User login
T1-hypointense count corrected by T2/FLAIR lesion volume indicates clinical severity in MS
Key clinical point: The number of T1-hypointense areas (T1-count), corrected for T2-fluid-attenuated inversion recovery (FLAIR)-hyperintense lesion volume, reflects the disease severity and activity in patients with multiple sclerosis (MS).
Major finding: T1-count (Spearman’s correlation coefficient [rho] = 0.51, P less than .001), gray-matter atrophy (rho = 0.40; P less than .01), and white-matter atrophy (rho = 0.49; P less than .001) significantly correlated with the expanded disability status scale. T1-count divided by FLAIR lesion volume correlated with the MS severity score (rho = 0.60; P less than .001).
Study details: This study included 42 patients with MS who were treated in a single university hospital in Japan; each patient underwent brain volumetry and was followed-up for more than 3 years until 2017.
Disclosures: Ichiro Nakashima was funded by JSPS KAKENHI. The authors declared no conflict of interest.
Citation: Akaishi T et al. PLoS One. 2020 Apr 3. doi: 10.1371/journal.pone.0231225.
Key clinical point: The number of T1-hypointense areas (T1-count), corrected for T2-fluid-attenuated inversion recovery (FLAIR)-hyperintense lesion volume, reflects the disease severity and activity in patients with multiple sclerosis (MS).
Major finding: T1-count (Spearman’s correlation coefficient [rho] = 0.51, P less than .001), gray-matter atrophy (rho = 0.40; P less than .01), and white-matter atrophy (rho = 0.49; P less than .001) significantly correlated with the expanded disability status scale. T1-count divided by FLAIR lesion volume correlated with the MS severity score (rho = 0.60; P less than .001).
Study details: This study included 42 patients with MS who were treated in a single university hospital in Japan; each patient underwent brain volumetry and was followed-up for more than 3 years until 2017.
Disclosures: Ichiro Nakashima was funded by JSPS KAKENHI. The authors declared no conflict of interest.
Citation: Akaishi T et al. PLoS One. 2020 Apr 3. doi: 10.1371/journal.pone.0231225.
Key clinical point: The number of T1-hypointense areas (T1-count), corrected for T2-fluid-attenuated inversion recovery (FLAIR)-hyperintense lesion volume, reflects the disease severity and activity in patients with multiple sclerosis (MS).
Major finding: T1-count (Spearman’s correlation coefficient [rho] = 0.51, P less than .001), gray-matter atrophy (rho = 0.40; P less than .01), and white-matter atrophy (rho = 0.49; P less than .001) significantly correlated with the expanded disability status scale. T1-count divided by FLAIR lesion volume correlated with the MS severity score (rho = 0.60; P less than .001).
Study details: This study included 42 patients with MS who were treated in a single university hospital in Japan; each patient underwent brain volumetry and was followed-up for more than 3 years until 2017.
Disclosures: Ichiro Nakashima was funded by JSPS KAKENHI. The authors declared no conflict of interest.
Citation: Akaishi T et al. PLoS One. 2020 Apr 3. doi: 10.1371/journal.pone.0231225.
Exclusive breastfeeding lowers the risk of postpartum MS relapse
Key clinical point: Most women diagnosed with multiple sclerosis (MS) can have children without incurring an increased risk of relapses and should be encouraged to breastfeed exclusively as this lowers the risk of postpartum relapses.
Major finding: The annualized relapse rates (ARRs) declined from 0.37 before pregnancy to 0.14 during pregnancy (P less than .0001), with no rebound disease activity in the postpartum period. ARR was found to be 0.27 at 3 months postpartum and 0.37 at 4-6 months, matching prepregnancy rates. Exclusive breastfeeding for at least 2 months after delivery reduced the risk of relapse in the first 6 months postpartum (adjusted hazard ratio, 0.37; P = .0093).
Study details: This study evaluated the electronic health records of 466 pregnancies among 375 women with MS and their infants at the Kaiser Permanente Southern and Northern California between 2008 and 2016.
Disclosures: This study was supported by the National Multiple Sclerosis Society. Annette Langer-Gould has received grant support and awards from the NIH, the Patient-Centered Outcomes Research Institute, and the National MS Society; and currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from the ICER. The remaining authors declared no conflict of interest.
Citation: Langer-Gould A et al. Neurology. 2020 Apr 13. doi: 10.1212/WNL.0000000000009374.
Key clinical point: Most women diagnosed with multiple sclerosis (MS) can have children without incurring an increased risk of relapses and should be encouraged to breastfeed exclusively as this lowers the risk of postpartum relapses.
Major finding: The annualized relapse rates (ARRs) declined from 0.37 before pregnancy to 0.14 during pregnancy (P less than .0001), with no rebound disease activity in the postpartum period. ARR was found to be 0.27 at 3 months postpartum and 0.37 at 4-6 months, matching prepregnancy rates. Exclusive breastfeeding for at least 2 months after delivery reduced the risk of relapse in the first 6 months postpartum (adjusted hazard ratio, 0.37; P = .0093).
Study details: This study evaluated the electronic health records of 466 pregnancies among 375 women with MS and their infants at the Kaiser Permanente Southern and Northern California between 2008 and 2016.
Disclosures: This study was supported by the National Multiple Sclerosis Society. Annette Langer-Gould has received grant support and awards from the NIH, the Patient-Centered Outcomes Research Institute, and the National MS Society; and currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from the ICER. The remaining authors declared no conflict of interest.
Citation: Langer-Gould A et al. Neurology. 2020 Apr 13. doi: 10.1212/WNL.0000000000009374.
Key clinical point: Most women diagnosed with multiple sclerosis (MS) can have children without incurring an increased risk of relapses and should be encouraged to breastfeed exclusively as this lowers the risk of postpartum relapses.
Major finding: The annualized relapse rates (ARRs) declined from 0.37 before pregnancy to 0.14 during pregnancy (P less than .0001), with no rebound disease activity in the postpartum period. ARR was found to be 0.27 at 3 months postpartum and 0.37 at 4-6 months, matching prepregnancy rates. Exclusive breastfeeding for at least 2 months after delivery reduced the risk of relapse in the first 6 months postpartum (adjusted hazard ratio, 0.37; P = .0093).
Study details: This study evaluated the electronic health records of 466 pregnancies among 375 women with MS and their infants at the Kaiser Permanente Southern and Northern California between 2008 and 2016.
Disclosures: This study was supported by the National Multiple Sclerosis Society. Annette Langer-Gould has received grant support and awards from the NIH, the Patient-Centered Outcomes Research Institute, and the National MS Society; and currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from the ICER. The remaining authors declared no conflict of interest.
Citation: Langer-Gould A et al. Neurology. 2020 Apr 13. doi: 10.1212/WNL.0000000000009374.
Neurologists’ pay gets a boost, most happy with career choice
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.
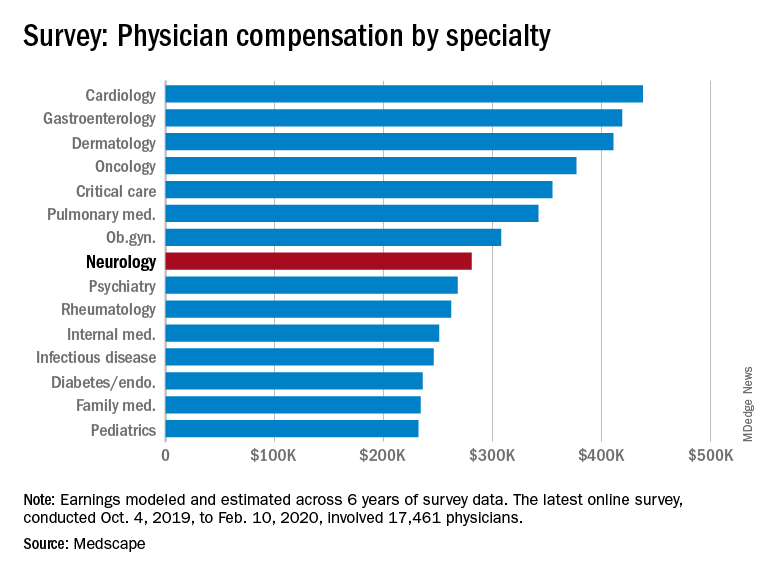
Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
Natalizumab bests fingolimod for relapsing-remitting MS
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
Serum NfL in early MS can help predict clinical course
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Initial high-efficacy MS therapy tied to less disability later
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Cautionary findings on acquired immunodeficiency from anti-CD20 MS therapy
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
REPORTING FROM AAN 2020
Five-year siponimod data support early MS treatment
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
FROM AAN 2020
Report details first case of PML with ocrelizumab alone
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
FROM AAN 2020
Database will collect data on COVID-19 in patients with MS
The COViMS (COVID-19 Infections in Multiple Sclerosis and Related Diseases) database is gathering information from patients throughout the United States and will soon gain access to Canadian data. Data from patients with CNS demyelinating diseases such as neuromyelitis optica and myelin oligodendrocyte glycoprotein antibody diseases also will be included in COViMS. Amber Salter, PhD, MPH, the director of the North American Research Committee on MS (NARCOMS) is supervising the data collection and analyses.
“COViMS will provide valuable insight on how COVID-19 affects people with MS, including if certain disease-modifying treatments incur special risks,” said June Halper, CEO of CMSC, in a press release.
The project began when CMSC and NMSS established independent registries of epidemiologic data related to MS and COVID-19. The two groups soon began communicating and included other researchers, who also were considering establishing registries, in their discussions. In addition, representatives of the Cleveland Clinic verbally agreed to share data that they have been collecting with the COViMS registry. “The fast-moving, almost parallel, efforts led to this collaboration,” said Gary Cutter, PhD, professor of biostatistics at the University of Alabama at Birmingham. “This in itself is noteworthy because all of this took place within an incredibly short time from inception to the initiation of data collection.”
The effects of SARS-CoV-2 infection on the health of patients with MS is little understood. In North America, no reporting system had been organized to gather information on these patients and track outcomes. Such a system could influence the treatment of people with MS who become infected with the novel coronavirus or other similar future viruses. The COViMS registry is intended to define the impact of COVID-19 on patients with MS and ascertain how factors such as age, comorbidities, and MS treatments affect outcomes of COVID-19. “The estimated median age of MS patients in the U.S. is about 52 years, thus putting many at increased risk just due to age,” said Dr. Cutter.
“People with MS and their health care providers need evidence-based guidance to provide optimal MS care during the COVID-19 pandemic, and the COViMS database will help answer the many pressing questions,” said Bruce Bebo, executive vice president of research for the NMSS, in a press release.
The two organizations encourage neurologists and other health care providers who treat patients with MS and documented COVID-19 infection to complete a Case Report Form on the COViMS website, which includes answers to frequently asked questions, a sample CRF, and other resources. The website will provide real-time data once registry participation is underway.
The COViMS (COVID-19 Infections in Multiple Sclerosis and Related Diseases) database is gathering information from patients throughout the United States and will soon gain access to Canadian data. Data from patients with CNS demyelinating diseases such as neuromyelitis optica and myelin oligodendrocyte glycoprotein antibody diseases also will be included in COViMS. Amber Salter, PhD, MPH, the director of the North American Research Committee on MS (NARCOMS) is supervising the data collection and analyses.
“COViMS will provide valuable insight on how COVID-19 affects people with MS, including if certain disease-modifying treatments incur special risks,” said June Halper, CEO of CMSC, in a press release.
The project began when CMSC and NMSS established independent registries of epidemiologic data related to MS and COVID-19. The two groups soon began communicating and included other researchers, who also were considering establishing registries, in their discussions. In addition, representatives of the Cleveland Clinic verbally agreed to share data that they have been collecting with the COViMS registry. “The fast-moving, almost parallel, efforts led to this collaboration,” said Gary Cutter, PhD, professor of biostatistics at the University of Alabama at Birmingham. “This in itself is noteworthy because all of this took place within an incredibly short time from inception to the initiation of data collection.”
The effects of SARS-CoV-2 infection on the health of patients with MS is little understood. In North America, no reporting system had been organized to gather information on these patients and track outcomes. Such a system could influence the treatment of people with MS who become infected with the novel coronavirus or other similar future viruses. The COViMS registry is intended to define the impact of COVID-19 on patients with MS and ascertain how factors such as age, comorbidities, and MS treatments affect outcomes of COVID-19. “The estimated median age of MS patients in the U.S. is about 52 years, thus putting many at increased risk just due to age,” said Dr. Cutter.
“People with MS and their health care providers need evidence-based guidance to provide optimal MS care during the COVID-19 pandemic, and the COViMS database will help answer the many pressing questions,” said Bruce Bebo, executive vice president of research for the NMSS, in a press release.
The two organizations encourage neurologists and other health care providers who treat patients with MS and documented COVID-19 infection to complete a Case Report Form on the COViMS website, which includes answers to frequently asked questions, a sample CRF, and other resources. The website will provide real-time data once registry participation is underway.
The COViMS (COVID-19 Infections in Multiple Sclerosis and Related Diseases) database is gathering information from patients throughout the United States and will soon gain access to Canadian data. Data from patients with CNS demyelinating diseases such as neuromyelitis optica and myelin oligodendrocyte glycoprotein antibody diseases also will be included in COViMS. Amber Salter, PhD, MPH, the director of the North American Research Committee on MS (NARCOMS) is supervising the data collection and analyses.
“COViMS will provide valuable insight on how COVID-19 affects people with MS, including if certain disease-modifying treatments incur special risks,” said June Halper, CEO of CMSC, in a press release.
The project began when CMSC and NMSS established independent registries of epidemiologic data related to MS and COVID-19. The two groups soon began communicating and included other researchers, who also were considering establishing registries, in their discussions. In addition, representatives of the Cleveland Clinic verbally agreed to share data that they have been collecting with the COViMS registry. “The fast-moving, almost parallel, efforts led to this collaboration,” said Gary Cutter, PhD, professor of biostatistics at the University of Alabama at Birmingham. “This in itself is noteworthy because all of this took place within an incredibly short time from inception to the initiation of data collection.”
The effects of SARS-CoV-2 infection on the health of patients with MS is little understood. In North America, no reporting system had been organized to gather information on these patients and track outcomes. Such a system could influence the treatment of people with MS who become infected with the novel coronavirus or other similar future viruses. The COViMS registry is intended to define the impact of COVID-19 on patients with MS and ascertain how factors such as age, comorbidities, and MS treatments affect outcomes of COVID-19. “The estimated median age of MS patients in the U.S. is about 52 years, thus putting many at increased risk just due to age,” said Dr. Cutter.
“People with MS and their health care providers need evidence-based guidance to provide optimal MS care during the COVID-19 pandemic, and the COViMS database will help answer the many pressing questions,” said Bruce Bebo, executive vice president of research for the NMSS, in a press release.
The two organizations encourage neurologists and other health care providers who treat patients with MS and documented COVID-19 infection to complete a Case Report Form on the COViMS website, which includes answers to frequently asked questions, a sample CRF, and other resources. The website will provide real-time data once registry participation is underway.

