User login
Heart Failure Diagnostic Alerts to Prompt Pharmacist Evaluation and Medication Optimization
Heart Failure Diagnostic Alerts to Prompt Pharmacist Evaluation and Medication Optimization
Heart failure (HF) is a prevalent disease in the United States affecting > 6.5 million adults and contributing to significant morbidity and mortality.1 The disease course associated with HF includes potential symptom improvement with intermittent periods of decompensation and possible clinical deterioration. Multiple therapies have been developed to improve outcomes in people with HF—to palliate HF symptoms, prevent hospitalizations, and reduce mortality.2 However, the risks of decompensation and hospitalization remain. HF decompensation development may precede clear actionable symptoms such as worsening dyspnea, noticeable edema, or weight gain. Tools to identify patient deterioration and trigger interventions to prevent HF admissions are clinically attractive compared with reliance on subjective factors alone.
Cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) devices made by Boston Scientific include the HeartLogic monitoring feature. Five main sensors produce an index risk score; an index score > 16 warns clinicians that the patient is at an increased risk for a HF event.3 The 5 sensors are thoracic impedance, first (S1) and third heart sounds (S3), night heart rate (NHR), respiratory rate (RR), and activity. Each sensor can draw attention to the primary driver of the alert and guide health care practitioners (HCPs) to the appropriate interventions.3 A HeartLogic alert example is shown in Figure 1.
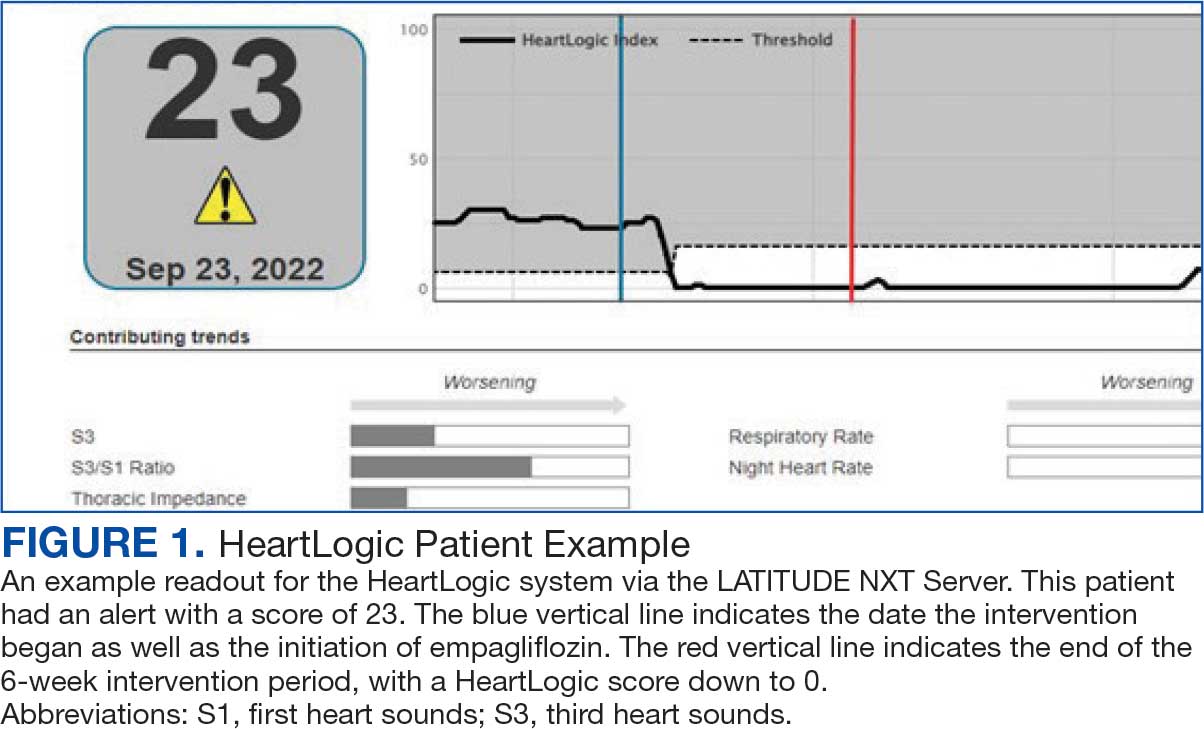
The S3 occurs during the early diastolic phase when blood moves into the ventricles. As HF worsens, with a combination of elevated filling pressures and reduced cardiac muscle compliance, S3 can become more pronounced.4 The S1 is correlated with the contractility of the left ventricle and will be reduced in patients at risk for HF events.5 Physical activity is a long-term prognostic marker in patients with HF; reduced activity is associated with mortality and increased risk of an HF event.6 Thoracic impedance is a sensor used to identify pulmonary congestion, pocket infections, pleural/pericardial effusion, and respiratory infections. The accumulation of intrathoracic fluid during pulmonary congestion increases conductance, causing a decrease in impedance.7 RR will increase as patients experience dyspnea with a more rapid, shallow breath and may trigger alerts closer to the actual HF event than other sensors. Nearly 90% of patients hospitalized for HF experience shortness of breath.8,9 NHR is used as a surrogate for resting heart rate (HR). A high resting HR is correlated with the progression of coronary atherosclerosis, harmful effects on left ventricular function, and increased risk of myocardial ischemia and ventricular arrhythmias.10
One of the challenges with preventing hospitalizations may be the lack of patient reported symptoms leading up to the event. The purpose of the sensors and HeartLogic index is to identify patients a median of 34 days before an HF event (HF admission or unscheduled intervention with intravenous treatment) with a sensitivity rate of 70%.3 According to real-word experience data, alerts have been found to precede HF symptoms by a median of 12 days and HF events such as hospitalizations by a median of 38 days, with an overall 67% reduction in HF hospitalizations when integrated into clinical care.11,12
MANAGE-HF evaluated 191 patients with HF with reduced ejection fraction (HFrEF) (< 35%), New York Heart Association class II-III symptoms, and who had an implanted CRT and/or ICD to develop an alert management guide to optimize medical treatment.12 It aimed to adjust patient regimen within 6 days of an elevated Heart- Logic index by either initiation, escalation, or maintenance of HF treatment depending on the index trend after the initial alert. This trial found that by focusing on such optimization, HF treatment was augmented during 74% of the 585 alert cases and during 54% of 3290 weekly alerts.
Initiation and uptitration of the 4 primary components of guideline-directed medical therapy (GDMT) are recommended by the 2022 Heart Failure Guidelines to reduce mortality and morbidity in patients with HFrEF.2 The 4 pillars of GDMT consist of -blockers (BB), sodium-glucose cotransporter type 2 inhibitors (SGLT2i), mineralocorticoid receptor antagonists (MRA), and renin-angiotensin-system inhibitors (RASi) including angiotensin II receptor blocker/neprilysin inhibitors (ARNi), angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) (Appendix 1). Obtaining and titrating to target doses wherever possible is recommended, as those were the doses that established safety and efficacy in patients with HFrEF in clinical trials.2 Pharmacists are adequately equipped to optimize HF GDMT and appropriately monitor drug response.
Through the use of HeartLogic in clinical practice, patients with HF have been shown to have improved clinical outcomes and are more likely to receive effective care; 80% of alerts were shown to provide new information to clinicians.13 This project sought to quantify the total number and types of pharmacist interventions driven by integration of HeartLogic index monitoring into practice.
Methods
The West Palm Beach Veterans Affairs Medical Center (WPBVAMC) Research Program Office approved this project and determined it was exempt from institutional review board oversight. Patients were screened retrospectively and prospectively from May 26, 2022, through December 31, 2022, by a cardiology clinical pharmacist practitioner (CPP) and a cardiology pharmacy resident using the local monitoring suite for the HeartLogic-compatible device, LATITUDE NXT. Read-only access to the local monitoring suit was granted by the National Cardiac Device Surveillance Program. Training for HeartLogic was completed through continuing education courses provided by Boston Scientific. Additional information was provided by Boston Scientific representative presentations and collaboration with WPBVAMC pacemaker clinic HCPs.
Individuals included were patients with HeartLogic-capable ICDs. A HeartLogic alert had to be present at initial patient contact. Patients were also contacted as part of routine clinical practice, but no formal number or frequency of calls to patients was required. The initial contact must be with a pharmacist for the patient to be included, but subsequent contact by other HCPs was included. Patients in the cardiology clinic are required to meet with a cardiologist at least annually; however, interim visits can be completed by advanced practice registered nurse practitioners, physicians assistants, or CPPs.
Patients in alert status were contacted by telephone and appropriate modifications of HF therapy were made by the CPP based on score metrics, medical record review, and patient interview. Information surrounding the initial alert, baseline patient data, medication and monitoring interventions made, and clinical outcomes such as hospitalization, symptom improvement, follow-up, and mortality were collected. Information for each encounter was collected until 42 days from the initial date of pharmacist contact.
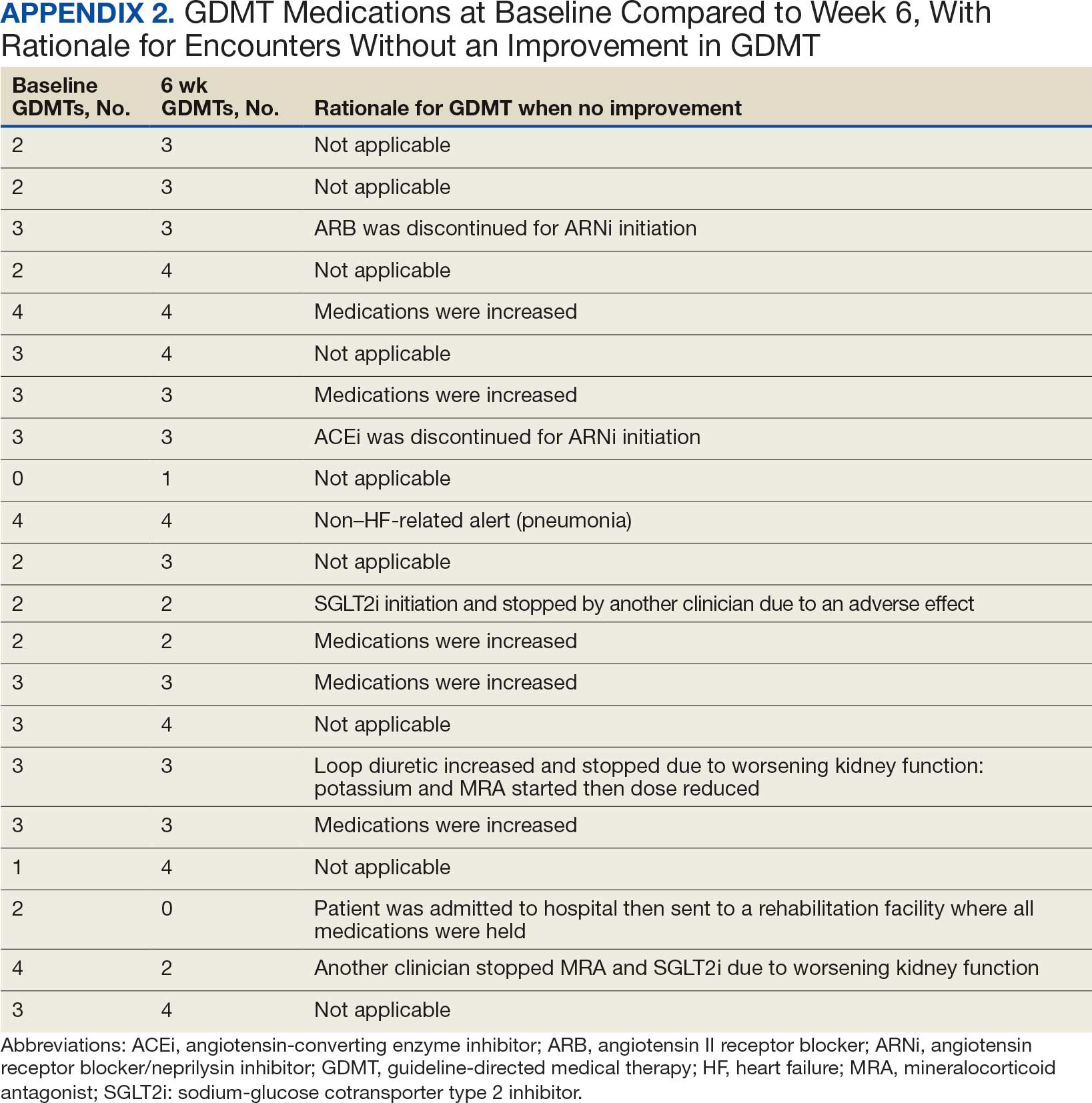
Clinically successful tolerability of intervention implementation was defined as tolerability, adherence, and lack of adverse effects (AEs) per patient report at follow-up or within 42 days from initial alert (Appendix 2). A decrease in dose was not counted as intolerance. A single patient may have been counted as multiple encounters if the original intervention resulted in treatment intolerance and the patient remained in alert or if an additional alert occurred after 42 days of the initial alert. There were no specific time criteria for follow-up, which occurred at the CPP’s discretion.
There was no mandated algorithm used to alter medications based on the Heart- Logic score, nor were there required minimum or maximum numbers of interventions after an alert. Patient contact by telephone initiated an encounter. The types of interventions included medication increases, decreases, initiation, discontinuation, or no medication change. Each medication change and rationale, if applicable, was recorded for the encounter ≤ 42 days after the initial contact date. If a medication with required monitoring parameters was augmented, the pharmacist was responsible for ordering laboratory testing and follow-up. Most interventions were completed by telephone; however, some patients had in-person visits in the HF CPP clinic.
Outcomes
The primary outcome was the number of pharmacist interventions made to optimize GDMT, defined as either an initiation or dose increase. Key intervention analysis included the use and dosing of the 4 primary components of HF GDMT: BB, SGLT2i, MRA, and ARNi/ARB/ACEi. In addition to the 4 primary components of GDMT, loop diuretic changes were also recorded and analyzed. Secondary endpoints were the number of HF hospitalizations ≤ 42 days after the initial alert, and the effect of medication interventions on device metrics, patient symptoms, and tolerability. Successful tolerability was defined as continued use of augmented GDMT without intolerance or discontinuation. The primary analysis was analyzed through descriptive statistics. Median changes in HeartLogic scores and metrics from baseline were analyzed using a paired, 2-sided t test with an α of .05 to detect significance.
Results
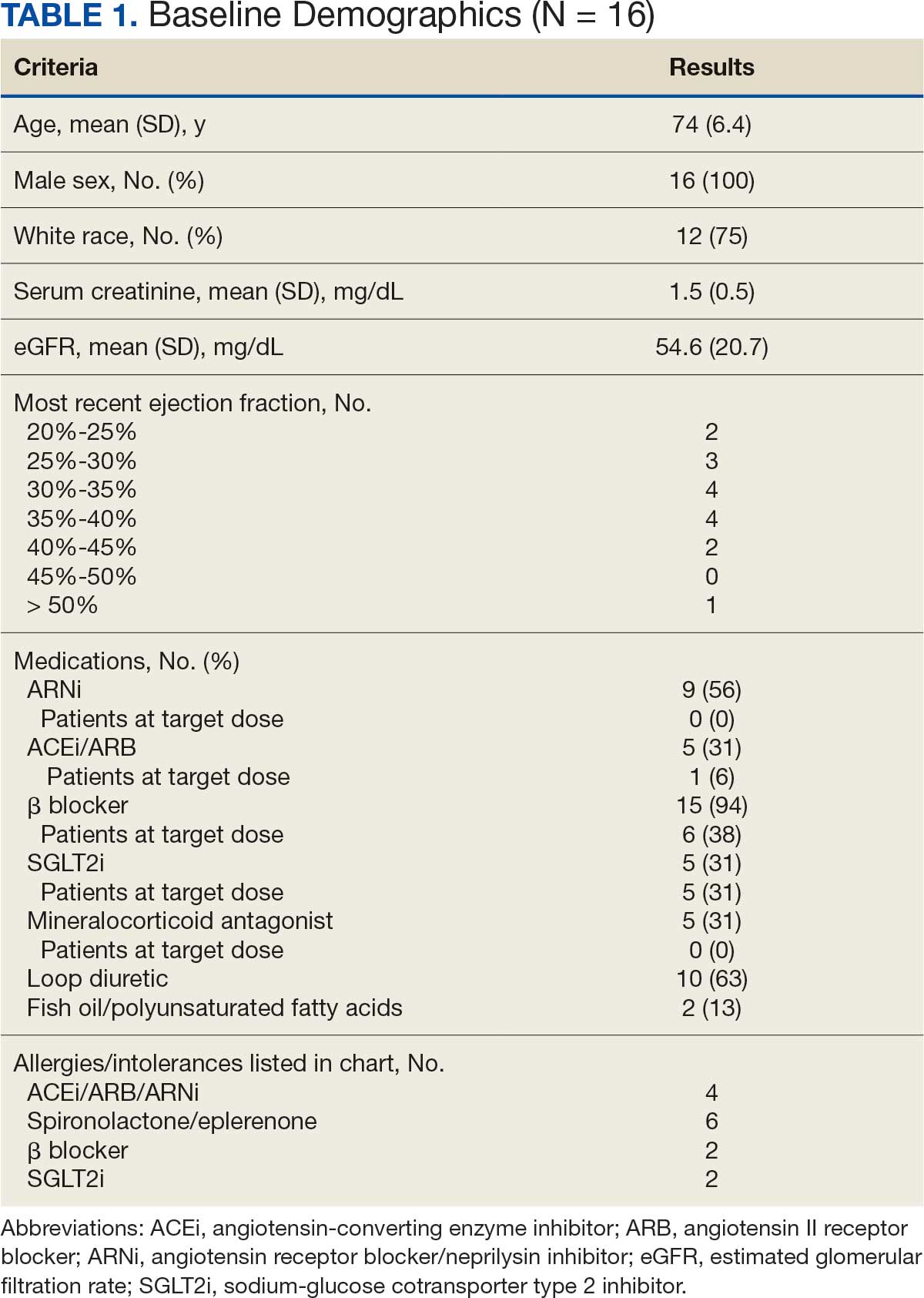
There were 39 WPBVAMC patients with a HeartLogic-capable device. Twenty-one alert encounters were analyzed in 16 patients (41%) over 31 weeks of data collection. The 16 patients at baseline had a mean age of 74 years, all were male, and 12 (75%) were White. Eight patients (50%) had a recent ejection fraction (EF) between 30% and 40%. Three patients had an EF ≥ 40%. At the time of alert, 15 patients used BB (94%), 10 used loop diuretics (63%), and 9 used ARNi (56%) (Table 1).
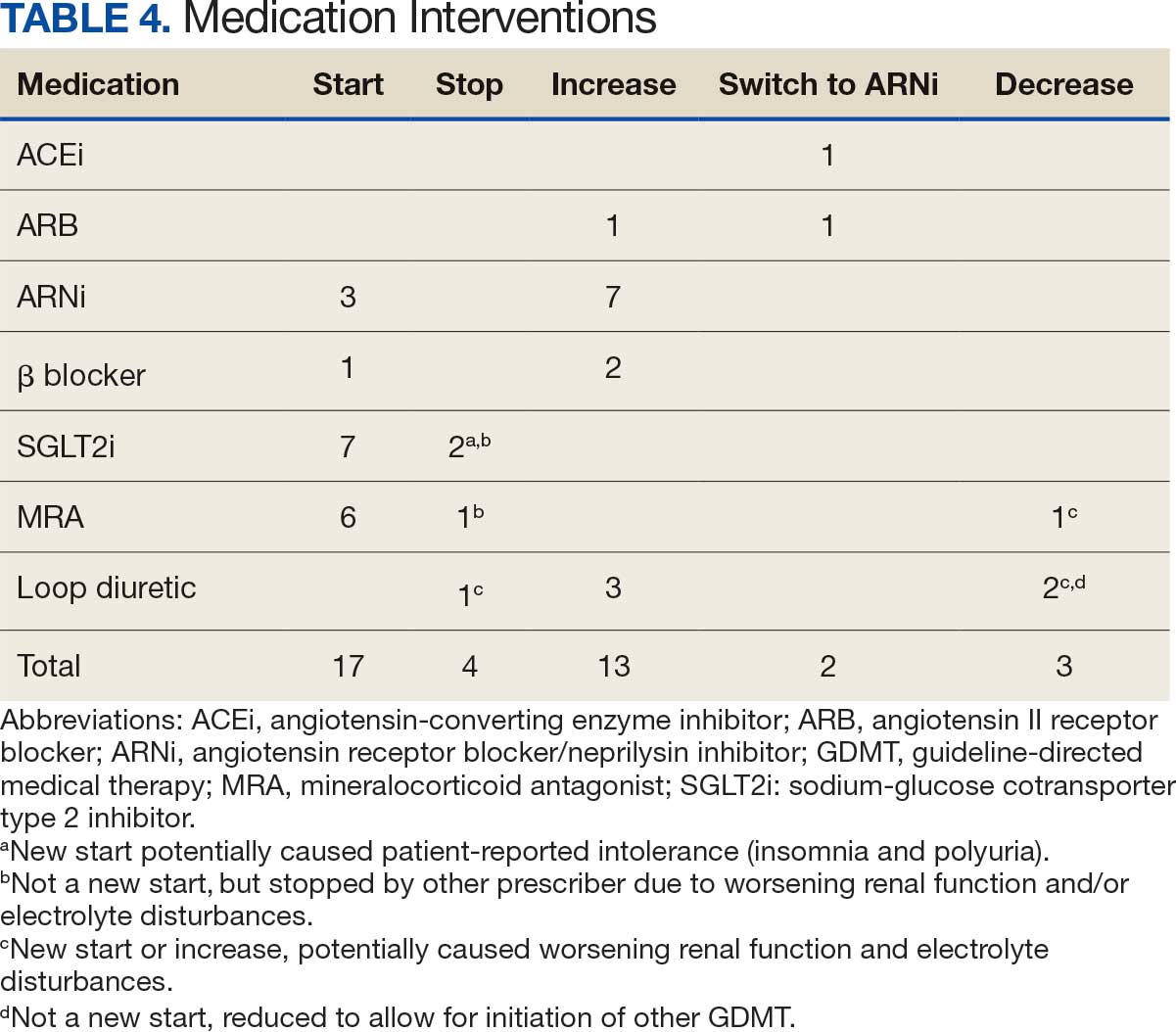
There were 23 medication changes made during initial contact. The most common change was starting an SGLT2i (30%; n = 7), followed by starting an MRA (22%; n = 5), and increasing the ARNi dose (22%; n = 5). At the initial contact, ≥ 1 medication optimization occurred in 95% (n = 20) of encounters. The CPP contacted patients a mean of 4.8 days after the initial alert.
Patients were taking a mean of 2.6 primary GDMT medications at baseline and 3.0 at 42 days. CPP encounters led to a mean of 1.8 medication changes over the 6-week period (range, 0-5). Seventeen medications were started, 13 medications were increased, 3 medications were decreased, and 4 medications were stopped (Table 2). One ACEi and 1 ARB were switched as a therapy escalation to an ARNi. One patient was on 1 of 4 primary GDMTs at baseline, which increased to 4 GDMT agents at 42 days.
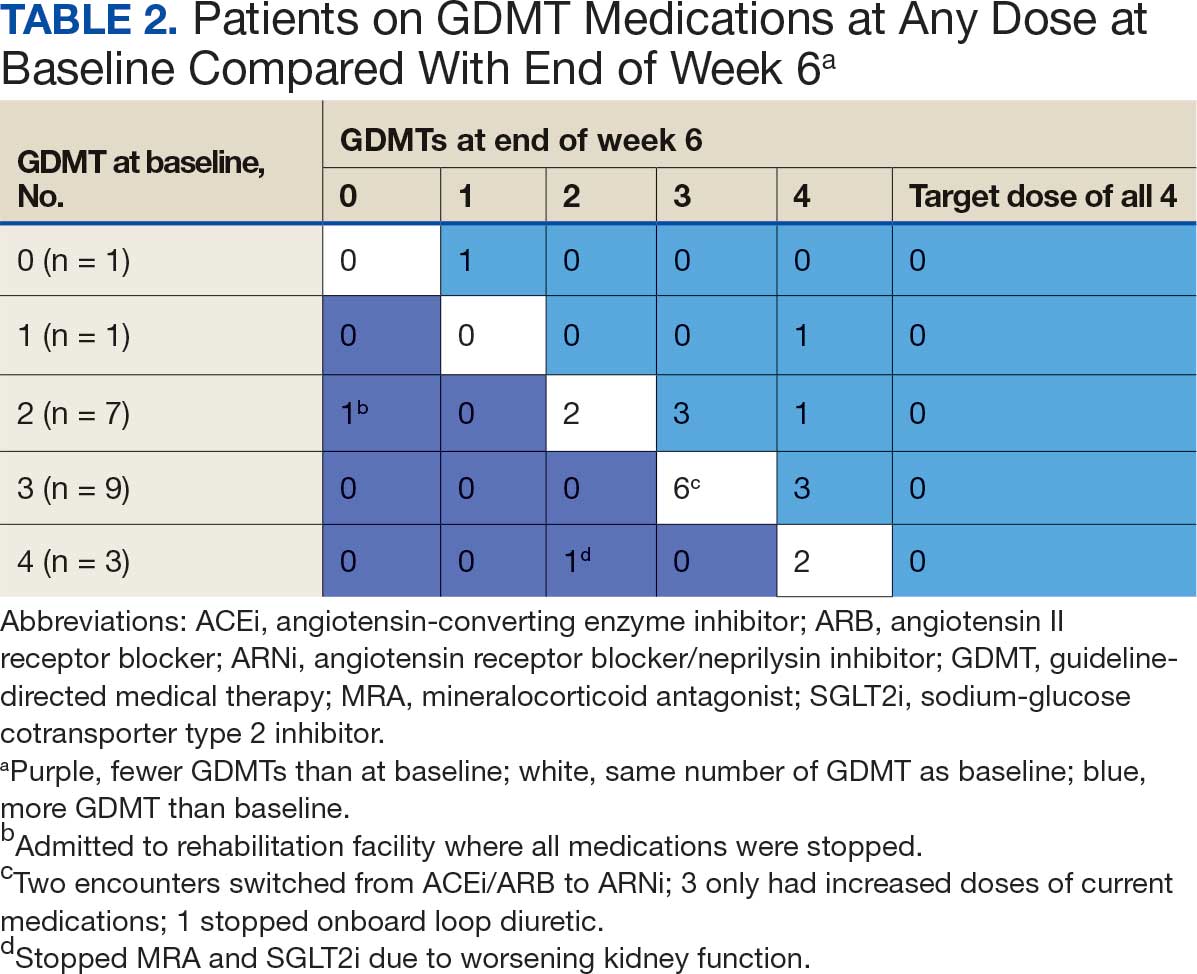
SGLT2 inhibitors were added most often at initial contact (54%) and throughout the 42-day period (41%). The most common successfully tolerated optimizations were RASi, followed by MRA, SGLT2 inhibitors, BB, and loop diuretics with 11, 6, 5, 3, and 2 patients, respectively. Interventions were tolerated by 90% of patients, and no HF hospitalization occurred during follow-up. All possible rationales for patients with the same or reduced number of GDMT at 42 days compared with baseline are shown in Appendix 2.
Device Metrics
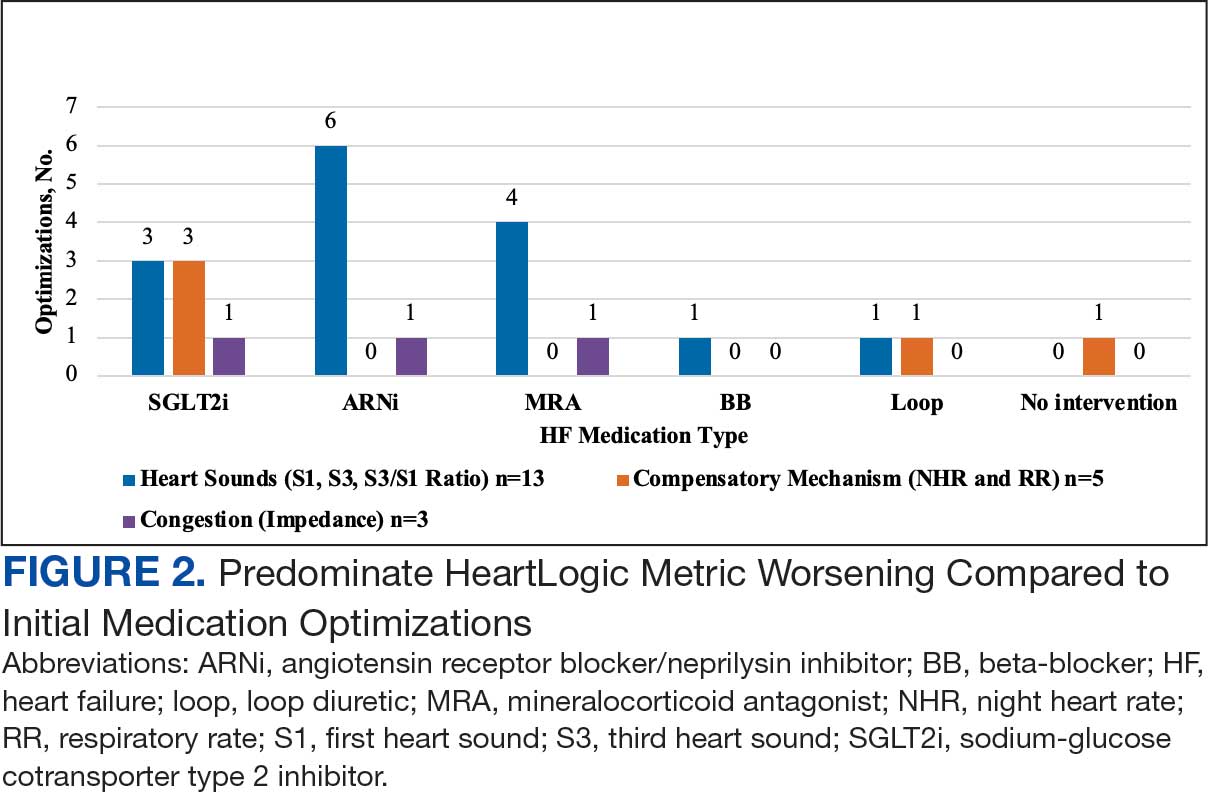
During initial contact, the most common HeartLogic metric category that was predominantly worsening were heart sounds (S1, S3, and S3/S1 ratio), followed by compensatory mechanism sensors (NHR and RR) and congestion (impedance) at rates of 61.9%, 23.8%, and 14.3%, respectively (Figure 2).
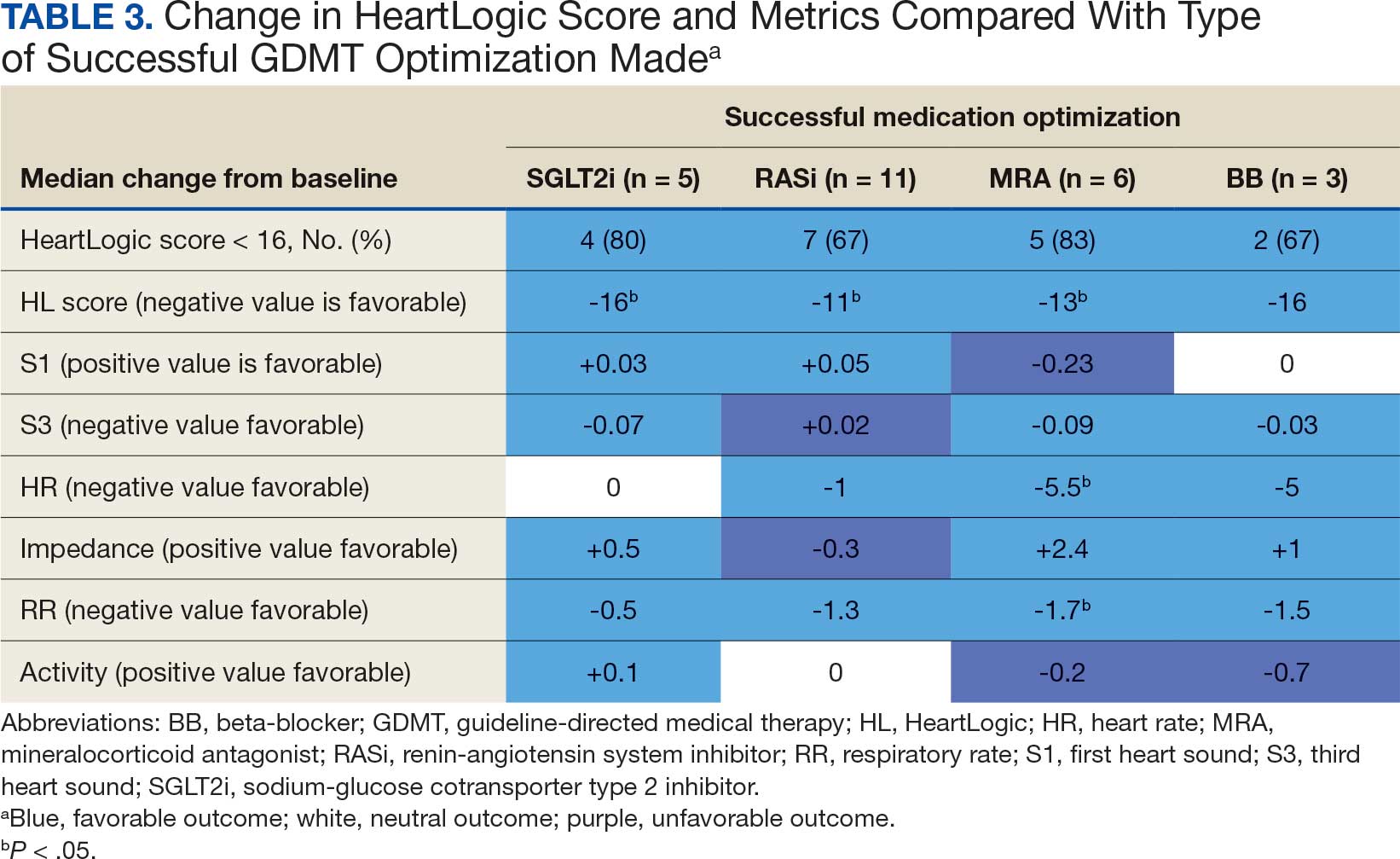
The median HeartLogic index score was 18 at baseline and 5 at the end of the follow-up period (P < .001). The changes in score and metrics were compared with the type of successfully tolerated GDMT optimization made (Table 3). The GDMT optimization analysis included SGLT2i, RASi, MRA, BB, and loop diuretics. All interventions reduced the overall HeartLogic index score, ranging from a 9.5-point reduction (loop diuretics) to a 16-point reduction (SGLT2i and BB). Optimization of SGLT2i, RASi, and loop diuretics had a positive impact on S1 score. For S3 score, SGLT2i, MRA, and BB had a positive impact. All medications, except for SGLT2i therapy, reduced the NHR score. Optimization of MRA, SGLT2i, and BB had positive impacts on the impedance score. All medications reduced RR from baseline. Only SGLT2i and loop diuretics had positive impacts on the activity score.
Clinical Outcomes and Adverse Effects
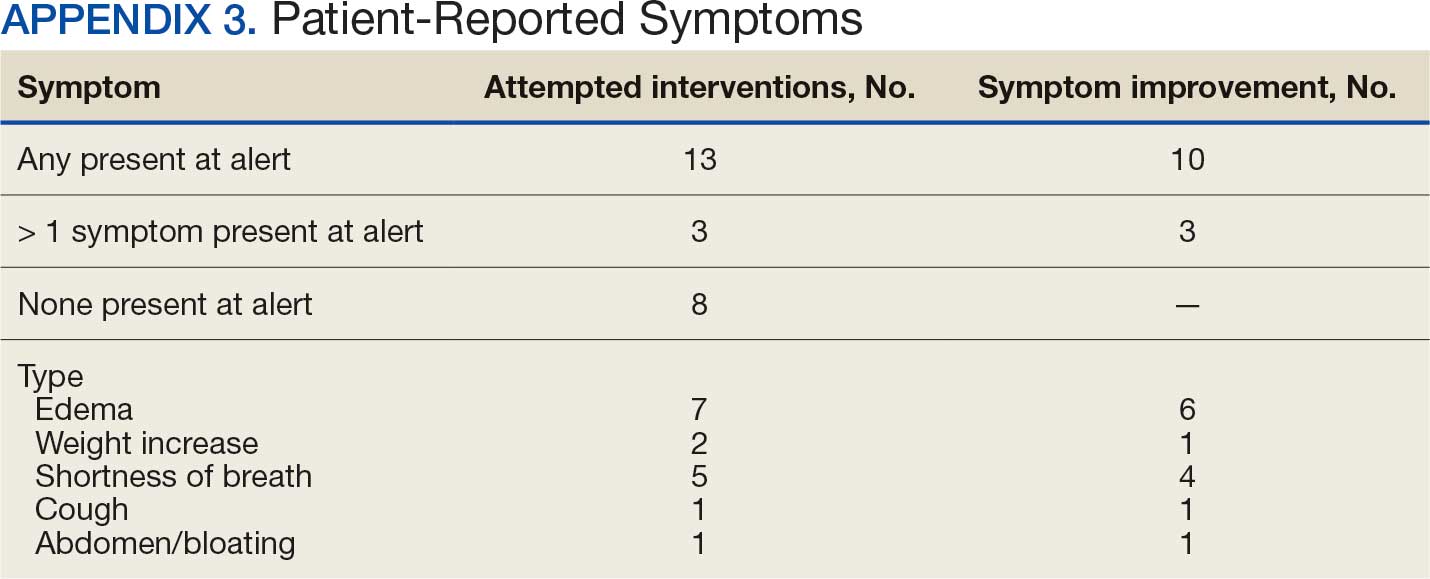
Within 42 days of contact, 17 encounters (81%) had ≥ 1 follow-up appointment with a CPP and all 21 patients had ≥ 1 follow-up health care team member. One patient had a HF-related hospitalization within 42 days of contact; however, that individual refused the recommended medication intervention. There were 13 encounters (62%) with reported symptoms at the time of the initial alert and 10 (77%) had subjective symptom improvement at 42 days (Appendix 3).
Of 30 medication optimizations, 27 primary GDMT medications were tolerated. Two medication intolerances led to discontinuation (1 SGLT2i and 1 loop diuretic) and 1 patient never started the SGLT2i (Table 4). There was only 1 known patient who did not follow the directions to adjust their medications. That individual was included because the patient agreed to the change during the CPP visit but later reported that he had never started the SGLT2i.
Discussion
The HeartLogic tool created a bridge for patients with HF to work with CPPs as soon as possible to optimize medication therapy to reduce HF events. This study highlights an additional area of expertise and service that CPPs may offer to their specialty HF clinic team. Over 31 weeks, 21 encounters and 30 medication optimizations were completed. These interventions led to significant reductions in HeartLogic scores, improvements in symptoms, and optimization of HF care, most of which were well tolerated.
Additional hemodynamic monitoring devices are available. Similar to HeartLogic, OptiVol is a tool embedded in select Medtronic implantable devices that monitors fluid status. 14 CardioMEMS is an implantable pulmonary artery pressure sensor used as a presymptomatic data point to alert clinicians when HF is worsening. In the CHAMPION trial, the use of CardioMEMS showed a 28% reduction in HF-related hospitalization at 6 months.15 Conversely, in the GUIDE-HF trial, monitoring with CardioMEMS did not significantly reduce the composite endpoint of mortality and total HF events.16 Therefore, remote hemodynamic monitoring has variable results and the use of these tools remains uncertain per the clinical guidelines.2
The MANAGE-HF study that contributed to the validation of the HeartLogic tool may provide a comparison with this smaller single-center project. The time to follow-up within 7 days of alert was noted in only 54% of the patients in MANAGE-HF.12 In this study, 86% of patients received follow-up within 7 days, with a mean of 4.8 days. The quick turnaround from the time of alert to intervention portrays pharmacists as readily available HCPs.
In MANAGE-HF, 89% of medication augmentation involved loop diuretics or thiazides; in our project, loop diuretics were the least frequently changed medication. Most optimizations in this project included ARNi, SGLT2i, BB, and MRA, which have been shown to reduce morbidity and mortality.2 Our project included use of SGLT2i therapy to affect HeartLogic metrics, which has not been evaluated previously. SGLT2i were the most commonly initiated medication after an alert. Of the 5 tolerated SGLT2i optimization encounters, 4 were out of alert at 42 days.
SGLT2i resulted in a significant decrease in HeartLogic index score from baseline and were the only class of medication that did not produce a negative change in any metric. In this study, CPPs utilizing and acting on HeartLogic alerts led to 1 (4.8%) hospitalization with HF as the primary reason for admission and no hospitalizations as a secondary cause in 42 days, compared to 37% and 7.9% in the MANAGE-HF in 1 year, respectively. An additional screening 1 year after the initial alert found that 2 (12.5%) of 12 patients had been admitted with 1 HF hospitalization each.
A strength of this study was the ability to use HeartLogic to identify high-risk patients, provide a source of patient contact and monitoring, interpret 5 cardiac sensors, and optimize all HF GDMT, not just volume management. By focusing efforts on making patient contact and pharmacotherapy interventions with morbidity and mortality benefit, remote hemodynamic monitoring may show a clear clinical benefit and become a vital part of HF care.
Limitations
Checking for adherence and tolerance to medications were mainly patient reported if there was a CPP follow-up within 42 days, or potentially through refill history when unclear. However, this limitation is reflective of current practice where patients may have multiple clinicians working to optimize HF care and where there is reliance on patients in order to guide continued therapy. Although unable to explicitly show a reduction in HF events given lack of comparator group, the interventions made are associated with improved outcomes and thus would be expected to improve patient outcomes. Changes in vital signs were not tracked as part of this project, however the main rationale for changes made were to optimize GDMT therapy, not specifically to impact vital sign measures.
HeartLogic alerts prompted identification of high-risk patients with HF, pharmacist evaluation and outreach, patient-focused pharmacotherapy care, and beneficial patient outcomes. With only 2 cardiology CPPs checking alerts once weekly, future studies may be needed with larger samples to create algorithms and protocols to increase the clinical utility of this tool on a greater scale.
Conclusions
Cardiology CPP-led HF interventions triggered by HeartLogic alerts lead to effective patient identification, increased access to care, reductions in HeartLogic scores, improvements in symptoms, and optimization of HF care. This project demonstrates the practical utility of the HeartLogic suite in conjunction with CPP care to prioritize treatment for highrisk patients with HF in an efficient manner. The data highlight the potential value of the HeartLogic tool and a CPP in HF care to facilitate initiation and optimization of GDMT to ultimately improve the morbidity and mortality in patients with HF.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93-e621. doi:10.1161/CIR.0000000000001123
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/ American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi:10.1161/CIR.0000000000001063
- Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. J Am Coll Cardiol HF. 2017;5:216-225. doi:10.1016/j.jchf.2016.12.011
- Cao M, Gardner RS, Hariharan R, et al. Ambulatory monitoring of heart sounds via an implanted device is superior to auscultation for prediction of heart failure events. J Card Fail. 2020;26:151-159. doi:10.1016/j.cardfail.2019.10.006
- Calò L, Capucci A, Santini L, et al. ICD-measured heart sounds and their correlation with echocardiographic indexes of systolic and diastolic function. J Interv Card Electrophysiol. 2020;58:95-101. doi:10.1007/s10840-019-00668
- Del Buono MG, Arena R, Borlaug BA, et al. Exercise intolerance in patients with heart failure: JACC state-of-the- art review. J Am Coll Cardiol. 2019;73:2209-2225. doi:10.1016/j.jacc.2019.01.072
- Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. doi:10.1161/CIRCULATIONAHA.104.492207
- Rials S, Aktas M, An Q, et al. Continuous respiratory rate is superior to routine outpatient dyspnea assessment for predicting heart failure events. J Card Fail. 2018;24:S45.
- Fonarow GC, ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(suppl 7):S21-S30. doi:10.1016/j.cardfail.2018.07.130
- Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823-830. doi:10.1016/j.jacc.2007.04.079
- De Ruvo E, Capucci A, Ammirati F, et al. Preliminary experience of remote management of heart failure patients with a multisensor ICD alert [abstract P1536]. Eur J Heart Fail. 2019;21(suppl S1):370.
- Hernandez AF, Albert NM, Allen LA, et al. Multiple cardiac sensors for management of heart failure (MANAGE- HF) - phase I evaluation of the integration and safety of the HeartLogic multisensor algorithm in patients with heart failure. J Card Fail. 2022;28:1245-1254. doi:10.1016/j.cardfail.2022.03.349
- Santini L, D’Onofrio A, Dello Russo A, et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin Cardiol. 2020;43:691-697. doi:10.1002/clc.23366
- Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. doi:10.1161/CIRCULATIONAHA.104.492207
- Adamson PB, Abraham WT, Stevenson LW, et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail. 2016;9:e002600. doi:10.1161/CIRCHEARTFAILURE.115.002600
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398:991-1001. doi:10.1016/S0140-6736(21)01754-2
Heart failure (HF) is a prevalent disease in the United States affecting > 6.5 million adults and contributing to significant morbidity and mortality.1 The disease course associated with HF includes potential symptom improvement with intermittent periods of decompensation and possible clinical deterioration. Multiple therapies have been developed to improve outcomes in people with HF—to palliate HF symptoms, prevent hospitalizations, and reduce mortality.2 However, the risks of decompensation and hospitalization remain. HF decompensation development may precede clear actionable symptoms such as worsening dyspnea, noticeable edema, or weight gain. Tools to identify patient deterioration and trigger interventions to prevent HF admissions are clinically attractive compared with reliance on subjective factors alone.
Cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) devices made by Boston Scientific include the HeartLogic monitoring feature. Five main sensors produce an index risk score; an index score > 16 warns clinicians that the patient is at an increased risk for a HF event.3 The 5 sensors are thoracic impedance, first (S1) and third heart sounds (S3), night heart rate (NHR), respiratory rate (RR), and activity. Each sensor can draw attention to the primary driver of the alert and guide health care practitioners (HCPs) to the appropriate interventions.3 A HeartLogic alert example is shown in Figure 1.

The S3 occurs during the early diastolic phase when blood moves into the ventricles. As HF worsens, with a combination of elevated filling pressures and reduced cardiac muscle compliance, S3 can become more pronounced.4 The S1 is correlated with the contractility of the left ventricle and will be reduced in patients at risk for HF events.5 Physical activity is a long-term prognostic marker in patients with HF; reduced activity is associated with mortality and increased risk of an HF event.6 Thoracic impedance is a sensor used to identify pulmonary congestion, pocket infections, pleural/pericardial effusion, and respiratory infections. The accumulation of intrathoracic fluid during pulmonary congestion increases conductance, causing a decrease in impedance.7 RR will increase as patients experience dyspnea with a more rapid, shallow breath and may trigger alerts closer to the actual HF event than other sensors. Nearly 90% of patients hospitalized for HF experience shortness of breath.8,9 NHR is used as a surrogate for resting heart rate (HR). A high resting HR is correlated with the progression of coronary atherosclerosis, harmful effects on left ventricular function, and increased risk of myocardial ischemia and ventricular arrhythmias.10
One of the challenges with preventing hospitalizations may be the lack of patient reported symptoms leading up to the event. The purpose of the sensors and HeartLogic index is to identify patients a median of 34 days before an HF event (HF admission or unscheduled intervention with intravenous treatment) with a sensitivity rate of 70%.3 According to real-word experience data, alerts have been found to precede HF symptoms by a median of 12 days and HF events such as hospitalizations by a median of 38 days, with an overall 67% reduction in HF hospitalizations when integrated into clinical care.11,12
MANAGE-HF evaluated 191 patients with HF with reduced ejection fraction (HFrEF) (< 35%), New York Heart Association class II-III symptoms, and who had an implanted CRT and/or ICD to develop an alert management guide to optimize medical treatment.12 It aimed to adjust patient regimen within 6 days of an elevated Heart- Logic index by either initiation, escalation, or maintenance of HF treatment depending on the index trend after the initial alert. This trial found that by focusing on such optimization, HF treatment was augmented during 74% of the 585 alert cases and during 54% of 3290 weekly alerts.
Initiation and uptitration of the 4 primary components of guideline-directed medical therapy (GDMT) are recommended by the 2022 Heart Failure Guidelines to reduce mortality and morbidity in patients with HFrEF.2 The 4 pillars of GDMT consist of -blockers (BB), sodium-glucose cotransporter type 2 inhibitors (SGLT2i), mineralocorticoid receptor antagonists (MRA), and renin-angiotensin-system inhibitors (RASi) including angiotensin II receptor blocker/neprilysin inhibitors (ARNi), angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) (Appendix 1). Obtaining and titrating to target doses wherever possible is recommended, as those were the doses that established safety and efficacy in patients with HFrEF in clinical trials.2 Pharmacists are adequately equipped to optimize HF GDMT and appropriately monitor drug response.

Through the use of HeartLogic in clinical practice, patients with HF have been shown to have improved clinical outcomes and are more likely to receive effective care; 80% of alerts were shown to provide new information to clinicians.13 This project sought to quantify the total number and types of pharmacist interventions driven by integration of HeartLogic index monitoring into practice.
Methods
The West Palm Beach Veterans Affairs Medical Center (WPBVAMC) Research Program Office approved this project and determined it was exempt from institutional review board oversight. Patients were screened retrospectively and prospectively from May 26, 2022, through December 31, 2022, by a cardiology clinical pharmacist practitioner (CPP) and a cardiology pharmacy resident using the local monitoring suite for the HeartLogic-compatible device, LATITUDE NXT. Read-only access to the local monitoring suit was granted by the National Cardiac Device Surveillance Program. Training for HeartLogic was completed through continuing education courses provided by Boston Scientific. Additional information was provided by Boston Scientific representative presentations and collaboration with WPBVAMC pacemaker clinic HCPs.
Individuals included were patients with HeartLogic-capable ICDs. A HeartLogic alert had to be present at initial patient contact. Patients were also contacted as part of routine clinical practice, but no formal number or frequency of calls to patients was required. The initial contact must be with a pharmacist for the patient to be included, but subsequent contact by other HCPs was included. Patients in the cardiology clinic are required to meet with a cardiologist at least annually; however, interim visits can be completed by advanced practice registered nurse practitioners, physicians assistants, or CPPs.
Patients in alert status were contacted by telephone and appropriate modifications of HF therapy were made by the CPP based on score metrics, medical record review, and patient interview. Information surrounding the initial alert, baseline patient data, medication and monitoring interventions made, and clinical outcomes such as hospitalization, symptom improvement, follow-up, and mortality were collected. Information for each encounter was collected until 42 days from the initial date of pharmacist contact.
Clinically successful tolerability of intervention implementation was defined as tolerability, adherence, and lack of adverse effects (AEs) per patient report at follow-up or within 42 days from initial alert (Appendix 2). A decrease in dose was not counted as intolerance. A single patient may have been counted as multiple encounters if the original intervention resulted in treatment intolerance and the patient remained in alert or if an additional alert occurred after 42 days of the initial alert. There were no specific time criteria for follow-up, which occurred at the CPP’s discretion.

There was no mandated algorithm used to alter medications based on the Heart- Logic score, nor were there required minimum or maximum numbers of interventions after an alert. Patient contact by telephone initiated an encounter. The types of interventions included medication increases, decreases, initiation, discontinuation, or no medication change. Each medication change and rationale, if applicable, was recorded for the encounter ≤ 42 days after the initial contact date. If a medication with required monitoring parameters was augmented, the pharmacist was responsible for ordering laboratory testing and follow-up. Most interventions were completed by telephone; however, some patients had in-person visits in the HF CPP clinic.
Outcomes
The primary outcome was the number of pharmacist interventions made to optimize GDMT, defined as either an initiation or dose increase. Key intervention analysis included the use and dosing of the 4 primary components of HF GDMT: BB, SGLT2i, MRA, and ARNi/ARB/ACEi. In addition to the 4 primary components of GDMT, loop diuretic changes were also recorded and analyzed. Secondary endpoints were the number of HF hospitalizations ≤ 42 days after the initial alert, and the effect of medication interventions on device metrics, patient symptoms, and tolerability. Successful tolerability was defined as continued use of augmented GDMT without intolerance or discontinuation. The primary analysis was analyzed through descriptive statistics. Median changes in HeartLogic scores and metrics from baseline were analyzed using a paired, 2-sided t test with an α of .05 to detect significance.
Results
There were 39 WPBVAMC patients with a HeartLogic-capable device. Twenty-one alert encounters were analyzed in 16 patients (41%) over 31 weeks of data collection. The 16 patients at baseline had a mean age of 74 years, all were male, and 12 (75%) were White. Eight patients (50%) had a recent ejection fraction (EF) between 30% and 40%. Three patients had an EF ≥ 40%. At the time of alert, 15 patients used BB (94%), 10 used loop diuretics (63%), and 9 used ARNi (56%) (Table 1).

There were 23 medication changes made during initial contact. The most common change was starting an SGLT2i (30%; n = 7), followed by starting an MRA (22%; n = 5), and increasing the ARNi dose (22%; n = 5). At the initial contact, ≥ 1 medication optimization occurred in 95% (n = 20) of encounters. The CPP contacted patients a mean of 4.8 days after the initial alert.
Patients were taking a mean of 2.6 primary GDMT medications at baseline and 3.0 at 42 days. CPP encounters led to a mean of 1.8 medication changes over the 6-week period (range, 0-5). Seventeen medications were started, 13 medications were increased, 3 medications were decreased, and 4 medications were stopped (Table 2). One ACEi and 1 ARB were switched as a therapy escalation to an ARNi. One patient was on 1 of 4 primary GDMTs at baseline, which increased to 4 GDMT agents at 42 days.

SGLT2 inhibitors were added most often at initial contact (54%) and throughout the 42-day period (41%). The most common successfully tolerated optimizations were RASi, followed by MRA, SGLT2 inhibitors, BB, and loop diuretics with 11, 6, 5, 3, and 2 patients, respectively. Interventions were tolerated by 90% of patients, and no HF hospitalization occurred during follow-up. All possible rationales for patients with the same or reduced number of GDMT at 42 days compared with baseline are shown in Appendix 2.
Device Metrics
During initial contact, the most common HeartLogic metric category that was predominantly worsening were heart sounds (S1, S3, and S3/S1 ratio), followed by compensatory mechanism sensors (NHR and RR) and congestion (impedance) at rates of 61.9%, 23.8%, and 14.3%, respectively (Figure 2).

The median HeartLogic index score was 18 at baseline and 5 at the end of the follow-up period (P < .001). The changes in score and metrics were compared with the type of successfully tolerated GDMT optimization made (Table 3). The GDMT optimization analysis included SGLT2i, RASi, MRA, BB, and loop diuretics. All interventions reduced the overall HeartLogic index score, ranging from a 9.5-point reduction (loop diuretics) to a 16-point reduction (SGLT2i and BB). Optimization of SGLT2i, RASi, and loop diuretics had a positive impact on S1 score. For S3 score, SGLT2i, MRA, and BB had a positive impact. All medications, except for SGLT2i therapy, reduced the NHR score. Optimization of MRA, SGLT2i, and BB had positive impacts on the impedance score. All medications reduced RR from baseline. Only SGLT2i and loop diuretics had positive impacts on the activity score.

Clinical Outcomes and Adverse Effects
Within 42 days of contact, 17 encounters (81%) had ≥ 1 follow-up appointment with a CPP and all 21 patients had ≥ 1 follow-up health care team member. One patient had a HF-related hospitalization within 42 days of contact; however, that individual refused the recommended medication intervention. There were 13 encounters (62%) with reported symptoms at the time of the initial alert and 10 (77%) had subjective symptom improvement at 42 days (Appendix 3).

Of 30 medication optimizations, 27 primary GDMT medications were tolerated. Two medication intolerances led to discontinuation (1 SGLT2i and 1 loop diuretic) and 1 patient never started the SGLT2i (Table 4). There was only 1 known patient who did not follow the directions to adjust their medications. That individual was included because the patient agreed to the change during the CPP visit but later reported that he had never started the SGLT2i.

Discussion
The HeartLogic tool created a bridge for patients with HF to work with CPPs as soon as possible to optimize medication therapy to reduce HF events. This study highlights an additional area of expertise and service that CPPs may offer to their specialty HF clinic team. Over 31 weeks, 21 encounters and 30 medication optimizations were completed. These interventions led to significant reductions in HeartLogic scores, improvements in symptoms, and optimization of HF care, most of which were well tolerated.
Additional hemodynamic monitoring devices are available. Similar to HeartLogic, OptiVol is a tool embedded in select Medtronic implantable devices that monitors fluid status. 14 CardioMEMS is an implantable pulmonary artery pressure sensor used as a presymptomatic data point to alert clinicians when HF is worsening. In the CHAMPION trial, the use of CardioMEMS showed a 28% reduction in HF-related hospitalization at 6 months.15 Conversely, in the GUIDE-HF trial, monitoring with CardioMEMS did not significantly reduce the composite endpoint of mortality and total HF events.16 Therefore, remote hemodynamic monitoring has variable results and the use of these tools remains uncertain per the clinical guidelines.2
The MANAGE-HF study that contributed to the validation of the HeartLogic tool may provide a comparison with this smaller single-center project. The time to follow-up within 7 days of alert was noted in only 54% of the patients in MANAGE-HF.12 In this study, 86% of patients received follow-up within 7 days, with a mean of 4.8 days. The quick turnaround from the time of alert to intervention portrays pharmacists as readily available HCPs.
In MANAGE-HF, 89% of medication augmentation involved loop diuretics or thiazides; in our project, loop diuretics were the least frequently changed medication. Most optimizations in this project included ARNi, SGLT2i, BB, and MRA, which have been shown to reduce morbidity and mortality.2 Our project included use of SGLT2i therapy to affect HeartLogic metrics, which has not been evaluated previously. SGLT2i were the most commonly initiated medication after an alert. Of the 5 tolerated SGLT2i optimization encounters, 4 were out of alert at 42 days.
SGLT2i resulted in a significant decrease in HeartLogic index score from baseline and were the only class of medication that did not produce a negative change in any metric. In this study, CPPs utilizing and acting on HeartLogic alerts led to 1 (4.8%) hospitalization with HF as the primary reason for admission and no hospitalizations as a secondary cause in 42 days, compared to 37% and 7.9% in the MANAGE-HF in 1 year, respectively. An additional screening 1 year after the initial alert found that 2 (12.5%) of 12 patients had been admitted with 1 HF hospitalization each.
A strength of this study was the ability to use HeartLogic to identify high-risk patients, provide a source of patient contact and monitoring, interpret 5 cardiac sensors, and optimize all HF GDMT, not just volume management. By focusing efforts on making patient contact and pharmacotherapy interventions with morbidity and mortality benefit, remote hemodynamic monitoring may show a clear clinical benefit and become a vital part of HF care.
Limitations
Checking for adherence and tolerance to medications were mainly patient reported if there was a CPP follow-up within 42 days, or potentially through refill history when unclear. However, this limitation is reflective of current practice where patients may have multiple clinicians working to optimize HF care and where there is reliance on patients in order to guide continued therapy. Although unable to explicitly show a reduction in HF events given lack of comparator group, the interventions made are associated with improved outcomes and thus would be expected to improve patient outcomes. Changes in vital signs were not tracked as part of this project, however the main rationale for changes made were to optimize GDMT therapy, not specifically to impact vital sign measures.
HeartLogic alerts prompted identification of high-risk patients with HF, pharmacist evaluation and outreach, patient-focused pharmacotherapy care, and beneficial patient outcomes. With only 2 cardiology CPPs checking alerts once weekly, future studies may be needed with larger samples to create algorithms and protocols to increase the clinical utility of this tool on a greater scale.
Conclusions
Cardiology CPP-led HF interventions triggered by HeartLogic alerts lead to effective patient identification, increased access to care, reductions in HeartLogic scores, improvements in symptoms, and optimization of HF care. This project demonstrates the practical utility of the HeartLogic suite in conjunction with CPP care to prioritize treatment for highrisk patients with HF in an efficient manner. The data highlight the potential value of the HeartLogic tool and a CPP in HF care to facilitate initiation and optimization of GDMT to ultimately improve the morbidity and mortality in patients with HF.
Heart failure (HF) is a prevalent disease in the United States affecting > 6.5 million adults and contributing to significant morbidity and mortality.1 The disease course associated with HF includes potential symptom improvement with intermittent periods of decompensation and possible clinical deterioration. Multiple therapies have been developed to improve outcomes in people with HF—to palliate HF symptoms, prevent hospitalizations, and reduce mortality.2 However, the risks of decompensation and hospitalization remain. HF decompensation development may precede clear actionable symptoms such as worsening dyspnea, noticeable edema, or weight gain. Tools to identify patient deterioration and trigger interventions to prevent HF admissions are clinically attractive compared with reliance on subjective factors alone.
Cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) devices made by Boston Scientific include the HeartLogic monitoring feature. Five main sensors produce an index risk score; an index score > 16 warns clinicians that the patient is at an increased risk for a HF event.3 The 5 sensors are thoracic impedance, first (S1) and third heart sounds (S3), night heart rate (NHR), respiratory rate (RR), and activity. Each sensor can draw attention to the primary driver of the alert and guide health care practitioners (HCPs) to the appropriate interventions.3 A HeartLogic alert example is shown in Figure 1.

The S3 occurs during the early diastolic phase when blood moves into the ventricles. As HF worsens, with a combination of elevated filling pressures and reduced cardiac muscle compliance, S3 can become more pronounced.4 The S1 is correlated with the contractility of the left ventricle and will be reduced in patients at risk for HF events.5 Physical activity is a long-term prognostic marker in patients with HF; reduced activity is associated with mortality and increased risk of an HF event.6 Thoracic impedance is a sensor used to identify pulmonary congestion, pocket infections, pleural/pericardial effusion, and respiratory infections. The accumulation of intrathoracic fluid during pulmonary congestion increases conductance, causing a decrease in impedance.7 RR will increase as patients experience dyspnea with a more rapid, shallow breath and may trigger alerts closer to the actual HF event than other sensors. Nearly 90% of patients hospitalized for HF experience shortness of breath.8,9 NHR is used as a surrogate for resting heart rate (HR). A high resting HR is correlated with the progression of coronary atherosclerosis, harmful effects on left ventricular function, and increased risk of myocardial ischemia and ventricular arrhythmias.10
One of the challenges with preventing hospitalizations may be the lack of patient reported symptoms leading up to the event. The purpose of the sensors and HeartLogic index is to identify patients a median of 34 days before an HF event (HF admission or unscheduled intervention with intravenous treatment) with a sensitivity rate of 70%.3 According to real-word experience data, alerts have been found to precede HF symptoms by a median of 12 days and HF events such as hospitalizations by a median of 38 days, with an overall 67% reduction in HF hospitalizations when integrated into clinical care.11,12
MANAGE-HF evaluated 191 patients with HF with reduced ejection fraction (HFrEF) (< 35%), New York Heart Association class II-III symptoms, and who had an implanted CRT and/or ICD to develop an alert management guide to optimize medical treatment.12 It aimed to adjust patient regimen within 6 days of an elevated Heart- Logic index by either initiation, escalation, or maintenance of HF treatment depending on the index trend after the initial alert. This trial found that by focusing on such optimization, HF treatment was augmented during 74% of the 585 alert cases and during 54% of 3290 weekly alerts.
Initiation and uptitration of the 4 primary components of guideline-directed medical therapy (GDMT) are recommended by the 2022 Heart Failure Guidelines to reduce mortality and morbidity in patients with HFrEF.2 The 4 pillars of GDMT consist of -blockers (BB), sodium-glucose cotransporter type 2 inhibitors (SGLT2i), mineralocorticoid receptor antagonists (MRA), and renin-angiotensin-system inhibitors (RASi) including angiotensin II receptor blocker/neprilysin inhibitors (ARNi), angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) (Appendix 1). Obtaining and titrating to target doses wherever possible is recommended, as those were the doses that established safety and efficacy in patients with HFrEF in clinical trials.2 Pharmacists are adequately equipped to optimize HF GDMT and appropriately monitor drug response.

Through the use of HeartLogic in clinical practice, patients with HF have been shown to have improved clinical outcomes and are more likely to receive effective care; 80% of alerts were shown to provide new information to clinicians.13 This project sought to quantify the total number and types of pharmacist interventions driven by integration of HeartLogic index monitoring into practice.
Methods
The West Palm Beach Veterans Affairs Medical Center (WPBVAMC) Research Program Office approved this project and determined it was exempt from institutional review board oversight. Patients were screened retrospectively and prospectively from May 26, 2022, through December 31, 2022, by a cardiology clinical pharmacist practitioner (CPP) and a cardiology pharmacy resident using the local monitoring suite for the HeartLogic-compatible device, LATITUDE NXT. Read-only access to the local monitoring suit was granted by the National Cardiac Device Surveillance Program. Training for HeartLogic was completed through continuing education courses provided by Boston Scientific. Additional information was provided by Boston Scientific representative presentations and collaboration with WPBVAMC pacemaker clinic HCPs.
Individuals included were patients with HeartLogic-capable ICDs. A HeartLogic alert had to be present at initial patient contact. Patients were also contacted as part of routine clinical practice, but no formal number or frequency of calls to patients was required. The initial contact must be with a pharmacist for the patient to be included, but subsequent contact by other HCPs was included. Patients in the cardiology clinic are required to meet with a cardiologist at least annually; however, interim visits can be completed by advanced practice registered nurse practitioners, physicians assistants, or CPPs.
Patients in alert status were contacted by telephone and appropriate modifications of HF therapy were made by the CPP based on score metrics, medical record review, and patient interview. Information surrounding the initial alert, baseline patient data, medication and monitoring interventions made, and clinical outcomes such as hospitalization, symptom improvement, follow-up, and mortality were collected. Information for each encounter was collected until 42 days from the initial date of pharmacist contact.
Clinically successful tolerability of intervention implementation was defined as tolerability, adherence, and lack of adverse effects (AEs) per patient report at follow-up or within 42 days from initial alert (Appendix 2). A decrease in dose was not counted as intolerance. A single patient may have been counted as multiple encounters if the original intervention resulted in treatment intolerance and the patient remained in alert or if an additional alert occurred after 42 days of the initial alert. There were no specific time criteria for follow-up, which occurred at the CPP’s discretion.

There was no mandated algorithm used to alter medications based on the Heart- Logic score, nor were there required minimum or maximum numbers of interventions after an alert. Patient contact by telephone initiated an encounter. The types of interventions included medication increases, decreases, initiation, discontinuation, or no medication change. Each medication change and rationale, if applicable, was recorded for the encounter ≤ 42 days after the initial contact date. If a medication with required monitoring parameters was augmented, the pharmacist was responsible for ordering laboratory testing and follow-up. Most interventions were completed by telephone; however, some patients had in-person visits in the HF CPP clinic.
Outcomes
The primary outcome was the number of pharmacist interventions made to optimize GDMT, defined as either an initiation or dose increase. Key intervention analysis included the use and dosing of the 4 primary components of HF GDMT: BB, SGLT2i, MRA, and ARNi/ARB/ACEi. In addition to the 4 primary components of GDMT, loop diuretic changes were also recorded and analyzed. Secondary endpoints were the number of HF hospitalizations ≤ 42 days after the initial alert, and the effect of medication interventions on device metrics, patient symptoms, and tolerability. Successful tolerability was defined as continued use of augmented GDMT without intolerance or discontinuation. The primary analysis was analyzed through descriptive statistics. Median changes in HeartLogic scores and metrics from baseline were analyzed using a paired, 2-sided t test with an α of .05 to detect significance.
Results
There were 39 WPBVAMC patients with a HeartLogic-capable device. Twenty-one alert encounters were analyzed in 16 patients (41%) over 31 weeks of data collection. The 16 patients at baseline had a mean age of 74 years, all were male, and 12 (75%) were White. Eight patients (50%) had a recent ejection fraction (EF) between 30% and 40%. Three patients had an EF ≥ 40%. At the time of alert, 15 patients used BB (94%), 10 used loop diuretics (63%), and 9 used ARNi (56%) (Table 1).

There were 23 medication changes made during initial contact. The most common change was starting an SGLT2i (30%; n = 7), followed by starting an MRA (22%; n = 5), and increasing the ARNi dose (22%; n = 5). At the initial contact, ≥ 1 medication optimization occurred in 95% (n = 20) of encounters. The CPP contacted patients a mean of 4.8 days after the initial alert.
Patients were taking a mean of 2.6 primary GDMT medications at baseline and 3.0 at 42 days. CPP encounters led to a mean of 1.8 medication changes over the 6-week period (range, 0-5). Seventeen medications were started, 13 medications were increased, 3 medications were decreased, and 4 medications were stopped (Table 2). One ACEi and 1 ARB were switched as a therapy escalation to an ARNi. One patient was on 1 of 4 primary GDMTs at baseline, which increased to 4 GDMT agents at 42 days.

SGLT2 inhibitors were added most often at initial contact (54%) and throughout the 42-day period (41%). The most common successfully tolerated optimizations were RASi, followed by MRA, SGLT2 inhibitors, BB, and loop diuretics with 11, 6, 5, 3, and 2 patients, respectively. Interventions were tolerated by 90% of patients, and no HF hospitalization occurred during follow-up. All possible rationales for patients with the same or reduced number of GDMT at 42 days compared with baseline are shown in Appendix 2.
Device Metrics
During initial contact, the most common HeartLogic metric category that was predominantly worsening were heart sounds (S1, S3, and S3/S1 ratio), followed by compensatory mechanism sensors (NHR and RR) and congestion (impedance) at rates of 61.9%, 23.8%, and 14.3%, respectively (Figure 2).

The median HeartLogic index score was 18 at baseline and 5 at the end of the follow-up period (P < .001). The changes in score and metrics were compared with the type of successfully tolerated GDMT optimization made (Table 3). The GDMT optimization analysis included SGLT2i, RASi, MRA, BB, and loop diuretics. All interventions reduced the overall HeartLogic index score, ranging from a 9.5-point reduction (loop diuretics) to a 16-point reduction (SGLT2i and BB). Optimization of SGLT2i, RASi, and loop diuretics had a positive impact on S1 score. For S3 score, SGLT2i, MRA, and BB had a positive impact. All medications, except for SGLT2i therapy, reduced the NHR score. Optimization of MRA, SGLT2i, and BB had positive impacts on the impedance score. All medications reduced RR from baseline. Only SGLT2i and loop diuretics had positive impacts on the activity score.

Clinical Outcomes and Adverse Effects
Within 42 days of contact, 17 encounters (81%) had ≥ 1 follow-up appointment with a CPP and all 21 patients had ≥ 1 follow-up health care team member. One patient had a HF-related hospitalization within 42 days of contact; however, that individual refused the recommended medication intervention. There were 13 encounters (62%) with reported symptoms at the time of the initial alert and 10 (77%) had subjective symptom improvement at 42 days (Appendix 3).

Of 30 medication optimizations, 27 primary GDMT medications were tolerated. Two medication intolerances led to discontinuation (1 SGLT2i and 1 loop diuretic) and 1 patient never started the SGLT2i (Table 4). There was only 1 known patient who did not follow the directions to adjust their medications. That individual was included because the patient agreed to the change during the CPP visit but later reported that he had never started the SGLT2i.

Discussion
The HeartLogic tool created a bridge for patients with HF to work with CPPs as soon as possible to optimize medication therapy to reduce HF events. This study highlights an additional area of expertise and service that CPPs may offer to their specialty HF clinic team. Over 31 weeks, 21 encounters and 30 medication optimizations were completed. These interventions led to significant reductions in HeartLogic scores, improvements in symptoms, and optimization of HF care, most of which were well tolerated.
Additional hemodynamic monitoring devices are available. Similar to HeartLogic, OptiVol is a tool embedded in select Medtronic implantable devices that monitors fluid status. 14 CardioMEMS is an implantable pulmonary artery pressure sensor used as a presymptomatic data point to alert clinicians when HF is worsening. In the CHAMPION trial, the use of CardioMEMS showed a 28% reduction in HF-related hospitalization at 6 months.15 Conversely, in the GUIDE-HF trial, monitoring with CardioMEMS did not significantly reduce the composite endpoint of mortality and total HF events.16 Therefore, remote hemodynamic monitoring has variable results and the use of these tools remains uncertain per the clinical guidelines.2
The MANAGE-HF study that contributed to the validation of the HeartLogic tool may provide a comparison with this smaller single-center project. The time to follow-up within 7 days of alert was noted in only 54% of the patients in MANAGE-HF.12 In this study, 86% of patients received follow-up within 7 days, with a mean of 4.8 days. The quick turnaround from the time of alert to intervention portrays pharmacists as readily available HCPs.
In MANAGE-HF, 89% of medication augmentation involved loop diuretics or thiazides; in our project, loop diuretics were the least frequently changed medication. Most optimizations in this project included ARNi, SGLT2i, BB, and MRA, which have been shown to reduce morbidity and mortality.2 Our project included use of SGLT2i therapy to affect HeartLogic metrics, which has not been evaluated previously. SGLT2i were the most commonly initiated medication after an alert. Of the 5 tolerated SGLT2i optimization encounters, 4 were out of alert at 42 days.
SGLT2i resulted in a significant decrease in HeartLogic index score from baseline and were the only class of medication that did not produce a negative change in any metric. In this study, CPPs utilizing and acting on HeartLogic alerts led to 1 (4.8%) hospitalization with HF as the primary reason for admission and no hospitalizations as a secondary cause in 42 days, compared to 37% and 7.9% in the MANAGE-HF in 1 year, respectively. An additional screening 1 year after the initial alert found that 2 (12.5%) of 12 patients had been admitted with 1 HF hospitalization each.
A strength of this study was the ability to use HeartLogic to identify high-risk patients, provide a source of patient contact and monitoring, interpret 5 cardiac sensors, and optimize all HF GDMT, not just volume management. By focusing efforts on making patient contact and pharmacotherapy interventions with morbidity and mortality benefit, remote hemodynamic monitoring may show a clear clinical benefit and become a vital part of HF care.
Limitations
Checking for adherence and tolerance to medications were mainly patient reported if there was a CPP follow-up within 42 days, or potentially through refill history when unclear. However, this limitation is reflective of current practice where patients may have multiple clinicians working to optimize HF care and where there is reliance on patients in order to guide continued therapy. Although unable to explicitly show a reduction in HF events given lack of comparator group, the interventions made are associated with improved outcomes and thus would be expected to improve patient outcomes. Changes in vital signs were not tracked as part of this project, however the main rationale for changes made were to optimize GDMT therapy, not specifically to impact vital sign measures.
HeartLogic alerts prompted identification of high-risk patients with HF, pharmacist evaluation and outreach, patient-focused pharmacotherapy care, and beneficial patient outcomes. With only 2 cardiology CPPs checking alerts once weekly, future studies may be needed with larger samples to create algorithms and protocols to increase the clinical utility of this tool on a greater scale.
Conclusions
Cardiology CPP-led HF interventions triggered by HeartLogic alerts lead to effective patient identification, increased access to care, reductions in HeartLogic scores, improvements in symptoms, and optimization of HF care. This project demonstrates the practical utility of the HeartLogic suite in conjunction with CPP care to prioritize treatment for highrisk patients with HF in an efficient manner. The data highlight the potential value of the HeartLogic tool and a CPP in HF care to facilitate initiation and optimization of GDMT to ultimately improve the morbidity and mortality in patients with HF.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93-e621. doi:10.1161/CIR.0000000000001123
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/ American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi:10.1161/CIR.0000000000001063
- Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. J Am Coll Cardiol HF. 2017;5:216-225. doi:10.1016/j.jchf.2016.12.011
- Cao M, Gardner RS, Hariharan R, et al. Ambulatory monitoring of heart sounds via an implanted device is superior to auscultation for prediction of heart failure events. J Card Fail. 2020;26:151-159. doi:10.1016/j.cardfail.2019.10.006
- Calò L, Capucci A, Santini L, et al. ICD-measured heart sounds and their correlation with echocardiographic indexes of systolic and diastolic function. J Interv Card Electrophysiol. 2020;58:95-101. doi:10.1007/s10840-019-00668
- Del Buono MG, Arena R, Borlaug BA, et al. Exercise intolerance in patients with heart failure: JACC state-of-the- art review. J Am Coll Cardiol. 2019;73:2209-2225. doi:10.1016/j.jacc.2019.01.072
- Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. doi:10.1161/CIRCULATIONAHA.104.492207
- Rials S, Aktas M, An Q, et al. Continuous respiratory rate is superior to routine outpatient dyspnea assessment for predicting heart failure events. J Card Fail. 2018;24:S45.
- Fonarow GC, ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(suppl 7):S21-S30. doi:10.1016/j.cardfail.2018.07.130
- Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823-830. doi:10.1016/j.jacc.2007.04.079
- De Ruvo E, Capucci A, Ammirati F, et al. Preliminary experience of remote management of heart failure patients with a multisensor ICD alert [abstract P1536]. Eur J Heart Fail. 2019;21(suppl S1):370.
- Hernandez AF, Albert NM, Allen LA, et al. Multiple cardiac sensors for management of heart failure (MANAGE- HF) - phase I evaluation of the integration and safety of the HeartLogic multisensor algorithm in patients with heart failure. J Card Fail. 2022;28:1245-1254. doi:10.1016/j.cardfail.2022.03.349
- Santini L, D’Onofrio A, Dello Russo A, et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin Cardiol. 2020;43:691-697. doi:10.1002/clc.23366
- Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. doi:10.1161/CIRCULATIONAHA.104.492207
- Adamson PB, Abraham WT, Stevenson LW, et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail. 2016;9:e002600. doi:10.1161/CIRCHEARTFAILURE.115.002600
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398:991-1001. doi:10.1016/S0140-6736(21)01754-2
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93-e621. doi:10.1161/CIR.0000000000001123
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/ American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi:10.1161/CIR.0000000000001063
- Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. J Am Coll Cardiol HF. 2017;5:216-225. doi:10.1016/j.jchf.2016.12.011
- Cao M, Gardner RS, Hariharan R, et al. Ambulatory monitoring of heart sounds via an implanted device is superior to auscultation for prediction of heart failure events. J Card Fail. 2020;26:151-159. doi:10.1016/j.cardfail.2019.10.006
- Calò L, Capucci A, Santini L, et al. ICD-measured heart sounds and their correlation with echocardiographic indexes of systolic and diastolic function. J Interv Card Electrophysiol. 2020;58:95-101. doi:10.1007/s10840-019-00668
- Del Buono MG, Arena R, Borlaug BA, et al. Exercise intolerance in patients with heart failure: JACC state-of-the- art review. J Am Coll Cardiol. 2019;73:2209-2225. doi:10.1016/j.jacc.2019.01.072
- Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. doi:10.1161/CIRCULATIONAHA.104.492207
- Rials S, Aktas M, An Q, et al. Continuous respiratory rate is superior to routine outpatient dyspnea assessment for predicting heart failure events. J Card Fail. 2018;24:S45.
- Fonarow GC, ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(suppl 7):S21-S30. doi:10.1016/j.cardfail.2018.07.130
- Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823-830. doi:10.1016/j.jacc.2007.04.079
- De Ruvo E, Capucci A, Ammirati F, et al. Preliminary experience of remote management of heart failure patients with a multisensor ICD alert [abstract P1536]. Eur J Heart Fail. 2019;21(suppl S1):370.
- Hernandez AF, Albert NM, Allen LA, et al. Multiple cardiac sensors for management of heart failure (MANAGE- HF) - phase I evaluation of the integration and safety of the HeartLogic multisensor algorithm in patients with heart failure. J Card Fail. 2022;28:1245-1254. doi:10.1016/j.cardfail.2022.03.349
- Santini L, D’Onofrio A, Dello Russo A, et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin Cardiol. 2020;43:691-697. doi:10.1002/clc.23366
- Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. doi:10.1161/CIRCULATIONAHA.104.492207
- Adamson PB, Abraham WT, Stevenson LW, et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail. 2016;9:e002600. doi:10.1161/CIRCHEARTFAILURE.115.002600
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398:991-1001. doi:10.1016/S0140-6736(21)01754-2
Heart Failure Diagnostic Alerts to Prompt Pharmacist Evaluation and Medication Optimization
Heart Failure Diagnostic Alerts to Prompt Pharmacist Evaluation and Medication Optimization
Veterans Face Long Delays in ATTR-CM Diagnosis After HF
TOPLINE:
Veterans received a diagnosis of transthyretin amyloid cardiomyopathy (ATTR-CM) a median of 490 days after heart failure (HF) diagnosis. Those who had atrial fibrillation, coronary artery disease, or chronic kidney disease experienced longer diagnostic delays, whereas older or Black patients experienced shorter delays.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Veterans Health Administration to quantify the time from HF diagnosis to ATTR-CM diagnosis and identify predictors of delays in diagnosis.
- They included veterans with HF and incident ATTR-CM diagnosed between 2016 and 2022; these veterans were identified using an algorithm based on diagnoses and medications.
- The final analysis included 2557 veterans, with a median age of 80.5 years; 99.5% were men, and 56.3% were White individuals.
- Assessments captured the time from the first HF diagnosis, the first outpatient prescription for a loop diuretic, and the first hospitalization for HF to the first ATTR-CM diagnosis, along with demographics and comorbidities.
- The primary outcome was the number of days from incident HF diagnosis to incident ATTR-CM diagnosis; a delay of > 6 months was considered meaningful.
TAKEAWAY:
- The median time from HF diagnosis to ATTR-CM diagnosis was 490 days. Overall, 65% of veterans experienced diagnostic delays > 6 months, and > 25% had delays longer than 3 years.
- Factors associated with a shorter time to ATTR-CM diagnosis included Black race (adjusted odds ratio [aOR], 0.71; 95% CI, 0.57-0.88) and older age (aOR per 10 years, 0.66; 95% CI, 0.59-0.73).
- The likelihood of longer diagnostic delays was higher in patients with coronary artery disease (aOR, 1.38; 95% CI, 1.15-1.64) and chronic kidney disease (aOR, 1.79; 95% CI, 1.50-2.15). Those with atrial fibrillation were more likely to have longer delays, although the lower bound of 95% CI was borderline (aOR, 1.21; 95% CI, 1.00-1.45).
- Among veterans with prior prescriptions of loop diuretics, the median time from the first prescription to ATTR-CM diagnosis was 835 days. Among those with a prior hospitalization for HF, the median time from hospitalization to ATTR-CM diagnosis was 300 days.
IN PRACTICE:
“This study supports the need for increased testing for ATTR-CM, thorough evaluation of cardiomyopathy etiologies at the time of HF diagnosis, and the need for new interventions that shorten the diagnostic delay in ATTR-CM,” the researchers reported.
SOURCE:
This study was led by Gabriela Spencer-Bonilla, MD, MSc, of Stanford University School of Medicine in Stanford, California. It was published online on JACC .
LIMITATIONS:
The analysis was limited to veterans engaged long enough for diagnoses of both HF and ATTR-CM. The findings may not be generalized to women given the predominantly male cohort. Echocardiography and ATTR subtype or genetic data were unavailable.
DISCLOSURES:
This study received funding from AstraZeneca. Four authors reported being the employees and stockholders of AstraZeneca. Several authors reported receiving research funding, consulting fees, and/or having equity in multiple pharmaceutical and healthcare companies, including Novo Nordisk, Bridge Bio, and Pfizer. Two authors reported receiving support from the American Heart Association, with one of them also receiving support from the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Veterans received a diagnosis of transthyretin amyloid cardiomyopathy (ATTR-CM) a median of 490 days after heart failure (HF) diagnosis. Those who had atrial fibrillation, coronary artery disease, or chronic kidney disease experienced longer diagnostic delays, whereas older or Black patients experienced shorter delays.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Veterans Health Administration to quantify the time from HF diagnosis to ATTR-CM diagnosis and identify predictors of delays in diagnosis.
- They included veterans with HF and incident ATTR-CM diagnosed between 2016 and 2022; these veterans were identified using an algorithm based on diagnoses and medications.
- The final analysis included 2557 veterans, with a median age of 80.5 years; 99.5% were men, and 56.3% were White individuals.
- Assessments captured the time from the first HF diagnosis, the first outpatient prescription for a loop diuretic, and the first hospitalization for HF to the first ATTR-CM diagnosis, along with demographics and comorbidities.
- The primary outcome was the number of days from incident HF diagnosis to incident ATTR-CM diagnosis; a delay of > 6 months was considered meaningful.
TAKEAWAY:
- The median time from HF diagnosis to ATTR-CM diagnosis was 490 days. Overall, 65% of veterans experienced diagnostic delays > 6 months, and > 25% had delays longer than 3 years.
- Factors associated with a shorter time to ATTR-CM diagnosis included Black race (adjusted odds ratio [aOR], 0.71; 95% CI, 0.57-0.88) and older age (aOR per 10 years, 0.66; 95% CI, 0.59-0.73).
- The likelihood of longer diagnostic delays was higher in patients with coronary artery disease (aOR, 1.38; 95% CI, 1.15-1.64) and chronic kidney disease (aOR, 1.79; 95% CI, 1.50-2.15). Those with atrial fibrillation were more likely to have longer delays, although the lower bound of 95% CI was borderline (aOR, 1.21; 95% CI, 1.00-1.45).
- Among veterans with prior prescriptions of loop diuretics, the median time from the first prescription to ATTR-CM diagnosis was 835 days. Among those with a prior hospitalization for HF, the median time from hospitalization to ATTR-CM diagnosis was 300 days.
IN PRACTICE:
“This study supports the need for increased testing for ATTR-CM, thorough evaluation of cardiomyopathy etiologies at the time of HF diagnosis, and the need for new interventions that shorten the diagnostic delay in ATTR-CM,” the researchers reported.
SOURCE:
This study was led by Gabriela Spencer-Bonilla, MD, MSc, of Stanford University School of Medicine in Stanford, California. It was published online on JACC .
LIMITATIONS:
The analysis was limited to veterans engaged long enough for diagnoses of both HF and ATTR-CM. The findings may not be generalized to women given the predominantly male cohort. Echocardiography and ATTR subtype or genetic data were unavailable.
DISCLOSURES:
This study received funding from AstraZeneca. Four authors reported being the employees and stockholders of AstraZeneca. Several authors reported receiving research funding, consulting fees, and/or having equity in multiple pharmaceutical and healthcare companies, including Novo Nordisk, Bridge Bio, and Pfizer. Two authors reported receiving support from the American Heart Association, with one of them also receiving support from the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Veterans received a diagnosis of transthyretin amyloid cardiomyopathy (ATTR-CM) a median of 490 days after heart failure (HF) diagnosis. Those who had atrial fibrillation, coronary artery disease, or chronic kidney disease experienced longer diagnostic delays, whereas older or Black patients experienced shorter delays.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Veterans Health Administration to quantify the time from HF diagnosis to ATTR-CM diagnosis and identify predictors of delays in diagnosis.
- They included veterans with HF and incident ATTR-CM diagnosed between 2016 and 2022; these veterans were identified using an algorithm based on diagnoses and medications.
- The final analysis included 2557 veterans, with a median age of 80.5 years; 99.5% were men, and 56.3% were White individuals.
- Assessments captured the time from the first HF diagnosis, the first outpatient prescription for a loop diuretic, and the first hospitalization for HF to the first ATTR-CM diagnosis, along with demographics and comorbidities.
- The primary outcome was the number of days from incident HF diagnosis to incident ATTR-CM diagnosis; a delay of > 6 months was considered meaningful.
TAKEAWAY:
- The median time from HF diagnosis to ATTR-CM diagnosis was 490 days. Overall, 65% of veterans experienced diagnostic delays > 6 months, and > 25% had delays longer than 3 years.
- Factors associated with a shorter time to ATTR-CM diagnosis included Black race (adjusted odds ratio [aOR], 0.71; 95% CI, 0.57-0.88) and older age (aOR per 10 years, 0.66; 95% CI, 0.59-0.73).
- The likelihood of longer diagnostic delays was higher in patients with coronary artery disease (aOR, 1.38; 95% CI, 1.15-1.64) and chronic kidney disease (aOR, 1.79; 95% CI, 1.50-2.15). Those with atrial fibrillation were more likely to have longer delays, although the lower bound of 95% CI was borderline (aOR, 1.21; 95% CI, 1.00-1.45).
- Among veterans with prior prescriptions of loop diuretics, the median time from the first prescription to ATTR-CM diagnosis was 835 days. Among those with a prior hospitalization for HF, the median time from hospitalization to ATTR-CM diagnosis was 300 days.
IN PRACTICE:
“This study supports the need for increased testing for ATTR-CM, thorough evaluation of cardiomyopathy etiologies at the time of HF diagnosis, and the need for new interventions that shorten the diagnostic delay in ATTR-CM,” the researchers reported.
SOURCE:
This study was led by Gabriela Spencer-Bonilla, MD, MSc, of Stanford University School of Medicine in Stanford, California. It was published online on JACC .
LIMITATIONS:
The analysis was limited to veterans engaged long enough for diagnoses of both HF and ATTR-CM. The findings may not be generalized to women given the predominantly male cohort. Echocardiography and ATTR subtype or genetic data were unavailable.
DISCLOSURES:
This study received funding from AstraZeneca. Four authors reported being the employees and stockholders of AstraZeneca. Several authors reported receiving research funding, consulting fees, and/or having equity in multiple pharmaceutical and healthcare companies, including Novo Nordisk, Bridge Bio, and Pfizer. Two authors reported receiving support from the American Heart Association, with one of them also receiving support from the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Hypereosinophilic Syndrome With Eosinophilic Endomyocarditis: A Rare Cardiac Manifestation
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Patients With Rheumatoid Arthritis Show Higher Risk for Heart Failure With Preserved Ejection Fraction
TOPLINE:
Patients with rheumatoid arthritis (RA) face a higher risk for heart failure (HF) than those without the condition, with the elevated risk primarily driven by HF with preserved ejection fraction (HFpEF).
METHODOLOGY:
- The researchers conducted a retrospective cohort study using data from the Mass General Brigham Biobank to investigate the risk for overall HF and its subtypes, particularly HF with reduced EF (HFrEF) and HFpEF, in patients with RA.
- They included 1445 patients newly diagnosed with RA (mean age, 51.4 years; 78.7% women) and 4335 matched comparators without RA.
- Patients with RA were identified using diagnosis codes and RA-related natural language processing concepts.
- HFpEF and HFrEF were defined as HF with an EF ≥ 50% and ≤ 40%, respectively; incidences for overall HF, HFpEF, and HFrEF were calculated per 1000 person-years.
TAKEAWAY:
- The study identified 92 incident HF cases in the RA cohort and 157 in the non-RA cohort over a median follow-up of 10.3 years per patient.
- HFpEF was the predominant HF subtype in both cohorts, with a higher incidence in patients with RA than in those without the condition (4.33 vs 2.11 per 1000 person-years).
- Patients with RA showed a 79% higher risk for HF than those without the condition (adjusted hazard ratio [aHR], 1.79; 95% CI, 1.38-2.32).
- Among the HF subtypes, patients with RA had a significantly increased risk for HFpEF (aHR, 1.99; 95% CI, 1.43-2.77) but not for HFrEF.
IN PRACTICE:
“RA can be considered a human model for inflammation, and findings from this study support the notion that chronic inflammation increases risk for HFpEF,” the authors wrote.
SOURCE:
This study was led by Yumeko Kawano, MD, Brigham and Women’s Hospital, Boston, Massachusetts, and was published online in Arthritis Care & Research.
LIMITATIONS:
This study was conducted within an academic tertiary hospital system and involved participants from a biobank, which may have introduced selection bias and limited generalizability. The study did not account for post-baseline variables that could mediate the observed associations, such as the chronic use of nonsteroidal anti-inflammatory drugs, steroids, or specific disease-modifying antirheumatic drugs. The study relied on the availability of clinically performed cardiology studies for HF subtyping, possibly introducing misclassification of HF.
DISCLOSURES:
This study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author received support from the Ruth L. Kirschstein Institutional National Research Service Award, National Institutes of Health.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with rheumatoid arthritis (RA) face a higher risk for heart failure (HF) than those without the condition, with the elevated risk primarily driven by HF with preserved ejection fraction (HFpEF).
METHODOLOGY:
- The researchers conducted a retrospective cohort study using data from the Mass General Brigham Biobank to investigate the risk for overall HF and its subtypes, particularly HF with reduced EF (HFrEF) and HFpEF, in patients with RA.
- They included 1445 patients newly diagnosed with RA (mean age, 51.4 years; 78.7% women) and 4335 matched comparators without RA.
- Patients with RA were identified using diagnosis codes and RA-related natural language processing concepts.
- HFpEF and HFrEF were defined as HF with an EF ≥ 50% and ≤ 40%, respectively; incidences for overall HF, HFpEF, and HFrEF were calculated per 1000 person-years.
TAKEAWAY:
- The study identified 92 incident HF cases in the RA cohort and 157 in the non-RA cohort over a median follow-up of 10.3 years per patient.
- HFpEF was the predominant HF subtype in both cohorts, with a higher incidence in patients with RA than in those without the condition (4.33 vs 2.11 per 1000 person-years).
- Patients with RA showed a 79% higher risk for HF than those without the condition (adjusted hazard ratio [aHR], 1.79; 95% CI, 1.38-2.32).
- Among the HF subtypes, patients with RA had a significantly increased risk for HFpEF (aHR, 1.99; 95% CI, 1.43-2.77) but not for HFrEF.
IN PRACTICE:
“RA can be considered a human model for inflammation, and findings from this study support the notion that chronic inflammation increases risk for HFpEF,” the authors wrote.
SOURCE:
This study was led by Yumeko Kawano, MD, Brigham and Women’s Hospital, Boston, Massachusetts, and was published online in Arthritis Care & Research.
LIMITATIONS:
This study was conducted within an academic tertiary hospital system and involved participants from a biobank, which may have introduced selection bias and limited generalizability. The study did not account for post-baseline variables that could mediate the observed associations, such as the chronic use of nonsteroidal anti-inflammatory drugs, steroids, or specific disease-modifying antirheumatic drugs. The study relied on the availability of clinically performed cardiology studies for HF subtyping, possibly introducing misclassification of HF.
DISCLOSURES:
This study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author received support from the Ruth L. Kirschstein Institutional National Research Service Award, National Institutes of Health.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with rheumatoid arthritis (RA) face a higher risk for heart failure (HF) than those without the condition, with the elevated risk primarily driven by HF with preserved ejection fraction (HFpEF).
METHODOLOGY:
- The researchers conducted a retrospective cohort study using data from the Mass General Brigham Biobank to investigate the risk for overall HF and its subtypes, particularly HF with reduced EF (HFrEF) and HFpEF, in patients with RA.
- They included 1445 patients newly diagnosed with RA (mean age, 51.4 years; 78.7% women) and 4335 matched comparators without RA.
- Patients with RA were identified using diagnosis codes and RA-related natural language processing concepts.
- HFpEF and HFrEF were defined as HF with an EF ≥ 50% and ≤ 40%, respectively; incidences for overall HF, HFpEF, and HFrEF were calculated per 1000 person-years.
TAKEAWAY:
- The study identified 92 incident HF cases in the RA cohort and 157 in the non-RA cohort over a median follow-up of 10.3 years per patient.
- HFpEF was the predominant HF subtype in both cohorts, with a higher incidence in patients with RA than in those without the condition (4.33 vs 2.11 per 1000 person-years).
- Patients with RA showed a 79% higher risk for HF than those without the condition (adjusted hazard ratio [aHR], 1.79; 95% CI, 1.38-2.32).
- Among the HF subtypes, patients with RA had a significantly increased risk for HFpEF (aHR, 1.99; 95% CI, 1.43-2.77) but not for HFrEF.
IN PRACTICE:
“RA can be considered a human model for inflammation, and findings from this study support the notion that chronic inflammation increases risk for HFpEF,” the authors wrote.
SOURCE:
This study was led by Yumeko Kawano, MD, Brigham and Women’s Hospital, Boston, Massachusetts, and was published online in Arthritis Care & Research.
LIMITATIONS:
This study was conducted within an academic tertiary hospital system and involved participants from a biobank, which may have introduced selection bias and limited generalizability. The study did not account for post-baseline variables that could mediate the observed associations, such as the chronic use of nonsteroidal anti-inflammatory drugs, steroids, or specific disease-modifying antirheumatic drugs. The study relied on the availability of clinically performed cardiology studies for HF subtyping, possibly introducing misclassification of HF.
DISCLOSURES:
This study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author received support from the Ruth L. Kirschstein Institutional National Research Service Award, National Institutes of Health.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Potassium Nitrate Fails to Boost Exercise Capacity in Patients With Heart Failure With Preserved Ejection Fraction
TOPLINE:
METHODOLOGY:
- This multicenter crossover trial, conducted across three centers in the United States, assessed the effect of administering KNO3 on exercise capacity and quality of life.
- It included 84 patients with symptomatic HFpEF (median age, 68 years; 69% women; 76% White) who had a left ventricular ejection fraction over 50% and elevated intracardiac pressures. Participants had obesity (mean body mass index, 36.22), with a high prevalence of hypertension, diabetes, and obstructive sleep apnea.
- Patients were randomly assigned to receive either 6 mmol KNO3 first (n = 41) or 6 mmol potassium chloride (KCl) first (n = 43) three times daily for 6 weeks, with a 1-week washout period in between.
- At the end of each intervention phase, a test of incremental cardiopulmonary exercise was conducted using a supine cycle ergometer.
- Primary endpoints were the difference in peak oxygen uptake and total work performed during the exercise test; secondary endpoints included quality of life, left ventricular systolic and diastolic function, exercise systemic vasodilatory reserve, and parameters related to pulsatile arterial load.
TAKEAWAY:
- The administration of KNO3 vs KCl increased the levels of serum metabolites of nitric oxide significantly after 6 weeks (418.44 vs 40.11 μM; P < .001).
- Peak oxygen uptake or the total work performed did not improve significantly with the administration of KNO3, compared with KCl. Quality of life also did not improve with the administration of KNO3.
- Mean arterial pressure at peak exercise was significantly lower after the administration of KNO3 than after KCl (122.5 vs 127.6 mm Hg; P = .04), but the vasodilatory reserve and resting and orthostatic blood pressure did not differ.
- Adverse events were mostly minor, with gastrointestinal issues being the most common side effects reported.
IN PRACTICE:
“In this randomized crossover trial, chronic KNO3 administration did not improve exercise capacity or quality of life, as compared with KCl among participants with HFpEF,” the authors of the study wrote.
SOURCE:
The study was led by Payman Zamani, MD, MTR, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. It was published online on December 18, 2024, in JAMA Cardiology.
LIMITATIONS:
The potential activation of compensatory mechanisms by the chronic inorganic nitrate administration may have neutralized the short-term benefits. Various abnormalities in oxygen transport may be present simultaneously in patients with HFpEF, suggesting a combination of interventions may be required to improve exercise capacity.
DISCLOSURES:
This trial was supported by the National Heart, Lung, and Blood Institute. The study was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Some authors reported receiving grants, personal fees, and consulting fees and having patents from various pharmaceutical and medical device companies and institutes. One author reported having full-time employment with a healthcare company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter crossover trial, conducted across three centers in the United States, assessed the effect of administering KNO3 on exercise capacity and quality of life.
- It included 84 patients with symptomatic HFpEF (median age, 68 years; 69% women; 76% White) who had a left ventricular ejection fraction over 50% and elevated intracardiac pressures. Participants had obesity (mean body mass index, 36.22), with a high prevalence of hypertension, diabetes, and obstructive sleep apnea.
- Patients were randomly assigned to receive either 6 mmol KNO3 first (n = 41) or 6 mmol potassium chloride (KCl) first (n = 43) three times daily for 6 weeks, with a 1-week washout period in between.
- At the end of each intervention phase, a test of incremental cardiopulmonary exercise was conducted using a supine cycle ergometer.
- Primary endpoints were the difference in peak oxygen uptake and total work performed during the exercise test; secondary endpoints included quality of life, left ventricular systolic and diastolic function, exercise systemic vasodilatory reserve, and parameters related to pulsatile arterial load.
TAKEAWAY:
- The administration of KNO3 vs KCl increased the levels of serum metabolites of nitric oxide significantly after 6 weeks (418.44 vs 40.11 μM; P < .001).
- Peak oxygen uptake or the total work performed did not improve significantly with the administration of KNO3, compared with KCl. Quality of life also did not improve with the administration of KNO3.
- Mean arterial pressure at peak exercise was significantly lower after the administration of KNO3 than after KCl (122.5 vs 127.6 mm Hg; P = .04), but the vasodilatory reserve and resting and orthostatic blood pressure did not differ.
- Adverse events were mostly minor, with gastrointestinal issues being the most common side effects reported.
IN PRACTICE:
“In this randomized crossover trial, chronic KNO3 administration did not improve exercise capacity or quality of life, as compared with KCl among participants with HFpEF,” the authors of the study wrote.
SOURCE:
The study was led by Payman Zamani, MD, MTR, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. It was published online on December 18, 2024, in JAMA Cardiology.
LIMITATIONS:
The potential activation of compensatory mechanisms by the chronic inorganic nitrate administration may have neutralized the short-term benefits. Various abnormalities in oxygen transport may be present simultaneously in patients with HFpEF, suggesting a combination of interventions may be required to improve exercise capacity.
DISCLOSURES:
This trial was supported by the National Heart, Lung, and Blood Institute. The study was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Some authors reported receiving grants, personal fees, and consulting fees and having patents from various pharmaceutical and medical device companies and institutes. One author reported having full-time employment with a healthcare company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter crossover trial, conducted across three centers in the United States, assessed the effect of administering KNO3 on exercise capacity and quality of life.
- It included 84 patients with symptomatic HFpEF (median age, 68 years; 69% women; 76% White) who had a left ventricular ejection fraction over 50% and elevated intracardiac pressures. Participants had obesity (mean body mass index, 36.22), with a high prevalence of hypertension, diabetes, and obstructive sleep apnea.
- Patients were randomly assigned to receive either 6 mmol KNO3 first (n = 41) or 6 mmol potassium chloride (KCl) first (n = 43) three times daily for 6 weeks, with a 1-week washout period in between.
- At the end of each intervention phase, a test of incremental cardiopulmonary exercise was conducted using a supine cycle ergometer.
- Primary endpoints were the difference in peak oxygen uptake and total work performed during the exercise test; secondary endpoints included quality of life, left ventricular systolic and diastolic function, exercise systemic vasodilatory reserve, and parameters related to pulsatile arterial load.
TAKEAWAY:
- The administration of KNO3 vs KCl increased the levels of serum metabolites of nitric oxide significantly after 6 weeks (418.44 vs 40.11 μM; P < .001).
- Peak oxygen uptake or the total work performed did not improve significantly with the administration of KNO3, compared with KCl. Quality of life also did not improve with the administration of KNO3.
- Mean arterial pressure at peak exercise was significantly lower after the administration of KNO3 than after KCl (122.5 vs 127.6 mm Hg; P = .04), but the vasodilatory reserve and resting and orthostatic blood pressure did not differ.
- Adverse events were mostly minor, with gastrointestinal issues being the most common side effects reported.
IN PRACTICE:
“In this randomized crossover trial, chronic KNO3 administration did not improve exercise capacity or quality of life, as compared with KCl among participants with HFpEF,” the authors of the study wrote.
SOURCE:
The study was led by Payman Zamani, MD, MTR, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. It was published online on December 18, 2024, in JAMA Cardiology.
LIMITATIONS:
The potential activation of compensatory mechanisms by the chronic inorganic nitrate administration may have neutralized the short-term benefits. Various abnormalities in oxygen transport may be present simultaneously in patients with HFpEF, suggesting a combination of interventions may be required to improve exercise capacity.
DISCLOSURES:
This trial was supported by the National Heart, Lung, and Blood Institute. The study was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Some authors reported receiving grants, personal fees, and consulting fees and having patents from various pharmaceutical and medical device companies and institutes. One author reported having full-time employment with a healthcare company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Monitoring Heart Health Crucial in Patients With Anorexia
TOPLINE:
Patients with anorexia nervosa are at significantly increased risk for cardiovascular conditions such as heart failure and cardiac arrest, compared with people without an eating disorder, researchers found. The risk for many of these conditions declines after 5 years of follow-up, whereas the risk for ischemic heart disease rises only after that period.
METHODOLOGY:
- Researchers conducted a longitudinal cohort study by analyzing the data from Taiwan’s National Health Insurance database to investigate the incidences and risk for cardiovascular conditions in patients with anorexia.
- They included 22,891 participants (mean age, 24.9 years; 91.3% women), of whom 2081 were diagnosed with anorexia between January 2010 and December 2021 and 20,810 were matched control participants without any eating disorder.
- The mean follow-up duration of this study was 5 years; investigators also assessed the risk for individual cardiovascular conditions during three periods after the diagnosis of anorexia: 0-24 months, between 24 and 60 months, and greater than 60 months.
- The primary outcomes were the occurrence of major adverse cardiovascular events (MACE) and any cardiovascular condition, including heart failure, stroke, ischemic heart diseases, conduction disorder, inflammatory heart disease, valve disease, cardiomyopathy, atherosclerosis, and cardiac arrest.
TAKEAWAY:
- Similarly, the incidence of any cardiovascular condition was higher in patients with anorexia than in those without (6.19% vs 2.27%), which translated to a nearly twofold increased risk (aHR, 1.93; 95% CI, 1.54-2.41).
- Patients with anorexia showed elevated risks for individual cardiovascular conditions such as cardiac arrest, structural heart disease, conduction disorder, and heart failure, but not stroke, atherosclerosis, ischemic heart disease, or inflammatory heart disease.
- The risks for congestive heart failure, structural heart disease, and conduction disorder increased in the first 24 months after the diagnosis of anorexia and disappeared after 5 years of follow-up, whereas the risk for ischemic heart disease increased only after 5 years of follow-up.
IN PRACTICE:
“Clinicians should monitor comorbid cardiovascular conditions among patients with [anorexia] at initial presentation, during treatment, and at follow-up,” the authors of the study wrote.
“In this study, most cardiovascular conditions were in remission after 5 years except ischemic heart disease,” the researchers noted. “This finding is corroborated by the recovery rate of 50%-70% in patients with [anorexia] after 4 years of follow-up in a recent meta-analysis, and in previous studies, most of the cardiac complications improved with weight restoration. Similarly, genome-wide association studies did not support elevated cardiovascular risk in patients with [anorexia] due to shared genetic mechanisms between [anorexia] and cardiovascular diseases, but they suggested that cardiovascular diseases were a downstream consequence” of the eating disorder.
SOURCE:
The study was led by Mei-Chih Meg Tseng, MD, PhD, of the Department of Psychiatry at Taipei Medical University in Taipei, Taiwan. It was published online on December 19, 2024, in JAMA Network Open.
LIMITATIONS:
The cardiovascular outcomes relied on the clinical diagnoses, and the validity of anorexia or its subtype was not confirmed. The study population was limited to patients seeking medical treatment, which may have led to the inclusion of patients with more severe symptoms. Key potential confounders such as body weight, nutritional status, lifestyle, drug use, and family history were unavailable in the claims dataset and could not be adjusted. The generalizability of the study may be limited as it involved only participants from a single ethnic group.
DISCLOSURES:
This study was supported by grants from the National Science and Technology Council, Taiwan, and Taipei Medical University. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with anorexia nervosa are at significantly increased risk for cardiovascular conditions such as heart failure and cardiac arrest, compared with people without an eating disorder, researchers found. The risk for many of these conditions declines after 5 years of follow-up, whereas the risk for ischemic heart disease rises only after that period.
METHODOLOGY:
- Researchers conducted a longitudinal cohort study by analyzing the data from Taiwan’s National Health Insurance database to investigate the incidences and risk for cardiovascular conditions in patients with anorexia.
- They included 22,891 participants (mean age, 24.9 years; 91.3% women), of whom 2081 were diagnosed with anorexia between January 2010 and December 2021 and 20,810 were matched control participants without any eating disorder.
- The mean follow-up duration of this study was 5 years; investigators also assessed the risk for individual cardiovascular conditions during three periods after the diagnosis of anorexia: 0-24 months, between 24 and 60 months, and greater than 60 months.
- The primary outcomes were the occurrence of major adverse cardiovascular events (MACE) and any cardiovascular condition, including heart failure, stroke, ischemic heart diseases, conduction disorder, inflammatory heart disease, valve disease, cardiomyopathy, atherosclerosis, and cardiac arrest.
TAKEAWAY:
- Similarly, the incidence of any cardiovascular condition was higher in patients with anorexia than in those without (6.19% vs 2.27%), which translated to a nearly twofold increased risk (aHR, 1.93; 95% CI, 1.54-2.41).
- Patients with anorexia showed elevated risks for individual cardiovascular conditions such as cardiac arrest, structural heart disease, conduction disorder, and heart failure, but not stroke, atherosclerosis, ischemic heart disease, or inflammatory heart disease.
- The risks for congestive heart failure, structural heart disease, and conduction disorder increased in the first 24 months after the diagnosis of anorexia and disappeared after 5 years of follow-up, whereas the risk for ischemic heart disease increased only after 5 years of follow-up.
IN PRACTICE:
“Clinicians should monitor comorbid cardiovascular conditions among patients with [anorexia] at initial presentation, during treatment, and at follow-up,” the authors of the study wrote.
“In this study, most cardiovascular conditions were in remission after 5 years except ischemic heart disease,” the researchers noted. “This finding is corroborated by the recovery rate of 50%-70% in patients with [anorexia] after 4 years of follow-up in a recent meta-analysis, and in previous studies, most of the cardiac complications improved with weight restoration. Similarly, genome-wide association studies did not support elevated cardiovascular risk in patients with [anorexia] due to shared genetic mechanisms between [anorexia] and cardiovascular diseases, but they suggested that cardiovascular diseases were a downstream consequence” of the eating disorder.
SOURCE:
The study was led by Mei-Chih Meg Tseng, MD, PhD, of the Department of Psychiatry at Taipei Medical University in Taipei, Taiwan. It was published online on December 19, 2024, in JAMA Network Open.
LIMITATIONS:
The cardiovascular outcomes relied on the clinical diagnoses, and the validity of anorexia or its subtype was not confirmed. The study population was limited to patients seeking medical treatment, which may have led to the inclusion of patients with more severe symptoms. Key potential confounders such as body weight, nutritional status, lifestyle, drug use, and family history were unavailable in the claims dataset and could not be adjusted. The generalizability of the study may be limited as it involved only participants from a single ethnic group.
DISCLOSURES:
This study was supported by grants from the National Science and Technology Council, Taiwan, and Taipei Medical University. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with anorexia nervosa are at significantly increased risk for cardiovascular conditions such as heart failure and cardiac arrest, compared with people without an eating disorder, researchers found. The risk for many of these conditions declines after 5 years of follow-up, whereas the risk for ischemic heart disease rises only after that period.
METHODOLOGY:
- Researchers conducted a longitudinal cohort study by analyzing the data from Taiwan’s National Health Insurance database to investigate the incidences and risk for cardiovascular conditions in patients with anorexia.
- They included 22,891 participants (mean age, 24.9 years; 91.3% women), of whom 2081 were diagnosed with anorexia between January 2010 and December 2021 and 20,810 were matched control participants without any eating disorder.
- The mean follow-up duration of this study was 5 years; investigators also assessed the risk for individual cardiovascular conditions during three periods after the diagnosis of anorexia: 0-24 months, between 24 and 60 months, and greater than 60 months.
- The primary outcomes were the occurrence of major adverse cardiovascular events (MACE) and any cardiovascular condition, including heart failure, stroke, ischemic heart diseases, conduction disorder, inflammatory heart disease, valve disease, cardiomyopathy, atherosclerosis, and cardiac arrest.
TAKEAWAY:
- Similarly, the incidence of any cardiovascular condition was higher in patients with anorexia than in those without (6.19% vs 2.27%), which translated to a nearly twofold increased risk (aHR, 1.93; 95% CI, 1.54-2.41).
- Patients with anorexia showed elevated risks for individual cardiovascular conditions such as cardiac arrest, structural heart disease, conduction disorder, and heart failure, but not stroke, atherosclerosis, ischemic heart disease, or inflammatory heart disease.
- The risks for congestive heart failure, structural heart disease, and conduction disorder increased in the first 24 months after the diagnosis of anorexia and disappeared after 5 years of follow-up, whereas the risk for ischemic heart disease increased only after 5 years of follow-up.
IN PRACTICE:
“Clinicians should monitor comorbid cardiovascular conditions among patients with [anorexia] at initial presentation, during treatment, and at follow-up,” the authors of the study wrote.
“In this study, most cardiovascular conditions were in remission after 5 years except ischemic heart disease,” the researchers noted. “This finding is corroborated by the recovery rate of 50%-70% in patients with [anorexia] after 4 years of follow-up in a recent meta-analysis, and in previous studies, most of the cardiac complications improved with weight restoration. Similarly, genome-wide association studies did not support elevated cardiovascular risk in patients with [anorexia] due to shared genetic mechanisms between [anorexia] and cardiovascular diseases, but they suggested that cardiovascular diseases were a downstream consequence” of the eating disorder.
SOURCE:
The study was led by Mei-Chih Meg Tseng, MD, PhD, of the Department of Psychiatry at Taipei Medical University in Taipei, Taiwan. It was published online on December 19, 2024, in JAMA Network Open.
LIMITATIONS:
The cardiovascular outcomes relied on the clinical diagnoses, and the validity of anorexia or its subtype was not confirmed. The study population was limited to patients seeking medical treatment, which may have led to the inclusion of patients with more severe symptoms. Key potential confounders such as body weight, nutritional status, lifestyle, drug use, and family history were unavailable in the claims dataset and could not be adjusted. The generalizability of the study may be limited as it involved only participants from a single ethnic group.
DISCLOSURES:
This study was supported by grants from the National Science and Technology Council, Taiwan, and Taipei Medical University. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Has Tirzepatide Scaled the HFpEF/Obesity SUMMIT?
The results of the SUMMIT trial of the long-acting agonist of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors, tirzepatide, in patients with heart failure with preserved ejection fraction (HFpEF) and obesity are positive. But the trial design leaves clinicians and regulators with big doses of uncertainty.
Known Facts About HFpEF
HFpEF has exceeded heart failure with reduced ejection fraction (HFrEF) as the most common form of heart failure. HFpEF differs from HFrEF in that patients with preserved ejection fraction often present later in life with more comorbidities.
Some of these comorbidities are on the causal pathway of heart failure. Obesity, for instance, both associates with HFpEF and surely causes the diastolic dysfunction central to the condition. This may be a direct effect via high excess adipose tissue or an indirect effect via pro-inflammatory pathways.
GLP-1 agonists and the dual-acting GIP/GLP1 agonist tirzepatide have proven efficacy for weight loss. Semaglutide has previously been shown to improve quality of life and physical functioning in two small trials of patients with HFpEF and obesity. Semaglutide also reduced hard clinical outcomes in patients with obesity and these other conditions: chronic kidney disease, diabetes, and established atherosclerotic vascular disease.
This class of drugs is costly. The combination of both high drug costs and highly prevalent conditions such as obesity and HFpEF forces clinicians to make both value and clinical judgments when translating evidence.
The SUMMIT Trial
The SUMMIT trial aimed to evaluate tirzepatide’s effect on typical heart failure events, health status and functional capacity in patients with obesity and HFpEF. A total of 731 patients were randomly assigned to receive to tirzepatide or placebo.
Investigators chose two co-primary endpoints. The first was a composite of cardiovascular (CV) death and worsening heart failure events—the latter could be a hospitalization for heart failure, a visit for intravenous diuretics, or intensification of oral diuretics. The idea behind this rather unique composite was to capture all heart failure events. The second co-primary endpoint was a change in baseline Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) at 1 year.
Characteristics of the patients included an average age of 65 years, 55% were female, the average body mass index was 38, and the mean left ventricular ejection fraction was 61% (the minimum for trial entry was 50%). Just under half had been hospitalized for heart failure in the year before trial entry.
Tirzepatide Results
The primary outcome of CV death and first heart failure event occurred in 36 patients (9.9%) in the tirzepatide group and 56 patients (15.3%) in the placebo group, for a hazard ratio of 0.62 (95% CI, 0.41-0.95; P =.026).
The 5.4% absolute risk reduction in the primary endpoint was completely driven by lower rates of heart failure events (8% vs 14.2%). CV death was actually higher in the tirzepatide arm, but the number of deaths was low in both arms (8 vs 5).
The rate of hospitalizations due to heart failure was lower with tirzepatide (3.3% vs 7.1%), as was intensification of oral diuretics (4.7% vs 5.7%).
The second co-primary endpoint of change from baseline in KCCQ-CSS favored tirzepatide.
Other secondary endpoints also favored tirzepatide: longer 6-minute walk distance, greater change in body weight (-11.6%), and lower high-sensitivity C-reactive protein levels and systolic blood pressure (-4.7 mm Hg).
Authors’ Conclusions and Expert Comments
At the American Heart Association Scientific Sessions, the primary investigator Milton Packer, MD, said SUMMIT was the first trial of patients with obesity and HFpEF that had major heart failure outcomes as the primary endpoint. And that tirzepatide changed the clinical trajectory of the disease.
Jennifer Ho, MD, associate professor of medicine at Harvard Medical School, Boston, Massachusetts, said, “This really is a practice-changing trial and cements this type of therapy as one of the cornerstones of obesity and HFpEF treatment.”
Other experts cited a recently published pooled analysis of semaglutide trials looking specifically at patients with HFpEF and found lower rates of HF events with the GLP-1 agonist.
The SUMMIT trial results were covered in 53 news outlets— nearly all with glowing headlines.
My Six Concerns With SUMMIT
The trial delivered statistically positive findings. What’s more, patients lost weight, and a greater than 11% weight loss difference is meaningful. Patients with a baseline weight of more than 100 kg who lose this much weight are bound to feel and function better.
The first problem comes when we ask whether the results are disease-modifying. There was no difference in CV death. And the number of hospitalizations for heart failure — the more standard endpoint — was low, at only 12 and 26, respectively. Contrast this with the DELIVER trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in HFpEF where there were nearly 750 hospitalizations for heart failure and PARAGON-HF of sacubitril-valsartan vs valsartan in HFpEF, where there were nearly 1500. SUMMIT simply had too few events to make conclusions — a point Packer has made regarding AF ablation trials in patients with heart failure.
I have previously called GLP-1 drugs disease-modifying in patients with obesity and atherosclerotic disease. This is because the SELECT trial of semaglutide randomized more than 17,000 patients and recorded a 20% reduction in hard outcomes. And there were more than 1200 primary outcome events. SUMMIT does not come close to this measure.
The second issue is short follow-up. These were 65-year-old patients and with only 2 years of follow-up, it is hard to make conclusions regarding whether or not these drugs can provide long-term benefit.
The third issue is that SUMMIT authors don’t tell us the number of all-cause hospitalizations. I was part of a recently published meta-analysis of more than 100 heart failure trials that raised questions regarding the value of hospitalizations for heart failure as a surrogate for heart failure outcomes.
For instance, we found that in large trials there was great variability in the ability of a reduction in HF hospitalizations to predict a reduction in all-cause hospitalization. In small trials, such as SUMMIT, it would likely be impossible to predict how the reduction in HF hospitalization would predict all-cause hospitalization. I believe all-cause hospitalization is a more inclusive endpoint because it is bias free; it captures benefits and potential harms of the therapy; and it is patient-centered, because patients probably do not care what type of hospitalization they avoid.
The fourth issue with SUMMIT is the difficulty in maintaining blinding, which reduces confidence in outcomes that require clinical decisions or patient judgments. Owing to gastrointestinal symptoms, decreased appetite, and weight loss, patients on this class of drugs are very likely to know their treatment assignment. This is a criticism of not only SUMMIT but all GLP-1 agonist trials. The fact that blinding is difficult to maintain argues for choosing endpoints less susceptible to bias, such as CV death or all-cause hospitalization.
Proponents of tirzepatide for this indication might argue that unblinding is less of an issue because of objective endpoints such as biomarkers. And they have a point, but nearly all other endpoints, especially the co-primary endpoint of KCCQ-CSS, are largely susceptible to bias.
The fifth and main problem comes in translating this evidence in the clinic. Should doctors give up on nondrug means of weight loss? All of the positive outcome trials in this class of drugs have also shown weight loss. I believe we should take these data and use them to re-invigorate our advocacy for weight loss without medication. I know the standard answer to this proposal is nihilism: It just will not work. And I cannot deny that we have failed previously in our efforts to help patients lose weight. But perhaps now, with the vast amount of data, we can be more persuasive. Imagine a world where key opinion leaders made weight loss the message rather than prescription of a drug.
Finally, if you approach SUMMIT from the view of a regulator, with its small numbers of outcome events and bias-susceptible endpoints, you cannot allow a disease-modifying claim. For that we would need a properly powered trial that shows that the drug reduces both CV death and all-cause hospitalization.
In the end, SUMMIT is not close to changing treatment norms in patients with HFpEF. As evidence-based clinicians, we should demand more from our partners in industry and academia.
Dr. Mandrola practices cardiac electrophysiology in Baptist Medical Associates, Louisville, Kentucky, and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results of the SUMMIT trial of the long-acting agonist of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors, tirzepatide, in patients with heart failure with preserved ejection fraction (HFpEF) and obesity are positive. But the trial design leaves clinicians and regulators with big doses of uncertainty.
Known Facts About HFpEF
HFpEF has exceeded heart failure with reduced ejection fraction (HFrEF) as the most common form of heart failure. HFpEF differs from HFrEF in that patients with preserved ejection fraction often present later in life with more comorbidities.
Some of these comorbidities are on the causal pathway of heart failure. Obesity, for instance, both associates with HFpEF and surely causes the diastolic dysfunction central to the condition. This may be a direct effect via high excess adipose tissue or an indirect effect via pro-inflammatory pathways.
GLP-1 agonists and the dual-acting GIP/GLP1 agonist tirzepatide have proven efficacy for weight loss. Semaglutide has previously been shown to improve quality of life and physical functioning in two small trials of patients with HFpEF and obesity. Semaglutide also reduced hard clinical outcomes in patients with obesity and these other conditions: chronic kidney disease, diabetes, and established atherosclerotic vascular disease.
This class of drugs is costly. The combination of both high drug costs and highly prevalent conditions such as obesity and HFpEF forces clinicians to make both value and clinical judgments when translating evidence.
The SUMMIT Trial
The SUMMIT trial aimed to evaluate tirzepatide’s effect on typical heart failure events, health status and functional capacity in patients with obesity and HFpEF. A total of 731 patients were randomly assigned to receive to tirzepatide or placebo.
Investigators chose two co-primary endpoints. The first was a composite of cardiovascular (CV) death and worsening heart failure events—the latter could be a hospitalization for heart failure, a visit for intravenous diuretics, or intensification of oral diuretics. The idea behind this rather unique composite was to capture all heart failure events. The second co-primary endpoint was a change in baseline Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) at 1 year.
Characteristics of the patients included an average age of 65 years, 55% were female, the average body mass index was 38, and the mean left ventricular ejection fraction was 61% (the minimum for trial entry was 50%). Just under half had been hospitalized for heart failure in the year before trial entry.
Tirzepatide Results
The primary outcome of CV death and first heart failure event occurred in 36 patients (9.9%) in the tirzepatide group and 56 patients (15.3%) in the placebo group, for a hazard ratio of 0.62 (95% CI, 0.41-0.95; P =.026).
The 5.4% absolute risk reduction in the primary endpoint was completely driven by lower rates of heart failure events (8% vs 14.2%). CV death was actually higher in the tirzepatide arm, but the number of deaths was low in both arms (8 vs 5).
The rate of hospitalizations due to heart failure was lower with tirzepatide (3.3% vs 7.1%), as was intensification of oral diuretics (4.7% vs 5.7%).
The second co-primary endpoint of change from baseline in KCCQ-CSS favored tirzepatide.
Other secondary endpoints also favored tirzepatide: longer 6-minute walk distance, greater change in body weight (-11.6%), and lower high-sensitivity C-reactive protein levels and systolic blood pressure (-4.7 mm Hg).
Authors’ Conclusions and Expert Comments
At the American Heart Association Scientific Sessions, the primary investigator Milton Packer, MD, said SUMMIT was the first trial of patients with obesity and HFpEF that had major heart failure outcomes as the primary endpoint. And that tirzepatide changed the clinical trajectory of the disease.
Jennifer Ho, MD, associate professor of medicine at Harvard Medical School, Boston, Massachusetts, said, “This really is a practice-changing trial and cements this type of therapy as one of the cornerstones of obesity and HFpEF treatment.”
Other experts cited a recently published pooled analysis of semaglutide trials looking specifically at patients with HFpEF and found lower rates of HF events with the GLP-1 agonist.
The SUMMIT trial results were covered in 53 news outlets— nearly all with glowing headlines.
My Six Concerns With SUMMIT
The trial delivered statistically positive findings. What’s more, patients lost weight, and a greater than 11% weight loss difference is meaningful. Patients with a baseline weight of more than 100 kg who lose this much weight are bound to feel and function better.
The first problem comes when we ask whether the results are disease-modifying. There was no difference in CV death. And the number of hospitalizations for heart failure — the more standard endpoint — was low, at only 12 and 26, respectively. Contrast this with the DELIVER trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in HFpEF where there were nearly 750 hospitalizations for heart failure and PARAGON-HF of sacubitril-valsartan vs valsartan in HFpEF, where there were nearly 1500. SUMMIT simply had too few events to make conclusions — a point Packer has made regarding AF ablation trials in patients with heart failure.
I have previously called GLP-1 drugs disease-modifying in patients with obesity and atherosclerotic disease. This is because the SELECT trial of semaglutide randomized more than 17,000 patients and recorded a 20% reduction in hard outcomes. And there were more than 1200 primary outcome events. SUMMIT does not come close to this measure.
The second issue is short follow-up. These were 65-year-old patients and with only 2 years of follow-up, it is hard to make conclusions regarding whether or not these drugs can provide long-term benefit.
The third issue is that SUMMIT authors don’t tell us the number of all-cause hospitalizations. I was part of a recently published meta-analysis of more than 100 heart failure trials that raised questions regarding the value of hospitalizations for heart failure as a surrogate for heart failure outcomes.
For instance, we found that in large trials there was great variability in the ability of a reduction in HF hospitalizations to predict a reduction in all-cause hospitalization. In small trials, such as SUMMIT, it would likely be impossible to predict how the reduction in HF hospitalization would predict all-cause hospitalization. I believe all-cause hospitalization is a more inclusive endpoint because it is bias free; it captures benefits and potential harms of the therapy; and it is patient-centered, because patients probably do not care what type of hospitalization they avoid.
The fourth issue with SUMMIT is the difficulty in maintaining blinding, which reduces confidence in outcomes that require clinical decisions or patient judgments. Owing to gastrointestinal symptoms, decreased appetite, and weight loss, patients on this class of drugs are very likely to know their treatment assignment. This is a criticism of not only SUMMIT but all GLP-1 agonist trials. The fact that blinding is difficult to maintain argues for choosing endpoints less susceptible to bias, such as CV death or all-cause hospitalization.
Proponents of tirzepatide for this indication might argue that unblinding is less of an issue because of objective endpoints such as biomarkers. And they have a point, but nearly all other endpoints, especially the co-primary endpoint of KCCQ-CSS, are largely susceptible to bias.
The fifth and main problem comes in translating this evidence in the clinic. Should doctors give up on nondrug means of weight loss? All of the positive outcome trials in this class of drugs have also shown weight loss. I believe we should take these data and use them to re-invigorate our advocacy for weight loss without medication. I know the standard answer to this proposal is nihilism: It just will not work. And I cannot deny that we have failed previously in our efforts to help patients lose weight. But perhaps now, with the vast amount of data, we can be more persuasive. Imagine a world where key opinion leaders made weight loss the message rather than prescription of a drug.
Finally, if you approach SUMMIT from the view of a regulator, with its small numbers of outcome events and bias-susceptible endpoints, you cannot allow a disease-modifying claim. For that we would need a properly powered trial that shows that the drug reduces both CV death and all-cause hospitalization.
In the end, SUMMIT is not close to changing treatment norms in patients with HFpEF. As evidence-based clinicians, we should demand more from our partners in industry and academia.
Dr. Mandrola practices cardiac electrophysiology in Baptist Medical Associates, Louisville, Kentucky, and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results of the SUMMIT trial of the long-acting agonist of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors, tirzepatide, in patients with heart failure with preserved ejection fraction (HFpEF) and obesity are positive. But the trial design leaves clinicians and regulators with big doses of uncertainty.
Known Facts About HFpEF
HFpEF has exceeded heart failure with reduced ejection fraction (HFrEF) as the most common form of heart failure. HFpEF differs from HFrEF in that patients with preserved ejection fraction often present later in life with more comorbidities.
Some of these comorbidities are on the causal pathway of heart failure. Obesity, for instance, both associates with HFpEF and surely causes the diastolic dysfunction central to the condition. This may be a direct effect via high excess adipose tissue or an indirect effect via pro-inflammatory pathways.
GLP-1 agonists and the dual-acting GIP/GLP1 agonist tirzepatide have proven efficacy for weight loss. Semaglutide has previously been shown to improve quality of life and physical functioning in two small trials of patients with HFpEF and obesity. Semaglutide also reduced hard clinical outcomes in patients with obesity and these other conditions: chronic kidney disease, diabetes, and established atherosclerotic vascular disease.
This class of drugs is costly. The combination of both high drug costs and highly prevalent conditions such as obesity and HFpEF forces clinicians to make both value and clinical judgments when translating evidence.
The SUMMIT Trial
The SUMMIT trial aimed to evaluate tirzepatide’s effect on typical heart failure events, health status and functional capacity in patients with obesity and HFpEF. A total of 731 patients were randomly assigned to receive to tirzepatide or placebo.
Investigators chose two co-primary endpoints. The first was a composite of cardiovascular (CV) death and worsening heart failure events—the latter could be a hospitalization for heart failure, a visit for intravenous diuretics, or intensification of oral diuretics. The idea behind this rather unique composite was to capture all heart failure events. The second co-primary endpoint was a change in baseline Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) at 1 year.
Characteristics of the patients included an average age of 65 years, 55% were female, the average body mass index was 38, and the mean left ventricular ejection fraction was 61% (the minimum for trial entry was 50%). Just under half had been hospitalized for heart failure in the year before trial entry.
Tirzepatide Results
The primary outcome of CV death and first heart failure event occurred in 36 patients (9.9%) in the tirzepatide group and 56 patients (15.3%) in the placebo group, for a hazard ratio of 0.62 (95% CI, 0.41-0.95; P =.026).
The 5.4% absolute risk reduction in the primary endpoint was completely driven by lower rates of heart failure events (8% vs 14.2%). CV death was actually higher in the tirzepatide arm, but the number of deaths was low in both arms (8 vs 5).
The rate of hospitalizations due to heart failure was lower with tirzepatide (3.3% vs 7.1%), as was intensification of oral diuretics (4.7% vs 5.7%).
The second co-primary endpoint of change from baseline in KCCQ-CSS favored tirzepatide.
Other secondary endpoints also favored tirzepatide: longer 6-minute walk distance, greater change in body weight (-11.6%), and lower high-sensitivity C-reactive protein levels and systolic blood pressure (-4.7 mm Hg).
Authors’ Conclusions and Expert Comments
At the American Heart Association Scientific Sessions, the primary investigator Milton Packer, MD, said SUMMIT was the first trial of patients with obesity and HFpEF that had major heart failure outcomes as the primary endpoint. And that tirzepatide changed the clinical trajectory of the disease.
Jennifer Ho, MD, associate professor of medicine at Harvard Medical School, Boston, Massachusetts, said, “This really is a practice-changing trial and cements this type of therapy as one of the cornerstones of obesity and HFpEF treatment.”
Other experts cited a recently published pooled analysis of semaglutide trials looking specifically at patients with HFpEF and found lower rates of HF events with the GLP-1 agonist.
The SUMMIT trial results were covered in 53 news outlets— nearly all with glowing headlines.
My Six Concerns With SUMMIT
The trial delivered statistically positive findings. What’s more, patients lost weight, and a greater than 11% weight loss difference is meaningful. Patients with a baseline weight of more than 100 kg who lose this much weight are bound to feel and function better.
The first problem comes when we ask whether the results are disease-modifying. There was no difference in CV death. And the number of hospitalizations for heart failure — the more standard endpoint — was low, at only 12 and 26, respectively. Contrast this with the DELIVER trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in HFpEF where there were nearly 750 hospitalizations for heart failure and PARAGON-HF of sacubitril-valsartan vs valsartan in HFpEF, where there were nearly 1500. SUMMIT simply had too few events to make conclusions — a point Packer has made regarding AF ablation trials in patients with heart failure.
I have previously called GLP-1 drugs disease-modifying in patients with obesity and atherosclerotic disease. This is because the SELECT trial of semaglutide randomized more than 17,000 patients and recorded a 20% reduction in hard outcomes. And there were more than 1200 primary outcome events. SUMMIT does not come close to this measure.
The second issue is short follow-up. These were 65-year-old patients and with only 2 years of follow-up, it is hard to make conclusions regarding whether or not these drugs can provide long-term benefit.
The third issue is that SUMMIT authors don’t tell us the number of all-cause hospitalizations. I was part of a recently published meta-analysis of more than 100 heart failure trials that raised questions regarding the value of hospitalizations for heart failure as a surrogate for heart failure outcomes.
For instance, we found that in large trials there was great variability in the ability of a reduction in HF hospitalizations to predict a reduction in all-cause hospitalization. In small trials, such as SUMMIT, it would likely be impossible to predict how the reduction in HF hospitalization would predict all-cause hospitalization. I believe all-cause hospitalization is a more inclusive endpoint because it is bias free; it captures benefits and potential harms of the therapy; and it is patient-centered, because patients probably do not care what type of hospitalization they avoid.
The fourth issue with SUMMIT is the difficulty in maintaining blinding, which reduces confidence in outcomes that require clinical decisions or patient judgments. Owing to gastrointestinal symptoms, decreased appetite, and weight loss, patients on this class of drugs are very likely to know their treatment assignment. This is a criticism of not only SUMMIT but all GLP-1 agonist trials. The fact that blinding is difficult to maintain argues for choosing endpoints less susceptible to bias, such as CV death or all-cause hospitalization.
Proponents of tirzepatide for this indication might argue that unblinding is less of an issue because of objective endpoints such as biomarkers. And they have a point, but nearly all other endpoints, especially the co-primary endpoint of KCCQ-CSS, are largely susceptible to bias.
The fifth and main problem comes in translating this evidence in the clinic. Should doctors give up on nondrug means of weight loss? All of the positive outcome trials in this class of drugs have also shown weight loss. I believe we should take these data and use them to re-invigorate our advocacy for weight loss without medication. I know the standard answer to this proposal is nihilism: It just will not work. And I cannot deny that we have failed previously in our efforts to help patients lose weight. But perhaps now, with the vast amount of data, we can be more persuasive. Imagine a world where key opinion leaders made weight loss the message rather than prescription of a drug.
Finally, if you approach SUMMIT from the view of a regulator, with its small numbers of outcome events and bias-susceptible endpoints, you cannot allow a disease-modifying claim. For that we would need a properly powered trial that shows that the drug reduces both CV death and all-cause hospitalization.
In the end, SUMMIT is not close to changing treatment norms in patients with HFpEF. As evidence-based clinicians, we should demand more from our partners in industry and academia.
Dr. Mandrola practices cardiac electrophysiology in Baptist Medical Associates, Louisville, Kentucky, and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
REALIZE-K: A New Potassium Binder to Help Keep Spiro on Board
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
Gout and SGLT2 Inhibitors: Evidence Points to Reduced Need for ULT, Flare Drugs
WASHINGTON — Use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) reduced the need for urate-lowering therapy (ULT) and gout flare therapies in people who had both type 2 diabetes (T2D) and gout, new research has found.
Data from a large US claims database showed that SGLT2i use was associated with a 31% lower rate of initiation of ULT. “This provides further support for the use of SLGT2i therapy in patients with gout, particularly those with high-risk multimorbidity and polypharmacy,” Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said in his presentation of the data at the annual meeting of the American College of Rheumatology.
The first agent of the SGLT2i class, dapagliflozin, was initially approved in the United States a decade ago for treating T2D. Since then, several other “flozins” have become available, and some have also received additional indications for heart failure and albuminuric chronic kidney disease. Several prior studies have linked SGLT2i use with lower rates of gout flares as well as lower likelihood of developing gout in the first place, although not all studies have found this benefit.
Asked about the clinical implications of the new data, Challener said in an interview that “I don’t think we’re quite at the point where this is changing gout management per se, but this just helps us understand that [SGT2is] may have a role at some point, maybe as a combination on top of another agent. Or, in some patients, it really may be enough if they’re already on an SGLT2i where we don’t need to jump to adding allopurinol. Maybe they have tophi, but they were just started on an SGLT2i and they’re not flaring. Typically, you would start those patients on allopurinol, but you could potentially just monitor them if they were just started on one of those [SGLT2i] agents.”
Asked to comment, session moderator J. Antonio Aviña-Zubieta, MD, PhD, head of the Division of Rheumatology at the University of British Columbia, Vancouver, Canada, and senior scientist at Arthritis Research Canada, said in an interview: “What I can see possibly happening when there’s more evidence is that SGLT2is may be used or even become standard of care as an adjuvant therapy to decrease flares, and by that, decrease the risk of complications.”
Reductions in ULT, Flares, and Healthcare Visits
The new study used administrative health data from the multicenter TriNetX Diamond network of electronic medical record and claims data from 92 healthcare sites with 212 million patients. Among those with both T2D and gout who were not taking ULT at baseline, a total of 16,104 initiated SGLT2is and 16,046 initiated glucagon-like peptide 1 receptor agonists (GLP-1 RA).
Propensity score matching was conducted for demographics including age, race, and sex; comorbidities; use of emergency, inpatient, and critical care services; medications; labs; and body mass index. That yielded 11,800 individuals each in the SGLT2i and GLP-1 RA groups.
Over 5 years, 9.9% of the SGLT2i group vs 13.4% of those using GLP-1 RA had initiated ULT, a significant difference with a hazard ratio (HR) of 0.69 (95% CI, 0.64-0.75). The risk for initiation of colchicine for gout flares was 4.7% with SGLT2i vs 6.0% for GLP-1 RA — also a significant difference with an HR of 0.74 (0.65-0.83).
Medical visits for gout occurred in 28.0% vs 28.4% of patients, which also reached statistical significance (HR, 0.94; 95% CI, 0.89-0.99).
Aviña-Zubieta, an author of one of the previous studies finding a reduction in gout flares with SGLT2i, said, “many patients do not want to start gout therapy until they start having more acute attacks. ... So, for a lot of people, it’s a burden taking another pill to prevent one attack. But, if you don’t treat it over time, the attacks come more often. So, can we still delay the initiation of therapy? If you’re not having that many flares, you’re decreasing the burden of the disease and polypharmacy, which I think is the potential benefit in the long run if you already have an indication for the therapy for diabetes. ... These data are supporting that.”
Indeed, Challener said these data can help in counseling patients. “Taking your SGLT2i for your heart failure and your diabetes is also providing some benefit for your gout, and we know that there is also cardiac benefit when gout is controlled.”
Challener and Aviña-Zubieta had no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — Use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) reduced the need for urate-lowering therapy (ULT) and gout flare therapies in people who had both type 2 diabetes (T2D) and gout, new research has found.
Data from a large US claims database showed that SGLT2i use was associated with a 31% lower rate of initiation of ULT. “This provides further support for the use of SLGT2i therapy in patients with gout, particularly those with high-risk multimorbidity and polypharmacy,” Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said in his presentation of the data at the annual meeting of the American College of Rheumatology.
The first agent of the SGLT2i class, dapagliflozin, was initially approved in the United States a decade ago for treating T2D. Since then, several other “flozins” have become available, and some have also received additional indications for heart failure and albuminuric chronic kidney disease. Several prior studies have linked SGLT2i use with lower rates of gout flares as well as lower likelihood of developing gout in the first place, although not all studies have found this benefit.
Asked about the clinical implications of the new data, Challener said in an interview that “I don’t think we’re quite at the point where this is changing gout management per se, but this just helps us understand that [SGT2is] may have a role at some point, maybe as a combination on top of another agent. Or, in some patients, it really may be enough if they’re already on an SGLT2i where we don’t need to jump to adding allopurinol. Maybe they have tophi, but they were just started on an SGLT2i and they’re not flaring. Typically, you would start those patients on allopurinol, but you could potentially just monitor them if they were just started on one of those [SGLT2i] agents.”
Asked to comment, session moderator J. Antonio Aviña-Zubieta, MD, PhD, head of the Division of Rheumatology at the University of British Columbia, Vancouver, Canada, and senior scientist at Arthritis Research Canada, said in an interview: “What I can see possibly happening when there’s more evidence is that SGLT2is may be used or even become standard of care as an adjuvant therapy to decrease flares, and by that, decrease the risk of complications.”
Reductions in ULT, Flares, and Healthcare Visits
The new study used administrative health data from the multicenter TriNetX Diamond network of electronic medical record and claims data from 92 healthcare sites with 212 million patients. Among those with both T2D and gout who were not taking ULT at baseline, a total of 16,104 initiated SGLT2is and 16,046 initiated glucagon-like peptide 1 receptor agonists (GLP-1 RA).
Propensity score matching was conducted for demographics including age, race, and sex; comorbidities; use of emergency, inpatient, and critical care services; medications; labs; and body mass index. That yielded 11,800 individuals each in the SGLT2i and GLP-1 RA groups.
Over 5 years, 9.9% of the SGLT2i group vs 13.4% of those using GLP-1 RA had initiated ULT, a significant difference with a hazard ratio (HR) of 0.69 (95% CI, 0.64-0.75). The risk for initiation of colchicine for gout flares was 4.7% with SGLT2i vs 6.0% for GLP-1 RA — also a significant difference with an HR of 0.74 (0.65-0.83).
Medical visits for gout occurred in 28.0% vs 28.4% of patients, which also reached statistical significance (HR, 0.94; 95% CI, 0.89-0.99).
Aviña-Zubieta, an author of one of the previous studies finding a reduction in gout flares with SGLT2i, said, “many patients do not want to start gout therapy until they start having more acute attacks. ... So, for a lot of people, it’s a burden taking another pill to prevent one attack. But, if you don’t treat it over time, the attacks come more often. So, can we still delay the initiation of therapy? If you’re not having that many flares, you’re decreasing the burden of the disease and polypharmacy, which I think is the potential benefit in the long run if you already have an indication for the therapy for diabetes. ... These data are supporting that.”
Indeed, Challener said these data can help in counseling patients. “Taking your SGLT2i for your heart failure and your diabetes is also providing some benefit for your gout, and we know that there is also cardiac benefit when gout is controlled.”
Challener and Aviña-Zubieta had no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — Use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) reduced the need for urate-lowering therapy (ULT) and gout flare therapies in people who had both type 2 diabetes (T2D) and gout, new research has found.
Data from a large US claims database showed that SGLT2i use was associated with a 31% lower rate of initiation of ULT. “This provides further support for the use of SLGT2i therapy in patients with gout, particularly those with high-risk multimorbidity and polypharmacy,” Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said in his presentation of the data at the annual meeting of the American College of Rheumatology.
The first agent of the SGLT2i class, dapagliflozin, was initially approved in the United States a decade ago for treating T2D. Since then, several other “flozins” have become available, and some have also received additional indications for heart failure and albuminuric chronic kidney disease. Several prior studies have linked SGLT2i use with lower rates of gout flares as well as lower likelihood of developing gout in the first place, although not all studies have found this benefit.
Asked about the clinical implications of the new data, Challener said in an interview that “I don’t think we’re quite at the point where this is changing gout management per se, but this just helps us understand that [SGT2is] may have a role at some point, maybe as a combination on top of another agent. Or, in some patients, it really may be enough if they’re already on an SGLT2i where we don’t need to jump to adding allopurinol. Maybe they have tophi, but they were just started on an SGLT2i and they’re not flaring. Typically, you would start those patients on allopurinol, but you could potentially just monitor them if they were just started on one of those [SGLT2i] agents.”
Asked to comment, session moderator J. Antonio Aviña-Zubieta, MD, PhD, head of the Division of Rheumatology at the University of British Columbia, Vancouver, Canada, and senior scientist at Arthritis Research Canada, said in an interview: “What I can see possibly happening when there’s more evidence is that SGLT2is may be used or even become standard of care as an adjuvant therapy to decrease flares, and by that, decrease the risk of complications.”
Reductions in ULT, Flares, and Healthcare Visits
The new study used administrative health data from the multicenter TriNetX Diamond network of electronic medical record and claims data from 92 healthcare sites with 212 million patients. Among those with both T2D and gout who were not taking ULT at baseline, a total of 16,104 initiated SGLT2is and 16,046 initiated glucagon-like peptide 1 receptor agonists (GLP-1 RA).
Propensity score matching was conducted for demographics including age, race, and sex; comorbidities; use of emergency, inpatient, and critical care services; medications; labs; and body mass index. That yielded 11,800 individuals each in the SGLT2i and GLP-1 RA groups.
Over 5 years, 9.9% of the SGLT2i group vs 13.4% of those using GLP-1 RA had initiated ULT, a significant difference with a hazard ratio (HR) of 0.69 (95% CI, 0.64-0.75). The risk for initiation of colchicine for gout flares was 4.7% with SGLT2i vs 6.0% for GLP-1 RA — also a significant difference with an HR of 0.74 (0.65-0.83).
Medical visits for gout occurred in 28.0% vs 28.4% of patients, which also reached statistical significance (HR, 0.94; 95% CI, 0.89-0.99).
Aviña-Zubieta, an author of one of the previous studies finding a reduction in gout flares with SGLT2i, said, “many patients do not want to start gout therapy until they start having more acute attacks. ... So, for a lot of people, it’s a burden taking another pill to prevent one attack. But, if you don’t treat it over time, the attacks come more often. So, can we still delay the initiation of therapy? If you’re not having that many flares, you’re decreasing the burden of the disease and polypharmacy, which I think is the potential benefit in the long run if you already have an indication for the therapy for diabetes. ... These data are supporting that.”
Indeed, Challener said these data can help in counseling patients. “Taking your SGLT2i for your heart failure and your diabetes is also providing some benefit for your gout, and we know that there is also cardiac benefit when gout is controlled.”
Challener and Aviña-Zubieta had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACR 2024
Angiotensin Receptor Blockers May Lead to Worse Outcomes in Celiac Disease
PHILADELPHIA — , according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
The association may be related to the similar pathophysiology between ARB-associated enteropathy and celiac disease, though additional research is needed.
“Based on our findings, people should take caution when prescribing angiotensin receptor blockers to people with celiac disease,” said lead author Isabel Hujoel, MD, clinical assistant professor of gastroenterology and clinic director of the Celiac Disease Center at the University of Washington, Seattle.
“When we see someone with nonresponsive celiac disease, meaning persistent symptoms despite a gluten-free diet, I do think we should review their medication list, and if they’re on an ARB, we should consider a trial off those medications to see if they respond,” she said. “A primary care provider may choose other hypertensives as well.”
Hujoel and co-author Margaux Hujoel, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston; Broad Institute, Cambridge; and Harvard Medical School, Boston, analyzed data from the National Institutes of Health’s All of Us, a large publicly available US longitudinal dataset.
The researchers conducted a survival analysis of time-to-first event after celiac disease diagnosis, allowing patients to have a time-dependent covariate of ARB use. They looked at outcomes such as iron deficiency, diarrhea, abdominal pain, vitamin deficiency, vitamin D deficiency, malabsorption, low hemoglobin, and weight loss.
The analysis included 1849 patients with celiac disease, including 1460 women and 389 men, with a median age of nearly 50 years at diagnosis. While the vast majority of patients (nearly 1600) didn’t take an ARB, 120 started one before celiac disease diagnosis and 142 started one after diagnosis.
Overall, taking an ARB was associated with increased hazard ratios [HRs] for low hemoglobin, iron deficiency, diarrhea, and abdominal pain. There weren’t increased risks for weight loss, malabsorption, or vitamin deficiencies.
When excluding those who had an ARB prescription before diagnosis, the HRs remained significantly higher for low hemoglobin (HR, 1.98) and iron deficiency (HR, 1.72) for those who started an ARB after diagnosis.
“The use of angiotensin receptor blockers may be associated with worse outcomes in the setting of celiac disease, specifically persistent symptoms and possibly poor small bowel healing as evidenced by malabsorption,” Hujoel said.
Future studies could look specifically at losartan, which was the most common ARB prescribed in this analysis, she said. Other studies could also analyze different patient outcomes, whether patients were on a gluten-free diet, medication adherence, and recurrence or persistence of symptoms rather than initial occurrence. The associations between ARB use and celiac disease could shift among patients who are in remission, for instance.
“ARBs are some of the most widely used medications, so studies like these can help people to understand that they may have symptoms but not know it’s related to their medication. Public awareness of this fact is key,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, Miami. Jones co-moderated the plenary session on small intestine, functional, and liver research.
“There are many types of antihypertensives, so while ARBs are used often, other options are available if people have symptoms, especially if they have worsening symptoms with celiac disease,” she said. “It’s important to make changes in your practice.”
The study was named an ACG Newsworthy Abstract. Isabel Hujoel and Patricia Jones reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
The association may be related to the similar pathophysiology between ARB-associated enteropathy and celiac disease, though additional research is needed.
“Based on our findings, people should take caution when prescribing angiotensin receptor blockers to people with celiac disease,” said lead author Isabel Hujoel, MD, clinical assistant professor of gastroenterology and clinic director of the Celiac Disease Center at the University of Washington, Seattle.
“When we see someone with nonresponsive celiac disease, meaning persistent symptoms despite a gluten-free diet, I do think we should review their medication list, and if they’re on an ARB, we should consider a trial off those medications to see if they respond,” she said. “A primary care provider may choose other hypertensives as well.”
Hujoel and co-author Margaux Hujoel, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston; Broad Institute, Cambridge; and Harvard Medical School, Boston, analyzed data from the National Institutes of Health’s All of Us, a large publicly available US longitudinal dataset.
The researchers conducted a survival analysis of time-to-first event after celiac disease diagnosis, allowing patients to have a time-dependent covariate of ARB use. They looked at outcomes such as iron deficiency, diarrhea, abdominal pain, vitamin deficiency, vitamin D deficiency, malabsorption, low hemoglobin, and weight loss.
The analysis included 1849 patients with celiac disease, including 1460 women and 389 men, with a median age of nearly 50 years at diagnosis. While the vast majority of patients (nearly 1600) didn’t take an ARB, 120 started one before celiac disease diagnosis and 142 started one after diagnosis.
Overall, taking an ARB was associated with increased hazard ratios [HRs] for low hemoglobin, iron deficiency, diarrhea, and abdominal pain. There weren’t increased risks for weight loss, malabsorption, or vitamin deficiencies.
When excluding those who had an ARB prescription before diagnosis, the HRs remained significantly higher for low hemoglobin (HR, 1.98) and iron deficiency (HR, 1.72) for those who started an ARB after diagnosis.
“The use of angiotensin receptor blockers may be associated with worse outcomes in the setting of celiac disease, specifically persistent symptoms and possibly poor small bowel healing as evidenced by malabsorption,” Hujoel said.
Future studies could look specifically at losartan, which was the most common ARB prescribed in this analysis, she said. Other studies could also analyze different patient outcomes, whether patients were on a gluten-free diet, medication adherence, and recurrence or persistence of symptoms rather than initial occurrence. The associations between ARB use and celiac disease could shift among patients who are in remission, for instance.
“ARBs are some of the most widely used medications, so studies like these can help people to understand that they may have symptoms but not know it’s related to their medication. Public awareness of this fact is key,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, Miami. Jones co-moderated the plenary session on small intestine, functional, and liver research.
“There are many types of antihypertensives, so while ARBs are used often, other options are available if people have symptoms, especially if they have worsening symptoms with celiac disease,” she said. “It’s important to make changes in your practice.”
The study was named an ACG Newsworthy Abstract. Isabel Hujoel and Patricia Jones reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
The association may be related to the similar pathophysiology between ARB-associated enteropathy and celiac disease, though additional research is needed.
“Based on our findings, people should take caution when prescribing angiotensin receptor blockers to people with celiac disease,” said lead author Isabel Hujoel, MD, clinical assistant professor of gastroenterology and clinic director of the Celiac Disease Center at the University of Washington, Seattle.
“When we see someone with nonresponsive celiac disease, meaning persistent symptoms despite a gluten-free diet, I do think we should review their medication list, and if they’re on an ARB, we should consider a trial off those medications to see if they respond,” she said. “A primary care provider may choose other hypertensives as well.”
Hujoel and co-author Margaux Hujoel, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston; Broad Institute, Cambridge; and Harvard Medical School, Boston, analyzed data from the National Institutes of Health’s All of Us, a large publicly available US longitudinal dataset.
The researchers conducted a survival analysis of time-to-first event after celiac disease diagnosis, allowing patients to have a time-dependent covariate of ARB use. They looked at outcomes such as iron deficiency, diarrhea, abdominal pain, vitamin deficiency, vitamin D deficiency, malabsorption, low hemoglobin, and weight loss.
The analysis included 1849 patients with celiac disease, including 1460 women and 389 men, with a median age of nearly 50 years at diagnosis. While the vast majority of patients (nearly 1600) didn’t take an ARB, 120 started one before celiac disease diagnosis and 142 started one after diagnosis.
Overall, taking an ARB was associated with increased hazard ratios [HRs] for low hemoglobin, iron deficiency, diarrhea, and abdominal pain. There weren’t increased risks for weight loss, malabsorption, or vitamin deficiencies.
When excluding those who had an ARB prescription before diagnosis, the HRs remained significantly higher for low hemoglobin (HR, 1.98) and iron deficiency (HR, 1.72) for those who started an ARB after diagnosis.
“The use of angiotensin receptor blockers may be associated with worse outcomes in the setting of celiac disease, specifically persistent symptoms and possibly poor small bowel healing as evidenced by malabsorption,” Hujoel said.
Future studies could look specifically at losartan, which was the most common ARB prescribed in this analysis, she said. Other studies could also analyze different patient outcomes, whether patients were on a gluten-free diet, medication adherence, and recurrence or persistence of symptoms rather than initial occurrence. The associations between ARB use and celiac disease could shift among patients who are in remission, for instance.
“ARBs are some of the most widely used medications, so studies like these can help people to understand that they may have symptoms but not know it’s related to their medication. Public awareness of this fact is key,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, Miami. Jones co-moderated the plenary session on small intestine, functional, and liver research.
“There are many types of antihypertensives, so while ARBs are used often, other options are available if people have symptoms, especially if they have worsening symptoms with celiac disease,” she said. “It’s important to make changes in your practice.”
The study was named an ACG Newsworthy Abstract. Isabel Hujoel and Patricia Jones reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACG 2024


