User login
Topical antibiotic decolonizes S. aureus in NICU infants
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
FROM PEDIATRICS
Meal programs for dual eligibles
Do food delivery programs reduce the use of costly health services and decrease medical spending in a population of patients dually eligible for Medicare and Medicaid?
Researchers in Massachusetts wanted to determine whether home meal delivery of either medically tailored food or nontailored food reduces the use of selected health care services and medical spending in a sample of adult “dual eligibles.”
“Compared with matched nonparticipants, participants had fewer emergency department visits in both the medically tailored meal program and the nontailored food program,” the investigators found. “Participants in the medically tailored meal program also had fewer inpatient admissions and lower medical spending. Participation in the nontailored food program was not associated with fewer inpatient admissions but was associated with lower medical spending.”
Reference
Berkowitz SA et al. Meal delivery programs reduce the use of costly health care in dually eligible Medicare and Medicaid beneficiaries. Health Aff (Millwood). 2018 Apr;37(4):535-42.
Do food delivery programs reduce the use of costly health services and decrease medical spending in a population of patients dually eligible for Medicare and Medicaid?
Researchers in Massachusetts wanted to determine whether home meal delivery of either medically tailored food or nontailored food reduces the use of selected health care services and medical spending in a sample of adult “dual eligibles.”
“Compared with matched nonparticipants, participants had fewer emergency department visits in both the medically tailored meal program and the nontailored food program,” the investigators found. “Participants in the medically tailored meal program also had fewer inpatient admissions and lower medical spending. Participation in the nontailored food program was not associated with fewer inpatient admissions but was associated with lower medical spending.”
Reference
Berkowitz SA et al. Meal delivery programs reduce the use of costly health care in dually eligible Medicare and Medicaid beneficiaries. Health Aff (Millwood). 2018 Apr;37(4):535-42.
Do food delivery programs reduce the use of costly health services and decrease medical spending in a population of patients dually eligible for Medicare and Medicaid?
Researchers in Massachusetts wanted to determine whether home meal delivery of either medically tailored food or nontailored food reduces the use of selected health care services and medical spending in a sample of adult “dual eligibles.”
“Compared with matched nonparticipants, participants had fewer emergency department visits in both the medically tailored meal program and the nontailored food program,” the investigators found. “Participants in the medically tailored meal program also had fewer inpatient admissions and lower medical spending. Participation in the nontailored food program was not associated with fewer inpatient admissions but was associated with lower medical spending.”
Reference
Berkowitz SA et al. Meal delivery programs reduce the use of costly health care in dually eligible Medicare and Medicaid beneficiaries. Health Aff (Millwood). 2018 Apr;37(4):535-42.
How to assess an Antimicrobial Stewardship Program
A study compares the merits of DOT and DOTA
The currently recommended method for hospital antimicrobial stewardship programs (ASPs) to measure antibiotic use is Days of Therapy/1,000 patient-days, but there are a few disadvantages of using the DOT, said Maryrose Laguio-Vila, MD, coauthor of a recent study on stewardship.
“For accurate measurement, it requires information technology (IT) support to assist an ASP in generating reports of antibiotic prescriptions and administrations to patients, often from an electronic medical record (EMR). In hospitals where there is no EMR, DOT is probably not easily done and would have to be manually extracted (a herculean task),” she said. “Second, DOT tends to be an aggregate measurement of antibiotics used at an institution or hospital location; if an ASP does a specific intervention targeting a group of antibiotics or infectious indication, changes in the hospital-wide DOT or drug-class DOT may not accurately reflect the exact impact of an ASP’s intervention.”
The paper offers an alternative/supplemental method for ASPs to quantify their impact on antibiotic use without using an EMR or needing IT support: Days of Therapy Avoided. “DOTA can be tracked prospectively (or retrospectively) with each intervention an ASP makes, and calculates an exact amount of antibiotic use avoided,” Dr. Laguio-Vila said. “If the ASP also tracks the types of antibiotic recommendations made according to infectious indication, comparison of DOTA between indications – such as pneumonia versus UTI [urinary tract infection] – can lead to ideas of which type of indication needs clinical guidelines development, or order set revision, or which type of infection the ASP should target to reduce high-risk antibiotics.”
Also, she added, because most ASPs have several types of interventions at once (such as education on pneumonia guidelines, as well as penicillin-allergy assessment), aggregate assessments of institutional antibiotic use like the DOT cannot quantify how much impact a specific intervention has accomplished. DOTA may offer a fairer assessment of the direct changes in antibiotic use resulting from specific ASP activities, because tracking DOTA is extracted from each specific patient intervention.
“Now that the Joint Commission has a requirement that all hospitals seeking JC accreditation have some form of an ASP in place and measure antibiotic use in some way at their institution, there may be numerous hospitals facing the same challenges with calculating a DOT. DOTA would meet these requirements, but in a ‘low tech’ way,” Dr. Laguio-Vila said. “For hospitalists with interests in being the antibiotic steward or champion for their institution, DOTA is an option for measuring antibiotic use.”
Reference
Datta S et al. Days of therapy avoided: A novel method for measuring the impact of an antimicrobial stewardship program to stop antibiotics. J Hosp Med. 2018 Feb 8. doi: 10.12788/jhm.2927.
A study compares the merits of DOT and DOTA
A study compares the merits of DOT and DOTA
The currently recommended method for hospital antimicrobial stewardship programs (ASPs) to measure antibiotic use is Days of Therapy/1,000 patient-days, but there are a few disadvantages of using the DOT, said Maryrose Laguio-Vila, MD, coauthor of a recent study on stewardship.
“For accurate measurement, it requires information technology (IT) support to assist an ASP in generating reports of antibiotic prescriptions and administrations to patients, often from an electronic medical record (EMR). In hospitals where there is no EMR, DOT is probably not easily done and would have to be manually extracted (a herculean task),” she said. “Second, DOT tends to be an aggregate measurement of antibiotics used at an institution or hospital location; if an ASP does a specific intervention targeting a group of antibiotics or infectious indication, changes in the hospital-wide DOT or drug-class DOT may not accurately reflect the exact impact of an ASP’s intervention.”
The paper offers an alternative/supplemental method for ASPs to quantify their impact on antibiotic use without using an EMR or needing IT support: Days of Therapy Avoided. “DOTA can be tracked prospectively (or retrospectively) with each intervention an ASP makes, and calculates an exact amount of antibiotic use avoided,” Dr. Laguio-Vila said. “If the ASP also tracks the types of antibiotic recommendations made according to infectious indication, comparison of DOTA between indications – such as pneumonia versus UTI [urinary tract infection] – can lead to ideas of which type of indication needs clinical guidelines development, or order set revision, or which type of infection the ASP should target to reduce high-risk antibiotics.”
Also, she added, because most ASPs have several types of interventions at once (such as education on pneumonia guidelines, as well as penicillin-allergy assessment), aggregate assessments of institutional antibiotic use like the DOT cannot quantify how much impact a specific intervention has accomplished. DOTA may offer a fairer assessment of the direct changes in antibiotic use resulting from specific ASP activities, because tracking DOTA is extracted from each specific patient intervention.
“Now that the Joint Commission has a requirement that all hospitals seeking JC accreditation have some form of an ASP in place and measure antibiotic use in some way at their institution, there may be numerous hospitals facing the same challenges with calculating a DOT. DOTA would meet these requirements, but in a ‘low tech’ way,” Dr. Laguio-Vila said. “For hospitalists with interests in being the antibiotic steward or champion for their institution, DOTA is an option for measuring antibiotic use.”
Reference
Datta S et al. Days of therapy avoided: A novel method for measuring the impact of an antimicrobial stewardship program to stop antibiotics. J Hosp Med. 2018 Feb 8. doi: 10.12788/jhm.2927.
The currently recommended method for hospital antimicrobial stewardship programs (ASPs) to measure antibiotic use is Days of Therapy/1,000 patient-days, but there are a few disadvantages of using the DOT, said Maryrose Laguio-Vila, MD, coauthor of a recent study on stewardship.
“For accurate measurement, it requires information technology (IT) support to assist an ASP in generating reports of antibiotic prescriptions and administrations to patients, often from an electronic medical record (EMR). In hospitals where there is no EMR, DOT is probably not easily done and would have to be manually extracted (a herculean task),” she said. “Second, DOT tends to be an aggregate measurement of antibiotics used at an institution or hospital location; if an ASP does a specific intervention targeting a group of antibiotics or infectious indication, changes in the hospital-wide DOT or drug-class DOT may not accurately reflect the exact impact of an ASP’s intervention.”
The paper offers an alternative/supplemental method for ASPs to quantify their impact on antibiotic use without using an EMR or needing IT support: Days of Therapy Avoided. “DOTA can be tracked prospectively (or retrospectively) with each intervention an ASP makes, and calculates an exact amount of antibiotic use avoided,” Dr. Laguio-Vila said. “If the ASP also tracks the types of antibiotic recommendations made according to infectious indication, comparison of DOTA between indications – such as pneumonia versus UTI [urinary tract infection] – can lead to ideas of which type of indication needs clinical guidelines development, or order set revision, or which type of infection the ASP should target to reduce high-risk antibiotics.”
Also, she added, because most ASPs have several types of interventions at once (such as education on pneumonia guidelines, as well as penicillin-allergy assessment), aggregate assessments of institutional antibiotic use like the DOT cannot quantify how much impact a specific intervention has accomplished. DOTA may offer a fairer assessment of the direct changes in antibiotic use resulting from specific ASP activities, because tracking DOTA is extracted from each specific patient intervention.
“Now that the Joint Commission has a requirement that all hospitals seeking JC accreditation have some form of an ASP in place and measure antibiotic use in some way at their institution, there may be numerous hospitals facing the same challenges with calculating a DOT. DOTA would meet these requirements, but in a ‘low tech’ way,” Dr. Laguio-Vila said. “For hospitalists with interests in being the antibiotic steward or champion for their institution, DOTA is an option for measuring antibiotic use.”
Reference
Datta S et al. Days of therapy avoided: A novel method for measuring the impact of an antimicrobial stewardship program to stop antibiotics. J Hosp Med. 2018 Feb 8. doi: 10.12788/jhm.2927.
CDC: Flu activity ‘high’ in nine states
according to the Centers for Disease Control and Prevention.
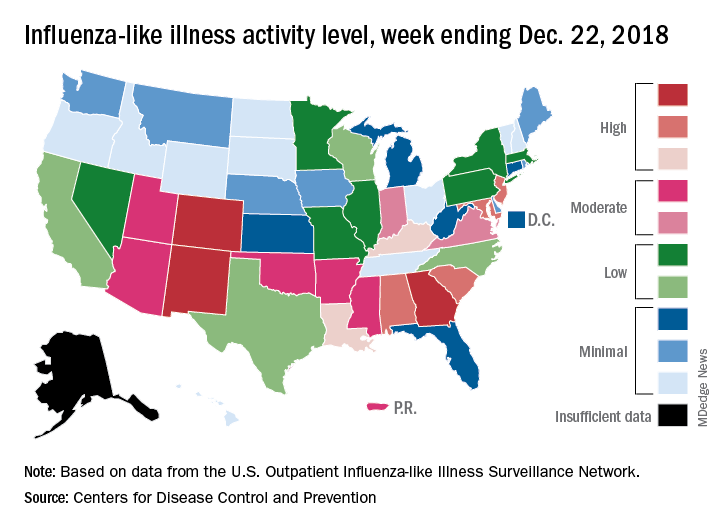
Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
according to the Centers for Disease Control and Prevention.

Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
according to the Centers for Disease Control and Prevention.

Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
No change in postoperative pain with restrictive opioid protocol
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Key clinical point: A ultrarestrictive postoperative opioid protocol is not associated with higher postoperative pain scores.
Major finding: The protocol achieves significant reductions in opioid use.
Study details: A case-control study in 1,231 women undergoing gynecologic surgery.
Disclosures: The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute, and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
Source: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
2018-2019 flu season starts in earnest
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.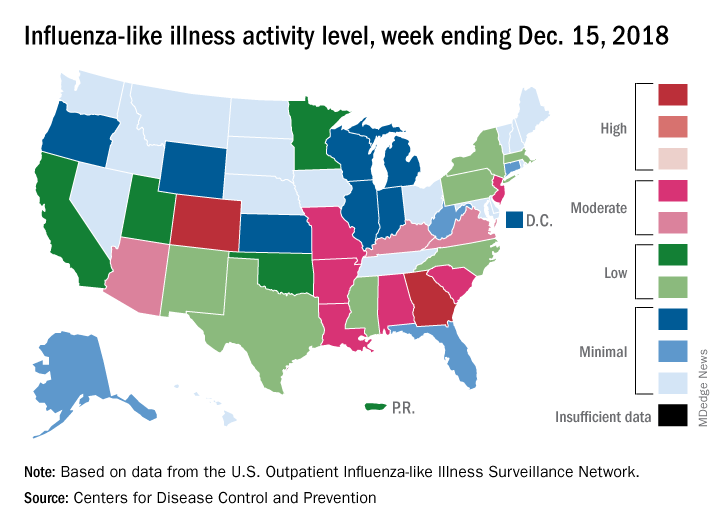
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
Can higher MAP post cardiac arrest improve neurologic outcomes?
CHICAGO – A European clinical trial that targeted a mean arterial blood pressure after cardiac arrest higher than what the existing guidelines recommend found that the approach was safe, improved blood flow and oxygen to the brain, helped patients recover quicker, and reduced the number of adverse cardiac events, although it did not reduce the extent of anoxic brain damage or improve functional outcomes, the lead investigator reported at the American Heart Association scientific sessions.
The Neuroprotect trial randomly assigned 112 adult survivors of an out-of-hospital cardiac arrest who were unconscious upon admission to two study groups: early goal-directed hemodynamic optimization (EGDHO), in which researchers used a targeted mean arterial pressure (MAP) of 85-100 mm Hg and mixed venous oxygen saturation between 65% and 75% during the first 36 hours after ICU admission; and the standard care group, in which they used the guideline-recommended MAP target of 65 mm Hg, said Koen Ameloot, MD, of East Limburg Hospital in Genk, Belgium.
“EGDHO clearly improved cerebral perfusion and oxygenation, thereby for the first time providing the proof of concept for this new hemodynamic target,” Dr. Ameloot said. “However, this did not result in the reduction of the extent of anoxic brain hemorrhage or effusion rate on MRI or an improvement in functional outcome at 180 days.”
He noted the trial was predicated on improving upon the so-called “two-hit” model of cardiac arrest sequelae: the first hit being the no-flow and low-flow period before achieving restoration of spontaneous circulation; the second hit being hypoperfusion and reperfusion injury during ICU stay.
Dr. Ameloot referenced a study in which he and other coauthors reported that patients with a MAP target of 65 mm Hg “experience a profound drop of cerebral oxygen saturation during the first 12 hours of ICU stay that may cause additional brain damage” (Resuscitation. 2018;123:92-7).
The researchers explored the question of what is the optimal MAP if a target of 65 mm Hg is too low, Dr. Ameloot said. “We showed that maximal brain oxygenation is achieved with a MAP of 100 mm Hg, while lower MAPs were associated with submaximal brain perfusion and higher MAPs with excessive after-load, a reduction in stroke volume, and suboptimal cerebral oxygenation.”
During the 36-hour intervention period, the EGDHO patients received higher doses of norepinephrine, Dr. Ameloot said. “This resulted in significant improvement of cerebral oxygenation during the first 12 hours and was paralleled by significantly higher cerebral perfusion in the subset of patients in whom Doppler measurements were performed,” he said. “While patients allocated to the MAP 65 mm Hg target experienced a profound drop of cerebral oxygenation during the critical first 6-12 hours of ICU stay, cerebral oxygenation was maintained at 67% in patients assigned to EGDHO.”
However, the rate of anoxic brain damage, measured as the percentage of irreversibly damaged anoxic voxels on diffusion-weighted MRI – the primary endpoint of the study – was actually higher in the EGDHO group, 16% vs. 12%, Dr. Ameloot said. “The percentage of anoxic voxels was only a poor predictor of favorable neurological outcome at 180 days, questioning the validity of the primary endpoint,” he said. He also noted that 23% of the trial participants did not have an MRI scan because of higher than expected 5-day rates of death.
“The percentage of patients with favorable neurological outcome tended to be somewhat higher in the intervention arm, although this did not reach statistical significance at ICU discharge and at 180 days,” Dr. Ameloot said. He noted that 42% of the intervention group and 33% of controls in the full-analysis set (P = .30) and 43% and 27%, respectively, in the per-protocol set (P = .15) had a favorable neurological outcome, as calculated using the Glasgow-Pittsburgh Cerebral Performance Category scores of 1 or 2, at 180 days.
The study did not reveal any noteworthy differences in ICU stay (7 vs. 8 days, P = .13) or days on mechanical ventilation (5 vs. 7, P = .31), although fewer patients in the EGDHO group required a tracheostomy (4% vs. 18%, P = .02). The intervention group also had lower rates of cardiac events, including recurrent cardiac arrest, limb ischemia, new atrial fibrillation, and pulmonary edema (13% vs. 33%; P = .02), Dr. Ameloot said.
Future post-hoc analyses of the data will explore the hypothesis that higher blood pressure leads to improved coronary perfusion and reduced infarct size, thus improving prognosis, he added.
“Should this trial therefore be the definite end to the promising hypothesis that improving brain oxygenation might reduce the second hit in post–cardiac arrest patients? I don’t think so,” Dr. Ameloot said. He noted a few limits to the study: that the perfusion rate on MRI was a poor predictor of 180-day outcome; that more patients than expected entered the trial without receiving basic life support and with nonshockable rhythms; and that there was possibly less extensive brain damage among controls at baseline. “Only an adequately powered clinical trial can provide an answer about the effects of EGDHO in post–cardiac arrest patients,” Dr. Ameloot said.
Dr. Ameloot had no financial relationships to disclose.
SOURCE: Ameloot K et al. AHA 2018, Abstract 18620
CHICAGO – A European clinical trial that targeted a mean arterial blood pressure after cardiac arrest higher than what the existing guidelines recommend found that the approach was safe, improved blood flow and oxygen to the brain, helped patients recover quicker, and reduced the number of adverse cardiac events, although it did not reduce the extent of anoxic brain damage or improve functional outcomes, the lead investigator reported at the American Heart Association scientific sessions.
The Neuroprotect trial randomly assigned 112 adult survivors of an out-of-hospital cardiac arrest who were unconscious upon admission to two study groups: early goal-directed hemodynamic optimization (EGDHO), in which researchers used a targeted mean arterial pressure (MAP) of 85-100 mm Hg and mixed venous oxygen saturation between 65% and 75% during the first 36 hours after ICU admission; and the standard care group, in which they used the guideline-recommended MAP target of 65 mm Hg, said Koen Ameloot, MD, of East Limburg Hospital in Genk, Belgium.
“EGDHO clearly improved cerebral perfusion and oxygenation, thereby for the first time providing the proof of concept for this new hemodynamic target,” Dr. Ameloot said. “However, this did not result in the reduction of the extent of anoxic brain hemorrhage or effusion rate on MRI or an improvement in functional outcome at 180 days.”
He noted the trial was predicated on improving upon the so-called “two-hit” model of cardiac arrest sequelae: the first hit being the no-flow and low-flow period before achieving restoration of spontaneous circulation; the second hit being hypoperfusion and reperfusion injury during ICU stay.
Dr. Ameloot referenced a study in which he and other coauthors reported that patients with a MAP target of 65 mm Hg “experience a profound drop of cerebral oxygen saturation during the first 12 hours of ICU stay that may cause additional brain damage” (Resuscitation. 2018;123:92-7).
The researchers explored the question of what is the optimal MAP if a target of 65 mm Hg is too low, Dr. Ameloot said. “We showed that maximal brain oxygenation is achieved with a MAP of 100 mm Hg, while lower MAPs were associated with submaximal brain perfusion and higher MAPs with excessive after-load, a reduction in stroke volume, and suboptimal cerebral oxygenation.”
During the 36-hour intervention period, the EGDHO patients received higher doses of norepinephrine, Dr. Ameloot said. “This resulted in significant improvement of cerebral oxygenation during the first 12 hours and was paralleled by significantly higher cerebral perfusion in the subset of patients in whom Doppler measurements were performed,” he said. “While patients allocated to the MAP 65 mm Hg target experienced a profound drop of cerebral oxygenation during the critical first 6-12 hours of ICU stay, cerebral oxygenation was maintained at 67% in patients assigned to EGDHO.”
However, the rate of anoxic brain damage, measured as the percentage of irreversibly damaged anoxic voxels on diffusion-weighted MRI – the primary endpoint of the study – was actually higher in the EGDHO group, 16% vs. 12%, Dr. Ameloot said. “The percentage of anoxic voxels was only a poor predictor of favorable neurological outcome at 180 days, questioning the validity of the primary endpoint,” he said. He also noted that 23% of the trial participants did not have an MRI scan because of higher than expected 5-day rates of death.
“The percentage of patients with favorable neurological outcome tended to be somewhat higher in the intervention arm, although this did not reach statistical significance at ICU discharge and at 180 days,” Dr. Ameloot said. He noted that 42% of the intervention group and 33% of controls in the full-analysis set (P = .30) and 43% and 27%, respectively, in the per-protocol set (P = .15) had a favorable neurological outcome, as calculated using the Glasgow-Pittsburgh Cerebral Performance Category scores of 1 or 2, at 180 days.
The study did not reveal any noteworthy differences in ICU stay (7 vs. 8 days, P = .13) or days on mechanical ventilation (5 vs. 7, P = .31), although fewer patients in the EGDHO group required a tracheostomy (4% vs. 18%, P = .02). The intervention group also had lower rates of cardiac events, including recurrent cardiac arrest, limb ischemia, new atrial fibrillation, and pulmonary edema (13% vs. 33%; P = .02), Dr. Ameloot said.
Future post-hoc analyses of the data will explore the hypothesis that higher blood pressure leads to improved coronary perfusion and reduced infarct size, thus improving prognosis, he added.
“Should this trial therefore be the definite end to the promising hypothesis that improving brain oxygenation might reduce the second hit in post–cardiac arrest patients? I don’t think so,” Dr. Ameloot said. He noted a few limits to the study: that the perfusion rate on MRI was a poor predictor of 180-day outcome; that more patients than expected entered the trial without receiving basic life support and with nonshockable rhythms; and that there was possibly less extensive brain damage among controls at baseline. “Only an adequately powered clinical trial can provide an answer about the effects of EGDHO in post–cardiac arrest patients,” Dr. Ameloot said.
Dr. Ameloot had no financial relationships to disclose.
SOURCE: Ameloot K et al. AHA 2018, Abstract 18620
CHICAGO – A European clinical trial that targeted a mean arterial blood pressure after cardiac arrest higher than what the existing guidelines recommend found that the approach was safe, improved blood flow and oxygen to the brain, helped patients recover quicker, and reduced the number of adverse cardiac events, although it did not reduce the extent of anoxic brain damage or improve functional outcomes, the lead investigator reported at the American Heart Association scientific sessions.
The Neuroprotect trial randomly assigned 112 adult survivors of an out-of-hospital cardiac arrest who were unconscious upon admission to two study groups: early goal-directed hemodynamic optimization (EGDHO), in which researchers used a targeted mean arterial pressure (MAP) of 85-100 mm Hg and mixed venous oxygen saturation between 65% and 75% during the first 36 hours after ICU admission; and the standard care group, in which they used the guideline-recommended MAP target of 65 mm Hg, said Koen Ameloot, MD, of East Limburg Hospital in Genk, Belgium.
“EGDHO clearly improved cerebral perfusion and oxygenation, thereby for the first time providing the proof of concept for this new hemodynamic target,” Dr. Ameloot said. “However, this did not result in the reduction of the extent of anoxic brain hemorrhage or effusion rate on MRI or an improvement in functional outcome at 180 days.”
He noted the trial was predicated on improving upon the so-called “two-hit” model of cardiac arrest sequelae: the first hit being the no-flow and low-flow period before achieving restoration of spontaneous circulation; the second hit being hypoperfusion and reperfusion injury during ICU stay.
Dr. Ameloot referenced a study in which he and other coauthors reported that patients with a MAP target of 65 mm Hg “experience a profound drop of cerebral oxygen saturation during the first 12 hours of ICU stay that may cause additional brain damage” (Resuscitation. 2018;123:92-7).
The researchers explored the question of what is the optimal MAP if a target of 65 mm Hg is too low, Dr. Ameloot said. “We showed that maximal brain oxygenation is achieved with a MAP of 100 mm Hg, while lower MAPs were associated with submaximal brain perfusion and higher MAPs with excessive after-load, a reduction in stroke volume, and suboptimal cerebral oxygenation.”
During the 36-hour intervention period, the EGDHO patients received higher doses of norepinephrine, Dr. Ameloot said. “This resulted in significant improvement of cerebral oxygenation during the first 12 hours and was paralleled by significantly higher cerebral perfusion in the subset of patients in whom Doppler measurements were performed,” he said. “While patients allocated to the MAP 65 mm Hg target experienced a profound drop of cerebral oxygenation during the critical first 6-12 hours of ICU stay, cerebral oxygenation was maintained at 67% in patients assigned to EGDHO.”
However, the rate of anoxic brain damage, measured as the percentage of irreversibly damaged anoxic voxels on diffusion-weighted MRI – the primary endpoint of the study – was actually higher in the EGDHO group, 16% vs. 12%, Dr. Ameloot said. “The percentage of anoxic voxels was only a poor predictor of favorable neurological outcome at 180 days, questioning the validity of the primary endpoint,” he said. He also noted that 23% of the trial participants did not have an MRI scan because of higher than expected 5-day rates of death.
“The percentage of patients with favorable neurological outcome tended to be somewhat higher in the intervention arm, although this did not reach statistical significance at ICU discharge and at 180 days,” Dr. Ameloot said. He noted that 42% of the intervention group and 33% of controls in the full-analysis set (P = .30) and 43% and 27%, respectively, in the per-protocol set (P = .15) had a favorable neurological outcome, as calculated using the Glasgow-Pittsburgh Cerebral Performance Category scores of 1 or 2, at 180 days.
The study did not reveal any noteworthy differences in ICU stay (7 vs. 8 days, P = .13) or days on mechanical ventilation (5 vs. 7, P = .31), although fewer patients in the EGDHO group required a tracheostomy (4% vs. 18%, P = .02). The intervention group also had lower rates of cardiac events, including recurrent cardiac arrest, limb ischemia, new atrial fibrillation, and pulmonary edema (13% vs. 33%; P = .02), Dr. Ameloot said.
Future post-hoc analyses of the data will explore the hypothesis that higher blood pressure leads to improved coronary perfusion and reduced infarct size, thus improving prognosis, he added.
“Should this trial therefore be the definite end to the promising hypothesis that improving brain oxygenation might reduce the second hit in post–cardiac arrest patients? I don’t think so,” Dr. Ameloot said. He noted a few limits to the study: that the perfusion rate on MRI was a poor predictor of 180-day outcome; that more patients than expected entered the trial without receiving basic life support and with nonshockable rhythms; and that there was possibly less extensive brain damage among controls at baseline. “Only an adequately powered clinical trial can provide an answer about the effects of EGDHO in post–cardiac arrest patients,” Dr. Ameloot said.
Dr. Ameloot had no financial relationships to disclose.
SOURCE: Ameloot K et al. AHA 2018, Abstract 18620
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Forty-three percent of patients in the intervention group had a favorable neurological outcome vs. 27% of controls (P = .15).
Study details: The Neuroprotect trial was a multicenter, randomized, open-label, assessor-blinded trial of 112 post–cardiac arrest patients.
Disclosures: Dr. Ameloot had no financial relationships to disclose.
Source: Ameloot K et al. AHA 2018, Abstract 18620
FDA aims to boost safety of platelets for transfusion
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
Guideline-concordant treatment still unlikely in nonchildren’s hospitals for pediatric CAP
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
FROM JAMA PEDIATRICS
In-hospital blood saving strategy appears safe with anemia
A blood management initiative that reduced RBC transfusions in the hospital did not adversely impact long-term outcomes after discharge, a retrospective analysis of an extensive patient database suggested.
Tolerating moderate in-hospital anemia did not increase subsequent RBC use, readmission, or mortality over the next 6 months, according to results of the study, which drew on nearly half a million patient records.
In fact, modest mortality decreases were seen over time for patients with moderate anemia, perhaps because of concomitant initiatives that targeted infectious and circulatory conditions, reported Nareg H. Roubinian, MD, of Kaiser Permanente Northern California in Oakland and the University of California, San Francisco, and coinvestigators.
“These data support the efficacy and safety of practice recommendations to limit red blood cell transfusion in patients with anemia during and after hospitalization,” Dr. Roubinian and colleagues wrote in their report, which appears in the Annals of Internal Medicine.
However, additional studies are needed to guide anemia management, they wrote, particularly since persistent anemia has impacts on quality of life that are “likely substantial” and linked to the severity of that anemia.
Dr. Roubinian and colleagues sought to evaluate the impact of blood management programs – initiated starting in 2010 – that included blood-sparing surgical and medical techniques, increased use of hemostatic and cell salvage agents, and treatment of suboptimal iron stores before surgery.
In previous retrospective cohort studies, the researchers had found that the blood conservation strategies did not impact in-hospital or 30-day mortality rates, which was consistent with short-term safety data from clinical trials and other observational studies.
Their latest report on longer-term outcomes was based on data from Kaiser Permanente Northern California for 445,371 adults who had 801,261 hospitalizations with discharges between 2010 and 2014. In this cohort, moderate anemia (hemoglobin between 7 g/dL and 10 g/dL) at discharge occurred in 119,489 patients (27%) and 187,440 hospitalizations overall (23%).
Over the 2010-2014 period, RBC transfusions decreased by more than 25% in the inpatient and outpatient settings; and in parallel, the prevalence of moderate anemia at hospital discharge increased from 20% to 25%.
However, the risks of subsequent RBC transfusions and rehospitalization after discharge with anemia decreased during the study period, and mortality rates stayed steady or decreased slightly.
Among patients with moderate anemia, the proportion with subsequent RBC transfusions within 6 months decreased from 18.9% in 2010 to 16.8% in 2014 (P less than .001), while the rate of rehospitalization within 6 months decreased from 36.5% to 32.8% over that same time period (P less than .001).
The adjusted 6-month mortality rate likewise decreased from 16.1% to 15.6% (P = .004) over that time period among patients with moderate anemia.
The study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Roubinian and several coauthors reported grants during the conduct of the study from the National Institutes of Health.
SOURCE: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
Some scrutiny is warranted of the observation of Roubinian et al. that long-term transfusion, readmission, and mortality outcomes were apparently unaffected by decreased in-hospital RBC transfusions, according to the authors of an accompanying editorial.
“Missing here is a wide spectrum of morbidity outcomes and issues related to diminished quality of life that do not reach the level of severity that would necessitate admission but nonetheless detract from patients’ health and well-being,” wrote Aryeh Shander, MD, and Lawrence Tim Goodnough, MD.
Moreover, transfusion rate is not a clinical outcome, they noted, adding that readmission and mortality are important outcomes but that they do not accurately or fully reflect patient well-being.
While blood management initiatives may be a safe practice, as Roubinian et al. found, proper management of anemia after discharge may actually improve outcomes, given the many consequences of anemia.
Instead of again testing whether restricting transfusions is acceptable because of lack of impact on outcomes, future studies could evaluate a “more sensible” hypothesis that proper anemia management – especially post discharge – could improve outcomes.
“Let’s increase efforts to prevent and treat anemia properly, rather than requiring patients to tolerate it,” they wrote.
Dr. Shander is with Englewood (N.J.) Hospital and Medical Center; Dr. Goodnough is with Stanford (Calif.) University. Dr. Shander reported consulting fees from Vifor and AMAG. Dr. Goodnough reported having no relevant financial disclosures. Their comments are taken from an accompanying editorial (Ann Intern Med. 2018 Dec 18. doi: 10.7326/M18-3145).
Some scrutiny is warranted of the observation of Roubinian et al. that long-term transfusion, readmission, and mortality outcomes were apparently unaffected by decreased in-hospital RBC transfusions, according to the authors of an accompanying editorial.
“Missing here is a wide spectrum of morbidity outcomes and issues related to diminished quality of life that do not reach the level of severity that would necessitate admission but nonetheless detract from patients’ health and well-being,” wrote Aryeh Shander, MD, and Lawrence Tim Goodnough, MD.
Moreover, transfusion rate is not a clinical outcome, they noted, adding that readmission and mortality are important outcomes but that they do not accurately or fully reflect patient well-being.
While blood management initiatives may be a safe practice, as Roubinian et al. found, proper management of anemia after discharge may actually improve outcomes, given the many consequences of anemia.
Instead of again testing whether restricting transfusions is acceptable because of lack of impact on outcomes, future studies could evaluate a “more sensible” hypothesis that proper anemia management – especially post discharge – could improve outcomes.
“Let’s increase efforts to prevent and treat anemia properly, rather than requiring patients to tolerate it,” they wrote.
Dr. Shander is with Englewood (N.J.) Hospital and Medical Center; Dr. Goodnough is with Stanford (Calif.) University. Dr. Shander reported consulting fees from Vifor and AMAG. Dr. Goodnough reported having no relevant financial disclosures. Their comments are taken from an accompanying editorial (Ann Intern Med. 2018 Dec 18. doi: 10.7326/M18-3145).
Some scrutiny is warranted of the observation of Roubinian et al. that long-term transfusion, readmission, and mortality outcomes were apparently unaffected by decreased in-hospital RBC transfusions, according to the authors of an accompanying editorial.
“Missing here is a wide spectrum of morbidity outcomes and issues related to diminished quality of life that do not reach the level of severity that would necessitate admission but nonetheless detract from patients’ health and well-being,” wrote Aryeh Shander, MD, and Lawrence Tim Goodnough, MD.
Moreover, transfusion rate is not a clinical outcome, they noted, adding that readmission and mortality are important outcomes but that they do not accurately or fully reflect patient well-being.
While blood management initiatives may be a safe practice, as Roubinian et al. found, proper management of anemia after discharge may actually improve outcomes, given the many consequences of anemia.
Instead of again testing whether restricting transfusions is acceptable because of lack of impact on outcomes, future studies could evaluate a “more sensible” hypothesis that proper anemia management – especially post discharge – could improve outcomes.
“Let’s increase efforts to prevent and treat anemia properly, rather than requiring patients to tolerate it,” they wrote.
Dr. Shander is with Englewood (N.J.) Hospital and Medical Center; Dr. Goodnough is with Stanford (Calif.) University. Dr. Shander reported consulting fees from Vifor and AMAG. Dr. Goodnough reported having no relevant financial disclosures. Their comments are taken from an accompanying editorial (Ann Intern Med. 2018 Dec 18. doi: 10.7326/M18-3145).
A blood management initiative that reduced RBC transfusions in the hospital did not adversely impact long-term outcomes after discharge, a retrospective analysis of an extensive patient database suggested.
Tolerating moderate in-hospital anemia did not increase subsequent RBC use, readmission, or mortality over the next 6 months, according to results of the study, which drew on nearly half a million patient records.
In fact, modest mortality decreases were seen over time for patients with moderate anemia, perhaps because of concomitant initiatives that targeted infectious and circulatory conditions, reported Nareg H. Roubinian, MD, of Kaiser Permanente Northern California in Oakland and the University of California, San Francisco, and coinvestigators.
“These data support the efficacy and safety of practice recommendations to limit red blood cell transfusion in patients with anemia during and after hospitalization,” Dr. Roubinian and colleagues wrote in their report, which appears in the Annals of Internal Medicine.
However, additional studies are needed to guide anemia management, they wrote, particularly since persistent anemia has impacts on quality of life that are “likely substantial” and linked to the severity of that anemia.
Dr. Roubinian and colleagues sought to evaluate the impact of blood management programs – initiated starting in 2010 – that included blood-sparing surgical and medical techniques, increased use of hemostatic and cell salvage agents, and treatment of suboptimal iron stores before surgery.
In previous retrospective cohort studies, the researchers had found that the blood conservation strategies did not impact in-hospital or 30-day mortality rates, which was consistent with short-term safety data from clinical trials and other observational studies.
Their latest report on longer-term outcomes was based on data from Kaiser Permanente Northern California for 445,371 adults who had 801,261 hospitalizations with discharges between 2010 and 2014. In this cohort, moderate anemia (hemoglobin between 7 g/dL and 10 g/dL) at discharge occurred in 119,489 patients (27%) and 187,440 hospitalizations overall (23%).
Over the 2010-2014 period, RBC transfusions decreased by more than 25% in the inpatient and outpatient settings; and in parallel, the prevalence of moderate anemia at hospital discharge increased from 20% to 25%.
However, the risks of subsequent RBC transfusions and rehospitalization after discharge with anemia decreased during the study period, and mortality rates stayed steady or decreased slightly.
Among patients with moderate anemia, the proportion with subsequent RBC transfusions within 6 months decreased from 18.9% in 2010 to 16.8% in 2014 (P less than .001), while the rate of rehospitalization within 6 months decreased from 36.5% to 32.8% over that same time period (P less than .001).
The adjusted 6-month mortality rate likewise decreased from 16.1% to 15.6% (P = .004) over that time period among patients with moderate anemia.
The study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Roubinian and several coauthors reported grants during the conduct of the study from the National Institutes of Health.
SOURCE: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
A blood management initiative that reduced RBC transfusions in the hospital did not adversely impact long-term outcomes after discharge, a retrospective analysis of an extensive patient database suggested.
Tolerating moderate in-hospital anemia did not increase subsequent RBC use, readmission, or mortality over the next 6 months, according to results of the study, which drew on nearly half a million patient records.
In fact, modest mortality decreases were seen over time for patients with moderate anemia, perhaps because of concomitant initiatives that targeted infectious and circulatory conditions, reported Nareg H. Roubinian, MD, of Kaiser Permanente Northern California in Oakland and the University of California, San Francisco, and coinvestigators.
“These data support the efficacy and safety of practice recommendations to limit red blood cell transfusion in patients with anemia during and after hospitalization,” Dr. Roubinian and colleagues wrote in their report, which appears in the Annals of Internal Medicine.
However, additional studies are needed to guide anemia management, they wrote, particularly since persistent anemia has impacts on quality of life that are “likely substantial” and linked to the severity of that anemia.
Dr. Roubinian and colleagues sought to evaluate the impact of blood management programs – initiated starting in 2010 – that included blood-sparing surgical and medical techniques, increased use of hemostatic and cell salvage agents, and treatment of suboptimal iron stores before surgery.
In previous retrospective cohort studies, the researchers had found that the blood conservation strategies did not impact in-hospital or 30-day mortality rates, which was consistent with short-term safety data from clinical trials and other observational studies.
Their latest report on longer-term outcomes was based on data from Kaiser Permanente Northern California for 445,371 adults who had 801,261 hospitalizations with discharges between 2010 and 2014. In this cohort, moderate anemia (hemoglobin between 7 g/dL and 10 g/dL) at discharge occurred in 119,489 patients (27%) and 187,440 hospitalizations overall (23%).
Over the 2010-2014 period, RBC transfusions decreased by more than 25% in the inpatient and outpatient settings; and in parallel, the prevalence of moderate anemia at hospital discharge increased from 20% to 25%.
However, the risks of subsequent RBC transfusions and rehospitalization after discharge with anemia decreased during the study period, and mortality rates stayed steady or decreased slightly.
Among patients with moderate anemia, the proportion with subsequent RBC transfusions within 6 months decreased from 18.9% in 2010 to 16.8% in 2014 (P less than .001), while the rate of rehospitalization within 6 months decreased from 36.5% to 32.8% over that same time period (P less than .001).
The adjusted 6-month mortality rate likewise decreased from 16.1% to 15.6% (P = .004) over that time period among patients with moderate anemia.
The study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Roubinian and several coauthors reported grants during the conduct of the study from the National Institutes of Health.
SOURCE: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: The adjusted 6-month mortality rate decreased from 16.1% to 15.6% (P = .004) in the 4-year period following implementation of blood conservation strategies.
Study details: A retrospective cohort study including 445,371 adults hospitalized and discharged between 2010 and 2014.
Disclosures: The study was supported by a grant from the National Heart, Lung, and Blood Institute. Several authors reported grants during the conduct of the study from the National Institutes of Health.
Source: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.