User login
Colonic Malignancy Risk Appears Low After Uncomplicated Diverticulitis
Clinical question: What is the benefit of routine colonic evaluation after an episode of acute diverticulitis?
Background: Currently accepted guidelines recommend routine colonic evaluation (colonoscopy, computed tomography (CT) colonography) after an episode of acute diverticulitis to confirm the diagnosis and exclude malignancy. Increased use of CT to confirm the diagnosis of acute diverticulitis and exclude associated complications has brought into question the recommendation for routine colonic evaluation after an episode of acute diverticulitis.
Study design: Meta-analysis.
Setting: Search of online databases and the Cochrane Library.
Synopsis: Eleven studies from seven countries included 1,970 patients who had a colonic evaluation after an episode of acute diverticulitis. The risk of finding a malignancy was 1.6%. Within this population, 1,497 patients were identified as having uncomplicated diverticulitis. Cancer was found in only five patients (proportional risk estimate 0.7%).
For the 79 patients identified as having complicated diverticulitis, the risk of finding a malignancy on subsequent screening was 10.8%.
Every systematic review is limited by the quality of the studies available for review and the differences in design and methodology of the studies. In this meta-analysis, the risk of finding cancer after an episode of uncomplicated diverticulitis appears to be low. Given the limited resources of the healthcare system and the small but real risk of morbidity and mortality associated with invasive colonic procedures, the routine recommendation for colon cancer screening after an episode of acute uncomplicated diverticulitis should be further evaluated.
Bottom line: The risk of malignancy after a radiologically proven episode of acute uncomplicated diverticulitis is low. In the absence of other indications, additional routine colonic evaluation may not be necessary.
Citation: Sharma PV, Eglinton T, Hider P, Frizelle F. Systematic review and meta-analysis of the role of routine colonic evaluation after radiologically confirmed acute diverticulitis. Ann Surg. 2014;259(2):263-272.
Clinical question: What is the benefit of routine colonic evaluation after an episode of acute diverticulitis?
Background: Currently accepted guidelines recommend routine colonic evaluation (colonoscopy, computed tomography (CT) colonography) after an episode of acute diverticulitis to confirm the diagnosis and exclude malignancy. Increased use of CT to confirm the diagnosis of acute diverticulitis and exclude associated complications has brought into question the recommendation for routine colonic evaluation after an episode of acute diverticulitis.
Study design: Meta-analysis.
Setting: Search of online databases and the Cochrane Library.
Synopsis: Eleven studies from seven countries included 1,970 patients who had a colonic evaluation after an episode of acute diverticulitis. The risk of finding a malignancy was 1.6%. Within this population, 1,497 patients were identified as having uncomplicated diverticulitis. Cancer was found in only five patients (proportional risk estimate 0.7%).
For the 79 patients identified as having complicated diverticulitis, the risk of finding a malignancy on subsequent screening was 10.8%.
Every systematic review is limited by the quality of the studies available for review and the differences in design and methodology of the studies. In this meta-analysis, the risk of finding cancer after an episode of uncomplicated diverticulitis appears to be low. Given the limited resources of the healthcare system and the small but real risk of morbidity and mortality associated with invasive colonic procedures, the routine recommendation for colon cancer screening after an episode of acute uncomplicated diverticulitis should be further evaluated.
Bottom line: The risk of malignancy after a radiologically proven episode of acute uncomplicated diverticulitis is low. In the absence of other indications, additional routine colonic evaluation may not be necessary.
Citation: Sharma PV, Eglinton T, Hider P, Frizelle F. Systematic review and meta-analysis of the role of routine colonic evaluation after radiologically confirmed acute diverticulitis. Ann Surg. 2014;259(2):263-272.
Clinical question: What is the benefit of routine colonic evaluation after an episode of acute diverticulitis?
Background: Currently accepted guidelines recommend routine colonic evaluation (colonoscopy, computed tomography (CT) colonography) after an episode of acute diverticulitis to confirm the diagnosis and exclude malignancy. Increased use of CT to confirm the diagnosis of acute diverticulitis and exclude associated complications has brought into question the recommendation for routine colonic evaluation after an episode of acute diverticulitis.
Study design: Meta-analysis.
Setting: Search of online databases and the Cochrane Library.
Synopsis: Eleven studies from seven countries included 1,970 patients who had a colonic evaluation after an episode of acute diverticulitis. The risk of finding a malignancy was 1.6%. Within this population, 1,497 patients were identified as having uncomplicated diverticulitis. Cancer was found in only five patients (proportional risk estimate 0.7%).
For the 79 patients identified as having complicated diverticulitis, the risk of finding a malignancy on subsequent screening was 10.8%.
Every systematic review is limited by the quality of the studies available for review and the differences in design and methodology of the studies. In this meta-analysis, the risk of finding cancer after an episode of uncomplicated diverticulitis appears to be low. Given the limited resources of the healthcare system and the small but real risk of morbidity and mortality associated with invasive colonic procedures, the routine recommendation for colon cancer screening after an episode of acute uncomplicated diverticulitis should be further evaluated.
Bottom line: The risk of malignancy after a radiologically proven episode of acute uncomplicated diverticulitis is low. In the absence of other indications, additional routine colonic evaluation may not be necessary.
Citation: Sharma PV, Eglinton T, Hider P, Frizelle F. Systematic review and meta-analysis of the role of routine colonic evaluation after radiologically confirmed acute diverticulitis. Ann Surg. 2014;259(2):263-272.
Physician Burnout Reduced with Intervention Groups
Clinical question: Does an intervention involving a facilitated physician small group result in improvement in well-being and reduction in burnout?
Background: Burnout affects nearly half of medical students, residents, and practicing physicians in the U.S.; however, very few interventions have been tested to address this problem.
Study design: Randomized controlled trial (RCT).
Setting: Department of Medicine at the Mayo Clinic, Rochester, Minn.
Synopsis: Practicing physicians were randomly assigned to facilitated, small-group intervention curriculum for one hour every two weeks (N=37) or control with unstructured, protected time for one hour every two weeks (N=37). A non-trial cohort of 350 practicing physicians was surveyed annually. This study showed a significant increase in empowerment and engagement at three months that was sustained for 12 months, and a significant decrease in high depersonalization scores was seen at both three and 12 months in the intervention group. There were no significant differences in stress, depression, quality of life, or job satisfaction.
Compared to the non-trial cohort, depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention arm and slightly in the control arm.
Sample size was small and results may not be generalizable. Topics covered included reflection, self-awareness, and mindfulness, with a combination of community building and skill acquisition to promote connectedness and meaning in work. It is not clear which elements of the curriculum were most effective.
Bottom line: A facilitated, small-group intervention with institution-provided protected time can improve physician empowerment and engagement and reduce depersonalization, an important component of burnout.
Citation: West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
Clinical question: Does an intervention involving a facilitated physician small group result in improvement in well-being and reduction in burnout?
Background: Burnout affects nearly half of medical students, residents, and practicing physicians in the U.S.; however, very few interventions have been tested to address this problem.
Study design: Randomized controlled trial (RCT).
Setting: Department of Medicine at the Mayo Clinic, Rochester, Minn.
Synopsis: Practicing physicians were randomly assigned to facilitated, small-group intervention curriculum for one hour every two weeks (N=37) or control with unstructured, protected time for one hour every two weeks (N=37). A non-trial cohort of 350 practicing physicians was surveyed annually. This study showed a significant increase in empowerment and engagement at three months that was sustained for 12 months, and a significant decrease in high depersonalization scores was seen at both three and 12 months in the intervention group. There were no significant differences in stress, depression, quality of life, or job satisfaction.
Compared to the non-trial cohort, depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention arm and slightly in the control arm.
Sample size was small and results may not be generalizable. Topics covered included reflection, self-awareness, and mindfulness, with a combination of community building and skill acquisition to promote connectedness and meaning in work. It is not clear which elements of the curriculum were most effective.
Bottom line: A facilitated, small-group intervention with institution-provided protected time can improve physician empowerment and engagement and reduce depersonalization, an important component of burnout.
Citation: West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
Clinical question: Does an intervention involving a facilitated physician small group result in improvement in well-being and reduction in burnout?
Background: Burnout affects nearly half of medical students, residents, and practicing physicians in the U.S.; however, very few interventions have been tested to address this problem.
Study design: Randomized controlled trial (RCT).
Setting: Department of Medicine at the Mayo Clinic, Rochester, Minn.
Synopsis: Practicing physicians were randomly assigned to facilitated, small-group intervention curriculum for one hour every two weeks (N=37) or control with unstructured, protected time for one hour every two weeks (N=37). A non-trial cohort of 350 practicing physicians was surveyed annually. This study showed a significant increase in empowerment and engagement at three months that was sustained for 12 months, and a significant decrease in high depersonalization scores was seen at both three and 12 months in the intervention group. There were no significant differences in stress, depression, quality of life, or job satisfaction.
Compared to the non-trial cohort, depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention arm and slightly in the control arm.
Sample size was small and results may not be generalizable. Topics covered included reflection, self-awareness, and mindfulness, with a combination of community building and skill acquisition to promote connectedness and meaning in work. It is not clear which elements of the curriculum were most effective.
Bottom line: A facilitated, small-group intervention with institution-provided protected time can improve physician empowerment and engagement and reduce depersonalization, an important component of burnout.
Citation: West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
Adult Hospital Medicine Boot Camp for Physician Assistants, Nurse Practitioners
Nurse practitioners and physician assistants are a critical part of the hospitalist care team. Together with the American Academy of Physician Assistants, SHM is hosting the annual Adult Hospital Medicine Boot Camp (www.aapa.org/bootcamp) specifically for nurse practitioners (NPs) and physician assistants (PAs).
The four-day program helps PAs and NPs stay up to date on the most common diagnoses, diseases, and treatments for hospitalized patients (27.75 hours Category 1 CME). A pre-course for PAs and NPs new to hospital medicine introduces them to the unique demands of inpatient care (eight hours Category 1 CME).
Adult Hospital Medicine Boot Camp October 2-5, 2014
The Westin Peachtree Plaza, Atlanta
Hospital Medicine 101
October 1, 2014
The Westin Peachtree Plaza, Atlanta
Nurse practitioners and physician assistants are a critical part of the hospitalist care team. Together with the American Academy of Physician Assistants, SHM is hosting the annual Adult Hospital Medicine Boot Camp (www.aapa.org/bootcamp) specifically for nurse practitioners (NPs) and physician assistants (PAs).
The four-day program helps PAs and NPs stay up to date on the most common diagnoses, diseases, and treatments for hospitalized patients (27.75 hours Category 1 CME). A pre-course for PAs and NPs new to hospital medicine introduces them to the unique demands of inpatient care (eight hours Category 1 CME).
Adult Hospital Medicine Boot Camp October 2-5, 2014
The Westin Peachtree Plaza, Atlanta
Hospital Medicine 101
October 1, 2014
The Westin Peachtree Plaza, Atlanta
Nurse practitioners and physician assistants are a critical part of the hospitalist care team. Together with the American Academy of Physician Assistants, SHM is hosting the annual Adult Hospital Medicine Boot Camp (www.aapa.org/bootcamp) specifically for nurse practitioners (NPs) and physician assistants (PAs).
The four-day program helps PAs and NPs stay up to date on the most common diagnoses, diseases, and treatments for hospitalized patients (27.75 hours Category 1 CME). A pre-course for PAs and NPs new to hospital medicine introduces them to the unique demands of inpatient care (eight hours Category 1 CME).
Adult Hospital Medicine Boot Camp October 2-5, 2014
The Westin Peachtree Plaza, Atlanta
Hospital Medicine 101
October 1, 2014
The Westin Peachtree Plaza, Atlanta
Deaf Hospitalist Focuses on Teaching, Co-Management, Patient-Centered Care
"What’s the bigger picture here?” Hospitalist Christopher Moreland, MD, MPH, FACP, drops his question neatly into the pause in resident Adrienne Victor, MD’s presentation of patient status and lab results.
We’re on the bustling 9th floor of University Hospital at the University of Texas Health Science Center (UTHSCSA) in San Antonio during fast-paced morning rounds. As attending physician, Dr. Moreland is focusing intently on Dr. Victor’s face, simultaneously monitoring the American Sign Language (ASL) interpretation of Todd Agan, CI/CT, BEI Master Interpreter. Immediately after his question to Dr. Victor, the discussion—conducted in both ASL and spoken English—shifts to the patient’s psychosocial issues and whether a palliative care consult would be advisable.
It’s clear that for Dr. Moreland, the work, not his lack of hearing, is the main point here. A hospitalist with the UTHSCSA team since 2010, Dr. Moreland quickly established himself not only as a valuable HM team member and educator, but also as a leader in other domains. For example, in addition to his academic appointment as assistant clinical professor of medicine, he previously was co-director of the medicine consult and co-management service at University Hospital and now serves as UTHSCSA’s associate program director for the internal medicine residency program.
Dr. Moreland’s question this morning is typical of his teaching, says Bret Simon, PhD, an educational development specialist and assistant professor with the division of hospital medicine at UTHSCSA.

–Christopher Moreland, MD, MPH, FACP
“He’s very good at using questions to teach, promoting reflection rather than simply telling the student what to do,” Dr. Simon explains.
Why Medicine?
Chris Moreland’s parents discovered their son was deaf at age two, by which time he had acquired very few spoken words. After multiple visits to healthcare professionals, a physician finally identified his deafness. The family then embarked on a bimodal approach to his education, using both signed and spoken English. He learned ASL in college. As a result, he communicates through a variety of channels: ASL with interpreters Agan and Keri Richardson, speech reading, and spoken English. When examining patients, he uses an electronic stethoscope that interfaces with his cochlear implant.
Medicine was not Dr. Moreland’s first academic choice.
“I went into college thinking I wanted to do computer science,” he says, speaking of his undergraduate studies at the University of Texas in Austin. When he realized computers were not for him, he switched his major to theater arts, continuing an interest he had had in high school. After that, research seemed appealing, and he became a research assistant in a lab in the Department of Anthropology. Finally, after shadowing a number of physicians, his interest in medical science was stimulated.
“Medicine,” he says, “became a nice culmination of everything I was interested in doing.” From computer science, he learned to appreciate an understanding of algorithms; from theater arts came the ability to understand where people are coming from; and from his link with research in linguistics and anthropology came the contribution of problem solving and methodology.
Fearless Communicator
Dr. Moreland says his deafness presents no impediments to his practice of medicine. “I grew up working with interpreters, so I’m used to that process,” he says. “It forces you to become less inhibited about what you’re doing. People have questions [‘who is that other person in the room?’], and you learn how to handle those questions quickly, without interfering with communication in order to advance the work.”
When Dr. Moreland started his clinical rotations as a third-year medical student, he grappled with the best way to introduce himself and his interpreter to patients. His first attempt at explaining the interpretive process “went on for quite a while” and was too much information. “It ended up overwhelming the patient,” he says.
The next time he chose not to introduce the interpreter but to simply address the patient directly. “That didn’t work either, because the patient’s eyes kept wandering to that other person in the room.”
Finally, “I realized that it wasn’t about me,” he says. “It was about the patient.” So he simply shortened the introduction to himself and the interpreter and asked the patients how they were doing.
“Once I became more professional about the situation, the more positive and patient-centered it became, and it went well.” He says he’s had no negative experiences since then, at least not related to his deafness. He approaches each new patient interaction proactively, and he and his interpreters become part of the flow of care.
Teaching’s Missing Pieces
As illustrated with his first question, Dr. Moreland intends for his trainees to learn to think globally about their patients.
“Although rote information has its role,” he explains later in the conference room, “I’m always afraid of overemphasizing it. When I trained in medical school, we didn’t learn that much about communication skills and teamwork. We talked a lot about information we use as physicians—the mechanism of disease, the drugs we use.
“What I try to emphasize with trainees is, what skills in communication, teamwork, and self-education can we develop so that we can use those skills continuously throughout our practice?”
Dr. Moreland takes setting resident-generated learning goals seriously, says Dr. Simon, for which he and trainees give him high marks.
“He is very supportive and encourages us to make our own management decisions,” Dr. Victor says. “Though, of course, he will let us know if something is likely the wrong choice, usually by discussing it first.”
Patrick S. Romano, MD, MPH, professor of general medicine and pediatrics and former director of the Primary Care Outcomes Research (PCOR) faculty development program at the University of California Davis, where Dr. Moreland was a resident and then a fellow, found his trainee was always “very thoughtful and conscientious, presenting different ways of looking at problems and asking the right questions. And, of course, that’s what we look for in teachers: people who know how to ask the right questions, because, then, of course, they are able to answer students’ questions.”
Transformational and Inspirational
For many of Dr. Moreland’s colleagues and trainees, working with him has been their first exposure to a hearing-impaired physician. Richard L. Kravitz, MD, MSPH, professor and co-vice chair of research in the department of medicine at UC Davis, supervised Dr. Moreland during his residency and later during his PCOR fellowship. The American Disabilities Act-mandated interpreter for Dr. Moreland introduced a “change in standard operating procedure,” Dr. Kravitz notes. “None of us knew what to expect when he came onboard the residency program. But, very quickly, any unease was put to rest because he was just so talented.”
For visitors, Dr. Moreland seamlessly addresses his hearing impairment and makes sure that everyone on the team is following the discussion. Luci K. Leykum, MD, MBA, MSc, hospital medicine division chief and associate dean for clinical affairs at UTHSCSA, says that Dr. Moreland has brought “a lot of positive energy to the group—and in ways I would not have expected.” She praised his talents as both a clinician and teacher.
John G. Rees, DBA, RN, patient care coordinator in the 5th Acute Care Unit, says that Dr. Moreland immediately “blended” with the staff on his service. “The rapport was perfect,” he adds.
Robert L. Talbert, PharmD, the SmithKline Centennial Professor of Pharmacy at the College of Pharmacy at the University of Texas at Austin, often participates in teaching rounds. Dr. Moreland, he says, “has an excellent fund of knowledge; he’s very rational and evidence-based in decisions he makes. He’s exactly what a physician should be.”
Watching interpreters Agan and Richardson during group meetings, Dr. Leykum believes, has influenced their group dynamics. “On a subtle level, having Chris in the group has made us more aware of how we interact with each other.”
Nilam Soni, MD, FHM associate professor in the department of medicine and leader of ultrasound education, has noticed that he has become attuned to Dr. Moreland’s way of communicating and often does not need the interpreters to decipher the conversation between them. Working with Dr. Moreland has given Dr. Soni “a better understanding of how to communicate effectively with patients that have difficulty hearing.”
After working with Dr. Moreland at UC Davis, Dr. Kravitz observed that employing physicians with hearing impairment or other disabilities brings additional benefits to the institution. Dr. Moreland’s presence “probably raised the level of understanding of the entire internal medicine staff, because it demonstrated that a disability is what you make of it,” he says. “One recognizes how porous the barriers are, provided that people with disabilities are supported appropriately. In that way, Chris was inspiring, and may have changed the way some of us look at this specific disability that he had, but also other disabilities.”
A bigger picture, indeed.
Gretchen Henkel is a freelance writer in California.
Reference
"What’s the bigger picture here?” Hospitalist Christopher Moreland, MD, MPH, FACP, drops his question neatly into the pause in resident Adrienne Victor, MD’s presentation of patient status and lab results.
We’re on the bustling 9th floor of University Hospital at the University of Texas Health Science Center (UTHSCSA) in San Antonio during fast-paced morning rounds. As attending physician, Dr. Moreland is focusing intently on Dr. Victor’s face, simultaneously monitoring the American Sign Language (ASL) interpretation of Todd Agan, CI/CT, BEI Master Interpreter. Immediately after his question to Dr. Victor, the discussion—conducted in both ASL and spoken English—shifts to the patient’s psychosocial issues and whether a palliative care consult would be advisable.
It’s clear that for Dr. Moreland, the work, not his lack of hearing, is the main point here. A hospitalist with the UTHSCSA team since 2010, Dr. Moreland quickly established himself not only as a valuable HM team member and educator, but also as a leader in other domains. For example, in addition to his academic appointment as assistant clinical professor of medicine, he previously was co-director of the medicine consult and co-management service at University Hospital and now serves as UTHSCSA’s associate program director for the internal medicine residency program.
Dr. Moreland’s question this morning is typical of his teaching, says Bret Simon, PhD, an educational development specialist and assistant professor with the division of hospital medicine at UTHSCSA.

–Christopher Moreland, MD, MPH, FACP
“He’s very good at using questions to teach, promoting reflection rather than simply telling the student what to do,” Dr. Simon explains.
Why Medicine?
Chris Moreland’s parents discovered their son was deaf at age two, by which time he had acquired very few spoken words. After multiple visits to healthcare professionals, a physician finally identified his deafness. The family then embarked on a bimodal approach to his education, using both signed and spoken English. He learned ASL in college. As a result, he communicates through a variety of channels: ASL with interpreters Agan and Keri Richardson, speech reading, and spoken English. When examining patients, he uses an electronic stethoscope that interfaces with his cochlear implant.
Medicine was not Dr. Moreland’s first academic choice.
“I went into college thinking I wanted to do computer science,” he says, speaking of his undergraduate studies at the University of Texas in Austin. When he realized computers were not for him, he switched his major to theater arts, continuing an interest he had had in high school. After that, research seemed appealing, and he became a research assistant in a lab in the Department of Anthropology. Finally, after shadowing a number of physicians, his interest in medical science was stimulated.
“Medicine,” he says, “became a nice culmination of everything I was interested in doing.” From computer science, he learned to appreciate an understanding of algorithms; from theater arts came the ability to understand where people are coming from; and from his link with research in linguistics and anthropology came the contribution of problem solving and methodology.
Fearless Communicator
Dr. Moreland says his deafness presents no impediments to his practice of medicine. “I grew up working with interpreters, so I’m used to that process,” he says. “It forces you to become less inhibited about what you’re doing. People have questions [‘who is that other person in the room?’], and you learn how to handle those questions quickly, without interfering with communication in order to advance the work.”
When Dr. Moreland started his clinical rotations as a third-year medical student, he grappled with the best way to introduce himself and his interpreter to patients. His first attempt at explaining the interpretive process “went on for quite a while” and was too much information. “It ended up overwhelming the patient,” he says.
The next time he chose not to introduce the interpreter but to simply address the patient directly. “That didn’t work either, because the patient’s eyes kept wandering to that other person in the room.”
Finally, “I realized that it wasn’t about me,” he says. “It was about the patient.” So he simply shortened the introduction to himself and the interpreter and asked the patients how they were doing.
“Once I became more professional about the situation, the more positive and patient-centered it became, and it went well.” He says he’s had no negative experiences since then, at least not related to his deafness. He approaches each new patient interaction proactively, and he and his interpreters become part of the flow of care.
Teaching’s Missing Pieces
As illustrated with his first question, Dr. Moreland intends for his trainees to learn to think globally about their patients.
“Although rote information has its role,” he explains later in the conference room, “I’m always afraid of overemphasizing it. When I trained in medical school, we didn’t learn that much about communication skills and teamwork. We talked a lot about information we use as physicians—the mechanism of disease, the drugs we use.
“What I try to emphasize with trainees is, what skills in communication, teamwork, and self-education can we develop so that we can use those skills continuously throughout our practice?”
Dr. Moreland takes setting resident-generated learning goals seriously, says Dr. Simon, for which he and trainees give him high marks.
“He is very supportive and encourages us to make our own management decisions,” Dr. Victor says. “Though, of course, he will let us know if something is likely the wrong choice, usually by discussing it first.”
Patrick S. Romano, MD, MPH, professor of general medicine and pediatrics and former director of the Primary Care Outcomes Research (PCOR) faculty development program at the University of California Davis, where Dr. Moreland was a resident and then a fellow, found his trainee was always “very thoughtful and conscientious, presenting different ways of looking at problems and asking the right questions. And, of course, that’s what we look for in teachers: people who know how to ask the right questions, because, then, of course, they are able to answer students’ questions.”
Transformational and Inspirational
For many of Dr. Moreland’s colleagues and trainees, working with him has been their first exposure to a hearing-impaired physician. Richard L. Kravitz, MD, MSPH, professor and co-vice chair of research in the department of medicine at UC Davis, supervised Dr. Moreland during his residency and later during his PCOR fellowship. The American Disabilities Act-mandated interpreter for Dr. Moreland introduced a “change in standard operating procedure,” Dr. Kravitz notes. “None of us knew what to expect when he came onboard the residency program. But, very quickly, any unease was put to rest because he was just so talented.”
For visitors, Dr. Moreland seamlessly addresses his hearing impairment and makes sure that everyone on the team is following the discussion. Luci K. Leykum, MD, MBA, MSc, hospital medicine division chief and associate dean for clinical affairs at UTHSCSA, says that Dr. Moreland has brought “a lot of positive energy to the group—and in ways I would not have expected.” She praised his talents as both a clinician and teacher.
John G. Rees, DBA, RN, patient care coordinator in the 5th Acute Care Unit, says that Dr. Moreland immediately “blended” with the staff on his service. “The rapport was perfect,” he adds.
Robert L. Talbert, PharmD, the SmithKline Centennial Professor of Pharmacy at the College of Pharmacy at the University of Texas at Austin, often participates in teaching rounds. Dr. Moreland, he says, “has an excellent fund of knowledge; he’s very rational and evidence-based in decisions he makes. He’s exactly what a physician should be.”
Watching interpreters Agan and Richardson during group meetings, Dr. Leykum believes, has influenced their group dynamics. “On a subtle level, having Chris in the group has made us more aware of how we interact with each other.”
Nilam Soni, MD, FHM associate professor in the department of medicine and leader of ultrasound education, has noticed that he has become attuned to Dr. Moreland’s way of communicating and often does not need the interpreters to decipher the conversation between them. Working with Dr. Moreland has given Dr. Soni “a better understanding of how to communicate effectively with patients that have difficulty hearing.”
After working with Dr. Moreland at UC Davis, Dr. Kravitz observed that employing physicians with hearing impairment or other disabilities brings additional benefits to the institution. Dr. Moreland’s presence “probably raised the level of understanding of the entire internal medicine staff, because it demonstrated that a disability is what you make of it,” he says. “One recognizes how porous the barriers are, provided that people with disabilities are supported appropriately. In that way, Chris was inspiring, and may have changed the way some of us look at this specific disability that he had, but also other disabilities.”
A bigger picture, indeed.
Gretchen Henkel is a freelance writer in California.
Reference
"What’s the bigger picture here?” Hospitalist Christopher Moreland, MD, MPH, FACP, drops his question neatly into the pause in resident Adrienne Victor, MD’s presentation of patient status and lab results.
We’re on the bustling 9th floor of University Hospital at the University of Texas Health Science Center (UTHSCSA) in San Antonio during fast-paced morning rounds. As attending physician, Dr. Moreland is focusing intently on Dr. Victor’s face, simultaneously monitoring the American Sign Language (ASL) interpretation of Todd Agan, CI/CT, BEI Master Interpreter. Immediately after his question to Dr. Victor, the discussion—conducted in both ASL and spoken English—shifts to the patient’s psychosocial issues and whether a palliative care consult would be advisable.
It’s clear that for Dr. Moreland, the work, not his lack of hearing, is the main point here. A hospitalist with the UTHSCSA team since 2010, Dr. Moreland quickly established himself not only as a valuable HM team member and educator, but also as a leader in other domains. For example, in addition to his academic appointment as assistant clinical professor of medicine, he previously was co-director of the medicine consult and co-management service at University Hospital and now serves as UTHSCSA’s associate program director for the internal medicine residency program.
Dr. Moreland’s question this morning is typical of his teaching, says Bret Simon, PhD, an educational development specialist and assistant professor with the division of hospital medicine at UTHSCSA.

–Christopher Moreland, MD, MPH, FACP
“He’s very good at using questions to teach, promoting reflection rather than simply telling the student what to do,” Dr. Simon explains.
Why Medicine?
Chris Moreland’s parents discovered their son was deaf at age two, by which time he had acquired very few spoken words. After multiple visits to healthcare professionals, a physician finally identified his deafness. The family then embarked on a bimodal approach to his education, using both signed and spoken English. He learned ASL in college. As a result, he communicates through a variety of channels: ASL with interpreters Agan and Keri Richardson, speech reading, and spoken English. When examining patients, he uses an electronic stethoscope that interfaces with his cochlear implant.
Medicine was not Dr. Moreland’s first academic choice.
“I went into college thinking I wanted to do computer science,” he says, speaking of his undergraduate studies at the University of Texas in Austin. When he realized computers were not for him, he switched his major to theater arts, continuing an interest he had had in high school. After that, research seemed appealing, and he became a research assistant in a lab in the Department of Anthropology. Finally, after shadowing a number of physicians, his interest in medical science was stimulated.
“Medicine,” he says, “became a nice culmination of everything I was interested in doing.” From computer science, he learned to appreciate an understanding of algorithms; from theater arts came the ability to understand where people are coming from; and from his link with research in linguistics and anthropology came the contribution of problem solving and methodology.
Fearless Communicator
Dr. Moreland says his deafness presents no impediments to his practice of medicine. “I grew up working with interpreters, so I’m used to that process,” he says. “It forces you to become less inhibited about what you’re doing. People have questions [‘who is that other person in the room?’], and you learn how to handle those questions quickly, without interfering with communication in order to advance the work.”
When Dr. Moreland started his clinical rotations as a third-year medical student, he grappled with the best way to introduce himself and his interpreter to patients. His first attempt at explaining the interpretive process “went on for quite a while” and was too much information. “It ended up overwhelming the patient,” he says.
The next time he chose not to introduce the interpreter but to simply address the patient directly. “That didn’t work either, because the patient’s eyes kept wandering to that other person in the room.”
Finally, “I realized that it wasn’t about me,” he says. “It was about the patient.” So he simply shortened the introduction to himself and the interpreter and asked the patients how they were doing.
“Once I became more professional about the situation, the more positive and patient-centered it became, and it went well.” He says he’s had no negative experiences since then, at least not related to his deafness. He approaches each new patient interaction proactively, and he and his interpreters become part of the flow of care.
Teaching’s Missing Pieces
As illustrated with his first question, Dr. Moreland intends for his trainees to learn to think globally about their patients.
“Although rote information has its role,” he explains later in the conference room, “I’m always afraid of overemphasizing it. When I trained in medical school, we didn’t learn that much about communication skills and teamwork. We talked a lot about information we use as physicians—the mechanism of disease, the drugs we use.
“What I try to emphasize with trainees is, what skills in communication, teamwork, and self-education can we develop so that we can use those skills continuously throughout our practice?”
Dr. Moreland takes setting resident-generated learning goals seriously, says Dr. Simon, for which he and trainees give him high marks.
“He is very supportive and encourages us to make our own management decisions,” Dr. Victor says. “Though, of course, he will let us know if something is likely the wrong choice, usually by discussing it first.”
Patrick S. Romano, MD, MPH, professor of general medicine and pediatrics and former director of the Primary Care Outcomes Research (PCOR) faculty development program at the University of California Davis, where Dr. Moreland was a resident and then a fellow, found his trainee was always “very thoughtful and conscientious, presenting different ways of looking at problems and asking the right questions. And, of course, that’s what we look for in teachers: people who know how to ask the right questions, because, then, of course, they are able to answer students’ questions.”
Transformational and Inspirational
For many of Dr. Moreland’s colleagues and trainees, working with him has been their first exposure to a hearing-impaired physician. Richard L. Kravitz, MD, MSPH, professor and co-vice chair of research in the department of medicine at UC Davis, supervised Dr. Moreland during his residency and later during his PCOR fellowship. The American Disabilities Act-mandated interpreter for Dr. Moreland introduced a “change in standard operating procedure,” Dr. Kravitz notes. “None of us knew what to expect when he came onboard the residency program. But, very quickly, any unease was put to rest because he was just so talented.”
For visitors, Dr. Moreland seamlessly addresses his hearing impairment and makes sure that everyone on the team is following the discussion. Luci K. Leykum, MD, MBA, MSc, hospital medicine division chief and associate dean for clinical affairs at UTHSCSA, says that Dr. Moreland has brought “a lot of positive energy to the group—and in ways I would not have expected.” She praised his talents as both a clinician and teacher.
John G. Rees, DBA, RN, patient care coordinator in the 5th Acute Care Unit, says that Dr. Moreland immediately “blended” with the staff on his service. “The rapport was perfect,” he adds.
Robert L. Talbert, PharmD, the SmithKline Centennial Professor of Pharmacy at the College of Pharmacy at the University of Texas at Austin, often participates in teaching rounds. Dr. Moreland, he says, “has an excellent fund of knowledge; he’s very rational and evidence-based in decisions he makes. He’s exactly what a physician should be.”
Watching interpreters Agan and Richardson during group meetings, Dr. Leykum believes, has influenced their group dynamics. “On a subtle level, having Chris in the group has made us more aware of how we interact with each other.”
Nilam Soni, MD, FHM associate professor in the department of medicine and leader of ultrasound education, has noticed that he has become attuned to Dr. Moreland’s way of communicating and often does not need the interpreters to decipher the conversation between them. Working with Dr. Moreland has given Dr. Soni “a better understanding of how to communicate effectively with patients that have difficulty hearing.”
After working with Dr. Moreland at UC Davis, Dr. Kravitz observed that employing physicians with hearing impairment or other disabilities brings additional benefits to the institution. Dr. Moreland’s presence “probably raised the level of understanding of the entire internal medicine staff, because it demonstrated that a disability is what you make of it,” he says. “One recognizes how porous the barriers are, provided that people with disabilities are supported appropriately. In that way, Chris was inspiring, and may have changed the way some of us look at this specific disability that he had, but also other disabilities.”
A bigger picture, indeed.
Gretchen Henkel is a freelance writer in California.
Reference
Hospitals Lose $45.9 Billion in Uncompensated Care in 2012
Dollar value of uncompensated care provided by U.S. hospitals in 2012, expressed in terms of actual costs, according to data from the American Hospital Association’s Annual Survey of Hospitals.6 This figure represents 6.1% of total costs, an increase of 11.7% from 2011. The total includes both bad debt and charity care provided to patients unable to pay for their care, AHA says, but does not include underpayments by Medicare and Medicaid.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
Dollar value of uncompensated care provided by U.S. hospitals in 2012, expressed in terms of actual costs, according to data from the American Hospital Association’s Annual Survey of Hospitals.6 This figure represents 6.1% of total costs, an increase of 11.7% from 2011. The total includes both bad debt and charity care provided to patients unable to pay for their care, AHA says, but does not include underpayments by Medicare and Medicaid.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
Dollar value of uncompensated care provided by U.S. hospitals in 2012, expressed in terms of actual costs, according to data from the American Hospital Association’s Annual Survey of Hospitals.6 This figure represents 6.1% of total costs, an increase of 11.7% from 2011. The total includes both bad debt and charity care provided to patients unable to pay for their care, AHA says, but does not include underpayments by Medicare and Medicaid.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
Yogurt May Reduce Clostridium Difficile Infection Rate
The Philadelphia Inquirer recently reported that Holy Redeemer Hospital in Meadowbrook, Pa., cut its incidence of Clostridium difficile by two-thirds after its nutritionists began encouraging hospitalized patients with orders for antibiotics for more than one day to eat a widely available brand of yogurt.2 Seventy-five C. diff cases were reported in the hospital during 2011 and only 23 in 2012. The facility won an innovation award for the program from the Hospital and Healthsystem Association of Pennsylvania.
Other hospitals dispense probiotic dietary supplements, shown to have benefits against C. diff, in granular form. Medical experts have expressed skepticism that dispensing yogurt made the difference in cutting C. diff infections, arguing that there has not been enough research yet to support its widespread implementation in hospital settings.
Nationally, 337,000 cases of C. diff are reported in hospitals each year. A recent study reports that its spores are still commonly carried on the hands of healthcare workers who rubbed alcohol on their hands shortly after providing routine care to patients.3 Risk factors independently associated with hand contamination among healthcare workers in the exposed group included high-risk contacts and at least one contact without the use of gloves.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
The Philadelphia Inquirer recently reported that Holy Redeemer Hospital in Meadowbrook, Pa., cut its incidence of Clostridium difficile by two-thirds after its nutritionists began encouraging hospitalized patients with orders for antibiotics for more than one day to eat a widely available brand of yogurt.2 Seventy-five C. diff cases were reported in the hospital during 2011 and only 23 in 2012. The facility won an innovation award for the program from the Hospital and Healthsystem Association of Pennsylvania.
Other hospitals dispense probiotic dietary supplements, shown to have benefits against C. diff, in granular form. Medical experts have expressed skepticism that dispensing yogurt made the difference in cutting C. diff infections, arguing that there has not been enough research yet to support its widespread implementation in hospital settings.
Nationally, 337,000 cases of C. diff are reported in hospitals each year. A recent study reports that its spores are still commonly carried on the hands of healthcare workers who rubbed alcohol on their hands shortly after providing routine care to patients.3 Risk factors independently associated with hand contamination among healthcare workers in the exposed group included high-risk contacts and at least one contact without the use of gloves.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
The Philadelphia Inquirer recently reported that Holy Redeemer Hospital in Meadowbrook, Pa., cut its incidence of Clostridium difficile by two-thirds after its nutritionists began encouraging hospitalized patients with orders for antibiotics for more than one day to eat a widely available brand of yogurt.2 Seventy-five C. diff cases were reported in the hospital during 2011 and only 23 in 2012. The facility won an innovation award for the program from the Hospital and Healthsystem Association of Pennsylvania.
Other hospitals dispense probiotic dietary supplements, shown to have benefits against C. diff, in granular form. Medical experts have expressed skepticism that dispensing yogurt made the difference in cutting C. diff infections, arguing that there has not been enough research yet to support its widespread implementation in hospital settings.
Nationally, 337,000 cases of C. diff are reported in hospitals each year. A recent study reports that its spores are still commonly carried on the hands of healthcare workers who rubbed alcohol on their hands shortly after providing routine care to patients.3 Risk factors independently associated with hand contamination among healthcare workers in the exposed group included high-risk contacts and at least one contact without the use of gloves.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
Home Hospice Providers Offer Best Practices for End-of-Life Care
New research from the Birmingham, Ala., Veterans Affairs Medical Center and the University of Alabama-Birmingham, published in the Journal of General Internal Medicine, finds that clinical techniques and care processes imported from home-based hospice professionals improved outcomes for hospitalized patients approaching the end of their lives.1
The project, conducted in six VA medical centers, employed a multi-modal strategy for improving end-of-life care processes, with staff training for all hospital providers in how to identify actively dying patients and then communicate this information to their families. Best clinical practices, supported by electronic order sets and paper-based educational materials, were implemented. Patients also were encouraged to eat what—and when—they wanted, to sit up in bed, and to receive family visitors at all hours.
“I started the project years ago, when I noticed that patients on hospice care at home often seemed more comfortable, while if I brought them into the hospital, they sometimes got worse,” says lead author F. Amos Bailey, MD. “We went out to the home to observe what the hospice nurses were doing and then came back to the hospital to write order sets to reflect that practice.”
Key quality endpoints included:
- Rates of orders for opioid pain medications;
- Anti-psychotic medications and scopolamine for death rattle;
- Completion of advance directives; and
- Consultations for palliative care and pastoral care.
Patients were more likely to have their pain relieved and symptoms addressed, according to chart reviews of 6,066 patients who died before or after the intervention was launched.
“All of the processes we measured moved in the direction of increased comfort,” Dr. Bailey says.
This is the first study to show that palliative care techniques developed in the home setting can have an impact on end-of-life care. That’s important, he adds, because most patients die in hospitals or nursing homes.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
New research from the Birmingham, Ala., Veterans Affairs Medical Center and the University of Alabama-Birmingham, published in the Journal of General Internal Medicine, finds that clinical techniques and care processes imported from home-based hospice professionals improved outcomes for hospitalized patients approaching the end of their lives.1
The project, conducted in six VA medical centers, employed a multi-modal strategy for improving end-of-life care processes, with staff training for all hospital providers in how to identify actively dying patients and then communicate this information to their families. Best clinical practices, supported by electronic order sets and paper-based educational materials, were implemented. Patients also were encouraged to eat what—and when—they wanted, to sit up in bed, and to receive family visitors at all hours.
“I started the project years ago, when I noticed that patients on hospice care at home often seemed more comfortable, while if I brought them into the hospital, they sometimes got worse,” says lead author F. Amos Bailey, MD. “We went out to the home to observe what the hospice nurses were doing and then came back to the hospital to write order sets to reflect that practice.”
Key quality endpoints included:
- Rates of orders for opioid pain medications;
- Anti-psychotic medications and scopolamine for death rattle;
- Completion of advance directives; and
- Consultations for palliative care and pastoral care.
Patients were more likely to have their pain relieved and symptoms addressed, according to chart reviews of 6,066 patients who died before or after the intervention was launched.
“All of the processes we measured moved in the direction of increased comfort,” Dr. Bailey says.
This is the first study to show that palliative care techniques developed in the home setting can have an impact on end-of-life care. That’s important, he adds, because most patients die in hospitals or nursing homes.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
New research from the Birmingham, Ala., Veterans Affairs Medical Center and the University of Alabama-Birmingham, published in the Journal of General Internal Medicine, finds that clinical techniques and care processes imported from home-based hospice professionals improved outcomes for hospitalized patients approaching the end of their lives.1
The project, conducted in six VA medical centers, employed a multi-modal strategy for improving end-of-life care processes, with staff training for all hospital providers in how to identify actively dying patients and then communicate this information to their families. Best clinical practices, supported by electronic order sets and paper-based educational materials, were implemented. Patients also were encouraged to eat what—and when—they wanted, to sit up in bed, and to receive family visitors at all hours.
“I started the project years ago, when I noticed that patients on hospice care at home often seemed more comfortable, while if I brought them into the hospital, they sometimes got worse,” says lead author F. Amos Bailey, MD. “We went out to the home to observe what the hospice nurses were doing and then came back to the hospital to write order sets to reflect that practice.”
Key quality endpoints included:
- Rates of orders for opioid pain medications;
- Anti-psychotic medications and scopolamine for death rattle;
- Completion of advance directives; and
- Consultations for palliative care and pastoral care.
Patients were more likely to have their pain relieved and symptoms addressed, according to chart reviews of 6,066 patients who died before or after the intervention was launched.
“All of the processes we measured moved in the direction of increased comfort,” Dr. Bailey says.
This is the first study to show that palliative care techniques developed in the home setting can have an impact on end-of-life care. That’s important, he adds, because most patients die in hospitals or nursing homes.
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life's end in inpatient settings: The BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
- Burling S. Yogurt a solution to hospital infection? Philadelphia Inquirer website. December 10, 2013. Available at: http://articles.philly.com/2013-12-10/news/44946926_1_holy-redeemer-probiotics-yogurt. Accessed June 5, 2014.
- Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Buisson CB. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol. 2014;35(1):10-15.
- Lewis K, Walker C. Development and application of information technology solutions to improve the quality and availability of discharge summaries. Journal of Hospital Medicine RIV abstracts website. Available at: http://www.shmabstracts.com/abstract.asp?MeetingID=793&id=104276&meeting=JHM201305. Published May 2013. Accessed June 14, 2014.
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement. American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976.
- American Hospital Association: Uncompensated hospital care cost fact sheet. January 2014. Available at: http://www.aha.org/content/14/14uncompensatedcare.pdf. Accessed June 5, 2014.
When Should You Suspect Kawasaki Disease as the Cause of Fever in an Infant?
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
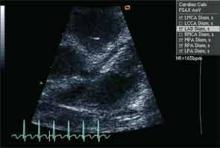
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Three Ways to Improve Quality of Patient Care in Your Hospital
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
American Board of Internal Medicine Foundation's Choosing Wisely Campaign Promotes Evidence-Based Patient Care
The American Board of Internal Medicine (ABIM) established the ABIM Foundation to advance professionalism in improving healthcare. The foundation initiated the Choosing Wisely campaign [www.choosingwisely.org] in April 2012 to promote conversations that help physicians guide patients in selecting care that is supported by evidence, not duplicative of other tests or procedures, not harmful, and truly necessary. In order to achieve this, national organizations representing medical specialists were asked to identify five common tests or procedures whose necessity should be questioned.
John Bulger, DO, MBA, SFHM, chief quality officer at Geisinger Health System in Danville, Pa., chaired SHM’s Choosing Wisely recommendations committee. He says the “proximal concern over these tests may be the unnecessary cost of the test itself, [but] there are other unintended consequences.
“False positive or false negative results of unsupported testing may cause unwarranted emotional harm for the patient or may give a false sense of security,” he adds. “The latter may also be true for physicians who may fail to further investigate other ailments based on a previous false negative test. Tests ordered with little evidence tend to lead to more tests ordered with little evidence.”
To date, more than 60 specialty societies and 17 consumer groups have joined the Choosing Wisely effort, citing more than 300 potentially harmful tests and procedures that physicians should discuss with patients. New lists will be published throughout 2014.
—Karen Appold
The American Board of Internal Medicine (ABIM) established the ABIM Foundation to advance professionalism in improving healthcare. The foundation initiated the Choosing Wisely campaign [www.choosingwisely.org] in April 2012 to promote conversations that help physicians guide patients in selecting care that is supported by evidence, not duplicative of other tests or procedures, not harmful, and truly necessary. In order to achieve this, national organizations representing medical specialists were asked to identify five common tests or procedures whose necessity should be questioned.
John Bulger, DO, MBA, SFHM, chief quality officer at Geisinger Health System in Danville, Pa., chaired SHM’s Choosing Wisely recommendations committee. He says the “proximal concern over these tests may be the unnecessary cost of the test itself, [but] there are other unintended consequences.
“False positive or false negative results of unsupported testing may cause unwarranted emotional harm for the patient or may give a false sense of security,” he adds. “The latter may also be true for physicians who may fail to further investigate other ailments based on a previous false negative test. Tests ordered with little evidence tend to lead to more tests ordered with little evidence.”
To date, more than 60 specialty societies and 17 consumer groups have joined the Choosing Wisely effort, citing more than 300 potentially harmful tests and procedures that physicians should discuss with patients. New lists will be published throughout 2014.
—Karen Appold
The American Board of Internal Medicine (ABIM) established the ABIM Foundation to advance professionalism in improving healthcare. The foundation initiated the Choosing Wisely campaign [www.choosingwisely.org] in April 2012 to promote conversations that help physicians guide patients in selecting care that is supported by evidence, not duplicative of other tests or procedures, not harmful, and truly necessary. In order to achieve this, national organizations representing medical specialists were asked to identify five common tests or procedures whose necessity should be questioned.
John Bulger, DO, MBA, SFHM, chief quality officer at Geisinger Health System in Danville, Pa., chaired SHM’s Choosing Wisely recommendations committee. He says the “proximal concern over these tests may be the unnecessary cost of the test itself, [but] there are other unintended consequences.
“False positive or false negative results of unsupported testing may cause unwarranted emotional harm for the patient or may give a false sense of security,” he adds. “The latter may also be true for physicians who may fail to further investigate other ailments based on a previous false negative test. Tests ordered with little evidence tend to lead to more tests ordered with little evidence.”
To date, more than 60 specialty societies and 17 consumer groups have joined the Choosing Wisely effort, citing more than 300 potentially harmful tests and procedures that physicians should discuss with patients. New lists will be published throughout 2014.
—Karen Appold