User login
From the Editors: Unexpected benefits
I am a member of several surgical societies, and more than once I’ve extolled the importance of going to meetings as part of my personal definition of a “good” surgeon.
While I mostly enjoy meetings nowadays, it hasn’t always been easy. My first experiences at surgical society meetings were painful. I was 32 years old, just out of training, and didn’t know anyone at the meetings. Receptions were the worst. My only friend sometimes was either my host or my wife. I was surrounded by these old guys, most of whom were famous.
Among the highlights of my life was being invited to join the Western Surgical Association. The year I was elected, J. David Richardson was the President. He gave such a rousing speech that at the President’s Dinner I rallied the courage to speak to the great man. When I called him “Dr. Richardson,” he waved that off and insisted I call him “Dave.” Little did I know that Dave would become one of my personal heroes and play an important part in the path that led to my being in a position to write a column such as this.
I never thought that being at a meeting might save my own life. As a devoted member of the Western Surgical Association, I breezed into Phoenix last year for the annual meeting. But I wasn’t feeling so well. Typical of a surgeon, I’d been denying that the symptoms in my abdomen and back were anything other than arthritis. Also, like most surgeons, I’d been pushing the accelerator pedal of life to the floorboard for some months. I arrived at the hotel at one in the morning with a stomach ache that clearly was becoming serious.
I lay in bed wondering what to do. Surgeons don’t get sick! Calling 911 seemed like an invitation to a 6-hour ED experience during which I would be demoted from surgeon to “the patient in Room 9” in a town where I didn’t know many people. As a surgeon, I knew that belly pain of this magnitude was not something I wanted to entrust to a nonsurgeon who was just trying to get through another long shift. This was personal.
Here’s where being a member of a surgical society became more than an academic exercise. I was in a hotel with many of the finest surgeons in the world – and I knew many of them! Among the attendees was my very good friend Margo Shoup, a first-rate cancer surgeon from Chicago. I knew she had come in that day because we were to have dinner together the next night. Rather than call her at 0300, I waited until 6. She came right over and examined me. We decided on a plan. As is always the case at a surgical meeting, one of the surgeon members is the local arrangement person. In this instance, it was none other than James Madura, MD, Chief of MIS GI surgery at Mayo Clinic, Scottsdale. We called James and when my abdomen decided to go nuclear with pain, he drove me to his hospital in his own car and got me in their ED.
The rest of the story is pretty mundane. My diagnosis turned out to be temporarily serious but benign. It was serious enough that without good people treating me, I could have done poorly. James and his fine team took superb care of me. Almost all of us go into surgery with the intent to help others. We take that extraseriously when it’s one of “us.” I can imagine the increased pressure on James when he was treating a colleague and the entire rest of the Western Surgical Association knew it. He never even broke a sweat. I am so glad he was my surgeon. His chief resident Ryan Day, MD, spent extra time with me. I saw a bunch of other residents as well. They reminded me of my fellow residents many years ago – eager, bright, and hyperdedicated. Several members of the Western came by the hospital to see me and even reviewed my images and diagnosis with me. I felt like I had a whole family of physicians who were both my friends and my support system a long way from home. I cannot thank James, Margo, and the rest enough for all they did. It’s great to feel back to normal again as well.
So, I would suggest you join and participate in the surgical societies that you think are a good fit for you. Develop those important professional relationships. Such activity will do your patients a world of good – and just might save your own life.
I am a member of several surgical societies, and more than once I’ve extolled the importance of going to meetings as part of my personal definition of a “good” surgeon.
While I mostly enjoy meetings nowadays, it hasn’t always been easy. My first experiences at surgical society meetings were painful. I was 32 years old, just out of training, and didn’t know anyone at the meetings. Receptions were the worst. My only friend sometimes was either my host or my wife. I was surrounded by these old guys, most of whom were famous.
Among the highlights of my life was being invited to join the Western Surgical Association. The year I was elected, J. David Richardson was the President. He gave such a rousing speech that at the President’s Dinner I rallied the courage to speak to the great man. When I called him “Dr. Richardson,” he waved that off and insisted I call him “Dave.” Little did I know that Dave would become one of my personal heroes and play an important part in the path that led to my being in a position to write a column such as this.
I never thought that being at a meeting might save my own life. As a devoted member of the Western Surgical Association, I breezed into Phoenix last year for the annual meeting. But I wasn’t feeling so well. Typical of a surgeon, I’d been denying that the symptoms in my abdomen and back were anything other than arthritis. Also, like most surgeons, I’d been pushing the accelerator pedal of life to the floorboard for some months. I arrived at the hotel at one in the morning with a stomach ache that clearly was becoming serious.
I lay in bed wondering what to do. Surgeons don’t get sick! Calling 911 seemed like an invitation to a 6-hour ED experience during which I would be demoted from surgeon to “the patient in Room 9” in a town where I didn’t know many people. As a surgeon, I knew that belly pain of this magnitude was not something I wanted to entrust to a nonsurgeon who was just trying to get through another long shift. This was personal.
Here’s where being a member of a surgical society became more than an academic exercise. I was in a hotel with many of the finest surgeons in the world – and I knew many of them! Among the attendees was my very good friend Margo Shoup, a first-rate cancer surgeon from Chicago. I knew she had come in that day because we were to have dinner together the next night. Rather than call her at 0300, I waited until 6. She came right over and examined me. We decided on a plan. As is always the case at a surgical meeting, one of the surgeon members is the local arrangement person. In this instance, it was none other than James Madura, MD, Chief of MIS GI surgery at Mayo Clinic, Scottsdale. We called James and when my abdomen decided to go nuclear with pain, he drove me to his hospital in his own car and got me in their ED.
The rest of the story is pretty mundane. My diagnosis turned out to be temporarily serious but benign. It was serious enough that without good people treating me, I could have done poorly. James and his fine team took superb care of me. Almost all of us go into surgery with the intent to help others. We take that extraseriously when it’s one of “us.” I can imagine the increased pressure on James when he was treating a colleague and the entire rest of the Western Surgical Association knew it. He never even broke a sweat. I am so glad he was my surgeon. His chief resident Ryan Day, MD, spent extra time with me. I saw a bunch of other residents as well. They reminded me of my fellow residents many years ago – eager, bright, and hyperdedicated. Several members of the Western came by the hospital to see me and even reviewed my images and diagnosis with me. I felt like I had a whole family of physicians who were both my friends and my support system a long way from home. I cannot thank James, Margo, and the rest enough for all they did. It’s great to feel back to normal again as well.
So, I would suggest you join and participate in the surgical societies that you think are a good fit for you. Develop those important professional relationships. Such activity will do your patients a world of good – and just might save your own life.
I am a member of several surgical societies, and more than once I’ve extolled the importance of going to meetings as part of my personal definition of a “good” surgeon.
While I mostly enjoy meetings nowadays, it hasn’t always been easy. My first experiences at surgical society meetings were painful. I was 32 years old, just out of training, and didn’t know anyone at the meetings. Receptions were the worst. My only friend sometimes was either my host or my wife. I was surrounded by these old guys, most of whom were famous.
Among the highlights of my life was being invited to join the Western Surgical Association. The year I was elected, J. David Richardson was the President. He gave such a rousing speech that at the President’s Dinner I rallied the courage to speak to the great man. When I called him “Dr. Richardson,” he waved that off and insisted I call him “Dave.” Little did I know that Dave would become one of my personal heroes and play an important part in the path that led to my being in a position to write a column such as this.
I never thought that being at a meeting might save my own life. As a devoted member of the Western Surgical Association, I breezed into Phoenix last year for the annual meeting. But I wasn’t feeling so well. Typical of a surgeon, I’d been denying that the symptoms in my abdomen and back were anything other than arthritis. Also, like most surgeons, I’d been pushing the accelerator pedal of life to the floorboard for some months. I arrived at the hotel at one in the morning with a stomach ache that clearly was becoming serious.
I lay in bed wondering what to do. Surgeons don’t get sick! Calling 911 seemed like an invitation to a 6-hour ED experience during which I would be demoted from surgeon to “the patient in Room 9” in a town where I didn’t know many people. As a surgeon, I knew that belly pain of this magnitude was not something I wanted to entrust to a nonsurgeon who was just trying to get through another long shift. This was personal.
Here’s where being a member of a surgical society became more than an academic exercise. I was in a hotel with many of the finest surgeons in the world – and I knew many of them! Among the attendees was my very good friend Margo Shoup, a first-rate cancer surgeon from Chicago. I knew she had come in that day because we were to have dinner together the next night. Rather than call her at 0300, I waited until 6. She came right over and examined me. We decided on a plan. As is always the case at a surgical meeting, one of the surgeon members is the local arrangement person. In this instance, it was none other than James Madura, MD, Chief of MIS GI surgery at Mayo Clinic, Scottsdale. We called James and when my abdomen decided to go nuclear with pain, he drove me to his hospital in his own car and got me in their ED.
The rest of the story is pretty mundane. My diagnosis turned out to be temporarily serious but benign. It was serious enough that without good people treating me, I could have done poorly. James and his fine team took superb care of me. Almost all of us go into surgery with the intent to help others. We take that extraseriously when it’s one of “us.” I can imagine the increased pressure on James when he was treating a colleague and the entire rest of the Western Surgical Association knew it. He never even broke a sweat. I am so glad he was my surgeon. His chief resident Ryan Day, MD, spent extra time with me. I saw a bunch of other residents as well. They reminded me of my fellow residents many years ago – eager, bright, and hyperdedicated. Several members of the Western came by the hospital to see me and even reviewed my images and diagnosis with me. I felt like I had a whole family of physicians who were both my friends and my support system a long way from home. I cannot thank James, Margo, and the rest enough for all they did. It’s great to feel back to normal again as well.
So, I would suggest you join and participate in the surgical societies that you think are a good fit for you. Develop those important professional relationships. Such activity will do your patients a world of good – and just might save your own life.
Deprescribing: When trying for less is more
Sometimes the answer is straightforward—the pills were started to reduce knee pain, but the pain is still there. But sometimes if I suggest stopping a drug, I get surprising pushback from the patient: “But the pain may be worse without those pills.” And it can be hard to assess whether a medication has attained its therapeutic goal, such as when a drug is given to prevent or reduce the occurrence of intermittent events (eg, hydroxychloroquine to reduce flares in lupus, aspirin to prevent transient ischemic attacks). Unless a medication has been given sufficient time to have its effect and has clearly failed, we often have to trust its efficacy because those events might be more frequent if the drug were stopped.
In this issue of the Journal, Kim and Factora discuss a difficult scenario—discontinuing cognitive-enhancing therapy in patients with Alzheimer dementia. The stakes, hopes, and anxiety are high for the patient and caregivers. These drugs have only modest efficacy, and it is often difficult to know if they are working. To complicate matters, dementia is progressive, but the rate of progression differs among individuals, making it harder to be convinced that the drugs have lost their efficacy and that discontinuation is warranted in order to reduce the side effects, cost, and pill burden. Similar challenges arise for similar reasons when considering discontinuation of antipsychotics or other drugs given for behavioral reasons to elderly patients with dementia.1
The issues surrounding downsizing medication lists—or deprescribing—extend far beyond patients with Alzheimer dementia. A disease may have progressed beyond the point where the drug can make a significant impact. Patients often develop tachyphylaxis to the newer protein drugs (biologics for rheumatoid arthritis, inflammatory bowel disease, psoriasis, or gout) due to the generation of neutralizing antidrug antibodies. At some point the patient’s life expectancy becomes a factor when considering medications directed at preventing long-term complications of a disease and the increased likelihood of significant adverse effects in patients as they age (eg, from aggressively prescribed antihypertensive and antidiabetic drugs). For drugs such as bisphosphonates, alkylating agents, and metoclopramide, complications of cumulative dosing over time should be considered a reason to discontinue therapy.
The take-home message from this article is to create opportunities to periodically revisit the rationale for all of a patient’s prescriptions, and to make sure patients are comfortable knowing why they should keep taking each of their medications. While revisiting a patient’s prescriptions may indeed reduce the medication burden, I believe it also enhances adherence to the remaining prescribed medications. The medication reconciliation process requires more than simply checking off the box in the EMR to indicate that the medications were “reviewed.”
- Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician 2018; 64(1):17–27.
Sometimes the answer is straightforward—the pills were started to reduce knee pain, but the pain is still there. But sometimes if I suggest stopping a drug, I get surprising pushback from the patient: “But the pain may be worse without those pills.” And it can be hard to assess whether a medication has attained its therapeutic goal, such as when a drug is given to prevent or reduce the occurrence of intermittent events (eg, hydroxychloroquine to reduce flares in lupus, aspirin to prevent transient ischemic attacks). Unless a medication has been given sufficient time to have its effect and has clearly failed, we often have to trust its efficacy because those events might be more frequent if the drug were stopped.
In this issue of the Journal, Kim and Factora discuss a difficult scenario—discontinuing cognitive-enhancing therapy in patients with Alzheimer dementia. The stakes, hopes, and anxiety are high for the patient and caregivers. These drugs have only modest efficacy, and it is often difficult to know if they are working. To complicate matters, dementia is progressive, but the rate of progression differs among individuals, making it harder to be convinced that the drugs have lost their efficacy and that discontinuation is warranted in order to reduce the side effects, cost, and pill burden. Similar challenges arise for similar reasons when considering discontinuation of antipsychotics or other drugs given for behavioral reasons to elderly patients with dementia.1
The issues surrounding downsizing medication lists—or deprescribing—extend far beyond patients with Alzheimer dementia. A disease may have progressed beyond the point where the drug can make a significant impact. Patients often develop tachyphylaxis to the newer protein drugs (biologics for rheumatoid arthritis, inflammatory bowel disease, psoriasis, or gout) due to the generation of neutralizing antidrug antibodies. At some point the patient’s life expectancy becomes a factor when considering medications directed at preventing long-term complications of a disease and the increased likelihood of significant adverse effects in patients as they age (eg, from aggressively prescribed antihypertensive and antidiabetic drugs). For drugs such as bisphosphonates, alkylating agents, and metoclopramide, complications of cumulative dosing over time should be considered a reason to discontinue therapy.
The take-home message from this article is to create opportunities to periodically revisit the rationale for all of a patient’s prescriptions, and to make sure patients are comfortable knowing why they should keep taking each of their medications. While revisiting a patient’s prescriptions may indeed reduce the medication burden, I believe it also enhances adherence to the remaining prescribed medications. The medication reconciliation process requires more than simply checking off the box in the EMR to indicate that the medications were “reviewed.”
Sometimes the answer is straightforward—the pills were started to reduce knee pain, but the pain is still there. But sometimes if I suggest stopping a drug, I get surprising pushback from the patient: “But the pain may be worse without those pills.” And it can be hard to assess whether a medication has attained its therapeutic goal, such as when a drug is given to prevent or reduce the occurrence of intermittent events (eg, hydroxychloroquine to reduce flares in lupus, aspirin to prevent transient ischemic attacks). Unless a medication has been given sufficient time to have its effect and has clearly failed, we often have to trust its efficacy because those events might be more frequent if the drug were stopped.
In this issue of the Journal, Kim and Factora discuss a difficult scenario—discontinuing cognitive-enhancing therapy in patients with Alzheimer dementia. The stakes, hopes, and anxiety are high for the patient and caregivers. These drugs have only modest efficacy, and it is often difficult to know if they are working. To complicate matters, dementia is progressive, but the rate of progression differs among individuals, making it harder to be convinced that the drugs have lost their efficacy and that discontinuation is warranted in order to reduce the side effects, cost, and pill burden. Similar challenges arise for similar reasons when considering discontinuation of antipsychotics or other drugs given for behavioral reasons to elderly patients with dementia.1
The issues surrounding downsizing medication lists—or deprescribing—extend far beyond patients with Alzheimer dementia. A disease may have progressed beyond the point where the drug can make a significant impact. Patients often develop tachyphylaxis to the newer protein drugs (biologics for rheumatoid arthritis, inflammatory bowel disease, psoriasis, or gout) due to the generation of neutralizing antidrug antibodies. At some point the patient’s life expectancy becomes a factor when considering medications directed at preventing long-term complications of a disease and the increased likelihood of significant adverse effects in patients as they age (eg, from aggressively prescribed antihypertensive and antidiabetic drugs). For drugs such as bisphosphonates, alkylating agents, and metoclopramide, complications of cumulative dosing over time should be considered a reason to discontinue therapy.
The take-home message from this article is to create opportunities to periodically revisit the rationale for all of a patient’s prescriptions, and to make sure patients are comfortable knowing why they should keep taking each of their medications. While revisiting a patient’s prescriptions may indeed reduce the medication burden, I believe it also enhances adherence to the remaining prescribed medications. The medication reconciliation process requires more than simply checking off the box in the EMR to indicate that the medications were “reviewed.”
- Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician 2018; 64(1):17–27.
- Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician 2018; 64(1):17–27.
Hidradenitis suppurativa: An underdiagnosed skin problem of women
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
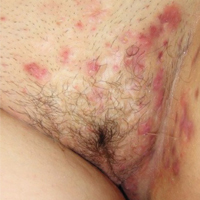
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9
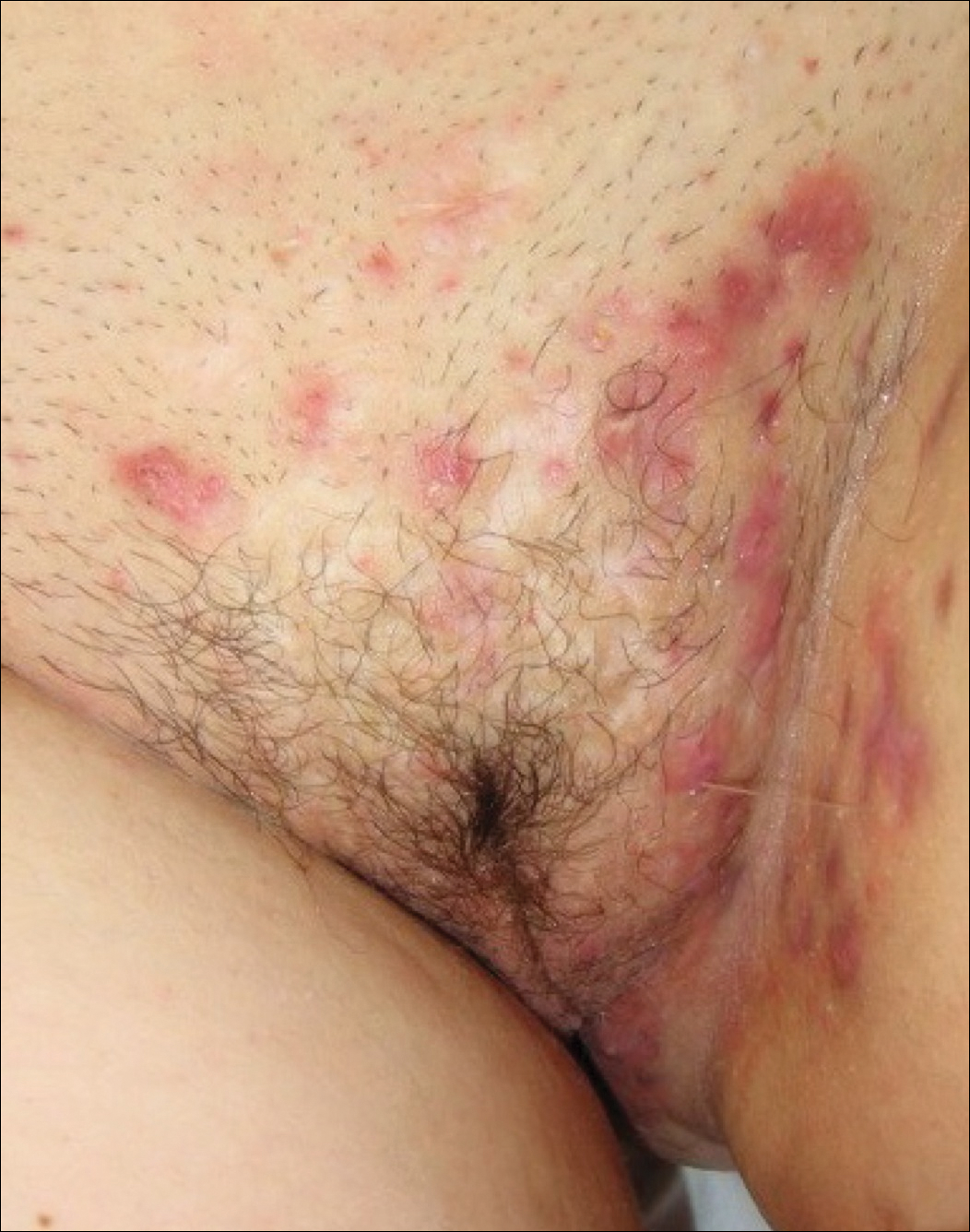
HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Balancing quality and cost of care with patient well-being
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
From the Editors: An unexpected call to action
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
A fantasy
The day had gone very well. The vascular surgeon woke early excited for a morning in the OR and then an afternoon in the office. Driving to the hospital, he had planned out his day. A patient with a fempop at 7:30, an AV fistula at 10:30 am, a quick bite in the doctor’s lounge, and then to the office for two phlebectomies, a few new consults, as well as some returning patients.
Fortunately, he had purchased an advanced electronic medical record so that reviewing old records and inputting new data went smoothly. He had been on call for the local hospital’s ER, but he received no calls, so his day was not impacted. After a dinner with his wife, also a surgeon, he helped put their youngest baby to sleep, played with his older children, took the dog out for a walk, read the latest JVS and went to sleep. Despite being on call, the phone never rang, and he had an uninterrupted sleep.
Now, what really happened!
The vascular surgeon woke early in preparation for a day in the OR and office. Traffic slowed him down, but he still arrived at the hospital just before his 7:30 start time. He expected his patient to be on the table prepped and ready for the procedure. But the OR supervisor informed him that new regulations required him to personally mark the site of surgery, update the H&P, and date and time the consent.
He was nonplussed. He had marked the patient last night and had signed the consent too. His PA had dictated a three-page H&P that was in the chart. However, the patient was still in the holding room. The surgeon rushed over, marked the leg again, and completed the required documentation.
“Well,” he thought, “I’ll run upstairs, discharge my carotid from yesterday, and by the time that’s done and I’ve changed into scrubs, my patient will be ready.” Impatiently he waited 5 minutes for the elevator, but it never arrived. So he elected to run up 10 floors and across to the other side of the hospital where the administrators had inconveniently placed the postop vascular patients. The patient was eager to leave. The vascular surgeon dictated the discharge note and signed into the hospital electronic medical record.
But the software insisted that he had to comply with numerous “safety” regulations before signing off. These required reviewing every medication and all discharge instructions. The patient was on 15 drugs, and the surgeon was unfamiliar with most. After 10 minutes of unsuccessfully trying to enter the relevant orders, he called a medical student over to help.
The patient was going to a skilled nursing facility. This required completing two more electronic forms. The software stubbornly refused to close the discharge section till he assigned the appropriate ICD-10 codes. After a few more frustrating minutes he finally clicked the proper boxes and completed the discharge.
It was 8:15 by the time he made the skin incision. The case went smoothly. He relaxed a little knowing that he probably would not run too late for the rest of his day. Finishing ahead of schedule, he dictated the note, spoke to the patient’s family, and went to preop his AV fistula scheduled for 10:30. Then back to the wards to complete rounds.
The first two patients were uncomplicated. The third had a fever requiring multiple orders in the EMR. Then heated conversations with the pharmacist and head of infection control, since the EMR would not allow him to prescribe the antibiotic of his choice. Back across the entire length of the hospital to see a patient with renal failure. But she was in dialysis at another distant location in the hospital.
At 10:30 he ran down to the OR ready to scrub. Again, this patient was still in the holding area. The patient’s potassium was 5.6, and the anesthesiologist wanted to run another blood test. Then the nurse had to go on break. Now there was confusion about whether a room would be available as another surgeon had a bump case.
Ultimately, he started at 11:30. During the procedure, his beeper went off constantly. There were already two consults in the ER. The fistula took a mere 30 minutes, but he had waited 90 minutes since finishing the fempop.
“Medicare should pay me for the time between cases, and I’ll do the procedure for free” he complained to a colleague as he passed her on the way to the ER to see the consults.
He sent the patient with the DVT home, but the patient with the infected foot would require later debridement. He admitted her and booked the OR for after office hours.
By the time he got to the doctors’ lounge all that was left was a half-eaten pack of Doritos and burned coffee.
He thought he would have a brief respite driving to the office. Then his surgeon wife called him in the car asking him to field a call from their son’s school since she was stuck in the OR.
He arrived late to the office. The waiting room was filled with hostile-looking patients one of whom made a point of holding up her watch as if to reinforce his tardiness. There were already three additions to his schedule. Further, his nurse told him that there was some issue with the internet connection to the server. Thus, despite his expensive EMR, no records were available. She had informed the patients that there would be a “little” delay.
While they were waiting she brought in reams of documents that had come in the prior day and needed his signatures. He also used the time “productively” to answer emails. When the EMR was back online, he returned to his patients who by now were seething.
A patient brought in a CD of a CTA. He loaded it up on a computer, but the disc kept spinning relentlessly. Cursing, he loaded it on a second computer. The instructions were indecipherable. He could get a picture up but could not scroll through the images. The program froze. By the time he had evaluated the disc, he had wasted over 20 minutes. He was running even further behind.
The next patient was a second opinion from a physician in another state. She brought in over 200 pages of medical records describing a multitude of prior procedures. Politely he explained he would need to read them first and rescheduled her.
The ER called again with a patient with a cold leg. He canceled the rest of the office and snuck out through a back door, afraid to witness the consternation in the waiting room.
At the hospital, he argued briefly with the anesthesiologist who was reluctant to anesthetize the patient who had eaten 5 hours before. So the harried surgeon read some vascular labs, and visited a few less stable patients. Then back to the OR to revascularize the ER patient’s leg and later to debride the earlier patient’s foot.
He got home at 8:30 pm. His wife had also been delayed by a long surgery. They put the baby to bed. There was no time to play with the other children. The surgical couple barely had the energy left to microwave leftovers for dinner. He was too tired to take the dog out for its nocturnal pee. He went to his study, picked up the JVS, and fell asleep in his chair. He woke up with a start as he felt the dog urinate on his leg.
Exhausted he climbed into bed. It had been a good day, he told himself. After all the ER had not been too disruptive. He drifted off into a deep sleep. And then the phone rang. Ruptured AAA in the ER.
The day had gone very well. The vascular surgeon woke early excited for a morning in the OR and then an afternoon in the office. Driving to the hospital, he had planned out his day. A patient with a fempop at 7:30, an AV fistula at 10:30 am, a quick bite in the doctor’s lounge, and then to the office for two phlebectomies, a few new consults, as well as some returning patients.
Fortunately, he had purchased an advanced electronic medical record so that reviewing old records and inputting new data went smoothly. He had been on call for the local hospital’s ER, but he received no calls, so his day was not impacted. After a dinner with his wife, also a surgeon, he helped put their youngest baby to sleep, played with his older children, took the dog out for a walk, read the latest JVS and went to sleep. Despite being on call, the phone never rang, and he had an uninterrupted sleep.
Now, what really happened!
The vascular surgeon woke early in preparation for a day in the OR and office. Traffic slowed him down, but he still arrived at the hospital just before his 7:30 start time. He expected his patient to be on the table prepped and ready for the procedure. But the OR supervisor informed him that new regulations required him to personally mark the site of surgery, update the H&P, and date and time the consent.
He was nonplussed. He had marked the patient last night and had signed the consent too. His PA had dictated a three-page H&P that was in the chart. However, the patient was still in the holding room. The surgeon rushed over, marked the leg again, and completed the required documentation.
“Well,” he thought, “I’ll run upstairs, discharge my carotid from yesterday, and by the time that’s done and I’ve changed into scrubs, my patient will be ready.” Impatiently he waited 5 minutes for the elevator, but it never arrived. So he elected to run up 10 floors and across to the other side of the hospital where the administrators had inconveniently placed the postop vascular patients. The patient was eager to leave. The vascular surgeon dictated the discharge note and signed into the hospital electronic medical record.
But the software insisted that he had to comply with numerous “safety” regulations before signing off. These required reviewing every medication and all discharge instructions. The patient was on 15 drugs, and the surgeon was unfamiliar with most. After 10 minutes of unsuccessfully trying to enter the relevant orders, he called a medical student over to help.
The patient was going to a skilled nursing facility. This required completing two more electronic forms. The software stubbornly refused to close the discharge section till he assigned the appropriate ICD-10 codes. After a few more frustrating minutes he finally clicked the proper boxes and completed the discharge.
It was 8:15 by the time he made the skin incision. The case went smoothly. He relaxed a little knowing that he probably would not run too late for the rest of his day. Finishing ahead of schedule, he dictated the note, spoke to the patient’s family, and went to preop his AV fistula scheduled for 10:30. Then back to the wards to complete rounds.
The first two patients were uncomplicated. The third had a fever requiring multiple orders in the EMR. Then heated conversations with the pharmacist and head of infection control, since the EMR would not allow him to prescribe the antibiotic of his choice. Back across the entire length of the hospital to see a patient with renal failure. But she was in dialysis at another distant location in the hospital.
At 10:30 he ran down to the OR ready to scrub. Again, this patient was still in the holding area. The patient’s potassium was 5.6, and the anesthesiologist wanted to run another blood test. Then the nurse had to go on break. Now there was confusion about whether a room would be available as another surgeon had a bump case.
Ultimately, he started at 11:30. During the procedure, his beeper went off constantly. There were already two consults in the ER. The fistula took a mere 30 minutes, but he had waited 90 minutes since finishing the fempop.
“Medicare should pay me for the time between cases, and I’ll do the procedure for free” he complained to a colleague as he passed her on the way to the ER to see the consults.
He sent the patient with the DVT home, but the patient with the infected foot would require later debridement. He admitted her and booked the OR for after office hours.
By the time he got to the doctors’ lounge all that was left was a half-eaten pack of Doritos and burned coffee.
He thought he would have a brief respite driving to the office. Then his surgeon wife called him in the car asking him to field a call from their son’s school since she was stuck in the OR.
He arrived late to the office. The waiting room was filled with hostile-looking patients one of whom made a point of holding up her watch as if to reinforce his tardiness. There were already three additions to his schedule. Further, his nurse told him that there was some issue with the internet connection to the server. Thus, despite his expensive EMR, no records were available. She had informed the patients that there would be a “little” delay.
While they were waiting she brought in reams of documents that had come in the prior day and needed his signatures. He also used the time “productively” to answer emails. When the EMR was back online, he returned to his patients who by now were seething.
A patient brought in a CD of a CTA. He loaded it up on a computer, but the disc kept spinning relentlessly. Cursing, he loaded it on a second computer. The instructions were indecipherable. He could get a picture up but could not scroll through the images. The program froze. By the time he had evaluated the disc, he had wasted over 20 minutes. He was running even further behind.
The next patient was a second opinion from a physician in another state. She brought in over 200 pages of medical records describing a multitude of prior procedures. Politely he explained he would need to read them first and rescheduled her.
The ER called again with a patient with a cold leg. He canceled the rest of the office and snuck out through a back door, afraid to witness the consternation in the waiting room.
At the hospital, he argued briefly with the anesthesiologist who was reluctant to anesthetize the patient who had eaten 5 hours before. So the harried surgeon read some vascular labs, and visited a few less stable patients. Then back to the OR to revascularize the ER patient’s leg and later to debride the earlier patient’s foot.
He got home at 8:30 pm. His wife had also been delayed by a long surgery. They put the baby to bed. There was no time to play with the other children. The surgical couple barely had the energy left to microwave leftovers for dinner. He was too tired to take the dog out for its nocturnal pee. He went to his study, picked up the JVS, and fell asleep in his chair. He woke up with a start as he felt the dog urinate on his leg.
Exhausted he climbed into bed. It had been a good day, he told himself. After all the ER had not been too disruptive. He drifted off into a deep sleep. And then the phone rang. Ruptured AAA in the ER.
The day had gone very well. The vascular surgeon woke early excited for a morning in the OR and then an afternoon in the office. Driving to the hospital, he had planned out his day. A patient with a fempop at 7:30, an AV fistula at 10:30 am, a quick bite in the doctor’s lounge, and then to the office for two phlebectomies, a few new consults, as well as some returning patients.
Fortunately, he had purchased an advanced electronic medical record so that reviewing old records and inputting new data went smoothly. He had been on call for the local hospital’s ER, but he received no calls, so his day was not impacted. After a dinner with his wife, also a surgeon, he helped put their youngest baby to sleep, played with his older children, took the dog out for a walk, read the latest JVS and went to sleep. Despite being on call, the phone never rang, and he had an uninterrupted sleep.
Now, what really happened!
The vascular surgeon woke early in preparation for a day in the OR and office. Traffic slowed him down, but he still arrived at the hospital just before his 7:30 start time. He expected his patient to be on the table prepped and ready for the procedure. But the OR supervisor informed him that new regulations required him to personally mark the site of surgery, update the H&P, and date and time the consent.
He was nonplussed. He had marked the patient last night and had signed the consent too. His PA had dictated a three-page H&P that was in the chart. However, the patient was still in the holding room. The surgeon rushed over, marked the leg again, and completed the required documentation.
“Well,” he thought, “I’ll run upstairs, discharge my carotid from yesterday, and by the time that’s done and I’ve changed into scrubs, my patient will be ready.” Impatiently he waited 5 minutes for the elevator, but it never arrived. So he elected to run up 10 floors and across to the other side of the hospital where the administrators had inconveniently placed the postop vascular patients. The patient was eager to leave. The vascular surgeon dictated the discharge note and signed into the hospital electronic medical record.
But the software insisted that he had to comply with numerous “safety” regulations before signing off. These required reviewing every medication and all discharge instructions. The patient was on 15 drugs, and the surgeon was unfamiliar with most. After 10 minutes of unsuccessfully trying to enter the relevant orders, he called a medical student over to help.
The patient was going to a skilled nursing facility. This required completing two more electronic forms. The software stubbornly refused to close the discharge section till he assigned the appropriate ICD-10 codes. After a few more frustrating minutes he finally clicked the proper boxes and completed the discharge.
It was 8:15 by the time he made the skin incision. The case went smoothly. He relaxed a little knowing that he probably would not run too late for the rest of his day. Finishing ahead of schedule, he dictated the note, spoke to the patient’s family, and went to preop his AV fistula scheduled for 10:30. Then back to the wards to complete rounds.
The first two patients were uncomplicated. The third had a fever requiring multiple orders in the EMR. Then heated conversations with the pharmacist and head of infection control, since the EMR would not allow him to prescribe the antibiotic of his choice. Back across the entire length of the hospital to see a patient with renal failure. But she was in dialysis at another distant location in the hospital.
At 10:30 he ran down to the OR ready to scrub. Again, this patient was still in the holding area. The patient’s potassium was 5.6, and the anesthesiologist wanted to run another blood test. Then the nurse had to go on break. Now there was confusion about whether a room would be available as another surgeon had a bump case.
Ultimately, he started at 11:30. During the procedure, his beeper went off constantly. There were already two consults in the ER. The fistula took a mere 30 minutes, but he had waited 90 minutes since finishing the fempop.
“Medicare should pay me for the time between cases, and I’ll do the procedure for free” he complained to a colleague as he passed her on the way to the ER to see the consults.
He sent the patient with the DVT home, but the patient with the infected foot would require later debridement. He admitted her and booked the OR for after office hours.
By the time he got to the doctors’ lounge all that was left was a half-eaten pack of Doritos and burned coffee.
He thought he would have a brief respite driving to the office. Then his surgeon wife called him in the car asking him to field a call from their son’s school since she was stuck in the OR.
He arrived late to the office. The waiting room was filled with hostile-looking patients one of whom made a point of holding up her watch as if to reinforce his tardiness. There were already three additions to his schedule. Further, his nurse told him that there was some issue with the internet connection to the server. Thus, despite his expensive EMR, no records were available. She had informed the patients that there would be a “little” delay.
While they were waiting she brought in reams of documents that had come in the prior day and needed his signatures. He also used the time “productively” to answer emails. When the EMR was back online, he returned to his patients who by now were seething.
A patient brought in a CD of a CTA. He loaded it up on a computer, but the disc kept spinning relentlessly. Cursing, he loaded it on a second computer. The instructions were indecipherable. He could get a picture up but could not scroll through the images. The program froze. By the time he had evaluated the disc, he had wasted over 20 minutes. He was running even further behind.
The next patient was a second opinion from a physician in another state. She brought in over 200 pages of medical records describing a multitude of prior procedures. Politely he explained he would need to read them first and rescheduled her.
The ER called again with a patient with a cold leg. He canceled the rest of the office and snuck out through a back door, afraid to witness the consternation in the waiting room.
At the hospital, he argued briefly with the anesthesiologist who was reluctant to anesthetize the patient who had eaten 5 hours before. So the harried surgeon read some vascular labs, and visited a few less stable patients. Then back to the OR to revascularize the ER patient’s leg and later to debride the earlier patient’s foot.
He got home at 8:30 pm. His wife had also been delayed by a long surgery. They put the baby to bed. There was no time to play with the other children. The surgical couple barely had the energy left to microwave leftovers for dinner. He was too tired to take the dog out for its nocturnal pee. He went to his study, picked up the JVS, and fell asleep in his chair. He woke up with a start as he felt the dog urinate on his leg.
Exhausted he climbed into bed. It had been a good day, he told himself. After all the ER had not been too disruptive. He drifted off into a deep sleep. And then the phone rang. Ruptured AAA in the ER.
Can a shared decision be wrong if made for the ‘right’ reasons?
It is difficult to provide enough information for informed consent and to ensure that the patient and his or her family fully understand what we are saying. Patients often come in with their own preferences and biases based on anecdote, dinner conversations, or the Internet. The physician must push hard to dispel a patient’s bias with the facts, while recognizing that we too regularly present “facts” and recommendations colored by our own biases based on anecdotal experience, professional ritual, and intellectual hubris.
Two articles in this issue of the Journal, one by Dr. Michael Rothberg1 and the other by Dr. Umesh Khot,2 examine percutaneous coronary intervention (PCI) in patients with stable chronic angina. Both discuss the findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial3 and how to use these findings in helping patients decide whether to undergo PCI.
Rothberg and Khot agree that in the COURAGE trial, PCI effectively if not completely reduced angina but did not decrease the likelihood of death or subsequent myocardial infarction (MI). Patients were excluded from the study if they had a likelihood of left main disease, heart failure, or severe angina. All underwent catheterization, and all were given optimal medical therapy. Thus, the trial results do not directly relate to every patient with stable angina.
While the patient may find it confusing that angina and the risk of MI are not reduced in parallel, since both are due to atherosclerosis, their dynamic pathophysiology is different. The COURAGE results are the mirror image of those in some early studies of aspirin in coronary disease, in which aspirin reduced the incidence of MI but did not significantly affect angina.
In view of the COURAGE results, Rothberg seems surprised that PCI continues to be frequently used in patients with stable angina. He points out that according to some surveys,4 not all cardiologists have embraced these (and other similar study) results. But as Khot notes, the use of PCI in stable angina has decreased. More interesting to me were the results of an online study conducted by Rothberg and colleagues in which participants were provided different background information about PCI.5 Even if given explicit information that PCI did not prevent MI, a fair number still said they would choose it and still believed it would prevent this outcome. Bias clearly influences what patients read and hear, and they bring these biases into the shared decision-making process.
While some patients may not fully understand PCI’s risks and putative benefits, others may choose it because of their personal knowledge of others’ experience or perhaps because the “softer” benefits demonstrated in COURAGE and other trials are important to them. As outlined by Khot, patients who underwent PCI had more rapid relief of angina symptoms, possibly experienced greater relief of symptoms even if incomplete, and needed less medication. More patients needed urgent revascularization in the medical group than in the PCI group. Rothberg appropriately notes that this did not “equate to a reduction in the rate of MI,” but to some patients (eg, international travelers, caregivers) this higher possibility of needing an urgent procedure may be enough to make them want the initial elective procedure. While patients should be told that many of the patients in the medical therapy group in COURAGE crossed over to get PCI (16% at 1 year, and about 1/3 after a median of 4.6 years of follow-up), a patient for whom avoiding invasive procedures is the highest priority will likely “hear” that he or she has a 2/3 likelihood of not needing PCI without being at increased risk of death or MI with medical therapy.
As Rothberg points out, “providing information alone is not enough.” The patient needs to recognize, verbalize, and perhaps rank his or her own biases, fears, and desires. Equally important, we need to recognize our own biases and not let them overshadow the patient’s concerns.
I urge you to read both articles, not only because they offer excellent critiques of the COURAGE results and what they mean in practice, but also because they should make us reflect on how often and well we engage in shared decision-making with our patients. Reading these made me realize that I need to better understand my patients’ concerns. Discussing my interpretation of clinical study results, no matter how sophisticated or correct, and then offering a recommendation without fully understanding the patient’s treatment goals is not shared decision-making. The seemingly “wrong” decision may be right for the patient.
- Rothberg M. PCI for stable angina: a missed opportunity for shared decision-making. Cleve Clin J Med 2018; 85:105–121.
- Khot UN. Having the COURAGE to include PCI in shared decision-making for stable angina. Cleve Clin J Med 2018; 85:124–127.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med 2007; 167:1604–1609.
- Rothberg MB, Scherer L, Kashef MA, et al. The effect of information presentation on beliefs about the benefits of elective percutaneous coronary intervention. JAMA Intern Med 2014; 174:1623–1629.
It is difficult to provide enough information for informed consent and to ensure that the patient and his or her family fully understand what we are saying. Patients often come in with their own preferences and biases based on anecdote, dinner conversations, or the Internet. The physician must push hard to dispel a patient’s bias with the facts, while recognizing that we too regularly present “facts” and recommendations colored by our own biases based on anecdotal experience, professional ritual, and intellectual hubris.
Two articles in this issue of the Journal, one by Dr. Michael Rothberg1 and the other by Dr. Umesh Khot,2 examine percutaneous coronary intervention (PCI) in patients with stable chronic angina. Both discuss the findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial3 and how to use these findings in helping patients decide whether to undergo PCI.
Rothberg and Khot agree that in the COURAGE trial, PCI effectively if not completely reduced angina but did not decrease the likelihood of death or subsequent myocardial infarction (MI). Patients were excluded from the study if they had a likelihood of left main disease, heart failure, or severe angina. All underwent catheterization, and all were given optimal medical therapy. Thus, the trial results do not directly relate to every patient with stable angina.
While the patient may find it confusing that angina and the risk of MI are not reduced in parallel, since both are due to atherosclerosis, their dynamic pathophysiology is different. The COURAGE results are the mirror image of those in some early studies of aspirin in coronary disease, in which aspirin reduced the incidence of MI but did not significantly affect angina.
In view of the COURAGE results, Rothberg seems surprised that PCI continues to be frequently used in patients with stable angina. He points out that according to some surveys,4 not all cardiologists have embraced these (and other similar study) results. But as Khot notes, the use of PCI in stable angina has decreased. More interesting to me were the results of an online study conducted by Rothberg and colleagues in which participants were provided different background information about PCI.5 Even if given explicit information that PCI did not prevent MI, a fair number still said they would choose it and still believed it would prevent this outcome. Bias clearly influences what patients read and hear, and they bring these biases into the shared decision-making process.
While some patients may not fully understand PCI’s risks and putative benefits, others may choose it because of their personal knowledge of others’ experience or perhaps because the “softer” benefits demonstrated in COURAGE and other trials are important to them. As outlined by Khot, patients who underwent PCI had more rapid relief of angina symptoms, possibly experienced greater relief of symptoms even if incomplete, and needed less medication. More patients needed urgent revascularization in the medical group than in the PCI group. Rothberg appropriately notes that this did not “equate to a reduction in the rate of MI,” but to some patients (eg, international travelers, caregivers) this higher possibility of needing an urgent procedure may be enough to make them want the initial elective procedure. While patients should be told that many of the patients in the medical therapy group in COURAGE crossed over to get PCI (16% at 1 year, and about 1/3 after a median of 4.6 years of follow-up), a patient for whom avoiding invasive procedures is the highest priority will likely “hear” that he or she has a 2/3 likelihood of not needing PCI without being at increased risk of death or MI with medical therapy.
As Rothberg points out, “providing information alone is not enough.” The patient needs to recognize, verbalize, and perhaps rank his or her own biases, fears, and desires. Equally important, we need to recognize our own biases and not let them overshadow the patient’s concerns.
I urge you to read both articles, not only because they offer excellent critiques of the COURAGE results and what they mean in practice, but also because they should make us reflect on how often and well we engage in shared decision-making with our patients. Reading these made me realize that I need to better understand my patients’ concerns. Discussing my interpretation of clinical study results, no matter how sophisticated or correct, and then offering a recommendation without fully understanding the patient’s treatment goals is not shared decision-making. The seemingly “wrong” decision may be right for the patient.
It is difficult to provide enough information for informed consent and to ensure that the patient and his or her family fully understand what we are saying. Patients often come in with their own preferences and biases based on anecdote, dinner conversations, or the Internet. The physician must push hard to dispel a patient’s bias with the facts, while recognizing that we too regularly present “facts” and recommendations colored by our own biases based on anecdotal experience, professional ritual, and intellectual hubris.
Two articles in this issue of the Journal, one by Dr. Michael Rothberg1 and the other by Dr. Umesh Khot,2 examine percutaneous coronary intervention (PCI) in patients with stable chronic angina. Both discuss the findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial3 and how to use these findings in helping patients decide whether to undergo PCI.
Rothberg and Khot agree that in the COURAGE trial, PCI effectively if not completely reduced angina but did not decrease the likelihood of death or subsequent myocardial infarction (MI). Patients were excluded from the study if they had a likelihood of left main disease, heart failure, or severe angina. All underwent catheterization, and all were given optimal medical therapy. Thus, the trial results do not directly relate to every patient with stable angina.
While the patient may find it confusing that angina and the risk of MI are not reduced in parallel, since both are due to atherosclerosis, their dynamic pathophysiology is different. The COURAGE results are the mirror image of those in some early studies of aspirin in coronary disease, in which aspirin reduced the incidence of MI but did not significantly affect angina.
In view of the COURAGE results, Rothberg seems surprised that PCI continues to be frequently used in patients with stable angina. He points out that according to some surveys,4 not all cardiologists have embraced these (and other similar study) results. But as Khot notes, the use of PCI in stable angina has decreased. More interesting to me were the results of an online study conducted by Rothberg and colleagues in which participants were provided different background information about PCI.5 Even if given explicit information that PCI did not prevent MI, a fair number still said they would choose it and still believed it would prevent this outcome. Bias clearly influences what patients read and hear, and they bring these biases into the shared decision-making process.
While some patients may not fully understand PCI’s risks and putative benefits, others may choose it because of their personal knowledge of others’ experience or perhaps because the “softer” benefits demonstrated in COURAGE and other trials are important to them. As outlined by Khot, patients who underwent PCI had more rapid relief of angina symptoms, possibly experienced greater relief of symptoms even if incomplete, and needed less medication. More patients needed urgent revascularization in the medical group than in the PCI group. Rothberg appropriately notes that this did not “equate to a reduction in the rate of MI,” but to some patients (eg, international travelers, caregivers) this higher possibility of needing an urgent procedure may be enough to make them want the initial elective procedure. While patients should be told that many of the patients in the medical therapy group in COURAGE crossed over to get PCI (16% at 1 year, and about 1/3 after a median of 4.6 years of follow-up), a patient for whom avoiding invasive procedures is the highest priority will likely “hear” that he or she has a 2/3 likelihood of not needing PCI without being at increased risk of death or MI with medical therapy.
As Rothberg points out, “providing information alone is not enough.” The patient needs to recognize, verbalize, and perhaps rank his or her own biases, fears, and desires. Equally important, we need to recognize our own biases and not let them overshadow the patient’s concerns.
I urge you to read both articles, not only because they offer excellent critiques of the COURAGE results and what they mean in practice, but also because they should make us reflect on how often and well we engage in shared decision-making with our patients. Reading these made me realize that I need to better understand my patients’ concerns. Discussing my interpretation of clinical study results, no matter how sophisticated or correct, and then offering a recommendation without fully understanding the patient’s treatment goals is not shared decision-making. The seemingly “wrong” decision may be right for the patient.
- Rothberg M. PCI for stable angina: a missed opportunity for shared decision-making. Cleve Clin J Med 2018; 85:105–121.
- Khot UN. Having the COURAGE to include PCI in shared decision-making for stable angina. Cleve Clin J Med 2018; 85:124–127.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med 2007; 167:1604–1609.
- Rothberg MB, Scherer L, Kashef MA, et al. The effect of information presentation on beliefs about the benefits of elective percutaneous coronary intervention. JAMA Intern Med 2014; 174:1623–1629.
- Rothberg M. PCI for stable angina: a missed opportunity for shared decision-making. Cleve Clin J Med 2018; 85:105–121.
- Khot UN. Having the COURAGE to include PCI in shared decision-making for stable angina. Cleve Clin J Med 2018; 85:124–127.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med 2007; 167:1604–1609.
- Rothberg MB, Scherer L, Kashef MA, et al. The effect of information presentation on beliefs about the benefits of elective percutaneous coronary intervention. JAMA Intern Med 2014; 174:1623–1629.