic pneumonias that are not all that idiopathic
Despite being defined as an individual entity for research purposes in 2015 (Fisher et al. Eur Respir J. 2015;46:976), interstitial pneumonias with autoimmune features (IPAF) remain a heterogeneous group of interstitial lung diseases that puzzle the clinician. Since the introduction of the IPAF definition, there have been attempts to validate the diagnostic criteria and study their prognostic implications. Some of these studies showed differential prognosis in patients who met the IPAF criteria (Oldham et al. Eur Respir J. 2016;47:1767).
Although the implications of the presence of autoimmune antibodies in idiopathic interstitial pneumonias (IIPs) is not fully understood, the treatment often entails immunosuppression, especially in those with non-UIP patterns of disease and/or clinical features of autoimmune disease. The stakes are high when IIPs are associated with antibodies correlated with rapidly progressive disease, such as MDA-5 antibody or antisynthetase antibodies. Pulmonologists often lack the clinical expertise to detect occult autoimmune disorders, though the role of the rheumatologist in facilitating the diagnosis and treatment of IPAF is not well delineated. Most health-care systems are not equipped with collaborative ILD-rheumatology clinics or even easy access to a rheumatologist. There is a need for real-world pragmatic studies to establish the optimal way to evaluate patients with ILD for autoimmune features and identify patients who would benefit most from an early referral to rheumatology to aid with diagnosis, treatment, and sometimes monitoring for extrapulmonary manifestations of autoimmune disorders.
Avanthika Thanushi Wynn, MD
Steering Committee Fellow-in-Training
Airways Disorders
Defining and treating early COPD: Can we make a difference?
There is growing evidence that early COPD—before currently accepted spirometric or symptomatic criteria are present—may be an important clinical entity. The primary pathobiologic mechanisms in early COPD development include both abnormal lung development and accelerated lung aging (Augustí et al. Am J Respir Crit Care Med. 2018 Oct 15;198:8:978).
Martinez and colleagues recently proposed defining early COPD as age <50 with 10+ pack-year smoking history and at least one of the following: (1) early airflow limitation (postbronchodilator FEV1/FVC < lower limit of normal), (2) compatible CT scan abnormalities, (3) rapid decline in FEV1 (≥60 mL/yr) that is accelerated relative to FVC (Martinez et al. Am J Respir Crit Care Med. 2018 Jun 15;197[12]:1540).
A novel multiresolution CT scan imaging protocol described by Koo and coworkers found that substantial loss of small airways— specifically the terminal and transitional bronchioles—occurs in patients with mild-to-moderate COPD even prior to the development of emphysema on CT scan. These findings show that significant destruction of the small airways has occurred prior to the development of mild COPD (Koo et al. Lancet Respir Med. 2018 Aug;6:591).
Pharmacologic treatment for COPD is targeted at the reduction of symptoms and risk of exacerbation, as there remains no conclusive evidence that existing therapies modify long-term decline in lung function. It is unknown if pharmacotherapy for “early COPD” will alter the disease course. While not directly addressing this subset, information may be gleaned from trials on younger, more mild GOLD Stage 1 or Stage 2 patients. The Tie-COPD trial, the largest powered study to date of mild-to-moderate COPD, found that among patients with GOLD stage 1 or 2 COPD treatment with tiotropium compared with placebo for 2 years resulted in significantly higher FEV1 before bronchodilator use (between group difference of 157 mL) and slowed annual decline in FEV1 after bronchodilator use (Zhou et al. N Engl J Med. 2017 Sep 7;377[10]:923).
As our understanding of heterogeneity within COPD increases, striving for improved outcomes from our therapies—an impact on lung function in addition to symptom and exacerbation risk—may need to begin with the study of earlier treatment.
Megan Conroy, MD
Steering Committee Fellow-in-Training
Allen J. Blaivas, DO, FCCP
Steering Committee Vice-Chair
Clinical Pulmonary Medicine
Asthma-COPD overlap: An underappreciated phenotype of obstructive airway disease (OAD)
Asthma-COPD overlap (ACO) is a common yet underappreciated clinical entity within the complex OAD spectrum. Currently, there is no consensus criteria to define ACO; however, a roundtable consensus from an international group (Sin et al. Eur Respir J. 2016 Sep;48:664) suggests using major and minor criteria, with key features being airflow limitation, asthma history, and cigarette or biomass exposure. Several studies have shown that patients with ACO have severe disease, faster lung function decline, greater morbidity and mortality, and lower QoL (Alshabanat et al. PLoS One. 2015 Sep 3;10:e0136065).
There is paucity of data on the pathophysiology, risk factors, and clinical management given exclusion of these patients from clinical trials of asthma and COPD. Indeed, clinicians and researchers now realize that ACO is an umbrella term for multiple subphenotypes, including patients who have predominant asthma with some COPD features and others with predominant COPD with some asthma features. Overall, IgE level, FeNO, sputum, and blood eosinophils are usually higher in ACO than in COPD and relatively similar compared with asthma (Kobayashi et al. Int J Chron Obs Pulmon Dis. 2016 May 26;11:2117).
Most recently, a longitudinal study looked at predictors of ACO among NY firefighters exposed to WTC dust (Singh et al. CHEST. 2018 Dec;154[6]:1301). Pre-exposure low lung function and elevated blood eosinophils and IL4 (T2 inflammatory cytokine) increased risk of developing ACO among those exposed to WTC dust. Further research is required to better understand the interaction of environmental exposure and risk factors in the pathophysiology of ACO. It may be more pragmatic to use the unifying term OAD, as originally proposed in the Dutch hypothesis, and further delineate how several phenotypes of airway disease can be classified by combining traditional approaches with molecular and genomic analysis.
Munish Luthra, MD, FCCP
Steering Committee Member
Samantha D’Annunzio, MD
Steering Committee Member
Critical Care
Mechanical ventilation: One size fits all?
Mechanical ventilation (MV) is a lifesaving intervention in the ICU, but it has been associated with numerous complications ranging from overuse of sedation, atelectasis, and baro or volutrauma.
After 2000, it became well known that using a low tidal volume (VT) strategy (6 mL/kg predicted body weight, PBW) in patients with ARDS produced lower mortality and more ventilator-free days (N Engl J Med. 2000 May 4;342[18]:1301). In addition, a meta-analysis in 2012 demonstrated a lower relative risk of new lung injury, mortality, and pulmonary infections with low VT in non-ARDS patients (Serpa et al. JAMA. 2012 Oct 24/31;308[16]:1651). However, the included studies varied widely in their use of VT (9-12 mL/kg), duration of MV, and in mixed settings (ICU or operating room).
Recently, a large randomized clinical trial compared the effect of low (4-6 mL/kg, PBW) vs intermediate (8-10 mL/kg, PBW) VT ventilation strategy in non-ARDS ICU patients. Interestingly, the study concluded that there is no significant difference in ventilator-free days (21 days in each group), median length ICU and hospital stay, ICU mortality rates, and 28- and 90-day mortality. Also, there was no difference in new-onset ARDS, severe atelectasis, sedation use, and delirium (JAMA. 2018; 320[18]:1872). This study suggests that in non-ARDS patients, MV should be individualized according to each patient’s clinical situation, the nature of the disease, and its effect on lung mechanics, especially in patients who cannot tolerate low tidal volumes.
Margaret A. Disselkamp, MD
Steering Committee Member
Mohammed A. Megri, MD
Fellow-in-Training Steering Committee Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Improving access to sleep medicine care for patients with NMD
Sleep-disordered breathing (SDB) occurs in up to 5% of children, with adverse implications for growth and development. Children with neuromuscular disease are at significantly higher risk than unaffected children (Chiang et al. Children. 2018;5:e78). Respiratory dysfunction that may present as SDB before daytime impairment in gas exchange is evident. Diagnosing and treating SDB (to include OSA, CSA, and hypoventilation syndromes) early can significantly improve morbidity and mortality.
Unfortunately, diagnostic sleep medicine resources are limited. Children may wait up to a year or more for definitive testing with in-laboratory, attended polysomnography (PSG). Among children with neuromuscular disease, fewer than 10% may undergo a sleep clinic evaluation, and, of those that do, they may have only one visit over a 3-year period of care (Rose et al. Pediatr Pulmonol. 2018 Oct;53:1378). Home sleep testing (HST) has been evaluated as an alternative to PSG given lower cost, availability, and advantage of the child sleeping in his/her own bed. Although HST is indicated in adults with a high pretest probability for moderate to severe OSA, it is not indicated in children, given the potential to underestimate disease severity or to miss the diagnosis entirely (Kirk et al. J Clin Sleep Med. 2017 Oct 15;13[10]:1199). HST lacks electroencephalogram (EEG) and capnography. Technical recording mishaps are more common in children, but in-lab PSG has the advantage of on-site troubleshooting by a technologist.
A recently published study by Fishman and colleagues attempted to compare gold standard in-lab PSG to HST with capnography (Fishman et al. J Clin Sleep Med. 2018 Dec 15;14[12]:2013). Despite a well-designed study with a carefully selected population, HST failed to reliably diagnose SDB. HST underestimated disease severity and, in some cases, missed the diagnosis of SDB entirely. The addition of end tidal CO2 monitoring failed to improve diagnostic accuracy, and HST and PSG-ETco2 values were poorly correlated.
Although children with neuromuscular disease face long wait times for sleep evaluations, HST is clearly not the solution for now. It remains to be seen if innovations in HST with extended monitoring (and transcutaneous CO2) become viable. In the meantime, finding ways to improve access to sleep medicine care for children with neuromuscular disease is a must.
Jacob Collen, MD, FCCP
Steering Committee Member
Interstitial and Diffuse Lung Disease
Idiopathic pneumonias that are not all that idiopathic
Despite being defined as an individual entity for research purposes in 2015 (Fisher et al. Eur Respir J. 2015;46:976), interstitial pneumonias with autoimmune features (IPAF) remain a heterogeneous group of interstitial lung diseases that puzzle the clinician. Since the introduction of the IPAF definition, there have been attempts to validate the diagnostic criteria and study their prognostic implications. Some of these studies showed differential prognosis in patients who met the IPAF criteria (Oldham et al. Eur Respir J. 2016;47:1767).
Although the implications of the presence of autoimmune antibodies in idiopathic interstitial pneumonias (IIPs) is not fully understood, the treatment often entails immunosuppression, especially in those with non-UIP patterns of disease and/or clinical features of autoimmune disease. The stakes are high when IIPs are associated with antibodies correlated with rapidly progressive disease, such as MDA-5 antibody or antisynthetase antibodies. Pulmonologists often lack the clinical expertise to detect occult autoimmune disorders, though the role of the rheumatologist in facilitating the diagnosis and treatment of IPAF is not well delineated. Most health-care systems are not equipped with collaborative ILD-rheumatology clinics or even easy access to a rheumatologist. There is a need for real-world pragmatic studies to establish the optimal way to evaluate patients with ILD for autoimmune features and identify patients who would benefit most from an early referral to rheumatology to aid with diagnosis, treatment, and sometimes monitoring for extrapulmonary manifestations of autoimmune disorders.
Avanthika Thanushi Wynn, MD
Steering Committee Fellow-in-Training