User login
True Benefit of Screening Colonoscopy for CRC Underestimated in NordICC
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
MMR/MSI Testing for CRC Climbs, But Variations Persist
TOPLINE:
with testing rates differing by cancer stage, individual hospital, patient sex, race, and insurance status.
METHODOLOGY:
- In 2017, the National Comprehensive Cancer Network (NCCN) recommended universal testing for MMR and MSI among patients with CRC, but studies suggest that testing may still be underused.
- To assess trends and factors associated with MMR/MSI testing in the United States, researchers evaluated 834,797 patients diagnosed with stage I-IV CRC between 2012 and 2021 across 1366 Commission on Cancer–accredited hospitals in the National Cancer Database.
- The variability in MMR/MSI testing was assessed in relation to both patient and hospital-level factors.
- Overall, 70.7% patients had colon cancer, 7.3% had rectosigmoid cancer, and 22.0% had rectal cancer. The median patient age was 66 years; just over half (53%) were men, 81.8% were White, and 11.9% were Black.
TAKEAWAY:
- Overall, 43.9% patients underwent MMR/MSI testing, but testing rates increased more than threefold between 2012 and 2021 — from 22.7% to 71.5%. Still, testing rates varied depending on a range of factors.
- About 22% variability in MMR/MSI testing was attributed to hospital-level variations, with the best vs worst performing hospitals reporting testing rates of 90% vs 2%. This hospital-level variation may be caused by testing protocol differences at individual institutions, the authors said.
- The likelihood of undergoing MMR/MSI testing was lower in patients with stage IV vs stage I disease (adjusted odds ratio [aOR], 0.78) but higher in those with stage II (aOR, 1.53) and III (aOR, 1.40) disease.
- The likelihood of undergoing MMR/MSI testing was slightly lower for men than for women (aOR, 0.98) and for Black patients than for White patients (aOR, 0.97). Having a lower household income, public or no insurance (vs private insurance), or living a longer distance (more than 5 miles) from the treatment facility was also associated with lower odds of testing.
IN PRACTICE:
“This cohort study indicated that MMR/MSI testing increased markedly, suggesting increased NCCN guideline adherence,” the authors said. However, variations still exist by cancer stage, hospital, and patient factors. Implementing “widespread institution-level reflexive testing for every initial diagnostic biopsy” can improve testing rates and reduce disparities, the authors suggested.
SOURCE:
This study, led by Totadri Dhimal, MD, University of Rochester Medical Center in New York, was published online in JAMA Oncology.
LIMITATIONS:
The study lacked clinical granularity, and potential coding inaccuracies and incomplete data could have affected the interpretation and generalizability of the findings.
DISCLOSURES:
No funding information was provided for the study. One author reported receiving author royalties from UpToDate outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with testing rates differing by cancer stage, individual hospital, patient sex, race, and insurance status.
METHODOLOGY:
- In 2017, the National Comprehensive Cancer Network (NCCN) recommended universal testing for MMR and MSI among patients with CRC, but studies suggest that testing may still be underused.
- To assess trends and factors associated with MMR/MSI testing in the United States, researchers evaluated 834,797 patients diagnosed with stage I-IV CRC between 2012 and 2021 across 1366 Commission on Cancer–accredited hospitals in the National Cancer Database.
- The variability in MMR/MSI testing was assessed in relation to both patient and hospital-level factors.
- Overall, 70.7% patients had colon cancer, 7.3% had rectosigmoid cancer, and 22.0% had rectal cancer. The median patient age was 66 years; just over half (53%) were men, 81.8% were White, and 11.9% were Black.
TAKEAWAY:
- Overall, 43.9% patients underwent MMR/MSI testing, but testing rates increased more than threefold between 2012 and 2021 — from 22.7% to 71.5%. Still, testing rates varied depending on a range of factors.
- About 22% variability in MMR/MSI testing was attributed to hospital-level variations, with the best vs worst performing hospitals reporting testing rates of 90% vs 2%. This hospital-level variation may be caused by testing protocol differences at individual institutions, the authors said.
- The likelihood of undergoing MMR/MSI testing was lower in patients with stage IV vs stage I disease (adjusted odds ratio [aOR], 0.78) but higher in those with stage II (aOR, 1.53) and III (aOR, 1.40) disease.
- The likelihood of undergoing MMR/MSI testing was slightly lower for men than for women (aOR, 0.98) and for Black patients than for White patients (aOR, 0.97). Having a lower household income, public or no insurance (vs private insurance), or living a longer distance (more than 5 miles) from the treatment facility was also associated with lower odds of testing.
IN PRACTICE:
“This cohort study indicated that MMR/MSI testing increased markedly, suggesting increased NCCN guideline adherence,” the authors said. However, variations still exist by cancer stage, hospital, and patient factors. Implementing “widespread institution-level reflexive testing for every initial diagnostic biopsy” can improve testing rates and reduce disparities, the authors suggested.
SOURCE:
This study, led by Totadri Dhimal, MD, University of Rochester Medical Center in New York, was published online in JAMA Oncology.
LIMITATIONS:
The study lacked clinical granularity, and potential coding inaccuracies and incomplete data could have affected the interpretation and generalizability of the findings.
DISCLOSURES:
No funding information was provided for the study. One author reported receiving author royalties from UpToDate outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with testing rates differing by cancer stage, individual hospital, patient sex, race, and insurance status.
METHODOLOGY:
- In 2017, the National Comprehensive Cancer Network (NCCN) recommended universal testing for MMR and MSI among patients with CRC, but studies suggest that testing may still be underused.
- To assess trends and factors associated with MMR/MSI testing in the United States, researchers evaluated 834,797 patients diagnosed with stage I-IV CRC between 2012 and 2021 across 1366 Commission on Cancer–accredited hospitals in the National Cancer Database.
- The variability in MMR/MSI testing was assessed in relation to both patient and hospital-level factors.
- Overall, 70.7% patients had colon cancer, 7.3% had rectosigmoid cancer, and 22.0% had rectal cancer. The median patient age was 66 years; just over half (53%) were men, 81.8% were White, and 11.9% were Black.
TAKEAWAY:
- Overall, 43.9% patients underwent MMR/MSI testing, but testing rates increased more than threefold between 2012 and 2021 — from 22.7% to 71.5%. Still, testing rates varied depending on a range of factors.
- About 22% variability in MMR/MSI testing was attributed to hospital-level variations, with the best vs worst performing hospitals reporting testing rates of 90% vs 2%. This hospital-level variation may be caused by testing protocol differences at individual institutions, the authors said.
- The likelihood of undergoing MMR/MSI testing was lower in patients with stage IV vs stage I disease (adjusted odds ratio [aOR], 0.78) but higher in those with stage II (aOR, 1.53) and III (aOR, 1.40) disease.
- The likelihood of undergoing MMR/MSI testing was slightly lower for men than for women (aOR, 0.98) and for Black patients than for White patients (aOR, 0.97). Having a lower household income, public or no insurance (vs private insurance), or living a longer distance (more than 5 miles) from the treatment facility was also associated with lower odds of testing.
IN PRACTICE:
“This cohort study indicated that MMR/MSI testing increased markedly, suggesting increased NCCN guideline adherence,” the authors said. However, variations still exist by cancer stage, hospital, and patient factors. Implementing “widespread institution-level reflexive testing for every initial diagnostic biopsy” can improve testing rates and reduce disparities, the authors suggested.
SOURCE:
This study, led by Totadri Dhimal, MD, University of Rochester Medical Center in New York, was published online in JAMA Oncology.
LIMITATIONS:
The study lacked clinical granularity, and potential coding inaccuracies and incomplete data could have affected the interpretation and generalizability of the findings.
DISCLOSURES:
No funding information was provided for the study. One author reported receiving author royalties from UpToDate outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Are Targeted Drugs the Future in Colorectal Cancer?
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
FDA OKs Next-Gen Cologuard Test for CRC Screening
Developed in collaboration with Mayo Clinic, the company noted in the news release announcing its approval that this noninvasive test “raises the performance bar.”
The company says the enhanced sensitivity will help minimize unnecessary follow-up colonoscopy procedures by reducing the odds of a false-positive screening test.
Enhanced sample stability components also will give patients more time to return their sample to the lab.
Cologuard Plus tests for three novel methylated DNA markers and fecal hemoglobin.
The BLUE-C Study
The FDA’s approval was based on the results of the BLUE-C study involving more than 20,000 adults at average risk for CRC that compared the next-generation mt-sDNA test with a fecal immunochemical test (FIT) and colonoscopy.
According to the BLUE-C results, the sensitivities of Cologuard Plus were 95% for CRC and 43% for advanced precancerous lesions, at 94% specificity with no findings on colonoscopy.
The BLUE-C results also showed that the test significantly outperformed FIT for sensitivity for CRC overall, CRC stages I-III, high-grade dysplasia, and advanced precancerous lesions.
“To meaningfully improve outcomes in colorectal cancer, we must catch cancer early — when it is most treatable — and find advanced precancers, which can prevent cases of this cancer,” Thomas F. Imperiale, MD, AGAF, professor of medicine at the Indiana University School of Medicine and research scientist at the Regenstrief Institute, said in the news release.
“The high colorectal cancer sensitivity and specificity of the Cologuard Plus test gives me confidence in the test’s ability to do just that while simultaneously maintaining a low risk of false positives. This makes the Cologuard Plus test a strong option for first-line screening of average risk patients,” said Dr. Imperiale, who served as principal investigator of the BLUE-C study.
The company plans to launch Cologuard Plus in 2025.
They anticipate that it will be covered by Medicare and included in the United States Preventive Services Task Force (USPSTF) guidelines and within quality measures.
A version of this article first appeared on Medscape.com.
Developed in collaboration with Mayo Clinic, the company noted in the news release announcing its approval that this noninvasive test “raises the performance bar.”
The company says the enhanced sensitivity will help minimize unnecessary follow-up colonoscopy procedures by reducing the odds of a false-positive screening test.
Enhanced sample stability components also will give patients more time to return their sample to the lab.
Cologuard Plus tests for three novel methylated DNA markers and fecal hemoglobin.
The BLUE-C Study
The FDA’s approval was based on the results of the BLUE-C study involving more than 20,000 adults at average risk for CRC that compared the next-generation mt-sDNA test with a fecal immunochemical test (FIT) and colonoscopy.
According to the BLUE-C results, the sensitivities of Cologuard Plus were 95% for CRC and 43% for advanced precancerous lesions, at 94% specificity with no findings on colonoscopy.
The BLUE-C results also showed that the test significantly outperformed FIT for sensitivity for CRC overall, CRC stages I-III, high-grade dysplasia, and advanced precancerous lesions.
“To meaningfully improve outcomes in colorectal cancer, we must catch cancer early — when it is most treatable — and find advanced precancers, which can prevent cases of this cancer,” Thomas F. Imperiale, MD, AGAF, professor of medicine at the Indiana University School of Medicine and research scientist at the Regenstrief Institute, said in the news release.
“The high colorectal cancer sensitivity and specificity of the Cologuard Plus test gives me confidence in the test’s ability to do just that while simultaneously maintaining a low risk of false positives. This makes the Cologuard Plus test a strong option for first-line screening of average risk patients,” said Dr. Imperiale, who served as principal investigator of the BLUE-C study.
The company plans to launch Cologuard Plus in 2025.
They anticipate that it will be covered by Medicare and included in the United States Preventive Services Task Force (USPSTF) guidelines and within quality measures.
A version of this article first appeared on Medscape.com.
Developed in collaboration with Mayo Clinic, the company noted in the news release announcing its approval that this noninvasive test “raises the performance bar.”
The company says the enhanced sensitivity will help minimize unnecessary follow-up colonoscopy procedures by reducing the odds of a false-positive screening test.
Enhanced sample stability components also will give patients more time to return their sample to the lab.
Cologuard Plus tests for three novel methylated DNA markers and fecal hemoglobin.
The BLUE-C Study
The FDA’s approval was based on the results of the BLUE-C study involving more than 20,000 adults at average risk for CRC that compared the next-generation mt-sDNA test with a fecal immunochemical test (FIT) and colonoscopy.
According to the BLUE-C results, the sensitivities of Cologuard Plus were 95% for CRC and 43% for advanced precancerous lesions, at 94% specificity with no findings on colonoscopy.
The BLUE-C results also showed that the test significantly outperformed FIT for sensitivity for CRC overall, CRC stages I-III, high-grade dysplasia, and advanced precancerous lesions.
“To meaningfully improve outcomes in colorectal cancer, we must catch cancer early — when it is most treatable — and find advanced precancers, which can prevent cases of this cancer,” Thomas F. Imperiale, MD, AGAF, professor of medicine at the Indiana University School of Medicine and research scientist at the Regenstrief Institute, said in the news release.
“The high colorectal cancer sensitivity and specificity of the Cologuard Plus test gives me confidence in the test’s ability to do just that while simultaneously maintaining a low risk of false positives. This makes the Cologuard Plus test a strong option for first-line screening of average risk patients,” said Dr. Imperiale, who served as principal investigator of the BLUE-C study.
The company plans to launch Cologuard Plus in 2025.
They anticipate that it will be covered by Medicare and included in the United States Preventive Services Task Force (USPSTF) guidelines and within quality measures.
A version of this article first appeared on Medscape.com.
CRC Screening After 75: Is Shared Decision-Making Helpful?
TOPLINE:
Physician training in shared decision-making does not increase the proportion of older adults who receive their preferred colorectal cancer (CRC) screening approach, new research suggests.
METHODOLOGY:
- Recent guidelines recommend that shared decision-making be employed when considering whether to stop or continue with CRC screening in adults older than 75 years of age.
- The impact of shared decision-making training on CRC decisions was assessed in 59 physicians and 449 patients (mean age, 80 years) across 36 primary care clinics in Massachusetts and Maine.
- Physicians received shared decision-making training plus pre-visit electronic reminders to discuss CRC screening (intervention) or only the reminders (comparator).
- Shared decision-making training focused on three options: stopping screening, switching to less invasive stool-based testing, and continuing colonoscopy.
- The primary outcome was concordance between patients’ preferred screening method and the screening they actually received, assessed over 12 months through surveys and electronic health records.
TAKEAWAY:
- Stool-based tests were preferred by 35% of patients, colonoscopy by 25%, and no further screening by 21%, whereas 16% were unsure and 4% did not provide a clear preference and were excluded.
- One year after the index visit, 39% of intervention patients and 29% of comparator patients completed CRC screening, a nonsignificant difference.
- Approximately 51% of patients in the intervention group received their preferred screening approach, as did 46% in the comparator group, a difference that was not statistically significant (P = .47).
- Two subgroups in the intervention group were significantly more likely to receive their desired screening: patients with a strong intention to follow through with their preferred approach and those who had longer discussions (5+ minutes) with their physicians about CRC screening.
IN PRACTICE:
“Although the [shared decision-making] training intervention did not make a statistically significant improvement in concordance in this sample, future work to refine and evaluate clinical decision support (in the form of an electronic advisory or reminder), as well as focused [shared decision-making] skills training for [primary care physicians], may promote high-quality, preference-concordant decisions about CRC testing for older adults,” the authors concluded.
SOURCE:
The study, with first author Karen R. Sepucha, PhD, Massachusetts General Hospital, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The study may have been underpowered to detect small differences in concordance rates. The limited racial and ethnic diversity and the high education level of the population restrict the generalizability of these results. The COVID-19 pandemic may have affected the ability of patients to follow through with CRC screening, potentially biasing the results.
DISCLOSURES:
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI). Several authors reported receiving grants from PCORI and other organizations.
A version of this article first appeared on Medscape.com.
TOPLINE:
Physician training in shared decision-making does not increase the proportion of older adults who receive their preferred colorectal cancer (CRC) screening approach, new research suggests.
METHODOLOGY:
- Recent guidelines recommend that shared decision-making be employed when considering whether to stop or continue with CRC screening in adults older than 75 years of age.
- The impact of shared decision-making training on CRC decisions was assessed in 59 physicians and 449 patients (mean age, 80 years) across 36 primary care clinics in Massachusetts and Maine.
- Physicians received shared decision-making training plus pre-visit electronic reminders to discuss CRC screening (intervention) or only the reminders (comparator).
- Shared decision-making training focused on three options: stopping screening, switching to less invasive stool-based testing, and continuing colonoscopy.
- The primary outcome was concordance between patients’ preferred screening method and the screening they actually received, assessed over 12 months through surveys and electronic health records.
TAKEAWAY:
- Stool-based tests were preferred by 35% of patients, colonoscopy by 25%, and no further screening by 21%, whereas 16% were unsure and 4% did not provide a clear preference and were excluded.
- One year after the index visit, 39% of intervention patients and 29% of comparator patients completed CRC screening, a nonsignificant difference.
- Approximately 51% of patients in the intervention group received their preferred screening approach, as did 46% in the comparator group, a difference that was not statistically significant (P = .47).
- Two subgroups in the intervention group were significantly more likely to receive their desired screening: patients with a strong intention to follow through with their preferred approach and those who had longer discussions (5+ minutes) with their physicians about CRC screening.
IN PRACTICE:
“Although the [shared decision-making] training intervention did not make a statistically significant improvement in concordance in this sample, future work to refine and evaluate clinical decision support (in the form of an electronic advisory or reminder), as well as focused [shared decision-making] skills training for [primary care physicians], may promote high-quality, preference-concordant decisions about CRC testing for older adults,” the authors concluded.
SOURCE:
The study, with first author Karen R. Sepucha, PhD, Massachusetts General Hospital, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The study may have been underpowered to detect small differences in concordance rates. The limited racial and ethnic diversity and the high education level of the population restrict the generalizability of these results. The COVID-19 pandemic may have affected the ability of patients to follow through with CRC screening, potentially biasing the results.
DISCLOSURES:
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI). Several authors reported receiving grants from PCORI and other organizations.
A version of this article first appeared on Medscape.com.
TOPLINE:
Physician training in shared decision-making does not increase the proportion of older adults who receive their preferred colorectal cancer (CRC) screening approach, new research suggests.
METHODOLOGY:
- Recent guidelines recommend that shared decision-making be employed when considering whether to stop or continue with CRC screening in adults older than 75 years of age.
- The impact of shared decision-making training on CRC decisions was assessed in 59 physicians and 449 patients (mean age, 80 years) across 36 primary care clinics in Massachusetts and Maine.
- Physicians received shared decision-making training plus pre-visit electronic reminders to discuss CRC screening (intervention) or only the reminders (comparator).
- Shared decision-making training focused on three options: stopping screening, switching to less invasive stool-based testing, and continuing colonoscopy.
- The primary outcome was concordance between patients’ preferred screening method and the screening they actually received, assessed over 12 months through surveys and electronic health records.
TAKEAWAY:
- Stool-based tests were preferred by 35% of patients, colonoscopy by 25%, and no further screening by 21%, whereas 16% were unsure and 4% did not provide a clear preference and were excluded.
- One year after the index visit, 39% of intervention patients and 29% of comparator patients completed CRC screening, a nonsignificant difference.
- Approximately 51% of patients in the intervention group received their preferred screening approach, as did 46% in the comparator group, a difference that was not statistically significant (P = .47).
- Two subgroups in the intervention group were significantly more likely to receive their desired screening: patients with a strong intention to follow through with their preferred approach and those who had longer discussions (5+ minutes) with their physicians about CRC screening.
IN PRACTICE:
“Although the [shared decision-making] training intervention did not make a statistically significant improvement in concordance in this sample, future work to refine and evaluate clinical decision support (in the form of an electronic advisory or reminder), as well as focused [shared decision-making] skills training for [primary care physicians], may promote high-quality, preference-concordant decisions about CRC testing for older adults,” the authors concluded.
SOURCE:
The study, with first author Karen R. Sepucha, PhD, Massachusetts General Hospital, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The study may have been underpowered to detect small differences in concordance rates. The limited racial and ethnic diversity and the high education level of the population restrict the generalizability of these results. The COVID-19 pandemic may have affected the ability of patients to follow through with CRC screening, potentially biasing the results.
DISCLOSURES:
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI). Several authors reported receiving grants from PCORI and other organizations.
A version of this article first appeared on Medscape.com.
Race Adjustments in Algorithms Boost CRC Risk Prediction
TOPLINE:
Accounting for racial disparities, including in the quality of family history data, enhanced the predictive performance of a colorectal cancer (CRC) risk prediction model.
METHODOLOGY:
- The medical community is reevaluating the use of race adjustments in clinical algorithms due to concerns about the exacerbation of health disparities, especially as reported family history data are known to vary by race.
- To understand how adjusting for race affects the accuracy of CRC prediction algorithms, researchers studied data from community health centers across 12 states as part of the Southern Community Cohort Study.
- Researchers compared two screening algorithms that modeled 10-year CRC risk: A race-blind algorithm and a race-adjusted algorithm that included Black race as a main effect and an interaction with family history.
- The primary outcome was the development of CRC within 10 years of enrollment, assessed using data collected from surveys at enrollment and follow-ups, cancer registry data, and National Death Index reports.
- The researchers compared the algorithms’ predictive performance using such measures as area under the receiver operating characteristic curve (AUC) and calibration and also assessed how adjusting for race changed the proportion of Black participants identified as being at high risk for CRC.
TAKEAWAY:
- The study sample included 77,836 adults aged 40-74 years with no history of CRC at baseline.
- Despite having higher cancer rates, Black participants were more likely to report unknown family history (odds ratio [OR], 1.69; P < .001) and less likely to report known positive family history (OR, 0.68; P < .001) than White participants.
- The interaction term between race and family history was 0.56, indicating that reported family history was less predictive of CRC risk in Black participants than in White participants (P = .010).
- Compared with the race-blinded algorithm, the race-adjusted algorithm increased the fraction of Black participants among the predicted high-risk group (66.1% vs 74.4%; P < .001), potentially enhancing access to screening.
- The race-adjusted algorithm improved the goodness of fit (P < .001) and showed a small improvement in AUC among Black participants (0.611 vs 0.608; P = .006).
IN PRACTICE:
“Our analysis found that removing race from colorectal screening predictors could reduce the number of Black patients recommended for screening, which would work against efforts to reduce disparities in colorectal cancer screening and outcomes,” the authors wrote.
SOURCE:
The study, led by Anna Zink, PhD, the University of Chicago Booth School of Business, Chicago, was published online in Proceedings of the National Academy of Sciences of the USA .
LIMITATIONS:
The study did not report any limitations.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Accounting for racial disparities, including in the quality of family history data, enhanced the predictive performance of a colorectal cancer (CRC) risk prediction model.
METHODOLOGY:
- The medical community is reevaluating the use of race adjustments in clinical algorithms due to concerns about the exacerbation of health disparities, especially as reported family history data are known to vary by race.
- To understand how adjusting for race affects the accuracy of CRC prediction algorithms, researchers studied data from community health centers across 12 states as part of the Southern Community Cohort Study.
- Researchers compared two screening algorithms that modeled 10-year CRC risk: A race-blind algorithm and a race-adjusted algorithm that included Black race as a main effect and an interaction with family history.
- The primary outcome was the development of CRC within 10 years of enrollment, assessed using data collected from surveys at enrollment and follow-ups, cancer registry data, and National Death Index reports.
- The researchers compared the algorithms’ predictive performance using such measures as area under the receiver operating characteristic curve (AUC) and calibration and also assessed how adjusting for race changed the proportion of Black participants identified as being at high risk for CRC.
TAKEAWAY:
- The study sample included 77,836 adults aged 40-74 years with no history of CRC at baseline.
- Despite having higher cancer rates, Black participants were more likely to report unknown family history (odds ratio [OR], 1.69; P < .001) and less likely to report known positive family history (OR, 0.68; P < .001) than White participants.
- The interaction term between race and family history was 0.56, indicating that reported family history was less predictive of CRC risk in Black participants than in White participants (P = .010).
- Compared with the race-blinded algorithm, the race-adjusted algorithm increased the fraction of Black participants among the predicted high-risk group (66.1% vs 74.4%; P < .001), potentially enhancing access to screening.
- The race-adjusted algorithm improved the goodness of fit (P < .001) and showed a small improvement in AUC among Black participants (0.611 vs 0.608; P = .006).
IN PRACTICE:
“Our analysis found that removing race from colorectal screening predictors could reduce the number of Black patients recommended for screening, which would work against efforts to reduce disparities in colorectal cancer screening and outcomes,” the authors wrote.
SOURCE:
The study, led by Anna Zink, PhD, the University of Chicago Booth School of Business, Chicago, was published online in Proceedings of the National Academy of Sciences of the USA .
LIMITATIONS:
The study did not report any limitations.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Accounting for racial disparities, including in the quality of family history data, enhanced the predictive performance of a colorectal cancer (CRC) risk prediction model.
METHODOLOGY:
- The medical community is reevaluating the use of race adjustments in clinical algorithms due to concerns about the exacerbation of health disparities, especially as reported family history data are known to vary by race.
- To understand how adjusting for race affects the accuracy of CRC prediction algorithms, researchers studied data from community health centers across 12 states as part of the Southern Community Cohort Study.
- Researchers compared two screening algorithms that modeled 10-year CRC risk: A race-blind algorithm and a race-adjusted algorithm that included Black race as a main effect and an interaction with family history.
- The primary outcome was the development of CRC within 10 years of enrollment, assessed using data collected from surveys at enrollment and follow-ups, cancer registry data, and National Death Index reports.
- The researchers compared the algorithms’ predictive performance using such measures as area under the receiver operating characteristic curve (AUC) and calibration and also assessed how adjusting for race changed the proportion of Black participants identified as being at high risk for CRC.
TAKEAWAY:
- The study sample included 77,836 adults aged 40-74 years with no history of CRC at baseline.
- Despite having higher cancer rates, Black participants were more likely to report unknown family history (odds ratio [OR], 1.69; P < .001) and less likely to report known positive family history (OR, 0.68; P < .001) than White participants.
- The interaction term between race and family history was 0.56, indicating that reported family history was less predictive of CRC risk in Black participants than in White participants (P = .010).
- Compared with the race-blinded algorithm, the race-adjusted algorithm increased the fraction of Black participants among the predicted high-risk group (66.1% vs 74.4%; P < .001), potentially enhancing access to screening.
- The race-adjusted algorithm improved the goodness of fit (P < .001) and showed a small improvement in AUC among Black participants (0.611 vs 0.608; P = .006).
IN PRACTICE:
“Our analysis found that removing race from colorectal screening predictors could reduce the number of Black patients recommended for screening, which would work against efforts to reduce disparities in colorectal cancer screening and outcomes,” the authors wrote.
SOURCE:
The study, led by Anna Zink, PhD, the University of Chicago Booth School of Business, Chicago, was published online in Proceedings of the National Academy of Sciences of the USA .
LIMITATIONS:
The study did not report any limitations.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A CRC Blood Test Is Here. What Does it Mean for Screening?
In July, the US Food and Drug Administration (FDA) approved the first blood-based test to screen for colorectal cancer (CRC).
The FDA’s approval of Shield (Guardant Health) marks a notable achievement, as individuals at average risk now have the option to receive a simple blood test for CRC screening, starting at age 45.
“No one has an excuse anymore not to be screened,” said John Marshall, MD, director of The Ruesch Center for the Cure of Gastrointestinal Cancers and chief medical officer of the Lombardi Comprehensive Cancer Center at the Georgetown University Medical Center in Washington, DC.
The approval was based on findings from the ECLIPSE study, which reported that Shield had 83% sensitivity for CRC and 90% specificity for advanced neoplasia, though only 13% sensitivity for advanced precancerous lesions.
While an exciting option, the test has its pros and cons.
The bad news, however, is that it does a poor job of detecting precancerous lesions. This could snowball if patients decide to replace a colonoscopy — which helps both detect and prevent CRC — with the blood test.
This news organization spoke to experts across three core specialties involved in the screening and treatment of CRC — primary care, gastroenterology, and oncology — to better understand both the potential value and potential pitfalls of this new option.
The interview responses have been condensed and edited for clarity.
What does this FDA approval mean for CRC screening?
David Lieberman, MD, gastroenterologist and professor emeritus at Oregon Health & Science University: Detecting circulating-free DNA associated with CRC in blood is a major scientific breakthrough. The ease of blood testing will appeal to patients and providers.
Folasade May, MD, director of the gastroenterology quality improvement program at the University of California, Los Angeles: The FDA approval means that we continue to broaden the scope of available tools to help reduce the impact of this largely preventable disease.
Dr. Marshall: Colonoscopy is still the gold standard, but we have to recognize that not everyone does it. And that not everyone wants to send their poop in the mail (with a stool-based test). Now there are no more excuses.
Alan Venook, MD, gastrointestinal medical oncologist at the University of California, San Francisco: Although it’s good to have a blood test that’s approved for CRC screening, I don’t think it moves the bar much in terms of screening. I worry about it overpromising and under-delivering. If it could find polyps or premalignant lesions, that would make a big difference; however, at 13%, that doesn’t really register, so this doesn’t really change anything.
Kenny Lin, MD, a family physician at Penn Medicine Lancaster General Health: I see this test as a good option for the 30% people of CRC screening age who are either not being screened or out of date for screening. I’m a little concerned about the people who are already getting recommended screening and may try to switch to this option.
William Golden, MD, internist and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, Arkansas: On a scale of 1-10, I give it a 2. It’s expensive ($900 per test without insurance). It’s also not sensitive for early cancers, which would be its main value. Frankly, there are better strategies to get patients engaged.
What do you see as the pros and cons of this test?
Dr. Lin: The pros are that it’s very convenient for patients, and it’s especially easy for physicians if they have a lab in their office and can avoid a referral where patients may never get the test. However, the data I saw were disappointing, with sensitivity and specificity falling short of the stool-based Cologuard test, which is also not invasive and less likely to miss early cancers, precancerous lesions, and polyps.
Dr. Lieberman: A major con is the detection rate of only 13% for advanced precancerous lesions, which means that this test is not likely to result in much cancer prevention. There is good evidence that if advanced precancerous lesions are detected and removed, many — if not most — CRCs can be prevented.
Dr. Marshall: Another issue is the potential for a false-positive result (which occurs for 1 in every 10 tests). With this result, you would do a scope but can’t find what’s going on. This is a big deal. It’s the first of the blood tests that will be used for cancer screening, and it could be scary for a patient to receive a positive result but not be able to figure out where it’s coming from.
Will you be recommending this test or relying on its results?
Dr. Lieberman: Patients need to understand that the blood test is inferior to every other screening test and, if selected, would result in less protection against developing CRC or dying from CRC than other screening tests. But models suggest that this test will perform better than no screening. Therefore, it is reasonable to offer the test to individuals who decline any other form of screening.
Dr. May: I will do what I’ve always done — after the FDA approval, I wait for the US Preventive Services Task Force (USPSTF) to endorse it. If it does, then I feel it’s my responsibility to tell my patients about all the options they have and stay up to date on how the tests perform, what the pros and cons are, and what reliable information will help patients make the best decision.
Dr. Venook: No, but I could potentially see us moving it into surveillance mode, where CRC survivors or patients undergoing therapy could take it, which might give us a unique second bite of the apple. The test could potentially be of value in identifying early relapse or recurrence, which might give us a heads-up or jump start on follow-up.
Are you concerned that patients won’t return for a colonoscopy after a positive result?
Dr. Golden: This concern is relevant for all tests, including fecal immunochemical test (FIT), but I’ve found that if the patient is willing to do the initial test and it comes back positive, most are willing to do the follow-up. Of course, some folks have issues with this, but now we’ll have a marker in their medical records and can re-engage them through outreach.
Dr. Lieberman: I am concerned that a patient who previously declined to have a colonoscopy may not follow up an abnormal blood test with a colonoscopy. If this occurs, it will render a blood test program ineffective for those patients. Patients should be told upfront that if the test is abnormal, a colonoscopy would be recommended.
Dr. May: This is a big concern that I have. We already have two-step screening processes with FIT, Cologuard, and CT colonography, and strong data show there is attrition. All doctors and companies will need to make it clear that if patients have an abnormal test result, they must undergo a colonoscopy. We must have activated and involved systems of patient follow-up and navigation.
Dr. Lin: I already have some concerns, given that some patients with positive FIT tests don’t get timely follow-up. I see it in my own practice where we call patients to get a colonoscopy, but they don’t take it seriously or their initial counseling wasn’t clear about the possibility of needing a follow-up colonoscopy. If people aren’t being screened for whatever reason in the first place and they get a positive result on the Shield blood test, they might be even less likely to get the necessary follow-up testing afterward.
What might this mean for insurance coverage and costs for patients?
Dr. May: This is an important question because if we don’t have equal access, we create or widen disparities. For insurers to cover Shield, it’ll need to be endorsed by major medical societies, including USPSTF. But what will happen in the beginning is that wealthy patients who can pay out of pocket will use it, while lower-income individuals won’t have access until insurers cover it.
Dr. Golden: I could do 70 (or more) FIT tests for the cost of this one blood test. A FIT test should be offered first. We’re advising the Medicaid program that physicians should be required to explain why a patient doesn’t want a FIT test, prior to covering this blood test.
Dr. Venook: It’s too early to say. Although it’s approved, we now have to look at the monetization factor. At the end of the day, we still need a colonoscopy. The science is impressive, but it doesn’t mean we need to spend $900 doing a blood test.
Dr. Lin: I could see the coverage trajectory being similar to that for Cologuard, which had little coverage when it came out 10 years ago, but eventually, Medicare and commercial coverage happened. With Shield, initially, there will be some coverage gaps, especially with commercial insurance, and I can see insurance companies having concerns, especially because the test is expensive compared with other tests and the return isn’t well known. It could also be a waste of money if people with positive tests don’t receive follow-up colonoscopies.
What else would you like to share that people may not have considered?
Dr. Marshall: These tests could pick up other genes from other cancers. My worry is that people could have another cancer detected but not find it on a colonoscopy and think the blood test must be wrong. Or they’ll do a scan, which could lead to more scans and tests.
Dr. Golden: This test has received a lot of attention and coverage that didn’t discuss other screening options, limitations, or nuances. Let’s face it — we’ll see lots of TV ads about it, but once we start dealing with the total cost of care and alternate payment models, it’s going to be hard for this test to find a niche.
Dr. Venook: This test has only been validated in a population of ages 45 years or older, which is the conventional screening population. We desperately need something that can work in younger people, where CRC rates are increasing. I’d like to see the research move in that direction.
Dr. Lin: I thought it was unique that the FDA Advisory Panel clearly stated this was better than nothing but also should be used as second-line screening. The agency took pains to say this is not a colonoscopy or even equivalent to the fecal tests in use. But they appropriately did approve it because a lot of people aren’t getting anything at all, which is the biggest problem with CRC screening.
A version of this article first appeared on Medscape.com.
In July, the US Food and Drug Administration (FDA) approved the first blood-based test to screen for colorectal cancer (CRC).
The FDA’s approval of Shield (Guardant Health) marks a notable achievement, as individuals at average risk now have the option to receive a simple blood test for CRC screening, starting at age 45.
“No one has an excuse anymore not to be screened,” said John Marshall, MD, director of The Ruesch Center for the Cure of Gastrointestinal Cancers and chief medical officer of the Lombardi Comprehensive Cancer Center at the Georgetown University Medical Center in Washington, DC.
The approval was based on findings from the ECLIPSE study, which reported that Shield had 83% sensitivity for CRC and 90% specificity for advanced neoplasia, though only 13% sensitivity for advanced precancerous lesions.
While an exciting option, the test has its pros and cons.
The bad news, however, is that it does a poor job of detecting precancerous lesions. This could snowball if patients decide to replace a colonoscopy — which helps both detect and prevent CRC — with the blood test.
This news organization spoke to experts across three core specialties involved in the screening and treatment of CRC — primary care, gastroenterology, and oncology — to better understand both the potential value and potential pitfalls of this new option.
The interview responses have been condensed and edited for clarity.
What does this FDA approval mean for CRC screening?
David Lieberman, MD, gastroenterologist and professor emeritus at Oregon Health & Science University: Detecting circulating-free DNA associated with CRC in blood is a major scientific breakthrough. The ease of blood testing will appeal to patients and providers.
Folasade May, MD, director of the gastroenterology quality improvement program at the University of California, Los Angeles: The FDA approval means that we continue to broaden the scope of available tools to help reduce the impact of this largely preventable disease.
Dr. Marshall: Colonoscopy is still the gold standard, but we have to recognize that not everyone does it. And that not everyone wants to send their poop in the mail (with a stool-based test). Now there are no more excuses.
Alan Venook, MD, gastrointestinal medical oncologist at the University of California, San Francisco: Although it’s good to have a blood test that’s approved for CRC screening, I don’t think it moves the bar much in terms of screening. I worry about it overpromising and under-delivering. If it could find polyps or premalignant lesions, that would make a big difference; however, at 13%, that doesn’t really register, so this doesn’t really change anything.
Kenny Lin, MD, a family physician at Penn Medicine Lancaster General Health: I see this test as a good option for the 30% people of CRC screening age who are either not being screened or out of date for screening. I’m a little concerned about the people who are already getting recommended screening and may try to switch to this option.
William Golden, MD, internist and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, Arkansas: On a scale of 1-10, I give it a 2. It’s expensive ($900 per test without insurance). It’s also not sensitive for early cancers, which would be its main value. Frankly, there are better strategies to get patients engaged.
What do you see as the pros and cons of this test?
Dr. Lin: The pros are that it’s very convenient for patients, and it’s especially easy for physicians if they have a lab in their office and can avoid a referral where patients may never get the test. However, the data I saw were disappointing, with sensitivity and specificity falling short of the stool-based Cologuard test, which is also not invasive and less likely to miss early cancers, precancerous lesions, and polyps.
Dr. Lieberman: A major con is the detection rate of only 13% for advanced precancerous lesions, which means that this test is not likely to result in much cancer prevention. There is good evidence that if advanced precancerous lesions are detected and removed, many — if not most — CRCs can be prevented.
Dr. Marshall: Another issue is the potential for a false-positive result (which occurs for 1 in every 10 tests). With this result, you would do a scope but can’t find what’s going on. This is a big deal. It’s the first of the blood tests that will be used for cancer screening, and it could be scary for a patient to receive a positive result but not be able to figure out where it’s coming from.
Will you be recommending this test or relying on its results?
Dr. Lieberman: Patients need to understand that the blood test is inferior to every other screening test and, if selected, would result in less protection against developing CRC or dying from CRC than other screening tests. But models suggest that this test will perform better than no screening. Therefore, it is reasonable to offer the test to individuals who decline any other form of screening.
Dr. May: I will do what I’ve always done — after the FDA approval, I wait for the US Preventive Services Task Force (USPSTF) to endorse it. If it does, then I feel it’s my responsibility to tell my patients about all the options they have and stay up to date on how the tests perform, what the pros and cons are, and what reliable information will help patients make the best decision.
Dr. Venook: No, but I could potentially see us moving it into surveillance mode, where CRC survivors or patients undergoing therapy could take it, which might give us a unique second bite of the apple. The test could potentially be of value in identifying early relapse or recurrence, which might give us a heads-up or jump start on follow-up.
Are you concerned that patients won’t return for a colonoscopy after a positive result?
Dr. Golden: This concern is relevant for all tests, including fecal immunochemical test (FIT), but I’ve found that if the patient is willing to do the initial test and it comes back positive, most are willing to do the follow-up. Of course, some folks have issues with this, but now we’ll have a marker in their medical records and can re-engage them through outreach.
Dr. Lieberman: I am concerned that a patient who previously declined to have a colonoscopy may not follow up an abnormal blood test with a colonoscopy. If this occurs, it will render a blood test program ineffective for those patients. Patients should be told upfront that if the test is abnormal, a colonoscopy would be recommended.
Dr. May: This is a big concern that I have. We already have two-step screening processes with FIT, Cologuard, and CT colonography, and strong data show there is attrition. All doctors and companies will need to make it clear that if patients have an abnormal test result, they must undergo a colonoscopy. We must have activated and involved systems of patient follow-up and navigation.
Dr. Lin: I already have some concerns, given that some patients with positive FIT tests don’t get timely follow-up. I see it in my own practice where we call patients to get a colonoscopy, but they don’t take it seriously or their initial counseling wasn’t clear about the possibility of needing a follow-up colonoscopy. If people aren’t being screened for whatever reason in the first place and they get a positive result on the Shield blood test, they might be even less likely to get the necessary follow-up testing afterward.
What might this mean for insurance coverage and costs for patients?
Dr. May: This is an important question because if we don’t have equal access, we create or widen disparities. For insurers to cover Shield, it’ll need to be endorsed by major medical societies, including USPSTF. But what will happen in the beginning is that wealthy patients who can pay out of pocket will use it, while lower-income individuals won’t have access until insurers cover it.
Dr. Golden: I could do 70 (or more) FIT tests for the cost of this one blood test. A FIT test should be offered first. We’re advising the Medicaid program that physicians should be required to explain why a patient doesn’t want a FIT test, prior to covering this blood test.
Dr. Venook: It’s too early to say. Although it’s approved, we now have to look at the monetization factor. At the end of the day, we still need a colonoscopy. The science is impressive, but it doesn’t mean we need to spend $900 doing a blood test.
Dr. Lin: I could see the coverage trajectory being similar to that for Cologuard, which had little coverage when it came out 10 years ago, but eventually, Medicare and commercial coverage happened. With Shield, initially, there will be some coverage gaps, especially with commercial insurance, and I can see insurance companies having concerns, especially because the test is expensive compared with other tests and the return isn’t well known. It could also be a waste of money if people with positive tests don’t receive follow-up colonoscopies.
What else would you like to share that people may not have considered?
Dr. Marshall: These tests could pick up other genes from other cancers. My worry is that people could have another cancer detected but not find it on a colonoscopy and think the blood test must be wrong. Or they’ll do a scan, which could lead to more scans and tests.
Dr. Golden: This test has received a lot of attention and coverage that didn’t discuss other screening options, limitations, or nuances. Let’s face it — we’ll see lots of TV ads about it, but once we start dealing with the total cost of care and alternate payment models, it’s going to be hard for this test to find a niche.
Dr. Venook: This test has only been validated in a population of ages 45 years or older, which is the conventional screening population. We desperately need something that can work in younger people, where CRC rates are increasing. I’d like to see the research move in that direction.
Dr. Lin: I thought it was unique that the FDA Advisory Panel clearly stated this was better than nothing but also should be used as second-line screening. The agency took pains to say this is not a colonoscopy or even equivalent to the fecal tests in use. But they appropriately did approve it because a lot of people aren’t getting anything at all, which is the biggest problem with CRC screening.
A version of this article first appeared on Medscape.com.
In July, the US Food and Drug Administration (FDA) approved the first blood-based test to screen for colorectal cancer (CRC).
The FDA’s approval of Shield (Guardant Health) marks a notable achievement, as individuals at average risk now have the option to receive a simple blood test for CRC screening, starting at age 45.
“No one has an excuse anymore not to be screened,” said John Marshall, MD, director of The Ruesch Center for the Cure of Gastrointestinal Cancers and chief medical officer of the Lombardi Comprehensive Cancer Center at the Georgetown University Medical Center in Washington, DC.
The approval was based on findings from the ECLIPSE study, which reported that Shield had 83% sensitivity for CRC and 90% specificity for advanced neoplasia, though only 13% sensitivity for advanced precancerous lesions.
While an exciting option, the test has its pros and cons.
The bad news, however, is that it does a poor job of detecting precancerous lesions. This could snowball if patients decide to replace a colonoscopy — which helps both detect and prevent CRC — with the blood test.
This news organization spoke to experts across three core specialties involved in the screening and treatment of CRC — primary care, gastroenterology, and oncology — to better understand both the potential value and potential pitfalls of this new option.
The interview responses have been condensed and edited for clarity.
What does this FDA approval mean for CRC screening?
David Lieberman, MD, gastroenterologist and professor emeritus at Oregon Health & Science University: Detecting circulating-free DNA associated with CRC in blood is a major scientific breakthrough. The ease of blood testing will appeal to patients and providers.
Folasade May, MD, director of the gastroenterology quality improvement program at the University of California, Los Angeles: The FDA approval means that we continue to broaden the scope of available tools to help reduce the impact of this largely preventable disease.
Dr. Marshall: Colonoscopy is still the gold standard, but we have to recognize that not everyone does it. And that not everyone wants to send their poop in the mail (with a stool-based test). Now there are no more excuses.
Alan Venook, MD, gastrointestinal medical oncologist at the University of California, San Francisco: Although it’s good to have a blood test that’s approved for CRC screening, I don’t think it moves the bar much in terms of screening. I worry about it overpromising and under-delivering. If it could find polyps or premalignant lesions, that would make a big difference; however, at 13%, that doesn’t really register, so this doesn’t really change anything.
Kenny Lin, MD, a family physician at Penn Medicine Lancaster General Health: I see this test as a good option for the 30% people of CRC screening age who are either not being screened or out of date for screening. I’m a little concerned about the people who are already getting recommended screening and may try to switch to this option.
William Golden, MD, internist and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, Arkansas: On a scale of 1-10, I give it a 2. It’s expensive ($900 per test without insurance). It’s also not sensitive for early cancers, which would be its main value. Frankly, there are better strategies to get patients engaged.
What do you see as the pros and cons of this test?
Dr. Lin: The pros are that it’s very convenient for patients, and it’s especially easy for physicians if they have a lab in their office and can avoid a referral where patients may never get the test. However, the data I saw were disappointing, with sensitivity and specificity falling short of the stool-based Cologuard test, which is also not invasive and less likely to miss early cancers, precancerous lesions, and polyps.
Dr. Lieberman: A major con is the detection rate of only 13% for advanced precancerous lesions, which means that this test is not likely to result in much cancer prevention. There is good evidence that if advanced precancerous lesions are detected and removed, many — if not most — CRCs can be prevented.
Dr. Marshall: Another issue is the potential for a false-positive result (which occurs for 1 in every 10 tests). With this result, you would do a scope but can’t find what’s going on. This is a big deal. It’s the first of the blood tests that will be used for cancer screening, and it could be scary for a patient to receive a positive result but not be able to figure out where it’s coming from.
Will you be recommending this test or relying on its results?
Dr. Lieberman: Patients need to understand that the blood test is inferior to every other screening test and, if selected, would result in less protection against developing CRC or dying from CRC than other screening tests. But models suggest that this test will perform better than no screening. Therefore, it is reasonable to offer the test to individuals who decline any other form of screening.
Dr. May: I will do what I’ve always done — after the FDA approval, I wait for the US Preventive Services Task Force (USPSTF) to endorse it. If it does, then I feel it’s my responsibility to tell my patients about all the options they have and stay up to date on how the tests perform, what the pros and cons are, and what reliable information will help patients make the best decision.
Dr. Venook: No, but I could potentially see us moving it into surveillance mode, where CRC survivors or patients undergoing therapy could take it, which might give us a unique second bite of the apple. The test could potentially be of value in identifying early relapse or recurrence, which might give us a heads-up or jump start on follow-up.
Are you concerned that patients won’t return for a colonoscopy after a positive result?
Dr. Golden: This concern is relevant for all tests, including fecal immunochemical test (FIT), but I’ve found that if the patient is willing to do the initial test and it comes back positive, most are willing to do the follow-up. Of course, some folks have issues with this, but now we’ll have a marker in their medical records and can re-engage them through outreach.
Dr. Lieberman: I am concerned that a patient who previously declined to have a colonoscopy may not follow up an abnormal blood test with a colonoscopy. If this occurs, it will render a blood test program ineffective for those patients. Patients should be told upfront that if the test is abnormal, a colonoscopy would be recommended.
Dr. May: This is a big concern that I have. We already have two-step screening processes with FIT, Cologuard, and CT colonography, and strong data show there is attrition. All doctors and companies will need to make it clear that if patients have an abnormal test result, they must undergo a colonoscopy. We must have activated and involved systems of patient follow-up and navigation.
Dr. Lin: I already have some concerns, given that some patients with positive FIT tests don’t get timely follow-up. I see it in my own practice where we call patients to get a colonoscopy, but they don’t take it seriously or their initial counseling wasn’t clear about the possibility of needing a follow-up colonoscopy. If people aren’t being screened for whatever reason in the first place and they get a positive result on the Shield blood test, they might be even less likely to get the necessary follow-up testing afterward.
What might this mean for insurance coverage and costs for patients?
Dr. May: This is an important question because if we don’t have equal access, we create or widen disparities. For insurers to cover Shield, it’ll need to be endorsed by major medical societies, including USPSTF. But what will happen in the beginning is that wealthy patients who can pay out of pocket will use it, while lower-income individuals won’t have access until insurers cover it.
Dr. Golden: I could do 70 (or more) FIT tests for the cost of this one blood test. A FIT test should be offered first. We’re advising the Medicaid program that physicians should be required to explain why a patient doesn’t want a FIT test, prior to covering this blood test.
Dr. Venook: It’s too early to say. Although it’s approved, we now have to look at the monetization factor. At the end of the day, we still need a colonoscopy. The science is impressive, but it doesn’t mean we need to spend $900 doing a blood test.
Dr. Lin: I could see the coverage trajectory being similar to that for Cologuard, which had little coverage when it came out 10 years ago, but eventually, Medicare and commercial coverage happened. With Shield, initially, there will be some coverage gaps, especially with commercial insurance, and I can see insurance companies having concerns, especially because the test is expensive compared with other tests and the return isn’t well known. It could also be a waste of money if people with positive tests don’t receive follow-up colonoscopies.
What else would you like to share that people may not have considered?
Dr. Marshall: These tests could pick up other genes from other cancers. My worry is that people could have another cancer detected but not find it on a colonoscopy and think the blood test must be wrong. Or they’ll do a scan, which could lead to more scans and tests.
Dr. Golden: This test has received a lot of attention and coverage that didn’t discuss other screening options, limitations, or nuances. Let’s face it — we’ll see lots of TV ads about it, but once we start dealing with the total cost of care and alternate payment models, it’s going to be hard for this test to find a niche.
Dr. Venook: This test has only been validated in a population of ages 45 years or older, which is the conventional screening population. We desperately need something that can work in younger people, where CRC rates are increasing. I’d like to see the research move in that direction.
Dr. Lin: I thought it was unique that the FDA Advisory Panel clearly stated this was better than nothing but also should be used as second-line screening. The agency took pains to say this is not a colonoscopy or even equivalent to the fecal tests in use. But they appropriately did approve it because a lot of people aren’t getting anything at all, which is the biggest problem with CRC screening.
A version of this article first appeared on Medscape.com.
Five Steps to Improved Colonoscopy Performance
According to several experts who spoke at the American Gastroenterological Association’s Postgraduate Course this spring, which was offered at Digestive Disease Week (DDW), gastroenterologists can take these five steps to improve their performance: Addressing poor bowel prep, improving polyp detection, following the best intervals for polyp surveillance, reducing the environmental impact of gastrointestinal (GI) practice, and implementing artificial intelligence (AI) tools for efficiency and quality.
Addressing Poor Prep
To improve bowel preparation rates, clinicians may consider identifying those at high risk for inadequate prep, which could include known risk factors such as age, body mass index, inpatient status, constipation, tobacco use, and hypertension. However, other variables tend to serve as bigger predictors of inadequate prep, such as the patient’s status regarding cirrhosis, Parkinson’s disease, dementia, diabetes, opioid use, gastroparesis, tricyclics, and colorectal surgery.
Although several prediction models are based on some of these factors — looking at comorbidities, antidepressant use, constipation, and prior abdominal or pelvic surgery — the data don’t indicate whether knowing about or addressing these risks actually leads to better bowel prep, said Brian Jacobson, MD, associate professor of medicine at Harvard Medical School, Boston, and director of program development for gastroenterology at Massachusetts General Hospital in Boston.
Instead, the biggest return-on-investment option is to maximize prep for all patients, he said, especially since every patient has at least some risk of poor prep, either due to the required diet changes, medication considerations, or purgative solution and timing.
To create a state-of-the-art bowel prep process, Dr. Jacobson recommended numerous tactics for all patients: Verbal and written instructions for all components of prep, patient navigation with phone or virtual messaging to guide patients through the process, a low-fiber or all-liquid diet on the day before colonoscopy, and a split-dose 2-L prep regimen. Patients should begin the second half of the split-dose regimen 4-6 hours before colonoscopy and complete it at least 2 hours before the procedure starts, and clinicians should use an irrigation pump during colonoscopy to improve visibility.
Beyond that, Dr. Jacobson noted, higher risk patients can take a split-dose 4-L prep regimen with bisacodyl, a low-fiber diet 2-3 days before colonoscopy, and a clear liquid diet the day before colonoscopy. Using simethicone as an adjunct solution can also reduce bubbles in the colon.
Future tech developments may help clinicians as well, he said, such as using AI to identify patients at high risk and modifying their prep process, creating a personalized prep on a digital platform with videos that guide patients through the process, and using a phone checklist tool to indicate when they’re ready for colonoscopy.
Improving Polyp Detection
Adenoma detection rates (ADR) can be highly variable due to different techniques, technical skills, pattern recognition, interpretation, and experience. New adjunct and AI-based tools can help improve ADR, especially if clinicians want to improve, receive training, and use best-practice techniques.
“In colonoscopy, it’s tricky because it’s not just a blood test or an x-ray. There’s really a lot of technique involved, both cognitive awareness and pattern recognition, as well as our technical skills,” said Tonya Kaltenbach, MD, professor of clinical medicine at the University of California San Francisco and director of advanced endoscopy at the San Francisco VA Health Care System in San Francisco.
For instance, multiple tools and techniques may be needed in real time to interpret a lesion, such as washing, retroflexing, and using better lighting, while paying attention to alerts and noting areas for further inspection and resection.
“This is not innate. It’s a learned skill,” she said. “It’s something we need to intentionally make efforts on and get feedback to improve.”
Improvement starts with using the right mindset for lesion detection, Dr. Kaltenbach said, by having a “reflexive recognition of deconstructed patterns of normal” — following the lines, vessels, and folds and looking for interruptions, abnormal thickness, and mucus caps. On top of that, adjunctive tools such as caps/cuffs and dye chromoendoscopy can help with proper ergonomics, irrigation, and mucosa exposure.
In the past 3 years, real-world studies using AI and computer-assisted detection have shown mixed results, with some demonstrating significant increases in ADR, while others haven’t, she said. However, being willing to try AI and other tools, such as the Endocuff cap, may help improve ADR, standardize interpretation, improve efficiency, and increase reproducibility.
“We’re always better with intentional feedback and deliberate practice,” she said. “Remember that if you improve, you’re protecting the patient from death and reducing interval cancer.”
Following Polyp Surveillance Intervals
The US Multi-Society Task Force on Colorectal Cancer’s recommendations for follow-up after colonoscopy and polypectomy provide valuable information and rationale for how to determine surveillance intervals for patients. However, clinicians still may be unsure what to recommend for some patients — or tell them to come back too soon, leading to unnecessary colonoscopy.
For instance, a 47-year-old woman who presents for her initial screening and has a single 6-mm polyp, which pathology returns as a single adenoma may be considered to be at average risk and suggested to return in 7-10 years. The guidelines seem more obvious for patients with one or two adenomas under 10 mm removed en bloc.
However, once the case details shift into gray areas and include three or four adenomas between 10 and 20 mm, or piecemeal removal, clinicians may differ on their recommendations, said Rajesh N. Keswani, MD, associate professor of medicine at the Northwestern University Feinberg School of Medicine and director of endoscopy for Northwestern Medicine in Chicago. At DDW 2024, Dr. Keswani presented several case examples, often finding various audience opinions.
In addition, he noted, recent studies have found that clinicians may estimate imprecise polyp measurements, struggle to identify sessile serrated polyposis syndrome, and often don’t follow evidence-based guidelines.
“Why do we ignore the guidelines? There’s this perception that a patient has risk factors that aren’t addressed by the guidelines, with regards to family history or a distant history of a large polyp that we don’t want to leave to the usual intervals,” he said. “We feel uncomfortable, even with our meticulous colonoscopy, telling people to come back in 10 years.”
To improve guideline adherence, Dr. Keswani suggested providing additional education, implementing an automated surveillance calculator, and using guidelines at the point of care. At Northwestern, for instance, clinicians use a hyperlink with an interpreted version of the guidelines with prior colonoscopy considerations. Overall though, practitioners should feel comfortable leaning toward longer surveillance intervals, he noted.
“More effort should be spent on getting unscreened patients in for colonoscopy than bringing back low-risk patients too early,” he said.
Reducing Environmental Effects
In recent waste audits of endoscopy rooms, providers generate 1-3 kg of waste per procedure, which would fill 117 soccer fields to a depth of 1 m, based on 18 million procedures in the United States per year. This waste comes from procedure-related equipment, administration, medications, travel of patients and staff, and infrastructure with systems such as air conditioning. Taking steps toward a green practice can reduce waste and the carbon footprint of healthcare.
“When we think about improving colonoscopy performance, the goal is to prevent colon cancer death, but when we expand that, we have to apply sustainable practices as a domain of quality,” said Heiko Pohl, MD, professor of medicine at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire, and a gastroenterologist at White River Junction VA Medical Center in White River Junction, Vermont.
The GI Multisociety Strategic Plan on Environmental Sustainability suggests a 5-year initiative to improve sustainability and reduce waste across seven domains — clinical setting, education, research, society efforts, intersociety efforts, industry, and advocacy.
For instance, clinicians can take the biggest step toward sustainability by avoiding unneeded colonoscopies, Dr. Pohl said, noting that between 20% and 30% aren’t appropriate or indicated. Instead, practitioners can implement longer surveillance intervals, adhere to guidelines, and consider alternative tests, such as the fecal immunochemical test, fecal DNA, blood-based tests, and CT colonography, where relevant.
Clinicians can also rethink their approach to resection, such as using a snare first instead of forceps to reduce single-instrument use, using clip closure only when it’s truly indicated, and implementing AI-assisted optical diagnosis to help with leaving rectosigmoid polyps in place.
In terms of physical waste, practices may also reconsider how they sort bins and biohazards, looking at new ways to dispose of regulated medical waste, sharps, recyclables, and typical trash. Waste audits can help find ways to reduce paper, combine procedures, and create more efficient use of endoscopy rooms.
“We are really in a very precarious situation,” Dr. Pohl said. “It’s our generation that has a responsibility to change the course for our children’s and grandchildren’s sake.”
AI for Quality And Efficiency
Moving forward, AI tools will likely become more popular in various parts of GI practice, by assisting with documentation, spotting polyps, tracking mucosal surfaces, providing optical histopathology, and supervising performance through high-quality feedback.
“Endoscopy has reached the limits of human visual capacity, where seeing more pixels won’t necessarily improve clinical diagnosis. What’s next for elevating the care of patients really is AI,” said Jason B. Samarasena, MD, professor of medicine and program director of the interventional endoscopy training program at the University of California Irvine in Irvine, California.
As practices adopt AI-based systems, however, clinicians should be cautious about a false sense of comfort or “alarm fatigue” if bounding boxes become distracting. Instead, new tools need to be adopted as a “physician-AI hybrid,” with the endoscopist in mind, particularly if helpful for performing a better exam by watching withdrawal time or endoscope slippage.
“In real-world practice, this is being implemented without attention to endoscopist inclination and behavior,” he said. “Having a better understanding of physician attitudes could yield more optimal results.”
Notably, AI-assisted tools should be viewed akin to spell-check, which signals to the endoscopist when to pay attention and double-check an area — but primarily relies on the expert to do a high-quality exam, said Aasma Shaukat, MD, professor of medicine and director of GI outcomes research at the NYU Grossman School of Medicine, New York City.
“This should be an adjunct or an additional tool, not a replacement tool,” she added. “This doesn’t mean to stop doing astute observation.”
Future tools show promise in terms of tracking additional data related to prep quality, cecal landmarks, polyp size, mucosa exposure, histology prediction, and complete resection. These automated reports could also link to real-time dashboards, hospital or national registries, and reimbursement systems, Dr. Shaukat noted.
“At the end of the day, our interests are aligned,” she said. “Everybody cares about quality, patient satisfaction, and reimbursement, and with that goal in mind, I think some of the tools can be applied to show how we can achieve those principles together.”
Dr. Jacobson, Dr. Kaltenbach, Dr. Keswani, Dr. Pohl, Dr. Samarasena, and Dr. Shaukat reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
According to several experts who spoke at the American Gastroenterological Association’s Postgraduate Course this spring, which was offered at Digestive Disease Week (DDW), gastroenterologists can take these five steps to improve their performance: Addressing poor bowel prep, improving polyp detection, following the best intervals for polyp surveillance, reducing the environmental impact of gastrointestinal (GI) practice, and implementing artificial intelligence (AI) tools for efficiency and quality.
Addressing Poor Prep
To improve bowel preparation rates, clinicians may consider identifying those at high risk for inadequate prep, which could include known risk factors such as age, body mass index, inpatient status, constipation, tobacco use, and hypertension. However, other variables tend to serve as bigger predictors of inadequate prep, such as the patient’s status regarding cirrhosis, Parkinson’s disease, dementia, diabetes, opioid use, gastroparesis, tricyclics, and colorectal surgery.
Although several prediction models are based on some of these factors — looking at comorbidities, antidepressant use, constipation, and prior abdominal or pelvic surgery — the data don’t indicate whether knowing about or addressing these risks actually leads to better bowel prep, said Brian Jacobson, MD, associate professor of medicine at Harvard Medical School, Boston, and director of program development for gastroenterology at Massachusetts General Hospital in Boston.
Instead, the biggest return-on-investment option is to maximize prep for all patients, he said, especially since every patient has at least some risk of poor prep, either due to the required diet changes, medication considerations, or purgative solution and timing.
To create a state-of-the-art bowel prep process, Dr. Jacobson recommended numerous tactics for all patients: Verbal and written instructions for all components of prep, patient navigation with phone or virtual messaging to guide patients through the process, a low-fiber or all-liquid diet on the day before colonoscopy, and a split-dose 2-L prep regimen. Patients should begin the second half of the split-dose regimen 4-6 hours before colonoscopy and complete it at least 2 hours before the procedure starts, and clinicians should use an irrigation pump during colonoscopy to improve visibility.
Beyond that, Dr. Jacobson noted, higher risk patients can take a split-dose 4-L prep regimen with bisacodyl, a low-fiber diet 2-3 days before colonoscopy, and a clear liquid diet the day before colonoscopy. Using simethicone as an adjunct solution can also reduce bubbles in the colon.
Future tech developments may help clinicians as well, he said, such as using AI to identify patients at high risk and modifying their prep process, creating a personalized prep on a digital platform with videos that guide patients through the process, and using a phone checklist tool to indicate when they’re ready for colonoscopy.
Improving Polyp Detection
Adenoma detection rates (ADR) can be highly variable due to different techniques, technical skills, pattern recognition, interpretation, and experience. New adjunct and AI-based tools can help improve ADR, especially if clinicians want to improve, receive training, and use best-practice techniques.
“In colonoscopy, it’s tricky because it’s not just a blood test or an x-ray. There’s really a lot of technique involved, both cognitive awareness and pattern recognition, as well as our technical skills,” said Tonya Kaltenbach, MD, professor of clinical medicine at the University of California San Francisco and director of advanced endoscopy at the San Francisco VA Health Care System in San Francisco.
For instance, multiple tools and techniques may be needed in real time to interpret a lesion, such as washing, retroflexing, and using better lighting, while paying attention to alerts and noting areas for further inspection and resection.
“This is not innate. It’s a learned skill,” she said. “It’s something we need to intentionally make efforts on and get feedback to improve.”
Improvement starts with using the right mindset for lesion detection, Dr. Kaltenbach said, by having a “reflexive recognition of deconstructed patterns of normal” — following the lines, vessels, and folds and looking for interruptions, abnormal thickness, and mucus caps. On top of that, adjunctive tools such as caps/cuffs and dye chromoendoscopy can help with proper ergonomics, irrigation, and mucosa exposure.
In the past 3 years, real-world studies using AI and computer-assisted detection have shown mixed results, with some demonstrating significant increases in ADR, while others haven’t, she said. However, being willing to try AI and other tools, such as the Endocuff cap, may help improve ADR, standardize interpretation, improve efficiency, and increase reproducibility.
“We’re always better with intentional feedback and deliberate practice,” she said. “Remember that if you improve, you’re protecting the patient from death and reducing interval cancer.”
Following Polyp Surveillance Intervals
The US Multi-Society Task Force on Colorectal Cancer’s recommendations for follow-up after colonoscopy and polypectomy provide valuable information and rationale for how to determine surveillance intervals for patients. However, clinicians still may be unsure what to recommend for some patients — or tell them to come back too soon, leading to unnecessary colonoscopy.
For instance, a 47-year-old woman who presents for her initial screening and has a single 6-mm polyp, which pathology returns as a single adenoma may be considered to be at average risk and suggested to return in 7-10 years. The guidelines seem more obvious for patients with one or two adenomas under 10 mm removed en bloc.
However, once the case details shift into gray areas and include three or four adenomas between 10 and 20 mm, or piecemeal removal, clinicians may differ on their recommendations, said Rajesh N. Keswani, MD, associate professor of medicine at the Northwestern University Feinberg School of Medicine and director of endoscopy for Northwestern Medicine in Chicago. At DDW 2024, Dr. Keswani presented several case examples, often finding various audience opinions.
In addition, he noted, recent studies have found that clinicians may estimate imprecise polyp measurements, struggle to identify sessile serrated polyposis syndrome, and often don’t follow evidence-based guidelines.
“Why do we ignore the guidelines? There’s this perception that a patient has risk factors that aren’t addressed by the guidelines, with regards to family history or a distant history of a large polyp that we don’t want to leave to the usual intervals,” he said. “We feel uncomfortable, even with our meticulous colonoscopy, telling people to come back in 10 years.”
To improve guideline adherence, Dr. Keswani suggested providing additional education, implementing an automated surveillance calculator, and using guidelines at the point of care. At Northwestern, for instance, clinicians use a hyperlink with an interpreted version of the guidelines with prior colonoscopy considerations. Overall though, practitioners should feel comfortable leaning toward longer surveillance intervals, he noted.
“More effort should be spent on getting unscreened patients in for colonoscopy than bringing back low-risk patients too early,” he said.
Reducing Environmental Effects
In recent waste audits of endoscopy rooms, providers generate 1-3 kg of waste per procedure, which would fill 117 soccer fields to a depth of 1 m, based on 18 million procedures in the United States per year. This waste comes from procedure-related equipment, administration, medications, travel of patients and staff, and infrastructure with systems such as air conditioning. Taking steps toward a green practice can reduce waste and the carbon footprint of healthcare.
“When we think about improving colonoscopy performance, the goal is to prevent colon cancer death, but when we expand that, we have to apply sustainable practices as a domain of quality,” said Heiko Pohl, MD, professor of medicine at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire, and a gastroenterologist at White River Junction VA Medical Center in White River Junction, Vermont.
The GI Multisociety Strategic Plan on Environmental Sustainability suggests a 5-year initiative to improve sustainability and reduce waste across seven domains — clinical setting, education, research, society efforts, intersociety efforts, industry, and advocacy.
For instance, clinicians can take the biggest step toward sustainability by avoiding unneeded colonoscopies, Dr. Pohl said, noting that between 20% and 30% aren’t appropriate or indicated. Instead, practitioners can implement longer surveillance intervals, adhere to guidelines, and consider alternative tests, such as the fecal immunochemical test, fecal DNA, blood-based tests, and CT colonography, where relevant.
Clinicians can also rethink their approach to resection, such as using a snare first instead of forceps to reduce single-instrument use, using clip closure only when it’s truly indicated, and implementing AI-assisted optical diagnosis to help with leaving rectosigmoid polyps in place.
In terms of physical waste, practices may also reconsider how they sort bins and biohazards, looking at new ways to dispose of regulated medical waste, sharps, recyclables, and typical trash. Waste audits can help find ways to reduce paper, combine procedures, and create more efficient use of endoscopy rooms.
“We are really in a very precarious situation,” Dr. Pohl said. “It’s our generation that has a responsibility to change the course for our children’s and grandchildren’s sake.”
AI for Quality And Efficiency
Moving forward, AI tools will likely become more popular in various parts of GI practice, by assisting with documentation, spotting polyps, tracking mucosal surfaces, providing optical histopathology, and supervising performance through high-quality feedback.
“Endoscopy has reached the limits of human visual capacity, where seeing more pixels won’t necessarily improve clinical diagnosis. What’s next for elevating the care of patients really is AI,” said Jason B. Samarasena, MD, professor of medicine and program director of the interventional endoscopy training program at the University of California Irvine in Irvine, California.
As practices adopt AI-based systems, however, clinicians should be cautious about a false sense of comfort or “alarm fatigue” if bounding boxes become distracting. Instead, new tools need to be adopted as a “physician-AI hybrid,” with the endoscopist in mind, particularly if helpful for performing a better exam by watching withdrawal time or endoscope slippage.
“In real-world practice, this is being implemented without attention to endoscopist inclination and behavior,” he said. “Having a better understanding of physician attitudes could yield more optimal results.”
Notably, AI-assisted tools should be viewed akin to spell-check, which signals to the endoscopist when to pay attention and double-check an area — but primarily relies on the expert to do a high-quality exam, said Aasma Shaukat, MD, professor of medicine and director of GI outcomes research at the NYU Grossman School of Medicine, New York City.
“This should be an adjunct or an additional tool, not a replacement tool,” she added. “This doesn’t mean to stop doing astute observation.”
Future tools show promise in terms of tracking additional data related to prep quality, cecal landmarks, polyp size, mucosa exposure, histology prediction, and complete resection. These automated reports could also link to real-time dashboards, hospital or national registries, and reimbursement systems, Dr. Shaukat noted.
“At the end of the day, our interests are aligned,” she said. “Everybody cares about quality, patient satisfaction, and reimbursement, and with that goal in mind, I think some of the tools can be applied to show how we can achieve those principles together.”
Dr. Jacobson, Dr. Kaltenbach, Dr. Keswani, Dr. Pohl, Dr. Samarasena, and Dr. Shaukat reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
According to several experts who spoke at the American Gastroenterological Association’s Postgraduate Course this spring, which was offered at Digestive Disease Week (DDW), gastroenterologists can take these five steps to improve their performance: Addressing poor bowel prep, improving polyp detection, following the best intervals for polyp surveillance, reducing the environmental impact of gastrointestinal (GI) practice, and implementing artificial intelligence (AI) tools for efficiency and quality.
Addressing Poor Prep
To improve bowel preparation rates, clinicians may consider identifying those at high risk for inadequate prep, which could include known risk factors such as age, body mass index, inpatient status, constipation, tobacco use, and hypertension. However, other variables tend to serve as bigger predictors of inadequate prep, such as the patient’s status regarding cirrhosis, Parkinson’s disease, dementia, diabetes, opioid use, gastroparesis, tricyclics, and colorectal surgery.
Although several prediction models are based on some of these factors — looking at comorbidities, antidepressant use, constipation, and prior abdominal or pelvic surgery — the data don’t indicate whether knowing about or addressing these risks actually leads to better bowel prep, said Brian Jacobson, MD, associate professor of medicine at Harvard Medical School, Boston, and director of program development for gastroenterology at Massachusetts General Hospital in Boston.
Instead, the biggest return-on-investment option is to maximize prep for all patients, he said, especially since every patient has at least some risk of poor prep, either due to the required diet changes, medication considerations, or purgative solution and timing.
To create a state-of-the-art bowel prep process, Dr. Jacobson recommended numerous tactics for all patients: Verbal and written instructions for all components of prep, patient navigation with phone or virtual messaging to guide patients through the process, a low-fiber or all-liquid diet on the day before colonoscopy, and a split-dose 2-L prep regimen. Patients should begin the second half of the split-dose regimen 4-6 hours before colonoscopy and complete it at least 2 hours before the procedure starts, and clinicians should use an irrigation pump during colonoscopy to improve visibility.
Beyond that, Dr. Jacobson noted, higher risk patients can take a split-dose 4-L prep regimen with bisacodyl, a low-fiber diet 2-3 days before colonoscopy, and a clear liquid diet the day before colonoscopy. Using simethicone as an adjunct solution can also reduce bubbles in the colon.
Future tech developments may help clinicians as well, he said, such as using AI to identify patients at high risk and modifying their prep process, creating a personalized prep on a digital platform with videos that guide patients through the process, and using a phone checklist tool to indicate when they’re ready for colonoscopy.
Improving Polyp Detection
Adenoma detection rates (ADR) can be highly variable due to different techniques, technical skills, pattern recognition, interpretation, and experience. New adjunct and AI-based tools can help improve ADR, especially if clinicians want to improve, receive training, and use best-practice techniques.
“In colonoscopy, it’s tricky because it’s not just a blood test or an x-ray. There’s really a lot of technique involved, both cognitive awareness and pattern recognition, as well as our technical skills,” said Tonya Kaltenbach, MD, professor of clinical medicine at the University of California San Francisco and director of advanced endoscopy at the San Francisco VA Health Care System in San Francisco.
For instance, multiple tools and techniques may be needed in real time to interpret a lesion, such as washing, retroflexing, and using better lighting, while paying attention to alerts and noting areas for further inspection and resection.
“This is not innate. It’s a learned skill,” she said. “It’s something we need to intentionally make efforts on and get feedback to improve.”
Improvement starts with using the right mindset for lesion detection, Dr. Kaltenbach said, by having a “reflexive recognition of deconstructed patterns of normal” — following the lines, vessels, and folds and looking for interruptions, abnormal thickness, and mucus caps. On top of that, adjunctive tools such as caps/cuffs and dye chromoendoscopy can help with proper ergonomics, irrigation, and mucosa exposure.
In the past 3 years, real-world studies using AI and computer-assisted detection have shown mixed results, with some demonstrating significant increases in ADR, while others haven’t, she said. However, being willing to try AI and other tools, such as the Endocuff cap, may help improve ADR, standardize interpretation, improve efficiency, and increase reproducibility.
“We’re always better with intentional feedback and deliberate practice,” she said. “Remember that if you improve, you’re protecting the patient from death and reducing interval cancer.”
Following Polyp Surveillance Intervals
The US Multi-Society Task Force on Colorectal Cancer’s recommendations for follow-up after colonoscopy and polypectomy provide valuable information and rationale for how to determine surveillance intervals for patients. However, clinicians still may be unsure what to recommend for some patients — or tell them to come back too soon, leading to unnecessary colonoscopy.
For instance, a 47-year-old woman who presents for her initial screening and has a single 6-mm polyp, which pathology returns as a single adenoma may be considered to be at average risk and suggested to return in 7-10 years. The guidelines seem more obvious for patients with one or two adenomas under 10 mm removed en bloc.
However, once the case details shift into gray areas and include three or four adenomas between 10 and 20 mm, or piecemeal removal, clinicians may differ on their recommendations, said Rajesh N. Keswani, MD, associate professor of medicine at the Northwestern University Feinberg School of Medicine and director of endoscopy for Northwestern Medicine in Chicago. At DDW 2024, Dr. Keswani presented several case examples, often finding various audience opinions.
In addition, he noted, recent studies have found that clinicians may estimate imprecise polyp measurements, struggle to identify sessile serrated polyposis syndrome, and often don’t follow evidence-based guidelines.
“Why do we ignore the guidelines? There’s this perception that a patient has risk factors that aren’t addressed by the guidelines, with regards to family history or a distant history of a large polyp that we don’t want to leave to the usual intervals,” he said. “We feel uncomfortable, even with our meticulous colonoscopy, telling people to come back in 10 years.”
To improve guideline adherence, Dr. Keswani suggested providing additional education, implementing an automated surveillance calculator, and using guidelines at the point of care. At Northwestern, for instance, clinicians use a hyperlink with an interpreted version of the guidelines with prior colonoscopy considerations. Overall though, practitioners should feel comfortable leaning toward longer surveillance intervals, he noted.
“More effort should be spent on getting unscreened patients in for colonoscopy than bringing back low-risk patients too early,” he said.
Reducing Environmental Effects
In recent waste audits of endoscopy rooms, providers generate 1-3 kg of waste per procedure, which would fill 117 soccer fields to a depth of 1 m, based on 18 million procedures in the United States per year. This waste comes from procedure-related equipment, administration, medications, travel of patients and staff, and infrastructure with systems such as air conditioning. Taking steps toward a green practice can reduce waste and the carbon footprint of healthcare.
“When we think about improving colonoscopy performance, the goal is to prevent colon cancer death, but when we expand that, we have to apply sustainable practices as a domain of quality,” said Heiko Pohl, MD, professor of medicine at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire, and a gastroenterologist at White River Junction VA Medical Center in White River Junction, Vermont.
The GI Multisociety Strategic Plan on Environmental Sustainability suggests a 5-year initiative to improve sustainability and reduce waste across seven domains — clinical setting, education, research, society efforts, intersociety efforts, industry, and advocacy.
For instance, clinicians can take the biggest step toward sustainability by avoiding unneeded colonoscopies, Dr. Pohl said, noting that between 20% and 30% aren’t appropriate or indicated. Instead, practitioners can implement longer surveillance intervals, adhere to guidelines, and consider alternative tests, such as the fecal immunochemical test, fecal DNA, blood-based tests, and CT colonography, where relevant.
Clinicians can also rethink their approach to resection, such as using a snare first instead of forceps to reduce single-instrument use, using clip closure only when it’s truly indicated, and implementing AI-assisted optical diagnosis to help with leaving rectosigmoid polyps in place.
In terms of physical waste, practices may also reconsider how they sort bins and biohazards, looking at new ways to dispose of regulated medical waste, sharps, recyclables, and typical trash. Waste audits can help find ways to reduce paper, combine procedures, and create more efficient use of endoscopy rooms.
“We are really in a very precarious situation,” Dr. Pohl said. “It’s our generation that has a responsibility to change the course for our children’s and grandchildren’s sake.”
AI for Quality And Efficiency
Moving forward, AI tools will likely become more popular in various parts of GI practice, by assisting with documentation, spotting polyps, tracking mucosal surfaces, providing optical histopathology, and supervising performance through high-quality feedback.
“Endoscopy has reached the limits of human visual capacity, where seeing more pixels won’t necessarily improve clinical diagnosis. What’s next for elevating the care of patients really is AI,” said Jason B. Samarasena, MD, professor of medicine and program director of the interventional endoscopy training program at the University of California Irvine in Irvine, California.
As practices adopt AI-based systems, however, clinicians should be cautious about a false sense of comfort or “alarm fatigue” if bounding boxes become distracting. Instead, new tools need to be adopted as a “physician-AI hybrid,” with the endoscopist in mind, particularly if helpful for performing a better exam by watching withdrawal time or endoscope slippage.
“In real-world practice, this is being implemented without attention to endoscopist inclination and behavior,” he said. “Having a better understanding of physician attitudes could yield more optimal results.”
Notably, AI-assisted tools should be viewed akin to spell-check, which signals to the endoscopist when to pay attention and double-check an area — but primarily relies on the expert to do a high-quality exam, said Aasma Shaukat, MD, professor of medicine and director of GI outcomes research at the NYU Grossman School of Medicine, New York City.
“This should be an adjunct or an additional tool, not a replacement tool,” she added. “This doesn’t mean to stop doing astute observation.”
Future tools show promise in terms of tracking additional data related to prep quality, cecal landmarks, polyp size, mucosa exposure, histology prediction, and complete resection. These automated reports could also link to real-time dashboards, hospital or national registries, and reimbursement systems, Dr. Shaukat noted.
“At the end of the day, our interests are aligned,” she said. “Everybody cares about quality, patient satisfaction, and reimbursement, and with that goal in mind, I think some of the tools can be applied to show how we can achieve those principles together.”
Dr. Jacobson, Dr. Kaltenbach, Dr. Keswani, Dr. Pohl, Dr. Samarasena, and Dr. Shaukat reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Fecal Immunochemical Test Performance for CRC Screening Varies Widely
, new research suggests.
In a comparative performance analysis of five commonly used FITs for colorectal cancer (CRC) screening, researchers found statistically significant differences in positivity rates, sensitivity, and specificity, as well as important differences in rates of unusable tests.
“Our findings have practical importance for FIT-based screening programs as these differences affect the need for repeated FIT, the yield of ACN detection, and the number of diagnostic colonoscopies that would be required to follow-up on abnormal findings,” wrote the researchers, led by Barcey T. Levy, MD, PhD, with University of Iowa, Iowa City.
The study was published online in Annals of Internal Medicine.
Wide Variation Found
Despite widespread use of FITs for CRC screening, there is limited data to help guide test selection. Understanding the comparative performance of different FITs is “crucial” for a successful FIT-based screening program, the researchers wrote.
Dr. Levy and colleagues directly compared the performance of five commercially available FITs — including four qualitative tests (Hemoccult ICT, Hemosure iFOB, OC-Light S FIT, and QuickVue iFOB) and one quantitative test (OC-Auto FIT) — using colonoscopy as the reference standard.
Participants included a diverse group of 3761 adults (mean age, 62 years; 63% women). Each participant was given all five tests and completed them using the same stool sample. They sent the tests by first class mail to a central location, where FITs were analyzed by a trained professional on the day of receipt.
The primary outcome was test performance (sensitivity and specificity) for ACN, defined as advanced polyps or CRC.
A total of 320 participants (8.5%) were found to have ACN based on colonoscopy results, including nine with CRC (0.2%) — rates that are similar to those found in other studies.
The sensitivity for detecting ACN ranged from 10.1% (Hemoccult ICT) to 36.7% (OC-Light S FIT), and specificity varied from 85.5% (OC-Light S FIT) to 96.6% (Hemoccult ICT).
“Given the variation in FIT cutoffs reported by manufacturers, it is not surprising that tests with lower cutoffs (such as OC-Light S FIT) had higher sensitivity than tests with higher cutoffs (such as Hemoccult ICT),” Dr. Levy and colleagues wrote.
Test positivity rates varied fourfold across FITs, from 3.9% for Hemoccult ICT to 16.4% for OC-Light S FIT.
The rates of tests deemed unevaluable (due to factors such as indeterminant results or user mistakes) ranged from 0.2% for OC-Auto FIT to 2.5% for QuickVue iFOB.
The highest positive predictive value (PPV) was observed with OC-Auto FIT (28.9%) and the lowest with Hemosure iFOB (18.2%). The negative predictive value was similar across tests, ranging from 92.2% to 93.3%, indicating consistent performance in ruling out disease.
The study also identified significant differences in test sensitivity based on factors such as the location of neoplasia (higher sensitivity for distal lesions) and patient characteristics (higher sensitivity in people with higher body mass index and lower income).
Dr. Levy and colleagues said their findings have implications both in terms of clinical benefits and cost-effectiveness of CRC screening using FITs.
“Tests with lower sensitivity will miss more patients with CRC and advanced polyps, and tests with higher sensitivity and lower PPV will require more colonoscopies to detect patients with actionable findings,” they wrote.
‘Jaw-Dropping’ Results
The sensitivity results are “jaw-dropping,” Robert Smith, PhD, senior vice-president for cancer screening at the American Cancer Society, said in an interview. “A patient should have at least a 50/50 chance of having their colorectal cancer detected with a stool test at the time of testing.”
“What these numbers show is that the level that the manufacturers believe their test is performing is not reproduced,” Dr. Smith added.
This study adds to “concerns that have been raised about the inherent limitations and the performance of these tests that have been cleared for use and that are supposed to be lifesaving,” he said.
Clearance by the US Food and Drug Administration should mean that there’s essentially “no risk to using the test in terms of the test itself being harmful,” Dr. Smith said. But that’s not the case with FITs “because it’s harmful if you have cancer and your test doesn’t find it.”
By way of study limitations, Dr. Levy and colleagues said it’s important to note that they did not evaluate the “programmatic” sensitivity of repeating FIT testing every 1-2 years, as is generally recommended in screening guidelines. Therefore, the sensitivity of a single FIT may be lower than that of a repeated FIT. Also, variability in the FIT collection process by participants might have affected the results.
The study had no commercial funding. Disclosures for authors are available with the original article. Dr. Smith had no relevant disclosures.
A version of this article appeared on Medscape.com.
, new research suggests.
In a comparative performance analysis of five commonly used FITs for colorectal cancer (CRC) screening, researchers found statistically significant differences in positivity rates, sensitivity, and specificity, as well as important differences in rates of unusable tests.
“Our findings have practical importance for FIT-based screening programs as these differences affect the need for repeated FIT, the yield of ACN detection, and the number of diagnostic colonoscopies that would be required to follow-up on abnormal findings,” wrote the researchers, led by Barcey T. Levy, MD, PhD, with University of Iowa, Iowa City.
The study was published online in Annals of Internal Medicine.
Wide Variation Found
Despite widespread use of FITs for CRC screening, there is limited data to help guide test selection. Understanding the comparative performance of different FITs is “crucial” for a successful FIT-based screening program, the researchers wrote.
Dr. Levy and colleagues directly compared the performance of five commercially available FITs — including four qualitative tests (Hemoccult ICT, Hemosure iFOB, OC-Light S FIT, and QuickVue iFOB) and one quantitative test (OC-Auto FIT) — using colonoscopy as the reference standard.
Participants included a diverse group of 3761 adults (mean age, 62 years; 63% women). Each participant was given all five tests and completed them using the same stool sample. They sent the tests by first class mail to a central location, where FITs were analyzed by a trained professional on the day of receipt.
The primary outcome was test performance (sensitivity and specificity) for ACN, defined as advanced polyps or CRC.
A total of 320 participants (8.5%) were found to have ACN based on colonoscopy results, including nine with CRC (0.2%) — rates that are similar to those found in other studies.
The sensitivity for detecting ACN ranged from 10.1% (Hemoccult ICT) to 36.7% (OC-Light S FIT), and specificity varied from 85.5% (OC-Light S FIT) to 96.6% (Hemoccult ICT).
“Given the variation in FIT cutoffs reported by manufacturers, it is not surprising that tests with lower cutoffs (such as OC-Light S FIT) had higher sensitivity than tests with higher cutoffs (such as Hemoccult ICT),” Dr. Levy and colleagues wrote.
Test positivity rates varied fourfold across FITs, from 3.9% for Hemoccult ICT to 16.4% for OC-Light S FIT.
The rates of tests deemed unevaluable (due to factors such as indeterminant results or user mistakes) ranged from 0.2% for OC-Auto FIT to 2.5% for QuickVue iFOB.
The highest positive predictive value (PPV) was observed with OC-Auto FIT (28.9%) and the lowest with Hemosure iFOB (18.2%). The negative predictive value was similar across tests, ranging from 92.2% to 93.3%, indicating consistent performance in ruling out disease.
The study also identified significant differences in test sensitivity based on factors such as the location of neoplasia (higher sensitivity for distal lesions) and patient characteristics (higher sensitivity in people with higher body mass index and lower income).
Dr. Levy and colleagues said their findings have implications both in terms of clinical benefits and cost-effectiveness of CRC screening using FITs.
“Tests with lower sensitivity will miss more patients with CRC and advanced polyps, and tests with higher sensitivity and lower PPV will require more colonoscopies to detect patients with actionable findings,” they wrote.
‘Jaw-Dropping’ Results
The sensitivity results are “jaw-dropping,” Robert Smith, PhD, senior vice-president for cancer screening at the American Cancer Society, said in an interview. “A patient should have at least a 50/50 chance of having their colorectal cancer detected with a stool test at the time of testing.”
“What these numbers show is that the level that the manufacturers believe their test is performing is not reproduced,” Dr. Smith added.
This study adds to “concerns that have been raised about the inherent limitations and the performance of these tests that have been cleared for use and that are supposed to be lifesaving,” he said.
Clearance by the US Food and Drug Administration should mean that there’s essentially “no risk to using the test in terms of the test itself being harmful,” Dr. Smith said. But that’s not the case with FITs “because it’s harmful if you have cancer and your test doesn’t find it.”
By way of study limitations, Dr. Levy and colleagues said it’s important to note that they did not evaluate the “programmatic” sensitivity of repeating FIT testing every 1-2 years, as is generally recommended in screening guidelines. Therefore, the sensitivity of a single FIT may be lower than that of a repeated FIT. Also, variability in the FIT collection process by participants might have affected the results.
The study had no commercial funding. Disclosures for authors are available with the original article. Dr. Smith had no relevant disclosures.
A version of this article appeared on Medscape.com.
, new research suggests.
In a comparative performance analysis of five commonly used FITs for colorectal cancer (CRC) screening, researchers found statistically significant differences in positivity rates, sensitivity, and specificity, as well as important differences in rates of unusable tests.
“Our findings have practical importance for FIT-based screening programs as these differences affect the need for repeated FIT, the yield of ACN detection, and the number of diagnostic colonoscopies that would be required to follow-up on abnormal findings,” wrote the researchers, led by Barcey T. Levy, MD, PhD, with University of Iowa, Iowa City.
The study was published online in Annals of Internal Medicine.
Wide Variation Found
Despite widespread use of FITs for CRC screening, there is limited data to help guide test selection. Understanding the comparative performance of different FITs is “crucial” for a successful FIT-based screening program, the researchers wrote.
Dr. Levy and colleagues directly compared the performance of five commercially available FITs — including four qualitative tests (Hemoccult ICT, Hemosure iFOB, OC-Light S FIT, and QuickVue iFOB) and one quantitative test (OC-Auto FIT) — using colonoscopy as the reference standard.
Participants included a diverse group of 3761 adults (mean age, 62 years; 63% women). Each participant was given all five tests and completed them using the same stool sample. They sent the tests by first class mail to a central location, where FITs were analyzed by a trained professional on the day of receipt.
The primary outcome was test performance (sensitivity and specificity) for ACN, defined as advanced polyps or CRC.
A total of 320 participants (8.5%) were found to have ACN based on colonoscopy results, including nine with CRC (0.2%) — rates that are similar to those found in other studies.
The sensitivity for detecting ACN ranged from 10.1% (Hemoccult ICT) to 36.7% (OC-Light S FIT), and specificity varied from 85.5% (OC-Light S FIT) to 96.6% (Hemoccult ICT).
“Given the variation in FIT cutoffs reported by manufacturers, it is not surprising that tests with lower cutoffs (such as OC-Light S FIT) had higher sensitivity than tests with higher cutoffs (such as Hemoccult ICT),” Dr. Levy and colleagues wrote.
Test positivity rates varied fourfold across FITs, from 3.9% for Hemoccult ICT to 16.4% for OC-Light S FIT.
The rates of tests deemed unevaluable (due to factors such as indeterminant results or user mistakes) ranged from 0.2% for OC-Auto FIT to 2.5% for QuickVue iFOB.
The highest positive predictive value (PPV) was observed with OC-Auto FIT (28.9%) and the lowest with Hemosure iFOB (18.2%). The negative predictive value was similar across tests, ranging from 92.2% to 93.3%, indicating consistent performance in ruling out disease.
The study also identified significant differences in test sensitivity based on factors such as the location of neoplasia (higher sensitivity for distal lesions) and patient characteristics (higher sensitivity in people with higher body mass index and lower income).
Dr. Levy and colleagues said their findings have implications both in terms of clinical benefits and cost-effectiveness of CRC screening using FITs.
“Tests with lower sensitivity will miss more patients with CRC and advanced polyps, and tests with higher sensitivity and lower PPV will require more colonoscopies to detect patients with actionable findings,” they wrote.
‘Jaw-Dropping’ Results
The sensitivity results are “jaw-dropping,” Robert Smith, PhD, senior vice-president for cancer screening at the American Cancer Society, said in an interview. “A patient should have at least a 50/50 chance of having their colorectal cancer detected with a stool test at the time of testing.”
“What these numbers show is that the level that the manufacturers believe their test is performing is not reproduced,” Dr. Smith added.
This study adds to “concerns that have been raised about the inherent limitations and the performance of these tests that have been cleared for use and that are supposed to be lifesaving,” he said.
Clearance by the US Food and Drug Administration should mean that there’s essentially “no risk to using the test in terms of the test itself being harmful,” Dr. Smith said. But that’s not the case with FITs “because it’s harmful if you have cancer and your test doesn’t find it.”
By way of study limitations, Dr. Levy and colleagues said it’s important to note that they did not evaluate the “programmatic” sensitivity of repeating FIT testing every 1-2 years, as is generally recommended in screening guidelines. Therefore, the sensitivity of a single FIT may be lower than that of a repeated FIT. Also, variability in the FIT collection process by participants might have affected the results.
The study had no commercial funding. Disclosures for authors are available with the original article. Dr. Smith had no relevant disclosures.
A version of this article appeared on Medscape.com.
Short Interval Repeat Colonoscopy After Inadequate Bowel Preparation Is Low Among Veterans
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.
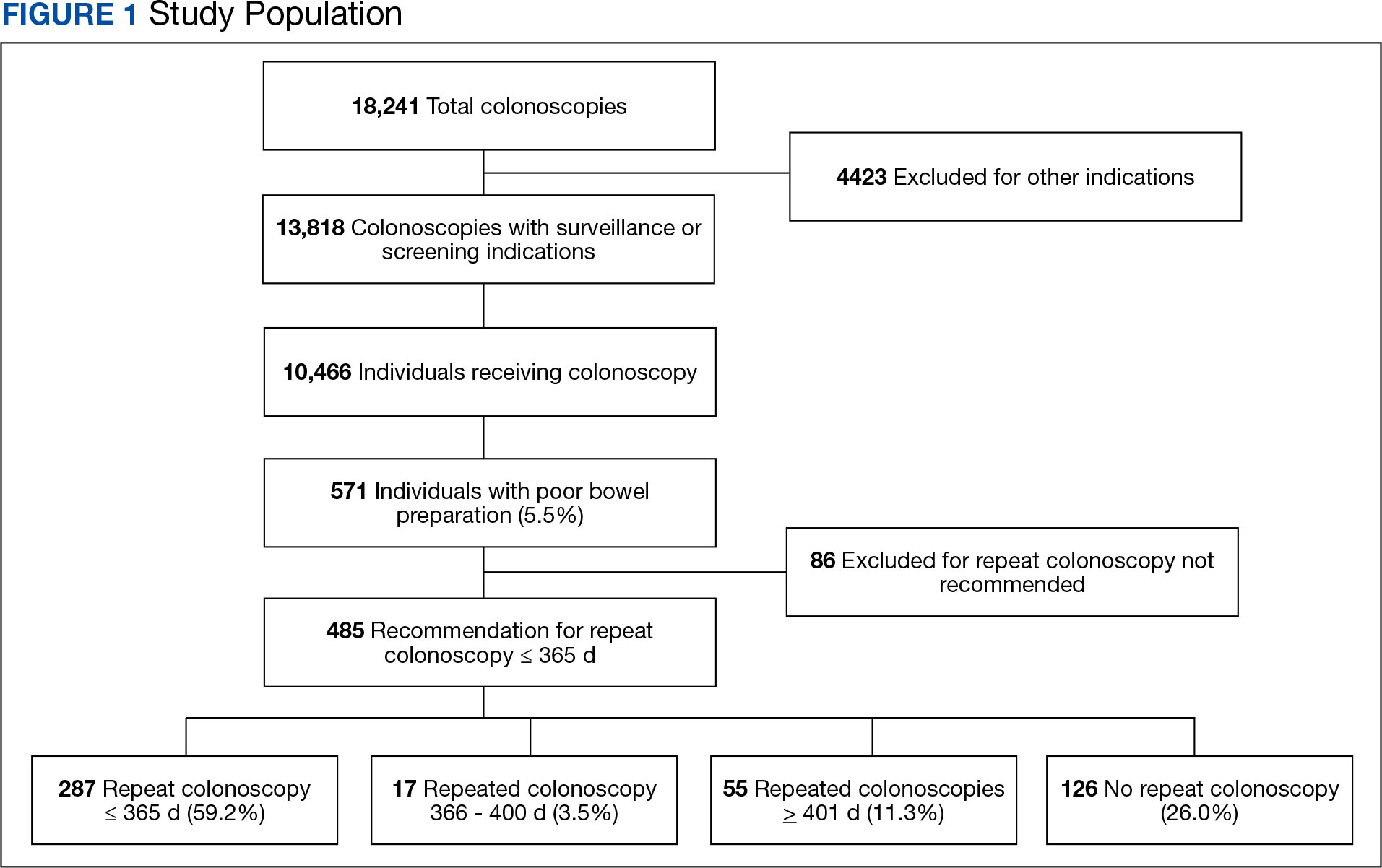
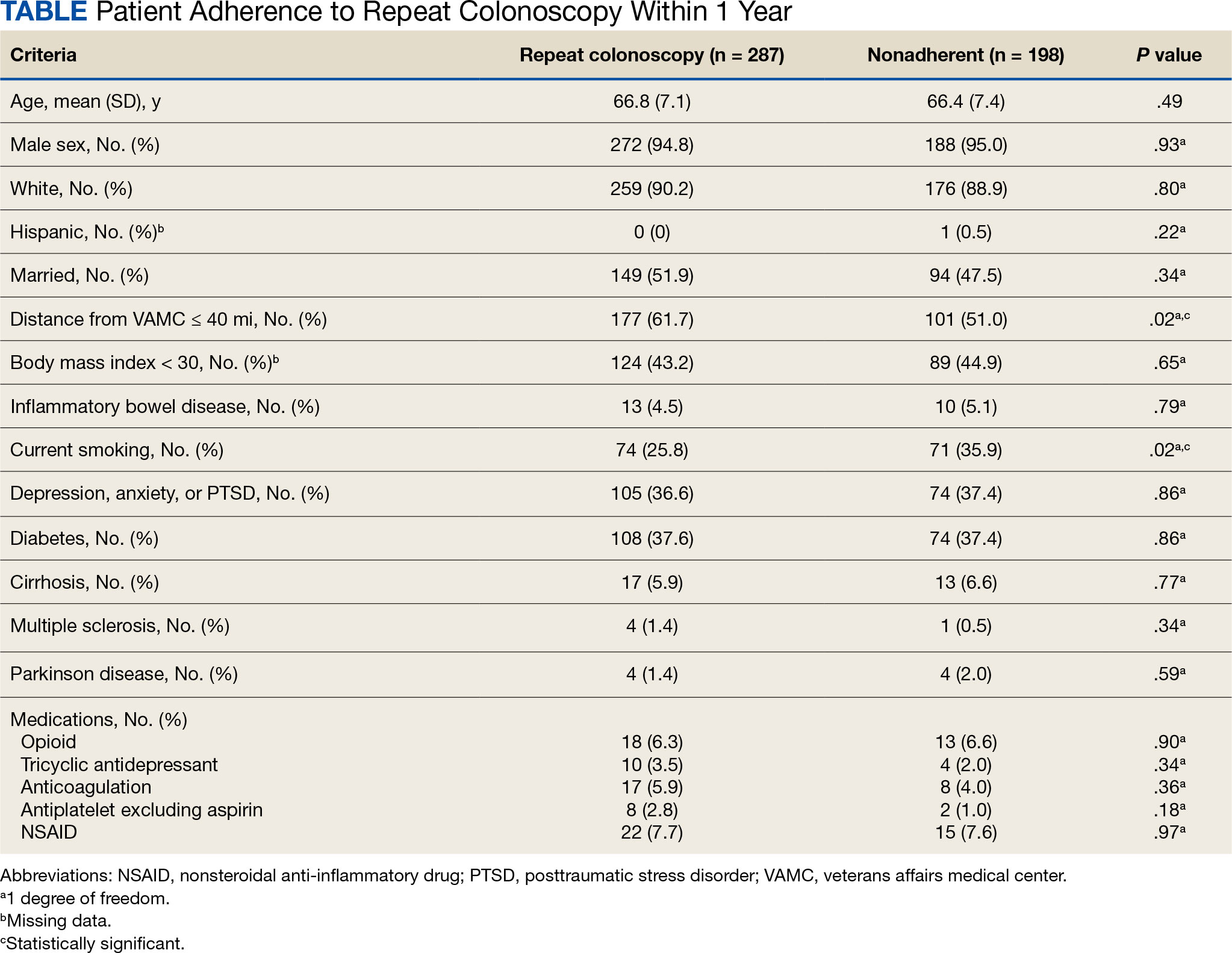
Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).
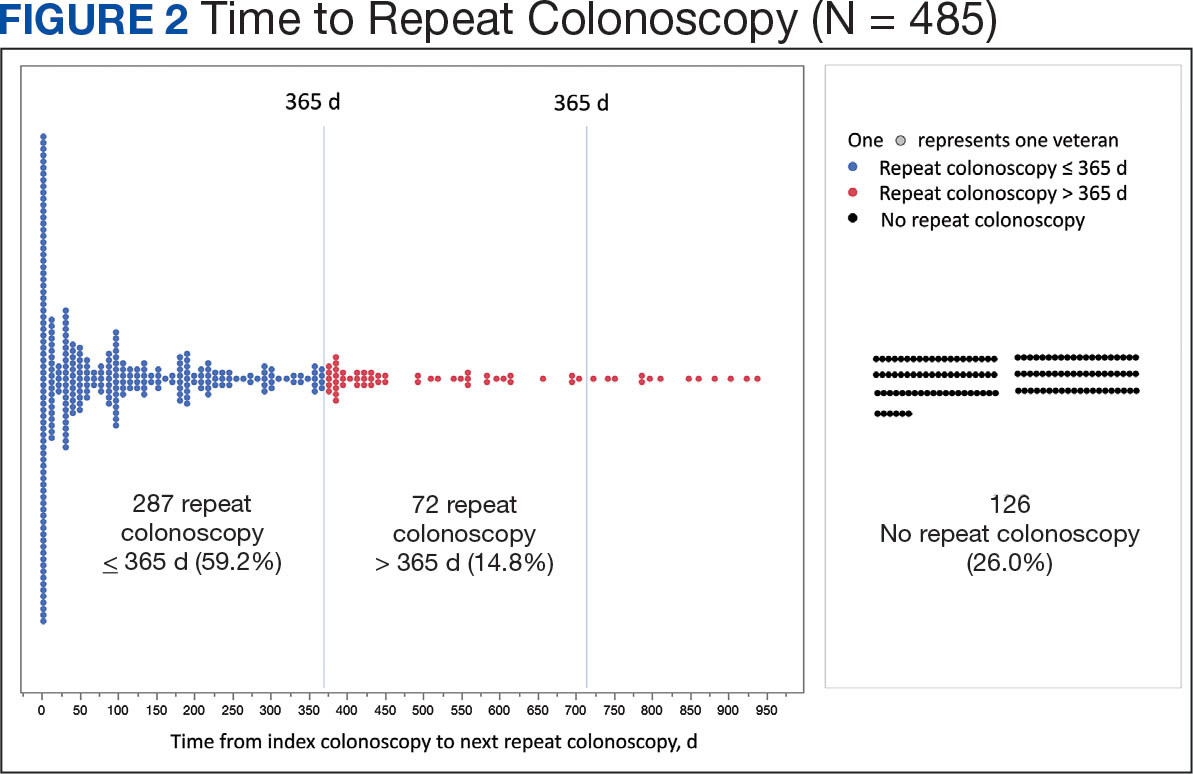
To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).
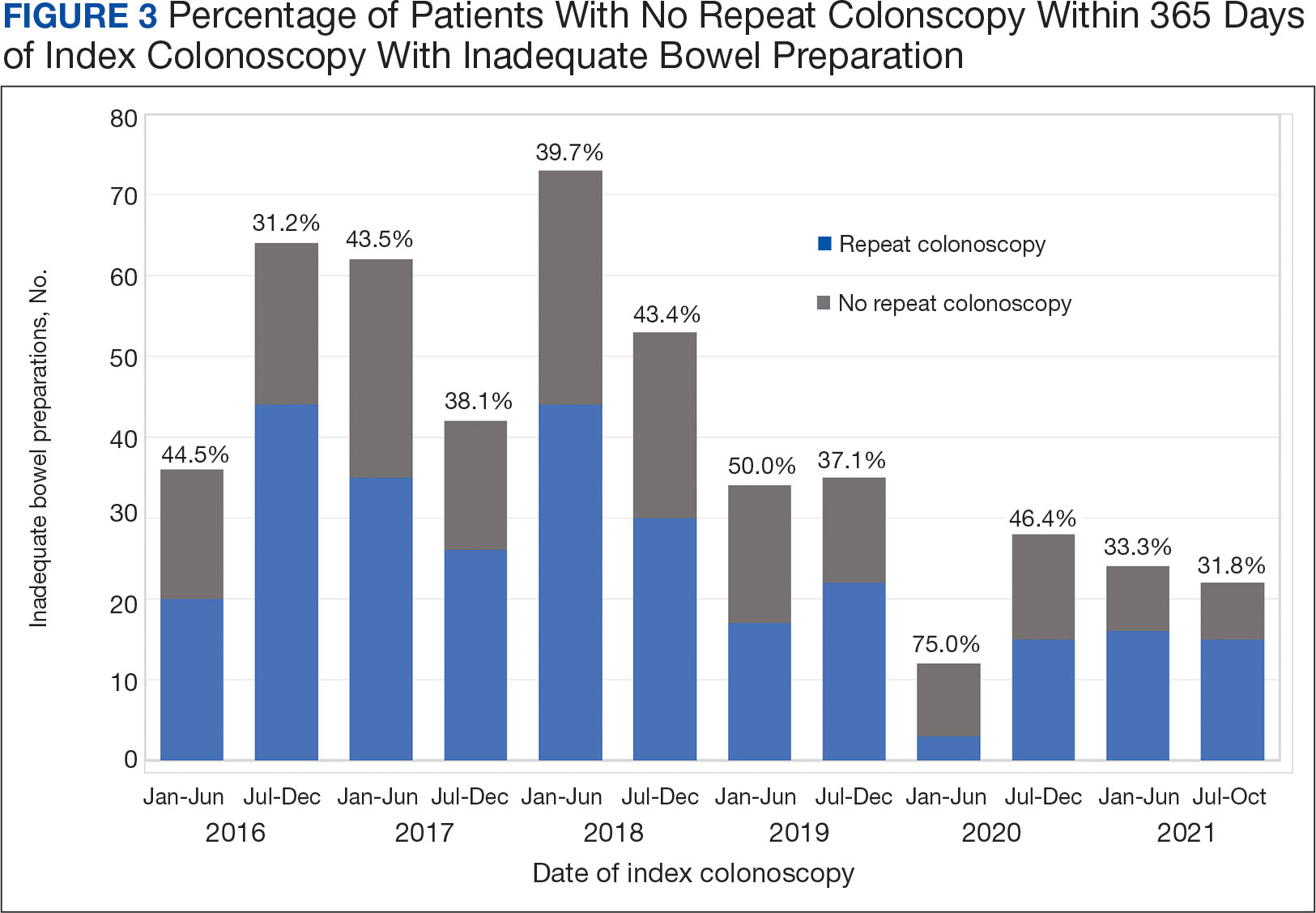
Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.


Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).

To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).

Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.


Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).

To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).

Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812








