User login
The AGA Research Foundation awards $2.66 million in research funding
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
Membership priorities shape the AGA advocacy agenda
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).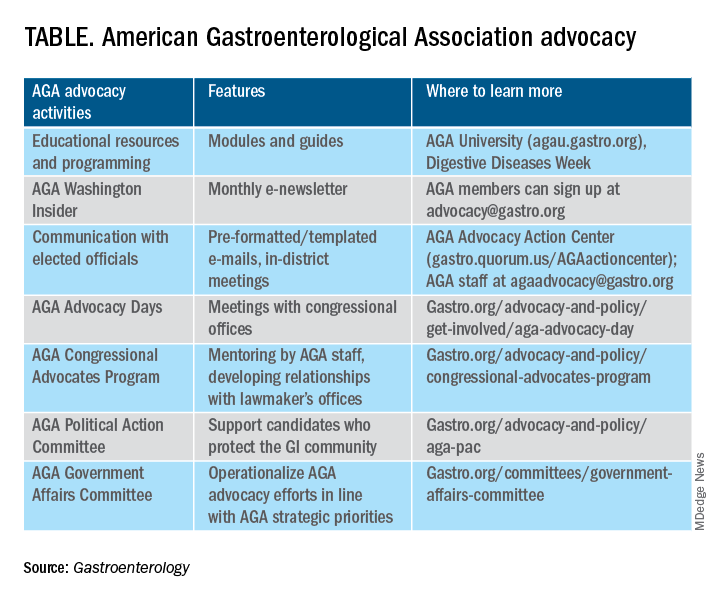
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
American Gastroenterological Association invests in unsedated transnasal endoscopy medical device company EvoEndo®
, a medical device company developing platforms for unsedated transnasal endoscopy (TNE).
“AGA is proud to support EvoEndo® and its innovative technology that has the potential to improve care, save time, resources, and cost for hospitals and the GI community at large,” said Michael L. Kochman, MD, AGAF, MASGE, Wilmott Family Professor of Medicine and Surgery, Center for Endoscopic Innovation, Research and Training, gastroenterology division, University of Pennsylvania Health System; fund manager and adviser, AGA GI Opportunity Fund.
The EvoEndo® Single-Use Endoscopy System received FDA 510(k) clearance in February 2022. The EvoEndo System includes a sterile, single-use, flexible gastroscope designed for unsedated transnasal upper endoscopy and a small portable video controller. The EvoEndo Comfort Kit (not part of the cleared EvoEndo System) includes virtual reality (VR) goggles for patient distraction during the unsedated transnasal endoscopy procedure. Unsedated TNE can be used to evaluate and diagnose a wide range of upper GI conditions that may require frequent monitoring, including eosinophilic esophagitis (EoE), dysphagia, celiac disease, gastroesophageal reflux disease, Barrett’s esophagus, malabsorption, and abdominal pain.
“We are grateful for the support of the AGA, which is a testament to our ongoing commitment to improving GI outcomes with our technology,” said Jonathan T. Hartmann, CEO at EvoEndo. “The AGA has always been at the forefront of improving GI care. Our team could not be more excited that they have recognized EvoEndo, and we look forward to continuing to expand adoption of our technology to the GI community, its physicians, and their patients.”
TNE enabled by EvoEndo’s Single-Use Endoscopy System allows hospitals to move endoscopy procedures from an ambulatory procedural suite to an office-based environment and allows the “traditional” sedation procedure rooms to be used for more complex, therapeutic cases.
“Expanding our fund’s portfolio to include technologies that can transform the pediatric GI landscape is particularly exciting for Varia Ventures,” said Andrea Vossler, cofounder and managing director at Varia Ventures. “EvoEndo® has made significant progress in the TNE category, and we are excited for what’s to come in the future.”
The EvoEndo® Model LE Gastroscope is intended for the visualization of the upper digestive tract in adults and pediatric patients, specifically for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenal bulb in patients over the age of five. The gastroscope is a sterile, single-use device and can be inserted orally or transnasally. The EvoEndo® Controller is intended for use with an EvoEndo® Endoscope for endoscopic diagnosis, treatment, and video observation. The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or scheduling demonstrations and training can contact the company here.
, a medical device company developing platforms for unsedated transnasal endoscopy (TNE).
“AGA is proud to support EvoEndo® and its innovative technology that has the potential to improve care, save time, resources, and cost for hospitals and the GI community at large,” said Michael L. Kochman, MD, AGAF, MASGE, Wilmott Family Professor of Medicine and Surgery, Center for Endoscopic Innovation, Research and Training, gastroenterology division, University of Pennsylvania Health System; fund manager and adviser, AGA GI Opportunity Fund.
The EvoEndo® Single-Use Endoscopy System received FDA 510(k) clearance in February 2022. The EvoEndo System includes a sterile, single-use, flexible gastroscope designed for unsedated transnasal upper endoscopy and a small portable video controller. The EvoEndo Comfort Kit (not part of the cleared EvoEndo System) includes virtual reality (VR) goggles for patient distraction during the unsedated transnasal endoscopy procedure. Unsedated TNE can be used to evaluate and diagnose a wide range of upper GI conditions that may require frequent monitoring, including eosinophilic esophagitis (EoE), dysphagia, celiac disease, gastroesophageal reflux disease, Barrett’s esophagus, malabsorption, and abdominal pain.
“We are grateful for the support of the AGA, which is a testament to our ongoing commitment to improving GI outcomes with our technology,” said Jonathan T. Hartmann, CEO at EvoEndo. “The AGA has always been at the forefront of improving GI care. Our team could not be more excited that they have recognized EvoEndo, and we look forward to continuing to expand adoption of our technology to the GI community, its physicians, and their patients.”
TNE enabled by EvoEndo’s Single-Use Endoscopy System allows hospitals to move endoscopy procedures from an ambulatory procedural suite to an office-based environment and allows the “traditional” sedation procedure rooms to be used for more complex, therapeutic cases.
“Expanding our fund’s portfolio to include technologies that can transform the pediatric GI landscape is particularly exciting for Varia Ventures,” said Andrea Vossler, cofounder and managing director at Varia Ventures. “EvoEndo® has made significant progress in the TNE category, and we are excited for what’s to come in the future.”
The EvoEndo® Model LE Gastroscope is intended for the visualization of the upper digestive tract in adults and pediatric patients, specifically for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenal bulb in patients over the age of five. The gastroscope is a sterile, single-use device and can be inserted orally or transnasally. The EvoEndo® Controller is intended for use with an EvoEndo® Endoscope for endoscopic diagnosis, treatment, and video observation. The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or scheduling demonstrations and training can contact the company here.
, a medical device company developing platforms for unsedated transnasal endoscopy (TNE).
“AGA is proud to support EvoEndo® and its innovative technology that has the potential to improve care, save time, resources, and cost for hospitals and the GI community at large,” said Michael L. Kochman, MD, AGAF, MASGE, Wilmott Family Professor of Medicine and Surgery, Center for Endoscopic Innovation, Research and Training, gastroenterology division, University of Pennsylvania Health System; fund manager and adviser, AGA GI Opportunity Fund.
The EvoEndo® Single-Use Endoscopy System received FDA 510(k) clearance in February 2022. The EvoEndo System includes a sterile, single-use, flexible gastroscope designed for unsedated transnasal upper endoscopy and a small portable video controller. The EvoEndo Comfort Kit (not part of the cleared EvoEndo System) includes virtual reality (VR) goggles for patient distraction during the unsedated transnasal endoscopy procedure. Unsedated TNE can be used to evaluate and diagnose a wide range of upper GI conditions that may require frequent monitoring, including eosinophilic esophagitis (EoE), dysphagia, celiac disease, gastroesophageal reflux disease, Barrett’s esophagus, malabsorption, and abdominal pain.
“We are grateful for the support of the AGA, which is a testament to our ongoing commitment to improving GI outcomes with our technology,” said Jonathan T. Hartmann, CEO at EvoEndo. “The AGA has always been at the forefront of improving GI care. Our team could not be more excited that they have recognized EvoEndo, and we look forward to continuing to expand adoption of our technology to the GI community, its physicians, and their patients.”
TNE enabled by EvoEndo’s Single-Use Endoscopy System allows hospitals to move endoscopy procedures from an ambulatory procedural suite to an office-based environment and allows the “traditional” sedation procedure rooms to be used for more complex, therapeutic cases.
“Expanding our fund’s portfolio to include technologies that can transform the pediatric GI landscape is particularly exciting for Varia Ventures,” said Andrea Vossler, cofounder and managing director at Varia Ventures. “EvoEndo® has made significant progress in the TNE category, and we are excited for what’s to come in the future.”
The EvoEndo® Model LE Gastroscope is intended for the visualization of the upper digestive tract in adults and pediatric patients, specifically for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenal bulb in patients over the age of five. The gastroscope is a sterile, single-use device and can be inserted orally or transnasally. The EvoEndo® Controller is intended for use with an EvoEndo® Endoscope for endoscopic diagnosis, treatment, and video observation. The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or scheduling demonstrations and training can contact the company here.
AGA guidelines, CPUs lead education at DDW® 2023
Below is a sampling of AGA’s invited-speaker sessions we’re excited about this year for clinical practitioners. To view other AGA program highlights, check out the DDW Preliminary Program.
- Guidelines Highlights 2023
- Clinical Practice Updates: Battle of the Heavyweights
- AGA Clinical Symposium
- Case Studies in Measuring Care and Improving Quality
- Optimizing Your GI Practice: Guidelines, Quality and Delivery
- AGA Postgraduate Course ($)
- Surviving the First Years in Clinical Practice: Roundtable With the Experts
Below is a sampling of AGA’s invited-speaker sessions we’re excited about this year for clinical practitioners. To view other AGA program highlights, check out the DDW Preliminary Program.
- Guidelines Highlights 2023
- Clinical Practice Updates: Battle of the Heavyweights
- AGA Clinical Symposium
- Case Studies in Measuring Care and Improving Quality
- Optimizing Your GI Practice: Guidelines, Quality and Delivery
- AGA Postgraduate Course ($)
- Surviving the First Years in Clinical Practice: Roundtable With the Experts
Below is a sampling of AGA’s invited-speaker sessions we’re excited about this year for clinical practitioners. To view other AGA program highlights, check out the DDW Preliminary Program.
- Guidelines Highlights 2023
- Clinical Practice Updates: Battle of the Heavyweights
- AGA Clinical Symposium
- Case Studies in Measuring Care and Improving Quality
- Optimizing Your GI Practice: Guidelines, Quality and Delivery
- AGA Postgraduate Course ($)
- Surviving the First Years in Clinical Practice: Roundtable With the Experts
Protect the next generation of GI investigators
Investing in research is the only way we will identify new diagnostics and treatments. However, at this time of unparalleled scientific and clinical opportunity, promising early stage investigators are leaving the field because of the instability of federal research funding.
Fortunately, the AGA Research Foundation has a proven track record of funding young investigators whose work advances the field of gastroenterology and hepatology.
Help the AGA build a community of investigators through the AGA Research Foundation.
Your donation to the AGA Research Foundation can fund future success stories by keeping young scientists working to advance our understanding of digestive diseases.
Donate today to help protect the GI research pipeline. Make a tax-deductible donation at www.foundation.gastro.org.
Investing in research is the only way we will identify new diagnostics and treatments. However, at this time of unparalleled scientific and clinical opportunity, promising early stage investigators are leaving the field because of the instability of federal research funding.
Fortunately, the AGA Research Foundation has a proven track record of funding young investigators whose work advances the field of gastroenterology and hepatology.
Help the AGA build a community of investigators through the AGA Research Foundation.
Your donation to the AGA Research Foundation can fund future success stories by keeping young scientists working to advance our understanding of digestive diseases.
Donate today to help protect the GI research pipeline. Make a tax-deductible donation at www.foundation.gastro.org.
Investing in research is the only way we will identify new diagnostics and treatments. However, at this time of unparalleled scientific and clinical opportunity, promising early stage investigators are leaving the field because of the instability of federal research funding.
Fortunately, the AGA Research Foundation has a proven track record of funding young investigators whose work advances the field of gastroenterology and hepatology.
Help the AGA build a community of investigators through the AGA Research Foundation.
Your donation to the AGA Research Foundation can fund future success stories by keeping young scientists working to advance our understanding of digestive diseases.
Donate today to help protect the GI research pipeline. Make a tax-deductible donation at www.foundation.gastro.org.
Reforming prior authorization remains AGA’s top policy priority
Reforming prior authorization polices to reduce red tape for physicians and help patients get the care they need in a timely manner is the AGA’s number one policy priority as it impacts every gastroenterologist regardless of practice setting. We have seen an increase in prior authorization policies from every major insurer. The most recent prior authorization program to impact gastroenterologists was announced by UnitedHealthcare (UHC) in March for implementation on June 1, 2023 and will require prior authorization for most colonoscopy and upper GI endoscopy procedures with the exception of screening colonoscopy.1 This policy is a step back at a time when payers should be developing innovative policies in collaboration with health care providers to improve patient care.
UHC’s GI prior authorization policy
AGA met with UHC in March to discuss their plan to require prior authorization for most GI endoscopy procedures. We stressed how this change will cause care delays for high-risk individuals, deter patients from undergoing medically recommended procedures, exacerbate existing sociodemographic disparities in care and outcomes, and add unnecessary paperwork burden to physicians who have mounting rates of burnout.
Linda Lee, MD, medical director of endoscopy at Brigham and Women’s Hospital, Boston, recently spoke of the impact this policy will have on gastroenterologists and their patients. “We all know that requiring prior authorizations really only leads to more bureaucracy within the insurance company, as well as within each health care provider’s practice, because we need people to fill out these prior authorization forms, waste time trying to get through to their 1-800 number to speak with someone who has no clinical knowledge, then be told we need to speak with someone else who actually does have some medical knowledge about why these procedures are necessary.”
However, Dr. Lee stressed that “most importantly, this will lead to poorer patient care with delays in care as we are struggling to wade through the morass of prior authorization while patients are bleeding, not able to swallow, vomiting, and more while waiting for their insurance company to approve their potentially life-saving procedures.”
We were particularly troubled that UHC announced this policy during Colorectal Cancer Awareness Month, given the need to screen more Americans for colorectal cancer which remains the nation’s number two cancer killer. The UHC program would require a PA on surveillance colonoscopy for those patients who have previously had polyps removed and are at a higher risk for developing colorectal cancer.
“We know that patients with high-risk adenomas or advanced sessile serrated lesions have a higher risk of developing colorectal cancer and timely access to the necessary surveillance colonoscopy is critical,” said David Lieberman, MD, past president of the AGA and chair of the AGA Executive Committee on the Screening Continuum.
AGA plans to meet with UHC again to ask them to reconsider this policy, but we need your advocacy now to tell United how this will impact you and your patients.
How you can help stop UHC’s prior authorization program
Write to UHC: Tell UHC how this policy would impact you and your patients. Contact their CEO using our customizable letter2 that outlines the impact of United’s GI endoscopy prior authorization program on gastroenterologists and their patients available on the AGA Advocacy Action Center.
Use social media: Tag United (@UHC) on Twitter and tell them how this burdensome program will cause delays for high-risk individuals, deter patients from seeking treatment, and exacerbate existing disparities in care, all while saddling physicians with even more paperwork. Once you’ve tweeted, tag your colleagues and encourage them to get involved.
AGA is working to reform prior authorization
The AGA has supported federal legislation that would streamline prior authorization processes in Medicare Advantage (MA), the private insurance plans that contract with the Medicare program, given the explosion of these policies over the past several years. The Improving Seniors Timely Access to Care Act, bipartisan, bicameral legislation, would reduce prior authorization burdens by:
- Establishing an electronic prior authorization (ePA) program and require MA plans to adopt ePA capabilities.
- Requiring the Secretary of Health and Human Services to establish a list of items and services eligible for real-time decisions under an MA ePA program.
- Standardizing and streamlining the prior authorization process for routinely approved items and services.
- Ensuring prior authorization requests are reviewed by qualified medical personnel.
- Increasing transparency around MA prior authorization requirements and their use.
- Protecting beneficiaries from any disruptions in care due to prior authorization requirements as they transition between MA plans.
The Centers for Medicare & Medicaid Services (CMS) has also recognized the impact that prior authorization is having on physician wellness and how it is contributing to physician burnout. The agency recently proposed implementing many of the provisions that are outlined in the legislation, and AGA has expressed our support for moving forward with many of their proposals.
Earlier this year, Shivan Mehta, MD, MPH, met with CMS administrator Chiquita Brooks-LaSure and Surgeon General Vivek Murthy, MD, MBA, to express AGA’s support for prior authorization reform and discussed how it impacts how patients with chronic conditions like inflammatory bowel disease maintain continuity of care. He also stressed how prior authorization further exacerbates health inequities since it creates an additional barrier to care when barriers already exist.
AGA is taking a multi-pronged approach to advocating for prior authorization reform and reducing paperwork through legislative advocacy, regulatory advocacy with the CMS, and payer advocacy. We can’t do this alone. Join our AGA Advocacy Center3 and get involved in our AGA Congressional Advocates Program.4The authors have no conflicts to declare.
References
1. UnitedHealthcare (2023 Mar 01) New requirements for gastroenterology services.
2. American Gastroenterological Association (n.d.) AGA Advocacy Action Center. Tell United to Stop New Prior Auth Requirements!
3. American Gastroenterological Association (n.d.) AGA Advocacy Action Center. Advocacy & Policy. Get Involved.
4. American Gastroenterological Association (n.d.) AGA Congressional Advocates Program.
Reforming prior authorization polices to reduce red tape for physicians and help patients get the care they need in a timely manner is the AGA’s number one policy priority as it impacts every gastroenterologist regardless of practice setting. We have seen an increase in prior authorization policies from every major insurer. The most recent prior authorization program to impact gastroenterologists was announced by UnitedHealthcare (UHC) in March for implementation on June 1, 2023 and will require prior authorization for most colonoscopy and upper GI endoscopy procedures with the exception of screening colonoscopy.1 This policy is a step back at a time when payers should be developing innovative policies in collaboration with health care providers to improve patient care.
UHC’s GI prior authorization policy
AGA met with UHC in March to discuss their plan to require prior authorization for most GI endoscopy procedures. We stressed how this change will cause care delays for high-risk individuals, deter patients from undergoing medically recommended procedures, exacerbate existing sociodemographic disparities in care and outcomes, and add unnecessary paperwork burden to physicians who have mounting rates of burnout.
Linda Lee, MD, medical director of endoscopy at Brigham and Women’s Hospital, Boston, recently spoke of the impact this policy will have on gastroenterologists and their patients. “We all know that requiring prior authorizations really only leads to more bureaucracy within the insurance company, as well as within each health care provider’s practice, because we need people to fill out these prior authorization forms, waste time trying to get through to their 1-800 number to speak with someone who has no clinical knowledge, then be told we need to speak with someone else who actually does have some medical knowledge about why these procedures are necessary.”
However, Dr. Lee stressed that “most importantly, this will lead to poorer patient care with delays in care as we are struggling to wade through the morass of prior authorization while patients are bleeding, not able to swallow, vomiting, and more while waiting for their insurance company to approve their potentially life-saving procedures.”
We were particularly troubled that UHC announced this policy during Colorectal Cancer Awareness Month, given the need to screen more Americans for colorectal cancer which remains the nation’s number two cancer killer. The UHC program would require a PA on surveillance colonoscopy for those patients who have previously had polyps removed and are at a higher risk for developing colorectal cancer.
“We know that patients with high-risk adenomas or advanced sessile serrated lesions have a higher risk of developing colorectal cancer and timely access to the necessary surveillance colonoscopy is critical,” said David Lieberman, MD, past president of the AGA and chair of the AGA Executive Committee on the Screening Continuum.
AGA plans to meet with UHC again to ask them to reconsider this policy, but we need your advocacy now to tell United how this will impact you and your patients.
How you can help stop UHC’s prior authorization program
Write to UHC: Tell UHC how this policy would impact you and your patients. Contact their CEO using our customizable letter2 that outlines the impact of United’s GI endoscopy prior authorization program on gastroenterologists and their patients available on the AGA Advocacy Action Center.
Use social media: Tag United (@UHC) on Twitter and tell them how this burdensome program will cause delays for high-risk individuals, deter patients from seeking treatment, and exacerbate existing disparities in care, all while saddling physicians with even more paperwork. Once you’ve tweeted, tag your colleagues and encourage them to get involved.
AGA is working to reform prior authorization
The AGA has supported federal legislation that would streamline prior authorization processes in Medicare Advantage (MA), the private insurance plans that contract with the Medicare program, given the explosion of these policies over the past several years. The Improving Seniors Timely Access to Care Act, bipartisan, bicameral legislation, would reduce prior authorization burdens by:
- Establishing an electronic prior authorization (ePA) program and require MA plans to adopt ePA capabilities.
- Requiring the Secretary of Health and Human Services to establish a list of items and services eligible for real-time decisions under an MA ePA program.
- Standardizing and streamlining the prior authorization process for routinely approved items and services.
- Ensuring prior authorization requests are reviewed by qualified medical personnel.
- Increasing transparency around MA prior authorization requirements and their use.
- Protecting beneficiaries from any disruptions in care due to prior authorization requirements as they transition between MA plans.
The Centers for Medicare & Medicaid Services (CMS) has also recognized the impact that prior authorization is having on physician wellness and how it is contributing to physician burnout. The agency recently proposed implementing many of the provisions that are outlined in the legislation, and AGA has expressed our support for moving forward with many of their proposals.
Earlier this year, Shivan Mehta, MD, MPH, met with CMS administrator Chiquita Brooks-LaSure and Surgeon General Vivek Murthy, MD, MBA, to express AGA’s support for prior authorization reform and discussed how it impacts how patients with chronic conditions like inflammatory bowel disease maintain continuity of care. He also stressed how prior authorization further exacerbates health inequities since it creates an additional barrier to care when barriers already exist.
AGA is taking a multi-pronged approach to advocating for prior authorization reform and reducing paperwork through legislative advocacy, regulatory advocacy with the CMS, and payer advocacy. We can’t do this alone. Join our AGA Advocacy Center3 and get involved in our AGA Congressional Advocates Program.4The authors have no conflicts to declare.
References
1. UnitedHealthcare (2023 Mar 01) New requirements for gastroenterology services.
2. American Gastroenterological Association (n.d.) AGA Advocacy Action Center. Tell United to Stop New Prior Auth Requirements!
3. American Gastroenterological Association (n.d.) AGA Advocacy Action Center. Advocacy & Policy. Get Involved.
4. American Gastroenterological Association (n.d.) AGA Congressional Advocates Program.
Reforming prior authorization polices to reduce red tape for physicians and help patients get the care they need in a timely manner is the AGA’s number one policy priority as it impacts every gastroenterologist regardless of practice setting. We have seen an increase in prior authorization policies from every major insurer. The most recent prior authorization program to impact gastroenterologists was announced by UnitedHealthcare (UHC) in March for implementation on June 1, 2023 and will require prior authorization for most colonoscopy and upper GI endoscopy procedures with the exception of screening colonoscopy.1 This policy is a step back at a time when payers should be developing innovative policies in collaboration with health care providers to improve patient care.
UHC’s GI prior authorization policy
AGA met with UHC in March to discuss their plan to require prior authorization for most GI endoscopy procedures. We stressed how this change will cause care delays for high-risk individuals, deter patients from undergoing medically recommended procedures, exacerbate existing sociodemographic disparities in care and outcomes, and add unnecessary paperwork burden to physicians who have mounting rates of burnout.
Linda Lee, MD, medical director of endoscopy at Brigham and Women’s Hospital, Boston, recently spoke of the impact this policy will have on gastroenterologists and their patients. “We all know that requiring prior authorizations really only leads to more bureaucracy within the insurance company, as well as within each health care provider’s practice, because we need people to fill out these prior authorization forms, waste time trying to get through to their 1-800 number to speak with someone who has no clinical knowledge, then be told we need to speak with someone else who actually does have some medical knowledge about why these procedures are necessary.”
However, Dr. Lee stressed that “most importantly, this will lead to poorer patient care with delays in care as we are struggling to wade through the morass of prior authorization while patients are bleeding, not able to swallow, vomiting, and more while waiting for their insurance company to approve their potentially life-saving procedures.”
We were particularly troubled that UHC announced this policy during Colorectal Cancer Awareness Month, given the need to screen more Americans for colorectal cancer which remains the nation’s number two cancer killer. The UHC program would require a PA on surveillance colonoscopy for those patients who have previously had polyps removed and are at a higher risk for developing colorectal cancer.
“We know that patients with high-risk adenomas or advanced sessile serrated lesions have a higher risk of developing colorectal cancer and timely access to the necessary surveillance colonoscopy is critical,” said David Lieberman, MD, past president of the AGA and chair of the AGA Executive Committee on the Screening Continuum.
AGA plans to meet with UHC again to ask them to reconsider this policy, but we need your advocacy now to tell United how this will impact you and your patients.
How you can help stop UHC’s prior authorization program
Write to UHC: Tell UHC how this policy would impact you and your patients. Contact their CEO using our customizable letter2 that outlines the impact of United’s GI endoscopy prior authorization program on gastroenterologists and their patients available on the AGA Advocacy Action Center.
Use social media: Tag United (@UHC) on Twitter and tell them how this burdensome program will cause delays for high-risk individuals, deter patients from seeking treatment, and exacerbate existing disparities in care, all while saddling physicians with even more paperwork. Once you’ve tweeted, tag your colleagues and encourage them to get involved.
AGA is working to reform prior authorization
The AGA has supported federal legislation that would streamline prior authorization processes in Medicare Advantage (MA), the private insurance plans that contract with the Medicare program, given the explosion of these policies over the past several years. The Improving Seniors Timely Access to Care Act, bipartisan, bicameral legislation, would reduce prior authorization burdens by:
- Establishing an electronic prior authorization (ePA) program and require MA plans to adopt ePA capabilities.
- Requiring the Secretary of Health and Human Services to establish a list of items and services eligible for real-time decisions under an MA ePA program.
- Standardizing and streamlining the prior authorization process for routinely approved items and services.
- Ensuring prior authorization requests are reviewed by qualified medical personnel.
- Increasing transparency around MA prior authorization requirements and their use.
- Protecting beneficiaries from any disruptions in care due to prior authorization requirements as they transition between MA plans.
The Centers for Medicare & Medicaid Services (CMS) has also recognized the impact that prior authorization is having on physician wellness and how it is contributing to physician burnout. The agency recently proposed implementing many of the provisions that are outlined in the legislation, and AGA has expressed our support for moving forward with many of their proposals.
Earlier this year, Shivan Mehta, MD, MPH, met with CMS administrator Chiquita Brooks-LaSure and Surgeon General Vivek Murthy, MD, MBA, to express AGA’s support for prior authorization reform and discussed how it impacts how patients with chronic conditions like inflammatory bowel disease maintain continuity of care. He also stressed how prior authorization further exacerbates health inequities since it creates an additional barrier to care when barriers already exist.
AGA is taking a multi-pronged approach to advocating for prior authorization reform and reducing paperwork through legislative advocacy, regulatory advocacy with the CMS, and payer advocacy. We can’t do this alone. Join our AGA Advocacy Center3 and get involved in our AGA Congressional Advocates Program.4The authors have no conflicts to declare.
References
1. UnitedHealthcare (2023 Mar 01) New requirements for gastroenterology services.
2. American Gastroenterological Association (n.d.) AGA Advocacy Action Center. Tell United to Stop New Prior Auth Requirements!
3. American Gastroenterological Association (n.d.) AGA Advocacy Action Center. Advocacy & Policy. Get Involved.
4. American Gastroenterological Association (n.d.) AGA Congressional Advocates Program.
New AGA guideline recommends blood and stool tests for monitoring ulcerative colitis
These guidelines were published in Gastroenterology.
The AGA guidelines outline use cases for three biomarkers that provide accurate insights into UC disease activity: serum C-reactive protein (CRP) (blood), fecal calprotectin (stool), and fecal lactoferrin (stool). AGA recommends a monitoring strategy that integrates noninvasive biomarkers for patients with UC in remission (no current symptoms) as well as those with current symptoms.
Patients with UC in symptomatic remission
- Perform interval biomarker monitoring every 6-12 months.
- AGA recommends stool-based biomarkers over blood testing.
- If biomarkers are normal, AGA suggests continuing biomarker monitoring and avoiding routine endoscopic assessment.
- If biomarkers are elevated, AGA suggests endoscopic assessment by a gastroenterologist.
- Listen to your body! Talk to your doctor about any new symptoms.
Patients with symptomatically active UC
- Biomarker testing should be the first step to determine the need for endoscopic assessment.
- For patients with mild symptoms who have normal or elevated biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms who have normal biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms and elevated biomarkers, AGA suggests treatment adjustment and avoiding endoscopic assessment.
With AGA guidelines guiding the use of noninvasive biomarkers, physicians can confidently offer a more convenient and closer monitoring option for their patients.
AGA will advocate for all insurers to cover the cost of biomarker testing in UC.
These guidelines were published in Gastroenterology.
The AGA guidelines outline use cases for three biomarkers that provide accurate insights into UC disease activity: serum C-reactive protein (CRP) (blood), fecal calprotectin (stool), and fecal lactoferrin (stool). AGA recommends a monitoring strategy that integrates noninvasive biomarkers for patients with UC in remission (no current symptoms) as well as those with current symptoms.
Patients with UC in symptomatic remission
- Perform interval biomarker monitoring every 6-12 months.
- AGA recommends stool-based biomarkers over blood testing.
- If biomarkers are normal, AGA suggests continuing biomarker monitoring and avoiding routine endoscopic assessment.
- If biomarkers are elevated, AGA suggests endoscopic assessment by a gastroenterologist.
- Listen to your body! Talk to your doctor about any new symptoms.
Patients with symptomatically active UC
- Biomarker testing should be the first step to determine the need for endoscopic assessment.
- For patients with mild symptoms who have normal or elevated biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms who have normal biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms and elevated biomarkers, AGA suggests treatment adjustment and avoiding endoscopic assessment.
With AGA guidelines guiding the use of noninvasive biomarkers, physicians can confidently offer a more convenient and closer monitoring option for their patients.
AGA will advocate for all insurers to cover the cost of biomarker testing in UC.
These guidelines were published in Gastroenterology.
The AGA guidelines outline use cases for three biomarkers that provide accurate insights into UC disease activity: serum C-reactive protein (CRP) (blood), fecal calprotectin (stool), and fecal lactoferrin (stool). AGA recommends a monitoring strategy that integrates noninvasive biomarkers for patients with UC in remission (no current symptoms) as well as those with current symptoms.
Patients with UC in symptomatic remission
- Perform interval biomarker monitoring every 6-12 months.
- AGA recommends stool-based biomarkers over blood testing.
- If biomarkers are normal, AGA suggests continuing biomarker monitoring and avoiding routine endoscopic assessment.
- If biomarkers are elevated, AGA suggests endoscopic assessment by a gastroenterologist.
- Listen to your body! Talk to your doctor about any new symptoms.
Patients with symptomatically active UC
- Biomarker testing should be the first step to determine the need for endoscopic assessment.
- For patients with mild symptoms who have normal or elevated biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms who have normal biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms and elevated biomarkers, AGA suggests treatment adjustment and avoiding endoscopic assessment.
With AGA guidelines guiding the use of noninvasive biomarkers, physicians can confidently offer a more convenient and closer monitoring option for their patients.
AGA will advocate for all insurers to cover the cost of biomarker testing in UC.
Medicare requires new modifier for CRC follow-on colonoscopy claims
To unlock this free benefit, providers must properly apply modifier KX.
What codes does this apply to?
Providers must append modifier KX (“requirements specified in the medical policy have been met”) to HCPCS codes G0105 and G0121 when the screening colonoscopy follows a positive result from one of the following noninvasive stool-based CRC screening tests:
- Screening Guaiac-based Fecal Occult Blood Test (gFOBT) (CPT 82270).
- Screening Immunoassay-based Fecal Occult Blood Test (iFOBT) (HCPCS G0328).
- Cologuard™ – Multi-target Stool DNA (sDNA) Test (CPT 81528).
What happens if I don’t use the KX modifier?
Medicare will return the screening colonoscopy claim as “unprocessable” and you will receive one of following messages:
CARC 16: “Claim/service lacks information or has submission billing error(s)” and RARC N822: “Missing Procedure Modifier(s)”
or
RARC N823: “Incomplete/Invalid Procedure Modifier”
Attach modifier KX and resubmit the claim to Medicare.
Should I use modifier KX if I remove polyps?
No. If you remove polyps during a screening colonoscopy following a positive noninvasive stool-based test, report the appropriate CPT code (for example, 45380, 45384, 45385, or 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare.
Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet when polypectomy is needed during a screening colonoscopy. Medicare beneficiary coinsurance responsibility is 15% of the cost of the procedure from 2023 to 2026. The coinsurance responsibility falls to 10% from 2027 to 2029 and by 2030 it will be covered 100% by Medicare.
Where can I find more information?
See the MLN Matters notice and the CMS Manual System.
To unlock this free benefit, providers must properly apply modifier KX.
What codes does this apply to?
Providers must append modifier KX (“requirements specified in the medical policy have been met”) to HCPCS codes G0105 and G0121 when the screening colonoscopy follows a positive result from one of the following noninvasive stool-based CRC screening tests:
- Screening Guaiac-based Fecal Occult Blood Test (gFOBT) (CPT 82270).
- Screening Immunoassay-based Fecal Occult Blood Test (iFOBT) (HCPCS G0328).
- Cologuard™ – Multi-target Stool DNA (sDNA) Test (CPT 81528).
What happens if I don’t use the KX modifier?
Medicare will return the screening colonoscopy claim as “unprocessable” and you will receive one of following messages:
CARC 16: “Claim/service lacks information or has submission billing error(s)” and RARC N822: “Missing Procedure Modifier(s)”
or
RARC N823: “Incomplete/Invalid Procedure Modifier”
Attach modifier KX and resubmit the claim to Medicare.
Should I use modifier KX if I remove polyps?
No. If you remove polyps during a screening colonoscopy following a positive noninvasive stool-based test, report the appropriate CPT code (for example, 45380, 45384, 45385, or 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare.
Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet when polypectomy is needed during a screening colonoscopy. Medicare beneficiary coinsurance responsibility is 15% of the cost of the procedure from 2023 to 2026. The coinsurance responsibility falls to 10% from 2027 to 2029 and by 2030 it will be covered 100% by Medicare.
Where can I find more information?
See the MLN Matters notice and the CMS Manual System.
To unlock this free benefit, providers must properly apply modifier KX.
What codes does this apply to?
Providers must append modifier KX (“requirements specified in the medical policy have been met”) to HCPCS codes G0105 and G0121 when the screening colonoscopy follows a positive result from one of the following noninvasive stool-based CRC screening tests:
- Screening Guaiac-based Fecal Occult Blood Test (gFOBT) (CPT 82270).
- Screening Immunoassay-based Fecal Occult Blood Test (iFOBT) (HCPCS G0328).
- Cologuard™ – Multi-target Stool DNA (sDNA) Test (CPT 81528).
What happens if I don’t use the KX modifier?
Medicare will return the screening colonoscopy claim as “unprocessable” and you will receive one of following messages:
CARC 16: “Claim/service lacks information or has submission billing error(s)” and RARC N822: “Missing Procedure Modifier(s)”
or
RARC N823: “Incomplete/Invalid Procedure Modifier”
Attach modifier KX and resubmit the claim to Medicare.
Should I use modifier KX if I remove polyps?
No. If you remove polyps during a screening colonoscopy following a positive noninvasive stool-based test, report the appropriate CPT code (for example, 45380, 45384, 45385, or 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare.
Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet when polypectomy is needed during a screening colonoscopy. Medicare beneficiary coinsurance responsibility is 15% of the cost of the procedure from 2023 to 2026. The coinsurance responsibility falls to 10% from 2027 to 2029 and by 2030 it will be covered 100% by Medicare.
Where can I find more information?
See the MLN Matters notice and the CMS Manual System.
A gift in your will: Getting started
A simple, flexible, and versatile way to ensure the AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable bequest, you need a current will or living trust.
After your lifetime, the AGA Research Foundation receives your gift.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise, and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
When planning a future gift, it’s sometimes difficult to determine what size donation will make sense. Emergencies happen, and you need to make sure your family is financially taken care of first. Including a bequest of a percentage of your estate ensures that your gift will remain proportionate no matter how your estate’s value fluctuates over the years.
Whether you would like to put your donation to work today or benefit us after your lifetime, you can find a charitable plan that lets you provide for your family and support the AGA Research Foundation.
Please contact us for more information at [email protected] or visit gastro.planmylegacy.org.
A simple, flexible, and versatile way to ensure the AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable bequest, you need a current will or living trust.
After your lifetime, the AGA Research Foundation receives your gift.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise, and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
When planning a future gift, it’s sometimes difficult to determine what size donation will make sense. Emergencies happen, and you need to make sure your family is financially taken care of first. Including a bequest of a percentage of your estate ensures that your gift will remain proportionate no matter how your estate’s value fluctuates over the years.
Whether you would like to put your donation to work today or benefit us after your lifetime, you can find a charitable plan that lets you provide for your family and support the AGA Research Foundation.
Please contact us for more information at [email protected] or visit gastro.planmylegacy.org.
A simple, flexible, and versatile way to ensure the AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable bequest, you need a current will or living trust.
After your lifetime, the AGA Research Foundation receives your gift.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise, and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
When planning a future gift, it’s sometimes difficult to determine what size donation will make sense. Emergencies happen, and you need to make sure your family is financially taken care of first. Including a bequest of a percentage of your estate ensures that your gift will remain proportionate no matter how your estate’s value fluctuates over the years.
Whether you would like to put your donation to work today or benefit us after your lifetime, you can find a charitable plan that lets you provide for your family and support the AGA Research Foundation.
Please contact us for more information at [email protected] or visit gastro.planmylegacy.org.
New coding policies to prevent surprise billing for CRC screening
The Departments of Labor, Health & Human Services, and the Treasury issued guidance in 2022 that plans and insurers “must cover and may not impose cost sharing with respect to a colonoscopy conducted after a positive non-invasive stool-based screening test” for plan or policy years1 beginning on or after May 31, 2022, and, further, “may not impose cost-sharing with respect to a polyp removal during a colonoscopy performed as a screening procedure.”2 So why are so many patients still being charged fees for these screening services? In many cases, the answer comes down to missing code modifiers.
Commercial insurers want you to use modifier 33
AGA spoke to Elevance (formerly Anthem), Cigna, Aetna, and Blue Cross Blue Shield Association about how physicians should report colorectal cancer screening procedures and tests. They said using the 33 modifier (preventive service) is essential for their systems to trigger the screening benefits for beneficiaries. Without the 33 modifier, the claim will be processed as a diagnostic service, and coinsurance may apply.
According to the CPT manual, modifier 33 should be used “when the primary purpose of the service is the delivery of an evidence-based service in accordance with a U.S. Preventive Services Task Force A or B rating in effect and other preventive services identified in preventive mandates (legislative or regulatory) ...” Use modifier 33 with colonoscopies that start out as screening procedures and with colonoscopies following a positive non-invasive stool-based test, like fecal immunochemical test (FIT) or Cologuard™ multi-target stool DNA test.
It is important to note that modifier 33 won’t ensure all screening colonoscopy claims are paid, because not all commercial plans are required to cover 100 percent of the costs of CRC screening tests and procedures. For example, employer-sponsored insurance plans and legacy plans can choose not to adopt the expanded CRC benefits. Patients who are covered under these plans may not be aware that their CRC test or procedure will not be fully covered. These patients may still receive a “surprise” bill if their screening colonoscopy requires removal of polyps or if they have a colonoscopy following a positive non-invasive CRC test.
Medicare wants you to use modifiers PT and KX, but not together
CMS uses Healthcare Common Procedural Coding System (HCPCS) codes to differentiate between screening and diagnostic colonoscopies to apply screening benefits. For Medicare beneficiaries who choose colonoscopy as their CRC screening, use HCPCS code G0105 (Colorectal cancer screening; colonoscopy on individual at high risk) or G0121 (Colorectal cancer screening; colonoscopy on individual not meeting the criteria for high risk) for screening colonoscopies as appropriate. No modifier is necessary with G0105 or G0121.
Effective for claims with dates of service on or after 1/1/2023, use the appropriate HCPCS codes G0105 or G0121 with the KX modifier for colonoscopy following a positive result for any of the following non-invasive stool-based CRC screening tests:
• Screening guaiac-based fecal occult blood test (gFOBT) (CPT 82270)
• Screening immunoassay-based fecal occult blood test (iFOBT) (HCPCS G0328)
• Cologuard™ – multi-target stool DNA (sDNA) test (CPT 81528)
According to the guidance in the CMS Manual System, if modifier KX is not added to G0105 or G0121 for colonoscopy following a positive non-invasive stool-based test, Medicare will return the screening colonoscopy claim as “unprocessable.”3 If this happens, add modifier KX and resubmit the claim.
If polyps are removed during a screening colonoscopy, use the appropriate CPT code (45380, 45384, 45385, 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare. However, it is important to note that if a polyp is removed during a screening colonoscopy, the Medicare beneficiary is responsible for 15% of the cost from 2023 to 2026. This falls to 10% of the cost from 2027 to 2029, and by 2030 it will be covered 100% by Medicare. Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet.

What to do if your patient gets an unexpected bill
If your patient gets an unexpected bill and you coded the procedure correctly with the correct modifier, direct them to the AGA GI Patient Care Center’s “Colorectal cancer screening: what to expect when paying” resource for help with next steps.4
The authors have no conflicts to declare.
References
1. U.S. Department of Labor (2022, Jan. 10) FAQs About Affordable Care Act Implementation Part 51. https://www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-51.pdf
2. Centers for Medicare and Medicaid Services (n.d.) Affordable Care Act Implementation FAQs - Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.
3. Centers for Medicare and Medicaid Services (2023, Jan. 27) CMS Manual System Pub 100-03 Medicare National Coverage Determinations Transmittal 11824. https://www.cms.gov/files/document/r11824ncd.pdf.
4. American Gastroenterological Association (2023, Feb. 21) AGA GI Patient Center Colorectal Cancer Screening: What to expect when paying. https://patient.gastro.org/paying-for-your-colonoscopy/.
The Departments of Labor, Health & Human Services, and the Treasury issued guidance in 2022 that plans and insurers “must cover and may not impose cost sharing with respect to a colonoscopy conducted after a positive non-invasive stool-based screening test” for plan or policy years1 beginning on or after May 31, 2022, and, further, “may not impose cost-sharing with respect to a polyp removal during a colonoscopy performed as a screening procedure.”2 So why are so many patients still being charged fees for these screening services? In many cases, the answer comes down to missing code modifiers.
Commercial insurers want you to use modifier 33
AGA spoke to Elevance (formerly Anthem), Cigna, Aetna, and Blue Cross Blue Shield Association about how physicians should report colorectal cancer screening procedures and tests. They said using the 33 modifier (preventive service) is essential for their systems to trigger the screening benefits for beneficiaries. Without the 33 modifier, the claim will be processed as a diagnostic service, and coinsurance may apply.
According to the CPT manual, modifier 33 should be used “when the primary purpose of the service is the delivery of an evidence-based service in accordance with a U.S. Preventive Services Task Force A or B rating in effect and other preventive services identified in preventive mandates (legislative or regulatory) ...” Use modifier 33 with colonoscopies that start out as screening procedures and with colonoscopies following a positive non-invasive stool-based test, like fecal immunochemical test (FIT) or Cologuard™ multi-target stool DNA test.
It is important to note that modifier 33 won’t ensure all screening colonoscopy claims are paid, because not all commercial plans are required to cover 100 percent of the costs of CRC screening tests and procedures. For example, employer-sponsored insurance plans and legacy plans can choose not to adopt the expanded CRC benefits. Patients who are covered under these plans may not be aware that their CRC test or procedure will not be fully covered. These patients may still receive a “surprise” bill if their screening colonoscopy requires removal of polyps or if they have a colonoscopy following a positive non-invasive CRC test.
Medicare wants you to use modifiers PT and KX, but not together
CMS uses Healthcare Common Procedural Coding System (HCPCS) codes to differentiate between screening and diagnostic colonoscopies to apply screening benefits. For Medicare beneficiaries who choose colonoscopy as their CRC screening, use HCPCS code G0105 (Colorectal cancer screening; colonoscopy on individual at high risk) or G0121 (Colorectal cancer screening; colonoscopy on individual not meeting the criteria for high risk) for screening colonoscopies as appropriate. No modifier is necessary with G0105 or G0121.
Effective for claims with dates of service on or after 1/1/2023, use the appropriate HCPCS codes G0105 or G0121 with the KX modifier for colonoscopy following a positive result for any of the following non-invasive stool-based CRC screening tests:
• Screening guaiac-based fecal occult blood test (gFOBT) (CPT 82270)
• Screening immunoassay-based fecal occult blood test (iFOBT) (HCPCS G0328)
• Cologuard™ – multi-target stool DNA (sDNA) test (CPT 81528)
According to the guidance in the CMS Manual System, if modifier KX is not added to G0105 or G0121 for colonoscopy following a positive non-invasive stool-based test, Medicare will return the screening colonoscopy claim as “unprocessable.”3 If this happens, add modifier KX and resubmit the claim.
If polyps are removed during a screening colonoscopy, use the appropriate CPT code (45380, 45384, 45385, 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare. However, it is important to note that if a polyp is removed during a screening colonoscopy, the Medicare beneficiary is responsible for 15% of the cost from 2023 to 2026. This falls to 10% of the cost from 2027 to 2029, and by 2030 it will be covered 100% by Medicare. Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet.

What to do if your patient gets an unexpected bill
If your patient gets an unexpected bill and you coded the procedure correctly with the correct modifier, direct them to the AGA GI Patient Care Center’s “Colorectal cancer screening: what to expect when paying” resource for help with next steps.4
The authors have no conflicts to declare.
References
1. U.S. Department of Labor (2022, Jan. 10) FAQs About Affordable Care Act Implementation Part 51. https://www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-51.pdf
2. Centers for Medicare and Medicaid Services (n.d.) Affordable Care Act Implementation FAQs - Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.
3. Centers for Medicare and Medicaid Services (2023, Jan. 27) CMS Manual System Pub 100-03 Medicare National Coverage Determinations Transmittal 11824. https://www.cms.gov/files/document/r11824ncd.pdf.
4. American Gastroenterological Association (2023, Feb. 21) AGA GI Patient Center Colorectal Cancer Screening: What to expect when paying. https://patient.gastro.org/paying-for-your-colonoscopy/.
The Departments of Labor, Health & Human Services, and the Treasury issued guidance in 2022 that plans and insurers “must cover and may not impose cost sharing with respect to a colonoscopy conducted after a positive non-invasive stool-based screening test” for plan or policy years1 beginning on or after May 31, 2022, and, further, “may not impose cost-sharing with respect to a polyp removal during a colonoscopy performed as a screening procedure.”2 So why are so many patients still being charged fees for these screening services? In many cases, the answer comes down to missing code modifiers.
Commercial insurers want you to use modifier 33
AGA spoke to Elevance (formerly Anthem), Cigna, Aetna, and Blue Cross Blue Shield Association about how physicians should report colorectal cancer screening procedures and tests. They said using the 33 modifier (preventive service) is essential for their systems to trigger the screening benefits for beneficiaries. Without the 33 modifier, the claim will be processed as a diagnostic service, and coinsurance may apply.
According to the CPT manual, modifier 33 should be used “when the primary purpose of the service is the delivery of an evidence-based service in accordance with a U.S. Preventive Services Task Force A or B rating in effect and other preventive services identified in preventive mandates (legislative or regulatory) ...” Use modifier 33 with colonoscopies that start out as screening procedures and with colonoscopies following a positive non-invasive stool-based test, like fecal immunochemical test (FIT) or Cologuard™ multi-target stool DNA test.
It is important to note that modifier 33 won’t ensure all screening colonoscopy claims are paid, because not all commercial plans are required to cover 100 percent of the costs of CRC screening tests and procedures. For example, employer-sponsored insurance plans and legacy plans can choose not to adopt the expanded CRC benefits. Patients who are covered under these plans may not be aware that their CRC test or procedure will not be fully covered. These patients may still receive a “surprise” bill if their screening colonoscopy requires removal of polyps or if they have a colonoscopy following a positive non-invasive CRC test.
Medicare wants you to use modifiers PT and KX, but not together
CMS uses Healthcare Common Procedural Coding System (HCPCS) codes to differentiate between screening and diagnostic colonoscopies to apply screening benefits. For Medicare beneficiaries who choose colonoscopy as their CRC screening, use HCPCS code G0105 (Colorectal cancer screening; colonoscopy on individual at high risk) or G0121 (Colorectal cancer screening; colonoscopy on individual not meeting the criteria for high risk) for screening colonoscopies as appropriate. No modifier is necessary with G0105 or G0121.
Effective for claims with dates of service on or after 1/1/2023, use the appropriate HCPCS codes G0105 or G0121 with the KX modifier for colonoscopy following a positive result for any of the following non-invasive stool-based CRC screening tests:
• Screening guaiac-based fecal occult blood test (gFOBT) (CPT 82270)
• Screening immunoassay-based fecal occult blood test (iFOBT) (HCPCS G0328)
• Cologuard™ – multi-target stool DNA (sDNA) test (CPT 81528)
According to the guidance in the CMS Manual System, if modifier KX is not added to G0105 or G0121 for colonoscopy following a positive non-invasive stool-based test, Medicare will return the screening colonoscopy claim as “unprocessable.”3 If this happens, add modifier KX and resubmit the claim.
If polyps are removed during a screening colonoscopy, use the appropriate CPT code (45380, 45384, 45385, 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare. However, it is important to note that if a polyp is removed during a screening colonoscopy, the Medicare beneficiary is responsible for 15% of the cost from 2023 to 2026. This falls to 10% of the cost from 2027 to 2029, and by 2030 it will be covered 100% by Medicare. Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet.

What to do if your patient gets an unexpected bill
If your patient gets an unexpected bill and you coded the procedure correctly with the correct modifier, direct them to the AGA GI Patient Care Center’s “Colorectal cancer screening: what to expect when paying” resource for help with next steps.4
The authors have no conflicts to declare.
References
1. U.S. Department of Labor (2022, Jan. 10) FAQs About Affordable Care Act Implementation Part 51. https://www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-51.pdf
2. Centers for Medicare and Medicaid Services (n.d.) Affordable Care Act Implementation FAQs - Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.
3. Centers for Medicare and Medicaid Services (2023, Jan. 27) CMS Manual System Pub 100-03 Medicare National Coverage Determinations Transmittal 11824. https://www.cms.gov/files/document/r11824ncd.pdf.
4. American Gastroenterological Association (2023, Feb. 21) AGA GI Patient Center Colorectal Cancer Screening: What to expect when paying. https://patient.gastro.org/paying-for-your-colonoscopy/.


