User login
Reducing guesswork in schizophrenia treatment
The Positive and Negative Syndrome Scale (PANSS) is moving from research into clinical practice as demand grows for objective rating scales. We see the PANSS becoming a treatment and planning tool for psychiatry, just as the electrocardiogram evolved into a measure of cardiac status in medical practice.
Based on our experience in co-authoring (LA Opler) and using the PANSS, we describe how you can use it to:
- identify psychotic symptoms for targeted treatment
- predict with greater accuracy how patients will respond to the treatment you provide.
Standardized Assessments
The PANSS first gained stature in studies that established the efficacy of second-generation antipsychotics (SGAs).1-6 But its authors7 also envisioned the scale as a useful tool to help practicing clinicians treat patients with schizophrenia and other psychotic disorders.
Twenty years of experience has shown the PANSS to be a reliable and valid severity symptom scale for schizophrenia, bipolar disorder, and other serious mental illnesses. It is particularly useful to track changes in positive and negative symptoms.8
Traditionally, psychiatric evaluation has been impressionistic and subjective, but standardized tools provide a common language while introducing objective, empiric measures of clinical status. Because patients with mental disorders are treated by providers from psychiatry, psychology, social work, nursing, and other mental health disciplines, having standardized benchmarks to assess symptom severity can facilitate an integrated approach. And because the PANSS has been translated into some 40 languages and is being adopted in clinical settings worldwide, it provides a universal means of communicating information about a patient’s clinical status.
Panss Scoring System
The PANSS includes 30 items, each rated from 1 (absent) to 7 (extreme). In theory, a patient rated “absent” (or 1) on all items would receive a total score of 30, and a patient rated “extreme” (or 7) on all items would receive a total score of 210. In the real world, though, no one sees these extremes. Stable outpatients usually score 60 to 80. Inpatients’ scores rarely exceed 80 to 150, even in “treatment refractory” cases.
The 30 items are arranged as 7 positive symptom subscale items (P1-P7), 7 negative symptom subscale items (N1-N7), and 16 general psychopathology symptom items (G1-G16) (Table 1). Each item has a definition and a basis for rating. The first question you need to answer when rating a patient is whether the item is absent or present.
How it works. For example, the PANSS defines delusions as “beliefs that are unfounded, unrealistic, and idiosyncratic,” and the basis for rating is “thought content expressed during the interview and its influence on the patient’s social relations and behavior as reported from primary care workers or family.” If the definition does not apply to your patient, you rate this item 1 or absent. If the definition does apply, “anchoring points” for each level of severity are provided (Table 2), and you decide which anchoring point best describes the patient’s functioning during the interview and the preceding week.
Time required. In research, gathering informant information, conducting the interview, and generating reliable ratings takes 45 to 60 minutes. In clinical settings, if you know your patient and can function as informant and interviewer, you probably can obtain accurate ratings in 30 to 45 minutes.
Ideally, you would use the Structured Clinical Interview for the PANSS (SCI-PANSS), though clinicians who know this instrument well may prefer a less structured interview that covers all areas of inquiry. Accurate PANSS scores are easy to generate on all 30 items by combining information from the interview with information about how the patient has functioned in the past week.
PANSS ratings are not meant to be obtained after every patient contact but rather as often as needed to guide clinical treatment. For example, you might obtain a PANSS rating:
- when an inpatient is first admitted
- before starting a new medication
- weeks or months later to gauge the new treatment’s effect.
The PANSS manual—a complete individual kit costs approximately $200—or licenses to use multiple copies are available from the copyright holder, MultiHealth Systems, Inc. (see Related resources).
Table 1
Subscales of the 30-item Positive and Negative Syndrome Scale (PANSS)
| 7 Positive symptom subscale items | 7 Negative symptom subscale items |
| P1. Delusions | N1. Blunted affect |
| P2. Conceptual disorganization | N2. Emotional withdrawal |
| P3. Hallucinatory behavior | N3. Poor rapport |
| P4. Excitement | N4. Passive/apathetic social withdrawal |
| P5. Grandiosity | N5. Difficulty in abstract thinking |
| P6. Suspiciousness/persecution | N6. Lack of spontaneity and flow of conversation |
| P7. Hostility | N7. Stereotyped thinking |
| 16 General psychopathology symptoms | |
| G1. Somatic concern | G9. Unusual thought content |
| G2. Anxiety | G10. Disorientation |
| G3. Guilt feelings | G11. Poor attention |
| G4. Tension | G12. Lack of judgment and insight |
| G5. Mannerisms and posturing | G13. Disturbance of volition |
| G6. Depression | G14. Poor impulse control |
| G7. Motor retardation | G15. Preoccupation |
| G8. Uncooperativeness | G16. Active social avoidance |
Table 2
7 levels of severity on the PANSS for characterizing delusions
| Severity level (“anchoring point”) | Description of patient function |
|---|---|
| 1 - Absent | The definition does not apply |
| 2 - Minimal | Questionable pathology; the patient may be at the upper extreme of normal limits |
| 3 - Mild | Presence of one or two delusions that are vague, uncrystallized, and not tenaciously held. The delusions do not interfere with the patient’s thinking, social relations, or behavior |
| 4 - Moderate | Presence of either a kaleidoscopic array of poorly formed, unstable delusions, or a few well-formed delusions that occasionally interfere with the patient’s thinking, social relations, or behavior |
| 5 - Moderate severe | Presence of numerous well-formed delusions that are tenaciously held and occasionally interfere with the patient’s thinking, social relations, or behavior |
| 6 - Severe | Presence of a stable set of delusions that are crystallized, possibly systematized, tenaciously held, and clearly interfere with the patient’s thinking, social relations, or behavior |
| 7 - Extreme | Presence of a stable set of delusions that are either highly systematized or very numerous, and that dominate major facets of the patient’s life. This behavior frequently results in inappropriate and irresponsible action that may jeopardize the safety of the patient or others |
Gauging Symptom Severity
Treatment planning. Clinicians at the Rochester (New York) Psychiatric Center use the PANSS to assess symptom severity in inpatients with schizophrenia and other psychotic disorders.
Within 1 week of admission, patients are evaluated on the 30 items by a team of experienced PANSS raters. Symptoms identified by the PANSS become targets in individualized treatment plans. Follow-up PANSS assessments help determine if treatment has improved the selected symptoms.
Tracking patient progress. Florida State Hospital uses the PANSS to track progress of patients with serious mental illnesses. Data collected over 8 years from >19,000 PANSS assessments in a multilingual, multicultural population suggests that the PANSS:
- aids in decision making for medical and nonmedical aspects of care for individual patients
- can help determine if changes in agency prescribing practices affect patient symptom profiles and severity, one indicator of how policy and guidelines translate into patient care.9
Predicting Outcomes
The PANSS has been shown to predict course of illness and treatment response, functional outcomes (including aggression), and long-term outcomes (including deterioration). Adjusting treatments to achieve optimal PANSS scores also can help clinicians achieve remission of their patients’ psychotic symptoms (Box).11,12
Remission. Achieving and maintaining remission of schizophrenia has been hampered by a lack of specificity in existing scales. Andreasen et al11 recommend using selected items from the PANSS and other rating scales, including the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS), and Scale for Assessment of Positive Symptoms (SAPS).
Creating agreed-upon criteria will mean that clinicians will know what is meant by symptom remission, allowing for better communication and a standard to achieve.
Costs. Eventually, rating scales such as PANSS may provide “financial prognoses” to predict treatment costs over time. Mohr et al12 used PANSS scores to group 663 patients from public and private psychiatric hospitals into eight categories based on symptom severity. When each disease state was correlated with annual treatment costs, baseline assessment was a significant predictor of annualized cost as well as clinical outcome.
Functional outcomes. Steinert et al14 used the PANSS to rate 199 inpatients within 24 hours of admission into an acute psychiatric ward. After discharge, each patient was assessed retrospectively for aggressive behavior. The conceptual disorganization and hostility items from the positive sub-scale could predict violent behaviors during inpatient treatment with statistical significance.
Long-term outcomes. White et al15 assessed older schizophrenia inpatients, using the PANSS at baseline and after 1 year. The researchers looked specifically at the “activation factor”—six PANSS items including hostility, poor impulse control, excitement, uncooperativeness, poor rapport, and tension. Poor outcome and low discharge rates were directly correlated with high baseline scores on the PANSS activation factor (PANSS-AF).
Deterioration. Goetz et al16 showed that residual positive symptoms were significantly related to deteriorating course of illness, even when patients adhered to their medications. These results suggest that even subtle symptom elevations as measured by the PANSS can predict deterioration.
- The PANSS Institute. Information on how to attain, maintain, and retain high reliability as a PANSS rater. www.panss.org.
- MultiHealth Systems, Inc. (publisher and copyright holder) to purchase the Positive and Negative Syndrome Scale (PANSS). www.mhs.com.
- Opler LA, Ramirez PM, Mougios VA. Measuring outcome in serious mental illness. In: IsHak WW, Burt T, Sederer L (eds). Outcome measurement in psychiatry: a critical review. Washington, DC: American Psychiatric Press; 2002.
Dr. Lewis A. Opler receives royalties from MultiHealth Systems, Inc. on sales of the Positive and Negative Syndrome Scale (PANSS) Manual, the Structured Clinical Interview for the PANSS (SCI-PANSS), and the Informant Questionnaire for the PANSS (IQ-PANSS).
Dr. Mark G. Opler is Executive Director of The PANSS Institute.
Acknowledgement
This work was supported in part by NIMH grant K24 MH01699 (DM).
1. van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1-2):168-75.
2. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 2003;64(5):580-8.
3. Duggan L, Fenton M, Rathbone J, et al. Olanzapine for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. Article No. CD001359.
4. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63(9):763-71.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004;83(2-3):263-75.
6. Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adol Psychopharmacol 2003;13(3):319-27.
7. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Toronto, Ontario: MultiHealth Systems, Inc.; 2006.
8. Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990;61(3):163-78.
9. Annis LV. Implementation of the Positive and Negative Syndrome Scale in a state psychiatric hospital: eight years of data and experience. Paper presented at: 16th Annual Conference on State Mental Health Agency Services Research, Program Evaluation and Policy, 2006.
10. Zalsman G, Posmanik S, Fisch T, et al. Psychosocial situations, quality of depression and schizophrenia in adolescents. Psychiatry Res 2003;129:149-157.
11. Andreason N, Carpenter W, Kane J, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441-9.
12. Mohr PE, Cheng CM, Claxton K, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
13. Hatta K, Nakamura H, Matsuzaki I. Acute-phase treatment in general hospitals: clinical psychopharmacologic evaluation in first-episode schizophrenia patients. Gen Hosp Psychiatry 2003;25:39-45.
14. Steinert T, Wolfle M, Gebhardt R-P. Measurement of violence during inpatient treatment and association with psychopathology. Acta Psychiatr Scand 2000;102:107-12.
15. White L, Opler L, Harvey P, et al. Activation symptoms and discharge in early chronic schizophrenia inpatients. J Nerv Ment Dis 2004;192(12):880-3.
16. Goetz D, Goetz R, Yale S, et al. Comparing early and chronic psychosis clinical characteristics. Schizophr Res 2004;70:120.-
The Positive and Negative Syndrome Scale (PANSS) is moving from research into clinical practice as demand grows for objective rating scales. We see the PANSS becoming a treatment and planning tool for psychiatry, just as the electrocardiogram evolved into a measure of cardiac status in medical practice.
Based on our experience in co-authoring (LA Opler) and using the PANSS, we describe how you can use it to:
- identify psychotic symptoms for targeted treatment
- predict with greater accuracy how patients will respond to the treatment you provide.
Standardized Assessments
The PANSS first gained stature in studies that established the efficacy of second-generation antipsychotics (SGAs).1-6 But its authors7 also envisioned the scale as a useful tool to help practicing clinicians treat patients with schizophrenia and other psychotic disorders.
Twenty years of experience has shown the PANSS to be a reliable and valid severity symptom scale for schizophrenia, bipolar disorder, and other serious mental illnesses. It is particularly useful to track changes in positive and negative symptoms.8
Traditionally, psychiatric evaluation has been impressionistic and subjective, but standardized tools provide a common language while introducing objective, empiric measures of clinical status. Because patients with mental disorders are treated by providers from psychiatry, psychology, social work, nursing, and other mental health disciplines, having standardized benchmarks to assess symptom severity can facilitate an integrated approach. And because the PANSS has been translated into some 40 languages and is being adopted in clinical settings worldwide, it provides a universal means of communicating information about a patient’s clinical status.
Panss Scoring System
The PANSS includes 30 items, each rated from 1 (absent) to 7 (extreme). In theory, a patient rated “absent” (or 1) on all items would receive a total score of 30, and a patient rated “extreme” (or 7) on all items would receive a total score of 210. In the real world, though, no one sees these extremes. Stable outpatients usually score 60 to 80. Inpatients’ scores rarely exceed 80 to 150, even in “treatment refractory” cases.
The 30 items are arranged as 7 positive symptom subscale items (P1-P7), 7 negative symptom subscale items (N1-N7), and 16 general psychopathology symptom items (G1-G16) (Table 1). Each item has a definition and a basis for rating. The first question you need to answer when rating a patient is whether the item is absent or present.
How it works. For example, the PANSS defines delusions as “beliefs that are unfounded, unrealistic, and idiosyncratic,” and the basis for rating is “thought content expressed during the interview and its influence on the patient’s social relations and behavior as reported from primary care workers or family.” If the definition does not apply to your patient, you rate this item 1 or absent. If the definition does apply, “anchoring points” for each level of severity are provided (Table 2), and you decide which anchoring point best describes the patient’s functioning during the interview and the preceding week.
Time required. In research, gathering informant information, conducting the interview, and generating reliable ratings takes 45 to 60 minutes. In clinical settings, if you know your patient and can function as informant and interviewer, you probably can obtain accurate ratings in 30 to 45 minutes.
Ideally, you would use the Structured Clinical Interview for the PANSS (SCI-PANSS), though clinicians who know this instrument well may prefer a less structured interview that covers all areas of inquiry. Accurate PANSS scores are easy to generate on all 30 items by combining information from the interview with information about how the patient has functioned in the past week.
PANSS ratings are not meant to be obtained after every patient contact but rather as often as needed to guide clinical treatment. For example, you might obtain a PANSS rating:
- when an inpatient is first admitted
- before starting a new medication
- weeks or months later to gauge the new treatment’s effect.
The PANSS manual—a complete individual kit costs approximately $200—or licenses to use multiple copies are available from the copyright holder, MultiHealth Systems, Inc. (see Related resources).
Table 1
Subscales of the 30-item Positive and Negative Syndrome Scale (PANSS)
| 7 Positive symptom subscale items | 7 Negative symptom subscale items |
| P1. Delusions | N1. Blunted affect |
| P2. Conceptual disorganization | N2. Emotional withdrawal |
| P3. Hallucinatory behavior | N3. Poor rapport |
| P4. Excitement | N4. Passive/apathetic social withdrawal |
| P5. Grandiosity | N5. Difficulty in abstract thinking |
| P6. Suspiciousness/persecution | N6. Lack of spontaneity and flow of conversation |
| P7. Hostility | N7. Stereotyped thinking |
| 16 General psychopathology symptoms | |
| G1. Somatic concern | G9. Unusual thought content |
| G2. Anxiety | G10. Disorientation |
| G3. Guilt feelings | G11. Poor attention |
| G4. Tension | G12. Lack of judgment and insight |
| G5. Mannerisms and posturing | G13. Disturbance of volition |
| G6. Depression | G14. Poor impulse control |
| G7. Motor retardation | G15. Preoccupation |
| G8. Uncooperativeness | G16. Active social avoidance |
Table 2
7 levels of severity on the PANSS for characterizing delusions
| Severity level (“anchoring point”) | Description of patient function |
|---|---|
| 1 - Absent | The definition does not apply |
| 2 - Minimal | Questionable pathology; the patient may be at the upper extreme of normal limits |
| 3 - Mild | Presence of one or two delusions that are vague, uncrystallized, and not tenaciously held. The delusions do not interfere with the patient’s thinking, social relations, or behavior |
| 4 - Moderate | Presence of either a kaleidoscopic array of poorly formed, unstable delusions, or a few well-formed delusions that occasionally interfere with the patient’s thinking, social relations, or behavior |
| 5 - Moderate severe | Presence of numerous well-formed delusions that are tenaciously held and occasionally interfere with the patient’s thinking, social relations, or behavior |
| 6 - Severe | Presence of a stable set of delusions that are crystallized, possibly systematized, tenaciously held, and clearly interfere with the patient’s thinking, social relations, or behavior |
| 7 - Extreme | Presence of a stable set of delusions that are either highly systematized or very numerous, and that dominate major facets of the patient’s life. This behavior frequently results in inappropriate and irresponsible action that may jeopardize the safety of the patient or others |
Gauging Symptom Severity
Treatment planning. Clinicians at the Rochester (New York) Psychiatric Center use the PANSS to assess symptom severity in inpatients with schizophrenia and other psychotic disorders.
Within 1 week of admission, patients are evaluated on the 30 items by a team of experienced PANSS raters. Symptoms identified by the PANSS become targets in individualized treatment plans. Follow-up PANSS assessments help determine if treatment has improved the selected symptoms.
Tracking patient progress. Florida State Hospital uses the PANSS to track progress of patients with serious mental illnesses. Data collected over 8 years from >19,000 PANSS assessments in a multilingual, multicultural population suggests that the PANSS:
- aids in decision making for medical and nonmedical aspects of care for individual patients
- can help determine if changes in agency prescribing practices affect patient symptom profiles and severity, one indicator of how policy and guidelines translate into patient care.9
Predicting Outcomes
The PANSS has been shown to predict course of illness and treatment response, functional outcomes (including aggression), and long-term outcomes (including deterioration). Adjusting treatments to achieve optimal PANSS scores also can help clinicians achieve remission of their patients’ psychotic symptoms (Box).11,12
Remission. Achieving and maintaining remission of schizophrenia has been hampered by a lack of specificity in existing scales. Andreasen et al11 recommend using selected items from the PANSS and other rating scales, including the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS), and Scale for Assessment of Positive Symptoms (SAPS).
Creating agreed-upon criteria will mean that clinicians will know what is meant by symptom remission, allowing for better communication and a standard to achieve.
Costs. Eventually, rating scales such as PANSS may provide “financial prognoses” to predict treatment costs over time. Mohr et al12 used PANSS scores to group 663 patients from public and private psychiatric hospitals into eight categories based on symptom severity. When each disease state was correlated with annual treatment costs, baseline assessment was a significant predictor of annualized cost as well as clinical outcome.
Functional outcomes. Steinert et al14 used the PANSS to rate 199 inpatients within 24 hours of admission into an acute psychiatric ward. After discharge, each patient was assessed retrospectively for aggressive behavior. The conceptual disorganization and hostility items from the positive sub-scale could predict violent behaviors during inpatient treatment with statistical significance.
Long-term outcomes. White et al15 assessed older schizophrenia inpatients, using the PANSS at baseline and after 1 year. The researchers looked specifically at the “activation factor”—six PANSS items including hostility, poor impulse control, excitement, uncooperativeness, poor rapport, and tension. Poor outcome and low discharge rates were directly correlated with high baseline scores on the PANSS activation factor (PANSS-AF).
Deterioration. Goetz et al16 showed that residual positive symptoms were significantly related to deteriorating course of illness, even when patients adhered to their medications. These results suggest that even subtle symptom elevations as measured by the PANSS can predict deterioration.
- The PANSS Institute. Information on how to attain, maintain, and retain high reliability as a PANSS rater. www.panss.org.
- MultiHealth Systems, Inc. (publisher and copyright holder) to purchase the Positive and Negative Syndrome Scale (PANSS). www.mhs.com.
- Opler LA, Ramirez PM, Mougios VA. Measuring outcome in serious mental illness. In: IsHak WW, Burt T, Sederer L (eds). Outcome measurement in psychiatry: a critical review. Washington, DC: American Psychiatric Press; 2002.
Dr. Lewis A. Opler receives royalties from MultiHealth Systems, Inc. on sales of the Positive and Negative Syndrome Scale (PANSS) Manual, the Structured Clinical Interview for the PANSS (SCI-PANSS), and the Informant Questionnaire for the PANSS (IQ-PANSS).
Dr. Mark G. Opler is Executive Director of The PANSS Institute.
Acknowledgement
This work was supported in part by NIMH grant K24 MH01699 (DM).
The Positive and Negative Syndrome Scale (PANSS) is moving from research into clinical practice as demand grows for objective rating scales. We see the PANSS becoming a treatment and planning tool for psychiatry, just as the electrocardiogram evolved into a measure of cardiac status in medical practice.
Based on our experience in co-authoring (LA Opler) and using the PANSS, we describe how you can use it to:
- identify psychotic symptoms for targeted treatment
- predict with greater accuracy how patients will respond to the treatment you provide.
Standardized Assessments
The PANSS first gained stature in studies that established the efficacy of second-generation antipsychotics (SGAs).1-6 But its authors7 also envisioned the scale as a useful tool to help practicing clinicians treat patients with schizophrenia and other psychotic disorders.
Twenty years of experience has shown the PANSS to be a reliable and valid severity symptom scale for schizophrenia, bipolar disorder, and other serious mental illnesses. It is particularly useful to track changes in positive and negative symptoms.8
Traditionally, psychiatric evaluation has been impressionistic and subjective, but standardized tools provide a common language while introducing objective, empiric measures of clinical status. Because patients with mental disorders are treated by providers from psychiatry, psychology, social work, nursing, and other mental health disciplines, having standardized benchmarks to assess symptom severity can facilitate an integrated approach. And because the PANSS has been translated into some 40 languages and is being adopted in clinical settings worldwide, it provides a universal means of communicating information about a patient’s clinical status.
Panss Scoring System
The PANSS includes 30 items, each rated from 1 (absent) to 7 (extreme). In theory, a patient rated “absent” (or 1) on all items would receive a total score of 30, and a patient rated “extreme” (or 7) on all items would receive a total score of 210. In the real world, though, no one sees these extremes. Stable outpatients usually score 60 to 80. Inpatients’ scores rarely exceed 80 to 150, even in “treatment refractory” cases.
The 30 items are arranged as 7 positive symptom subscale items (P1-P7), 7 negative symptom subscale items (N1-N7), and 16 general psychopathology symptom items (G1-G16) (Table 1). Each item has a definition and a basis for rating. The first question you need to answer when rating a patient is whether the item is absent or present.
How it works. For example, the PANSS defines delusions as “beliefs that are unfounded, unrealistic, and idiosyncratic,” and the basis for rating is “thought content expressed during the interview and its influence on the patient’s social relations and behavior as reported from primary care workers or family.” If the definition does not apply to your patient, you rate this item 1 or absent. If the definition does apply, “anchoring points” for each level of severity are provided (Table 2), and you decide which anchoring point best describes the patient’s functioning during the interview and the preceding week.
Time required. In research, gathering informant information, conducting the interview, and generating reliable ratings takes 45 to 60 minutes. In clinical settings, if you know your patient and can function as informant and interviewer, you probably can obtain accurate ratings in 30 to 45 minutes.
Ideally, you would use the Structured Clinical Interview for the PANSS (SCI-PANSS), though clinicians who know this instrument well may prefer a less structured interview that covers all areas of inquiry. Accurate PANSS scores are easy to generate on all 30 items by combining information from the interview with information about how the patient has functioned in the past week.
PANSS ratings are not meant to be obtained after every patient contact but rather as often as needed to guide clinical treatment. For example, you might obtain a PANSS rating:
- when an inpatient is first admitted
- before starting a new medication
- weeks or months later to gauge the new treatment’s effect.
The PANSS manual—a complete individual kit costs approximately $200—or licenses to use multiple copies are available from the copyright holder, MultiHealth Systems, Inc. (see Related resources).
Table 1
Subscales of the 30-item Positive and Negative Syndrome Scale (PANSS)
| 7 Positive symptom subscale items | 7 Negative symptom subscale items |
| P1. Delusions | N1. Blunted affect |
| P2. Conceptual disorganization | N2. Emotional withdrawal |
| P3. Hallucinatory behavior | N3. Poor rapport |
| P4. Excitement | N4. Passive/apathetic social withdrawal |
| P5. Grandiosity | N5. Difficulty in abstract thinking |
| P6. Suspiciousness/persecution | N6. Lack of spontaneity and flow of conversation |
| P7. Hostility | N7. Stereotyped thinking |
| 16 General psychopathology symptoms | |
| G1. Somatic concern | G9. Unusual thought content |
| G2. Anxiety | G10. Disorientation |
| G3. Guilt feelings | G11. Poor attention |
| G4. Tension | G12. Lack of judgment and insight |
| G5. Mannerisms and posturing | G13. Disturbance of volition |
| G6. Depression | G14. Poor impulse control |
| G7. Motor retardation | G15. Preoccupation |
| G8. Uncooperativeness | G16. Active social avoidance |
Table 2
7 levels of severity on the PANSS for characterizing delusions
| Severity level (“anchoring point”) | Description of patient function |
|---|---|
| 1 - Absent | The definition does not apply |
| 2 - Minimal | Questionable pathology; the patient may be at the upper extreme of normal limits |
| 3 - Mild | Presence of one or two delusions that are vague, uncrystallized, and not tenaciously held. The delusions do not interfere with the patient’s thinking, social relations, or behavior |
| 4 - Moderate | Presence of either a kaleidoscopic array of poorly formed, unstable delusions, or a few well-formed delusions that occasionally interfere with the patient’s thinking, social relations, or behavior |
| 5 - Moderate severe | Presence of numerous well-formed delusions that are tenaciously held and occasionally interfere with the patient’s thinking, social relations, or behavior |
| 6 - Severe | Presence of a stable set of delusions that are crystallized, possibly systematized, tenaciously held, and clearly interfere with the patient’s thinking, social relations, or behavior |
| 7 - Extreme | Presence of a stable set of delusions that are either highly systematized or very numerous, and that dominate major facets of the patient’s life. This behavior frequently results in inappropriate and irresponsible action that may jeopardize the safety of the patient or others |
Gauging Symptom Severity
Treatment planning. Clinicians at the Rochester (New York) Psychiatric Center use the PANSS to assess symptom severity in inpatients with schizophrenia and other psychotic disorders.
Within 1 week of admission, patients are evaluated on the 30 items by a team of experienced PANSS raters. Symptoms identified by the PANSS become targets in individualized treatment plans. Follow-up PANSS assessments help determine if treatment has improved the selected symptoms.
Tracking patient progress. Florida State Hospital uses the PANSS to track progress of patients with serious mental illnesses. Data collected over 8 years from >19,000 PANSS assessments in a multilingual, multicultural population suggests that the PANSS:
- aids in decision making for medical and nonmedical aspects of care for individual patients
- can help determine if changes in agency prescribing practices affect patient symptom profiles and severity, one indicator of how policy and guidelines translate into patient care.9
Predicting Outcomes
The PANSS has been shown to predict course of illness and treatment response, functional outcomes (including aggression), and long-term outcomes (including deterioration). Adjusting treatments to achieve optimal PANSS scores also can help clinicians achieve remission of their patients’ psychotic symptoms (Box).11,12
Remission. Achieving and maintaining remission of schizophrenia has been hampered by a lack of specificity in existing scales. Andreasen et al11 recommend using selected items from the PANSS and other rating scales, including the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS), and Scale for Assessment of Positive Symptoms (SAPS).
Creating agreed-upon criteria will mean that clinicians will know what is meant by symptom remission, allowing for better communication and a standard to achieve.
Costs. Eventually, rating scales such as PANSS may provide “financial prognoses” to predict treatment costs over time. Mohr et al12 used PANSS scores to group 663 patients from public and private psychiatric hospitals into eight categories based on symptom severity. When each disease state was correlated with annual treatment costs, baseline assessment was a significant predictor of annualized cost as well as clinical outcome.
Functional outcomes. Steinert et al14 used the PANSS to rate 199 inpatients within 24 hours of admission into an acute psychiatric ward. After discharge, each patient was assessed retrospectively for aggressive behavior. The conceptual disorganization and hostility items from the positive sub-scale could predict violent behaviors during inpatient treatment with statistical significance.
Long-term outcomes. White et al15 assessed older schizophrenia inpatients, using the PANSS at baseline and after 1 year. The researchers looked specifically at the “activation factor”—six PANSS items including hostility, poor impulse control, excitement, uncooperativeness, poor rapport, and tension. Poor outcome and low discharge rates were directly correlated with high baseline scores on the PANSS activation factor (PANSS-AF).
Deterioration. Goetz et al16 showed that residual positive symptoms were significantly related to deteriorating course of illness, even when patients adhered to their medications. These results suggest that even subtle symptom elevations as measured by the PANSS can predict deterioration.
- The PANSS Institute. Information on how to attain, maintain, and retain high reliability as a PANSS rater. www.panss.org.
- MultiHealth Systems, Inc. (publisher and copyright holder) to purchase the Positive and Negative Syndrome Scale (PANSS). www.mhs.com.
- Opler LA, Ramirez PM, Mougios VA. Measuring outcome in serious mental illness. In: IsHak WW, Burt T, Sederer L (eds). Outcome measurement in psychiatry: a critical review. Washington, DC: American Psychiatric Press; 2002.
Dr. Lewis A. Opler receives royalties from MultiHealth Systems, Inc. on sales of the Positive and Negative Syndrome Scale (PANSS) Manual, the Structured Clinical Interview for the PANSS (SCI-PANSS), and the Informant Questionnaire for the PANSS (IQ-PANSS).
Dr. Mark G. Opler is Executive Director of The PANSS Institute.
Acknowledgement
This work was supported in part by NIMH grant K24 MH01699 (DM).
1. van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1-2):168-75.
2. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 2003;64(5):580-8.
3. Duggan L, Fenton M, Rathbone J, et al. Olanzapine for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. Article No. CD001359.
4. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63(9):763-71.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004;83(2-3):263-75.
6. Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adol Psychopharmacol 2003;13(3):319-27.
7. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Toronto, Ontario: MultiHealth Systems, Inc.; 2006.
8. Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990;61(3):163-78.
9. Annis LV. Implementation of the Positive and Negative Syndrome Scale in a state psychiatric hospital: eight years of data and experience. Paper presented at: 16th Annual Conference on State Mental Health Agency Services Research, Program Evaluation and Policy, 2006.
10. Zalsman G, Posmanik S, Fisch T, et al. Psychosocial situations, quality of depression and schizophrenia in adolescents. Psychiatry Res 2003;129:149-157.
11. Andreason N, Carpenter W, Kane J, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441-9.
12. Mohr PE, Cheng CM, Claxton K, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
13. Hatta K, Nakamura H, Matsuzaki I. Acute-phase treatment in general hospitals: clinical psychopharmacologic evaluation in first-episode schizophrenia patients. Gen Hosp Psychiatry 2003;25:39-45.
14. Steinert T, Wolfle M, Gebhardt R-P. Measurement of violence during inpatient treatment and association with psychopathology. Acta Psychiatr Scand 2000;102:107-12.
15. White L, Opler L, Harvey P, et al. Activation symptoms and discharge in early chronic schizophrenia inpatients. J Nerv Ment Dis 2004;192(12):880-3.
16. Goetz D, Goetz R, Yale S, et al. Comparing early and chronic psychosis clinical characteristics. Schizophr Res 2004;70:120.-
1. van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1-2):168-75.
2. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 2003;64(5):580-8.
3. Duggan L, Fenton M, Rathbone J, et al. Olanzapine for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. Article No. CD001359.
4. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63(9):763-71.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004;83(2-3):263-75.
6. Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adol Psychopharmacol 2003;13(3):319-27.
7. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Toronto, Ontario: MultiHealth Systems, Inc.; 2006.
8. Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990;61(3):163-78.
9. Annis LV. Implementation of the Positive and Negative Syndrome Scale in a state psychiatric hospital: eight years of data and experience. Paper presented at: 16th Annual Conference on State Mental Health Agency Services Research, Program Evaluation and Policy, 2006.
10. Zalsman G, Posmanik S, Fisch T, et al. Psychosocial situations, quality of depression and schizophrenia in adolescents. Psychiatry Res 2003;129:149-157.
11. Andreason N, Carpenter W, Kane J, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441-9.
12. Mohr PE, Cheng CM, Claxton K, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
13. Hatta K, Nakamura H, Matsuzaki I. Acute-phase treatment in general hospitals: clinical psychopharmacologic evaluation in first-episode schizophrenia patients. Gen Hosp Psychiatry 2003;25:39-45.
14. Steinert T, Wolfle M, Gebhardt R-P. Measurement of violence during inpatient treatment and association with psychopathology. Acta Psychiatr Scand 2000;102:107-12.
15. White L, Opler L, Harvey P, et al. Activation symptoms and discharge in early chronic schizophrenia inpatients. J Nerv Ment Dis 2004;192(12):880-3.
16. Goetz D, Goetz R, Yale S, et al. Comparing early and chronic psychosis clinical characteristics. Schizophr Res 2004;70:120.-
Hepatitis C and interferon: Watch for hostility, impulsivity
Pegylated interferon-alpha with ribavirin is the most effective therapy for chronic hepatitis C infection,1,2 but psychiatric patients often discontinue IFN-alpha because of its mood side effects.3 Most studies describe depressive states, although manic symptoms—irritability, aggression, anger, hostility, emotional lability, anxiety, panic attacks, and insomnia—also have been reported.4-6
To help you manage adverse mood changes and prevent IFN-alpha treatment discontinuation in patients with hepatitis C, this article:
- reviews studies of patients with a history mood disorders who were treated with IFN-alpha
- explains how to recognize and treat IFN-alpha-induced mood disturbances when antidepressants are contraindicated.
Psychiatric Patients and IFN Therapy
Chronic hepatitis C infection is common among psychiatric patients. Routine screening among 1,556 patients admitted to a U.S. public psychiatric hospital across 3 years identified 133 patients (8.5%) who were positive for hepatitis C virus.7
Patients with psychopathologic symptoms before starting IFN therapy may suffer more-severe adverse psychiatric effects during treatment than those without psychopathology.8 In fact, mood disorders were considered an absolute contraindication to IFN therapy until recently.
Now that the National Institutes of Health (NIH) has recommended extending hepatitis C research and treatment to psychiatric patients, IFN-alpha-induced mental illness could become more common in clinical practice. Before the 2002 NIH consensus statement, patients with mental illness and substance use disorders—who represent >50% of candidates who need IFN therapy—were excluded from research protocols.
Safely using IFN. Some reports and studies suggest that patients with past or existing psychiatric disorders can be treated safely and effectively with IFN-alpha.
In a prospective open-label study, 29 of 31 patients with co-existing chronic hepatitis C and psychiatric illness completed 6 months of IFN therapy, 5 million units (MU) three times/week or 5 MU daily. Patients continued maintenance psychotropics during IFN treatment, and a psychiatrist monitored psychiatric symptoms.
Psychiatric illness worsened in four patients, and two discontinued IFN treatment. Serum alanine aminotransferase returned to normal in 22 patients (71%), and hepatitis C virus RNA cleared from the sera of 15 (48%).9
Another prospective study of 50 patients treated with IFN for chronic hepatitis found that those with a pre-existing mood or anxiety disorder were not more likely than others to discontinue IFN therapy.10
Unique to hepatitis therapy? IFN-induced mood disturbances are probably different in patients with chronic hepatitis C than in those receiving IFN for other diseases because of differences in regimens and effects of the underlying pathologies. For example, prescribing a preventative antidepressant before starting IFN treatment might help cancer patients but not patients with hepatitis C.11
This distinction could be particularly important when giving IFN-alpha to patients who are vulnerable to psychiatric illness with impulsive features. To emphasize this point, we describe clinical features and treatment response in patients with hepatitis C who were treated in our department.
IFN-Alpha-Induced Moods
In our prospective study of 93 patients, 30 (32%) developed IFN-alpha-induced mood disorders during the first 12 weeks of treatment.12 Contrary to previous studies focusing on depression, most of our patients had a mix of manic/hypomanic and depressive symptoms. Twenty-four (13 women and 11 men, mean age 43) accepted referral to a psychiatrist specializing in mood disorders to characterize their symptoms.
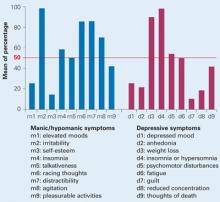
Mood characteristics. Using DSM-IV criteria, the psychiatrist determined that IFN-alpha induced both manic/hypomanic and depressive symptoms in many patients, and the manic/hypomanic features predominated. Five manic symptoms and three depressive symptoms were present in >50% of patients (Figure 1, Table 1).
Three patients presented with a manic episode,15 with hypomania with prevalent irritability and dysphoric features, and 6 with a mixed depressive state (major depressive episode with at least 3 hypomania symptoms).13 Four patients (17%) suffered a relapse of alcohol or cannabis abuse.
Nearly all (84%) reported paroxysmal anxiety, and all had emotional hyperreactivity that the psychiatrist described as a main symptom of a manic or mixed state.14 Most felt extremely impulsive and feared losing control. Two faced legal difficulties, and one was in prison.
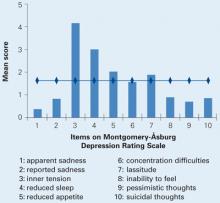
The patients’ mean Montgomery-Åsburg Depression Rating Scale (MADRS) score was 16.12 (±1.6), indicating a mild to moderate depressive state. The highest-scoring items were inner tension, reduced sleep, reduced appetite, and lassitude (Figure 2).
Their mean Bech-Rafaelsen Mania Scale15 score was 14.33 (±1.3), indicating hypomanic or moderate manic symptoms. Hostility was by far the predominant manic symptom (mean score 3.2/4). Other manic symptom scores ranged from 0.2/4 to 2.1/4 (Figure 3).
Figure 1 Mood symptoms identified in 24 patients after 12 weeks of IFN-alpha treatment
IFN: Interferon
Source: Reference 12
Figure 2 Depressive symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12
Figure 3 Manic symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12Table 1
Most-prevalent IFN-induced mood symptoms in 24 patients treated for hepatitis C
| 5 manic symptoms (% of patients) | 3 depressive symptoms (% of patients) |
| Irritability (100%) | Insomnia or hypersomnia (100%) |
| Racing thoughts (87%) | Poor appetite or weight loss (92%) |
| Distractibility (87%) | Psychomotor agitation or retardation (54%) |
| Insomnia (58%) | |
| Agitation (70%) | |
| IFN: Interferon | |
| Source: Reference 12 | |
Treatment. Given the predominance of manic/hypomanic symptoms, we treated the 24 patients with low-to-moderate dosages of an atypical antipsychotic (amisulpride, 100 to 600 mg/d). This medication—not available in the United States—would be similar to using risperidone, 1 to 6 mg/d. Low-dose benzodiazepines (clonazepam or alprazolam) were added as needed to manage insomnia. Antipsychotic treatment enabled 23 of 24 patients (96%) to continue antiviral therapy.
Five patients had received selective serotonin reuptake inhibitors (SSRIs) for 1 to 4 weeks before referral to the psychiatrist. Antidepressants worsened their mood symptoms, which included impulsivity, agitation, and insomnia. Emotional hyper-reactivity, irritability, hostility, and impulsiveness improved in all 5 patients within 1 to 2 weeks of stopping SSRIs and starting the atypical antipsychotic.
Discussion
Accurately characterizing IFN-induced mood states confirmed our published data showing a mix of manic/hypomanic and depressive symptoms in psychiatric patients treated for chronic hepatitis C. Irritability and hostility were the two most prominent symptoms.
These findings differ from those of investigations that identified depressive symptoms as the hallmark of IFN’s psychiatric side effects.6 Our data were thoroughly characterized according to DSM-IV-TR criteria, two mood-rating scales, and a psychiatrist experienced in mood disorders.
Why is mania missed? One possible explanation for these different findings is that IFN-alpha-induced fatigue and flu-like symptoms could be misinterpreted as depressive symptoms. Also, most researchers have used depression rating scales—but not mania scales—and have not considered other psychiatric diagnostics.16
Self-report questionnaires for evaluating depression also take into account somatic side effects, resulting in higher rating scores. Moreover, few studies have included clinical psychiatric interviews and even fewer diagnostic confirmation by a psychiatrist.
Finally, clinical experience indicates that patients who present with both manic/hypomanic and depressive symptoms are more likely to complain about depression than about manic symptoms. Therefore, clinicians may miss manic symptoms if they don’t actively seek them.
Irritability and hostility. Previous studies have described irritability, mood lability, and anger/hostility as frequent symptoms4,5 but failed to consider them as possible manifestations of mania or hypomania. Many of our patients reported irritability severe enough to interfere with their work, social, and family relationships.
Hostility was the symptom with the highest score on the Bech-Rafaelsen Mania Scale. This objective evidence suggests that investigating the consequences to patients of increased irritability, impulsivity, or hostility might reveal some frightening behavior
How to treat these patients. Considerable evidence from characterizing IFN-induced side effects in patients with hepatitis C points to a syndrome of depressive and manic/hypomanic symptoms13,17-19 that does not fulfill criteria for a mixed state. This disorder is not described in DSM-IV-TR and, unfortunately, usually is misdiagnosed as a depressive state.
Antidepressants can worsen depression by causing agitation and impulsivity and can increase risk of suicide.17,18,20 By contrast, atypical antipsychotics have been shown to improve bipolar depression.21,22
We used an atypical antipsychotic to treat patients with prevalent manic or hypomanic symptoms and those who did not respond to SSRIs. Their IFN-induced mood disorders improved rapidly on low dosages of amisulpride, and antiviral therapy discontinuation rates were low (1/24; 4%). By comparison, other studies have reported antiviral treatment discontinuation rates of 30% to 40% in patients taking antidepressants.23,24
The atypical antipsychotic did not improve our patients’ fatigue and other neurovegetative symptoms, but antidepressants likewise do not improve these symptoms.8
Recommendations
This study leads us to warn clinicians that antidepressants can worsen IFN-alpha-induced mood disorders and to recommend that antipsychotics be considered:
- at least before you decide to discontinue a hepatitis C patient’s IFN-alpha therapy
- or in patients with high irritability and hostility.
To determine whether to use an antidepressant or antimanic agent as first-line treatment, carefully diagnose the patient’s symptoms as a manic/hypomanic state, depressive mixed state, or depression. Successful IFN therapy requires:
- psychological support
- medication for psychiatric adverse effects
- collaboration between the psychiatrist and hepatologist.
IFN-alpha-induced mood disorders are triggered exogenously, do not correspond to typical features of any classic psychiatric disorder, and require further study. IFN-induced impulsivity and hostility also need to be better characterized, particularly as more patients with pre-existing psychiatric disorders are treated for hepatitis C.
Related resources
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345(1):41-52.
- Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry 2004;161:3.
Drug brand names
- Alprazolam • Xanax
- Amisulpride • (not available in the United States)
- Clonazepam • Klonopin
- Risperidone • Risperdal
Acknowledgment
This study was conducted with support from the Centre d’Investigation Clinique INSERM/CHU de Bordeaux.
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
2. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
3. Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207-11.
4. Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (INF-alpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
5. Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316-26.
6. Kraus MR, Schäfer A, Faler H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2B therapy. J Clin Psychiatry. 2003;64:6.-
7. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-4.
8. Capuron L, Gummick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.
9. Van Thiel DH, Friedlander L, Molloy PJ, et al. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7(2):165-8.
10. Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347(2):148-9.
11. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon-alfa. N Engl J Med. 2001;344(13):961-6.
12. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms J Clin Psychiatry. 2005;66;8:1050-7.
13. Benazzi F. Major depressive episodes with hypomanic symptoms are common among depressed outpatients. Comprehensive Psychiatry. 2001;42(2):139-43.
14. Henry C, Swendsen J, Van Den Bulke D, et al. Emotional hyperreactivity as the fundamental mood characteristic of manic and mixed states. Eur Psychiat. 2003;18:124-8.
15. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology. 1978;17:430-1.
16. Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study (abstract). Hepatology. 2003;38 (suppl.1):735A.-
17. Koukopoulos A, Koukopoulos A. Agitated depression as a mixed state and the problem of melancholia. Bipolarity: beyond classic mania. Psychiatr Clin North Am. 1999;22(3):564-74.
18. Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry. 2004;19:85-90.
19. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network. A sex-specific phenomenon. Arch Gen Psychiatry. 2005;62:1089-96.
20. Henry C, Demotes-Mainard J. Avoiding drug-induced switching in patients with bipolar depression. Drug Safety. 2003;26:337-51.
21. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079-88.
22. Calabrese JR, Keck PE, Jr, MacFadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression, Am J Psychiatry. 2005;162(7):1351-60.
23. Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-9.
24. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-7.
Pegylated interferon-alpha with ribavirin is the most effective therapy for chronic hepatitis C infection,1,2 but psychiatric patients often discontinue IFN-alpha because of its mood side effects.3 Most studies describe depressive states, although manic symptoms—irritability, aggression, anger, hostility, emotional lability, anxiety, panic attacks, and insomnia—also have been reported.4-6
To help you manage adverse mood changes and prevent IFN-alpha treatment discontinuation in patients with hepatitis C, this article:
- reviews studies of patients with a history mood disorders who were treated with IFN-alpha
- explains how to recognize and treat IFN-alpha-induced mood disturbances when antidepressants are contraindicated.
Psychiatric Patients and IFN Therapy
Chronic hepatitis C infection is common among psychiatric patients. Routine screening among 1,556 patients admitted to a U.S. public psychiatric hospital across 3 years identified 133 patients (8.5%) who were positive for hepatitis C virus.7
Patients with psychopathologic symptoms before starting IFN therapy may suffer more-severe adverse psychiatric effects during treatment than those without psychopathology.8 In fact, mood disorders were considered an absolute contraindication to IFN therapy until recently.
Now that the National Institutes of Health (NIH) has recommended extending hepatitis C research and treatment to psychiatric patients, IFN-alpha-induced mental illness could become more common in clinical practice. Before the 2002 NIH consensus statement, patients with mental illness and substance use disorders—who represent >50% of candidates who need IFN therapy—were excluded from research protocols.
Safely using IFN. Some reports and studies suggest that patients with past or existing psychiatric disorders can be treated safely and effectively with IFN-alpha.
In a prospective open-label study, 29 of 31 patients with co-existing chronic hepatitis C and psychiatric illness completed 6 months of IFN therapy, 5 million units (MU) three times/week or 5 MU daily. Patients continued maintenance psychotropics during IFN treatment, and a psychiatrist monitored psychiatric symptoms.
Psychiatric illness worsened in four patients, and two discontinued IFN treatment. Serum alanine aminotransferase returned to normal in 22 patients (71%), and hepatitis C virus RNA cleared from the sera of 15 (48%).9
Another prospective study of 50 patients treated with IFN for chronic hepatitis found that those with a pre-existing mood or anxiety disorder were not more likely than others to discontinue IFN therapy.10
Unique to hepatitis therapy? IFN-induced mood disturbances are probably different in patients with chronic hepatitis C than in those receiving IFN for other diseases because of differences in regimens and effects of the underlying pathologies. For example, prescribing a preventative antidepressant before starting IFN treatment might help cancer patients but not patients with hepatitis C.11
This distinction could be particularly important when giving IFN-alpha to patients who are vulnerable to psychiatric illness with impulsive features. To emphasize this point, we describe clinical features and treatment response in patients with hepatitis C who were treated in our department.
IFN-Alpha-Induced Moods
In our prospective study of 93 patients, 30 (32%) developed IFN-alpha-induced mood disorders during the first 12 weeks of treatment.12 Contrary to previous studies focusing on depression, most of our patients had a mix of manic/hypomanic and depressive symptoms. Twenty-four (13 women and 11 men, mean age 43) accepted referral to a psychiatrist specializing in mood disorders to characterize their symptoms.
Mood characteristics. Using DSM-IV criteria, the psychiatrist determined that IFN-alpha induced both manic/hypomanic and depressive symptoms in many patients, and the manic/hypomanic features predominated. Five manic symptoms and three depressive symptoms were present in >50% of patients (Figure 1, Table 1).
Three patients presented with a manic episode,15 with hypomania with prevalent irritability and dysphoric features, and 6 with a mixed depressive state (major depressive episode with at least 3 hypomania symptoms).13 Four patients (17%) suffered a relapse of alcohol or cannabis abuse.
Nearly all (84%) reported paroxysmal anxiety, and all had emotional hyperreactivity that the psychiatrist described as a main symptom of a manic or mixed state.14 Most felt extremely impulsive and feared losing control. Two faced legal difficulties, and one was in prison.
The patients’ mean Montgomery-Åsburg Depression Rating Scale (MADRS) score was 16.12 (±1.6), indicating a mild to moderate depressive state. The highest-scoring items were inner tension, reduced sleep, reduced appetite, and lassitude (Figure 2).
Their mean Bech-Rafaelsen Mania Scale15 score was 14.33 (±1.3), indicating hypomanic or moderate manic symptoms. Hostility was by far the predominant manic symptom (mean score 3.2/4). Other manic symptom scores ranged from 0.2/4 to 2.1/4 (Figure 3).
Figure 1 Mood symptoms identified in 24 patients after 12 weeks of IFN-alpha treatment
IFN: Interferon
Source: Reference 12
Figure 2 Depressive symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12
Figure 3 Manic symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12Table 1
Most-prevalent IFN-induced mood symptoms in 24 patients treated for hepatitis C
| 5 manic symptoms (% of patients) | 3 depressive symptoms (% of patients) |
| Irritability (100%) | Insomnia or hypersomnia (100%) |
| Racing thoughts (87%) | Poor appetite or weight loss (92%) |
| Distractibility (87%) | Psychomotor agitation or retardation (54%) |
| Insomnia (58%) | |
| Agitation (70%) | |
| IFN: Interferon | |
| Source: Reference 12 | |
Treatment. Given the predominance of manic/hypomanic symptoms, we treated the 24 patients with low-to-moderate dosages of an atypical antipsychotic (amisulpride, 100 to 600 mg/d). This medication—not available in the United States—would be similar to using risperidone, 1 to 6 mg/d. Low-dose benzodiazepines (clonazepam or alprazolam) were added as needed to manage insomnia. Antipsychotic treatment enabled 23 of 24 patients (96%) to continue antiviral therapy.
Five patients had received selective serotonin reuptake inhibitors (SSRIs) for 1 to 4 weeks before referral to the psychiatrist. Antidepressants worsened their mood symptoms, which included impulsivity, agitation, and insomnia. Emotional hyper-reactivity, irritability, hostility, and impulsiveness improved in all 5 patients within 1 to 2 weeks of stopping SSRIs and starting the atypical antipsychotic.
Discussion
Accurately characterizing IFN-induced mood states confirmed our published data showing a mix of manic/hypomanic and depressive symptoms in psychiatric patients treated for chronic hepatitis C. Irritability and hostility were the two most prominent symptoms.
These findings differ from those of investigations that identified depressive symptoms as the hallmark of IFN’s psychiatric side effects.6 Our data were thoroughly characterized according to DSM-IV-TR criteria, two mood-rating scales, and a psychiatrist experienced in mood disorders.
Why is mania missed? One possible explanation for these different findings is that IFN-alpha-induced fatigue and flu-like symptoms could be misinterpreted as depressive symptoms. Also, most researchers have used depression rating scales—but not mania scales—and have not considered other psychiatric diagnostics.16
Self-report questionnaires for evaluating depression also take into account somatic side effects, resulting in higher rating scores. Moreover, few studies have included clinical psychiatric interviews and even fewer diagnostic confirmation by a psychiatrist.
Finally, clinical experience indicates that patients who present with both manic/hypomanic and depressive symptoms are more likely to complain about depression than about manic symptoms. Therefore, clinicians may miss manic symptoms if they don’t actively seek them.
Irritability and hostility. Previous studies have described irritability, mood lability, and anger/hostility as frequent symptoms4,5 but failed to consider them as possible manifestations of mania or hypomania. Many of our patients reported irritability severe enough to interfere with their work, social, and family relationships.
Hostility was the symptom with the highest score on the Bech-Rafaelsen Mania Scale. This objective evidence suggests that investigating the consequences to patients of increased irritability, impulsivity, or hostility might reveal some frightening behavior
How to treat these patients. Considerable evidence from characterizing IFN-induced side effects in patients with hepatitis C points to a syndrome of depressive and manic/hypomanic symptoms13,17-19 that does not fulfill criteria for a mixed state. This disorder is not described in DSM-IV-TR and, unfortunately, usually is misdiagnosed as a depressive state.
Antidepressants can worsen depression by causing agitation and impulsivity and can increase risk of suicide.17,18,20 By contrast, atypical antipsychotics have been shown to improve bipolar depression.21,22
We used an atypical antipsychotic to treat patients with prevalent manic or hypomanic symptoms and those who did not respond to SSRIs. Their IFN-induced mood disorders improved rapidly on low dosages of amisulpride, and antiviral therapy discontinuation rates were low (1/24; 4%). By comparison, other studies have reported antiviral treatment discontinuation rates of 30% to 40% in patients taking antidepressants.23,24
The atypical antipsychotic did not improve our patients’ fatigue and other neurovegetative symptoms, but antidepressants likewise do not improve these symptoms.8
Recommendations
This study leads us to warn clinicians that antidepressants can worsen IFN-alpha-induced mood disorders and to recommend that antipsychotics be considered:
- at least before you decide to discontinue a hepatitis C patient’s IFN-alpha therapy
- or in patients with high irritability and hostility.
To determine whether to use an antidepressant or antimanic agent as first-line treatment, carefully diagnose the patient’s symptoms as a manic/hypomanic state, depressive mixed state, or depression. Successful IFN therapy requires:
- psychological support
- medication for psychiatric adverse effects
- collaboration between the psychiatrist and hepatologist.
IFN-alpha-induced mood disorders are triggered exogenously, do not correspond to typical features of any classic psychiatric disorder, and require further study. IFN-induced impulsivity and hostility also need to be better characterized, particularly as more patients with pre-existing psychiatric disorders are treated for hepatitis C.
Related resources
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345(1):41-52.
- Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry 2004;161:3.
Drug brand names
- Alprazolam • Xanax
- Amisulpride • (not available in the United States)
- Clonazepam • Klonopin
- Risperidone • Risperdal
Acknowledgment
This study was conducted with support from the Centre d’Investigation Clinique INSERM/CHU de Bordeaux.
Pegylated interferon-alpha with ribavirin is the most effective therapy for chronic hepatitis C infection,1,2 but psychiatric patients often discontinue IFN-alpha because of its mood side effects.3 Most studies describe depressive states, although manic symptoms—irritability, aggression, anger, hostility, emotional lability, anxiety, panic attacks, and insomnia—also have been reported.4-6
To help you manage adverse mood changes and prevent IFN-alpha treatment discontinuation in patients with hepatitis C, this article:
- reviews studies of patients with a history mood disorders who were treated with IFN-alpha
- explains how to recognize and treat IFN-alpha-induced mood disturbances when antidepressants are contraindicated.
Psychiatric Patients and IFN Therapy
Chronic hepatitis C infection is common among psychiatric patients. Routine screening among 1,556 patients admitted to a U.S. public psychiatric hospital across 3 years identified 133 patients (8.5%) who were positive for hepatitis C virus.7
Patients with psychopathologic symptoms before starting IFN therapy may suffer more-severe adverse psychiatric effects during treatment than those without psychopathology.8 In fact, mood disorders were considered an absolute contraindication to IFN therapy until recently.
Now that the National Institutes of Health (NIH) has recommended extending hepatitis C research and treatment to psychiatric patients, IFN-alpha-induced mental illness could become more common in clinical practice. Before the 2002 NIH consensus statement, patients with mental illness and substance use disorders—who represent >50% of candidates who need IFN therapy—were excluded from research protocols.
Safely using IFN. Some reports and studies suggest that patients with past or existing psychiatric disorders can be treated safely and effectively with IFN-alpha.
In a prospective open-label study, 29 of 31 patients with co-existing chronic hepatitis C and psychiatric illness completed 6 months of IFN therapy, 5 million units (MU) three times/week or 5 MU daily. Patients continued maintenance psychotropics during IFN treatment, and a psychiatrist monitored psychiatric symptoms.
Psychiatric illness worsened in four patients, and two discontinued IFN treatment. Serum alanine aminotransferase returned to normal in 22 patients (71%), and hepatitis C virus RNA cleared from the sera of 15 (48%).9
Another prospective study of 50 patients treated with IFN for chronic hepatitis found that those with a pre-existing mood or anxiety disorder were not more likely than others to discontinue IFN therapy.10
Unique to hepatitis therapy? IFN-induced mood disturbances are probably different in patients with chronic hepatitis C than in those receiving IFN for other diseases because of differences in regimens and effects of the underlying pathologies. For example, prescribing a preventative antidepressant before starting IFN treatment might help cancer patients but not patients with hepatitis C.11
This distinction could be particularly important when giving IFN-alpha to patients who are vulnerable to psychiatric illness with impulsive features. To emphasize this point, we describe clinical features and treatment response in patients with hepatitis C who were treated in our department.
IFN-Alpha-Induced Moods
In our prospective study of 93 patients, 30 (32%) developed IFN-alpha-induced mood disorders during the first 12 weeks of treatment.12 Contrary to previous studies focusing on depression, most of our patients had a mix of manic/hypomanic and depressive symptoms. Twenty-four (13 women and 11 men, mean age 43) accepted referral to a psychiatrist specializing in mood disorders to characterize their symptoms.
Mood characteristics. Using DSM-IV criteria, the psychiatrist determined that IFN-alpha induced both manic/hypomanic and depressive symptoms in many patients, and the manic/hypomanic features predominated. Five manic symptoms and three depressive symptoms were present in >50% of patients (Figure 1, Table 1).
Three patients presented with a manic episode,15 with hypomania with prevalent irritability and dysphoric features, and 6 with a mixed depressive state (major depressive episode with at least 3 hypomania symptoms).13 Four patients (17%) suffered a relapse of alcohol or cannabis abuse.
Nearly all (84%) reported paroxysmal anxiety, and all had emotional hyperreactivity that the psychiatrist described as a main symptom of a manic or mixed state.14 Most felt extremely impulsive and feared losing control. Two faced legal difficulties, and one was in prison.
The patients’ mean Montgomery-Åsburg Depression Rating Scale (MADRS) score was 16.12 (±1.6), indicating a mild to moderate depressive state. The highest-scoring items were inner tension, reduced sleep, reduced appetite, and lassitude (Figure 2).
Their mean Bech-Rafaelsen Mania Scale15 score was 14.33 (±1.3), indicating hypomanic or moderate manic symptoms. Hostility was by far the predominant manic symptom (mean score 3.2/4). Other manic symptom scores ranged from 0.2/4 to 2.1/4 (Figure 3).
Figure 1 Mood symptoms identified in 24 patients after 12 weeks of IFN-alpha treatment
IFN: Interferon
Source: Reference 12
Figure 2 Depressive symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12
Figure 3 Manic symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12Table 1
Most-prevalent IFN-induced mood symptoms in 24 patients treated for hepatitis C
| 5 manic symptoms (% of patients) | 3 depressive symptoms (% of patients) |
| Irritability (100%) | Insomnia or hypersomnia (100%) |
| Racing thoughts (87%) | Poor appetite or weight loss (92%) |
| Distractibility (87%) | Psychomotor agitation or retardation (54%) |
| Insomnia (58%) | |
| Agitation (70%) | |
| IFN: Interferon | |
| Source: Reference 12 | |
Treatment. Given the predominance of manic/hypomanic symptoms, we treated the 24 patients with low-to-moderate dosages of an atypical antipsychotic (amisulpride, 100 to 600 mg/d). This medication—not available in the United States—would be similar to using risperidone, 1 to 6 mg/d. Low-dose benzodiazepines (clonazepam or alprazolam) were added as needed to manage insomnia. Antipsychotic treatment enabled 23 of 24 patients (96%) to continue antiviral therapy.
Five patients had received selective serotonin reuptake inhibitors (SSRIs) for 1 to 4 weeks before referral to the psychiatrist. Antidepressants worsened their mood symptoms, which included impulsivity, agitation, and insomnia. Emotional hyper-reactivity, irritability, hostility, and impulsiveness improved in all 5 patients within 1 to 2 weeks of stopping SSRIs and starting the atypical antipsychotic.
Discussion
Accurately characterizing IFN-induced mood states confirmed our published data showing a mix of manic/hypomanic and depressive symptoms in psychiatric patients treated for chronic hepatitis C. Irritability and hostility were the two most prominent symptoms.
These findings differ from those of investigations that identified depressive symptoms as the hallmark of IFN’s psychiatric side effects.6 Our data were thoroughly characterized according to DSM-IV-TR criteria, two mood-rating scales, and a psychiatrist experienced in mood disorders.
Why is mania missed? One possible explanation for these different findings is that IFN-alpha-induced fatigue and flu-like symptoms could be misinterpreted as depressive symptoms. Also, most researchers have used depression rating scales—but not mania scales—and have not considered other psychiatric diagnostics.16
Self-report questionnaires for evaluating depression also take into account somatic side effects, resulting in higher rating scores. Moreover, few studies have included clinical psychiatric interviews and even fewer diagnostic confirmation by a psychiatrist.
Finally, clinical experience indicates that patients who present with both manic/hypomanic and depressive symptoms are more likely to complain about depression than about manic symptoms. Therefore, clinicians may miss manic symptoms if they don’t actively seek them.
Irritability and hostility. Previous studies have described irritability, mood lability, and anger/hostility as frequent symptoms4,5 but failed to consider them as possible manifestations of mania or hypomania. Many of our patients reported irritability severe enough to interfere with their work, social, and family relationships.
Hostility was the symptom with the highest score on the Bech-Rafaelsen Mania Scale. This objective evidence suggests that investigating the consequences to patients of increased irritability, impulsivity, or hostility might reveal some frightening behavior
How to treat these patients. Considerable evidence from characterizing IFN-induced side effects in patients with hepatitis C points to a syndrome of depressive and manic/hypomanic symptoms13,17-19 that does not fulfill criteria for a mixed state. This disorder is not described in DSM-IV-TR and, unfortunately, usually is misdiagnosed as a depressive state.
Antidepressants can worsen depression by causing agitation and impulsivity and can increase risk of suicide.17,18,20 By contrast, atypical antipsychotics have been shown to improve bipolar depression.21,22
We used an atypical antipsychotic to treat patients with prevalent manic or hypomanic symptoms and those who did not respond to SSRIs. Their IFN-induced mood disorders improved rapidly on low dosages of amisulpride, and antiviral therapy discontinuation rates were low (1/24; 4%). By comparison, other studies have reported antiviral treatment discontinuation rates of 30% to 40% in patients taking antidepressants.23,24
The atypical antipsychotic did not improve our patients’ fatigue and other neurovegetative symptoms, but antidepressants likewise do not improve these symptoms.8
Recommendations
This study leads us to warn clinicians that antidepressants can worsen IFN-alpha-induced mood disorders and to recommend that antipsychotics be considered:
- at least before you decide to discontinue a hepatitis C patient’s IFN-alpha therapy
- or in patients with high irritability and hostility.
To determine whether to use an antidepressant or antimanic agent as first-line treatment, carefully diagnose the patient’s symptoms as a manic/hypomanic state, depressive mixed state, or depression. Successful IFN therapy requires:
- psychological support
- medication for psychiatric adverse effects
- collaboration between the psychiatrist and hepatologist.
IFN-alpha-induced mood disorders are triggered exogenously, do not correspond to typical features of any classic psychiatric disorder, and require further study. IFN-induced impulsivity and hostility also need to be better characterized, particularly as more patients with pre-existing psychiatric disorders are treated for hepatitis C.
Related resources
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345(1):41-52.
- Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry 2004;161:3.
Drug brand names
- Alprazolam • Xanax
- Amisulpride • (not available in the United States)
- Clonazepam • Klonopin
- Risperidone • Risperdal
Acknowledgment
This study was conducted with support from the Centre d’Investigation Clinique INSERM/CHU de Bordeaux.
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
2. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
3. Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207-11.
4. Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (INF-alpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
5. Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316-26.
6. Kraus MR, Schäfer A, Faler H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2B therapy. J Clin Psychiatry. 2003;64:6.-
7. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-4.
8. Capuron L, Gummick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.
9. Van Thiel DH, Friedlander L, Molloy PJ, et al. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7(2):165-8.
10. Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347(2):148-9.
11. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon-alfa. N Engl J Med. 2001;344(13):961-6.
12. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms J Clin Psychiatry. 2005;66;8:1050-7.
13. Benazzi F. Major depressive episodes with hypomanic symptoms are common among depressed outpatients. Comprehensive Psychiatry. 2001;42(2):139-43.
14. Henry C, Swendsen J, Van Den Bulke D, et al. Emotional hyperreactivity as the fundamental mood characteristic of manic and mixed states. Eur Psychiat. 2003;18:124-8.
15. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology. 1978;17:430-1.
16. Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study (abstract). Hepatology. 2003;38 (suppl.1):735A.-
17. Koukopoulos A, Koukopoulos A. Agitated depression as a mixed state and the problem of melancholia. Bipolarity: beyond classic mania. Psychiatr Clin North Am. 1999;22(3):564-74.
18. Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry. 2004;19:85-90.
19. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network. A sex-specific phenomenon. Arch Gen Psychiatry. 2005;62:1089-96.
20. Henry C, Demotes-Mainard J. Avoiding drug-induced switching in patients with bipolar depression. Drug Safety. 2003;26:337-51.
21. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079-88.
22. Calabrese JR, Keck PE, Jr, MacFadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression, Am J Psychiatry. 2005;162(7):1351-60.
23. Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-9.
24. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-7.
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
2. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
3. Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207-11.
4. Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (INF-alpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
5. Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316-26.
6. Kraus MR, Schäfer A, Faler H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2B therapy. J Clin Psychiatry. 2003;64:6.-
7. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-4.
8. Capuron L, Gummick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.
9. Van Thiel DH, Friedlander L, Molloy PJ, et al. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7(2):165-8.
10. Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347(2):148-9.
11. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon-alfa. N Engl J Med. 2001;344(13):961-6.
12. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms J Clin Psychiatry. 2005;66;8:1050-7.
13. Benazzi F. Major depressive episodes with hypomanic symptoms are common among depressed outpatients. Comprehensive Psychiatry. 2001;42(2):139-43.
14. Henry C, Swendsen J, Van Den Bulke D, et al. Emotional hyperreactivity as the fundamental mood characteristic of manic and mixed states. Eur Psychiat. 2003;18:124-8.
15. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology. 1978;17:430-1.
16. Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study (abstract). Hepatology. 2003;38 (suppl.1):735A.-
17. Koukopoulos A, Koukopoulos A. Agitated depression as a mixed state and the problem of melancholia. Bipolarity: beyond classic mania. Psychiatr Clin North Am. 1999;22(3):564-74.
18. Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry. 2004;19:85-90.
19. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network. A sex-specific phenomenon. Arch Gen Psychiatry. 2005;62:1089-96.
20. Henry C, Demotes-Mainard J. Avoiding drug-induced switching in patients with bipolar depression. Drug Safety. 2003;26:337-51.
21. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079-88.
22. Calabrese JR, Keck PE, Jr, MacFadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression, Am J Psychiatry. 2005;162(7):1351-60.
23. Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-9.
24. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-7.
Is this patient not guilty by reason of insanity?
Police find Mr. B, age 45, at home after he called 911 to report that he killed his wife. Covered in blood, he confesses immediately and is holding the knife he used to stab her. Police arrest him without resistance and charge him with murder.
Three months later, Mr. B presents for a sanity evaluation. He has a history of schizoaffective disorder and has required three past psychiatric hospitalizations. Urine and serum toxicology studies the day of the killing were negative for alcohol and drugs.
Was Mr. B legally sane or insane when he committed this offense? As psychiatrists, we are often called on to assess competence to stand trial and sanity at the time of a crime. In a previous article (Current Psychiatry, June 2006), we described how to evaluate whether a mentally ill criminal court defendant is competent to stand trial. This article introduces the process for conducting a sanity evaluation.
What is sanity?
“Sanity” is a legal—not clinical—term related to a plea of “not guilty by reason of insanity.” A sanity evaluation—a mental health professional’s specialized assessment—may be entered into evidence at a criminal trial to help a judge or jury determine whether a defendant is criminally responsible for an alleged offense.
Approximately 1 in every 100 defendants charged with a felony raise an insanity defense.1 A criminal defendant who pleads not guilty by reason of insanity asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred. Competence to stand trial, by comparison, focuses on the defendant’s present mental state (Table 1).
Before starting a sanity evaluation, determine the standard that applies in the jurisdiction where the alleged offense occurred. Federal and state courts have restricted insanity standards the past 20 years. Some states, including Idaho and Nevada, abolished the insanity defense. Others adopted “guilty but mentally ill” standards, which hold mentally-ill defendants criminally responsible for their actions.2
All insanity standards require that the defendant had a mental disease or defect at the time of the offense, but the terms “mental disease” and “mental defect” do not equate with particular DSM-IV-TR “mental disorders.” Rather, courts can interpret which diagnoses qualify for determining sanity.
Courts usually rule that serious psychotic and mood disorders qualify as a mental disease and mental retardation qualifies as a mental defect. Mental disorders that usually do not qualify include personality disorders, paraphilias, and voluntary intoxication.
Table 1
How competency and sanity assessments differ
| Competency | Sanity | |
|---|---|---|
| Presence of mental illness | Yes | Yes |
| Mental status | Current mental state | Mental state at the time of the offense |
| Purpose of evaluation | Ability to stand trial | Criminal responsibility |
| Variation in laws by jurisdiction | Minor variation | Great variation |
History of insanity defense
Many early codes of law provided exceptions to criminal responsibility for the mentally ill. Modern insanity standards are based on English common law (Table 2).
Table 2
From ‘wild beast’ to ‘irresistible impulse’: Milestones in the insanity defense
| Standard | Year | Description |
|---|---|---|
| Wild beast | 1724 | Most strict standard; required total deprivation of memory and understanding |
| Irresistible impulse | 1840 | More liberal standard; required that “…some controlling disease was…the acting power within him which he could not resist…” |
| M’Naughten rule | 1843 | Required that the defendant not know the nature/quality or the wrongfulness of the offense. |
| American Law Institute’s Model Penal Code standard | 1955 | Combined M’Naughten Rule with irresistible impulse |
| Federal Insanity Defense Reform Act | 1984 | Stricter standard that dropped the irresistible impulse standard after attempted assassination of President Reagan |
To be found insane under the wild beast standard, the defendant had to be “totally deprived of his understanding and memory, so as to not know what he is doing, no more than an infant, a brute or a wild beast.”3
‘Irresistible impulse.’ The “irresistible impulse” standard was first used successfully in 1840 in the trial of Edward Oxford, who attempted to assassinate Queen Victoria. In Regina v. Oxford, the court recognized that “if some controlling disease was…the acting power within him which he could not resist, then he will not be responsible.”4
The M’Naughten rule—perhaps the most famous standard—was established in 1843. M’Naughten suffered from paranoid delusions that the prime minister of England was plotting against him; he planned to kill the prime minister but mistakenly killed his secretary. The examiners who evaluated M’Naughten testified that he was insane, and the jury concurred. The public and royal family were incensed, however, and appellate judges reviewed the verdict and insanity standard.
The appeals court issued the M’Naughten rule,5 by which a mentally ill defendant may be considered insane if, at the time of the act, he:
- did not understand the nature and quality of the act
- or did not know the wrongfulness of the act.
- appreciate the criminality of his act
- or conform his conduct to the requirements of the law.
How to evaluate insanity
Prepare. Review the defendant’s medical/psychiatric records and materials pertaining to the offense, including the police report and other legal or medical documents. Indications of a prior psychiatric diagnosis may support or rebut the presence of a mental disease or defect at the time account of the of the alleged offense.
To assess the defendant’s mental state at the time of the act, note any recorded observations of his or her behavior during or around that time. You may wish to collect this information from collateral sources, as well. Look for bizarre behavior or other evidence that the defendant suffered from delusions, hallucinations, or other symptoms of a severe mental disease or defect.
Police records may contain:
- reports by witnesses, victims, and police officers about the defendant’s statements or behavior during or soon after the offense
- a defendant’s statement to the arresting officers.
Interview the defendant. Next, conduct a thorough standard psychiatric interview in person. Inform the defendant that the interview is not confidential, and that any information he or she provides may be included in a written report to the court or disclosed during trial testimony.
Consider psychiatric symptoms the defendant experienced during the offense, medication adherence, and use of alcohol or other mood-altering substances. Remember that voluntary intoxication does not provide grounds for an insanity defense, even in states that allow the irresistible impulse defense.
Obtain a detailed account of the event from the defendant. Look for:
- symptoms of a mental disease or defect when the offense occurred
- the defendant’s knowledge (or lack thereof) that the offense was wrong at that time (Table 3)
Signs that a defendant knew an act was wrong
| Efforts to avoid detection | Wearing gloves or a mask during the offense |
| Concealing a weapon | |
| Falsifying information (using an alias or creating a passport) | |
| Committing the act in the dark | |
| Disposal of evidence | Washing away blood |
| Removing fingerprints | |
| Discarding the weapon | |
| Hiding the body | |
| Efforts to avoid apprehension | Fleeing |
| Lying to authorities |
Indications that the defendant was aware at the time of the offense that his actions were wrong may include:
- behaviors during or immediately after the event, such as hiding evidence, lying to authorities, or fleeing the scene
- a rational motive such as jealousy, revenge, or personal gain.
Caveats. The defendant’s mental status during the interview may differ vastly from that at the time of the offense. Don’t be swayed if a defendant with a history of psychosis now appears symptom-free. Recent treatment may explain his or her lack of symptoms. Also be aware that the defendant may be malingering mental illness to support an insanity defense and escape criminal responsibility.7
Outcomes. Approximately 15% to 25% of criminal defendants who plead insanity are adjudicated insane. Technically, a defendant who is found legally insane has been acquitted of the offense. The insanity defense is less likely to succeed in jury than in nonjury trials.8
Although the defendant may not be punished once acquitted, he or she may be committed to a mental institution to ensure treatment compliance and protect the public.
Table 4
Irresistible impulse test for sanity: A modern interpretation
| Inability to defer the act |
| Inability to ignore specific instructions |
| Must not be caused by rage or intoxication |
| Magnitude, likelihood, and imminence of consequences if act is not performed |
| Attempted alternatives to the act |
| Genuineness of command hallucinations and the ability to ignore them |
Case continued: seeing red
When you interview Mr. B 3 months after his arrest, he is not psychotic. He says he ran out of his medications 6 months before he killed his wife and resumed taking them while in jail awaiting trial.
Mr. B relates that in the months before the offense he grew concerned that his wife was involved in “ritualistic sexual perversions” commanded by the devil. He tried to discuss this with his mother-in-law and minister but did not get a satisfactory response.
On the evening of the killing, Mr. B was particularly agitated while waiting for his wife to return home from work. She walked in wearing a red sweater, which indicated to him that she had had sex with 17 different men at work that day. To save her from eternal damnation for adultery, Mr. B believed he had to stab her 17 times before sunrise.
He becomes tearful during the interview and says he wishes he “could go back in time and fix things.”
Mr. B. shows clear evidence of a severe mental disease during the offense (psychosis, medication nonadherence) without a personality or substance use disorder.
Factors that indicate he did not know his actions were wrong at the time of the event include:
- his delusional belief that he was saving his wife from damnation
- lack of a rational motive
- lack of effort to conceal the offense
- his ready confession to 911 operators and police officers
- his cooperation with police.
Mr. B’s attempts at alternate solutions (discussions with clergy and his mother-in-law) and the perceived deadline (the need to kill his wife before sunrise to prevent damnation) indicate that he had an irresistible impulse.
Open to interpretation
As this case suggests, a defendant’s sanity or insanity is determined by many factors and is often open to interpretation. In court, the prosecution and defense aim to answer two complex questions:
- Did the defendant suffer from a mental illness? (This may be clear in patients with schizophrenia but more difficult to determine in others, such as in substance-induced mood disorder.)
- Did this mental illness alter the defendant’s judgment to such a degree that he or she no longer knew the offense was wrongful?
Recent cases. Despite her plea of not guilty by reason of insanity, Andrea Yates was convicted of murder in June 2002 for drowning her five children in the bathtub of their home. Though most would agree the Texas housewife suffered from a severe mental illness, prosecutors convinced the jury that she knew the wrongfulness of her actions. An appeal was granted earlier this year, and Yates returned to court in June.
Also this year, the U.S. Supreme Court heard a case contesting Arizona’s insanity defense on grounds that it violated a defendant’s right to due process. In late June, the court sided with the state, continuing to allow each to state to establish its own insanity defense standard.
Related resources
- American Academy of Psychiatry and the Law. www.aapl.org,
- Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL practice guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law 2002;30(2 suppl):S3-40.
- Noffsinger SG, Resnick PJ. Insanity defense evaluations. Directions in Psychiatry 1999;19:325-38.
1. Callahan L, Meyer C, et al. Insanity defense reform in the United States-post-Hinckley. Ment Phys Disabil Law Rep 1987;11:54-9.
2. Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL practice guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law 2002;30(2 suppl):S3-40.
3. Rex v. Arnold, 16 How. St. Tr. 695 (1724).
4. Regina v. Oxford, 9 Car. and P. 525, 546 (1840).
5. M’Naughten’s case, 8 Eng Rep. 718 (1843).
6. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am 1999;22:159-72.
7. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry 2005;4(11):13-25.
8. Roger JL, Bloom JD, Manson SM. Insanity defenses: contested or concealed? Am J Psychiatry 1984;141:885-8.
Police find Mr. B, age 45, at home after he called 911 to report that he killed his wife. Covered in blood, he confesses immediately and is holding the knife he used to stab her. Police arrest him without resistance and charge him with murder.
Three months later, Mr. B presents for a sanity evaluation. He has a history of schizoaffective disorder and has required three past psychiatric hospitalizations. Urine and serum toxicology studies the day of the killing were negative for alcohol and drugs.
Was Mr. B legally sane or insane when he committed this offense? As psychiatrists, we are often called on to assess competence to stand trial and sanity at the time of a crime. In a previous article (Current Psychiatry, June 2006), we described how to evaluate whether a mentally ill criminal court defendant is competent to stand trial. This article introduces the process for conducting a sanity evaluation.
What is sanity?
“Sanity” is a legal—not clinical—term related to a plea of “not guilty by reason of insanity.” A sanity evaluation—a mental health professional’s specialized assessment—may be entered into evidence at a criminal trial to help a judge or jury determine whether a defendant is criminally responsible for an alleged offense.
Approximately 1 in every 100 defendants charged with a felony raise an insanity defense.1 A criminal defendant who pleads not guilty by reason of insanity asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred. Competence to stand trial, by comparison, focuses on the defendant’s present mental state (Table 1).
Before starting a sanity evaluation, determine the standard that applies in the jurisdiction where the alleged offense occurred. Federal and state courts have restricted insanity standards the past 20 years. Some states, including Idaho and Nevada, abolished the insanity defense. Others adopted “guilty but mentally ill” standards, which hold mentally-ill defendants criminally responsible for their actions.2
All insanity standards require that the defendant had a mental disease or defect at the time of the offense, but the terms “mental disease” and “mental defect” do not equate with particular DSM-IV-TR “mental disorders.” Rather, courts can interpret which diagnoses qualify for determining sanity.
Courts usually rule that serious psychotic and mood disorders qualify as a mental disease and mental retardation qualifies as a mental defect. Mental disorders that usually do not qualify include personality disorders, paraphilias, and voluntary intoxication.
Table 1
How competency and sanity assessments differ
| Competency | Sanity | |
|---|---|---|
| Presence of mental illness | Yes | Yes |
| Mental status | Current mental state | Mental state at the time of the offense |
| Purpose of evaluation | Ability to stand trial | Criminal responsibility |
| Variation in laws by jurisdiction | Minor variation | Great variation |
History of insanity defense
Many early codes of law provided exceptions to criminal responsibility for the mentally ill. Modern insanity standards are based on English common law (Table 2).
Table 2
From ‘wild beast’ to ‘irresistible impulse’: Milestones in the insanity defense
| Standard | Year | Description |
|---|---|---|
| Wild beast | 1724 | Most strict standard; required total deprivation of memory and understanding |
| Irresistible impulse | 1840 | More liberal standard; required that “…some controlling disease was…the acting power within him which he could not resist…” |
| M’Naughten rule | 1843 | Required that the defendant not know the nature/quality or the wrongfulness of the offense. |
| American Law Institute’s Model Penal Code standard | 1955 | Combined M’Naughten Rule with irresistible impulse |
| Federal Insanity Defense Reform Act | 1984 | Stricter standard that dropped the irresistible impulse standard after attempted assassination of President Reagan |
To be found insane under the wild beast standard, the defendant had to be “totally deprived of his understanding and memory, so as to not know what he is doing, no more than an infant, a brute or a wild beast.”3
‘Irresistible impulse.’ The “irresistible impulse” standard was first used successfully in 1840 in the trial of Edward Oxford, who attempted to assassinate Queen Victoria. In Regina v. Oxford, the court recognized that “if some controlling disease was…the acting power within him which he could not resist, then he will not be responsible.”4
The M’Naughten rule—perhaps the most famous standard—was established in 1843. M’Naughten suffered from paranoid delusions that the prime minister of England was plotting against him; he planned to kill the prime minister but mistakenly killed his secretary. The examiners who evaluated M’Naughten testified that he was insane, and the jury concurred. The public and royal family were incensed, however, and appellate judges reviewed the verdict and insanity standard.
The appeals court issued the M’Naughten rule,5 by which a mentally ill defendant may be considered insane if, at the time of the act, he:
- did not understand the nature and quality of the act
- or did not know the wrongfulness of the act.
- appreciate the criminality of his act
- or conform his conduct to the requirements of the law.
How to evaluate insanity
Prepare. Review the defendant’s medical/psychiatric records and materials pertaining to the offense, including the police report and other legal or medical documents. Indications of a prior psychiatric diagnosis may support or rebut the presence of a mental disease or defect at the time account of the of the alleged offense.
To assess the defendant’s mental state at the time of the act, note any recorded observations of his or her behavior during or around that time. You may wish to collect this information from collateral sources, as well. Look for bizarre behavior or other evidence that the defendant suffered from delusions, hallucinations, or other symptoms of a severe mental disease or defect.
Police records may contain:
- reports by witnesses, victims, and police officers about the defendant’s statements or behavior during or soon after the offense
- a defendant’s statement to the arresting officers.
Interview the defendant. Next, conduct a thorough standard psychiatric interview in person. Inform the defendant that the interview is not confidential, and that any information he or she provides may be included in a written report to the court or disclosed during trial testimony.
Consider psychiatric symptoms the defendant experienced during the offense, medication adherence, and use of alcohol or other mood-altering substances. Remember that voluntary intoxication does not provide grounds for an insanity defense, even in states that allow the irresistible impulse defense.
Obtain a detailed account of the event from the defendant. Look for:
- symptoms of a mental disease or defect when the offense occurred
- the defendant’s knowledge (or lack thereof) that the offense was wrong at that time (Table 3)
Signs that a defendant knew an act was wrong
| Efforts to avoid detection | Wearing gloves or a mask during the offense |
| Concealing a weapon | |
| Falsifying information (using an alias or creating a passport) | |
| Committing the act in the dark | |
| Disposal of evidence | Washing away blood |
| Removing fingerprints | |
| Discarding the weapon | |
| Hiding the body | |
| Efforts to avoid apprehension | Fleeing |
| Lying to authorities |
Indications that the defendant was aware at the time of the offense that his actions were wrong may include:
- behaviors during or immediately after the event, such as hiding evidence, lying to authorities, or fleeing the scene
- a rational motive such as jealousy, revenge, or personal gain.
Caveats. The defendant’s mental status during the interview may differ vastly from that at the time of the offense. Don’t be swayed if a defendant with a history of psychosis now appears symptom-free. Recent treatment may explain his or her lack of symptoms. Also be aware that the defendant may be malingering mental illness to support an insanity defense and escape criminal responsibility.7
Outcomes. Approximately 15% to 25% of criminal defendants who plead insanity are adjudicated insane. Technically, a defendant who is found legally insane has been acquitted of the offense. The insanity defense is less likely to succeed in jury than in nonjury trials.8
Although the defendant may not be punished once acquitted, he or she may be committed to a mental institution to ensure treatment compliance and protect the public.
Table 4
Irresistible impulse test for sanity: A modern interpretation
| Inability to defer the act |
| Inability to ignore specific instructions |
| Must not be caused by rage or intoxication |
| Magnitude, likelihood, and imminence of consequences if act is not performed |
| Attempted alternatives to the act |
| Genuineness of command hallucinations and the ability to ignore them |
Case continued: seeing red
When you interview Mr. B 3 months after his arrest, he is not psychotic. He says he ran out of his medications 6 months before he killed his wife and resumed taking them while in jail awaiting trial.
Mr. B relates that in the months before the offense he grew concerned that his wife was involved in “ritualistic sexual perversions” commanded by the devil. He tried to discuss this with his mother-in-law and minister but did not get a satisfactory response.
On the evening of the killing, Mr. B was particularly agitated while waiting for his wife to return home from work. She walked in wearing a red sweater, which indicated to him that she had had sex with 17 different men at work that day. To save her from eternal damnation for adultery, Mr. B believed he had to stab her 17 times before sunrise.
He becomes tearful during the interview and says he wishes he “could go back in time and fix things.”
Mr. B. shows clear evidence of a severe mental disease during the offense (psychosis, medication nonadherence) without a personality or substance use disorder.
Factors that indicate he did not know his actions were wrong at the time of the event include:
- his delusional belief that he was saving his wife from damnation
- lack of a rational motive
- lack of effort to conceal the offense
- his ready confession to 911 operators and police officers
- his cooperation with police.
Mr. B’s attempts at alternate solutions (discussions with clergy and his mother-in-law) and the perceived deadline (the need to kill his wife before sunrise to prevent damnation) indicate that he had an irresistible impulse.
Open to interpretation
As this case suggests, a defendant’s sanity or insanity is determined by many factors and is often open to interpretation. In court, the prosecution and defense aim to answer two complex questions:
- Did the defendant suffer from a mental illness? (This may be clear in patients with schizophrenia but more difficult to determine in others, such as in substance-induced mood disorder.)
- Did this mental illness alter the defendant’s judgment to such a degree that he or she no longer knew the offense was wrongful?
Recent cases. Despite her plea of not guilty by reason of insanity, Andrea Yates was convicted of murder in June 2002 for drowning her five children in the bathtub of their home. Though most would agree the Texas housewife suffered from a severe mental illness, prosecutors convinced the jury that she knew the wrongfulness of her actions. An appeal was granted earlier this year, and Yates returned to court in June.
Also this year, the U.S. Supreme Court heard a case contesting Arizona’s insanity defense on grounds that it violated a defendant’s right to due process. In late June, the court sided with the state, continuing to allow each to state to establish its own insanity defense standard.
Related resources
- American Academy of Psychiatry and the Law. www.aapl.org,
- Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL practice guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law 2002;30(2 suppl):S3-40.
- Noffsinger SG, Resnick PJ. Insanity defense evaluations. Directions in Psychiatry 1999;19:325-38.
Police find Mr. B, age 45, at home after he called 911 to report that he killed his wife. Covered in blood, he confesses immediately and is holding the knife he used to stab her. Police arrest him without resistance and charge him with murder.
Three months later, Mr. B presents for a sanity evaluation. He has a history of schizoaffective disorder and has required three past psychiatric hospitalizations. Urine and serum toxicology studies the day of the killing were negative for alcohol and drugs.
Was Mr. B legally sane or insane when he committed this offense? As psychiatrists, we are often called on to assess competence to stand trial and sanity at the time of a crime. In a previous article (Current Psychiatry, June 2006), we described how to evaluate whether a mentally ill criminal court defendant is competent to stand trial. This article introduces the process for conducting a sanity evaluation.
What is sanity?
“Sanity” is a legal—not clinical—term related to a plea of “not guilty by reason of insanity.” A sanity evaluation—a mental health professional’s specialized assessment—may be entered into evidence at a criminal trial to help a judge or jury determine whether a defendant is criminally responsible for an alleged offense.
Approximately 1 in every 100 defendants charged with a felony raise an insanity defense.1 A criminal defendant who pleads not guilty by reason of insanity asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred. Competence to stand trial, by comparison, focuses on the defendant’s present mental state (Table 1).
Before starting a sanity evaluation, determine the standard that applies in the jurisdiction where the alleged offense occurred. Federal and state courts have restricted insanity standards the past 20 years. Some states, including Idaho and Nevada, abolished the insanity defense. Others adopted “guilty but mentally ill” standards, which hold mentally-ill defendants criminally responsible for their actions.2
All insanity standards require that the defendant had a mental disease or defect at the time of the offense, but the terms “mental disease” and “mental defect” do not equate with particular DSM-IV-TR “mental disorders.” Rather, courts can interpret which diagnoses qualify for determining sanity.
Courts usually rule that serious psychotic and mood disorders qualify as a mental disease and mental retardation qualifies as a mental defect. Mental disorders that usually do not qualify include personality disorders, paraphilias, and voluntary intoxication.
Table 1
How competency and sanity assessments differ
| Competency | Sanity | |
|---|---|---|
| Presence of mental illness | Yes | Yes |
| Mental status | Current mental state | Mental state at the time of the offense |
| Purpose of evaluation | Ability to stand trial | Criminal responsibility |
| Variation in laws by jurisdiction | Minor variation | Great variation |
History of insanity defense
Many early codes of law provided exceptions to criminal responsibility for the mentally ill. Modern insanity standards are based on English common law (Table 2).
Table 2
From ‘wild beast’ to ‘irresistible impulse’: Milestones in the insanity defense
| Standard | Year | Description |
|---|---|---|
| Wild beast | 1724 | Most strict standard; required total deprivation of memory and understanding |
| Irresistible impulse | 1840 | More liberal standard; required that “…some controlling disease was…the acting power within him which he could not resist…” |
| M’Naughten rule | 1843 | Required that the defendant not know the nature/quality or the wrongfulness of the offense. |
| American Law Institute’s Model Penal Code standard | 1955 | Combined M’Naughten Rule with irresistible impulse |
| Federal Insanity Defense Reform Act | 1984 | Stricter standard that dropped the irresistible impulse standard after attempted assassination of President Reagan |
To be found insane under the wild beast standard, the defendant had to be “totally deprived of his understanding and memory, so as to not know what he is doing, no more than an infant, a brute or a wild beast.”3
‘Irresistible impulse.’ The “irresistible impulse” standard was first used successfully in 1840 in the trial of Edward Oxford, who attempted to assassinate Queen Victoria. In Regina v. Oxford, the court recognized that “if some controlling disease was…the acting power within him which he could not resist, then he will not be responsible.”4
The M’Naughten rule—perhaps the most famous standard—was established in 1843. M’Naughten suffered from paranoid delusions that the prime minister of England was plotting against him; he planned to kill the prime minister but mistakenly killed his secretary. The examiners who evaluated M’Naughten testified that he was insane, and the jury concurred. The public and royal family were incensed, however, and appellate judges reviewed the verdict and insanity standard.
The appeals court issued the M’Naughten rule,5 by which a mentally ill defendant may be considered insane if, at the time of the act, he:
- did not understand the nature and quality of the act
- or did not know the wrongfulness of the act.
- appreciate the criminality of his act
- or conform his conduct to the requirements of the law.
How to evaluate insanity
Prepare. Review the defendant’s medical/psychiatric records and materials pertaining to the offense, including the police report and other legal or medical documents. Indications of a prior psychiatric diagnosis may support or rebut the presence of a mental disease or defect at the time account of the of the alleged offense.
To assess the defendant’s mental state at the time of the act, note any recorded observations of his or her behavior during or around that time. You may wish to collect this information from collateral sources, as well. Look for bizarre behavior or other evidence that the defendant suffered from delusions, hallucinations, or other symptoms of a severe mental disease or defect.
Police records may contain:
- reports by witnesses, victims, and police officers about the defendant’s statements or behavior during or soon after the offense
- a defendant’s statement to the arresting officers.
Interview the defendant. Next, conduct a thorough standard psychiatric interview in person. Inform the defendant that the interview is not confidential, and that any information he or she provides may be included in a written report to the court or disclosed during trial testimony.
Consider psychiatric symptoms the defendant experienced during the offense, medication adherence, and use of alcohol or other mood-altering substances. Remember that voluntary intoxication does not provide grounds for an insanity defense, even in states that allow the irresistible impulse defense.
Obtain a detailed account of the event from the defendant. Look for:
- symptoms of a mental disease or defect when the offense occurred
- the defendant’s knowledge (or lack thereof) that the offense was wrong at that time (Table 3)
Signs that a defendant knew an act was wrong
| Efforts to avoid detection | Wearing gloves or a mask during the offense |
| Concealing a weapon | |
| Falsifying information (using an alias or creating a passport) | |
| Committing the act in the dark | |
| Disposal of evidence | Washing away blood |
| Removing fingerprints | |
| Discarding the weapon | |
| Hiding the body | |
| Efforts to avoid apprehension | Fleeing |
| Lying to authorities |
Indications that the defendant was aware at the time of the offense that his actions were wrong may include:
- behaviors during or immediately after the event, such as hiding evidence, lying to authorities, or fleeing the scene
- a rational motive such as jealousy, revenge, or personal gain.
Caveats. The defendant’s mental status during the interview may differ vastly from that at the time of the offense. Don’t be swayed if a defendant with a history of psychosis now appears symptom-free. Recent treatment may explain his or her lack of symptoms. Also be aware that the defendant may be malingering mental illness to support an insanity defense and escape criminal responsibility.7
Outcomes. Approximately 15% to 25% of criminal defendants who plead insanity are adjudicated insane. Technically, a defendant who is found legally insane has been acquitted of the offense. The insanity defense is less likely to succeed in jury than in nonjury trials.8
Although the defendant may not be punished once acquitted, he or she may be committed to a mental institution to ensure treatment compliance and protect the public.
Table 4
Irresistible impulse test for sanity: A modern interpretation
| Inability to defer the act |
| Inability to ignore specific instructions |
| Must not be caused by rage or intoxication |
| Magnitude, likelihood, and imminence of consequences if act is not performed |
| Attempted alternatives to the act |
| Genuineness of command hallucinations and the ability to ignore them |
Case continued: seeing red
When you interview Mr. B 3 months after his arrest, he is not psychotic. He says he ran out of his medications 6 months before he killed his wife and resumed taking them while in jail awaiting trial.
Mr. B relates that in the months before the offense he grew concerned that his wife was involved in “ritualistic sexual perversions” commanded by the devil. He tried to discuss this with his mother-in-law and minister but did not get a satisfactory response.
On the evening of the killing, Mr. B was particularly agitated while waiting for his wife to return home from work. She walked in wearing a red sweater, which indicated to him that she had had sex with 17 different men at work that day. To save her from eternal damnation for adultery, Mr. B believed he had to stab her 17 times before sunrise.
He becomes tearful during the interview and says he wishes he “could go back in time and fix things.”
Mr. B. shows clear evidence of a severe mental disease during the offense (psychosis, medication nonadherence) without a personality or substance use disorder.
Factors that indicate he did not know his actions were wrong at the time of the event include:
- his delusional belief that he was saving his wife from damnation
- lack of a rational motive
- lack of effort to conceal the offense
- his ready confession to 911 operators and police officers
- his cooperation with police.
Mr. B’s attempts at alternate solutions (discussions with clergy and his mother-in-law) and the perceived deadline (the need to kill his wife before sunrise to prevent damnation) indicate that he had an irresistible impulse.
Open to interpretation
As this case suggests, a defendant’s sanity or insanity is determined by many factors and is often open to interpretation. In court, the prosecution and defense aim to answer two complex questions:
- Did the defendant suffer from a mental illness? (This may be clear in patients with schizophrenia but more difficult to determine in others, such as in substance-induced mood disorder.)
- Did this mental illness alter the defendant’s judgment to such a degree that he or she no longer knew the offense was wrongful?
Recent cases. Despite her plea of not guilty by reason of insanity, Andrea Yates was convicted of murder in June 2002 for drowning her five children in the bathtub of their home. Though most would agree the Texas housewife suffered from a severe mental illness, prosecutors convinced the jury that she knew the wrongfulness of her actions. An appeal was granted earlier this year, and Yates returned to court in June.
Also this year, the U.S. Supreme Court heard a case contesting Arizona’s insanity defense on grounds that it violated a defendant’s right to due process. In late June, the court sided with the state, continuing to allow each to state to establish its own insanity defense standard.
Related resources
- American Academy of Psychiatry and the Law. www.aapl.org,
- Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL practice guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law 2002;30(2 suppl):S3-40.
- Noffsinger SG, Resnick PJ. Insanity defense evaluations. Directions in Psychiatry 1999;19:325-38.
1. Callahan L, Meyer C, et al. Insanity defense reform in the United States-post-Hinckley. Ment Phys Disabil Law Rep 1987;11:54-9.
2. Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL practice guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law 2002;30(2 suppl):S3-40.
3. Rex v. Arnold, 16 How. St. Tr. 695 (1724).
4. Regina v. Oxford, 9 Car. and P. 525, 546 (1840).
5. M’Naughten’s case, 8 Eng Rep. 718 (1843).
6. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am 1999;22:159-72.
7. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry 2005;4(11):13-25.
8. Roger JL, Bloom JD, Manson SM. Insanity defenses: contested or concealed? Am J Psychiatry 1984;141:885-8.
1. Callahan L, Meyer C, et al. Insanity defense reform in the United States-post-Hinckley. Ment Phys Disabil Law Rep 1987;11:54-9.
2. Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL practice guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law 2002;30(2 suppl):S3-40.
3. Rex v. Arnold, 16 How. St. Tr. 695 (1724).
4. Regina v. Oxford, 9 Car. and P. 525, 546 (1840).
5. M’Naughten’s case, 8 Eng Rep. 718 (1843).
6. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am 1999;22:159-72.
7. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry 2005;4(11):13-25.
8. Roger JL, Bloom JD, Manson SM. Insanity defenses: contested or concealed? Am J Psychiatry 1984;141:885-8.
For women only: Hormones may prevent addiction relapse
Women become dependent more rapidly than men after initial cocaine, opioid, or alcohol use and may be more sensitive to drugs’ adverse health effects.1 And although men and women relapse to substance use at similar rates, ovulating women may be particularly vulnerable to relapse at certain times of the month.
Understanding the hormonal influences that increase women’s relapse risk can help you intervene more effectively. This article describes:
- how women’s relapse patterns differ from men’s
- why psychotherapy and hormone regulation may be preferred for relapse prevention in women with substance use disorders.
Case report: will she relapse again?
Ms. H, age 46, is in her third month of an alcohol and drug residential rehabilitation program. She has a 10-year history of alcohol and crack cocaine dependence and is battling cravings to use again. These feelings are usually triggered by being in places or with people associated with her drug use, but this time she is committed to staying sober.
She started smoking cigarettes in her teens and using drugs and alcohol in her mid 20s. She feels that her dependency has been out of control in the 10 years since her son was born.
She has tried to quit many times on her own but has managed no more than 1 month of abstinence. She often has relapsed in response to feeling anxious or depressed about being unemployed or after arguing with her partner.
Mechanisms of relapse
Dopamine release is essential for encoding learned associations. When a drug is used in the early dependency state, dopamine release produces pleasure that reinforces continued drug use. Once the behavior is learned, environmental stimuli can trigger dopamine and turn on the brain circuits for this familiar, highly rewarding behavior. Dopamine also is the primary cause of long-lasting brain changes that make it difficult for substance-dependent persons such as Ms. H to control desire for the drug.2
Early relapse—caused by dopamine’s and other neurotransmitters’ effects on various brain regions—is triggered by environmental stimuli such as:
- re-exposure to a small amount of the drug
- exposure to an environment or cues associated with past drug use
- exposure to stressful events.
Table 1
3 stages of relapse and their neurobiologic components
| Relapse stage | Key neurotransmitters | Brain regions involved |
|---|---|---|
| Early relapse triggered by: | ||
| • exposure to a small amount of the drug | Dopamine | Ventral tegmental area |
| Nucleus accumbens core | ||
| Prefrontal cortex | ||
| • environmental cues that re-trigger learned associations | Dopamine | Basolateral amygdala |
| Nucleus accumbens core | ||
| • stressful events and disappointments | Norepinephrine, corticotropin-releasing factor | Extended amygdala |
| Bed nucleus of the stria terminalis | ||
| Craving | Multiple, undetermined | Prefrontal cortex circuitry involving the anterior cingulate and orbitofrontal cortices |
| Relapse | Glutamate, dopamine | Prefrontal cortex |
| Nucleus accumbens core | ||
| Ventral pallidum | ||
| Source: References 2-4 | ||
Emotions, stress trigger relapse
Ms. H. reports increased irritability and impulsivity along with depressed mood—especially during the 3 to 4 days preceding her menstrual period. Her periods are regular, and these mood symptoms recur each month. She does not meet criteria for major depressive disorder.
Emotional reactions play a larger role in relapse for women than for men. Women report higher levels of craving and depressed mood during abstinence and experience stronger urges to drink and smoke when depressed. Women also are more likely to report substance use relapse in response to specific stressful events, disappointments, or depressed mood.1 This is consistent with evidence that women have heightened physiologic responses to social rejection and social stressors.5
A lower density of brain serotonin transporter has been associated with a higher risk of depression in women. Because estrogen and progesterone affect expression of the serotonin transporter, changes in these hormone levels might alter the risk of depression.6 Thus, ovarian hormones’ effect on the serotonin system may contribute to the higher rate of emotionally triggered relapse in women versus men.
Menstrual cycle phases. How men and women respond to stress may contribute to differences in their relapse behaviors (Table 2).
During the first 2 weeks of the menstrual cycle—the follicular phase—women show lower physiologic reactivity (as seen in blood pressure and catecholamine measurements) and lower cortisol responsiveness than men do in response to psychosocial stress. Estrogen contributes to this effect by attenuating sympathoadrenal responsiveness.5
During the latter 2 weeks of the menstrual cycle—the luteal phase—the ovulating woman’s hypothalamic-pituitary-adrenal (HPA) axis response increases and increases her sensitivity to stress. In this phase, progesterone’s presence reverses estrogen’s effect and makes the brain more reactive to emotions and stressors.
Higher stress responsiveness is associated with increased cocaine craving.7,8 A 20-year literature review of the role of substance abuse in depression indicates that HPA axis responsiveness of depressed women exceeds that of depressed men.9
Summary. Women may be more susceptible than men to emotionally triggered relapse, especially during the menstrual cycle’s luteal phase. Women may also be more susceptible than men to relapse in response to nicotine and cocaine cues.
Table 2
Substance use relapse patterns: Women versus men
| Emotions and mood state play a greater role in driving relapse in women |
| Craving. Women have greater craving than men in response to nicotine and cocaine drug cues |
| Nicotine dependency. Women are more likely to relapse to cigarette use |
| Abstinence. Women have shorter abstinence periods after cocaine treatment |
| Residential treatment. Women have a better prognosis than men 6 months after residential cocaine treatment |
| Premenstrual hormone changes increase women’s relapse risk |
Menstruation and relapse patterns
Research into a correlation between menstrual cycle phases and dependency behavior is in its infancy. Early nicotine, alcohol, and stimulant addiction studies have shown inconsistent results.
Nicotine. A naturalistic study of women smokers ages 20 to 39 showed that they smoked more cigarettes per day during the late luteal phase, but their nicotine boost and mood states were similar throughout the menstrual cycle.10
Some—but not all—studies suggest that nicotine withdrawal symptoms increase during the luteal phase.11,12 In an outpatient study designed to assess hormonal effects on nicotine response, 30 female smokers acutely abstinent of nicotine were randomly assigned by menstrual cycle phase to receive transdermal nicotine or a placebo patch. Both premenstrual and nicotine withdrawal symptoms intensified in the women’s late luteal phase, compared with the follicular phase.12
Conversely, the same researchers found that menstrual cycle phase did not affect withdrawal symptoms in 21 nicotine-dependent female inpatients, even though premenstrual changes occurred in the late luteal phase.
As the authors observed, drawing conclusions can be difficult when menstrual cycle hormone withdrawal and nicotine withdrawal symptoms overlap.12-16
Alcohol. Premenstrual syndrome (PMS) increases a woman’s risk of alcohol abuse. Alcohol and allopregnenolone—progesterone’s neuroactive metabolite—both facilitate gamma-aminobutyric acid ionotropic type A (GABAA) receptor activity. Thus, women with PMS and alcohol dependence may have a genetically more-sensitive GABAA system.17 Unfortunately, aside from one study that shows increased alcohol intake during the luteal phase, no studies have examined alcohol withdrawal, craving, and relapse across the menstrual cycle.18
Stimulants. Stimulant craving and relapse have not been examined in women at different menstrual cycle phases. Some authors speculate that women may have a higher subjective response to stimulants during the early follicular phase—when estrogen levels are higher and progesterone levels are lower—compared with the luteal phase.19
Sex hormones and relapse
Estrogen. Preclinical studies suggest that estrogen facilitates substance dependence by enhancing dopaminergic activity (Table 3).1,20-26 Because reinstatement (the animal model of relapse) is driven partially by dopaminergic activity in the striatum, one could hypothesize that:
- estrogen’s dopamine-enhancing effects facilitate dependence
- women with stimulant dependence are at higher risk of relapse in the follicular phase—when estrogen levels are higher—than in the luteal phase.
Table 3
Preclinical findings: How hormones may influence addictive behavior
| Hormone | Neurobiologic effect | Mechanism | Behavioral effect |
|---|---|---|---|
| Estrogen | Facilitates dopamine | Decreases inhibitory GABAB activity, regulates D2 autoreceptor expression, alters dopamine reuptake, modulates glutamate activity | Enhances reward, self-administration, sensitization,* and stimulant dependence; facilitates reinstatement† |
| Progesterone | Facilitates dopamine when given intermittently; might facilitate or inhibit estrogen’s effects on dopamine | Unclear | Attenuates response to stress, anxiety, pain, aggressiveness |
| Allopregnenolone‡ | Facilitates GABAA | GABAA-positive allosteric modulator, such as ethanol | Enhances ethanol consumption, promotes ethanol reinstatement |
| GABAA/GABAB: gamma-aminobutyric acid, ionotropic types A and B | |||
| * Sensitization: Repeated exposure to psychostimulants results in drug-seeking response to subsequent exposure, which plays an important role in addiction and craving. | |||
| † Reinstatement is the animal model of relapse. | |||
| ‡ Progesterone’s neuroactive metabolite | |||
| Source: References 1, 20-26 | |||
Treatment implications
Psychotherapy. Evidence suggests that emotions and mood states may play a larger role in triggering substance abuse relapse in women than in men. Psychotherapy and residential treatment therefore are particularly important components of women’s treatment.
In a 6-month follow-up outpatient study of cocaine dependence, women responded better than men did to behavioral treatment, even though the women had more-severe disorders at entry.27,28
A more-recent inpatient study followed 64 men and 37 women hospitalized for treatment of cocaine dependence. Researchers compared the patients’ drug use histories, psychiatric diagnoses, and Addiction Severity Index (ASI) scores during hospitalization and their cocaine use and ASI scores 6 months later. In initial evaluations, women had significantly more-severe family and social problems. At follow-up, however, significantly more women than men were abstinent from cocaine use, and their family/social problems had diminshed.28
Notably, a recent functional MRI study comparing 17 male and 10 female abstinent cocaine-dependent subjects indicated that the women more often used verbal coping strategies to decrease cocaine craving.29 This finding supports the potential benefit of psychotherapy to prevent relapse in women with a history of substance dependence.
Hormone regulation. For many women, continuous oral contraceptives (OCPs) can improve affect variability across the menstrual cycle and diminish negative mood. Others, however, experience negative changes in mood or affect while taking OCPs. Risk factors for a negative response include:
- history of depression or other psychological distress symptoms
- dysmenorrhea
- PMS
- history of pregnancy-related mood symptoms
- family history of OCP-related mood complaints
- being in the postpartum
- age 30
Continuous OCPs can be given so that women have only two to three menstrual periods per year. Formulations with ethinyl estradiol and norethindrone—such as Necon 0.5/35 or 1.0/35—may stabilize mood more effectively than others.
Give a 2-month trial, then re-evaluate progress. Because of the increased risk of clotting, only nonsmokers and women without a history of blood clots should take OCPs.
Case report: Fighting the cravings
Eight months ago, when Ms. H was still using cocaine, her primary care physician prescribed fluoxetine, 20 mg/d, for depressive symptoms. Her mood has not improved, nor has her menstrual cycle-related depression or irritability. She asks if anything else might stop her premenstrual cravings.
Because of Ms. H’s reported PMS, we counsel her to be especially vigilant for alcohol cravings around the luteal and late luteal phases of her menstrual cycle (Table 4). We discuss with her:
- the need to watch for signs of relapse
- the importance of aggressive treatment, including psychotherapy, group therapy, and residential treatment, as needed.
She agrees to a 2-month trial, and we schedule a follow-up appointment to re-evaluate her progress.
Table 4
Interventions to prevent relapse in women with addiction disorders
| Track craving and mood symptoms in relation to the patient’s menstrual cycle |
| Educate her about triggers for relapse |
| Provide psychotherapy to bolster her coping strategies for stressful life events |
| Screen for comorbid mood or psychiatric disorders and treat them aggressively |
| Treat premenstrual mood symptoms with a selective serotonin reuptake inhibitor and/or by regulating hormone levels with a continuous oral contraceptive |
- Carroll ME, Lynch WJ, Roth ME, et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci 2004;25(5):273-9.
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 2004;28(6):533-46.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8(11):1481-9.
- Fluoxetine • Prozac
- Ethinyl estradiol and norethindrone oral contraceptive • Necon, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121-37.
2. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403-13.
3. Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44-56.
4. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex decision-making and drug addiction. Trends Neurosci 2006;29(2):116-24.
5. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31(2):151-78.
6. Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59(1):40-7.
7. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 2006;63(3):324-31.
8. Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 2005;29(7):1351-5.
9. Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry 2002;63(7):616-27.
10. Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25(7):677-91.
11. O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 1989;14(6):595-600.
12. Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res 2000;2(3):231-41.
13. Allen SS, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res 1999;1(2):129-42.
14. Franklin TR, Napier K, Ehrman R, et al. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res 2004;6(1):171-5.
15. Pomerleau CS, Mehringer AM, Marks JL, et al. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav 2000;25(4):483-97.
16. Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relapse and withdrawal symptoms. In: Keefe FJ (ed). Thirteenth annual proceedings for the Society of Behavioral Medicine. Rockville, MD, 1992:107.
17. Backstrom T, Andersson A, Andree L, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 2003;1007:42-53.
18. Harvey SM, Beckman LJ. Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 1985;9(5):465-7.
19. White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73(4):729-41.
20. Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 2004;29(1):81-5.
21. Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 2004;46(5):672-87.
22. Lynch WJ, Roth ME, Mickelberg JL, Carroll M. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001;68(4):641-6.
23. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med 2004;71(Suppl 2):S4-10.
24. Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004;19(6):344-53.
25. Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 2003;30(1):1-7.
26. Nie H, Janak, PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1-2):222-8.
27. Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63-6.
28. Weiss RD, Martinez-Raga J, Griffin ML, et al. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 1997;44(1):35-40.
29. Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:487-94.
30. Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord 2002;70(3):229-40.
31. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am 2004;27(4):627-48.
Women become dependent more rapidly than men after initial cocaine, opioid, or alcohol use and may be more sensitive to drugs’ adverse health effects.1 And although men and women relapse to substance use at similar rates, ovulating women may be particularly vulnerable to relapse at certain times of the month.
Understanding the hormonal influences that increase women’s relapse risk can help you intervene more effectively. This article describes:
- how women’s relapse patterns differ from men’s
- why psychotherapy and hormone regulation may be preferred for relapse prevention in women with substance use disorders.
Case report: will she relapse again?
Ms. H, age 46, is in her third month of an alcohol and drug residential rehabilitation program. She has a 10-year history of alcohol and crack cocaine dependence and is battling cravings to use again. These feelings are usually triggered by being in places or with people associated with her drug use, but this time she is committed to staying sober.
She started smoking cigarettes in her teens and using drugs and alcohol in her mid 20s. She feels that her dependency has been out of control in the 10 years since her son was born.
She has tried to quit many times on her own but has managed no more than 1 month of abstinence. She often has relapsed in response to feeling anxious or depressed about being unemployed or after arguing with her partner.
Mechanisms of relapse
Dopamine release is essential for encoding learned associations. When a drug is used in the early dependency state, dopamine release produces pleasure that reinforces continued drug use. Once the behavior is learned, environmental stimuli can trigger dopamine and turn on the brain circuits for this familiar, highly rewarding behavior. Dopamine also is the primary cause of long-lasting brain changes that make it difficult for substance-dependent persons such as Ms. H to control desire for the drug.2
Early relapse—caused by dopamine’s and other neurotransmitters’ effects on various brain regions—is triggered by environmental stimuli such as:
- re-exposure to a small amount of the drug
- exposure to an environment or cues associated with past drug use
- exposure to stressful events.
Table 1
3 stages of relapse and their neurobiologic components
| Relapse stage | Key neurotransmitters | Brain regions involved |
|---|---|---|
| Early relapse triggered by: | ||
| • exposure to a small amount of the drug | Dopamine | Ventral tegmental area |
| Nucleus accumbens core | ||
| Prefrontal cortex | ||
| • environmental cues that re-trigger learned associations | Dopamine | Basolateral amygdala |
| Nucleus accumbens core | ||
| • stressful events and disappointments | Norepinephrine, corticotropin-releasing factor | Extended amygdala |
| Bed nucleus of the stria terminalis | ||
| Craving | Multiple, undetermined | Prefrontal cortex circuitry involving the anterior cingulate and orbitofrontal cortices |
| Relapse | Glutamate, dopamine | Prefrontal cortex |
| Nucleus accumbens core | ||
| Ventral pallidum | ||
| Source: References 2-4 | ||
Emotions, stress trigger relapse
Ms. H. reports increased irritability and impulsivity along with depressed mood—especially during the 3 to 4 days preceding her menstrual period. Her periods are regular, and these mood symptoms recur each month. She does not meet criteria for major depressive disorder.
Emotional reactions play a larger role in relapse for women than for men. Women report higher levels of craving and depressed mood during abstinence and experience stronger urges to drink and smoke when depressed. Women also are more likely to report substance use relapse in response to specific stressful events, disappointments, or depressed mood.1 This is consistent with evidence that women have heightened physiologic responses to social rejection and social stressors.5
A lower density of brain serotonin transporter has been associated with a higher risk of depression in women. Because estrogen and progesterone affect expression of the serotonin transporter, changes in these hormone levels might alter the risk of depression.6 Thus, ovarian hormones’ effect on the serotonin system may contribute to the higher rate of emotionally triggered relapse in women versus men.
Menstrual cycle phases. How men and women respond to stress may contribute to differences in their relapse behaviors (Table 2).
During the first 2 weeks of the menstrual cycle—the follicular phase—women show lower physiologic reactivity (as seen in blood pressure and catecholamine measurements) and lower cortisol responsiveness than men do in response to psychosocial stress. Estrogen contributes to this effect by attenuating sympathoadrenal responsiveness.5
During the latter 2 weeks of the menstrual cycle—the luteal phase—the ovulating woman’s hypothalamic-pituitary-adrenal (HPA) axis response increases and increases her sensitivity to stress. In this phase, progesterone’s presence reverses estrogen’s effect and makes the brain more reactive to emotions and stressors.
Higher stress responsiveness is associated with increased cocaine craving.7,8 A 20-year literature review of the role of substance abuse in depression indicates that HPA axis responsiveness of depressed women exceeds that of depressed men.9
Summary. Women may be more susceptible than men to emotionally triggered relapse, especially during the menstrual cycle’s luteal phase. Women may also be more susceptible than men to relapse in response to nicotine and cocaine cues.
Table 2
Substance use relapse patterns: Women versus men
| Emotions and mood state play a greater role in driving relapse in women |
| Craving. Women have greater craving than men in response to nicotine and cocaine drug cues |
| Nicotine dependency. Women are more likely to relapse to cigarette use |
| Abstinence. Women have shorter abstinence periods after cocaine treatment |
| Residential treatment. Women have a better prognosis than men 6 months after residential cocaine treatment |
| Premenstrual hormone changes increase women’s relapse risk |
Menstruation and relapse patterns
Research into a correlation between menstrual cycle phases and dependency behavior is in its infancy. Early nicotine, alcohol, and stimulant addiction studies have shown inconsistent results.
Nicotine. A naturalistic study of women smokers ages 20 to 39 showed that they smoked more cigarettes per day during the late luteal phase, but their nicotine boost and mood states were similar throughout the menstrual cycle.10
Some—but not all—studies suggest that nicotine withdrawal symptoms increase during the luteal phase.11,12 In an outpatient study designed to assess hormonal effects on nicotine response, 30 female smokers acutely abstinent of nicotine were randomly assigned by menstrual cycle phase to receive transdermal nicotine or a placebo patch. Both premenstrual and nicotine withdrawal symptoms intensified in the women’s late luteal phase, compared with the follicular phase.12
Conversely, the same researchers found that menstrual cycle phase did not affect withdrawal symptoms in 21 nicotine-dependent female inpatients, even though premenstrual changes occurred in the late luteal phase.
As the authors observed, drawing conclusions can be difficult when menstrual cycle hormone withdrawal and nicotine withdrawal symptoms overlap.12-16
Alcohol. Premenstrual syndrome (PMS) increases a woman’s risk of alcohol abuse. Alcohol and allopregnenolone—progesterone’s neuroactive metabolite—both facilitate gamma-aminobutyric acid ionotropic type A (GABAA) receptor activity. Thus, women with PMS and alcohol dependence may have a genetically more-sensitive GABAA system.17 Unfortunately, aside from one study that shows increased alcohol intake during the luteal phase, no studies have examined alcohol withdrawal, craving, and relapse across the menstrual cycle.18
Stimulants. Stimulant craving and relapse have not been examined in women at different menstrual cycle phases. Some authors speculate that women may have a higher subjective response to stimulants during the early follicular phase—when estrogen levels are higher and progesterone levels are lower—compared with the luteal phase.19
Sex hormones and relapse
Estrogen. Preclinical studies suggest that estrogen facilitates substance dependence by enhancing dopaminergic activity (Table 3).1,20-26 Because reinstatement (the animal model of relapse) is driven partially by dopaminergic activity in the striatum, one could hypothesize that:
- estrogen’s dopamine-enhancing effects facilitate dependence
- women with stimulant dependence are at higher risk of relapse in the follicular phase—when estrogen levels are higher—than in the luteal phase.
Table 3
Preclinical findings: How hormones may influence addictive behavior
| Hormone | Neurobiologic effect | Mechanism | Behavioral effect |
|---|---|---|---|
| Estrogen | Facilitates dopamine | Decreases inhibitory GABAB activity, regulates D2 autoreceptor expression, alters dopamine reuptake, modulates glutamate activity | Enhances reward, self-administration, sensitization,* and stimulant dependence; facilitates reinstatement† |
| Progesterone | Facilitates dopamine when given intermittently; might facilitate or inhibit estrogen’s effects on dopamine | Unclear | Attenuates response to stress, anxiety, pain, aggressiveness |
| Allopregnenolone‡ | Facilitates GABAA | GABAA-positive allosteric modulator, such as ethanol | Enhances ethanol consumption, promotes ethanol reinstatement |
| GABAA/GABAB: gamma-aminobutyric acid, ionotropic types A and B | |||
| * Sensitization: Repeated exposure to psychostimulants results in drug-seeking response to subsequent exposure, which plays an important role in addiction and craving. | |||
| † Reinstatement is the animal model of relapse. | |||
| ‡ Progesterone’s neuroactive metabolite | |||
| Source: References 1, 20-26 | |||
Treatment implications
Psychotherapy. Evidence suggests that emotions and mood states may play a larger role in triggering substance abuse relapse in women than in men. Psychotherapy and residential treatment therefore are particularly important components of women’s treatment.
In a 6-month follow-up outpatient study of cocaine dependence, women responded better than men did to behavioral treatment, even though the women had more-severe disorders at entry.27,28
A more-recent inpatient study followed 64 men and 37 women hospitalized for treatment of cocaine dependence. Researchers compared the patients’ drug use histories, psychiatric diagnoses, and Addiction Severity Index (ASI) scores during hospitalization and their cocaine use and ASI scores 6 months later. In initial evaluations, women had significantly more-severe family and social problems. At follow-up, however, significantly more women than men were abstinent from cocaine use, and their family/social problems had diminshed.28
Notably, a recent functional MRI study comparing 17 male and 10 female abstinent cocaine-dependent subjects indicated that the women more often used verbal coping strategies to decrease cocaine craving.29 This finding supports the potential benefit of psychotherapy to prevent relapse in women with a history of substance dependence.
Hormone regulation. For many women, continuous oral contraceptives (OCPs) can improve affect variability across the menstrual cycle and diminish negative mood. Others, however, experience negative changes in mood or affect while taking OCPs. Risk factors for a negative response include:
- history of depression or other psychological distress symptoms
- dysmenorrhea
- PMS
- history of pregnancy-related mood symptoms
- family history of OCP-related mood complaints
- being in the postpartum
- age 30
Continuous OCPs can be given so that women have only two to three menstrual periods per year. Formulations with ethinyl estradiol and norethindrone—such as Necon 0.5/35 or 1.0/35—may stabilize mood more effectively than others.
Give a 2-month trial, then re-evaluate progress. Because of the increased risk of clotting, only nonsmokers and women without a history of blood clots should take OCPs.
Case report: Fighting the cravings
Eight months ago, when Ms. H was still using cocaine, her primary care physician prescribed fluoxetine, 20 mg/d, for depressive symptoms. Her mood has not improved, nor has her menstrual cycle-related depression or irritability. She asks if anything else might stop her premenstrual cravings.
Because of Ms. H’s reported PMS, we counsel her to be especially vigilant for alcohol cravings around the luteal and late luteal phases of her menstrual cycle (Table 4). We discuss with her:
- the need to watch for signs of relapse
- the importance of aggressive treatment, including psychotherapy, group therapy, and residential treatment, as needed.
She agrees to a 2-month trial, and we schedule a follow-up appointment to re-evaluate her progress.
Table 4
Interventions to prevent relapse in women with addiction disorders
| Track craving and mood symptoms in relation to the patient’s menstrual cycle |
| Educate her about triggers for relapse |
| Provide psychotherapy to bolster her coping strategies for stressful life events |
| Screen for comorbid mood or psychiatric disorders and treat them aggressively |
| Treat premenstrual mood symptoms with a selective serotonin reuptake inhibitor and/or by regulating hormone levels with a continuous oral contraceptive |
- Carroll ME, Lynch WJ, Roth ME, et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci 2004;25(5):273-9.
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 2004;28(6):533-46.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8(11):1481-9.
- Fluoxetine • Prozac
- Ethinyl estradiol and norethindrone oral contraceptive • Necon, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Women become dependent more rapidly than men after initial cocaine, opioid, or alcohol use and may be more sensitive to drugs’ adverse health effects.1 And although men and women relapse to substance use at similar rates, ovulating women may be particularly vulnerable to relapse at certain times of the month.
Understanding the hormonal influences that increase women’s relapse risk can help you intervene more effectively. This article describes:
- how women’s relapse patterns differ from men’s
- why psychotherapy and hormone regulation may be preferred for relapse prevention in women with substance use disorders.
Case report: will she relapse again?
Ms. H, age 46, is in her third month of an alcohol and drug residential rehabilitation program. She has a 10-year history of alcohol and crack cocaine dependence and is battling cravings to use again. These feelings are usually triggered by being in places or with people associated with her drug use, but this time she is committed to staying sober.
She started smoking cigarettes in her teens and using drugs and alcohol in her mid 20s. She feels that her dependency has been out of control in the 10 years since her son was born.
She has tried to quit many times on her own but has managed no more than 1 month of abstinence. She often has relapsed in response to feeling anxious or depressed about being unemployed or after arguing with her partner.
Mechanisms of relapse
Dopamine release is essential for encoding learned associations. When a drug is used in the early dependency state, dopamine release produces pleasure that reinforces continued drug use. Once the behavior is learned, environmental stimuli can trigger dopamine and turn on the brain circuits for this familiar, highly rewarding behavior. Dopamine also is the primary cause of long-lasting brain changes that make it difficult for substance-dependent persons such as Ms. H to control desire for the drug.2
Early relapse—caused by dopamine’s and other neurotransmitters’ effects on various brain regions—is triggered by environmental stimuli such as:
- re-exposure to a small amount of the drug
- exposure to an environment or cues associated with past drug use
- exposure to stressful events.
Table 1
3 stages of relapse and their neurobiologic components
| Relapse stage | Key neurotransmitters | Brain regions involved |
|---|---|---|
| Early relapse triggered by: | ||
| • exposure to a small amount of the drug | Dopamine | Ventral tegmental area |
| Nucleus accumbens core | ||
| Prefrontal cortex | ||
| • environmental cues that re-trigger learned associations | Dopamine | Basolateral amygdala |
| Nucleus accumbens core | ||
| • stressful events and disappointments | Norepinephrine, corticotropin-releasing factor | Extended amygdala |
| Bed nucleus of the stria terminalis | ||
| Craving | Multiple, undetermined | Prefrontal cortex circuitry involving the anterior cingulate and orbitofrontal cortices |
| Relapse | Glutamate, dopamine | Prefrontal cortex |
| Nucleus accumbens core | ||
| Ventral pallidum | ||
| Source: References 2-4 | ||
Emotions, stress trigger relapse
Ms. H. reports increased irritability and impulsivity along with depressed mood—especially during the 3 to 4 days preceding her menstrual period. Her periods are regular, and these mood symptoms recur each month. She does not meet criteria for major depressive disorder.
Emotional reactions play a larger role in relapse for women than for men. Women report higher levels of craving and depressed mood during abstinence and experience stronger urges to drink and smoke when depressed. Women also are more likely to report substance use relapse in response to specific stressful events, disappointments, or depressed mood.1 This is consistent with evidence that women have heightened physiologic responses to social rejection and social stressors.5
A lower density of brain serotonin transporter has been associated with a higher risk of depression in women. Because estrogen and progesterone affect expression of the serotonin transporter, changes in these hormone levels might alter the risk of depression.6 Thus, ovarian hormones’ effect on the serotonin system may contribute to the higher rate of emotionally triggered relapse in women versus men.
Menstrual cycle phases. How men and women respond to stress may contribute to differences in their relapse behaviors (Table 2).
During the first 2 weeks of the menstrual cycle—the follicular phase—women show lower physiologic reactivity (as seen in blood pressure and catecholamine measurements) and lower cortisol responsiveness than men do in response to psychosocial stress. Estrogen contributes to this effect by attenuating sympathoadrenal responsiveness.5
During the latter 2 weeks of the menstrual cycle—the luteal phase—the ovulating woman’s hypothalamic-pituitary-adrenal (HPA) axis response increases and increases her sensitivity to stress. In this phase, progesterone’s presence reverses estrogen’s effect and makes the brain more reactive to emotions and stressors.
Higher stress responsiveness is associated with increased cocaine craving.7,8 A 20-year literature review of the role of substance abuse in depression indicates that HPA axis responsiveness of depressed women exceeds that of depressed men.9
Summary. Women may be more susceptible than men to emotionally triggered relapse, especially during the menstrual cycle’s luteal phase. Women may also be more susceptible than men to relapse in response to nicotine and cocaine cues.
Table 2
Substance use relapse patterns: Women versus men
| Emotions and mood state play a greater role in driving relapse in women |
| Craving. Women have greater craving than men in response to nicotine and cocaine drug cues |
| Nicotine dependency. Women are more likely to relapse to cigarette use |
| Abstinence. Women have shorter abstinence periods after cocaine treatment |
| Residential treatment. Women have a better prognosis than men 6 months after residential cocaine treatment |
| Premenstrual hormone changes increase women’s relapse risk |
Menstruation and relapse patterns
Research into a correlation between menstrual cycle phases and dependency behavior is in its infancy. Early nicotine, alcohol, and stimulant addiction studies have shown inconsistent results.
Nicotine. A naturalistic study of women smokers ages 20 to 39 showed that they smoked more cigarettes per day during the late luteal phase, but their nicotine boost and mood states were similar throughout the menstrual cycle.10
Some—but not all—studies suggest that nicotine withdrawal symptoms increase during the luteal phase.11,12 In an outpatient study designed to assess hormonal effects on nicotine response, 30 female smokers acutely abstinent of nicotine were randomly assigned by menstrual cycle phase to receive transdermal nicotine or a placebo patch. Both premenstrual and nicotine withdrawal symptoms intensified in the women’s late luteal phase, compared with the follicular phase.12
Conversely, the same researchers found that menstrual cycle phase did not affect withdrawal symptoms in 21 nicotine-dependent female inpatients, even though premenstrual changes occurred in the late luteal phase.
As the authors observed, drawing conclusions can be difficult when menstrual cycle hormone withdrawal and nicotine withdrawal symptoms overlap.12-16
Alcohol. Premenstrual syndrome (PMS) increases a woman’s risk of alcohol abuse. Alcohol and allopregnenolone—progesterone’s neuroactive metabolite—both facilitate gamma-aminobutyric acid ionotropic type A (GABAA) receptor activity. Thus, women with PMS and alcohol dependence may have a genetically more-sensitive GABAA system.17 Unfortunately, aside from one study that shows increased alcohol intake during the luteal phase, no studies have examined alcohol withdrawal, craving, and relapse across the menstrual cycle.18
Stimulants. Stimulant craving and relapse have not been examined in women at different menstrual cycle phases. Some authors speculate that women may have a higher subjective response to stimulants during the early follicular phase—when estrogen levels are higher and progesterone levels are lower—compared with the luteal phase.19
Sex hormones and relapse
Estrogen. Preclinical studies suggest that estrogen facilitates substance dependence by enhancing dopaminergic activity (Table 3).1,20-26 Because reinstatement (the animal model of relapse) is driven partially by dopaminergic activity in the striatum, one could hypothesize that:
- estrogen’s dopamine-enhancing effects facilitate dependence
- women with stimulant dependence are at higher risk of relapse in the follicular phase—when estrogen levels are higher—than in the luteal phase.
Table 3
Preclinical findings: How hormones may influence addictive behavior
| Hormone | Neurobiologic effect | Mechanism | Behavioral effect |
|---|---|---|---|
| Estrogen | Facilitates dopamine | Decreases inhibitory GABAB activity, regulates D2 autoreceptor expression, alters dopamine reuptake, modulates glutamate activity | Enhances reward, self-administration, sensitization,* and stimulant dependence; facilitates reinstatement† |
| Progesterone | Facilitates dopamine when given intermittently; might facilitate or inhibit estrogen’s effects on dopamine | Unclear | Attenuates response to stress, anxiety, pain, aggressiveness |
| Allopregnenolone‡ | Facilitates GABAA | GABAA-positive allosteric modulator, such as ethanol | Enhances ethanol consumption, promotes ethanol reinstatement |
| GABAA/GABAB: gamma-aminobutyric acid, ionotropic types A and B | |||
| * Sensitization: Repeated exposure to psychostimulants results in drug-seeking response to subsequent exposure, which plays an important role in addiction and craving. | |||
| † Reinstatement is the animal model of relapse. | |||
| ‡ Progesterone’s neuroactive metabolite | |||
| Source: References 1, 20-26 | |||
Treatment implications
Psychotherapy. Evidence suggests that emotions and mood states may play a larger role in triggering substance abuse relapse in women than in men. Psychotherapy and residential treatment therefore are particularly important components of women’s treatment.
In a 6-month follow-up outpatient study of cocaine dependence, women responded better than men did to behavioral treatment, even though the women had more-severe disorders at entry.27,28
A more-recent inpatient study followed 64 men and 37 women hospitalized for treatment of cocaine dependence. Researchers compared the patients’ drug use histories, psychiatric diagnoses, and Addiction Severity Index (ASI) scores during hospitalization and their cocaine use and ASI scores 6 months later. In initial evaluations, women had significantly more-severe family and social problems. At follow-up, however, significantly more women than men were abstinent from cocaine use, and their family/social problems had diminshed.28
Notably, a recent functional MRI study comparing 17 male and 10 female abstinent cocaine-dependent subjects indicated that the women more often used verbal coping strategies to decrease cocaine craving.29 This finding supports the potential benefit of psychotherapy to prevent relapse in women with a history of substance dependence.
Hormone regulation. For many women, continuous oral contraceptives (OCPs) can improve affect variability across the menstrual cycle and diminish negative mood. Others, however, experience negative changes in mood or affect while taking OCPs. Risk factors for a negative response include:
- history of depression or other psychological distress symptoms
- dysmenorrhea
- PMS
- history of pregnancy-related mood symptoms
- family history of OCP-related mood complaints
- being in the postpartum
- age 30
Continuous OCPs can be given so that women have only two to three menstrual periods per year. Formulations with ethinyl estradiol and norethindrone—such as Necon 0.5/35 or 1.0/35—may stabilize mood more effectively than others.
Give a 2-month trial, then re-evaluate progress. Because of the increased risk of clotting, only nonsmokers and women without a history of blood clots should take OCPs.
Case report: Fighting the cravings
Eight months ago, when Ms. H was still using cocaine, her primary care physician prescribed fluoxetine, 20 mg/d, for depressive symptoms. Her mood has not improved, nor has her menstrual cycle-related depression or irritability. She asks if anything else might stop her premenstrual cravings.
Because of Ms. H’s reported PMS, we counsel her to be especially vigilant for alcohol cravings around the luteal and late luteal phases of her menstrual cycle (Table 4). We discuss with her:
- the need to watch for signs of relapse
- the importance of aggressive treatment, including psychotherapy, group therapy, and residential treatment, as needed.
She agrees to a 2-month trial, and we schedule a follow-up appointment to re-evaluate her progress.
Table 4
Interventions to prevent relapse in women with addiction disorders
| Track craving and mood symptoms in relation to the patient’s menstrual cycle |
| Educate her about triggers for relapse |
| Provide psychotherapy to bolster her coping strategies for stressful life events |
| Screen for comorbid mood or psychiatric disorders and treat them aggressively |
| Treat premenstrual mood symptoms with a selective serotonin reuptake inhibitor and/or by regulating hormone levels with a continuous oral contraceptive |
- Carroll ME, Lynch WJ, Roth ME, et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci 2004;25(5):273-9.
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 2004;28(6):533-46.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8(11):1481-9.
- Fluoxetine • Prozac
- Ethinyl estradiol and norethindrone oral contraceptive • Necon, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121-37.
2. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403-13.
3. Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44-56.
4. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex decision-making and drug addiction. Trends Neurosci 2006;29(2):116-24.
5. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31(2):151-78.
6. Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59(1):40-7.
7. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 2006;63(3):324-31.
8. Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 2005;29(7):1351-5.
9. Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry 2002;63(7):616-27.
10. Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25(7):677-91.
11. O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 1989;14(6):595-600.
12. Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res 2000;2(3):231-41.
13. Allen SS, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res 1999;1(2):129-42.
14. Franklin TR, Napier K, Ehrman R, et al. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res 2004;6(1):171-5.
15. Pomerleau CS, Mehringer AM, Marks JL, et al. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav 2000;25(4):483-97.
16. Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relapse and withdrawal symptoms. In: Keefe FJ (ed). Thirteenth annual proceedings for the Society of Behavioral Medicine. Rockville, MD, 1992:107.
17. Backstrom T, Andersson A, Andree L, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 2003;1007:42-53.
18. Harvey SM, Beckman LJ. Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 1985;9(5):465-7.
19. White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73(4):729-41.
20. Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 2004;29(1):81-5.
21. Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 2004;46(5):672-87.
22. Lynch WJ, Roth ME, Mickelberg JL, Carroll M. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001;68(4):641-6.
23. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med 2004;71(Suppl 2):S4-10.
24. Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004;19(6):344-53.
25. Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 2003;30(1):1-7.
26. Nie H, Janak, PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1-2):222-8.
27. Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63-6.
28. Weiss RD, Martinez-Raga J, Griffin ML, et al. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 1997;44(1):35-40.
29. Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:487-94.
30. Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord 2002;70(3):229-40.
31. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am 2004;27(4):627-48.
1. Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121-37.
2. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403-13.
3. Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44-56.
4. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex decision-making and drug addiction. Trends Neurosci 2006;29(2):116-24.
5. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31(2):151-78.
6. Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59(1):40-7.
7. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 2006;63(3):324-31.
8. Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 2005;29(7):1351-5.
9. Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry 2002;63(7):616-27.
10. Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25(7):677-91.
11. O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 1989;14(6):595-600.
12. Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res 2000;2(3):231-41.
13. Allen SS, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res 1999;1(2):129-42.
14. Franklin TR, Napier K, Ehrman R, et al. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res 2004;6(1):171-5.
15. Pomerleau CS, Mehringer AM, Marks JL, et al. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav 2000;25(4):483-97.
16. Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relapse and withdrawal symptoms. In: Keefe FJ (ed). Thirteenth annual proceedings for the Society of Behavioral Medicine. Rockville, MD, 1992:107.
17. Backstrom T, Andersson A, Andree L, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 2003;1007:42-53.
18. Harvey SM, Beckman LJ. Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 1985;9(5):465-7.
19. White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73(4):729-41.
20. Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 2004;29(1):81-5.
21. Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 2004;46(5):672-87.
22. Lynch WJ, Roth ME, Mickelberg JL, Carroll M. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001;68(4):641-6.
23. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med 2004;71(Suppl 2):S4-10.
24. Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004;19(6):344-53.
25. Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 2003;30(1):1-7.
26. Nie H, Janak, PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1-2):222-8.
27. Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63-6.
28. Weiss RD, Martinez-Raga J, Griffin ML, et al. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 1997;44(1):35-40.
29. Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:487-94.
30. Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord 2002;70(3):229-40.
31. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am 2004;27(4):627-48.
Demystifying CBT: Effective, easy-to-use treatment for depression and anxiety
Whether you are in training or an experienced practitioner, you need more than a rudimentary understanding of cognitive-behavioral therapy (CBT). This easy-to-use psychotherapy has broad empiric support, is a first-choice treatment,1 and can help patients cope with depression, anxiety, and other psychological problems.
Psychiatrists who learn CBT well can alleviate many patients’ distress by creatively applying its tools and techniques. For example, the cognitive approach to panic disorder compares favorably to medication1-3 (Box).
Aaron beck’s CBT
Negative thoughts, biased processing. The greater your fidelity to CBT’s guiding principles (Table 1), the more effective the therapy becomes.4 Beck designed CBT to address his observation that depressed persons hold unrealistically negative views about themselves, the world, and the future.5,6 A distorted information-processing system prevents them from correcting underlying negative beliefs. Negative thoughts predominate their cognitions and seem to arise spontaneously, reflexively, and unremittingly. These “automatic thoughts,” as he called them, reflect underlying themes about the self that can be identified as:
- intermediate beliefs (conditional assumptions, attitudes, and rules)
- core beliefs (fundamental, often global, and absolute rules).7
using CBT to experientially disconfirm catastrophic cognitions is the psychotherapy of choice for anxiety disorders.1 Cognitive models exist for each anxiety disorder2 and include psychoeducation, self-monitoring, evaluation of anxious cognitions, and testing cognitions interoceptively (within the body) and in vivo (within the environment). The therapist strategically uses adjunctive measures such as relaxation training, controlled breathing, visualization, and distraction.
Panic disorder is characterized by catastrophic misinterpretations of benign bodily sensations that accompany a fear response.2,3 Disability occurs when patients avoid situations or activities they believe will activate bodily sensations such as dizziness or breathlessness. Using environmental manipulations—spinning, hyperventilation, or straw-breathing, to name a few—CBT aims to disconfirm patients’ catastrophic thoughts by deliberately exposing them to feared somatic sensations.2
When used to treat panic disorder, CBT is associated with remission rates similar to those achieved by medication and much lower relapse rates.1
Guiding principles of cognitive-behavioral therapy
|
- external (environmental) stress that carries symbolic value
- internal (physiologic) stress that activates the affective valence of the underlying schema.1,5,6
CBT’s scientific method. CBT teaches a person the skills to identify this cognitive material and to recognize biases that affect how he or she processes information. You can help patients understand:
- the bidirectional relationship between thoughts, feelings, and behaviors
- that they can influence their emotions by changing their thoughts and behavior.
You provide empiric tools to help them explore the validity or usefulness of their thoughts and the impact of their behaviors. When patients disprove a negative cognition through this process of “experiential disconfirmation,” you help them to change that cognition. Homework is a key ingredient (Table 2); patients who do their CBT homework are more likely to improve than those who don’t.8
Table 2
Structure of a typical CBT session
| Step | What therapist may say or do to introduce this step |
|---|---|
| Collaborate in setting the agenda | ‘What would you like to put on the agenda for today’s session? If we could address one or two items, what would they be?’ |
| Link to previous session via feedback | Review and comment on the patient’s feedback form |
| Check target symptoms | ‘How would you rate your level of (depression, anxiety, etc.) this week on a scale of 0 to 100?’ |
| Therapist also can review standardized rating scales, such as Beck Depression Inventory II | |
| Check medication | ‘Are there any concerns this week about your medication?’ |
| Review week/scheduling | ‘Could you update me about your week?’ |
| ‘What would be important to focus on this coming week?’ | |
| Review homework | ‘Let’s have a look at the homework/self-help work you did this week’ |
| Set new agenda items | ‘This issue sounds important; would you like to add it to today’s agenda?’ |
| Collaborate in developing new homework | ‘I’d like to work on this further next week; let’s decide together what would be doable’ |
| Feedback | ‘How did you feel about today’s session?’ |
| ‘Is there anything you would like to be sure to remember after you leave today?’ | |
| ‘Anything you want to put on the agenda for next session?’ |
Evaluating cognitive distortions
Thought records. Automatic thoughts are the cognitive content that runs through our minds moment to moment and that we can access by asking ourselves, “What was going through my mind when I felt (emotion)?” Automatic thoughts can exist as:
- verbal messages (“I can’t believe this!” or “I’m such a loser”)
- or images (“I picture my boss screaming at me”).
Self-monitoring also facilitates “decentering,” or viewing one’s emotional experience from a distance, which may be crucial to therapeutic success.9 By using Socratic questioning (Table 3) and guided discovery, you teach patients to evaluate their automatic thoughts as hypotheses to be tested.
If patients discover that their automatic thoughts are inaccurate, you can help them construct more-balanced or alternate appraisals. Conversely, hypothesis testing may help generate new solutions if the process validates the patient’s initial interpretation of a situation.10
Table 3
Examples of Socratic and non-Socratic questioning
Socratic questioning
|
- overgeneralization (“Nothing ever works out for me”)
- all-or-nothing thinking (“I failed again” [after getting 95% on an exam])
- mind-reading (“My boss thinks I’m incompetent”)
- catastrophization (“My heart is racing; I think I’m having a heart attack!”).11
- attitudes (“Weakness is contemptible”)
- rules (“I will not let others take advantage of me”)
- conditional assumptions (“If I let others take advantage of me, I’m a thoroughly weak person”).
Framing the intermediate belief as a conditional assumption can help accomplish this goal. Presumably, the patient was distressed about loaning money to his friend. When pressed, he says he didn’t want to lend the money but felt he couldn’t say no. He identifies his automatic thought as “I’ve been taken advantage of.” Asked what this means if it is true, he replies, “If I get taken advantage of, it means I’m weak.”
Core beliefs are deeper cognitive structures that are not always immediately accessible, although they may occasionally emerge spontaneously as automatic thoughts. They are overgeneralized, absolute statements that fall into one of two categories:
- affiliation (“I am bad,” “I am unlovable”)
- competence/vulnerability (“I am weak,” “I am helpless”).
Very often, intermediate and core beliefs must be defined in a measurable way before you can help the patient test them. For the man feeling distressed about loaning money, for example, you might ask him to define “being taken advantage of ” or define “weak” by listing all the characteristics he associates with this label.
Restructuring negative beliefs
A variety of techniques can be used to restructure negative beliefs.
- Cost-benefit analysis involves exploring advantages and disadvantages of maintaining a negative belief or a more-balanced alternate belief.
- Core belief logs12 can track day-to-day evidence that suggests a core belief is not 100% true. You and the patient can scrutinize evidence that supports the core belief and reframe the evidence in a more-rational manner.
- Life review10,12 involves asking the patient to re-evaluate a core belief ’s historical underpinnings and to reframe these events from an adult perspective.
- rational-emotional role play
- empty chair or two-chair dialogue
- restructuring early memories with directed imagery.
Adjunctive Behavioral Strategies
Behavioral interventions are used in CBT to combat anergia, increase socialization, diminish avoidance, and accumulate data to challenge negative beliefs. Common strategies are designed to enhance self-esteem and confidence and build therapeutic momentum as patients gain energy, feel better, and disconfirm negative beliefs.
Activity monitoring and scheduling. Instruct anergic or avoidant patients to monitor daily activities for the week and to rate the degree of pleasure and accomplishment each activity yields on a scale of 1 to 10. As patients become aware of how much time they spend on low-yield activities, help them gradually replace low-yield with higher-yield activities.
Schedule into the week behavioral goals patients identified at the beginning of therapy. Schedule avoided tasks such as household chores, and link them to pleasurable activities as rewards. To evaluate the accuracy of their thinking, ask patients to predict how much pleasure or mastery they will achieve with scheduled activities, then compare their predictions with actual results.
Also ask patients to anticipate obstacles to achieving their goals, to challenge those obstacles, and to develop contingency plans. Reviewing the patient’s week and its bright spots can disconfirm negatively biased recall such as, “My week was terrible,” or, “I don’t have the energy to do anything anymore.”13
Graded task assignments. When scheduling activities, improve success rates by helping patients break down large, unrealistic goals into smaller, more manageable pieces. Ask them to consider realistically what they can accomplish now, not what they could have accomplished before they became ill.
Exposure. Anxious patients avoid feared situations because of catastrophic beliefs that experiencing those situations will harm them. A man with panic disorder may avoid exercise, for example, because he perceives lightheadedness and rapid heart rate as signs of imminent heart attack. Avoiding exercise to prevent the feared symptoms perpetuates his catastrophic beliefs.
Exposure to feared symptoms—while initially arousing high anxiety—allows the patient to experientially disconfirm his beliefs. As he remains well after lightheadedness and rapid heart rate are induced interoceptively (by climbing several flights of stairs, for example), he comes to recognize the situational symptoms as manifestations of anxiety rather than evidence of life-threatening illness.2,3
In vivo exposure entails confronting the patient with the avoided object or situation. For example, you may show a woman with needle phobia pictures of needles, followed by actual needles themselves, then ask her to touch a needle, hold a needle, etc., until her anxiety gradually diminishes.
Imaginal exposure involves asking the patient to imagine himself in a feared situation and manipulating the images to build his sense of mastery. If he stops the image at the moment of highest arousal, instruct him to “continue to play the film forward” by asking, “What happens next?” This approach shows him that he can cope with difficult situations.
Related resources
For clinicians
- Persons JB. Cognitive therapy in practice: a case formulation approach. New York: WW Norton and Co.; 1989.
- Academy of Cognitive Therapy. Training, certification as a cognitive therapist. www.academyofct.org.
- Behavior Online. Gathering site for mental health professionals. www.behavior.net.
- MySelfHelp.com. Interactive programs and moderated discussion designed as treatment adjuncts for patients with depression, stress, eating disorders, etc. Funded by the National Institute of Mental Health ($20/month program access fee). www.MySelfHelp.com.
- Antony M, Swinson R. When perfect isn’t good enough. Oakland, CA: New Harbinger Press; 1998.
- Antony M, Swinson R. The shyness and social anxiety workbook. Oakland, CA: New Harbinger Press; 2000.
- Young J, Klosko J. Reinventing your life: how to break free from negative life patterns. New York: Dutton; 1993.
- Davis M, Eshelman E, McKay M. The relaxation and stress reduction workbook. New York: New Harbinger Press; 1995.
1. Hollon SD, Beck AT. Cognitive and cognitive behavioral therapies. In: Lambert, MJ (ed). Bergin and Garfield’s handbook of psychotherapy and behavior change, 5th ed. New York: John Wiley and Sons; 2004:447-92.
2. Wells A. Cognitive therapy of anxiety disorders: a practice manual and conceptual guide. West Sussex, UK: John Wiley and Sons; 1997.
3. Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH (ed). Clinical handbook of psychological disorders, 3rd ed. New York: Guilford Press; 1991:1-59.
4. Rosenbaum M, Ronen T. Clinical supervision from the standpoint of cognitive-behavior therapy. Psychotherapy 1998;35(2):220-30.
5. Beck AT. The current state of cognitive therapy. A 40-year retrospective. Arch Gen Psychiatry 2005;62:953-9.
6. Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979.
7. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry, 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1247.
8. Rees CS, McEvoy P, Nathan PR. Relationship between homework completion and outcome in cognitive behaviour therapy. Cognit Behav Ther 2005;34(4):242-7.
9. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275-87.
10. Greenberger D, Padesky CA. Mind over mood: a cognitive therapy treatment manual for clients.New York: Guilford Press; 1995.
11. Burns DD. Feeling good handbook. New York: Penguin; 1999.
12. Beck J. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995.
13. Lau MA, Segal ZV, Zaretsky AE. Cognitive-behavioral therapies for the medical clinic. In: Moss D, McGrady A, Davies TC, Wickramasekera I (eds). Handbook of mind-body medicine for primary care. Thousand Oaks, CA: Sage Publications; 2003:167-79.
Whether you are in training or an experienced practitioner, you need more than a rudimentary understanding of cognitive-behavioral therapy (CBT). This easy-to-use psychotherapy has broad empiric support, is a first-choice treatment,1 and can help patients cope with depression, anxiety, and other psychological problems.
Psychiatrists who learn CBT well can alleviate many patients’ distress by creatively applying its tools and techniques. For example, the cognitive approach to panic disorder compares favorably to medication1-3 (Box).
Aaron beck’s CBT
Negative thoughts, biased processing. The greater your fidelity to CBT’s guiding principles (Table 1), the more effective the therapy becomes.4 Beck designed CBT to address his observation that depressed persons hold unrealistically negative views about themselves, the world, and the future.5,6 A distorted information-processing system prevents them from correcting underlying negative beliefs. Negative thoughts predominate their cognitions and seem to arise spontaneously, reflexively, and unremittingly. These “automatic thoughts,” as he called them, reflect underlying themes about the self that can be identified as:
- intermediate beliefs (conditional assumptions, attitudes, and rules)
- core beliefs (fundamental, often global, and absolute rules).7
using CBT to experientially disconfirm catastrophic cognitions is the psychotherapy of choice for anxiety disorders.1 Cognitive models exist for each anxiety disorder2 and include psychoeducation, self-monitoring, evaluation of anxious cognitions, and testing cognitions interoceptively (within the body) and in vivo (within the environment). The therapist strategically uses adjunctive measures such as relaxation training, controlled breathing, visualization, and distraction.
Panic disorder is characterized by catastrophic misinterpretations of benign bodily sensations that accompany a fear response.2,3 Disability occurs when patients avoid situations or activities they believe will activate bodily sensations such as dizziness or breathlessness. Using environmental manipulations—spinning, hyperventilation, or straw-breathing, to name a few—CBT aims to disconfirm patients’ catastrophic thoughts by deliberately exposing them to feared somatic sensations.2
When used to treat panic disorder, CBT is associated with remission rates similar to those achieved by medication and much lower relapse rates.1
Guiding principles of cognitive-behavioral therapy
|
- external (environmental) stress that carries symbolic value
- internal (physiologic) stress that activates the affective valence of the underlying schema.1,5,6
CBT’s scientific method. CBT teaches a person the skills to identify this cognitive material and to recognize biases that affect how he or she processes information. You can help patients understand:
- the bidirectional relationship between thoughts, feelings, and behaviors
- that they can influence their emotions by changing their thoughts and behavior.
You provide empiric tools to help them explore the validity or usefulness of their thoughts and the impact of their behaviors. When patients disprove a negative cognition through this process of “experiential disconfirmation,” you help them to change that cognition. Homework is a key ingredient (Table 2); patients who do their CBT homework are more likely to improve than those who don’t.8
Table 2
Structure of a typical CBT session
| Step | What therapist may say or do to introduce this step |
|---|---|
| Collaborate in setting the agenda | ‘What would you like to put on the agenda for today’s session? If we could address one or two items, what would they be?’ |
| Link to previous session via feedback | Review and comment on the patient’s feedback form |
| Check target symptoms | ‘How would you rate your level of (depression, anxiety, etc.) this week on a scale of 0 to 100?’ |
| Therapist also can review standardized rating scales, such as Beck Depression Inventory II | |
| Check medication | ‘Are there any concerns this week about your medication?’ |
| Review week/scheduling | ‘Could you update me about your week?’ |
| ‘What would be important to focus on this coming week?’ | |
| Review homework | ‘Let’s have a look at the homework/self-help work you did this week’ |
| Set new agenda items | ‘This issue sounds important; would you like to add it to today’s agenda?’ |
| Collaborate in developing new homework | ‘I’d like to work on this further next week; let’s decide together what would be doable’ |
| Feedback | ‘How did you feel about today’s session?’ |
| ‘Is there anything you would like to be sure to remember after you leave today?’ | |
| ‘Anything you want to put on the agenda for next session?’ |
Evaluating cognitive distortions
Thought records. Automatic thoughts are the cognitive content that runs through our minds moment to moment and that we can access by asking ourselves, “What was going through my mind when I felt (emotion)?” Automatic thoughts can exist as:
- verbal messages (“I can’t believe this!” or “I’m such a loser”)
- or images (“I picture my boss screaming at me”).
Self-monitoring also facilitates “decentering,” or viewing one’s emotional experience from a distance, which may be crucial to therapeutic success.9 By using Socratic questioning (Table 3) and guided discovery, you teach patients to evaluate their automatic thoughts as hypotheses to be tested.
If patients discover that their automatic thoughts are inaccurate, you can help them construct more-balanced or alternate appraisals. Conversely, hypothesis testing may help generate new solutions if the process validates the patient’s initial interpretation of a situation.10
Table 3
Examples of Socratic and non-Socratic questioning
Socratic questioning
|
- overgeneralization (“Nothing ever works out for me”)
- all-or-nothing thinking (“I failed again” [after getting 95% on an exam])
- mind-reading (“My boss thinks I’m incompetent”)
- catastrophization (“My heart is racing; I think I’m having a heart attack!”).11
- attitudes (“Weakness is contemptible”)
- rules (“I will not let others take advantage of me”)
- conditional assumptions (“If I let others take advantage of me, I’m a thoroughly weak person”).
Framing the intermediate belief as a conditional assumption can help accomplish this goal. Presumably, the patient was distressed about loaning money to his friend. When pressed, he says he didn’t want to lend the money but felt he couldn’t say no. He identifies his automatic thought as “I’ve been taken advantage of.” Asked what this means if it is true, he replies, “If I get taken advantage of, it means I’m weak.”
Core beliefs are deeper cognitive structures that are not always immediately accessible, although they may occasionally emerge spontaneously as automatic thoughts. They are overgeneralized, absolute statements that fall into one of two categories:
- affiliation (“I am bad,” “I am unlovable”)
- competence/vulnerability (“I am weak,” “I am helpless”).
Very often, intermediate and core beliefs must be defined in a measurable way before you can help the patient test them. For the man feeling distressed about loaning money, for example, you might ask him to define “being taken advantage of ” or define “weak” by listing all the characteristics he associates with this label.
Restructuring negative beliefs
A variety of techniques can be used to restructure negative beliefs.
- Cost-benefit analysis involves exploring advantages and disadvantages of maintaining a negative belief or a more-balanced alternate belief.
- Core belief logs12 can track day-to-day evidence that suggests a core belief is not 100% true. You and the patient can scrutinize evidence that supports the core belief and reframe the evidence in a more-rational manner.
- Life review10,12 involves asking the patient to re-evaluate a core belief ’s historical underpinnings and to reframe these events from an adult perspective.
- rational-emotional role play
- empty chair or two-chair dialogue
- restructuring early memories with directed imagery.
Adjunctive Behavioral Strategies
Behavioral interventions are used in CBT to combat anergia, increase socialization, diminish avoidance, and accumulate data to challenge negative beliefs. Common strategies are designed to enhance self-esteem and confidence and build therapeutic momentum as patients gain energy, feel better, and disconfirm negative beliefs.
Activity monitoring and scheduling. Instruct anergic or avoidant patients to monitor daily activities for the week and to rate the degree of pleasure and accomplishment each activity yields on a scale of 1 to 10. As patients become aware of how much time they spend on low-yield activities, help them gradually replace low-yield with higher-yield activities.
Schedule into the week behavioral goals patients identified at the beginning of therapy. Schedule avoided tasks such as household chores, and link them to pleasurable activities as rewards. To evaluate the accuracy of their thinking, ask patients to predict how much pleasure or mastery they will achieve with scheduled activities, then compare their predictions with actual results.
Also ask patients to anticipate obstacles to achieving their goals, to challenge those obstacles, and to develop contingency plans. Reviewing the patient’s week and its bright spots can disconfirm negatively biased recall such as, “My week was terrible,” or, “I don’t have the energy to do anything anymore.”13
Graded task assignments. When scheduling activities, improve success rates by helping patients break down large, unrealistic goals into smaller, more manageable pieces. Ask them to consider realistically what they can accomplish now, not what they could have accomplished before they became ill.
Exposure. Anxious patients avoid feared situations because of catastrophic beliefs that experiencing those situations will harm them. A man with panic disorder may avoid exercise, for example, because he perceives lightheadedness and rapid heart rate as signs of imminent heart attack. Avoiding exercise to prevent the feared symptoms perpetuates his catastrophic beliefs.
Exposure to feared symptoms—while initially arousing high anxiety—allows the patient to experientially disconfirm his beliefs. As he remains well after lightheadedness and rapid heart rate are induced interoceptively (by climbing several flights of stairs, for example), he comes to recognize the situational symptoms as manifestations of anxiety rather than evidence of life-threatening illness.2,3
In vivo exposure entails confronting the patient with the avoided object or situation. For example, you may show a woman with needle phobia pictures of needles, followed by actual needles themselves, then ask her to touch a needle, hold a needle, etc., until her anxiety gradually diminishes.
Imaginal exposure involves asking the patient to imagine himself in a feared situation and manipulating the images to build his sense of mastery. If he stops the image at the moment of highest arousal, instruct him to “continue to play the film forward” by asking, “What happens next?” This approach shows him that he can cope with difficult situations.
Related resources
For clinicians
- Persons JB. Cognitive therapy in practice: a case formulation approach. New York: WW Norton and Co.; 1989.
- Academy of Cognitive Therapy. Training, certification as a cognitive therapist. www.academyofct.org.
- Behavior Online. Gathering site for mental health professionals. www.behavior.net.
- MySelfHelp.com. Interactive programs and moderated discussion designed as treatment adjuncts for patients with depression, stress, eating disorders, etc. Funded by the National Institute of Mental Health ($20/month program access fee). www.MySelfHelp.com.
- Antony M, Swinson R. When perfect isn’t good enough. Oakland, CA: New Harbinger Press; 1998.
- Antony M, Swinson R. The shyness and social anxiety workbook. Oakland, CA: New Harbinger Press; 2000.
- Young J, Klosko J. Reinventing your life: how to break free from negative life patterns. New York: Dutton; 1993.
- Davis M, Eshelman E, McKay M. The relaxation and stress reduction workbook. New York: New Harbinger Press; 1995.
Whether you are in training or an experienced practitioner, you need more than a rudimentary understanding of cognitive-behavioral therapy (CBT). This easy-to-use psychotherapy has broad empiric support, is a first-choice treatment,1 and can help patients cope with depression, anxiety, and other psychological problems.
Psychiatrists who learn CBT well can alleviate many patients’ distress by creatively applying its tools and techniques. For example, the cognitive approach to panic disorder compares favorably to medication1-3 (Box).
Aaron beck’s CBT
Negative thoughts, biased processing. The greater your fidelity to CBT’s guiding principles (Table 1), the more effective the therapy becomes.4 Beck designed CBT to address his observation that depressed persons hold unrealistically negative views about themselves, the world, and the future.5,6 A distorted information-processing system prevents them from correcting underlying negative beliefs. Negative thoughts predominate their cognitions and seem to arise spontaneously, reflexively, and unremittingly. These “automatic thoughts,” as he called them, reflect underlying themes about the self that can be identified as:
- intermediate beliefs (conditional assumptions, attitudes, and rules)
- core beliefs (fundamental, often global, and absolute rules).7
using CBT to experientially disconfirm catastrophic cognitions is the psychotherapy of choice for anxiety disorders.1 Cognitive models exist for each anxiety disorder2 and include psychoeducation, self-monitoring, evaluation of anxious cognitions, and testing cognitions interoceptively (within the body) and in vivo (within the environment). The therapist strategically uses adjunctive measures such as relaxation training, controlled breathing, visualization, and distraction.
Panic disorder is characterized by catastrophic misinterpretations of benign bodily sensations that accompany a fear response.2,3 Disability occurs when patients avoid situations or activities they believe will activate bodily sensations such as dizziness or breathlessness. Using environmental manipulations—spinning, hyperventilation, or straw-breathing, to name a few—CBT aims to disconfirm patients’ catastrophic thoughts by deliberately exposing them to feared somatic sensations.2
When used to treat panic disorder, CBT is associated with remission rates similar to those achieved by medication and much lower relapse rates.1
Guiding principles of cognitive-behavioral therapy
|
- external (environmental) stress that carries symbolic value
- internal (physiologic) stress that activates the affective valence of the underlying schema.1,5,6
CBT’s scientific method. CBT teaches a person the skills to identify this cognitive material and to recognize biases that affect how he or she processes information. You can help patients understand:
- the bidirectional relationship between thoughts, feelings, and behaviors
- that they can influence their emotions by changing their thoughts and behavior.
You provide empiric tools to help them explore the validity or usefulness of their thoughts and the impact of their behaviors. When patients disprove a negative cognition through this process of “experiential disconfirmation,” you help them to change that cognition. Homework is a key ingredient (Table 2); patients who do their CBT homework are more likely to improve than those who don’t.8
Table 2
Structure of a typical CBT session
| Step | What therapist may say or do to introduce this step |
|---|---|
| Collaborate in setting the agenda | ‘What would you like to put on the agenda for today’s session? If we could address one or two items, what would they be?’ |
| Link to previous session via feedback | Review and comment on the patient’s feedback form |
| Check target symptoms | ‘How would you rate your level of (depression, anxiety, etc.) this week on a scale of 0 to 100?’ |
| Therapist also can review standardized rating scales, such as Beck Depression Inventory II | |
| Check medication | ‘Are there any concerns this week about your medication?’ |
| Review week/scheduling | ‘Could you update me about your week?’ |
| ‘What would be important to focus on this coming week?’ | |
| Review homework | ‘Let’s have a look at the homework/self-help work you did this week’ |
| Set new agenda items | ‘This issue sounds important; would you like to add it to today’s agenda?’ |
| Collaborate in developing new homework | ‘I’d like to work on this further next week; let’s decide together what would be doable’ |
| Feedback | ‘How did you feel about today’s session?’ |
| ‘Is there anything you would like to be sure to remember after you leave today?’ | |
| ‘Anything you want to put on the agenda for next session?’ |
Evaluating cognitive distortions
Thought records. Automatic thoughts are the cognitive content that runs through our minds moment to moment and that we can access by asking ourselves, “What was going through my mind when I felt (emotion)?” Automatic thoughts can exist as:
- verbal messages (“I can’t believe this!” or “I’m such a loser”)
- or images (“I picture my boss screaming at me”).
Self-monitoring also facilitates “decentering,” or viewing one’s emotional experience from a distance, which may be crucial to therapeutic success.9 By using Socratic questioning (Table 3) and guided discovery, you teach patients to evaluate their automatic thoughts as hypotheses to be tested.
If patients discover that their automatic thoughts are inaccurate, you can help them construct more-balanced or alternate appraisals. Conversely, hypothesis testing may help generate new solutions if the process validates the patient’s initial interpretation of a situation.10
Table 3
Examples of Socratic and non-Socratic questioning
Socratic questioning
|
- overgeneralization (“Nothing ever works out for me”)
- all-or-nothing thinking (“I failed again” [after getting 95% on an exam])
- mind-reading (“My boss thinks I’m incompetent”)
- catastrophization (“My heart is racing; I think I’m having a heart attack!”).11
- attitudes (“Weakness is contemptible”)
- rules (“I will not let others take advantage of me”)
- conditional assumptions (“If I let others take advantage of me, I’m a thoroughly weak person”).
Framing the intermediate belief as a conditional assumption can help accomplish this goal. Presumably, the patient was distressed about loaning money to his friend. When pressed, he says he didn’t want to lend the money but felt he couldn’t say no. He identifies his automatic thought as “I’ve been taken advantage of.” Asked what this means if it is true, he replies, “If I get taken advantage of, it means I’m weak.”
Core beliefs are deeper cognitive structures that are not always immediately accessible, although they may occasionally emerge spontaneously as automatic thoughts. They are overgeneralized, absolute statements that fall into one of two categories:
- affiliation (“I am bad,” “I am unlovable”)
- competence/vulnerability (“I am weak,” “I am helpless”).
Very often, intermediate and core beliefs must be defined in a measurable way before you can help the patient test them. For the man feeling distressed about loaning money, for example, you might ask him to define “being taken advantage of ” or define “weak” by listing all the characteristics he associates with this label.
Restructuring negative beliefs
A variety of techniques can be used to restructure negative beliefs.
- Cost-benefit analysis involves exploring advantages and disadvantages of maintaining a negative belief or a more-balanced alternate belief.
- Core belief logs12 can track day-to-day evidence that suggests a core belief is not 100% true. You and the patient can scrutinize evidence that supports the core belief and reframe the evidence in a more-rational manner.
- Life review10,12 involves asking the patient to re-evaluate a core belief ’s historical underpinnings and to reframe these events from an adult perspective.
- rational-emotional role play
- empty chair or two-chair dialogue
- restructuring early memories with directed imagery.
Adjunctive Behavioral Strategies
Behavioral interventions are used in CBT to combat anergia, increase socialization, diminish avoidance, and accumulate data to challenge negative beliefs. Common strategies are designed to enhance self-esteem and confidence and build therapeutic momentum as patients gain energy, feel better, and disconfirm negative beliefs.
Activity monitoring and scheduling. Instruct anergic or avoidant patients to monitor daily activities for the week and to rate the degree of pleasure and accomplishment each activity yields on a scale of 1 to 10. As patients become aware of how much time they spend on low-yield activities, help them gradually replace low-yield with higher-yield activities.
Schedule into the week behavioral goals patients identified at the beginning of therapy. Schedule avoided tasks such as household chores, and link them to pleasurable activities as rewards. To evaluate the accuracy of their thinking, ask patients to predict how much pleasure or mastery they will achieve with scheduled activities, then compare their predictions with actual results.
Also ask patients to anticipate obstacles to achieving their goals, to challenge those obstacles, and to develop contingency plans. Reviewing the patient’s week and its bright spots can disconfirm negatively biased recall such as, “My week was terrible,” or, “I don’t have the energy to do anything anymore.”13
Graded task assignments. When scheduling activities, improve success rates by helping patients break down large, unrealistic goals into smaller, more manageable pieces. Ask them to consider realistically what they can accomplish now, not what they could have accomplished before they became ill.
Exposure. Anxious patients avoid feared situations because of catastrophic beliefs that experiencing those situations will harm them. A man with panic disorder may avoid exercise, for example, because he perceives lightheadedness and rapid heart rate as signs of imminent heart attack. Avoiding exercise to prevent the feared symptoms perpetuates his catastrophic beliefs.
Exposure to feared symptoms—while initially arousing high anxiety—allows the patient to experientially disconfirm his beliefs. As he remains well after lightheadedness and rapid heart rate are induced interoceptively (by climbing several flights of stairs, for example), he comes to recognize the situational symptoms as manifestations of anxiety rather than evidence of life-threatening illness.2,3
In vivo exposure entails confronting the patient with the avoided object or situation. For example, you may show a woman with needle phobia pictures of needles, followed by actual needles themselves, then ask her to touch a needle, hold a needle, etc., until her anxiety gradually diminishes.
Imaginal exposure involves asking the patient to imagine himself in a feared situation and manipulating the images to build his sense of mastery. If he stops the image at the moment of highest arousal, instruct him to “continue to play the film forward” by asking, “What happens next?” This approach shows him that he can cope with difficult situations.
Related resources
For clinicians
- Persons JB. Cognitive therapy in practice: a case formulation approach. New York: WW Norton and Co.; 1989.
- Academy of Cognitive Therapy. Training, certification as a cognitive therapist. www.academyofct.org.
- Behavior Online. Gathering site for mental health professionals. www.behavior.net.
- MySelfHelp.com. Interactive programs and moderated discussion designed as treatment adjuncts for patients with depression, stress, eating disorders, etc. Funded by the National Institute of Mental Health ($20/month program access fee). www.MySelfHelp.com.
- Antony M, Swinson R. When perfect isn’t good enough. Oakland, CA: New Harbinger Press; 1998.
- Antony M, Swinson R. The shyness and social anxiety workbook. Oakland, CA: New Harbinger Press; 2000.
- Young J, Klosko J. Reinventing your life: how to break free from negative life patterns. New York: Dutton; 1993.
- Davis M, Eshelman E, McKay M. The relaxation and stress reduction workbook. New York: New Harbinger Press; 1995.
1. Hollon SD, Beck AT. Cognitive and cognitive behavioral therapies. In: Lambert, MJ (ed). Bergin and Garfield’s handbook of psychotherapy and behavior change, 5th ed. New York: John Wiley and Sons; 2004:447-92.
2. Wells A. Cognitive therapy of anxiety disorders: a practice manual and conceptual guide. West Sussex, UK: John Wiley and Sons; 1997.
3. Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH (ed). Clinical handbook of psychological disorders, 3rd ed. New York: Guilford Press; 1991:1-59.
4. Rosenbaum M, Ronen T. Clinical supervision from the standpoint of cognitive-behavior therapy. Psychotherapy 1998;35(2):220-30.
5. Beck AT. The current state of cognitive therapy. A 40-year retrospective. Arch Gen Psychiatry 2005;62:953-9.
6. Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979.
7. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry, 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1247.
8. Rees CS, McEvoy P, Nathan PR. Relationship between homework completion and outcome in cognitive behaviour therapy. Cognit Behav Ther 2005;34(4):242-7.
9. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275-87.
10. Greenberger D, Padesky CA. Mind over mood: a cognitive therapy treatment manual for clients.New York: Guilford Press; 1995.
11. Burns DD. Feeling good handbook. New York: Penguin; 1999.
12. Beck J. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995.
13. Lau MA, Segal ZV, Zaretsky AE. Cognitive-behavioral therapies for the medical clinic. In: Moss D, McGrady A, Davies TC, Wickramasekera I (eds). Handbook of mind-body medicine for primary care. Thousand Oaks, CA: Sage Publications; 2003:167-79.
1. Hollon SD, Beck AT. Cognitive and cognitive behavioral therapies. In: Lambert, MJ (ed). Bergin and Garfield’s handbook of psychotherapy and behavior change, 5th ed. New York: John Wiley and Sons; 2004:447-92.
2. Wells A. Cognitive therapy of anxiety disorders: a practice manual and conceptual guide. West Sussex, UK: John Wiley and Sons; 1997.
3. Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH (ed). Clinical handbook of psychological disorders, 3rd ed. New York: Guilford Press; 1991:1-59.
4. Rosenbaum M, Ronen T. Clinical supervision from the standpoint of cognitive-behavior therapy. Psychotherapy 1998;35(2):220-30.
5. Beck AT. The current state of cognitive therapy. A 40-year retrospective. Arch Gen Psychiatry 2005;62:953-9.
6. Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979.
7. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry, 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1247.
8. Rees CS, McEvoy P, Nathan PR. Relationship between homework completion and outcome in cognitive behaviour therapy. Cognit Behav Ther 2005;34(4):242-7.
9. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275-87.
10. Greenberger D, Padesky CA. Mind over mood: a cognitive therapy treatment manual for clients.New York: Guilford Press; 1995.
11. Burns DD. Feeling good handbook. New York: Penguin; 1999.
12. Beck J. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995.
13. Lau MA, Segal ZV, Zaretsky AE. Cognitive-behavioral therapies for the medical clinic. In: Moss D, McGrady A, Davies TC, Wickramasekera I (eds). Handbook of mind-body medicine for primary care. Thousand Oaks, CA: Sage Publications; 2003:167-79.
How to take a sexual history (without blushing)
When you address sexuality, you open a window to the patient’s psychology. Talking about sex may illuminate important clues about the individual’s capacity to:
- give and receive pleasure
- love and be loved
- be psychologically intimate
- manage expected and unexpected changes throughout adulthood.1
The opportunity to listen to sexual histories over time will help you become proficient in generating causal hypotheses and using them to help your patients.
Patients think—often erroneously—that all psychiatrists are knowledgeable, skillful, and interested in addressing sexual concerns. But psychiatric practice has turned away from clinical sexuality, and most of us learn on our own how to take a sexual history and to bring up relevant topics during subsequent sessions.
These activities are not particularly difficult,2 but they often bump up against one or more clinician fears (Table 1).3 You can master these apprehensions by:
- identifying them in yourself
- thinking about them rationally
- learning about the broad range of human sexual expression
- understanding professional boundaries.4
Table 1
Clinicians’ 5 worst fears about taking sexual histories
| Personal or patient sexual arousal while talking about sex |
| Not knowing what questions to ask |
| Not knowing how to help with patients’ sexual problems |
| Sudden awareness of one’s own sexual concerns |
| Having the patient see our moral repugnance about certain sexual practices |
| Source: Reference 3 |
Assessing sexual complaints
Sexual behavior—normal and abnormal, masturbatory and partnered—rests upon biological, psychological, and interpersonal elements, and cultural concepts of normality and morality.5 These four components also are the sources of sexual problems (Table 2).6,7
To accurately assess individuals’ and couples’ sexual problems, we must consider the four components’ present and past contributions in every case. We may declare a hypothesis of cause after one or two sessions, but the explanation usually evolves and becomes more complex with time.8
Outside of sexuality clinics, we usually learn about a patient’s sexual complaint during therapy for another problem:
- Individuals may bring up cross-dressing, anxiety about possibly being homosexual, concern about violent sexual fantasies, or other issues of sexual identity. Sexual function concerns may include new difficulty attaining orgasm, aversion to intercourse, painful intercourse, too-rapid ejaculation, episodic inability to maintain an erection, or longstanding inability to ejaculate while with a partner.
- Couples may present with difficulty orchestrating their sexual lives. Their complaints may involve discrepancies in sexual desire, inability to bring a young wife to orgasm, cessation of sex, infidelity, dyspareunia, erectile dysfunction in a newly married couple in their 60s, or a wife’s distress over her husband’s use of Internet pornography.
- Referrals may come from a social agency about an individual whose sexual behavior clashes with social values or laws. Judges, lawyers, state boards, clergy, or medical chiefs-of-staff may request assistance with individuals who cross sexual boundaries at work, are accused of sex crimes, or have been sexually victimized.5
4 components of sexual behavior: Where sexual problems may arise
| Component | Related sexual problems (examples) |
|---|---|
| Biological elements | Congenital androgen receptor disorder,9 undiagnosed prolactinoma,10 medical disorders (such as multiple sclerosis), medication side effects, heroin abuse |
| Psychological elements | Developmental processes (neglect, lack of warmth, or physical and sexual abuse from childhood caretakers) or present states (affect disorder, paranoia) |
| Interpersonal elements | Lack of psychological intimacy, marital alienation, disapproval of spouse’s behavior (such as gambling or excessive shopping), disrespect for spouse’s parenting style, past infidelity |
| Cultural concepts of normality and morality | Inability to free oneself of antisexual religious attitudes, homophobia, or belief that masturbation or oral-genital contact is abnormal sexual behavior |
Ask about sexual identity
By the end of adolescence, most individuals have stably in place the three self labels that encompass sexual identity:
- gender identity—the degree of comfort with the self as masculine or feminine
- orientation—the gender of those who attract or repel us for romantic and sexual purposes
- intention—what we want to do with our bodies and our partners’ bodies during sexual behavior.9
Conventional sexual identities do not pose a countertransference problem for most professionals after they become accustomed to discussing sexual matters. But unconventional identities—such as a gender identity disorder, homosexuality, or a paraphilia—can cause anxiety and avoidance for sexually conventional psychiatrists.
Table 3
Ask about 3 components of sexual identity
| Component | Sample questions |
|---|---|
| Gender identify | Are you happy that you are a male (female)? |
| Do you privately feel sufficiently masculine (feminine)? | |
| Orientation | Are you sexually and romantically attracted primarily to males, females, or both? |
| Intention | Are your sexual fantasies focused on unconventional images involving sadism, masochism, exhibitionism, voyeurism, clothing, animals, or children? |
Paraphilia. The most upsetting paraphilia to learn about is pedophilia. The patient typically is nervous about revealing his (or rarely her) fantasy focus on boys, girls, or both. Pedophiles may be exclusively interested in particular age groups—such as preschoolers or grade school children—or be preoccupied with children while also having more conventional adult sexual interests.
Learn to ask about erotic fantasies, knowing that occasionally you will encounter behaviors or thoughts that are contrary to your own values. Knowing that you will encounter paraphilia enables you to anticipate and work through any private moral outrage before you meet the patient.3
Ask about sexual function
Desire, arousal, and orgasm are the three dimensions of sexual function listed in DSM-IV-TR. Sexual dysfunction may be classified as lifelong (since onset of sexual activity) or acquired (after a symptom-free period). If a patient’s sexual dysfunction is acquired, determine whether it occurs in all sexual encounters or is situational (with only one partner or present sometimes with a partner). These distinctions allow you to rationally pursue the cause (Table 4).
If a patient complains of loss of desire for sex, determine if it is manifested by:
- absence of sexual thoughts, fantasies, attractions, or masturbation (as might be seen in acquired hypogonadal states)
- lost motivation to approach his or her partner for sex (as commonly occurs when partners become alienated).10
Common sexual dysfunctions such as premature ejaculation, female anorgasmia, hypoactive sexual desire disorder, and arousal dysfunctions often have no significant genital findings. Erectile dysfunction in middle-aged and older men indicates the need to do a workup for early vascular disease and metabolic syndrome.
Desire versus arousal. Differentiating sexual desire and arousal can be complicated because they overlap, particularly during middle age or as individuals settle down with a consistent partner. Desire is also complicated by a vital gender difference.11 Most women in monogamous relationships eventually notice that the arousal stimulated by sexual behavior precedes their intense desire for sex, whereas most men report that their desire for sex precedes their arousal through much of the life cycle. Understanding these concepts will shape your follow-up questions about desire and arousal experiences.
Table 4
Ask about 3 components of sexual function
| Component | Sample questions |
|---|---|
| Desire | Are you ever “horny”—that is, have spontaneous feelings of mild sexual arousal? |
| Tell me what motivates you to have sexual behavior with your partner | |
| Arousal | Explain what is it like for you during lovemaking. |
| Do you get excited? Do you stay excited? | |
| Orgasm | Please tell me about your concerns about attaining orgasm |
Adult Sex Life: 6 Stages
Sexual dysfunction symptoms may be the same throughout the life cycle, but their meanings to patients vary dramatically. For example:
- A psychological stress that creates erectile dysfunction in a 60-year-old might not affect a 25-year-old because biological capacities for arousal are different at these life stages.
- Anorgasmia in a 22-year-old does not have the same psychological and biological sources as anorgasmia in a 62-year-old.
Stage 1: Sexual unfolding usually corresponds with adolescence and single adulthood. It is characterized by growing awareness of individual identity and functional characteristics and experiments in managing sexual drives, sexual opportunities, and relationships through masturbation and partner sex. It ends when a person depends on one partner for sexual expression.
Stage 2: Sexual equilibrium is established as part of a monogamous partnership. This equilibrium, which shapes the couple’s unique pattern of sexual expression, is formed by the interaction of their individual identity, desire, arousal, and orgasmic attainment characteristics.
The power of this interaction can be seen in previously functional men and women who quickly become dysfunctional in a new equilibrium because they discern their partner’s displeasure, lack of satisfaction, or lack of interest in particular sexual acts. Their perception of their partner’s unhappiness can quickly induce performance anxiety during sex, anger about sex, or a sense of hopelessness about getting one’s needs met.
The sexual equilibrium’s power is evident in previously dysfunctional individuals who quickly become comfortable and capable when they sense that their partners are pleased with them as partners. Inhibitions gradually lessen, and the couple’s sexual life begins on a good footing.
Stage 3: Preservation of sexual behavior refers to maintaining partnered sexual activity as life becomes more complex because of expected events such as pregnancy, rearing children, new job responsibilities, illness in parents, etc. The couple’s ability to preserve their sexual relationship rests on their capacity to:
- manage disappointment over emerging knowledge of the partner’s character
- resolve periodic nonsexual disagreements
- re-attain psychological intimacy
- understand how important sex is as a means to erase anger, reduce extramarital temptation, reaffirm the couple’s bond, and have fun.
Stage 4: The physiologic downturn in midlife for women begins during perimenopause and is characterized by diminished drive, vaginal dryness, and reduced vulvar and breast erotic sensitivity.12 Men’s physiologic decline—usually apparent to them by their mid-50s—is characterized by reduced drive and less-firm penile tumescence.13
Stage 5: Aging effects emerge gradually as individuals move into their 60s. Both sexes usually find orgasm more difficult to attain. Women may say they have sex primarily to please their partners, and men may notice less consistent potency.
Both sexes rely on motivational aspects of desire to have sex, not on sex drive per se. A man may say he wants to have sex, for example, because “it is normal to want to have sex as long as the body is willing; it makes me feel manly.” Women who maintained natural vaginal lubrication during their 50s often now use lubricants.14
Stage 6: The era of serious illness—whether psychiatric or physical—can occur any time in the life cycle. Illnesses ranging from congestive heart failure to complicated grief can limit a person’s sexual activities. Some changes can increase the frequency of sex—such as hypomania or mania, the new appreciation of a now-impaired spouse, or substance abuse that decreases sexual restraints—but most serious illnesses diminish the patient’s or partner’s sexual desire and arousal.
When death, divorce, or other separations disrupt relationships and individuals find themselves unattached, they return to stage 1: unfolding. With new partners, they will have different desire, arousal, and orgasmic characteristics than they did when last unattached. Their next sexual equilibrium will be different from the one before.
Related resources
- Levine SB. Sexual life: a clinician’s guide. New York: Plenum; 1992.
- Risen CB. Listening to sexual stories. In: Levine SB, Risen CB, Althof SE (eds). Handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003:1-20.
1. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
2. Maurice WL. Sexual medicine in primary care. Philadelphia: Mosby; 1999.
3. Risen CB. Listening to sexual stories. In: Levine SB, Risen CB, Althof SE (eds). Handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003:1-20.
4. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry (pamphlet). Washington, DC: American Psychiatric Association; 1993.
5. Levine SB. A reintroduction to clinical sexuality. Focus 2005;III(4):526-31.
6. Diamond M, Watson LA. Androgen insensitivity syndrome and Klinefelter’s syndrome: sex and gender considerations. Child Adolesc Psychiatr Clin North Am 2004;13(3):623-40.
7. Schlechte JA. Prolactinoma. N Engl J Med 2003;349(21):2035-41.
8. Gabbard GO. Mind, brain and personality disorders. Am J Psychiatry 2005;162(4):648-55.
9. Kafka MP. The paraphilia-related disorders: nonparaphillic hyper-sexuality and sexual compulsivity/addiction. In: Leiblum SR, Rosen RC (eds). Principles and practices of sex therapy. New York: Guilford Press; 2000:471-503.
10. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
11. Basson R. Human sex response cycles. J Sex Marital Ther 2001;27(1):33-43.
12. Dennerstein L. The sexual impact of menopause. In: Levine SB, Risen CB, Althof SE (eds). The handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003.
13. Schiavi RC, Schreiner-Engel P, Mandeli J. Healthy aging and male sexual function. Am J Psychiatry 1990;147(6):766-71.
14. Kellett JM. Older adult sexuality. In: Szuchman LT, Muscarella F (eds). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:355-82.
When you address sexuality, you open a window to the patient’s psychology. Talking about sex may illuminate important clues about the individual’s capacity to:
- give and receive pleasure
- love and be loved
- be psychologically intimate
- manage expected and unexpected changes throughout adulthood.1
The opportunity to listen to sexual histories over time will help you become proficient in generating causal hypotheses and using them to help your patients.
Patients think—often erroneously—that all psychiatrists are knowledgeable, skillful, and interested in addressing sexual concerns. But psychiatric practice has turned away from clinical sexuality, and most of us learn on our own how to take a sexual history and to bring up relevant topics during subsequent sessions.
These activities are not particularly difficult,2 but they often bump up against one or more clinician fears (Table 1).3 You can master these apprehensions by:
- identifying them in yourself
- thinking about them rationally
- learning about the broad range of human sexual expression
- understanding professional boundaries.4
Table 1
Clinicians’ 5 worst fears about taking sexual histories
| Personal or patient sexual arousal while talking about sex |
| Not knowing what questions to ask |
| Not knowing how to help with patients’ sexual problems |
| Sudden awareness of one’s own sexual concerns |
| Having the patient see our moral repugnance about certain sexual practices |
| Source: Reference 3 |
Assessing sexual complaints
Sexual behavior—normal and abnormal, masturbatory and partnered—rests upon biological, psychological, and interpersonal elements, and cultural concepts of normality and morality.5 These four components also are the sources of sexual problems (Table 2).6,7
To accurately assess individuals’ and couples’ sexual problems, we must consider the four components’ present and past contributions in every case. We may declare a hypothesis of cause after one or two sessions, but the explanation usually evolves and becomes more complex with time.8
Outside of sexuality clinics, we usually learn about a patient’s sexual complaint during therapy for another problem:
- Individuals may bring up cross-dressing, anxiety about possibly being homosexual, concern about violent sexual fantasies, or other issues of sexual identity. Sexual function concerns may include new difficulty attaining orgasm, aversion to intercourse, painful intercourse, too-rapid ejaculation, episodic inability to maintain an erection, or longstanding inability to ejaculate while with a partner.
- Couples may present with difficulty orchestrating their sexual lives. Their complaints may involve discrepancies in sexual desire, inability to bring a young wife to orgasm, cessation of sex, infidelity, dyspareunia, erectile dysfunction in a newly married couple in their 60s, or a wife’s distress over her husband’s use of Internet pornography.
- Referrals may come from a social agency about an individual whose sexual behavior clashes with social values or laws. Judges, lawyers, state boards, clergy, or medical chiefs-of-staff may request assistance with individuals who cross sexual boundaries at work, are accused of sex crimes, or have been sexually victimized.5
4 components of sexual behavior: Where sexual problems may arise
| Component | Related sexual problems (examples) |
|---|---|
| Biological elements | Congenital androgen receptor disorder,9 undiagnosed prolactinoma,10 medical disorders (such as multiple sclerosis), medication side effects, heroin abuse |
| Psychological elements | Developmental processes (neglect, lack of warmth, or physical and sexual abuse from childhood caretakers) or present states (affect disorder, paranoia) |
| Interpersonal elements | Lack of psychological intimacy, marital alienation, disapproval of spouse’s behavior (such as gambling or excessive shopping), disrespect for spouse’s parenting style, past infidelity |
| Cultural concepts of normality and morality | Inability to free oneself of antisexual religious attitudes, homophobia, or belief that masturbation or oral-genital contact is abnormal sexual behavior |
Ask about sexual identity
By the end of adolescence, most individuals have stably in place the three self labels that encompass sexual identity:
- gender identity—the degree of comfort with the self as masculine or feminine
- orientation—the gender of those who attract or repel us for romantic and sexual purposes
- intention—what we want to do with our bodies and our partners’ bodies during sexual behavior.9
Conventional sexual identities do not pose a countertransference problem for most professionals after they become accustomed to discussing sexual matters. But unconventional identities—such as a gender identity disorder, homosexuality, or a paraphilia—can cause anxiety and avoidance for sexually conventional psychiatrists.
Table 3
Ask about 3 components of sexual identity
| Component | Sample questions |
|---|---|
| Gender identify | Are you happy that you are a male (female)? |
| Do you privately feel sufficiently masculine (feminine)? | |
| Orientation | Are you sexually and romantically attracted primarily to males, females, or both? |
| Intention | Are your sexual fantasies focused on unconventional images involving sadism, masochism, exhibitionism, voyeurism, clothing, animals, or children? |
Paraphilia. The most upsetting paraphilia to learn about is pedophilia. The patient typically is nervous about revealing his (or rarely her) fantasy focus on boys, girls, or both. Pedophiles may be exclusively interested in particular age groups—such as preschoolers or grade school children—or be preoccupied with children while also having more conventional adult sexual interests.
Learn to ask about erotic fantasies, knowing that occasionally you will encounter behaviors or thoughts that are contrary to your own values. Knowing that you will encounter paraphilia enables you to anticipate and work through any private moral outrage before you meet the patient.3
Ask about sexual function
Desire, arousal, and orgasm are the three dimensions of sexual function listed in DSM-IV-TR. Sexual dysfunction may be classified as lifelong (since onset of sexual activity) or acquired (after a symptom-free period). If a patient’s sexual dysfunction is acquired, determine whether it occurs in all sexual encounters or is situational (with only one partner or present sometimes with a partner). These distinctions allow you to rationally pursue the cause (Table 4).
If a patient complains of loss of desire for sex, determine if it is manifested by:
- absence of sexual thoughts, fantasies, attractions, or masturbation (as might be seen in acquired hypogonadal states)
- lost motivation to approach his or her partner for sex (as commonly occurs when partners become alienated).10
Common sexual dysfunctions such as premature ejaculation, female anorgasmia, hypoactive sexual desire disorder, and arousal dysfunctions often have no significant genital findings. Erectile dysfunction in middle-aged and older men indicates the need to do a workup for early vascular disease and metabolic syndrome.
Desire versus arousal. Differentiating sexual desire and arousal can be complicated because they overlap, particularly during middle age or as individuals settle down with a consistent partner. Desire is also complicated by a vital gender difference.11 Most women in monogamous relationships eventually notice that the arousal stimulated by sexual behavior precedes their intense desire for sex, whereas most men report that their desire for sex precedes their arousal through much of the life cycle. Understanding these concepts will shape your follow-up questions about desire and arousal experiences.
Table 4
Ask about 3 components of sexual function
| Component | Sample questions |
|---|---|
| Desire | Are you ever “horny”—that is, have spontaneous feelings of mild sexual arousal? |
| Tell me what motivates you to have sexual behavior with your partner | |
| Arousal | Explain what is it like for you during lovemaking. |
| Do you get excited? Do you stay excited? | |
| Orgasm | Please tell me about your concerns about attaining orgasm |
Adult Sex Life: 6 Stages
Sexual dysfunction symptoms may be the same throughout the life cycle, but their meanings to patients vary dramatically. For example:
- A psychological stress that creates erectile dysfunction in a 60-year-old might not affect a 25-year-old because biological capacities for arousal are different at these life stages.
- Anorgasmia in a 22-year-old does not have the same psychological and biological sources as anorgasmia in a 62-year-old.
Stage 1: Sexual unfolding usually corresponds with adolescence and single adulthood. It is characterized by growing awareness of individual identity and functional characteristics and experiments in managing sexual drives, sexual opportunities, and relationships through masturbation and partner sex. It ends when a person depends on one partner for sexual expression.
Stage 2: Sexual equilibrium is established as part of a monogamous partnership. This equilibrium, which shapes the couple’s unique pattern of sexual expression, is formed by the interaction of their individual identity, desire, arousal, and orgasmic attainment characteristics.
The power of this interaction can be seen in previously functional men and women who quickly become dysfunctional in a new equilibrium because they discern their partner’s displeasure, lack of satisfaction, or lack of interest in particular sexual acts. Their perception of their partner’s unhappiness can quickly induce performance anxiety during sex, anger about sex, or a sense of hopelessness about getting one’s needs met.
The sexual equilibrium’s power is evident in previously dysfunctional individuals who quickly become comfortable and capable when they sense that their partners are pleased with them as partners. Inhibitions gradually lessen, and the couple’s sexual life begins on a good footing.
Stage 3: Preservation of sexual behavior refers to maintaining partnered sexual activity as life becomes more complex because of expected events such as pregnancy, rearing children, new job responsibilities, illness in parents, etc. The couple’s ability to preserve their sexual relationship rests on their capacity to:
- manage disappointment over emerging knowledge of the partner’s character
- resolve periodic nonsexual disagreements
- re-attain psychological intimacy
- understand how important sex is as a means to erase anger, reduce extramarital temptation, reaffirm the couple’s bond, and have fun.
Stage 4: The physiologic downturn in midlife for women begins during perimenopause and is characterized by diminished drive, vaginal dryness, and reduced vulvar and breast erotic sensitivity.12 Men’s physiologic decline—usually apparent to them by their mid-50s—is characterized by reduced drive and less-firm penile tumescence.13
Stage 5: Aging effects emerge gradually as individuals move into their 60s. Both sexes usually find orgasm more difficult to attain. Women may say they have sex primarily to please their partners, and men may notice less consistent potency.
Both sexes rely on motivational aspects of desire to have sex, not on sex drive per se. A man may say he wants to have sex, for example, because “it is normal to want to have sex as long as the body is willing; it makes me feel manly.” Women who maintained natural vaginal lubrication during their 50s often now use lubricants.14
Stage 6: The era of serious illness—whether psychiatric or physical—can occur any time in the life cycle. Illnesses ranging from congestive heart failure to complicated grief can limit a person’s sexual activities. Some changes can increase the frequency of sex—such as hypomania or mania, the new appreciation of a now-impaired spouse, or substance abuse that decreases sexual restraints—but most serious illnesses diminish the patient’s or partner’s sexual desire and arousal.
When death, divorce, or other separations disrupt relationships and individuals find themselves unattached, they return to stage 1: unfolding. With new partners, they will have different desire, arousal, and orgasmic characteristics than they did when last unattached. Their next sexual equilibrium will be different from the one before.
Related resources
- Levine SB. Sexual life: a clinician’s guide. New York: Plenum; 1992.
- Risen CB. Listening to sexual stories. In: Levine SB, Risen CB, Althof SE (eds). Handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003:1-20.
When you address sexuality, you open a window to the patient’s psychology. Talking about sex may illuminate important clues about the individual’s capacity to:
- give and receive pleasure
- love and be loved
- be psychologically intimate
- manage expected and unexpected changes throughout adulthood.1
The opportunity to listen to sexual histories over time will help you become proficient in generating causal hypotheses and using them to help your patients.
Patients think—often erroneously—that all psychiatrists are knowledgeable, skillful, and interested in addressing sexual concerns. But psychiatric practice has turned away from clinical sexuality, and most of us learn on our own how to take a sexual history and to bring up relevant topics during subsequent sessions.
These activities are not particularly difficult,2 but they often bump up against one or more clinician fears (Table 1).3 You can master these apprehensions by:
- identifying them in yourself
- thinking about them rationally
- learning about the broad range of human sexual expression
- understanding professional boundaries.4
Table 1
Clinicians’ 5 worst fears about taking sexual histories
| Personal or patient sexual arousal while talking about sex |
| Not knowing what questions to ask |
| Not knowing how to help with patients’ sexual problems |
| Sudden awareness of one’s own sexual concerns |
| Having the patient see our moral repugnance about certain sexual practices |
| Source: Reference 3 |
Assessing sexual complaints
Sexual behavior—normal and abnormal, masturbatory and partnered—rests upon biological, psychological, and interpersonal elements, and cultural concepts of normality and morality.5 These four components also are the sources of sexual problems (Table 2).6,7
To accurately assess individuals’ and couples’ sexual problems, we must consider the four components’ present and past contributions in every case. We may declare a hypothesis of cause after one or two sessions, but the explanation usually evolves and becomes more complex with time.8
Outside of sexuality clinics, we usually learn about a patient’s sexual complaint during therapy for another problem:
- Individuals may bring up cross-dressing, anxiety about possibly being homosexual, concern about violent sexual fantasies, or other issues of sexual identity. Sexual function concerns may include new difficulty attaining orgasm, aversion to intercourse, painful intercourse, too-rapid ejaculation, episodic inability to maintain an erection, or longstanding inability to ejaculate while with a partner.
- Couples may present with difficulty orchestrating their sexual lives. Their complaints may involve discrepancies in sexual desire, inability to bring a young wife to orgasm, cessation of sex, infidelity, dyspareunia, erectile dysfunction in a newly married couple in their 60s, or a wife’s distress over her husband’s use of Internet pornography.
- Referrals may come from a social agency about an individual whose sexual behavior clashes with social values or laws. Judges, lawyers, state boards, clergy, or medical chiefs-of-staff may request assistance with individuals who cross sexual boundaries at work, are accused of sex crimes, or have been sexually victimized.5
4 components of sexual behavior: Where sexual problems may arise
| Component | Related sexual problems (examples) |
|---|---|
| Biological elements | Congenital androgen receptor disorder,9 undiagnosed prolactinoma,10 medical disorders (such as multiple sclerosis), medication side effects, heroin abuse |
| Psychological elements | Developmental processes (neglect, lack of warmth, or physical and sexual abuse from childhood caretakers) or present states (affect disorder, paranoia) |
| Interpersonal elements | Lack of psychological intimacy, marital alienation, disapproval of spouse’s behavior (such as gambling or excessive shopping), disrespect for spouse’s parenting style, past infidelity |
| Cultural concepts of normality and morality | Inability to free oneself of antisexual religious attitudes, homophobia, or belief that masturbation or oral-genital contact is abnormal sexual behavior |
Ask about sexual identity
By the end of adolescence, most individuals have stably in place the three self labels that encompass sexual identity:
- gender identity—the degree of comfort with the self as masculine or feminine
- orientation—the gender of those who attract or repel us for romantic and sexual purposes
- intention—what we want to do with our bodies and our partners’ bodies during sexual behavior.9
Conventional sexual identities do not pose a countertransference problem for most professionals after they become accustomed to discussing sexual matters. But unconventional identities—such as a gender identity disorder, homosexuality, or a paraphilia—can cause anxiety and avoidance for sexually conventional psychiatrists.
Table 3
Ask about 3 components of sexual identity
| Component | Sample questions |
|---|---|
| Gender identify | Are you happy that you are a male (female)? |
| Do you privately feel sufficiently masculine (feminine)? | |
| Orientation | Are you sexually and romantically attracted primarily to males, females, or both? |
| Intention | Are your sexual fantasies focused on unconventional images involving sadism, masochism, exhibitionism, voyeurism, clothing, animals, or children? |
Paraphilia. The most upsetting paraphilia to learn about is pedophilia. The patient typically is nervous about revealing his (or rarely her) fantasy focus on boys, girls, or both. Pedophiles may be exclusively interested in particular age groups—such as preschoolers or grade school children—or be preoccupied with children while also having more conventional adult sexual interests.
Learn to ask about erotic fantasies, knowing that occasionally you will encounter behaviors or thoughts that are contrary to your own values. Knowing that you will encounter paraphilia enables you to anticipate and work through any private moral outrage before you meet the patient.3
Ask about sexual function
Desire, arousal, and orgasm are the three dimensions of sexual function listed in DSM-IV-TR. Sexual dysfunction may be classified as lifelong (since onset of sexual activity) or acquired (after a symptom-free period). If a patient’s sexual dysfunction is acquired, determine whether it occurs in all sexual encounters or is situational (with only one partner or present sometimes with a partner). These distinctions allow you to rationally pursue the cause (Table 4).
If a patient complains of loss of desire for sex, determine if it is manifested by:
- absence of sexual thoughts, fantasies, attractions, or masturbation (as might be seen in acquired hypogonadal states)
- lost motivation to approach his or her partner for sex (as commonly occurs when partners become alienated).10
Common sexual dysfunctions such as premature ejaculation, female anorgasmia, hypoactive sexual desire disorder, and arousal dysfunctions often have no significant genital findings. Erectile dysfunction in middle-aged and older men indicates the need to do a workup for early vascular disease and metabolic syndrome.
Desire versus arousal. Differentiating sexual desire and arousal can be complicated because they overlap, particularly during middle age or as individuals settle down with a consistent partner. Desire is also complicated by a vital gender difference.11 Most women in monogamous relationships eventually notice that the arousal stimulated by sexual behavior precedes their intense desire for sex, whereas most men report that their desire for sex precedes their arousal through much of the life cycle. Understanding these concepts will shape your follow-up questions about desire and arousal experiences.
Table 4
Ask about 3 components of sexual function
| Component | Sample questions |
|---|---|
| Desire | Are you ever “horny”—that is, have spontaneous feelings of mild sexual arousal? |
| Tell me what motivates you to have sexual behavior with your partner | |
| Arousal | Explain what is it like for you during lovemaking. |
| Do you get excited? Do you stay excited? | |
| Orgasm | Please tell me about your concerns about attaining orgasm |
Adult Sex Life: 6 Stages
Sexual dysfunction symptoms may be the same throughout the life cycle, but their meanings to patients vary dramatically. For example:
- A psychological stress that creates erectile dysfunction in a 60-year-old might not affect a 25-year-old because biological capacities for arousal are different at these life stages.
- Anorgasmia in a 22-year-old does not have the same psychological and biological sources as anorgasmia in a 62-year-old.
Stage 1: Sexual unfolding usually corresponds with adolescence and single adulthood. It is characterized by growing awareness of individual identity and functional characteristics and experiments in managing sexual drives, sexual opportunities, and relationships through masturbation and partner sex. It ends when a person depends on one partner for sexual expression.
Stage 2: Sexual equilibrium is established as part of a monogamous partnership. This equilibrium, which shapes the couple’s unique pattern of sexual expression, is formed by the interaction of their individual identity, desire, arousal, and orgasmic attainment characteristics.
The power of this interaction can be seen in previously functional men and women who quickly become dysfunctional in a new equilibrium because they discern their partner’s displeasure, lack of satisfaction, or lack of interest in particular sexual acts. Their perception of their partner’s unhappiness can quickly induce performance anxiety during sex, anger about sex, or a sense of hopelessness about getting one’s needs met.
The sexual equilibrium’s power is evident in previously dysfunctional individuals who quickly become comfortable and capable when they sense that their partners are pleased with them as partners. Inhibitions gradually lessen, and the couple’s sexual life begins on a good footing.
Stage 3: Preservation of sexual behavior refers to maintaining partnered sexual activity as life becomes more complex because of expected events such as pregnancy, rearing children, new job responsibilities, illness in parents, etc. The couple’s ability to preserve their sexual relationship rests on their capacity to:
- manage disappointment over emerging knowledge of the partner’s character
- resolve periodic nonsexual disagreements
- re-attain psychological intimacy
- understand how important sex is as a means to erase anger, reduce extramarital temptation, reaffirm the couple’s bond, and have fun.
Stage 4: The physiologic downturn in midlife for women begins during perimenopause and is characterized by diminished drive, vaginal dryness, and reduced vulvar and breast erotic sensitivity.12 Men’s physiologic decline—usually apparent to them by their mid-50s—is characterized by reduced drive and less-firm penile tumescence.13
Stage 5: Aging effects emerge gradually as individuals move into their 60s. Both sexes usually find orgasm more difficult to attain. Women may say they have sex primarily to please their partners, and men may notice less consistent potency.
Both sexes rely on motivational aspects of desire to have sex, not on sex drive per se. A man may say he wants to have sex, for example, because “it is normal to want to have sex as long as the body is willing; it makes me feel manly.” Women who maintained natural vaginal lubrication during their 50s often now use lubricants.14
Stage 6: The era of serious illness—whether psychiatric or physical—can occur any time in the life cycle. Illnesses ranging from congestive heart failure to complicated grief can limit a person’s sexual activities. Some changes can increase the frequency of sex—such as hypomania or mania, the new appreciation of a now-impaired spouse, or substance abuse that decreases sexual restraints—but most serious illnesses diminish the patient’s or partner’s sexual desire and arousal.
When death, divorce, or other separations disrupt relationships and individuals find themselves unattached, they return to stage 1: unfolding. With new partners, they will have different desire, arousal, and orgasmic characteristics than they did when last unattached. Their next sexual equilibrium will be different from the one before.
Related resources
- Levine SB. Sexual life: a clinician’s guide. New York: Plenum; 1992.
- Risen CB. Listening to sexual stories. In: Levine SB, Risen CB, Althof SE (eds). Handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003:1-20.
1. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
2. Maurice WL. Sexual medicine in primary care. Philadelphia: Mosby; 1999.
3. Risen CB. Listening to sexual stories. In: Levine SB, Risen CB, Althof SE (eds). Handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003:1-20.
4. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry (pamphlet). Washington, DC: American Psychiatric Association; 1993.
5. Levine SB. A reintroduction to clinical sexuality. Focus 2005;III(4):526-31.
6. Diamond M, Watson LA. Androgen insensitivity syndrome and Klinefelter’s syndrome: sex and gender considerations. Child Adolesc Psychiatr Clin North Am 2004;13(3):623-40.
7. Schlechte JA. Prolactinoma. N Engl J Med 2003;349(21):2035-41.
8. Gabbard GO. Mind, brain and personality disorders. Am J Psychiatry 2005;162(4):648-55.
9. Kafka MP. The paraphilia-related disorders: nonparaphillic hyper-sexuality and sexual compulsivity/addiction. In: Leiblum SR, Rosen RC (eds). Principles and practices of sex therapy. New York: Guilford Press; 2000:471-503.
10. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
11. Basson R. Human sex response cycles. J Sex Marital Ther 2001;27(1):33-43.
12. Dennerstein L. The sexual impact of menopause. In: Levine SB, Risen CB, Althof SE (eds). The handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003.
13. Schiavi RC, Schreiner-Engel P, Mandeli J. Healthy aging and male sexual function. Am J Psychiatry 1990;147(6):766-71.
14. Kellett JM. Older adult sexuality. In: Szuchman LT, Muscarella F (eds). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:355-82.
1. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
2. Maurice WL. Sexual medicine in primary care. Philadelphia: Mosby; 1999.
3. Risen CB. Listening to sexual stories. In: Levine SB, Risen CB, Althof SE (eds). Handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003:1-20.
4. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry (pamphlet). Washington, DC: American Psychiatric Association; 1993.
5. Levine SB. A reintroduction to clinical sexuality. Focus 2005;III(4):526-31.
6. Diamond M, Watson LA. Androgen insensitivity syndrome and Klinefelter’s syndrome: sex and gender considerations. Child Adolesc Psychiatr Clin North Am 2004;13(3):623-40.
7. Schlechte JA. Prolactinoma. N Engl J Med 2003;349(21):2035-41.
8. Gabbard GO. Mind, brain and personality disorders. Am J Psychiatry 2005;162(4):648-55.
9. Kafka MP. The paraphilia-related disorders: nonparaphillic hyper-sexuality and sexual compulsivity/addiction. In: Leiblum SR, Rosen RC (eds). Principles and practices of sex therapy. New York: Guilford Press; 2000:471-503.
10. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
11. Basson R. Human sex response cycles. J Sex Marital Ther 2001;27(1):33-43.
12. Dennerstein L. The sexual impact of menopause. In: Levine SB, Risen CB, Althof SE (eds). The handbook of clinical sexuality for mental health professionals. New York: Brunner/Routledge; 2003.
13. Schiavi RC, Schreiner-Engel P, Mandeli J. Healthy aging and male sexual function. Am J Psychiatry 1990;147(6):766-71.
14. Kellett JM. Older adult sexuality. In: Szuchman LT, Muscarella F (eds). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:355-82.
Getting too many e-alerts?
Are e-alerts cluttering your in box?
These indispensable e-mails offer immediate access to current clinical articles. But with so many e-alerts and search services available, useless alerts and irrelevant search results can clog your client—in the bargain slowing access to the data you need.
This article explains how to customize your subscriptions and choose e-alerts that most closely meet your needs.
Which e-alert is best for you?
When new issues appear online, journals typically send out:
- Publication alerts, which provide a link to the journal’s home page or current table of contents.
- Tables of contents—or eTOCs. Whereas publication alerts contain a few links, eTOCs list the current issue’s full TOC with links to abstracts and full-text versions of each article.
- Content alerts, which consolidate links to new articles on a specific topic. Content alerts are sent each time a keyword on your interest list is included within a new article’s title, keywords, or abstract.
- Citation alerts when an article on your list is cited. This helps you track how often authors are citing an article and alerts you to other articles in your field of interest.
- Author alerts when an article by a selected author is published, helping you consolidate links to current data from thought leaders on a given topic. Author alerts also help you track articles you’ve published.
- Saved search alerts, which retrieve a prior search each time the publisher’s database is updated. This can help you avoid having to restart searches.
E-alerts usually are free, but a paid subscription to the journal often is required to access full-text articles.
Psychiatric journals offer many types of e-alerts:
- Archives of General Psychiatry offers free eTOCs, content, author, and saved search alerts, as well as links to “early release” articles that are published online before they appear in print.
- The American Journal of Psychiatry offers these alerts through HighWire Press as well as PDA services, which each month download TOCs, citations, and abstracts to your personal digital assistant. You need a (free) HighWire account to subscribe to these services.
Publisher alerts. Some journal publishers—including American Psychiatric Publishing, Elsevier, Karger, and Springer—offer free publisher alerts that announce online updates to all journals in their groups. Elsevier offers a range of e-alert services, including free issue, citation, and saved search alerts, and a quarterly alert listing the “25 hottest articles.”
Publisher alerts contain links to several journals in one message. You don’t need to subscribe to any one journal to receive a publisher alert, but the information is limited to that publisher’s journals. American Psychiatric Publishing’s alert, for example, offers links to The American Journal of Psychiatry and the publisher’s other journals, but you need a separate alert for Journal of Clinical Psychiatry.
Choosing an e-alert service
Independent e-alert services, which scan the medical literature for links to pertinent articles, offer substantially more links than do publication or group e-alerts
HighWire Press, a division of Stanford University Libraries, hosts a searchable repository of full-text online articles from more than 900 journals—many of which can be accessed at no charge. HighWire also offers:
- eTOCs
- CiteTrack, an alert on new journal content based on search criteria (topics/keywords, authors, or articles being referenced or cited)
- PDA Channels, which delivers alerts to Palm OS and Pocket PC handhelds
- RSS (really simple syndication) feeds—compilations of alerts and headlines from various publications that must be viewed using RSS Reader software
- Subscription alerts, which warn users a few weeks before that their subscription will expire.
PubMed Central. Users can open MyNCBI accounts to save searches and receive alerts for new content, authors, journals, and saved searches.
AMEDEO offers weekly updates of journals by topic and alerts providing information on various medical fields. AMEDEO’s “psychiatric disorders” link, however, lists articles only on depression and schizophrenia.
MDLinx offers daily updates of journal and medical news by specialty and subspecialty. “PsychLinx” provides links to psychiatry articles, by subspecialty or topic, and direct links to journals.
IngentaConnect sends users free monthly eTOCs from five journals. Institutions that purchase an institutional alerting license from IngentaConnect can receive unlimited new-issue alerts and free search alerts.
We find that using HighWire with PubMed Central offers an optimal yield of psychiatric articles. Paid alert services, such as Ovid or Web of Science, require an institutional or personal subscription and offer no significant advantage over free services.
You can customize your e-alert based on specialty and specific search criteria. By scrolling to “Alert Sources” on the HighWire site, for example, you can choose to scan all PubMed content (abstracts), all HighWire-hosted content (abstracts and full text), or psychiatry journals only by clicking on “View list by topic” and checking the “Psychiatry” box.
Remember that e-alerts—no matter how effective they are or how many you receive—cannot link you to every article you need. Web searches will be necessary at times.
Avoiding spam, viruses, other problems
Before you subscribe to an e-alert, ask these questions to prevent unwanted e-mails:
Is the e-alert secure? As with any online service, giving your e-mail address when subscribing to an e-alert could open your client to spam or—worse—a virus transmitted via an unwanted message.
To reduce the risk, update your subscription form as needed to confirm your identity, and change your password yearly to guard your privacy. Most publishers/services enforce privacy policies that ensure safe transmission.
Is the journal site easy to navigate? The site should offer advanced search capabilities that allow you to customize searches. Instructions or answers to frequently asked questions about alert frequency, managing and refining alerts, and other issues should be clear and accessible.
Will my search terms work? Use specific terms to filter information. Whereas a broad search term such as “bipolar disorder” would yield a long list of irrelevant articles, a more specific term such as “bipolar maintenance therapy” would substantially narrow the search. Adjust and refine your search terms and update your interest list as needed to ensure an optimal flow of information.
Is the journal’s Webmaster accessible? Contact the Webmaster if an e-alert is not meeting your needs or if you have suggestions for improvement. A link to the Webmaster should be listed on the “contact us” page.
Related resources
Cuddy C. HighWire Press and its journey to become the world’s largest full-text STM online journal collection. Journal of Electronic Resources in Medical Libraries 2005;2:1-13.
Drs. Lapid and Kung report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
To comment on this article or share your experience with e-alerts, click here.
Are e-alerts cluttering your in box?
These indispensable e-mails offer immediate access to current clinical articles. But with so many e-alerts and search services available, useless alerts and irrelevant search results can clog your client—in the bargain slowing access to the data you need.
This article explains how to customize your subscriptions and choose e-alerts that most closely meet your needs.
Which e-alert is best for you?
When new issues appear online, journals typically send out:
- Publication alerts, which provide a link to the journal’s home page or current table of contents.
- Tables of contents—or eTOCs. Whereas publication alerts contain a few links, eTOCs list the current issue’s full TOC with links to abstracts and full-text versions of each article.
- Content alerts, which consolidate links to new articles on a specific topic. Content alerts are sent each time a keyword on your interest list is included within a new article’s title, keywords, or abstract.
- Citation alerts when an article on your list is cited. This helps you track how often authors are citing an article and alerts you to other articles in your field of interest.
- Author alerts when an article by a selected author is published, helping you consolidate links to current data from thought leaders on a given topic. Author alerts also help you track articles you’ve published.
- Saved search alerts, which retrieve a prior search each time the publisher’s database is updated. This can help you avoid having to restart searches.
E-alerts usually are free, but a paid subscription to the journal often is required to access full-text articles.
Psychiatric journals offer many types of e-alerts:
- Archives of General Psychiatry offers free eTOCs, content, author, and saved search alerts, as well as links to “early release” articles that are published online before they appear in print.
- The American Journal of Psychiatry offers these alerts through HighWire Press as well as PDA services, which each month download TOCs, citations, and abstracts to your personal digital assistant. You need a (free) HighWire account to subscribe to these services.
Publisher alerts. Some journal publishers—including American Psychiatric Publishing, Elsevier, Karger, and Springer—offer free publisher alerts that announce online updates to all journals in their groups. Elsevier offers a range of e-alert services, including free issue, citation, and saved search alerts, and a quarterly alert listing the “25 hottest articles.”
Publisher alerts contain links to several journals in one message. You don’t need to subscribe to any one journal to receive a publisher alert, but the information is limited to that publisher’s journals. American Psychiatric Publishing’s alert, for example, offers links to The American Journal of Psychiatry and the publisher’s other journals, but you need a separate alert for Journal of Clinical Psychiatry.
Choosing an e-alert service
Independent e-alert services, which scan the medical literature for links to pertinent articles, offer substantially more links than do publication or group e-alerts
HighWire Press, a division of Stanford University Libraries, hosts a searchable repository of full-text online articles from more than 900 journals—many of which can be accessed at no charge. HighWire also offers:
- eTOCs
- CiteTrack, an alert on new journal content based on search criteria (topics/keywords, authors, or articles being referenced or cited)
- PDA Channels, which delivers alerts to Palm OS and Pocket PC handhelds
- RSS (really simple syndication) feeds—compilations of alerts and headlines from various publications that must be viewed using RSS Reader software
- Subscription alerts, which warn users a few weeks before that their subscription will expire.
PubMed Central. Users can open MyNCBI accounts to save searches and receive alerts for new content, authors, journals, and saved searches.
AMEDEO offers weekly updates of journals by topic and alerts providing information on various medical fields. AMEDEO’s “psychiatric disorders” link, however, lists articles only on depression and schizophrenia.
MDLinx offers daily updates of journal and medical news by specialty and subspecialty. “PsychLinx” provides links to psychiatry articles, by subspecialty or topic, and direct links to journals.
IngentaConnect sends users free monthly eTOCs from five journals. Institutions that purchase an institutional alerting license from IngentaConnect can receive unlimited new-issue alerts and free search alerts.
We find that using HighWire with PubMed Central offers an optimal yield of psychiatric articles. Paid alert services, such as Ovid or Web of Science, require an institutional or personal subscription and offer no significant advantage over free services.
You can customize your e-alert based on specialty and specific search criteria. By scrolling to “Alert Sources” on the HighWire site, for example, you can choose to scan all PubMed content (abstracts), all HighWire-hosted content (abstracts and full text), or psychiatry journals only by clicking on “View list by topic” and checking the “Psychiatry” box.
Remember that e-alerts—no matter how effective they are or how many you receive—cannot link you to every article you need. Web searches will be necessary at times.
Avoiding spam, viruses, other problems
Before you subscribe to an e-alert, ask these questions to prevent unwanted e-mails:
Is the e-alert secure? As with any online service, giving your e-mail address when subscribing to an e-alert could open your client to spam or—worse—a virus transmitted via an unwanted message.
To reduce the risk, update your subscription form as needed to confirm your identity, and change your password yearly to guard your privacy. Most publishers/services enforce privacy policies that ensure safe transmission.
Is the journal site easy to navigate? The site should offer advanced search capabilities that allow you to customize searches. Instructions or answers to frequently asked questions about alert frequency, managing and refining alerts, and other issues should be clear and accessible.
Will my search terms work? Use specific terms to filter information. Whereas a broad search term such as “bipolar disorder” would yield a long list of irrelevant articles, a more specific term such as “bipolar maintenance therapy” would substantially narrow the search. Adjust and refine your search terms and update your interest list as needed to ensure an optimal flow of information.
Is the journal’s Webmaster accessible? Contact the Webmaster if an e-alert is not meeting your needs or if you have suggestions for improvement. A link to the Webmaster should be listed on the “contact us” page.
Related resources
Cuddy C. HighWire Press and its journey to become the world’s largest full-text STM online journal collection. Journal of Electronic Resources in Medical Libraries 2005;2:1-13.
Drs. Lapid and Kung report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
To comment on this article or share your experience with e-alerts, click here.
Are e-alerts cluttering your in box?
These indispensable e-mails offer immediate access to current clinical articles. But with so many e-alerts and search services available, useless alerts and irrelevant search results can clog your client—in the bargain slowing access to the data you need.
This article explains how to customize your subscriptions and choose e-alerts that most closely meet your needs.
Which e-alert is best for you?
When new issues appear online, journals typically send out:
- Publication alerts, which provide a link to the journal’s home page or current table of contents.
- Tables of contents—or eTOCs. Whereas publication alerts contain a few links, eTOCs list the current issue’s full TOC with links to abstracts and full-text versions of each article.
- Content alerts, which consolidate links to new articles on a specific topic. Content alerts are sent each time a keyword on your interest list is included within a new article’s title, keywords, or abstract.
- Citation alerts when an article on your list is cited. This helps you track how often authors are citing an article and alerts you to other articles in your field of interest.
- Author alerts when an article by a selected author is published, helping you consolidate links to current data from thought leaders on a given topic. Author alerts also help you track articles you’ve published.
- Saved search alerts, which retrieve a prior search each time the publisher’s database is updated. This can help you avoid having to restart searches.
E-alerts usually are free, but a paid subscription to the journal often is required to access full-text articles.
Psychiatric journals offer many types of e-alerts:
- Archives of General Psychiatry offers free eTOCs, content, author, and saved search alerts, as well as links to “early release” articles that are published online before they appear in print.
- The American Journal of Psychiatry offers these alerts through HighWire Press as well as PDA services, which each month download TOCs, citations, and abstracts to your personal digital assistant. You need a (free) HighWire account to subscribe to these services.
Publisher alerts. Some journal publishers—including American Psychiatric Publishing, Elsevier, Karger, and Springer—offer free publisher alerts that announce online updates to all journals in their groups. Elsevier offers a range of e-alert services, including free issue, citation, and saved search alerts, and a quarterly alert listing the “25 hottest articles.”
Publisher alerts contain links to several journals in one message. You don’t need to subscribe to any one journal to receive a publisher alert, but the information is limited to that publisher’s journals. American Psychiatric Publishing’s alert, for example, offers links to The American Journal of Psychiatry and the publisher’s other journals, but you need a separate alert for Journal of Clinical Psychiatry.
Choosing an e-alert service
Independent e-alert services, which scan the medical literature for links to pertinent articles, offer substantially more links than do publication or group e-alerts
HighWire Press, a division of Stanford University Libraries, hosts a searchable repository of full-text online articles from more than 900 journals—many of which can be accessed at no charge. HighWire also offers:
- eTOCs
- CiteTrack, an alert on new journal content based on search criteria (topics/keywords, authors, or articles being referenced or cited)
- PDA Channels, which delivers alerts to Palm OS and Pocket PC handhelds
- RSS (really simple syndication) feeds—compilations of alerts and headlines from various publications that must be viewed using RSS Reader software
- Subscription alerts, which warn users a few weeks before that their subscription will expire.
PubMed Central. Users can open MyNCBI accounts to save searches and receive alerts for new content, authors, journals, and saved searches.
AMEDEO offers weekly updates of journals by topic and alerts providing information on various medical fields. AMEDEO’s “psychiatric disorders” link, however, lists articles only on depression and schizophrenia.
MDLinx offers daily updates of journal and medical news by specialty and subspecialty. “PsychLinx” provides links to psychiatry articles, by subspecialty or topic, and direct links to journals.
IngentaConnect sends users free monthly eTOCs from five journals. Institutions that purchase an institutional alerting license from IngentaConnect can receive unlimited new-issue alerts and free search alerts.
We find that using HighWire with PubMed Central offers an optimal yield of psychiatric articles. Paid alert services, such as Ovid or Web of Science, require an institutional or personal subscription and offer no significant advantage over free services.
You can customize your e-alert based on specialty and specific search criteria. By scrolling to “Alert Sources” on the HighWire site, for example, you can choose to scan all PubMed content (abstracts), all HighWire-hosted content (abstracts and full text), or psychiatry journals only by clicking on “View list by topic” and checking the “Psychiatry” box.
Remember that e-alerts—no matter how effective they are or how many you receive—cannot link you to every article you need. Web searches will be necessary at times.
Avoiding spam, viruses, other problems
Before you subscribe to an e-alert, ask these questions to prevent unwanted e-mails:
Is the e-alert secure? As with any online service, giving your e-mail address when subscribing to an e-alert could open your client to spam or—worse—a virus transmitted via an unwanted message.
To reduce the risk, update your subscription form as needed to confirm your identity, and change your password yearly to guard your privacy. Most publishers/services enforce privacy policies that ensure safe transmission.
Is the journal site easy to navigate? The site should offer advanced search capabilities that allow you to customize searches. Instructions or answers to frequently asked questions about alert frequency, managing and refining alerts, and other issues should be clear and accessible.
Will my search terms work? Use specific terms to filter information. Whereas a broad search term such as “bipolar disorder” would yield a long list of irrelevant articles, a more specific term such as “bipolar maintenance therapy” would substantially narrow the search. Adjust and refine your search terms and update your interest list as needed to ensure an optimal flow of information.
Is the journal’s Webmaster accessible? Contact the Webmaster if an e-alert is not meeting your needs or if you have suggestions for improvement. A link to the Webmaster should be listed on the “contact us” page.
Related resources
Cuddy C. HighWire Press and its journey to become the world’s largest full-text STM online journal collection. Journal of Electronic Resources in Medical Libraries 2005;2:1-13.
Drs. Lapid and Kung report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
To comment on this article or share your experience with e-alerts, click here.
Female sexual dysfunction: Don’t assume it’s a side effect
Antidepressants are one of many possible causes of sexual dysfunction in women. Sifting through the medical, psychological, and gynecologic possibilities can be daunting, but missing the cause can aggravate the sexual disorder and—worse—delay appropriate treatment.
To help you zero in more quickly, this article explains how to:
- accurately classify the sexual disorder based on presenting complaints
- perform a targeted yet thorough medical, gynecologic, and psychiatric history
- determine which lab and medical tests to order and where to refer the patient.
Case: Depressed with sexual problems
Ms. S, age 42, has major depressive disorder. Her medical history is unremarkable and menstruation is regular, but she reports discord with her husband.
The patient had been taking paroxetine, 40 mg/d for 2 years, but stopped taking it 6 months ago because she thought she no longer needed it. During that time, Ms. S says, her depressive symptoms (reduced interest and energy, insomnia, loss of appetite with weight loss, and inability to concentrate) have resurfaced. She says she sometimes wishes she were dead but denies intent to harm herself. We restart paroxetine at 20 mg/d and increase the dosage over 2 weeks to 40 mg/d.
Five weeks after reaching the target dosage, Ms. S reports delayed orgasms and reduced libido, which she attributes to paroxetine. She insists that we switch her to bupropion because it is less likely than other antidepressants to reduce sexual function.Sexual dysfunction: What’s love got to do with it?”). Women who have been sexually, physically, or emotionally abused tend to avoid sexual relationships. Some women become less sexually active with age, as fewer potential sexual partners become available. Others may have lost a sexual partner because of divorce, infidelity, disability, or death.
Is there a psychiatric cause?
Obtain a detailed psychiatric history, which should include:
Relationship status. Does the patient have a steady partner? If so, are she and her partner equally interested in sex? Are she and her partner fighting? If she is married, has she or her husband had an extramarital affair?6
If the patient does not have a steady partner, does she have sex? If yes, how often? Is she pleased with the experience?
Relationship history. Has the patient been in an abusive relationship?
Substance use. Does the patient use drugs or alcohol, which can impair sexual function? If yes, how often and how much?
Psychosocial history. Find out whether cultural or religious values have influenced the patient’s attitude toward sex.8 Cultural restrictions against openly discussing sex can inhibit a person’s view of sex or foster a belief that sex is not permissible.
Sexual orientation. Be nonjudgmental, but watch for signs of gender identity disorder, which requires more-focused interventions.
Past and current psychiatric disorders. Women with depression are more likely than nondepressed women to lose sexual desire and complain of anorgasmia.8
Sexual dysfunction can also accompany symptoms of posttraumatic stress disorder or anxiety, particularly if past sexual trauma caused these symptoms.
Case Continued: A turn for the worse
Several months later, Ms. S’ depressive symptoms have worsened, and she develops psychosis (primarily persecutory delusions). We hospitalize her and diagnose major depressive disorder with psychotic features. We increase paroxetine to 60 mg/d over 3 days. We also add risperidone, 1 mg nightly, and titrate over 1 week to 2 mg nightly.
After 2 weeks, we discharge Ms. S when she reports improved mood with no suicidal thoughts or psychotic symptoms. She says her marriage is stable, but her sexual interest is again diminished. Continued psychotherapy has not alleviated her sexual symptoms, nor has reducing paroxetine to 40 mg/d. Risperidone dosage is the same.
Six weeks after discharge, Ms. S reports irregular menses and galactorrhea that have been present for 1 month. She fears she is entering menopause.
Discussion. Ms. S’ case shows how convergent psychological, medical, and pharmacologic risk factors for sexual dysfunction can confound diagnosis in women with depression.
Paroxetine—like other selective serotonin reuptake inhibitors—can reduce sexual desire,9 but Ms. S’ sexual symptoms persisted after we reduced the dosage or stopped the medication. Psychodynamic psychotherapy saved her marriage but did not improve her sexual function.
- Is Ms. S starting menopause?
- Is risperidone—which also can decrease libido3—a contributing factor?
- Do galactorrhea and irregular menses signal a serious medical problem?
Is there a medical problem?
Because so many medical conditions can contribute to one or more sexual dysfunction disorders, the presenting symptoms should guide the medical history (Table 3). Ask about:
- personal or family history of common medical conditions that cause sexual dysfunction, such as diabetes mellitus and hypertension (Table 4). Vascular complications stemming from these disorders can block arousal. Osteoarthritis—which is probably ruled out for Ms. S but is prevalent in postmenopausal women—may cause discomfort or pain that obliterates sexual desire.6
- current or past gynecologic conditions. Many gynecologic disorders can impair sexual function.
Be aware that some women may have comorbid disorders, any of which could decrease sexual function. For these women, finding the root cause is essential to ensuring appropriate, targeted treatment. For example, ask a patient who complains of decreased arousal to explain how she feels during sex. Her answers might reveal symptoms that suggest dyspareunia. Similarly, an older woman who loses interest in sex could have a sexual aversion disorder or could be starting menopause (Box).
Finally, ask how long the sexual dysfunction has persisted, as this will help you rule out causes. For example, diabetes progressively depletes sexual function, whereas medication might precipitate a more acute or abrupt change.
Ms. A, age 63, reached menopause at age 55. She reports no other medical problems when she presents for an annual checkup.
For 6 months, she says, she has had little desire for sex with her husband. During additional questioning, she reports vaginal discomfort and lack of pleasure.
Pelvic exam reveals vaginal wall atrophy and vaginal vault dryness. We suspect that vaginal atrophy secondary to menopause has diminished Ms. A’s libido, as other physical exam and laboratory findings are normal.
Sexual dysfunction is more frequent and is more often irreversible among women who are experiencing or have gone through menopause, compared with younger women.4
Genital tissues have abundant estrogen receptors in the epithelial, endothelial, and smooth muscle cells. Ovarian estradiol production ceases with menopause onset, subjecting genital tissues to atrophy; this in turn decreases lubrication and vaginal moisture. Loss of moisture causes dyspareunia, prompting women to avoid or fear sexual intercourse. Decreased sexual activity leads to further vaginal atrophy, creating a cycle of sexual dysfunction.
Iatrogenic causes, such as bilateral oophorectomy and chemotherapy, also can trigger menopause. In such cases, sexual dysfunction caused by lack of androgen production can be severe.10
As women approach or have completed menopause, hormonal changes—including FSH, LH, estrogen, and progesterone—and chronic medical illnesses such as diabetes and hypertension, are more likely to contribute to sexual dysfunction. In younger women, workup may focus on thyroid dysfunction and medications, such as oral contraceptives.
Low interest? Delayed orgasm? Vaginal pain?
Here’s what you need to find out
| If patient reports… | Ask:* |
|---|---|
| Lack of interest in sex | Has this happened before? |
| Have you started any new medications or changed dosages? | |
| Dreading the prospect of sex with her partner | Are there any physical reasons why this happens? |
| Has this happened before? | |
| What is the nature of your relationship with your partner? | |
| Have you had problems like this with previous partners? | |
| Difficulty becoming aroused | Does this happen only with your partner? |
| Can you become aroused with self-stimulation or use of other objects? | |
| Difficulty reaching or maintaining orgasm | Does this happen only with your partner? |
| Are you able to achieve orgasm with self-stimulation or use of other objects? | |
| Has this happened with other partners? | |
| Vaginal pain during intercourse | Have you noticed vaginal dryness? |
| Has this happened before? | |
| Does this happen with self-stimulation or use of other objects? | |
| * Patient’s answers to questions about specific sexual dysfunction symptoms could suggest concurrent sexual dysfunction disorders. | |
Female sexual dysfunction: 5 medical causes you should not miss
| Diabetes |
| Hypertension |
| Stroke |
| Urinary incontinence |
| Urinary tract infections |
Ordering additional tests
An introductory laboratory evaluation, performed in concert with the patient’s primary care physician, can help assess for common medical causes of sexual dysfunction. Watch for:
- elevated blood pressure, which suggests hypertension
- overweight, which can lead to hyperlipidemia or osteoarthritis
- glycosylated hemoglobin >7%, which could signal diabetes
- prolactin >20 ng/mL, which suggests pituitary dysfunction
- low estrogen and/or follicle-stimulating hormone (FSH) 40 to 200 mlU/mL, which suggest menopause
- elevated estrogen or FSH >5 mlU/mL, which could signal amenorrhea
- low free and total testosterone readings, which may signal an androgen problem
- Elevated thyroid-simulating hormone (TSH), which could point to hypothyroidism.
- Low TSH, which could signal hyperthyroidism.8
Case Continued: Telling test results
We measure Ms. S’ prolactin because she is taking risperidone, which can cause hyperprolactinemia. Prolactin is 61.86 ng/mL, suggesting medication-induced prolactinemia or a pituitary abnormality. We consult with endocrinology and refer the patient to a radiologist for brain MRI, which reveals a prolactin-secreting tumor in the pituitary gland.
Tapering and discontinuing risperidone over 1 week reduce but do not normalize Ms. S’ prolactin. Although dopamine agonists are first-line therapy for prolactin-secreting tumors, we fear such drugs would activate Ms. S’ psychotic symptoms. She instead undergoes surgery to remove the adenoma. Her sexual symptoms, galactorrhea, and irregular menses eventually resolve.
Related resources
- Hitt E. Female sexual dysfunction common. WebMD. Available at: http://www.webmd.com/content/Article/64/72281.htm. Accessed June 6, 2006.
- Basson R, Leiblum S, Brotto L, et al. Revised definitions of women’s sexual dysfunction. J Sex Med 2004;1(1):40-8.
- Dennerstein L, Hayes R. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med 2005;suppl 3:118-32.
- Michelson D, Bancroft J, Targum S et al. Female sexual dysfunction associated with antidepressant administration: a randomized placebo-controlled study of pharmacologic intervention. Am J Psychiatry 2000;157:239-43.
- Bupropion SA • Wellbutrin SR
- Clonidine • Catapres
- Paroxetine • Paxil, Pevexa
- Risperidone • Risperdal
- Spironolactone • Aldactone
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Coleman CC, King BR, Bolden-Watson C, et al. A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clin Ther 2001;23:1040-58.
2. Croft H, Settle E, Jr, Houser T, et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther 1999;21:643-58.
3. Knegtering H, Boks M, Blijd C, et al. A randomized open-label comparison of the impact of olanzapine versus risperidone on sexual functioning. J Sex Marital Ther 2006;32:315-26.
4. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med 2004;1:24-34.
5. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:539-66.
6. Phillips N. Female sexual dysfunction: evaluation and treatment. Am Fam Physician 2000;62:127-42.
7. Brigham and Women’s Hospital Female sexual dysfunction. Available at: http://healthgate.partners.org/browsing/browseContent.asp?fileName=11866.xml&title=Female%20Sexual%20Dysfunction. Accessed June 9, 2006.
8. Bartlik B, Goldstein M. Maintaining sexual health after menopause. Psychiatr Serv 2000;51:751-3.
9. Balon R, Yeragani VK, Pohl R, Remesh C. Sexual dysfunction during antidepressant treatment. J Clin Psych 1993;54:209-12.
10. Goldstein I, Alexander J. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med 2005;Suppl 3:154-65.
Antidepressants are one of many possible causes of sexual dysfunction in women. Sifting through the medical, psychological, and gynecologic possibilities can be daunting, but missing the cause can aggravate the sexual disorder and—worse—delay appropriate treatment.
To help you zero in more quickly, this article explains how to:
- accurately classify the sexual disorder based on presenting complaints
- perform a targeted yet thorough medical, gynecologic, and psychiatric history
- determine which lab and medical tests to order and where to refer the patient.
Case: Depressed with sexual problems
Ms. S, age 42, has major depressive disorder. Her medical history is unremarkable and menstruation is regular, but she reports discord with her husband.
The patient had been taking paroxetine, 40 mg/d for 2 years, but stopped taking it 6 months ago because she thought she no longer needed it. During that time, Ms. S says, her depressive symptoms (reduced interest and energy, insomnia, loss of appetite with weight loss, and inability to concentrate) have resurfaced. She says she sometimes wishes she were dead but denies intent to harm herself. We restart paroxetine at 20 mg/d and increase the dosage over 2 weeks to 40 mg/d.
Five weeks after reaching the target dosage, Ms. S reports delayed orgasms and reduced libido, which she attributes to paroxetine. She insists that we switch her to bupropion because it is less likely than other antidepressants to reduce sexual function.Sexual dysfunction: What’s love got to do with it?”). Women who have been sexually, physically, or emotionally abused tend to avoid sexual relationships. Some women become less sexually active with age, as fewer potential sexual partners become available. Others may have lost a sexual partner because of divorce, infidelity, disability, or death.
Is there a psychiatric cause?
Obtain a detailed psychiatric history, which should include:
Relationship status. Does the patient have a steady partner? If so, are she and her partner equally interested in sex? Are she and her partner fighting? If she is married, has she or her husband had an extramarital affair?6
If the patient does not have a steady partner, does she have sex? If yes, how often? Is she pleased with the experience?
Relationship history. Has the patient been in an abusive relationship?
Substance use. Does the patient use drugs or alcohol, which can impair sexual function? If yes, how often and how much?
Psychosocial history. Find out whether cultural or religious values have influenced the patient’s attitude toward sex.8 Cultural restrictions against openly discussing sex can inhibit a person’s view of sex or foster a belief that sex is not permissible.
Sexual orientation. Be nonjudgmental, but watch for signs of gender identity disorder, which requires more-focused interventions.
Past and current psychiatric disorders. Women with depression are more likely than nondepressed women to lose sexual desire and complain of anorgasmia.8
Sexual dysfunction can also accompany symptoms of posttraumatic stress disorder or anxiety, particularly if past sexual trauma caused these symptoms.
Case Continued: A turn for the worse
Several months later, Ms. S’ depressive symptoms have worsened, and she develops psychosis (primarily persecutory delusions). We hospitalize her and diagnose major depressive disorder with psychotic features. We increase paroxetine to 60 mg/d over 3 days. We also add risperidone, 1 mg nightly, and titrate over 1 week to 2 mg nightly.
After 2 weeks, we discharge Ms. S when she reports improved mood with no suicidal thoughts or psychotic symptoms. She says her marriage is stable, but her sexual interest is again diminished. Continued psychotherapy has not alleviated her sexual symptoms, nor has reducing paroxetine to 40 mg/d. Risperidone dosage is the same.
Six weeks after discharge, Ms. S reports irregular menses and galactorrhea that have been present for 1 month. She fears she is entering menopause.
Discussion. Ms. S’ case shows how convergent psychological, medical, and pharmacologic risk factors for sexual dysfunction can confound diagnosis in women with depression.
Paroxetine—like other selective serotonin reuptake inhibitors—can reduce sexual desire,9 but Ms. S’ sexual symptoms persisted after we reduced the dosage or stopped the medication. Psychodynamic psychotherapy saved her marriage but did not improve her sexual function.
- Is Ms. S starting menopause?
- Is risperidone—which also can decrease libido3—a contributing factor?
- Do galactorrhea and irregular menses signal a serious medical problem?
Is there a medical problem?
Because so many medical conditions can contribute to one or more sexual dysfunction disorders, the presenting symptoms should guide the medical history (Table 3). Ask about:
- personal or family history of common medical conditions that cause sexual dysfunction, such as diabetes mellitus and hypertension (Table 4). Vascular complications stemming from these disorders can block arousal. Osteoarthritis—which is probably ruled out for Ms. S but is prevalent in postmenopausal women—may cause discomfort or pain that obliterates sexual desire.6
- current or past gynecologic conditions. Many gynecologic disorders can impair sexual function.
Be aware that some women may have comorbid disorders, any of which could decrease sexual function. For these women, finding the root cause is essential to ensuring appropriate, targeted treatment. For example, ask a patient who complains of decreased arousal to explain how she feels during sex. Her answers might reveal symptoms that suggest dyspareunia. Similarly, an older woman who loses interest in sex could have a sexual aversion disorder or could be starting menopause (Box).
Finally, ask how long the sexual dysfunction has persisted, as this will help you rule out causes. For example, diabetes progressively depletes sexual function, whereas medication might precipitate a more acute or abrupt change.
Ms. A, age 63, reached menopause at age 55. She reports no other medical problems when she presents for an annual checkup.
For 6 months, she says, she has had little desire for sex with her husband. During additional questioning, she reports vaginal discomfort and lack of pleasure.
Pelvic exam reveals vaginal wall atrophy and vaginal vault dryness. We suspect that vaginal atrophy secondary to menopause has diminished Ms. A’s libido, as other physical exam and laboratory findings are normal.
Sexual dysfunction is more frequent and is more often irreversible among women who are experiencing or have gone through menopause, compared with younger women.4
Genital tissues have abundant estrogen receptors in the epithelial, endothelial, and smooth muscle cells. Ovarian estradiol production ceases with menopause onset, subjecting genital tissues to atrophy; this in turn decreases lubrication and vaginal moisture. Loss of moisture causes dyspareunia, prompting women to avoid or fear sexual intercourse. Decreased sexual activity leads to further vaginal atrophy, creating a cycle of sexual dysfunction.
Iatrogenic causes, such as bilateral oophorectomy and chemotherapy, also can trigger menopause. In such cases, sexual dysfunction caused by lack of androgen production can be severe.10
As women approach or have completed menopause, hormonal changes—including FSH, LH, estrogen, and progesterone—and chronic medical illnesses such as diabetes and hypertension, are more likely to contribute to sexual dysfunction. In younger women, workup may focus on thyroid dysfunction and medications, such as oral contraceptives.
Low interest? Delayed orgasm? Vaginal pain?
Here’s what you need to find out
| If patient reports… | Ask:* |
|---|---|
| Lack of interest in sex | Has this happened before? |
| Have you started any new medications or changed dosages? | |
| Dreading the prospect of sex with her partner | Are there any physical reasons why this happens? |
| Has this happened before? | |
| What is the nature of your relationship with your partner? | |
| Have you had problems like this with previous partners? | |
| Difficulty becoming aroused | Does this happen only with your partner? |
| Can you become aroused with self-stimulation or use of other objects? | |
| Difficulty reaching or maintaining orgasm | Does this happen only with your partner? |
| Are you able to achieve orgasm with self-stimulation or use of other objects? | |
| Has this happened with other partners? | |
| Vaginal pain during intercourse | Have you noticed vaginal dryness? |
| Has this happened before? | |
| Does this happen with self-stimulation or use of other objects? | |
| * Patient’s answers to questions about specific sexual dysfunction symptoms could suggest concurrent sexual dysfunction disorders. | |
Female sexual dysfunction: 5 medical causes you should not miss
| Diabetes |
| Hypertension |
| Stroke |
| Urinary incontinence |
| Urinary tract infections |
Ordering additional tests
An introductory laboratory evaluation, performed in concert with the patient’s primary care physician, can help assess for common medical causes of sexual dysfunction. Watch for:
- elevated blood pressure, which suggests hypertension
- overweight, which can lead to hyperlipidemia or osteoarthritis
- glycosylated hemoglobin >7%, which could signal diabetes
- prolactin >20 ng/mL, which suggests pituitary dysfunction
- low estrogen and/or follicle-stimulating hormone (FSH) 40 to 200 mlU/mL, which suggest menopause
- elevated estrogen or FSH >5 mlU/mL, which could signal amenorrhea
- low free and total testosterone readings, which may signal an androgen problem
- Elevated thyroid-simulating hormone (TSH), which could point to hypothyroidism.
- Low TSH, which could signal hyperthyroidism.8
Case Continued: Telling test results
We measure Ms. S’ prolactin because she is taking risperidone, which can cause hyperprolactinemia. Prolactin is 61.86 ng/mL, suggesting medication-induced prolactinemia or a pituitary abnormality. We consult with endocrinology and refer the patient to a radiologist for brain MRI, which reveals a prolactin-secreting tumor in the pituitary gland.
Tapering and discontinuing risperidone over 1 week reduce but do not normalize Ms. S’ prolactin. Although dopamine agonists are first-line therapy for prolactin-secreting tumors, we fear such drugs would activate Ms. S’ psychotic symptoms. She instead undergoes surgery to remove the adenoma. Her sexual symptoms, galactorrhea, and irregular menses eventually resolve.
Related resources
- Hitt E. Female sexual dysfunction common. WebMD. Available at: http://www.webmd.com/content/Article/64/72281.htm. Accessed June 6, 2006.
- Basson R, Leiblum S, Brotto L, et al. Revised definitions of women’s sexual dysfunction. J Sex Med 2004;1(1):40-8.
- Dennerstein L, Hayes R. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med 2005;suppl 3:118-32.
- Michelson D, Bancroft J, Targum S et al. Female sexual dysfunction associated with antidepressant administration: a randomized placebo-controlled study of pharmacologic intervention. Am J Psychiatry 2000;157:239-43.
- Bupropion SA • Wellbutrin SR
- Clonidine • Catapres
- Paroxetine • Paxil, Pevexa
- Risperidone • Risperdal
- Spironolactone • Aldactone
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Antidepressants are one of many possible causes of sexual dysfunction in women. Sifting through the medical, psychological, and gynecologic possibilities can be daunting, but missing the cause can aggravate the sexual disorder and—worse—delay appropriate treatment.
To help you zero in more quickly, this article explains how to:
- accurately classify the sexual disorder based on presenting complaints
- perform a targeted yet thorough medical, gynecologic, and psychiatric history
- determine which lab and medical tests to order and where to refer the patient.
Case: Depressed with sexual problems
Ms. S, age 42, has major depressive disorder. Her medical history is unremarkable and menstruation is regular, but she reports discord with her husband.
The patient had been taking paroxetine, 40 mg/d for 2 years, but stopped taking it 6 months ago because she thought she no longer needed it. During that time, Ms. S says, her depressive symptoms (reduced interest and energy, insomnia, loss of appetite with weight loss, and inability to concentrate) have resurfaced. She says she sometimes wishes she were dead but denies intent to harm herself. We restart paroxetine at 20 mg/d and increase the dosage over 2 weeks to 40 mg/d.
Five weeks after reaching the target dosage, Ms. S reports delayed orgasms and reduced libido, which she attributes to paroxetine. She insists that we switch her to bupropion because it is less likely than other antidepressants to reduce sexual function.Sexual dysfunction: What’s love got to do with it?”). Women who have been sexually, physically, or emotionally abused tend to avoid sexual relationships. Some women become less sexually active with age, as fewer potential sexual partners become available. Others may have lost a sexual partner because of divorce, infidelity, disability, or death.
Is there a psychiatric cause?
Obtain a detailed psychiatric history, which should include:
Relationship status. Does the patient have a steady partner? If so, are she and her partner equally interested in sex? Are she and her partner fighting? If she is married, has she or her husband had an extramarital affair?6
If the patient does not have a steady partner, does she have sex? If yes, how often? Is she pleased with the experience?
Relationship history. Has the patient been in an abusive relationship?
Substance use. Does the patient use drugs or alcohol, which can impair sexual function? If yes, how often and how much?
Psychosocial history. Find out whether cultural or religious values have influenced the patient’s attitude toward sex.8 Cultural restrictions against openly discussing sex can inhibit a person’s view of sex or foster a belief that sex is not permissible.
Sexual orientation. Be nonjudgmental, but watch for signs of gender identity disorder, which requires more-focused interventions.
Past and current psychiatric disorders. Women with depression are more likely than nondepressed women to lose sexual desire and complain of anorgasmia.8
Sexual dysfunction can also accompany symptoms of posttraumatic stress disorder or anxiety, particularly if past sexual trauma caused these symptoms.
Case Continued: A turn for the worse
Several months later, Ms. S’ depressive symptoms have worsened, and she develops psychosis (primarily persecutory delusions). We hospitalize her and diagnose major depressive disorder with psychotic features. We increase paroxetine to 60 mg/d over 3 days. We also add risperidone, 1 mg nightly, and titrate over 1 week to 2 mg nightly.
After 2 weeks, we discharge Ms. S when she reports improved mood with no suicidal thoughts or psychotic symptoms. She says her marriage is stable, but her sexual interest is again diminished. Continued psychotherapy has not alleviated her sexual symptoms, nor has reducing paroxetine to 40 mg/d. Risperidone dosage is the same.
Six weeks after discharge, Ms. S reports irregular menses and galactorrhea that have been present for 1 month. She fears she is entering menopause.
Discussion. Ms. S’ case shows how convergent psychological, medical, and pharmacologic risk factors for sexual dysfunction can confound diagnosis in women with depression.
Paroxetine—like other selective serotonin reuptake inhibitors—can reduce sexual desire,9 but Ms. S’ sexual symptoms persisted after we reduced the dosage or stopped the medication. Psychodynamic psychotherapy saved her marriage but did not improve her sexual function.
- Is Ms. S starting menopause?
- Is risperidone—which also can decrease libido3—a contributing factor?
- Do galactorrhea and irregular menses signal a serious medical problem?
Is there a medical problem?
Because so many medical conditions can contribute to one or more sexual dysfunction disorders, the presenting symptoms should guide the medical history (Table 3). Ask about:
- personal or family history of common medical conditions that cause sexual dysfunction, such as diabetes mellitus and hypertension (Table 4). Vascular complications stemming from these disorders can block arousal. Osteoarthritis—which is probably ruled out for Ms. S but is prevalent in postmenopausal women—may cause discomfort or pain that obliterates sexual desire.6
- current or past gynecologic conditions. Many gynecologic disorders can impair sexual function.
Be aware that some women may have comorbid disorders, any of which could decrease sexual function. For these women, finding the root cause is essential to ensuring appropriate, targeted treatment. For example, ask a patient who complains of decreased arousal to explain how she feels during sex. Her answers might reveal symptoms that suggest dyspareunia. Similarly, an older woman who loses interest in sex could have a sexual aversion disorder or could be starting menopause (Box).
Finally, ask how long the sexual dysfunction has persisted, as this will help you rule out causes. For example, diabetes progressively depletes sexual function, whereas medication might precipitate a more acute or abrupt change.
Ms. A, age 63, reached menopause at age 55. She reports no other medical problems when she presents for an annual checkup.
For 6 months, she says, she has had little desire for sex with her husband. During additional questioning, she reports vaginal discomfort and lack of pleasure.
Pelvic exam reveals vaginal wall atrophy and vaginal vault dryness. We suspect that vaginal atrophy secondary to menopause has diminished Ms. A’s libido, as other physical exam and laboratory findings are normal.
Sexual dysfunction is more frequent and is more often irreversible among women who are experiencing or have gone through menopause, compared with younger women.4
Genital tissues have abundant estrogen receptors in the epithelial, endothelial, and smooth muscle cells. Ovarian estradiol production ceases with menopause onset, subjecting genital tissues to atrophy; this in turn decreases lubrication and vaginal moisture. Loss of moisture causes dyspareunia, prompting women to avoid or fear sexual intercourse. Decreased sexual activity leads to further vaginal atrophy, creating a cycle of sexual dysfunction.
Iatrogenic causes, such as bilateral oophorectomy and chemotherapy, also can trigger menopause. In such cases, sexual dysfunction caused by lack of androgen production can be severe.10
As women approach or have completed menopause, hormonal changes—including FSH, LH, estrogen, and progesterone—and chronic medical illnesses such as diabetes and hypertension, are more likely to contribute to sexual dysfunction. In younger women, workup may focus on thyroid dysfunction and medications, such as oral contraceptives.
Low interest? Delayed orgasm? Vaginal pain?
Here’s what you need to find out
| If patient reports… | Ask:* |
|---|---|
| Lack of interest in sex | Has this happened before? |
| Have you started any new medications or changed dosages? | |
| Dreading the prospect of sex with her partner | Are there any physical reasons why this happens? |
| Has this happened before? | |
| What is the nature of your relationship with your partner? | |
| Have you had problems like this with previous partners? | |
| Difficulty becoming aroused | Does this happen only with your partner? |
| Can you become aroused with self-stimulation or use of other objects? | |
| Difficulty reaching or maintaining orgasm | Does this happen only with your partner? |
| Are you able to achieve orgasm with self-stimulation or use of other objects? | |
| Has this happened with other partners? | |
| Vaginal pain during intercourse | Have you noticed vaginal dryness? |
| Has this happened before? | |
| Does this happen with self-stimulation or use of other objects? | |
| * Patient’s answers to questions about specific sexual dysfunction symptoms could suggest concurrent sexual dysfunction disorders. | |
Female sexual dysfunction: 5 medical causes you should not miss
| Diabetes |
| Hypertension |
| Stroke |
| Urinary incontinence |
| Urinary tract infections |
Ordering additional tests
An introductory laboratory evaluation, performed in concert with the patient’s primary care physician, can help assess for common medical causes of sexual dysfunction. Watch for:
- elevated blood pressure, which suggests hypertension
- overweight, which can lead to hyperlipidemia or osteoarthritis
- glycosylated hemoglobin >7%, which could signal diabetes
- prolactin >20 ng/mL, which suggests pituitary dysfunction
- low estrogen and/or follicle-stimulating hormone (FSH) 40 to 200 mlU/mL, which suggest menopause
- elevated estrogen or FSH >5 mlU/mL, which could signal amenorrhea
- low free and total testosterone readings, which may signal an androgen problem
- Elevated thyroid-simulating hormone (TSH), which could point to hypothyroidism.
- Low TSH, which could signal hyperthyroidism.8
Case Continued: Telling test results
We measure Ms. S’ prolactin because she is taking risperidone, which can cause hyperprolactinemia. Prolactin is 61.86 ng/mL, suggesting medication-induced prolactinemia or a pituitary abnormality. We consult with endocrinology and refer the patient to a radiologist for brain MRI, which reveals a prolactin-secreting tumor in the pituitary gland.
Tapering and discontinuing risperidone over 1 week reduce but do not normalize Ms. S’ prolactin. Although dopamine agonists are first-line therapy for prolactin-secreting tumors, we fear such drugs would activate Ms. S’ psychotic symptoms. She instead undergoes surgery to remove the adenoma. Her sexual symptoms, galactorrhea, and irregular menses eventually resolve.
Related resources
- Hitt E. Female sexual dysfunction common. WebMD. Available at: http://www.webmd.com/content/Article/64/72281.htm. Accessed June 6, 2006.
- Basson R, Leiblum S, Brotto L, et al. Revised definitions of women’s sexual dysfunction. J Sex Med 2004;1(1):40-8.
- Dennerstein L, Hayes R. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med 2005;suppl 3:118-32.
- Michelson D, Bancroft J, Targum S et al. Female sexual dysfunction associated with antidepressant administration: a randomized placebo-controlled study of pharmacologic intervention. Am J Psychiatry 2000;157:239-43.
- Bupropion SA • Wellbutrin SR
- Clonidine • Catapres
- Paroxetine • Paxil, Pevexa
- Risperidone • Risperdal
- Spironolactone • Aldactone
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Coleman CC, King BR, Bolden-Watson C, et al. A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clin Ther 2001;23:1040-58.
2. Croft H, Settle E, Jr, Houser T, et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther 1999;21:643-58.
3. Knegtering H, Boks M, Blijd C, et al. A randomized open-label comparison of the impact of olanzapine versus risperidone on sexual functioning. J Sex Marital Ther 2006;32:315-26.
4. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med 2004;1:24-34.
5. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:539-66.
6. Phillips N. Female sexual dysfunction: evaluation and treatment. Am Fam Physician 2000;62:127-42.
7. Brigham and Women’s Hospital Female sexual dysfunction. Available at: http://healthgate.partners.org/browsing/browseContent.asp?fileName=11866.xml&title=Female%20Sexual%20Dysfunction. Accessed June 9, 2006.
8. Bartlik B, Goldstein M. Maintaining sexual health after menopause. Psychiatr Serv 2000;51:751-3.
9. Balon R, Yeragani VK, Pohl R, Remesh C. Sexual dysfunction during antidepressant treatment. J Clin Psych 1993;54:209-12.
10. Goldstein I, Alexander J. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med 2005;Suppl 3:154-65.
1. Coleman CC, King BR, Bolden-Watson C, et al. A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clin Ther 2001;23:1040-58.
2. Croft H, Settle E, Jr, Houser T, et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther 1999;21:643-58.
3. Knegtering H, Boks M, Blijd C, et al. A randomized open-label comparison of the impact of olanzapine versus risperidone on sexual functioning. J Sex Marital Ther 2006;32:315-26.
4. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med 2004;1:24-34.
5. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:539-66.
6. Phillips N. Female sexual dysfunction: evaluation and treatment. Am Fam Physician 2000;62:127-42.
7. Brigham and Women’s Hospital Female sexual dysfunction. Available at: http://healthgate.partners.org/browsing/browseContent.asp?fileName=11866.xml&title=Female%20Sexual%20Dysfunction. Accessed June 9, 2006.
8. Bartlik B, Goldstein M. Maintaining sexual health after menopause. Psychiatr Serv 2000;51:751-3.
9. Balon R, Yeragani VK, Pohl R, Remesh C. Sexual dysfunction during antidepressant treatment. J Clin Psych 1993;54:209-12.
10. Goldstein I, Alexander J. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med 2005;Suppl 3:154-65.
Beware ictal activity that mimics psychiatric illness
Nonconvulsive status epilepticus (NCSE) is marked by neurobehavioral disturbances that resemble primary psychiatric disorders. Mistaken diagnosis and delayed treatment increase the risk of neurologic damage, so recognizing NCSE symptoms early is important.
To help you make a timely diagnosis, this article describes:
- neuropsychiatric manifestations of NCSE
- how to narrow the differential diagnosis by reviewing clinical symptoms and using electroencephalography (EEG)
- techniques used to rapidly halt ictal activity.
Status epilepticus (SE) is an acute medical emergency. Both forms—convulsive (CSE) and nonconvulsive (NCSE)—require early recognition and treatment. In the United States, 60 SE cases occur per 100,000 population/year, with mortality rates of 20% in adults and 38% in the elderly.1,2
Mortality risk. Data suggest patients with NCSE are unlikely to die unless NCSE co-occurs with CSE or severe medical illness such as delirium or acute complications. Mortality risk does not appear linked with a type of EEG discharge.3
Neurologic injury risk. Prolonged NCSE may cause permanent neurologic damage.4 Transient memory impairment has been reported after cessation of complex partial status epilepticus (CPSE).5 CPSE also has resulted in prolonged neurologic deficits, although concomitant medical illnesses might have contributed to the deficits.6 In one study, some patients gradually returned to baseline cognitive function after CPSE stopped, but they were not tested with standardized neuropsychological tools.7
No significant postictal memory impairment was observed on neuropsychological testing in patients with NCSE of frontal origin.8 A >5-year follow-up study of absence status epilepticus (ASE) found no evidence of long-term cognitive or behavioral decline, even though most patients had recurrent ASE.9 Similarly, no long-term sequelae were seen in patients with ASE.10,11
Triggers, neurologic symptoms
NCSE is an acute but treatable medical emergency that calls for assessing and supporting cardiac and respiratory function, monitoring vital signs, temperature reduction, and fluid replacement. Prognosis is usually good unless NCSE is associated with a serious medical illness (Box).1-11
Many metabolic, neurologic, pharmacologic, and medical abnormalities can precipitate NCSE (Table 1). The most common causes are hypoxia/anoxia, stroke, infection, subtherapeutic antiepileptic levels, alcohol and benzodiazepine intoxication/withdrawal, and metabolic abnormalities.4,7,10,12
NCSE manifests as absence status epilepticus (ASE) or complex partial status epilepticus (CPSE). A generally accepted diagnostic definition is ≥30 minutes of behavioral change from baseline, with diagnostic EEG findings.4,13 EEG is indispensable because the clinical manifestations of NCSE are predominantly behavioral, with minimal or no motor activity.
Table 1
Clinical factors that may precipitate NCSE
| Medical | Recent infection, hyperventilation, trauma, menstruation, pregnancy, renal dialysis, postoperative period, sleep deprivation |
| Metabolic | Hypoparathyroidism, renal failure, hyper/hyponatremia, hyper/hypoglycemia, hypocalcemia |
| Neurologic | Mental retardation, dementia, stroke |
| Pharmacologic | Low serum levels or abrupt discontinuation of anticonvulsants, alcohol intoxication/withdrawal, benzodiazepine withdrawal lithium and neuroleptic use, psychotropic overdose |
| Source : References 9,10,12,16 | |
ASE is reported primarily in children, although de novo cases have been described in elderly patients with no history of epilepsy.10,14
CPSE is usually associated with a history of focal epilepsy and vascular disease. CPSE has a focal onset, with subsequent secondary generalization. Onset is usually temporal in origin but also can be extratemporal.
Patients with CPSE often cycle between an “epileptic twilight state” with confusion and complete unresponsiveness with stereotyped automatisms. It can present with marked behavioral fluctuation or a change in mental status and is generally followed by a prolonged postictal state.4,7,13-15 Several NCSE cases have occurred in patients with no history of seizures.9,10,16
Historically, CPSE was reported to be less common than ASE, but this misconception was most likely caused by failure to recognize CPSE’s clinical presentation and rapid generalization on EEG.7,15
Neuropsychiatric features
Patients with NCSE may be referred for evaluation of an array of behavioral changes commonly seen in psychiatric practice. The differential diagnosis is extensive (Table 2) and includes neurologic and medical conditions often associated with catatonic syndrome.17,18
In a retrospective study, Kaplan12 assessed clinical presentations and reasons for diagnostic delay in 23 adults eventually diagnosed with NCSE. Presenting symptoms included:
- confusion, agitation, aggressive behavior
- lethargy, mutism, verbal perseveration, echolalia
- delirium, blinking, staring, chewing or picking behaviors
- tremulousness or myoclonus
- bizarre behavior (inappropriate laughing, crying, or singing)
- rigidity with waxy flexibility
- delusions, hallucinations.
A prospective study of 22 patients with NCSE found that 7 had a history of psychotic depression, schizophrenia, self-mutilation, bipolar disorder, or episodic severe aggression; 12 of 18 with ASE had a history of epilepsy, and 3 of 4 with CPSE had experienced seizures associated with cerebrovascular accident, right cerebral embolus, and thiazide-induced hyponatremia, respectively.16
Table 2
Differential diagnosis of NCSE
| Metabolic disorders | Hypo/hyperglycemia, hypercalcemia, Addison’s disease, Cushing’s disease, uremia |
| Neurologic disorders | Stroke, CNS tumors, closed head trauma, transient global amnesia, seizures, inflammatory and infectious encephalopathies |
| Psychiatric disorders | Schizophrenia, mood disorders, catatonia, malignant catatonia, somatoform disorders, conversion disorder, Asperger’s syndrome, malingering |
| Toxic disorders | Toxic encephalopathy, neuroleptic malignant syndrome, serotonin syndrome, alcohol and sedative-hypnotic withdrawal, drugs (lithium toxicity, tricyclics, baclofen, tiagabine, overdose) |
| Source: Reference 17,18 | |
Cerebrovascular disease, tumors, and trauma are the most common causes of late-life NCSE.4,19 De novo NCSE occasionally presents:
- after benzodiazepine withdrawal
- with neuroleptic, tricyclic antidepressant, or lithium treatment10,16
- with metabolic abnormalities and nonpsychotropic medications.10
Clinical symptoms
Clinical features of NCSE include cognitive changes, speech abnormalities, affective disturbances, psychosis, poor impulse control, and bizarre behaviors (Table 3). Some patients develop ictal phenomena resembling catatonia or clinical and EEG changes that mimic neuroleptic malignant syndrome (NMS).20-23
Table 3
Clinical features that raise suspicion of NCSE
| Domain | Features |
|---|---|
| Cognitive changes | Prolonged confusion, executive dysfunction, obtundation, attention/memory difficulties, lack of initiative, perseveration, stupor |
| Speech | Poverty of speech with monosyllabic answers, verbal perseveration, echolalia, palilalia, aphasia, paraphasic errors, confabulation, mutism |
| Affective | Prolonged fear, affective indifferent state with blank facial expression, hypomania, psychotic depression, inappropriate laughing and crying, anxiety states |
| Psychosis | Visual, auditory and cenesthetic hallucinations, delusions |
| Impulse control | Hostility, agitation, violence, groping, genital manipulation, picking, posturing |
| Others | Catatonic signs, autonomic disturbances |
| Source: References 5,7-9,12,15-17,20-23 | |
Among 29 patients with acute catatonic syndromes, epileptic activity was identified in 4. One patient with absence status was diagnosed with NMS during the catatonic period.26 Conversely, the commonality of clinical features has led to misdiagnosis of psychogenic catatonia as NCSE. EEG is necessary to exclude NCSE in these cases.
NMS. Yoshino et al27 described two patients taking neuroleptics who met criteria for NMS and had EEG changes consistent with NCSE. They later reported another patient with NCSE complicating NMS; the point at which NCSE developed was unknown, however, because EEG activity was not recorded at NMS onset.28 Based on NMS diagnostic criteria proposed by Caroff et al,29 these patients could have developed NCSE mimicking NMS.
EEG for diagnosis
Candidates. Because differentiating NCSE from similar conditions can be difficult, use EEG to confirm your clinical observations. No guidelines exist, but consider EEG when the patient’s history suggests NCSE. Ask the patient or family about:
- changes in mental status from baseline, especially new-onset catatonia or unexplained altered consciousness
- duration of events
- presence or absence of motor activity
- behavioral fluctuations
- presence or absence of automatisms or blinking.
EEG patterns. Table 4 summarized NCSE diagnostic criteria. NCSE shows characteristic patterns in ASE and CPSE,9,10,16,23 and EEG changes can be continuous or nearly continuous in both.
Table 4
EEG findings that support a clinical diagnosis of NCSE
| Clear-cut criteria |
|---|
| Frequent or continuous focal seizures, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution |
| Frequent or continuous generalized spike wave discharges: |
|
| Periodic lateralized epileptiform discharges (“PLEDs”) or bilateral periodic epileptiform discharges (“biPEDs") occurring in patients with coma from generalized tonic-clonic status epilepticus (subtle SE) |
| Probable (equivocal) criteria |
| Patients with acute cerebral damage who also show frequent or continuous EEG abnormalities without previous similar findings |
| Patients with epilepsy who show frequent or continuous generalized EEG abnormalities and similar interictal EEG patterns but whose clinical symptoms suggest NCSE |
| Source: References 4,12-14,17 |
31,32
In CPSE, less-synchronous epileptiform activity has been described, including rhythmical slow, rhythmic spikes, or rhythmic spike and slow waves. Two types of CPSE of frontal origin have been described:
- Type 1 presents clinically with mood disturbance and minimal confusion. EEG shows a frontal focus with a normal background.
- Type 2 presents clinically with confusion. EEG shows bilateral asymmetric frontal discharges.8
- generalized in 69%
- diffuse with focal predominance in 18%
- focal in 13%.
Distinguish between ictal and interictal EEG findings with epileptiform activity, because only the former is diagnostic for NCSE. Intravenous benzodiazepines might be necessary during EEG to verify the diagnosis.33
NCSE has developed after electroconvulsive therapy (ECT), but a cause-effect relationship is debatable. Interictal and abnormal EEG findings after ECT may be misdiagnosed as NCSE.34
Neuroimaging has limited clinical value because of the need for patient cooperation and specialized equipment.4 Head CT or MRI can exclude structural abnormalities. PET and SPECT show increased metabolism and blood flow, respectively, in NCSE. MR spectroscopy shows elevated lactate and decreased N-acetyl aspartate.
Halting ictal activity
To rapidly stop ictal activity—the main goal of treatment—recognizing and correcting precipitant factors is vital:
- Consider discontinuing medications that could lower the seizure threshold.
- Order a complete blood count, serum electrolytes, calcium, arterial-blood gas, liver and renal function tests, urine toxicology screen, and serum antiepileptic drug concentrations.
- When possible, obtain neuroimaging and EEG in the emergency room for accurate diagnosis and prompt treatment.12
Response to benzodiazepines might be transient, lasting only hours or days. For instance, diazepam’s anticonvulsant effect may last
Newer antiepileptics—such as lamotrigine, levetiracetam, or topiramate—have been used with varying results, and their role in first-line treatment of NCSE is evolving. Rarely, the antiepileptic tiagabine precipitates or worsens NCSE.4,13,14
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals (888) 667-8367. www.nmsis.org
- Carbamazepine • Tegretol, Carbatrol
- Clonazepam • Klonopin
- Diazepam • Valium
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium carbonate • Lithobid, Eskalith CR
- Lorazepam • Ativan
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Acknowledgment
Dr. Goveas was a geriatric psychiatry fellow, University of Pennsylvania, when he wrote this article in collaboration with his mentors, Drs. Caroff and Riggio.
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-35.
2. Shorvon S. Status epilepticus: Its clinical features and treatment in children and adults Cambridge, UK: Cambridge University Press, 1994.
3. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73.
4. Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord 2005;7(3):53-296.
5. Engel J, Ludwig BI, Fetell M. Prolonged partial complex status epilepticus: EEG and behavioral observations. Neurology 1978;28:863-9.
6. Krumholz A, Sung GY, Fisher RS, et al. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995;45:1499-1504.
7. Ballenger CE, King DW, Gallagher BB. Partial complex status epilepticus. Neurology 1983;33:1545-52.
8. Thomas P, Zifkin B, Migneco O, et al. Nonconvulsive status epilepticus of frontal origin. Neurology 1999;52:1174-83.
9. Guberman A, Cantu-Reyna G, Stuss D, Broughton R. Nonconvulsive generalized status epilepticus: Clinical features, neuropsychological testing, and long-term follow-up. Neurology 1986;36:1284-91.
10. Thomas P, Beaumanoir A, Genton P, et al. ‘De novo’ absence status of late onset: Report of 11 cases. Neurology 1992;42:104-10.
11. Andermann F, Robb J. Absence status: a reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177-87.
12. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37(7):643-50.
13. Riggio S. Nonconvulsive status epilepticus: Clinical features and diagnostic challenges. Psychiatr Clin N Am 2005;28(3):653-64.
14. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000;1(5):301-14.
15. Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: Thirty-two consecutive patients from a general hospital population. Epilepsia 1992;3(5):829-35.
16. Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: A prospective study in an adult general hospital. Q J Med 1987;62(238):117-26.
17. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav 2002;3(2):122-39.
18. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc, 2003:121-43.
19. Sung CY, Chu NS. Status epilepticus in elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989;80:51-6.
20. McLachlan RS, Blume WT. Isolated fear in complex partial status epilepticus. Ann Neurol 1980;8:639-41.
21. Walls MJ, Bowers TC, Dilsaver SC, Swann AC. Catatonia associated with depression secondary to complex partial epilepsy. J Clin Psychiatry 1993;54(2):73.-
22. Wells CE. Transient ictal psychosis. Arch Gen Psychiatry 1975;32:1201-3.
23. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: Diagnostic and syndromic considerations. Epilepsia 1998;39(12):1265-76.
24. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986;49:833-6.
25. Drury I, Klass DW, Westmoreland BF, Sharbrough FW. An acute syndrome with psychiatric symptoms and EEG abnormalities. Neurology 1985;35(6):911-14.
26. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry 1994;57(11):1419-22.
27. Yoshino A, Yoshimasu H, Tatsuzawa Y, et al. Nonconvulsive status epilepticus in two patients with neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):347-9.
28. Yoshino A, Yoshimasu H. Nonconvulsive status epilepticus complicating neuroleptic malignant syndrome improved by intravenous diazepam. J Clin Psychopharmacol 2000;20(3):389-90.
29. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions, 2nd ed. Washington, DC: American Psychiatric Publishing; 2003:1-44.
30. Lob H, Roger J, Soulayrol R. Les etats de mal generalizes a expression confusionelle. In: Gastaut H, Roger J, Lob H, eds. Les etats de mal epileptiques. Paris: Masson; 1967:91-109.
31. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia 1994;35(1):42-7.
32. Niedermeyer E, Fineyre F, Riley T, Uematsu S. Absence status (petit mal status) with focal characteristics. Arch Neurol 1979;36:417-21.
33. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66.
34. Povlsen UJ, Wildschiodtz G, Hogenhaven H, Bolwig TG. Nonconvulsive status epilepticus after electroconvulsive therapy. J ECT 2003;19(3):164-9.
Nonconvulsive status epilepticus (NCSE) is marked by neurobehavioral disturbances that resemble primary psychiatric disorders. Mistaken diagnosis and delayed treatment increase the risk of neurologic damage, so recognizing NCSE symptoms early is important.
To help you make a timely diagnosis, this article describes:
- neuropsychiatric manifestations of NCSE
- how to narrow the differential diagnosis by reviewing clinical symptoms and using electroencephalography (EEG)
- techniques used to rapidly halt ictal activity.
Status epilepticus (SE) is an acute medical emergency. Both forms—convulsive (CSE) and nonconvulsive (NCSE)—require early recognition and treatment. In the United States, 60 SE cases occur per 100,000 population/year, with mortality rates of 20% in adults and 38% in the elderly.1,2
Mortality risk. Data suggest patients with NCSE are unlikely to die unless NCSE co-occurs with CSE or severe medical illness such as delirium or acute complications. Mortality risk does not appear linked with a type of EEG discharge.3
Neurologic injury risk. Prolonged NCSE may cause permanent neurologic damage.4 Transient memory impairment has been reported after cessation of complex partial status epilepticus (CPSE).5 CPSE also has resulted in prolonged neurologic deficits, although concomitant medical illnesses might have contributed to the deficits.6 In one study, some patients gradually returned to baseline cognitive function after CPSE stopped, but they were not tested with standardized neuropsychological tools.7
No significant postictal memory impairment was observed on neuropsychological testing in patients with NCSE of frontal origin.8 A >5-year follow-up study of absence status epilepticus (ASE) found no evidence of long-term cognitive or behavioral decline, even though most patients had recurrent ASE.9 Similarly, no long-term sequelae were seen in patients with ASE.10,11
Triggers, neurologic symptoms
NCSE is an acute but treatable medical emergency that calls for assessing and supporting cardiac and respiratory function, monitoring vital signs, temperature reduction, and fluid replacement. Prognosis is usually good unless NCSE is associated with a serious medical illness (Box).1-11
Many metabolic, neurologic, pharmacologic, and medical abnormalities can precipitate NCSE (Table 1). The most common causes are hypoxia/anoxia, stroke, infection, subtherapeutic antiepileptic levels, alcohol and benzodiazepine intoxication/withdrawal, and metabolic abnormalities.4,7,10,12
NCSE manifests as absence status epilepticus (ASE) or complex partial status epilepticus (CPSE). A generally accepted diagnostic definition is ≥30 minutes of behavioral change from baseline, with diagnostic EEG findings.4,13 EEG is indispensable because the clinical manifestations of NCSE are predominantly behavioral, with minimal or no motor activity.
Table 1
Clinical factors that may precipitate NCSE
| Medical | Recent infection, hyperventilation, trauma, menstruation, pregnancy, renal dialysis, postoperative period, sleep deprivation |
| Metabolic | Hypoparathyroidism, renal failure, hyper/hyponatremia, hyper/hypoglycemia, hypocalcemia |
| Neurologic | Mental retardation, dementia, stroke |
| Pharmacologic | Low serum levels or abrupt discontinuation of anticonvulsants, alcohol intoxication/withdrawal, benzodiazepine withdrawal lithium and neuroleptic use, psychotropic overdose |
| Source : References 9,10,12,16 | |
ASE is reported primarily in children, although de novo cases have been described in elderly patients with no history of epilepsy.10,14
CPSE is usually associated with a history of focal epilepsy and vascular disease. CPSE has a focal onset, with subsequent secondary generalization. Onset is usually temporal in origin but also can be extratemporal.
Patients with CPSE often cycle between an “epileptic twilight state” with confusion and complete unresponsiveness with stereotyped automatisms. It can present with marked behavioral fluctuation or a change in mental status and is generally followed by a prolonged postictal state.4,7,13-15 Several NCSE cases have occurred in patients with no history of seizures.9,10,16
Historically, CPSE was reported to be less common than ASE, but this misconception was most likely caused by failure to recognize CPSE’s clinical presentation and rapid generalization on EEG.7,15
Neuropsychiatric features
Patients with NCSE may be referred for evaluation of an array of behavioral changes commonly seen in psychiatric practice. The differential diagnosis is extensive (Table 2) and includes neurologic and medical conditions often associated with catatonic syndrome.17,18
In a retrospective study, Kaplan12 assessed clinical presentations and reasons for diagnostic delay in 23 adults eventually diagnosed with NCSE. Presenting symptoms included:
- confusion, agitation, aggressive behavior
- lethargy, mutism, verbal perseveration, echolalia
- delirium, blinking, staring, chewing or picking behaviors
- tremulousness or myoclonus
- bizarre behavior (inappropriate laughing, crying, or singing)
- rigidity with waxy flexibility
- delusions, hallucinations.
A prospective study of 22 patients with NCSE found that 7 had a history of psychotic depression, schizophrenia, self-mutilation, bipolar disorder, or episodic severe aggression; 12 of 18 with ASE had a history of epilepsy, and 3 of 4 with CPSE had experienced seizures associated with cerebrovascular accident, right cerebral embolus, and thiazide-induced hyponatremia, respectively.16
Table 2
Differential diagnosis of NCSE
| Metabolic disorders | Hypo/hyperglycemia, hypercalcemia, Addison’s disease, Cushing’s disease, uremia |
| Neurologic disorders | Stroke, CNS tumors, closed head trauma, transient global amnesia, seizures, inflammatory and infectious encephalopathies |
| Psychiatric disorders | Schizophrenia, mood disorders, catatonia, malignant catatonia, somatoform disorders, conversion disorder, Asperger’s syndrome, malingering |
| Toxic disorders | Toxic encephalopathy, neuroleptic malignant syndrome, serotonin syndrome, alcohol and sedative-hypnotic withdrawal, drugs (lithium toxicity, tricyclics, baclofen, tiagabine, overdose) |
| Source: Reference 17,18 | |
Cerebrovascular disease, tumors, and trauma are the most common causes of late-life NCSE.4,19 De novo NCSE occasionally presents:
- after benzodiazepine withdrawal
- with neuroleptic, tricyclic antidepressant, or lithium treatment10,16
- with metabolic abnormalities and nonpsychotropic medications.10
Clinical symptoms
Clinical features of NCSE include cognitive changes, speech abnormalities, affective disturbances, psychosis, poor impulse control, and bizarre behaviors (Table 3). Some patients develop ictal phenomena resembling catatonia or clinical and EEG changes that mimic neuroleptic malignant syndrome (NMS).20-23
Table 3
Clinical features that raise suspicion of NCSE
| Domain | Features |
|---|---|
| Cognitive changes | Prolonged confusion, executive dysfunction, obtundation, attention/memory difficulties, lack of initiative, perseveration, stupor |
| Speech | Poverty of speech with monosyllabic answers, verbal perseveration, echolalia, palilalia, aphasia, paraphasic errors, confabulation, mutism |
| Affective | Prolonged fear, affective indifferent state with blank facial expression, hypomania, psychotic depression, inappropriate laughing and crying, anxiety states |
| Psychosis | Visual, auditory and cenesthetic hallucinations, delusions |
| Impulse control | Hostility, agitation, violence, groping, genital manipulation, picking, posturing |
| Others | Catatonic signs, autonomic disturbances |
| Source: References 5,7-9,12,15-17,20-23 | |
Among 29 patients with acute catatonic syndromes, epileptic activity was identified in 4. One patient with absence status was diagnosed with NMS during the catatonic period.26 Conversely, the commonality of clinical features has led to misdiagnosis of psychogenic catatonia as NCSE. EEG is necessary to exclude NCSE in these cases.
NMS. Yoshino et al27 described two patients taking neuroleptics who met criteria for NMS and had EEG changes consistent with NCSE. They later reported another patient with NCSE complicating NMS; the point at which NCSE developed was unknown, however, because EEG activity was not recorded at NMS onset.28 Based on NMS diagnostic criteria proposed by Caroff et al,29 these patients could have developed NCSE mimicking NMS.
EEG for diagnosis
Candidates. Because differentiating NCSE from similar conditions can be difficult, use EEG to confirm your clinical observations. No guidelines exist, but consider EEG when the patient’s history suggests NCSE. Ask the patient or family about:
- changes in mental status from baseline, especially new-onset catatonia or unexplained altered consciousness
- duration of events
- presence or absence of motor activity
- behavioral fluctuations
- presence or absence of automatisms or blinking.
EEG patterns. Table 4 summarized NCSE diagnostic criteria. NCSE shows characteristic patterns in ASE and CPSE,9,10,16,23 and EEG changes can be continuous or nearly continuous in both.
Table 4
EEG findings that support a clinical diagnosis of NCSE
| Clear-cut criteria |
|---|
| Frequent or continuous focal seizures, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution |
| Frequent or continuous generalized spike wave discharges: |
|
| Periodic lateralized epileptiform discharges (“PLEDs”) or bilateral periodic epileptiform discharges (“biPEDs") occurring in patients with coma from generalized tonic-clonic status epilepticus (subtle SE) |
| Probable (equivocal) criteria |
| Patients with acute cerebral damage who also show frequent or continuous EEG abnormalities without previous similar findings |
| Patients with epilepsy who show frequent or continuous generalized EEG abnormalities and similar interictal EEG patterns but whose clinical symptoms suggest NCSE |
| Source: References 4,12-14,17 |
31,32
In CPSE, less-synchronous epileptiform activity has been described, including rhythmical slow, rhythmic spikes, or rhythmic spike and slow waves. Two types of CPSE of frontal origin have been described:
- Type 1 presents clinically with mood disturbance and minimal confusion. EEG shows a frontal focus with a normal background.
- Type 2 presents clinically with confusion. EEG shows bilateral asymmetric frontal discharges.8
- generalized in 69%
- diffuse with focal predominance in 18%
- focal in 13%.
Distinguish between ictal and interictal EEG findings with epileptiform activity, because only the former is diagnostic for NCSE. Intravenous benzodiazepines might be necessary during EEG to verify the diagnosis.33
NCSE has developed after electroconvulsive therapy (ECT), but a cause-effect relationship is debatable. Interictal and abnormal EEG findings after ECT may be misdiagnosed as NCSE.34
Neuroimaging has limited clinical value because of the need for patient cooperation and specialized equipment.4 Head CT or MRI can exclude structural abnormalities. PET and SPECT show increased metabolism and blood flow, respectively, in NCSE. MR spectroscopy shows elevated lactate and decreased N-acetyl aspartate.
Halting ictal activity
To rapidly stop ictal activity—the main goal of treatment—recognizing and correcting precipitant factors is vital:
- Consider discontinuing medications that could lower the seizure threshold.
- Order a complete blood count, serum electrolytes, calcium, arterial-blood gas, liver and renal function tests, urine toxicology screen, and serum antiepileptic drug concentrations.
- When possible, obtain neuroimaging and EEG in the emergency room for accurate diagnosis and prompt treatment.12
Response to benzodiazepines might be transient, lasting only hours or days. For instance, diazepam’s anticonvulsant effect may last
Newer antiepileptics—such as lamotrigine, levetiracetam, or topiramate—have been used with varying results, and their role in first-line treatment of NCSE is evolving. Rarely, the antiepileptic tiagabine precipitates or worsens NCSE.4,13,14
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals (888) 667-8367. www.nmsis.org
- Carbamazepine • Tegretol, Carbatrol
- Clonazepam • Klonopin
- Diazepam • Valium
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium carbonate • Lithobid, Eskalith CR
- Lorazepam • Ativan
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Acknowledgment
Dr. Goveas was a geriatric psychiatry fellow, University of Pennsylvania, when he wrote this article in collaboration with his mentors, Drs. Caroff and Riggio.
Nonconvulsive status epilepticus (NCSE) is marked by neurobehavioral disturbances that resemble primary psychiatric disorders. Mistaken diagnosis and delayed treatment increase the risk of neurologic damage, so recognizing NCSE symptoms early is important.
To help you make a timely diagnosis, this article describes:
- neuropsychiatric manifestations of NCSE
- how to narrow the differential diagnosis by reviewing clinical symptoms and using electroencephalography (EEG)
- techniques used to rapidly halt ictal activity.
Status epilepticus (SE) is an acute medical emergency. Both forms—convulsive (CSE) and nonconvulsive (NCSE)—require early recognition and treatment. In the United States, 60 SE cases occur per 100,000 population/year, with mortality rates of 20% in adults and 38% in the elderly.1,2
Mortality risk. Data suggest patients with NCSE are unlikely to die unless NCSE co-occurs with CSE or severe medical illness such as delirium or acute complications. Mortality risk does not appear linked with a type of EEG discharge.3
Neurologic injury risk. Prolonged NCSE may cause permanent neurologic damage.4 Transient memory impairment has been reported after cessation of complex partial status epilepticus (CPSE).5 CPSE also has resulted in prolonged neurologic deficits, although concomitant medical illnesses might have contributed to the deficits.6 In one study, some patients gradually returned to baseline cognitive function after CPSE stopped, but they were not tested with standardized neuropsychological tools.7
No significant postictal memory impairment was observed on neuropsychological testing in patients with NCSE of frontal origin.8 A >5-year follow-up study of absence status epilepticus (ASE) found no evidence of long-term cognitive or behavioral decline, even though most patients had recurrent ASE.9 Similarly, no long-term sequelae were seen in patients with ASE.10,11
Triggers, neurologic symptoms
NCSE is an acute but treatable medical emergency that calls for assessing and supporting cardiac and respiratory function, monitoring vital signs, temperature reduction, and fluid replacement. Prognosis is usually good unless NCSE is associated with a serious medical illness (Box).1-11
Many metabolic, neurologic, pharmacologic, and medical abnormalities can precipitate NCSE (Table 1). The most common causes are hypoxia/anoxia, stroke, infection, subtherapeutic antiepileptic levels, alcohol and benzodiazepine intoxication/withdrawal, and metabolic abnormalities.4,7,10,12
NCSE manifests as absence status epilepticus (ASE) or complex partial status epilepticus (CPSE). A generally accepted diagnostic definition is ≥30 minutes of behavioral change from baseline, with diagnostic EEG findings.4,13 EEG is indispensable because the clinical manifestations of NCSE are predominantly behavioral, with minimal or no motor activity.
Table 1
Clinical factors that may precipitate NCSE
| Medical | Recent infection, hyperventilation, trauma, menstruation, pregnancy, renal dialysis, postoperative period, sleep deprivation |
| Metabolic | Hypoparathyroidism, renal failure, hyper/hyponatremia, hyper/hypoglycemia, hypocalcemia |
| Neurologic | Mental retardation, dementia, stroke |
| Pharmacologic | Low serum levels or abrupt discontinuation of anticonvulsants, alcohol intoxication/withdrawal, benzodiazepine withdrawal lithium and neuroleptic use, psychotropic overdose |
| Source : References 9,10,12,16 | |
ASE is reported primarily in children, although de novo cases have been described in elderly patients with no history of epilepsy.10,14
CPSE is usually associated with a history of focal epilepsy and vascular disease. CPSE has a focal onset, with subsequent secondary generalization. Onset is usually temporal in origin but also can be extratemporal.
Patients with CPSE often cycle between an “epileptic twilight state” with confusion and complete unresponsiveness with stereotyped automatisms. It can present with marked behavioral fluctuation or a change in mental status and is generally followed by a prolonged postictal state.4,7,13-15 Several NCSE cases have occurred in patients with no history of seizures.9,10,16
Historically, CPSE was reported to be less common than ASE, but this misconception was most likely caused by failure to recognize CPSE’s clinical presentation and rapid generalization on EEG.7,15
Neuropsychiatric features
Patients with NCSE may be referred for evaluation of an array of behavioral changes commonly seen in psychiatric practice. The differential diagnosis is extensive (Table 2) and includes neurologic and medical conditions often associated with catatonic syndrome.17,18
In a retrospective study, Kaplan12 assessed clinical presentations and reasons for diagnostic delay in 23 adults eventually diagnosed with NCSE. Presenting symptoms included:
- confusion, agitation, aggressive behavior
- lethargy, mutism, verbal perseveration, echolalia
- delirium, blinking, staring, chewing or picking behaviors
- tremulousness or myoclonus
- bizarre behavior (inappropriate laughing, crying, or singing)
- rigidity with waxy flexibility
- delusions, hallucinations.
A prospective study of 22 patients with NCSE found that 7 had a history of psychotic depression, schizophrenia, self-mutilation, bipolar disorder, or episodic severe aggression; 12 of 18 with ASE had a history of epilepsy, and 3 of 4 with CPSE had experienced seizures associated with cerebrovascular accident, right cerebral embolus, and thiazide-induced hyponatremia, respectively.16
Table 2
Differential diagnosis of NCSE
| Metabolic disorders | Hypo/hyperglycemia, hypercalcemia, Addison’s disease, Cushing’s disease, uremia |
| Neurologic disorders | Stroke, CNS tumors, closed head trauma, transient global amnesia, seizures, inflammatory and infectious encephalopathies |
| Psychiatric disorders | Schizophrenia, mood disorders, catatonia, malignant catatonia, somatoform disorders, conversion disorder, Asperger’s syndrome, malingering |
| Toxic disorders | Toxic encephalopathy, neuroleptic malignant syndrome, serotonin syndrome, alcohol and sedative-hypnotic withdrawal, drugs (lithium toxicity, tricyclics, baclofen, tiagabine, overdose) |
| Source: Reference 17,18 | |
Cerebrovascular disease, tumors, and trauma are the most common causes of late-life NCSE.4,19 De novo NCSE occasionally presents:
- after benzodiazepine withdrawal
- with neuroleptic, tricyclic antidepressant, or lithium treatment10,16
- with metabolic abnormalities and nonpsychotropic medications.10
Clinical symptoms
Clinical features of NCSE include cognitive changes, speech abnormalities, affective disturbances, psychosis, poor impulse control, and bizarre behaviors (Table 3). Some patients develop ictal phenomena resembling catatonia or clinical and EEG changes that mimic neuroleptic malignant syndrome (NMS).20-23
Table 3
Clinical features that raise suspicion of NCSE
| Domain | Features |
|---|---|
| Cognitive changes | Prolonged confusion, executive dysfunction, obtundation, attention/memory difficulties, lack of initiative, perseveration, stupor |
| Speech | Poverty of speech with monosyllabic answers, verbal perseveration, echolalia, palilalia, aphasia, paraphasic errors, confabulation, mutism |
| Affective | Prolonged fear, affective indifferent state with blank facial expression, hypomania, psychotic depression, inappropriate laughing and crying, anxiety states |
| Psychosis | Visual, auditory and cenesthetic hallucinations, delusions |
| Impulse control | Hostility, agitation, violence, groping, genital manipulation, picking, posturing |
| Others | Catatonic signs, autonomic disturbances |
| Source: References 5,7-9,12,15-17,20-23 | |
Among 29 patients with acute catatonic syndromes, epileptic activity was identified in 4. One patient with absence status was diagnosed with NMS during the catatonic period.26 Conversely, the commonality of clinical features has led to misdiagnosis of psychogenic catatonia as NCSE. EEG is necessary to exclude NCSE in these cases.
NMS. Yoshino et al27 described two patients taking neuroleptics who met criteria for NMS and had EEG changes consistent with NCSE. They later reported another patient with NCSE complicating NMS; the point at which NCSE developed was unknown, however, because EEG activity was not recorded at NMS onset.28 Based on NMS diagnostic criteria proposed by Caroff et al,29 these patients could have developed NCSE mimicking NMS.
EEG for diagnosis
Candidates. Because differentiating NCSE from similar conditions can be difficult, use EEG to confirm your clinical observations. No guidelines exist, but consider EEG when the patient’s history suggests NCSE. Ask the patient or family about:
- changes in mental status from baseline, especially new-onset catatonia or unexplained altered consciousness
- duration of events
- presence or absence of motor activity
- behavioral fluctuations
- presence or absence of automatisms or blinking.
EEG patterns. Table 4 summarized NCSE diagnostic criteria. NCSE shows characteristic patterns in ASE and CPSE,9,10,16,23 and EEG changes can be continuous or nearly continuous in both.
Table 4
EEG findings that support a clinical diagnosis of NCSE
| Clear-cut criteria |
|---|
| Frequent or continuous focal seizures, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution |
| Frequent or continuous generalized spike wave discharges: |
|
| Periodic lateralized epileptiform discharges (“PLEDs”) or bilateral periodic epileptiform discharges (“biPEDs") occurring in patients with coma from generalized tonic-clonic status epilepticus (subtle SE) |
| Probable (equivocal) criteria |
| Patients with acute cerebral damage who also show frequent or continuous EEG abnormalities without previous similar findings |
| Patients with epilepsy who show frequent or continuous generalized EEG abnormalities and similar interictal EEG patterns but whose clinical symptoms suggest NCSE |
| Source: References 4,12-14,17 |
31,32
In CPSE, less-synchronous epileptiform activity has been described, including rhythmical slow, rhythmic spikes, or rhythmic spike and slow waves. Two types of CPSE of frontal origin have been described:
- Type 1 presents clinically with mood disturbance and minimal confusion. EEG shows a frontal focus with a normal background.
- Type 2 presents clinically with confusion. EEG shows bilateral asymmetric frontal discharges.8
- generalized in 69%
- diffuse with focal predominance in 18%
- focal in 13%.
Distinguish between ictal and interictal EEG findings with epileptiform activity, because only the former is diagnostic for NCSE. Intravenous benzodiazepines might be necessary during EEG to verify the diagnosis.33
NCSE has developed after electroconvulsive therapy (ECT), but a cause-effect relationship is debatable. Interictal and abnormal EEG findings after ECT may be misdiagnosed as NCSE.34
Neuroimaging has limited clinical value because of the need for patient cooperation and specialized equipment.4 Head CT or MRI can exclude structural abnormalities. PET and SPECT show increased metabolism and blood flow, respectively, in NCSE. MR spectroscopy shows elevated lactate and decreased N-acetyl aspartate.
Halting ictal activity
To rapidly stop ictal activity—the main goal of treatment—recognizing and correcting precipitant factors is vital:
- Consider discontinuing medications that could lower the seizure threshold.
- Order a complete blood count, serum electrolytes, calcium, arterial-blood gas, liver and renal function tests, urine toxicology screen, and serum antiepileptic drug concentrations.
- When possible, obtain neuroimaging and EEG in the emergency room for accurate diagnosis and prompt treatment.12
Response to benzodiazepines might be transient, lasting only hours or days. For instance, diazepam’s anticonvulsant effect may last
Newer antiepileptics—such as lamotrigine, levetiracetam, or topiramate—have been used with varying results, and their role in first-line treatment of NCSE is evolving. Rarely, the antiepileptic tiagabine precipitates or worsens NCSE.4,13,14
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals (888) 667-8367. www.nmsis.org
- Carbamazepine • Tegretol, Carbatrol
- Clonazepam • Klonopin
- Diazepam • Valium
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium carbonate • Lithobid, Eskalith CR
- Lorazepam • Ativan
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Acknowledgment
Dr. Goveas was a geriatric psychiatry fellow, University of Pennsylvania, when he wrote this article in collaboration with his mentors, Drs. Caroff and Riggio.
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-35.
2. Shorvon S. Status epilepticus: Its clinical features and treatment in children and adults Cambridge, UK: Cambridge University Press, 1994.
3. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73.
4. Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord 2005;7(3):53-296.
5. Engel J, Ludwig BI, Fetell M. Prolonged partial complex status epilepticus: EEG and behavioral observations. Neurology 1978;28:863-9.
6. Krumholz A, Sung GY, Fisher RS, et al. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995;45:1499-1504.
7. Ballenger CE, King DW, Gallagher BB. Partial complex status epilepticus. Neurology 1983;33:1545-52.
8. Thomas P, Zifkin B, Migneco O, et al. Nonconvulsive status epilepticus of frontal origin. Neurology 1999;52:1174-83.
9. Guberman A, Cantu-Reyna G, Stuss D, Broughton R. Nonconvulsive generalized status epilepticus: Clinical features, neuropsychological testing, and long-term follow-up. Neurology 1986;36:1284-91.
10. Thomas P, Beaumanoir A, Genton P, et al. ‘De novo’ absence status of late onset: Report of 11 cases. Neurology 1992;42:104-10.
11. Andermann F, Robb J. Absence status: a reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177-87.
12. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37(7):643-50.
13. Riggio S. Nonconvulsive status epilepticus: Clinical features and diagnostic challenges. Psychiatr Clin N Am 2005;28(3):653-64.
14. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000;1(5):301-14.
15. Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: Thirty-two consecutive patients from a general hospital population. Epilepsia 1992;3(5):829-35.
16. Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: A prospective study in an adult general hospital. Q J Med 1987;62(238):117-26.
17. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav 2002;3(2):122-39.
18. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc, 2003:121-43.
19. Sung CY, Chu NS. Status epilepticus in elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989;80:51-6.
20. McLachlan RS, Blume WT. Isolated fear in complex partial status epilepticus. Ann Neurol 1980;8:639-41.
21. Walls MJ, Bowers TC, Dilsaver SC, Swann AC. Catatonia associated with depression secondary to complex partial epilepsy. J Clin Psychiatry 1993;54(2):73.-
22. Wells CE. Transient ictal psychosis. Arch Gen Psychiatry 1975;32:1201-3.
23. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: Diagnostic and syndromic considerations. Epilepsia 1998;39(12):1265-76.
24. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986;49:833-6.
25. Drury I, Klass DW, Westmoreland BF, Sharbrough FW. An acute syndrome with psychiatric symptoms and EEG abnormalities. Neurology 1985;35(6):911-14.
26. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry 1994;57(11):1419-22.
27. Yoshino A, Yoshimasu H, Tatsuzawa Y, et al. Nonconvulsive status epilepticus in two patients with neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):347-9.
28. Yoshino A, Yoshimasu H. Nonconvulsive status epilepticus complicating neuroleptic malignant syndrome improved by intravenous diazepam. J Clin Psychopharmacol 2000;20(3):389-90.
29. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions, 2nd ed. Washington, DC: American Psychiatric Publishing; 2003:1-44.
30. Lob H, Roger J, Soulayrol R. Les etats de mal generalizes a expression confusionelle. In: Gastaut H, Roger J, Lob H, eds. Les etats de mal epileptiques. Paris: Masson; 1967:91-109.
31. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia 1994;35(1):42-7.
32. Niedermeyer E, Fineyre F, Riley T, Uematsu S. Absence status (petit mal status) with focal characteristics. Arch Neurol 1979;36:417-21.
33. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66.
34. Povlsen UJ, Wildschiodtz G, Hogenhaven H, Bolwig TG. Nonconvulsive status epilepticus after electroconvulsive therapy. J ECT 2003;19(3):164-9.
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-35.
2. Shorvon S. Status epilepticus: Its clinical features and treatment in children and adults Cambridge, UK: Cambridge University Press, 1994.
3. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73.
4. Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord 2005;7(3):53-296.
5. Engel J, Ludwig BI, Fetell M. Prolonged partial complex status epilepticus: EEG and behavioral observations. Neurology 1978;28:863-9.
6. Krumholz A, Sung GY, Fisher RS, et al. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995;45:1499-1504.
7. Ballenger CE, King DW, Gallagher BB. Partial complex status epilepticus. Neurology 1983;33:1545-52.
8. Thomas P, Zifkin B, Migneco O, et al. Nonconvulsive status epilepticus of frontal origin. Neurology 1999;52:1174-83.
9. Guberman A, Cantu-Reyna G, Stuss D, Broughton R. Nonconvulsive generalized status epilepticus: Clinical features, neuropsychological testing, and long-term follow-up. Neurology 1986;36:1284-91.
10. Thomas P, Beaumanoir A, Genton P, et al. ‘De novo’ absence status of late onset: Report of 11 cases. Neurology 1992;42:104-10.
11. Andermann F, Robb J. Absence status: a reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177-87.
12. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37(7):643-50.
13. Riggio S. Nonconvulsive status epilepticus: Clinical features and diagnostic challenges. Psychiatr Clin N Am 2005;28(3):653-64.
14. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000;1(5):301-14.
15. Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: Thirty-two consecutive patients from a general hospital population. Epilepsia 1992;3(5):829-35.
16. Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: A prospective study in an adult general hospital. Q J Med 1987;62(238):117-26.
17. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav 2002;3(2):122-39.
18. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc, 2003:121-43.
19. Sung CY, Chu NS. Status epilepticus in elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989;80:51-6.
20. McLachlan RS, Blume WT. Isolated fear in complex partial status epilepticus. Ann Neurol 1980;8:639-41.
21. Walls MJ, Bowers TC, Dilsaver SC, Swann AC. Catatonia associated with depression secondary to complex partial epilepsy. J Clin Psychiatry 1993;54(2):73.-
22. Wells CE. Transient ictal psychosis. Arch Gen Psychiatry 1975;32:1201-3.
23. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: Diagnostic and syndromic considerations. Epilepsia 1998;39(12):1265-76.
24. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986;49:833-6.
25. Drury I, Klass DW, Westmoreland BF, Sharbrough FW. An acute syndrome with psychiatric symptoms and EEG abnormalities. Neurology 1985;35(6):911-14.
26. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry 1994;57(11):1419-22.
27. Yoshino A, Yoshimasu H, Tatsuzawa Y, et al. Nonconvulsive status epilepticus in two patients with neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):347-9.
28. Yoshino A, Yoshimasu H. Nonconvulsive status epilepticus complicating neuroleptic malignant syndrome improved by intravenous diazepam. J Clin Psychopharmacol 2000;20(3):389-90.
29. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A, eds. Neuroleptic malignant syndrome and related conditions, 2nd ed. Washington, DC: American Psychiatric Publishing; 2003:1-44.
30. Lob H, Roger J, Soulayrol R. Les etats de mal generalizes a expression confusionelle. In: Gastaut H, Roger J, Lob H, eds. Les etats de mal epileptiques. Paris: Masson; 1967:91-109.
31. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia 1994;35(1):42-7.
32. Niedermeyer E, Fineyre F, Riley T, Uematsu S. Absence status (petit mal status) with focal characteristics. Arch Neurol 1979;36:417-21.
33. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66.
34. Povlsen UJ, Wildschiodtz G, Hogenhaven H, Bolwig TG. Nonconvulsive status epilepticus after electroconvulsive therapy. J ECT 2003;19(3):164-9.
Sexual dysfunction: What’s love got to do with it?
Concepts of love and sexual desire lurk around clinical discussions of sexual dysfunction. Love is frequently dismissed as hopelessly unscientific, whereas desire is simplified as if it were a thing called libido. Decreased libido per se tells us little about a patient’s sexual complaints; the key is to differentiate between:
- those with sexual drive but no motivation for their partners
- those with no driveFemale sexual dysfunction”).
Psychiatrists avoid talking about love; it has too many meanings and nuances, too many avenues of defeat, and is too abstract. All you have to say to a patient is, “Tell me about your marriage,” and listen closely as he or she comes to grip with love’s complexity.
This article’s aim is to help you counsel patients more effectively about relationship and sexual problems by exploring two questions: “What is love?” and “What is sexual desire?”
What is love?
Mrs. C, age 41, is being treated for depression and wonders why she has lost desire for her husband. The antidepressant she is taking improves her mood and diminishes her considerable anxiety but makes her feel sexually dead. “My husband doesn’t mind how I feel, as long as he can have sex,” she says.
After adjusting her medication, you explore other problems that might be contributing to her sexual dysfunction. She expresses uncertainty about what love is. Though faithful and committed to her husband, she has stopped enjoying the way he interacts with her, their two grade-school children, her family, and friends.
Love is the usual context within which sexual activities are viewed. Among adults, unhappiness in love predisposes to sexual concerns, and sexual concerns interfere with loving and being loved.
Our patients’ expectations for feeling and receiving love and experiencing satisfying sex are disappointed through a myriad of avenues. Clinicians may overlook it, but demoralization about love can precede the onset of anxiety, panic attacks, and lingering depression.2 Sexual love is expected to begin with connecting with a partner and to evolve for 65 or more years. Most individuals harbor the secret that they are not certain what love is (Table 1) or are surprised by their lack of words to explain it.
1. Love as transient emotion. The assumption that love is a feeling leads too many people waiting to experience the pure feeling. But unlike sadness, fear, anger, or shame, love does not indicate a discrete feeling. Saying, “I love you,” connotes at least two feelings: pleasure and interest.
- Pleasure begins with pleasantness and moves up through delight to exhilaration.
- Interest ranges from mild curiosity to preoccupying fascination.
Most events simultaneously provoke more than one feeling. Discovering that your beloved wants to marry you usually brings about at least happiness, pride, gratitude, and awe. Even if only one feeling is produced, our attitude towards that feeling complicates the experience. When a child is taught that feeling envy is wrong, for example, his experience of it evokes anxiety (from the guilt) and shame (if someone is watching).
After the family, culture, and the person have worked on a simple feeling, it becomes a layered complexity called an emotion. Love, the emotion, is quickly layered with attitudes (which are the product of feelings and defenses against them) based on the person’s sense of safety stemming from earlier attachments.3 When someone says “I love you,” he or she knows the motive for saying it and hopes for a particular response from the listener.
Sexual desire is an ingredient of love’s emotional complexity. Because “I love you” can create sexual arousal in the listener, the speaker can use the phrase when his or her primary pleasure and interest in the person is the anticipation of sex.
Meanings and motives for expressing love change all the time. When someone tells us “I love you,” we have to discern both meaning and motive. Love’s emotions and their expression to another person are always complicated by past, present, and future considerations.
2. Love as an ambition. Love is so intensely celebrated in every culture that few people grow up without longing to realize it. Table 2 shows one version of the ambition to love and be loved.4 Many clinical declarations of love for a partner signify that the person has not yet given up on this ambition.
3. Love as an arrangement. All adult sexual relationships are quid pro quo exchanges of hopes, expectations, and assets. During courtship, both people are preoccupied with answering the question: “What will this person bring to my life?”
The question has many dimensions: social, economic, aesthetic, recreational, sexual, medical, time-to-death, and more. In their first romantic relationships, people generally prefer not to think in these terms. Their embarrassment dissipates with experience.
This ordinary process can be more clearly perceived after a relationship ends by breakup, divorce, or death and the person begins anew with someone. The person then can deliberately weigh the factors that will determine his or her involvement. When an arrangement is worked out, each person perceives what has been offered by the partner. Of course, perceptions vary in accuracy.
Anticipating making a deal can be very exciting, and once the deal is formally accepted, people often feel a celebratory degree of pleasure, interest, and sexual desire. They think that life is good. In cultures where parents make the deal, the couple courts in the hope that they will fall in love by early marriage.
4. Love is an attachment. Love also means the presence of a bond or attachment. People weave their psyches together and begin to feel a hunger to be with the other person. They think of themselves as belonging with and to the other.
Sexual activities—particularly those that lead to orgasm—facilitate attachment, but the bonds within each partner’s mind do not necessarily develop at the same time or solidify at the same rate. Thus, some people are unable to answer, “I love you, too,” when the partner reveals feelings that are summarized as love.
5. Love as a moral commitment. The rituals that sanctify marriage emphasize clearly that love is a commitment for couples to try to realize the grand idealized ambition (see “Love as an ambition”). The rituals are public promises to honor and cherish each other through all of life’s vicissitudes.
This love as moral commitment instantly restructures life by generating a new set of obligations. Many hostile, disappointed, and seemingly asexual spouses who have not felt pleasure and interest in a partner for a long time will tell their doctors they love the partner. They mean they remain bound by their moral commitment.
6. Love as a mental struggle. Love’s original emotions are stimulated by an idealized version of the partner. This image is internalized early in the relationship. As time passes, discovering our partner’s limitations gradually attenuates our idealization. We think of our earlier appraisals as naïve. Even so, disappointment does not quickly cancel our commitment because of our:
- ambition to love
- obligation to live through bad moments
- ability to love the idealized version of the partner
- moral commitments to raising our children.
The moral commitment to love can sustain people for a lifetime, despite grave disappointments. It also explains the persistent guilt many feel as they contemplate extramarital affairs, divorce, and the agonizing dilemma between their commitment to live with their children and their wish to be free of unhappiness with their partner.
“I love my partner, but I am not in love with him/her,” means, “although I’m still committed, I have lost my ability to idealize my partner.”
7. Love as a force of nature. Love is a force in nature that creates a unity from two individuals. It casts our fates together, organizes reproduction, and remains vital to adult growth and development and to the maturation of children. This love is a backbone that supports the sexual and non-sexual processes of our lives.5
Among older couples, “I love my partner but I am no longer in love with her/him” may mean, “We have shared so much of our lives that my partner is an inextricable part of me. I could never be free of my partner, even though most of the pleasure is gone.”
8. Love as an illusion. We create love for our partner by internal private processes, maintain it by prudent diplomatic dishonesties, and can lose it without the partner’s knowing. To remain in an intimate relationship, the processes of love require defensive distortions of a person’s feelings, thoughts, and perceptions.
As individuals gain experience, many look back and see that their assumptions about love were self-serving illusions. When entire relationships are dismissed with “what was I thinking?” the person usually means that now I can perceive that I created illusions so as not to admit to the depth of my disappointment with my partner.
9. Love as a stop sign. When a person says, “I love you,” the listener is challenged to discern its meaning. The emotions and motives behind the sentence can be very difficult to accurately perceive. Some love relationships, after all, are deceptions.
At any particular moment, we may know what we mean by “I love you” and why we are saying it. We may not be willing, however, to have our motives, meanings, and emotions fully known by the listener. In fact, the motive for saying “I love you” is often to obscure the view:
- Lover A: I love you.
- Lover B: Why do you love me?
- Lover A: I don’t know, I just do.
- Doctor: Why do you put up with this behavior from your spouse?
- Patient: Because I love him.
- Doctor: What does that mean?
- Patient: I don’t know.
What is the meaning of ‘I love you’? Love is…
| A transient emotion |
| An ambition |
| An arrangement |
| An attachment |
| A moral commitment |
| A mental struggle |
| A force of nature |
| An illusion |
| A stop sign |
Love as ambition: 7 ideals for loving relationships
| Mutual respect |
| Behavioral reliability |
| Enjoyment of one another |
| Sexual fidelity |
| Psychological intimacy |
| Sexual pleasure |
| A comfortable balance of individuality and couplehood |
| Source: Reference 4 |
What is sexual desire?
Sexual desire—at any given moment—is the sum of biological, psychological, interpersonal, and cultural forces that incline us toward and away from sexual behavior.6 Understanding desire can help you:
- ask patients insightful questions about their relationship concerns
- formulate a hypothesis to explain how drive, motivation, and values contribute to a patient’s sexual dysfunction.
Motivation is the degree of willingness an individual has to enter into sexual behavior with a particular partner at a moment in time. Sexual motivation is a psychological force that is influenced by:
- affective states, such as joy or sorrow
- interpersonal states, such as mutual affection, disagreement, or disrespect
- relationship stage, such as short or long duration
- cognitive states, such as moral disapproval.7
Values serve an evaluative function as our minds screen personal sexual behaviors with two questions:
- Is the behavior normal or abnormal?
- Is it morally acceptable or unacceptable?
In talking with Mrs. C, for example, you learn that her family reinforced the religious prohibition against extramarital sexual expression. “When I was a teenager, my father told me not to come home if I got pregnant before I was married,” she relates.
Values augment or diminish desire by affecting our willingness to engage in sexual behaviors. Values are camouflaged as motivation; Mrs. C may not realize that values she acquired at home early in life continue to influence her and may contribute to her lack of desire for nonreproductive sex.
Related resources
- Regan PC: Love relationships. In: Muscarella F (ed). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:232-82.
- Aron A, Fisher H, Mashek DJ. Reward, motivation, and emotion systems associated with early stage intense romantic love. J Neurophysiology 2005;94:327-37.
Singer Tina Turner recorded “What’s Love Got To Do With It?” in 1984.
1. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
2. Levine SB. What is love anyway? J Sex Marital Ther 2005;31(2):143-51.
3. Bowlby J. The making and breaking of affectional bonds. London: Routledge; 1989.
4. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
5. Lear J. Love and its place in nature: a philosophical interpretation of Freudian psychoanalysis. New York: Farrar, Straus & Giroux; 1990.
6. Levine SB. The nature of sexual desire: a clinician’s perspective. Arch Sex Behav 2003;32(3):279-85.
7. Clement U. Sex in long-term relationships: a systemic approach to sexual desire problems. Arch Sex Behav 2002;31(3):241-6.
Concepts of love and sexual desire lurk around clinical discussions of sexual dysfunction. Love is frequently dismissed as hopelessly unscientific, whereas desire is simplified as if it were a thing called libido. Decreased libido per se tells us little about a patient’s sexual complaints; the key is to differentiate between:
- those with sexual drive but no motivation for their partners
- those with no driveFemale sexual dysfunction”).
Psychiatrists avoid talking about love; it has too many meanings and nuances, too many avenues of defeat, and is too abstract. All you have to say to a patient is, “Tell me about your marriage,” and listen closely as he or she comes to grip with love’s complexity.
This article’s aim is to help you counsel patients more effectively about relationship and sexual problems by exploring two questions: “What is love?” and “What is sexual desire?”
What is love?
Mrs. C, age 41, is being treated for depression and wonders why she has lost desire for her husband. The antidepressant she is taking improves her mood and diminishes her considerable anxiety but makes her feel sexually dead. “My husband doesn’t mind how I feel, as long as he can have sex,” she says.
After adjusting her medication, you explore other problems that might be contributing to her sexual dysfunction. She expresses uncertainty about what love is. Though faithful and committed to her husband, she has stopped enjoying the way he interacts with her, their two grade-school children, her family, and friends.
Love is the usual context within which sexual activities are viewed. Among adults, unhappiness in love predisposes to sexual concerns, and sexual concerns interfere with loving and being loved.
Our patients’ expectations for feeling and receiving love and experiencing satisfying sex are disappointed through a myriad of avenues. Clinicians may overlook it, but demoralization about love can precede the onset of anxiety, panic attacks, and lingering depression.2 Sexual love is expected to begin with connecting with a partner and to evolve for 65 or more years. Most individuals harbor the secret that they are not certain what love is (Table 1) or are surprised by their lack of words to explain it.
1. Love as transient emotion. The assumption that love is a feeling leads too many people waiting to experience the pure feeling. But unlike sadness, fear, anger, or shame, love does not indicate a discrete feeling. Saying, “I love you,” connotes at least two feelings: pleasure and interest.
- Pleasure begins with pleasantness and moves up through delight to exhilaration.
- Interest ranges from mild curiosity to preoccupying fascination.
Most events simultaneously provoke more than one feeling. Discovering that your beloved wants to marry you usually brings about at least happiness, pride, gratitude, and awe. Even if only one feeling is produced, our attitude towards that feeling complicates the experience. When a child is taught that feeling envy is wrong, for example, his experience of it evokes anxiety (from the guilt) and shame (if someone is watching).
After the family, culture, and the person have worked on a simple feeling, it becomes a layered complexity called an emotion. Love, the emotion, is quickly layered with attitudes (which are the product of feelings and defenses against them) based on the person’s sense of safety stemming from earlier attachments.3 When someone says “I love you,” he or she knows the motive for saying it and hopes for a particular response from the listener.
Sexual desire is an ingredient of love’s emotional complexity. Because “I love you” can create sexual arousal in the listener, the speaker can use the phrase when his or her primary pleasure and interest in the person is the anticipation of sex.
Meanings and motives for expressing love change all the time. When someone tells us “I love you,” we have to discern both meaning and motive. Love’s emotions and their expression to another person are always complicated by past, present, and future considerations.
2. Love as an ambition. Love is so intensely celebrated in every culture that few people grow up without longing to realize it. Table 2 shows one version of the ambition to love and be loved.4 Many clinical declarations of love for a partner signify that the person has not yet given up on this ambition.
3. Love as an arrangement. All adult sexual relationships are quid pro quo exchanges of hopes, expectations, and assets. During courtship, both people are preoccupied with answering the question: “What will this person bring to my life?”
The question has many dimensions: social, economic, aesthetic, recreational, sexual, medical, time-to-death, and more. In their first romantic relationships, people generally prefer not to think in these terms. Their embarrassment dissipates with experience.
This ordinary process can be more clearly perceived after a relationship ends by breakup, divorce, or death and the person begins anew with someone. The person then can deliberately weigh the factors that will determine his or her involvement. When an arrangement is worked out, each person perceives what has been offered by the partner. Of course, perceptions vary in accuracy.
Anticipating making a deal can be very exciting, and once the deal is formally accepted, people often feel a celebratory degree of pleasure, interest, and sexual desire. They think that life is good. In cultures where parents make the deal, the couple courts in the hope that they will fall in love by early marriage.
4. Love is an attachment. Love also means the presence of a bond or attachment. People weave their psyches together and begin to feel a hunger to be with the other person. They think of themselves as belonging with and to the other.
Sexual activities—particularly those that lead to orgasm—facilitate attachment, but the bonds within each partner’s mind do not necessarily develop at the same time or solidify at the same rate. Thus, some people are unable to answer, “I love you, too,” when the partner reveals feelings that are summarized as love.
5. Love as a moral commitment. The rituals that sanctify marriage emphasize clearly that love is a commitment for couples to try to realize the grand idealized ambition (see “Love as an ambition”). The rituals are public promises to honor and cherish each other through all of life’s vicissitudes.
This love as moral commitment instantly restructures life by generating a new set of obligations. Many hostile, disappointed, and seemingly asexual spouses who have not felt pleasure and interest in a partner for a long time will tell their doctors they love the partner. They mean they remain bound by their moral commitment.
6. Love as a mental struggle. Love’s original emotions are stimulated by an idealized version of the partner. This image is internalized early in the relationship. As time passes, discovering our partner’s limitations gradually attenuates our idealization. We think of our earlier appraisals as naïve. Even so, disappointment does not quickly cancel our commitment because of our:
- ambition to love
- obligation to live through bad moments
- ability to love the idealized version of the partner
- moral commitments to raising our children.
The moral commitment to love can sustain people for a lifetime, despite grave disappointments. It also explains the persistent guilt many feel as they contemplate extramarital affairs, divorce, and the agonizing dilemma between their commitment to live with their children and their wish to be free of unhappiness with their partner.
“I love my partner, but I am not in love with him/her,” means, “although I’m still committed, I have lost my ability to idealize my partner.”
7. Love as a force of nature. Love is a force in nature that creates a unity from two individuals. It casts our fates together, organizes reproduction, and remains vital to adult growth and development and to the maturation of children. This love is a backbone that supports the sexual and non-sexual processes of our lives.5
Among older couples, “I love my partner but I am no longer in love with her/him” may mean, “We have shared so much of our lives that my partner is an inextricable part of me. I could never be free of my partner, even though most of the pleasure is gone.”
8. Love as an illusion. We create love for our partner by internal private processes, maintain it by prudent diplomatic dishonesties, and can lose it without the partner’s knowing. To remain in an intimate relationship, the processes of love require defensive distortions of a person’s feelings, thoughts, and perceptions.
As individuals gain experience, many look back and see that their assumptions about love were self-serving illusions. When entire relationships are dismissed with “what was I thinking?” the person usually means that now I can perceive that I created illusions so as not to admit to the depth of my disappointment with my partner.
9. Love as a stop sign. When a person says, “I love you,” the listener is challenged to discern its meaning. The emotions and motives behind the sentence can be very difficult to accurately perceive. Some love relationships, after all, are deceptions.
At any particular moment, we may know what we mean by “I love you” and why we are saying it. We may not be willing, however, to have our motives, meanings, and emotions fully known by the listener. In fact, the motive for saying “I love you” is often to obscure the view:
- Lover A: I love you.
- Lover B: Why do you love me?
- Lover A: I don’t know, I just do.
- Doctor: Why do you put up with this behavior from your spouse?
- Patient: Because I love him.
- Doctor: What does that mean?
- Patient: I don’t know.
What is the meaning of ‘I love you’? Love is…
| A transient emotion |
| An ambition |
| An arrangement |
| An attachment |
| A moral commitment |
| A mental struggle |
| A force of nature |
| An illusion |
| A stop sign |
Love as ambition: 7 ideals for loving relationships
| Mutual respect |
| Behavioral reliability |
| Enjoyment of one another |
| Sexual fidelity |
| Psychological intimacy |
| Sexual pleasure |
| A comfortable balance of individuality and couplehood |
| Source: Reference 4 |
What is sexual desire?
Sexual desire—at any given moment—is the sum of biological, psychological, interpersonal, and cultural forces that incline us toward and away from sexual behavior.6 Understanding desire can help you:
- ask patients insightful questions about their relationship concerns
- formulate a hypothesis to explain how drive, motivation, and values contribute to a patient’s sexual dysfunction.
Motivation is the degree of willingness an individual has to enter into sexual behavior with a particular partner at a moment in time. Sexual motivation is a psychological force that is influenced by:
- affective states, such as joy or sorrow
- interpersonal states, such as mutual affection, disagreement, or disrespect
- relationship stage, such as short or long duration
- cognitive states, such as moral disapproval.7
Values serve an evaluative function as our minds screen personal sexual behaviors with two questions:
- Is the behavior normal or abnormal?
- Is it morally acceptable or unacceptable?
In talking with Mrs. C, for example, you learn that her family reinforced the religious prohibition against extramarital sexual expression. “When I was a teenager, my father told me not to come home if I got pregnant before I was married,” she relates.
Values augment or diminish desire by affecting our willingness to engage in sexual behaviors. Values are camouflaged as motivation; Mrs. C may not realize that values she acquired at home early in life continue to influence her and may contribute to her lack of desire for nonreproductive sex.
Related resources
- Regan PC: Love relationships. In: Muscarella F (ed). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:232-82.
- Aron A, Fisher H, Mashek DJ. Reward, motivation, and emotion systems associated with early stage intense romantic love. J Neurophysiology 2005;94:327-37.
Singer Tina Turner recorded “What’s Love Got To Do With It?” in 1984.
Concepts of love and sexual desire lurk around clinical discussions of sexual dysfunction. Love is frequently dismissed as hopelessly unscientific, whereas desire is simplified as if it were a thing called libido. Decreased libido per se tells us little about a patient’s sexual complaints; the key is to differentiate between:
- those with sexual drive but no motivation for their partners
- those with no driveFemale sexual dysfunction”).
Psychiatrists avoid talking about love; it has too many meanings and nuances, too many avenues of defeat, and is too abstract. All you have to say to a patient is, “Tell me about your marriage,” and listen closely as he or she comes to grip with love’s complexity.
This article’s aim is to help you counsel patients more effectively about relationship and sexual problems by exploring two questions: “What is love?” and “What is sexual desire?”
What is love?
Mrs. C, age 41, is being treated for depression and wonders why she has lost desire for her husband. The antidepressant she is taking improves her mood and diminishes her considerable anxiety but makes her feel sexually dead. “My husband doesn’t mind how I feel, as long as he can have sex,” she says.
After adjusting her medication, you explore other problems that might be contributing to her sexual dysfunction. She expresses uncertainty about what love is. Though faithful and committed to her husband, she has stopped enjoying the way he interacts with her, their two grade-school children, her family, and friends.
Love is the usual context within which sexual activities are viewed. Among adults, unhappiness in love predisposes to sexual concerns, and sexual concerns interfere with loving and being loved.
Our patients’ expectations for feeling and receiving love and experiencing satisfying sex are disappointed through a myriad of avenues. Clinicians may overlook it, but demoralization about love can precede the onset of anxiety, panic attacks, and lingering depression.2 Sexual love is expected to begin with connecting with a partner and to evolve for 65 or more years. Most individuals harbor the secret that they are not certain what love is (Table 1) or are surprised by their lack of words to explain it.
1. Love as transient emotion. The assumption that love is a feeling leads too many people waiting to experience the pure feeling. But unlike sadness, fear, anger, or shame, love does not indicate a discrete feeling. Saying, “I love you,” connotes at least two feelings: pleasure and interest.
- Pleasure begins with pleasantness and moves up through delight to exhilaration.
- Interest ranges from mild curiosity to preoccupying fascination.
Most events simultaneously provoke more than one feeling. Discovering that your beloved wants to marry you usually brings about at least happiness, pride, gratitude, and awe. Even if only one feeling is produced, our attitude towards that feeling complicates the experience. When a child is taught that feeling envy is wrong, for example, his experience of it evokes anxiety (from the guilt) and shame (if someone is watching).
After the family, culture, and the person have worked on a simple feeling, it becomes a layered complexity called an emotion. Love, the emotion, is quickly layered with attitudes (which are the product of feelings and defenses against them) based on the person’s sense of safety stemming from earlier attachments.3 When someone says “I love you,” he or she knows the motive for saying it and hopes for a particular response from the listener.
Sexual desire is an ingredient of love’s emotional complexity. Because “I love you” can create sexual arousal in the listener, the speaker can use the phrase when his or her primary pleasure and interest in the person is the anticipation of sex.
Meanings and motives for expressing love change all the time. When someone tells us “I love you,” we have to discern both meaning and motive. Love’s emotions and their expression to another person are always complicated by past, present, and future considerations.
2. Love as an ambition. Love is so intensely celebrated in every culture that few people grow up without longing to realize it. Table 2 shows one version of the ambition to love and be loved.4 Many clinical declarations of love for a partner signify that the person has not yet given up on this ambition.
3. Love as an arrangement. All adult sexual relationships are quid pro quo exchanges of hopes, expectations, and assets. During courtship, both people are preoccupied with answering the question: “What will this person bring to my life?”
The question has many dimensions: social, economic, aesthetic, recreational, sexual, medical, time-to-death, and more. In their first romantic relationships, people generally prefer not to think in these terms. Their embarrassment dissipates with experience.
This ordinary process can be more clearly perceived after a relationship ends by breakup, divorce, or death and the person begins anew with someone. The person then can deliberately weigh the factors that will determine his or her involvement. When an arrangement is worked out, each person perceives what has been offered by the partner. Of course, perceptions vary in accuracy.
Anticipating making a deal can be very exciting, and once the deal is formally accepted, people often feel a celebratory degree of pleasure, interest, and sexual desire. They think that life is good. In cultures where parents make the deal, the couple courts in the hope that they will fall in love by early marriage.
4. Love is an attachment. Love also means the presence of a bond or attachment. People weave their psyches together and begin to feel a hunger to be with the other person. They think of themselves as belonging with and to the other.
Sexual activities—particularly those that lead to orgasm—facilitate attachment, but the bonds within each partner’s mind do not necessarily develop at the same time or solidify at the same rate. Thus, some people are unable to answer, “I love you, too,” when the partner reveals feelings that are summarized as love.
5. Love as a moral commitment. The rituals that sanctify marriage emphasize clearly that love is a commitment for couples to try to realize the grand idealized ambition (see “Love as an ambition”). The rituals are public promises to honor and cherish each other through all of life’s vicissitudes.
This love as moral commitment instantly restructures life by generating a new set of obligations. Many hostile, disappointed, and seemingly asexual spouses who have not felt pleasure and interest in a partner for a long time will tell their doctors they love the partner. They mean they remain bound by their moral commitment.
6. Love as a mental struggle. Love’s original emotions are stimulated by an idealized version of the partner. This image is internalized early in the relationship. As time passes, discovering our partner’s limitations gradually attenuates our idealization. We think of our earlier appraisals as naïve. Even so, disappointment does not quickly cancel our commitment because of our:
- ambition to love
- obligation to live through bad moments
- ability to love the idealized version of the partner
- moral commitments to raising our children.
The moral commitment to love can sustain people for a lifetime, despite grave disappointments. It also explains the persistent guilt many feel as they contemplate extramarital affairs, divorce, and the agonizing dilemma between their commitment to live with their children and their wish to be free of unhappiness with their partner.
“I love my partner, but I am not in love with him/her,” means, “although I’m still committed, I have lost my ability to idealize my partner.”
7. Love as a force of nature. Love is a force in nature that creates a unity from two individuals. It casts our fates together, organizes reproduction, and remains vital to adult growth and development and to the maturation of children. This love is a backbone that supports the sexual and non-sexual processes of our lives.5
Among older couples, “I love my partner but I am no longer in love with her/him” may mean, “We have shared so much of our lives that my partner is an inextricable part of me. I could never be free of my partner, even though most of the pleasure is gone.”
8. Love as an illusion. We create love for our partner by internal private processes, maintain it by prudent diplomatic dishonesties, and can lose it without the partner’s knowing. To remain in an intimate relationship, the processes of love require defensive distortions of a person’s feelings, thoughts, and perceptions.
As individuals gain experience, many look back and see that their assumptions about love were self-serving illusions. When entire relationships are dismissed with “what was I thinking?” the person usually means that now I can perceive that I created illusions so as not to admit to the depth of my disappointment with my partner.
9. Love as a stop sign. When a person says, “I love you,” the listener is challenged to discern its meaning. The emotions and motives behind the sentence can be very difficult to accurately perceive. Some love relationships, after all, are deceptions.
At any particular moment, we may know what we mean by “I love you” and why we are saying it. We may not be willing, however, to have our motives, meanings, and emotions fully known by the listener. In fact, the motive for saying “I love you” is often to obscure the view:
- Lover A: I love you.
- Lover B: Why do you love me?
- Lover A: I don’t know, I just do.
- Doctor: Why do you put up with this behavior from your spouse?
- Patient: Because I love him.
- Doctor: What does that mean?
- Patient: I don’t know.
What is the meaning of ‘I love you’? Love is…
| A transient emotion |
| An ambition |
| An arrangement |
| An attachment |
| A moral commitment |
| A mental struggle |
| A force of nature |
| An illusion |
| A stop sign |
Love as ambition: 7 ideals for loving relationships
| Mutual respect |
| Behavioral reliability |
| Enjoyment of one another |
| Sexual fidelity |
| Psychological intimacy |
| Sexual pleasure |
| A comfortable balance of individuality and couplehood |
| Source: Reference 4 |
What is sexual desire?
Sexual desire—at any given moment—is the sum of biological, psychological, interpersonal, and cultural forces that incline us toward and away from sexual behavior.6 Understanding desire can help you:
- ask patients insightful questions about their relationship concerns
- formulate a hypothesis to explain how drive, motivation, and values contribute to a patient’s sexual dysfunction.
Motivation is the degree of willingness an individual has to enter into sexual behavior with a particular partner at a moment in time. Sexual motivation is a psychological force that is influenced by:
- affective states, such as joy or sorrow
- interpersonal states, such as mutual affection, disagreement, or disrespect
- relationship stage, such as short or long duration
- cognitive states, such as moral disapproval.7
Values serve an evaluative function as our minds screen personal sexual behaviors with two questions:
- Is the behavior normal or abnormal?
- Is it morally acceptable or unacceptable?
In talking with Mrs. C, for example, you learn that her family reinforced the religious prohibition against extramarital sexual expression. “When I was a teenager, my father told me not to come home if I got pregnant before I was married,” she relates.
Values augment or diminish desire by affecting our willingness to engage in sexual behaviors. Values are camouflaged as motivation; Mrs. C may not realize that values she acquired at home early in life continue to influence her and may contribute to her lack of desire for nonreproductive sex.
Related resources
- Regan PC: Love relationships. In: Muscarella F (ed). Psychological perspectives on human sexuality. New York: John Wiley & Sons; 2000:232-82.
- Aron A, Fisher H, Mashek DJ. Reward, motivation, and emotion systems associated with early stage intense romantic love. J Neurophysiology 2005;94:327-37.
Singer Tina Turner recorded “What’s Love Got To Do With It?” in 1984.
1. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
2. Levine SB. What is love anyway? J Sex Marital Ther 2005;31(2):143-51.
3. Bowlby J. The making and breaking of affectional bonds. London: Routledge; 1989.
4. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
5. Lear J. Love and its place in nature: a philosophical interpretation of Freudian psychoanalysis. New York: Farrar, Straus & Giroux; 1990.
6. Levine SB. The nature of sexual desire: a clinician’s perspective. Arch Sex Behav 2003;32(3):279-85.
7. Clement U. Sex in long-term relationships: a systemic approach to sexual desire problems. Arch Sex Behav 2002;31(3):241-6.
1. Basson R. Sexual desire and arousal disorders in women. N Engl J Med 2006;354(14):1497-1506.
2. Levine SB. What is love anyway? J Sex Marital Ther 2005;31(2):143-51.
3. Bowlby J. The making and breaking of affectional bonds. London: Routledge; 1989.
4. Levine SB. Sexuality in mid-life. New York: Plenum; 1998.
5. Lear J. Love and its place in nature: a philosophical interpretation of Freudian psychoanalysis. New York: Farrar, Straus & Giroux; 1990.
6. Levine SB. The nature of sexual desire: a clinician’s perspective. Arch Sex Behav 2003;32(3):279-85.
7. Clement U. Sex in long-term relationships: a systemic approach to sexual desire problems. Arch Sex Behav 2002;31(3):241-6.