User login
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.

THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).


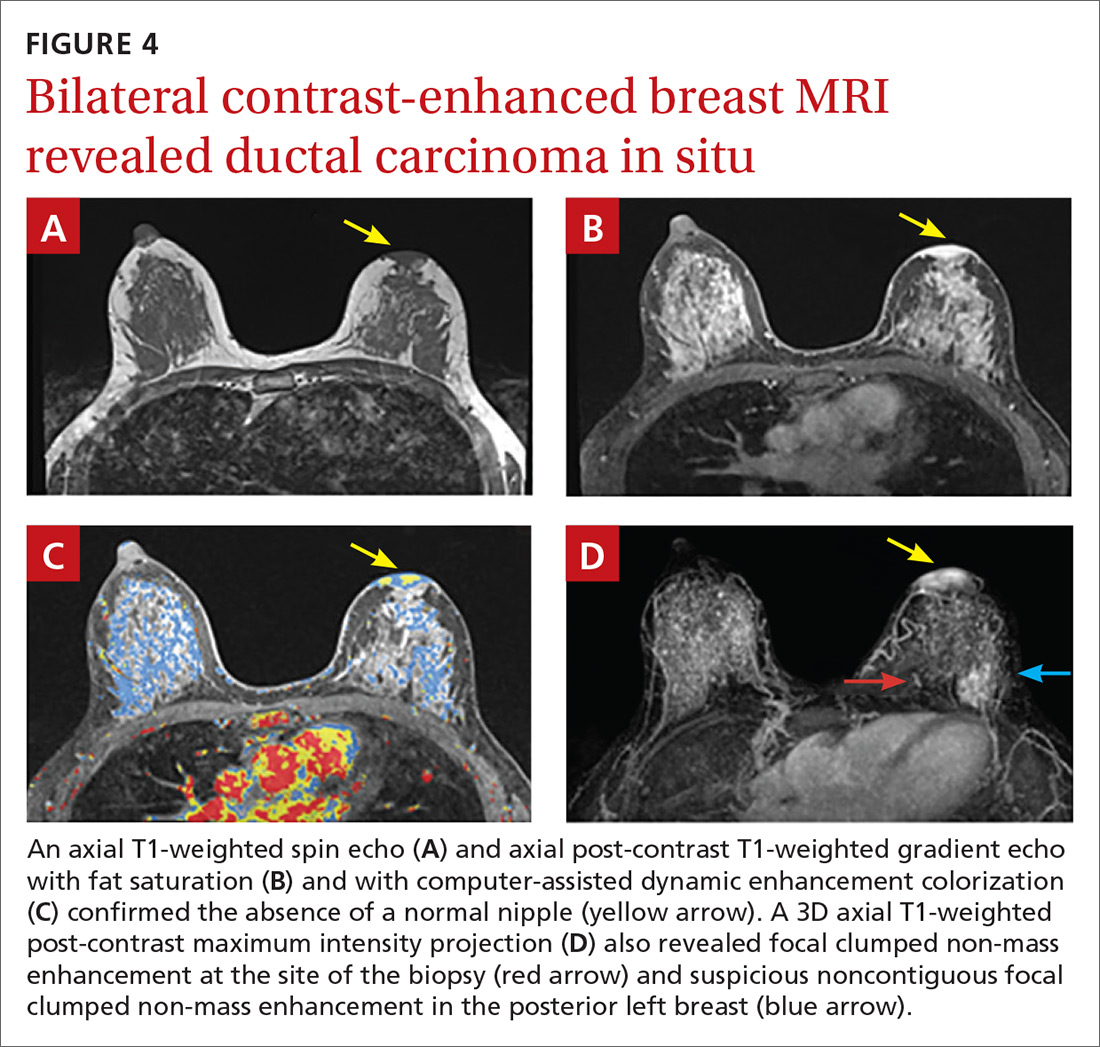
Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.
DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.

THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).


Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.

DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.

THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).


Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.

DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
