User login
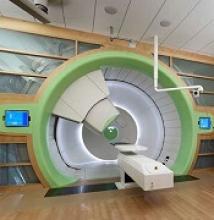
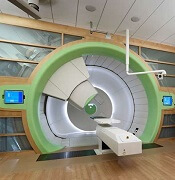
Guidelines for proton therapy in mediastinal lymphomas
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but the treatment should only be used in patients expected to derive the most benefit, according to new guidelines from the International Lymphoma Radiation Oncology Group.
The guidelines note that proton therapy reduces the radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart.
However, the advantages of proton therapy are not always clear in other situations, such as when the target spans the right side of the heart or when the target is above the heart with no axillary involvement.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” said guideline author Bouthaina Dabaja, MD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues.
The group’s guidelines were published in Blood.
The guidelines note that proton therapy—like intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy—presents an opportunity for more conformal dose distribution and better sparing of organs at risk.
Proton therapy can greatly benefit certain patients with mediastinal disease, including:
- Young female patients in whom proton therapy would reduce the breast dose and decrease the risk of secondary breast cancer
- Patients at high risk of radiation-related toxicity due to previous treatment
- Patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines.
However, the consideration of proton therapy needs to factor in the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy is the least complex delivery technique, it is challenging because beams can conform only to one side of the target. In contrast, active mode pencil beam scanning proton therapy potentially provides better conformality and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said.
However, “motion management is of prime importance” with pencil beam scanning proton therapy, which is more sensitive to density changes in the beam path than is passive scattering proton therapy.
To that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
Dr. Dabaja and her coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but the treatment should only be used in patients expected to derive the most benefit, according to new guidelines from the International Lymphoma Radiation Oncology Group.
The guidelines note that proton therapy reduces the radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart.
However, the advantages of proton therapy are not always clear in other situations, such as when the target spans the right side of the heart or when the target is above the heart with no axillary involvement.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” said guideline author Bouthaina Dabaja, MD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues.
The group’s guidelines were published in Blood.
The guidelines note that proton therapy—like intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy—presents an opportunity for more conformal dose distribution and better sparing of organs at risk.
Proton therapy can greatly benefit certain patients with mediastinal disease, including:
- Young female patients in whom proton therapy would reduce the breast dose and decrease the risk of secondary breast cancer
- Patients at high risk of radiation-related toxicity due to previous treatment
- Patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines.
However, the consideration of proton therapy needs to factor in the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy is the least complex delivery technique, it is challenging because beams can conform only to one side of the target. In contrast, active mode pencil beam scanning proton therapy potentially provides better conformality and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said.
However, “motion management is of prime importance” with pencil beam scanning proton therapy, which is more sensitive to density changes in the beam path than is passive scattering proton therapy.
To that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
Dr. Dabaja and her coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but the treatment should only be used in patients expected to derive the most benefit, according to new guidelines from the International Lymphoma Radiation Oncology Group.
The guidelines note that proton therapy reduces the radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart.
However, the advantages of proton therapy are not always clear in other situations, such as when the target spans the right side of the heart or when the target is above the heart with no axillary involvement.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” said guideline author Bouthaina Dabaja, MD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues.
The group’s guidelines were published in Blood.
The guidelines note that proton therapy—like intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy—presents an opportunity for more conformal dose distribution and better sparing of organs at risk.
Proton therapy can greatly benefit certain patients with mediastinal disease, including:
- Young female patients in whom proton therapy would reduce the breast dose and decrease the risk of secondary breast cancer
- Patients at high risk of radiation-related toxicity due to previous treatment
- Patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines.
However, the consideration of proton therapy needs to factor in the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy is the least complex delivery technique, it is challenging because beams can conform only to one side of the target. In contrast, active mode pencil beam scanning proton therapy potentially provides better conformality and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said.
However, “motion management is of prime importance” with pencil beam scanning proton therapy, which is more sensitive to density changes in the beam path than is passive scattering proton therapy.
To that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
Dr. Dabaja and her coauthors reported no funding or conflicts of interest.
AKT inhibitor exhibits activity against MM
Preclinical research suggests an AKT inhibitor could be effective against multiple myeloma (MM).
The inhibitor, HS1793, is a derivative of the antioxidant compound resveratrol.
Investigators found that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in MM cells.
HS1793 was cytotoxic to MM cells in a mouse model of human metastatic myeloma and in human MM cells in vitro.
Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues reported these results in Cancer Letters.
“AKT is frequently activated in MM cells, and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They had screened 400 compounds and identified HS1793 as the most promising AKT inhibitor. Experiments suggested that HS1793 inhibits AKT activation by interfering with the interaction between AKT and its promoter, HSP90.
HS1793 exhibited antimyeloma activity in human MM cell lines. The investigators said this appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In additional experiments, the team found that HS1793’s inhibition of AKT/HSP90 interaction results in cell death by suppressing NF-κB signaling. HS1793-induced cell death was caused by the direct inhibition of AKT that, in turn, suppressed NF-κB activation.
The investigators found that HS1793 “dramatically decreased” lytic skull and femur lesions in a mouse model of MM metastatic to bone. And mice that received HS1793 had superior survival, when compared to mice that received vehicle control.
The investigators also showed that HS1793 was cytotoxic to MM cells but not normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” the authors wrote.
This research was supported by grants from the Korean government. The investigators reported no potential conflicts of interest.
Preclinical research suggests an AKT inhibitor could be effective against multiple myeloma (MM).
The inhibitor, HS1793, is a derivative of the antioxidant compound resveratrol.
Investigators found that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in MM cells.
HS1793 was cytotoxic to MM cells in a mouse model of human metastatic myeloma and in human MM cells in vitro.
Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues reported these results in Cancer Letters.
“AKT is frequently activated in MM cells, and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They had screened 400 compounds and identified HS1793 as the most promising AKT inhibitor. Experiments suggested that HS1793 inhibits AKT activation by interfering with the interaction between AKT and its promoter, HSP90.
HS1793 exhibited antimyeloma activity in human MM cell lines. The investigators said this appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In additional experiments, the team found that HS1793’s inhibition of AKT/HSP90 interaction results in cell death by suppressing NF-κB signaling. HS1793-induced cell death was caused by the direct inhibition of AKT that, in turn, suppressed NF-κB activation.
The investigators found that HS1793 “dramatically decreased” lytic skull and femur lesions in a mouse model of MM metastatic to bone. And mice that received HS1793 had superior survival, when compared to mice that received vehicle control.
The investigators also showed that HS1793 was cytotoxic to MM cells but not normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” the authors wrote.
This research was supported by grants from the Korean government. The investigators reported no potential conflicts of interest.
Preclinical research suggests an AKT inhibitor could be effective against multiple myeloma (MM).
The inhibitor, HS1793, is a derivative of the antioxidant compound resveratrol.
Investigators found that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in MM cells.
HS1793 was cytotoxic to MM cells in a mouse model of human metastatic myeloma and in human MM cells in vitro.
Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues reported these results in Cancer Letters.
“AKT is frequently activated in MM cells, and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They had screened 400 compounds and identified HS1793 as the most promising AKT inhibitor. Experiments suggested that HS1793 inhibits AKT activation by interfering with the interaction between AKT and its promoter, HSP90.
HS1793 exhibited antimyeloma activity in human MM cell lines. The investigators said this appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In additional experiments, the team found that HS1793’s inhibition of AKT/HSP90 interaction results in cell death by suppressing NF-κB signaling. HS1793-induced cell death was caused by the direct inhibition of AKT that, in turn, suppressed NF-κB activation.
The investigators found that HS1793 “dramatically decreased” lytic skull and femur lesions in a mouse model of MM metastatic to bone. And mice that received HS1793 had superior survival, when compared to mice that received vehicle control.
The investigators also showed that HS1793 was cytotoxic to MM cells but not normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” the authors wrote.
This research was supported by grants from the Korean government. The investigators reported no potential conflicts of interest.
Risks of watchful waiting in follicular lymphoma
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
Sequencing informs prognosis after HSCT in MDS
Gene sequencing early after transplant may provide important prognostic information in patients with myelodysplastic syndromes (MDS), according to a new study.
Patients who had disease-associated mutations in the bone marrow 30 days after hematopoietic stem cell transplant (HSCT) were significantly more likely to experience disease progression and have lower rates of progression-free survival (PFS) at 1 year.
“Using our sequencing method, we’re identifying residual tumor cells before a pathologist could see them under the microscope and before a patient develops symptoms,” said Matthew J. Walter, MD, of Washington University in St. Louis, Mo.
“At that moment, there may be time to intervene in ways that could delay the cancer from coming back or potentially prevent it completely.”
Dr. Walter and his colleagues described results with their sequencing method in The New England Journal of Medicine.
The researchers sequenced bone marrow and skin (control) samples from 90 adults with MDS who underwent allogeneic HSCT.
The team used enhanced exome sequencing to detect mutations before HSCT and evaluated mutation clearance using error-corrected sequencing to genotype mutations in bone marrow samples collected 30 days after HSCT.
The researchers detected at least one validated somatic mutation in the pre-HSCT samples from 86 of 90 patients.
Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after HSCT. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the researchers explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not progress (P<0.001).
Progression occurred in 53.1% of patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days, whereas progression occurred in 13% of patients who did not have such mutations. After adjusting for conditioning regimen, the hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P<0.001).
The 1-year PFS rate was 31.3% in patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days and 59.3% in patients who did not have the mutations. After adjusting for conditioning, the HR for progression or death was 2.22 (P=0.005).
The researchers noted that PFS was lower in patients who had received reduced-intensity conditioning and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 (P≤0.001), when compared to other combinations of conditioning regimen and mutation status.
In multivariable analyses, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than four-fold risk of progression (HR, 4.48; P<0.001) and a more than two-fold risk of progression or death (HR, 2.39; P=0.002).
“Now that we have detected mutations early and shown that it predicts a higher risk of recurrence, we want to determine the best course of action for those high-risk patients,” Dr. Walter said.
He and his colleagues acknowledged that the high-coverage exome sequencing technique used for this study is not routinely available in the clinic. Therefore, the researchers also analyzed samples using a subset of genes that are usually included in gene sequencing panels for MDS and acute myeloid leukemia.
The researchers noted that this 40-gene panel revealed fewer patients (n=68; 79%) with mutations, but “the prognostic value of detection of measurable residual disease was still highly clinically significant.”
With this approach, the presence of at least one mutation with a variant allele frequency of at least 0.5% 30 days after HSCT was associated with a higher risk of disease progression at 1 year (HR, 3.39; P=0.001) and a higher risk of progression or death at 1 year (HR, 2.09; P=0.02).
This study was supported by grants from the Leukemia and Lymphoma Society and other groups.
Gene sequencing early after transplant may provide important prognostic information in patients with myelodysplastic syndromes (MDS), according to a new study.
Patients who had disease-associated mutations in the bone marrow 30 days after hematopoietic stem cell transplant (HSCT) were significantly more likely to experience disease progression and have lower rates of progression-free survival (PFS) at 1 year.
“Using our sequencing method, we’re identifying residual tumor cells before a pathologist could see them under the microscope and before a patient develops symptoms,” said Matthew J. Walter, MD, of Washington University in St. Louis, Mo.
“At that moment, there may be time to intervene in ways that could delay the cancer from coming back or potentially prevent it completely.”
Dr. Walter and his colleagues described results with their sequencing method in The New England Journal of Medicine.
The researchers sequenced bone marrow and skin (control) samples from 90 adults with MDS who underwent allogeneic HSCT.
The team used enhanced exome sequencing to detect mutations before HSCT and evaluated mutation clearance using error-corrected sequencing to genotype mutations in bone marrow samples collected 30 days after HSCT.
The researchers detected at least one validated somatic mutation in the pre-HSCT samples from 86 of 90 patients.
Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after HSCT. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the researchers explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not progress (P<0.001).
Progression occurred in 53.1% of patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days, whereas progression occurred in 13% of patients who did not have such mutations. After adjusting for conditioning regimen, the hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P<0.001).
The 1-year PFS rate was 31.3% in patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days and 59.3% in patients who did not have the mutations. After adjusting for conditioning, the HR for progression or death was 2.22 (P=0.005).
The researchers noted that PFS was lower in patients who had received reduced-intensity conditioning and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 (P≤0.001), when compared to other combinations of conditioning regimen and mutation status.
In multivariable analyses, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than four-fold risk of progression (HR, 4.48; P<0.001) and a more than two-fold risk of progression or death (HR, 2.39; P=0.002).
“Now that we have detected mutations early and shown that it predicts a higher risk of recurrence, we want to determine the best course of action for those high-risk patients,” Dr. Walter said.
He and his colleagues acknowledged that the high-coverage exome sequencing technique used for this study is not routinely available in the clinic. Therefore, the researchers also analyzed samples using a subset of genes that are usually included in gene sequencing panels for MDS and acute myeloid leukemia.
The researchers noted that this 40-gene panel revealed fewer patients (n=68; 79%) with mutations, but “the prognostic value of detection of measurable residual disease was still highly clinically significant.”
With this approach, the presence of at least one mutation with a variant allele frequency of at least 0.5% 30 days after HSCT was associated with a higher risk of disease progression at 1 year (HR, 3.39; P=0.001) and a higher risk of progression or death at 1 year (HR, 2.09; P=0.02).
This study was supported by grants from the Leukemia and Lymphoma Society and other groups.
Gene sequencing early after transplant may provide important prognostic information in patients with myelodysplastic syndromes (MDS), according to a new study.
Patients who had disease-associated mutations in the bone marrow 30 days after hematopoietic stem cell transplant (HSCT) were significantly more likely to experience disease progression and have lower rates of progression-free survival (PFS) at 1 year.
“Using our sequencing method, we’re identifying residual tumor cells before a pathologist could see them under the microscope and before a patient develops symptoms,” said Matthew J. Walter, MD, of Washington University in St. Louis, Mo.
“At that moment, there may be time to intervene in ways that could delay the cancer from coming back or potentially prevent it completely.”
Dr. Walter and his colleagues described results with their sequencing method in The New England Journal of Medicine.
The researchers sequenced bone marrow and skin (control) samples from 90 adults with MDS who underwent allogeneic HSCT.
The team used enhanced exome sequencing to detect mutations before HSCT and evaluated mutation clearance using error-corrected sequencing to genotype mutations in bone marrow samples collected 30 days after HSCT.
The researchers detected at least one validated somatic mutation in the pre-HSCT samples from 86 of 90 patients.
Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after HSCT. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the researchers explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not progress (P<0.001).
Progression occurred in 53.1% of patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days, whereas progression occurred in 13% of patients who did not have such mutations. After adjusting for conditioning regimen, the hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P<0.001).
The 1-year PFS rate was 31.3% in patients who had one or more mutations with a variant allele frequency of at least 0.5% at 30 days and 59.3% in patients who did not have the mutations. After adjusting for conditioning, the HR for progression or death was 2.22 (P=0.005).
The researchers noted that PFS was lower in patients who had received reduced-intensity conditioning and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 (P≤0.001), when compared to other combinations of conditioning regimen and mutation status.
In multivariable analyses, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than four-fold risk of progression (HR, 4.48; P<0.001) and a more than two-fold risk of progression or death (HR, 2.39; P=0.002).
“Now that we have detected mutations early and shown that it predicts a higher risk of recurrence, we want to determine the best course of action for those high-risk patients,” Dr. Walter said.
He and his colleagues acknowledged that the high-coverage exome sequencing technique used for this study is not routinely available in the clinic. Therefore, the researchers also analyzed samples using a subset of genes that are usually included in gene sequencing panels for MDS and acute myeloid leukemia.
The researchers noted that this 40-gene panel revealed fewer patients (n=68; 79%) with mutations, but “the prognostic value of detection of measurable residual disease was still highly clinically significant.”
With this approach, the presence of at least one mutation with a variant allele frequency of at least 0.5% 30 days after HSCT was associated with a higher risk of disease progression at 1 year (HR, 3.39; P=0.001) and a higher risk of progression or death at 1 year (HR, 2.09; P=0.02).
This study was supported by grants from the Leukemia and Lymphoma Society and other groups.
ASCO addresses financial barriers to cancer clinical trials
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
Physician burnout linked to patient safety
Physician burnout may jeopardize patient care, according to research published in JAMA Internal Medicine.
A review and meta-analysis suggested that physician burnout was associated with a higher risk of patient safety incidents, reduced patient satisfaction, and low professionalism.
Burnout was defined as “a response to prolonged exposure to occupational stress encompassing feelings of emotional exhaustion, depersonalization, and reduced professional efficacy.”
This research was conducted by Maria Panagioti, PhD, of the University of Manchester in the U.K., and her colleagues.
The researchers analyzed 47 studies on the topics of physician burnout and patient care, which included data from a pooled cohort of 42,473 physicians.
Nearly 45% of studies included physicians in their residency or early career (up to 5 years post-residency), and 55.3% included more experienced physicians. The studies included physicians in a hospital setting (63.8%), primary care setting (27.7%), or a mix of health care settings (8.5%).
The data indicated that physician burnout was significantly associated with:
- An increased risk of patient safety incidents—odds ratio [OR], 1.96 (P<0.001)
- Low professionalism—OR, 2.31 (P<0.001)
- Reduced patient satisfaction—OR, 2.28 (P<0.001).
The researchers noted that the reporting method had an impact on the results. According to physician report, burnout was associated with significantly increased risks of safety incidents (OR, 2.07) and low professionalism (OR, 2.67).
However, according to system reports, there was no significant association between physician burnout and safety incidents (OR, 1.00) or low professionalism (OR, 1.15).
Another factor that impacted results was physician experience. The association between burnout and low professionalism was significantly larger in studies of residents and early career physicians (OR, 3.39) than in studies of middle- and late-career physicians (OR, 1.73).
The researchers noted that this review had its limitations, including variation in outcomes across studies, heterogeneity among studies, potential selection bias by excluding gray literature, and the inability to establish causal links from findings because of the cross-sectional nature of the studies analyzed.
This research was funded by the United Kingdom National Institute for Health Research (NIHR) School for Primary Care Research and the NIHR Greater Manchester Patient Safety Translational Research Centre. Study authors reported no relevant conflicts of interest.
Physician burnout may jeopardize patient care, according to research published in JAMA Internal Medicine.
A review and meta-analysis suggested that physician burnout was associated with a higher risk of patient safety incidents, reduced patient satisfaction, and low professionalism.
Burnout was defined as “a response to prolonged exposure to occupational stress encompassing feelings of emotional exhaustion, depersonalization, and reduced professional efficacy.”
This research was conducted by Maria Panagioti, PhD, of the University of Manchester in the U.K., and her colleagues.
The researchers analyzed 47 studies on the topics of physician burnout and patient care, which included data from a pooled cohort of 42,473 physicians.
Nearly 45% of studies included physicians in their residency or early career (up to 5 years post-residency), and 55.3% included more experienced physicians. The studies included physicians in a hospital setting (63.8%), primary care setting (27.7%), or a mix of health care settings (8.5%).
The data indicated that physician burnout was significantly associated with:
- An increased risk of patient safety incidents—odds ratio [OR], 1.96 (P<0.001)
- Low professionalism—OR, 2.31 (P<0.001)
- Reduced patient satisfaction—OR, 2.28 (P<0.001).
The researchers noted that the reporting method had an impact on the results. According to physician report, burnout was associated with significantly increased risks of safety incidents (OR, 2.07) and low professionalism (OR, 2.67).
However, according to system reports, there was no significant association between physician burnout and safety incidents (OR, 1.00) or low professionalism (OR, 1.15).
Another factor that impacted results was physician experience. The association between burnout and low professionalism was significantly larger in studies of residents and early career physicians (OR, 3.39) than in studies of middle- and late-career physicians (OR, 1.73).
The researchers noted that this review had its limitations, including variation in outcomes across studies, heterogeneity among studies, potential selection bias by excluding gray literature, and the inability to establish causal links from findings because of the cross-sectional nature of the studies analyzed.
This research was funded by the United Kingdom National Institute for Health Research (NIHR) School for Primary Care Research and the NIHR Greater Manchester Patient Safety Translational Research Centre. Study authors reported no relevant conflicts of interest.
Physician burnout may jeopardize patient care, according to research published in JAMA Internal Medicine.
A review and meta-analysis suggested that physician burnout was associated with a higher risk of patient safety incidents, reduced patient satisfaction, and low professionalism.
Burnout was defined as “a response to prolonged exposure to occupational stress encompassing feelings of emotional exhaustion, depersonalization, and reduced professional efficacy.”
This research was conducted by Maria Panagioti, PhD, of the University of Manchester in the U.K., and her colleagues.
The researchers analyzed 47 studies on the topics of physician burnout and patient care, which included data from a pooled cohort of 42,473 physicians.
Nearly 45% of studies included physicians in their residency or early career (up to 5 years post-residency), and 55.3% included more experienced physicians. The studies included physicians in a hospital setting (63.8%), primary care setting (27.7%), or a mix of health care settings (8.5%).
The data indicated that physician burnout was significantly associated with:
- An increased risk of patient safety incidents—odds ratio [OR], 1.96 (P<0.001)
- Low professionalism—OR, 2.31 (P<0.001)
- Reduced patient satisfaction—OR, 2.28 (P<0.001).
The researchers noted that the reporting method had an impact on the results. According to physician report, burnout was associated with significantly increased risks of safety incidents (OR, 2.07) and low professionalism (OR, 2.67).
However, according to system reports, there was no significant association between physician burnout and safety incidents (OR, 1.00) or low professionalism (OR, 1.15).
Another factor that impacted results was physician experience. The association between burnout and low professionalism was significantly larger in studies of residents and early career physicians (OR, 3.39) than in studies of middle- and late-career physicians (OR, 1.73).
The researchers noted that this review had its limitations, including variation in outcomes across studies, heterogeneity among studies, potential selection bias by excluding gray literature, and the inability to establish causal links from findings because of the cross-sectional nature of the studies analyzed.
This research was funded by the United Kingdom National Institute for Health Research (NIHR) School for Primary Care Research and the NIHR Greater Manchester Patient Safety Translational Research Centre. Study authors reported no relevant conflicts of interest.
Doc reports ‘very encouraging’ results in penta-refractory MM
HOUSTON—Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma (MM), according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%.
The median progression-free survival was 3.7 months, and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Mount Sinai School of Medicine in New York, N.Y., presented these results at the Society of Hematologic Oncology (SOHO) 2018 Annual Meeting as abstract MM-255.*
“The additional phase 2b clinical results ... are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said.
“Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM study.”
The study (NCT02336815) included 122 patients with penta-refractory MM. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of 7 (range, 3-18) prior treatment regimens. Their median age was 65 (range, 40-86), 58% were male, and 53% had high-risk cytogenetics.
Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.
Response and survival
Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10-6 and one at 1 x 10-4.
Six patients (4.9%) had very good partial responses, 24 (19.7%) had partial responses (PRs), 16 (13.1%) had minimal responses (MRs), and 48 (39.3%) had stable disease (SD).
Sixteen patients (13.1%) had progressive disease (PD), and 10 (8.2%) were not evaluable for response.
The overall response rate (PR or better) was 26.2% (n=32), the clinical benefit rate (MR or better) was 39.3% (n=48), and the disease control rate (SD or better) was 78.7% (n=98).
The median duration of response was 4.4 months (range, <1 to 12.2 months).
The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had PD or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. It was 15.6 months in patients with an MR or better and 1.7 months in patients who had PD or were not evaluable (P<0.0001).
Safety
The most common non-hematologic treatment-related adverse events (AEs) were fatigue/asthenia (69.9%), nausea (69.1%), anorexia (52.0%), weight loss (47.2%), vomiting (35.0%), diarrhea (33.3%), and hyponatremia (30.9%).
Hematologic treatment-related AEs included thrombocytopenia (67.5%), anemia (48.0%), neutropenia (35.8%), leukopenia (29.3%), and lymphopenia (13.8%).
The “most important” grade 3/4 AEs, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%).
Twenty-three patients (19.5%) discontinued treatment due to a related AE.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GSK.
*Information in the abstract differs from the presentation.
HOUSTON—Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma (MM), according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%.
The median progression-free survival was 3.7 months, and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Mount Sinai School of Medicine in New York, N.Y., presented these results at the Society of Hematologic Oncology (SOHO) 2018 Annual Meeting as abstract MM-255.*
“The additional phase 2b clinical results ... are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said.
“Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM study.”
The study (NCT02336815) included 122 patients with penta-refractory MM. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of 7 (range, 3-18) prior treatment regimens. Their median age was 65 (range, 40-86), 58% were male, and 53% had high-risk cytogenetics.
Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.
Response and survival
Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10-6 and one at 1 x 10-4.
Six patients (4.9%) had very good partial responses, 24 (19.7%) had partial responses (PRs), 16 (13.1%) had minimal responses (MRs), and 48 (39.3%) had stable disease (SD).
Sixteen patients (13.1%) had progressive disease (PD), and 10 (8.2%) were not evaluable for response.
The overall response rate (PR or better) was 26.2% (n=32), the clinical benefit rate (MR or better) was 39.3% (n=48), and the disease control rate (SD or better) was 78.7% (n=98).
The median duration of response was 4.4 months (range, <1 to 12.2 months).
The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had PD or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. It was 15.6 months in patients with an MR or better and 1.7 months in patients who had PD or were not evaluable (P<0.0001).
Safety
The most common non-hematologic treatment-related adverse events (AEs) were fatigue/asthenia (69.9%), nausea (69.1%), anorexia (52.0%), weight loss (47.2%), vomiting (35.0%), diarrhea (33.3%), and hyponatremia (30.9%).
Hematologic treatment-related AEs included thrombocytopenia (67.5%), anemia (48.0%), neutropenia (35.8%), leukopenia (29.3%), and lymphopenia (13.8%).
The “most important” grade 3/4 AEs, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%).
Twenty-three patients (19.5%) discontinued treatment due to a related AE.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GSK.
*Information in the abstract differs from the presentation.
HOUSTON—Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma (MM), according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%.
The median progression-free survival was 3.7 months, and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Mount Sinai School of Medicine in New York, N.Y., presented these results at the Society of Hematologic Oncology (SOHO) 2018 Annual Meeting as abstract MM-255.*
“The additional phase 2b clinical results ... are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said.
“Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM study.”
The study (NCT02336815) included 122 patients with penta-refractory MM. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of 7 (range, 3-18) prior treatment regimens. Their median age was 65 (range, 40-86), 58% were male, and 53% had high-risk cytogenetics.
Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.
Response and survival
Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10-6 and one at 1 x 10-4.
Six patients (4.9%) had very good partial responses, 24 (19.7%) had partial responses (PRs), 16 (13.1%) had minimal responses (MRs), and 48 (39.3%) had stable disease (SD).
Sixteen patients (13.1%) had progressive disease (PD), and 10 (8.2%) were not evaluable for response.
The overall response rate (PR or better) was 26.2% (n=32), the clinical benefit rate (MR or better) was 39.3% (n=48), and the disease control rate (SD or better) was 78.7% (n=98).
The median duration of response was 4.4 months (range, <1 to 12.2 months).
The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had PD or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. It was 15.6 months in patients with an MR or better and 1.7 months in patients who had PD or were not evaluable (P<0.0001).
Safety
The most common non-hematologic treatment-related adverse events (AEs) were fatigue/asthenia (69.9%), nausea (69.1%), anorexia (52.0%), weight loss (47.2%), vomiting (35.0%), diarrhea (33.3%), and hyponatremia (30.9%).
Hematologic treatment-related AEs included thrombocytopenia (67.5%), anemia (48.0%), neutropenia (35.8%), leukopenia (29.3%), and lymphopenia (13.8%).
The “most important” grade 3/4 AEs, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%).
Twenty-three patients (19.5%) discontinued treatment due to a related AE.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GSK.
*Information in the abstract differs from the presentation.
CAR T-cell therapy elicits responses in MM
BOSTON—Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma (MM).
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome, but the condition resolved without use of tociluzimab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated MM. They had a median of six prior therapies. Their median age was 60, and 73% were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51×106 (n=3), 152×106 (n=7), and 430×106 (n=1).
As of August 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and 5 had thrombocytopenia.
Researchers suspected cytokine release syndrome in one patient, but the condition resolved without tociluzimab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was only evaluable by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
“We believe our advantages of a purified product, where all cells express the CAR molecule, and a product with high levels of stem cell memory T cells, producing a more gradual and prolonged immune response against tumor cells, provide a significantly better therapeutic index when compared with other CAR-T therapeutics. We are also encouraged that P-BCMA-101 is demonstrating significant efficacy even at doses that have been ineffective for other anti-BCMA CAR-T therapies and that our response rates continue to improve as the dose increases.”
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
BOSTON—Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma (MM).
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome, but the condition resolved without use of tociluzimab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated MM. They had a median of six prior therapies. Their median age was 60, and 73% were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51×106 (n=3), 152×106 (n=7), and 430×106 (n=1).
As of August 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and 5 had thrombocytopenia.
Researchers suspected cytokine release syndrome in one patient, but the condition resolved without tociluzimab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was only evaluable by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
“We believe our advantages of a purified product, where all cells express the CAR molecule, and a product with high levels of stem cell memory T cells, producing a more gradual and prolonged immune response against tumor cells, provide a significantly better therapeutic index when compared with other CAR-T therapeutics. We are also encouraged that P-BCMA-101 is demonstrating significant efficacy even at doses that have been ineffective for other anti-BCMA CAR-T therapies and that our response rates continue to improve as the dose increases.”
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
BOSTON—Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma (MM).
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome, but the condition resolved without use of tociluzimab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated MM. They had a median of six prior therapies. Their median age was 60, and 73% were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51×106 (n=3), 152×106 (n=7), and 430×106 (n=1).
As of August 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and 5 had thrombocytopenia.
Researchers suspected cytokine release syndrome in one patient, but the condition resolved without tociluzimab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was only evaluable by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
“We believe our advantages of a purified product, where all cells express the CAR molecule, and a product with high levels of stem cell memory T cells, producing a more gradual and prolonged immune response against tumor cells, provide a significantly better therapeutic index when compared with other CAR-T therapeutics. We are also encouraged that P-BCMA-101 is demonstrating significant efficacy even at doses that have been ineffective for other anti-BCMA CAR-T therapies and that our response rates continue to improve as the dose increases.”
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
Kids with BCP-ALL exhibit immunological disparities at birth
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, a PhD student at Statens Serum Institut in Copenhagen, Denmark.
“This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
“Importantly, our study does not inform about the nature of the associations observed—i.e., whether they are causal or consequential. Accordingly, further studies are needed both to confirm the findings and to identify the underlying mechanisms.”
For this study, Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1 to 9.
The inflammatory markers assessed were interleukin (IL)-6, its soluble receptor sIL-6Rα, IL-8, IL-10, IL-12, IL-17, IL-18, transforming growth factor (TGF)-β1, monocyte chemotactic protein (MCP)-1, and C-reactive protein (CRP).
Results
Compared to controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of sIL-6Rα, IL-8, TGF-β1, MCP-1, and CRP were significantly lower among the BCP-ALL patients. The adjusted odds ratios (adjusted for birth weight and maternal age) of BCP-ALL were 0.82 for sIL-6Rα, 0.84 for IL-8, 0.83 for TGF-β1, 0.68 for MCP-1, and 0.83 for CRP.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls. The adjusted odds ratios were 1.19 for IL-6, 1.12 for IL-17, and 1.08 for IL-18.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls (31% to 61%) had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors—namely, birth order, gestational age, and sex—were associated with the neonatal concentrations of inflammatory markers,” Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6Rα and TGF-β1 concentrations and higher CRP concentrations. And males had significantly lower sIL-6Rα and IL-8 concentrations and higher CRP concentrations than females.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact due to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL. In future studies, we will further characterize the relation between immune constitution at birth and risk of childhood ALL with the ultimate goal of developing preventive strategies targeting predisposed children.”
Søegaard noted that this study had its limitations, including the small number of inflammatory markers studied. In addition, the limited sample size made it impossible to detect potential differences between BCP-ALL subtypes.
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. There were no conflicts of interest disclosed.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, a PhD student at Statens Serum Institut in Copenhagen, Denmark.
“This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
“Importantly, our study does not inform about the nature of the associations observed—i.e., whether they are causal or consequential. Accordingly, further studies are needed both to confirm the findings and to identify the underlying mechanisms.”
For this study, Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1 to 9.
The inflammatory markers assessed were interleukin (IL)-6, its soluble receptor sIL-6Rα, IL-8, IL-10, IL-12, IL-17, IL-18, transforming growth factor (TGF)-β1, monocyte chemotactic protein (MCP)-1, and C-reactive protein (CRP).
Results
Compared to controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of sIL-6Rα, IL-8, TGF-β1, MCP-1, and CRP were significantly lower among the BCP-ALL patients. The adjusted odds ratios (adjusted for birth weight and maternal age) of BCP-ALL were 0.82 for sIL-6Rα, 0.84 for IL-8, 0.83 for TGF-β1, 0.68 for MCP-1, and 0.83 for CRP.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls. The adjusted odds ratios were 1.19 for IL-6, 1.12 for IL-17, and 1.08 for IL-18.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls (31% to 61%) had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors—namely, birth order, gestational age, and sex—were associated with the neonatal concentrations of inflammatory markers,” Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6Rα and TGF-β1 concentrations and higher CRP concentrations. And males had significantly lower sIL-6Rα and IL-8 concentrations and higher CRP concentrations than females.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact due to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL. In future studies, we will further characterize the relation between immune constitution at birth and risk of childhood ALL with the ultimate goal of developing preventive strategies targeting predisposed children.”
Søegaard noted that this study had its limitations, including the small number of inflammatory markers studied. In addition, the limited sample size made it impossible to detect potential differences between BCP-ALL subtypes.
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. There were no conflicts of interest disclosed.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, a PhD student at Statens Serum Institut in Copenhagen, Denmark.
“This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
“Importantly, our study does not inform about the nature of the associations observed—i.e., whether they are causal or consequential. Accordingly, further studies are needed both to confirm the findings and to identify the underlying mechanisms.”
For this study, Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1 to 9.
The inflammatory markers assessed were interleukin (IL)-6, its soluble receptor sIL-6Rα, IL-8, IL-10, IL-12, IL-17, IL-18, transforming growth factor (TGF)-β1, monocyte chemotactic protein (MCP)-1, and C-reactive protein (CRP).
Results
Compared to controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of sIL-6Rα, IL-8, TGF-β1, MCP-1, and CRP were significantly lower among the BCP-ALL patients. The adjusted odds ratios (adjusted for birth weight and maternal age) of BCP-ALL were 0.82 for sIL-6Rα, 0.84 for IL-8, 0.83 for TGF-β1, 0.68 for MCP-1, and 0.83 for CRP.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls. The adjusted odds ratios were 1.19 for IL-6, 1.12 for IL-17, and 1.08 for IL-18.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls (31% to 61%) had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors—namely, birth order, gestational age, and sex—were associated with the neonatal concentrations of inflammatory markers,” Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6Rα and TGF-β1 concentrations and higher CRP concentrations. And males had significantly lower sIL-6Rα and IL-8 concentrations and higher CRP concentrations than females.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact due to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL. In future studies, we will further characterize the relation between immune constitution at birth and risk of childhood ALL with the ultimate goal of developing preventive strategies targeting predisposed children.”
Søegaard noted that this study had its limitations, including the small number of inflammatory markers studied. In addition, the limited sample size made it impossible to detect potential differences between BCP-ALL subtypes.
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. There were no conflicts of interest disclosed.
FDA approves drug for hairy cell leukemia
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.