User login
Potential therapeutic target for leukemia, other cancers

Photo by Thomas Semkow
Preclinical research indicates that a member of the Mediator protein complex plays a key role in hematopoiesis.
Investigators found that MED12 was required for the survival of hematopoietic stem and progenitor cells (HSPCs).
The team said this finding, along with the fact that MED12 mutations have been linked to leukemia and solid tumor malignancies, suggests that targeting MED12 hyperactivity might be a useful strategy for treating cancers.
“Because MED12 appears to be so essential to hematopoiesis, our study points to it as a possible target for future anticancer therapies for both chronic and acute forms of leukemia,” said Iannis Aifantis, PhD, of NYU Langone Medical Center in New York.
“Our study also suggests that MED12 hyperactivation or loss of control is a possible explanation for what factors may trigger these cancers and other solid tumors.”
Dr Aifantis and his colleagues described their study in Cell Stem Cell.
The investigators first analyzed the effects of MED12 deletion in mice. Mice bred to lack MED12 died within 2 weeks of birth and showed evidence of aberrant hematopoiesis—namely, a “severe reduction of bone marrow and thymus cellularity.”
Adult mice that were engineered to lose expression of MED12 after the injection of an activating molecule experienced a “rapid” reduction in bone marrow cellularity, as well as reductions in spleen and thymus size. These mice also had low white blood cell and platelet counts and died within 3 weeks of MED12 deletion.
Subsequent analyses of the animals’ bone marrow showed that estimates of HSPCs in each mouse fell from nearly 150,000 to 15,000 within 4 days of injection. Within 10 days, there were no HSPCs left.
Deleting MED12 was also lethal for human HSPCs. Colonies of CD34+ cells dropped from an average of 25 per plate to 5 per plate within 10 days of MED12 deletion.
On the other hand, MED12 did not affect the survival of other cell types. For example, MED12 deletion did not impact mouse embryonic fibroblasts, embryonic stem cells, or hair follicle stem cells.
In addition, deleting members of the Mediator kinase module besides MED12—MED13, CDK8, or CYCLIN C—did not have a significant effect on HSPCs and did not kill mice. The investigators said this provides further evidence that MED12—by loss of its function alone—is essential for hematopoiesis.
The team found that MED12 deletion destabilizes P300 binding at lineage-specific enhancers, which results in H3K27Ac depletion, enhancer de-activation, and the consequent loss of hematopoietic stem cell gene expression signatures.
As a next step, the investigators plan to screen blood samples from cancer patients for signs of MED12 mutations and uncontrolled HSPC development.
The team also hopes to determine the biological mechanisms involved in MED12 hyperactivation and identify drug molecules that could block MED12 hyperactivity and serve as potential MED12 inhibitors. ![]()

Photo by Thomas Semkow
Preclinical research indicates that a member of the Mediator protein complex plays a key role in hematopoiesis.
Investigators found that MED12 was required for the survival of hematopoietic stem and progenitor cells (HSPCs).
The team said this finding, along with the fact that MED12 mutations have been linked to leukemia and solid tumor malignancies, suggests that targeting MED12 hyperactivity might be a useful strategy for treating cancers.
“Because MED12 appears to be so essential to hematopoiesis, our study points to it as a possible target for future anticancer therapies for both chronic and acute forms of leukemia,” said Iannis Aifantis, PhD, of NYU Langone Medical Center in New York.
“Our study also suggests that MED12 hyperactivation or loss of control is a possible explanation for what factors may trigger these cancers and other solid tumors.”
Dr Aifantis and his colleagues described their study in Cell Stem Cell.
The investigators first analyzed the effects of MED12 deletion in mice. Mice bred to lack MED12 died within 2 weeks of birth and showed evidence of aberrant hematopoiesis—namely, a “severe reduction of bone marrow and thymus cellularity.”
Adult mice that were engineered to lose expression of MED12 after the injection of an activating molecule experienced a “rapid” reduction in bone marrow cellularity, as well as reductions in spleen and thymus size. These mice also had low white blood cell and platelet counts and died within 3 weeks of MED12 deletion.
Subsequent analyses of the animals’ bone marrow showed that estimates of HSPCs in each mouse fell from nearly 150,000 to 15,000 within 4 days of injection. Within 10 days, there were no HSPCs left.
Deleting MED12 was also lethal for human HSPCs. Colonies of CD34+ cells dropped from an average of 25 per plate to 5 per plate within 10 days of MED12 deletion.
On the other hand, MED12 did not affect the survival of other cell types. For example, MED12 deletion did not impact mouse embryonic fibroblasts, embryonic stem cells, or hair follicle stem cells.
In addition, deleting members of the Mediator kinase module besides MED12—MED13, CDK8, or CYCLIN C—did not have a significant effect on HSPCs and did not kill mice. The investigators said this provides further evidence that MED12—by loss of its function alone—is essential for hematopoiesis.
The team found that MED12 deletion destabilizes P300 binding at lineage-specific enhancers, which results in H3K27Ac depletion, enhancer de-activation, and the consequent loss of hematopoietic stem cell gene expression signatures.
As a next step, the investigators plan to screen blood samples from cancer patients for signs of MED12 mutations and uncontrolled HSPC development.
The team also hopes to determine the biological mechanisms involved in MED12 hyperactivation and identify drug molecules that could block MED12 hyperactivity and serve as potential MED12 inhibitors. ![]()

Photo by Thomas Semkow
Preclinical research indicates that a member of the Mediator protein complex plays a key role in hematopoiesis.
Investigators found that MED12 was required for the survival of hematopoietic stem and progenitor cells (HSPCs).
The team said this finding, along with the fact that MED12 mutations have been linked to leukemia and solid tumor malignancies, suggests that targeting MED12 hyperactivity might be a useful strategy for treating cancers.
“Because MED12 appears to be so essential to hematopoiesis, our study points to it as a possible target for future anticancer therapies for both chronic and acute forms of leukemia,” said Iannis Aifantis, PhD, of NYU Langone Medical Center in New York.
“Our study also suggests that MED12 hyperactivation or loss of control is a possible explanation for what factors may trigger these cancers and other solid tumors.”
Dr Aifantis and his colleagues described their study in Cell Stem Cell.
The investigators first analyzed the effects of MED12 deletion in mice. Mice bred to lack MED12 died within 2 weeks of birth and showed evidence of aberrant hematopoiesis—namely, a “severe reduction of bone marrow and thymus cellularity.”
Adult mice that were engineered to lose expression of MED12 after the injection of an activating molecule experienced a “rapid” reduction in bone marrow cellularity, as well as reductions in spleen and thymus size. These mice also had low white blood cell and platelet counts and died within 3 weeks of MED12 deletion.
Subsequent analyses of the animals’ bone marrow showed that estimates of HSPCs in each mouse fell from nearly 150,000 to 15,000 within 4 days of injection. Within 10 days, there were no HSPCs left.
Deleting MED12 was also lethal for human HSPCs. Colonies of CD34+ cells dropped from an average of 25 per plate to 5 per plate within 10 days of MED12 deletion.
On the other hand, MED12 did not affect the survival of other cell types. For example, MED12 deletion did not impact mouse embryonic fibroblasts, embryonic stem cells, or hair follicle stem cells.
In addition, deleting members of the Mediator kinase module besides MED12—MED13, CDK8, or CYCLIN C—did not have a significant effect on HSPCs and did not kill mice. The investigators said this provides further evidence that MED12—by loss of its function alone—is essential for hematopoiesis.
The team found that MED12 deletion destabilizes P300 binding at lineage-specific enhancers, which results in H3K27Ac depletion, enhancer de-activation, and the consequent loss of hematopoietic stem cell gene expression signatures.
As a next step, the investigators plan to screen blood samples from cancer patients for signs of MED12 mutations and uncontrolled HSPC development.
The team also hopes to determine the biological mechanisms involved in MED12 hyperactivation and identify drug molecules that could block MED12 hyperactivity and serve as potential MED12 inhibitors. ![]()
NICE approves bosutinib for routine NHS use
The National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending that bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML), be made available through the National Health Service (NHS).
This means patients will no longer have to apply to the Cancer Drugs Fund (CDF) to obtain bosutinib.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
NICE previously considered making bosutinib available through the NHS in 2013 but decided the drug was not cost-effective. So bosutinib was made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib. ![]()

The National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending that bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML), be made available through the National Health Service (NHS).
This means patients will no longer have to apply to the Cancer Drugs Fund (CDF) to obtain bosutinib.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
NICE previously considered making bosutinib available through the NHS in 2013 but decided the drug was not cost-effective. So bosutinib was made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib. ![]()

The National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending that bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML), be made available through the National Health Service (NHS).
This means patients will no longer have to apply to the Cancer Drugs Fund (CDF) to obtain bosutinib.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
NICE previously considered making bosutinib available through the NHS in 2013 but decided the drug was not cost-effective. So bosutinib was made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib. ![]()
FDA grants drug orphan designation for CLL

The US Food and Drug Administration (FDA) has granted orphan drug designation to the PI3K delta inhibitor TGR-1202 for the treatment of chronic lymphocytic leukemia (CLL).
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases.
This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved.
About TGR-1202
TG Therapeutics, Inc. is developing TGR-1202 as a treatment for hematologic malignancies.
The drug is currently being evaluated in the phase 3 UNITY-CLL trial (NCT02612311), which includes patients with previously treated and untreated CLL. Patients are receiving TGR-1202 plus ublituximab, obinutuzumab plus chlorambucil, ublituximab alone, or TGR-1202 alone.
At EHA 2016, researchers reported preliminary results of a phase 1/1b study (NCT02268851) of TGR-1202 in combination with ibrutinib in patients with relapsed/refractory CLL or mantle cell lymphoma.
At ASCO 2016, researchers reported long-term follow-up of 2 studies of TGR-1202.
The first (TGR-1202-101, NCT01767766) is a phase 1 study of TGR-1202 in patients with relapsed or refractory hematologic malignancies.
The second (UTX-TGR-103, NCT02006485) is a phase 1/1b trial evaluating the combination of ublituximab and TGR-1202 in patients with relapsed or refractory non-Hodgkin lymphoma or CLL.
At ASH 2015, researchers reported results of a phase 1 trial (TGR-GA-106, NCT02100852) of TGR-1202 in combination with obinutuzumab and chlorambucil in patients with CLL. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to the PI3K delta inhibitor TGR-1202 for the treatment of chronic lymphocytic leukemia (CLL).
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases.
This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved.
About TGR-1202
TG Therapeutics, Inc. is developing TGR-1202 as a treatment for hematologic malignancies.
The drug is currently being evaluated in the phase 3 UNITY-CLL trial (NCT02612311), which includes patients with previously treated and untreated CLL. Patients are receiving TGR-1202 plus ublituximab, obinutuzumab plus chlorambucil, ublituximab alone, or TGR-1202 alone.
At EHA 2016, researchers reported preliminary results of a phase 1/1b study (NCT02268851) of TGR-1202 in combination with ibrutinib in patients with relapsed/refractory CLL or mantle cell lymphoma.
At ASCO 2016, researchers reported long-term follow-up of 2 studies of TGR-1202.
The first (TGR-1202-101, NCT01767766) is a phase 1 study of TGR-1202 in patients with relapsed or refractory hematologic malignancies.
The second (UTX-TGR-103, NCT02006485) is a phase 1/1b trial evaluating the combination of ublituximab and TGR-1202 in patients with relapsed or refractory non-Hodgkin lymphoma or CLL.
At ASH 2015, researchers reported results of a phase 1 trial (TGR-GA-106, NCT02100852) of TGR-1202 in combination with obinutuzumab and chlorambucil in patients with CLL. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to the PI3K delta inhibitor TGR-1202 for the treatment of chronic lymphocytic leukemia (CLL).
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases.
This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved.
About TGR-1202
TG Therapeutics, Inc. is developing TGR-1202 as a treatment for hematologic malignancies.
The drug is currently being evaluated in the phase 3 UNITY-CLL trial (NCT02612311), which includes patients with previously treated and untreated CLL. Patients are receiving TGR-1202 plus ublituximab, obinutuzumab plus chlorambucil, ublituximab alone, or TGR-1202 alone.
At EHA 2016, researchers reported preliminary results of a phase 1/1b study (NCT02268851) of TGR-1202 in combination with ibrutinib in patients with relapsed/refractory CLL or mantle cell lymphoma.
At ASCO 2016, researchers reported long-term follow-up of 2 studies of TGR-1202.
The first (TGR-1202-101, NCT01767766) is a phase 1 study of TGR-1202 in patients with relapsed or refractory hematologic malignancies.
The second (UTX-TGR-103, NCT02006485) is a phase 1/1b trial evaluating the combination of ublituximab and TGR-1202 in patients with relapsed or refractory non-Hodgkin lymphoma or CLL.
At ASH 2015, researchers reported results of a phase 1 trial (TGR-GA-106, NCT02100852) of TGR-1202 in combination with obinutuzumab and chlorambucil in patients with CLL. ![]()
Excess weight linked to myeloma, other cancers

An analysis of more than 1000 studies suggests multiple myeloma (MM) and 12 other cancers are associated with excess weight.
The data suggest that limiting weight gain over time could help reduce a person’s risk of developing these cancers.
A working group convened by the International Agency for Research on Cancer (IARC) conducted this analysis and reported the results in NEJM.
“The burden of cancer due to being overweight or obese is more extensive than what has been assumed,” said Graham Colditz, MD, DrPH, who chaired the IARC working group.
“Many of the newly identified cancers linked to excess weight haven’t been on people’s radar screens as having a weight component.”
In 2002, an IARC working group reported finding sufficient evidence linking excess weight to higher risks of colon, esophageal, kidney, breast, and uterine cancers.
Now, another IARC working group has found evidence linking excess weight and additional cancers.
The group reviewed more than 1000 epidemiologic studies. Most of the studies provided cancer risk estimates for adult body mass index (BMI), although some provided estimates for BMI or body shape in childhood/adolescence, changes in BMI or weight over time, or other indicators of adiposity.
The IARC working group reported the relative risk (RR) of developing various cancers for the highest BMI category evaluated, versus a normal BMI.
The group said there was sufficient evidence linking excess weight to the following cancers: adenocarcinoma (RR=4.8), gastric cardia (RR=1.8), colon and rectal cancer (RR=1.3), liver cancer (RR=1.8), gallbladder cancer (RR=1.3), pancreatic cancer (RR=1.5), postmenopausal breast cancer (RR=1.1), corpus uteri (RR=7.1), ovarian cancer (RR=1.1), renal cell cancer (RR=1.8), meningioma (RR=1.5), thyroid cancer (RR=1.1), and MM (RR=1.5).
Looking more closely at MM, the RR was 1.2 for adults who were overweight (BMI 25-29.9), 1.2 for those with class 1 obesity (BMI 30-34.9), and 1.5 for those with class 2 or 3 obesity (BMI 35-40+).
For most of the cancers, there was positive dose-response relationship; in other words, the higher the BMI, the greater the cancer risk.
In addition, the cancer risks associated with excess weight were similar for men and women and were consistent across geographic regions—North America, Europe, Asia, and the Middle East—where data were available.
“Significant numbers of the US and the world’s population are overweight,” Dr Colditz noted. “This is another wake-up call. It’s time to take our health and our diets seriously.”
Dr Colditz conceded that losing weight can be difficult. Therefore, he recommended that people who struggle with weight loss should focus on avoiding weight gain to reduce their risk of developing certain cancers. ![]()

An analysis of more than 1000 studies suggests multiple myeloma (MM) and 12 other cancers are associated with excess weight.
The data suggest that limiting weight gain over time could help reduce a person’s risk of developing these cancers.
A working group convened by the International Agency for Research on Cancer (IARC) conducted this analysis and reported the results in NEJM.
“The burden of cancer due to being overweight or obese is more extensive than what has been assumed,” said Graham Colditz, MD, DrPH, who chaired the IARC working group.
“Many of the newly identified cancers linked to excess weight haven’t been on people’s radar screens as having a weight component.”
In 2002, an IARC working group reported finding sufficient evidence linking excess weight to higher risks of colon, esophageal, kidney, breast, and uterine cancers.
Now, another IARC working group has found evidence linking excess weight and additional cancers.
The group reviewed more than 1000 epidemiologic studies. Most of the studies provided cancer risk estimates for adult body mass index (BMI), although some provided estimates for BMI or body shape in childhood/adolescence, changes in BMI or weight over time, or other indicators of adiposity.
The IARC working group reported the relative risk (RR) of developing various cancers for the highest BMI category evaluated, versus a normal BMI.
The group said there was sufficient evidence linking excess weight to the following cancers: adenocarcinoma (RR=4.8), gastric cardia (RR=1.8), colon and rectal cancer (RR=1.3), liver cancer (RR=1.8), gallbladder cancer (RR=1.3), pancreatic cancer (RR=1.5), postmenopausal breast cancer (RR=1.1), corpus uteri (RR=7.1), ovarian cancer (RR=1.1), renal cell cancer (RR=1.8), meningioma (RR=1.5), thyroid cancer (RR=1.1), and MM (RR=1.5).
Looking more closely at MM, the RR was 1.2 for adults who were overweight (BMI 25-29.9), 1.2 for those with class 1 obesity (BMI 30-34.9), and 1.5 for those with class 2 or 3 obesity (BMI 35-40+).
For most of the cancers, there was positive dose-response relationship; in other words, the higher the BMI, the greater the cancer risk.
In addition, the cancer risks associated with excess weight were similar for men and women and were consistent across geographic regions—North America, Europe, Asia, and the Middle East—where data were available.
“Significant numbers of the US and the world’s population are overweight,” Dr Colditz noted. “This is another wake-up call. It’s time to take our health and our diets seriously.”
Dr Colditz conceded that losing weight can be difficult. Therefore, he recommended that people who struggle with weight loss should focus on avoiding weight gain to reduce their risk of developing certain cancers. ![]()

An analysis of more than 1000 studies suggests multiple myeloma (MM) and 12 other cancers are associated with excess weight.
The data suggest that limiting weight gain over time could help reduce a person’s risk of developing these cancers.
A working group convened by the International Agency for Research on Cancer (IARC) conducted this analysis and reported the results in NEJM.
“The burden of cancer due to being overweight or obese is more extensive than what has been assumed,” said Graham Colditz, MD, DrPH, who chaired the IARC working group.
“Many of the newly identified cancers linked to excess weight haven’t been on people’s radar screens as having a weight component.”
In 2002, an IARC working group reported finding sufficient evidence linking excess weight to higher risks of colon, esophageal, kidney, breast, and uterine cancers.
Now, another IARC working group has found evidence linking excess weight and additional cancers.
The group reviewed more than 1000 epidemiologic studies. Most of the studies provided cancer risk estimates for adult body mass index (BMI), although some provided estimates for BMI or body shape in childhood/adolescence, changes in BMI or weight over time, or other indicators of adiposity.
The IARC working group reported the relative risk (RR) of developing various cancers for the highest BMI category evaluated, versus a normal BMI.
The group said there was sufficient evidence linking excess weight to the following cancers: adenocarcinoma (RR=4.8), gastric cardia (RR=1.8), colon and rectal cancer (RR=1.3), liver cancer (RR=1.8), gallbladder cancer (RR=1.3), pancreatic cancer (RR=1.5), postmenopausal breast cancer (RR=1.1), corpus uteri (RR=7.1), ovarian cancer (RR=1.1), renal cell cancer (RR=1.8), meningioma (RR=1.5), thyroid cancer (RR=1.1), and MM (RR=1.5).
Looking more closely at MM, the RR was 1.2 for adults who were overweight (BMI 25-29.9), 1.2 for those with class 1 obesity (BMI 30-34.9), and 1.5 for those with class 2 or 3 obesity (BMI 35-40+).
For most of the cancers, there was positive dose-response relationship; in other words, the higher the BMI, the greater the cancer risk.
In addition, the cancer risks associated with excess weight were similar for men and women and were consistent across geographic regions—North America, Europe, Asia, and the Middle East—where data were available.
“Significant numbers of the US and the world’s population are overweight,” Dr Colditz noted. “This is another wake-up call. It’s time to take our health and our diets seriously.”
Dr Colditz conceded that losing weight can be difficult. Therefore, he recommended that people who struggle with weight loss should focus on avoiding weight gain to reduce their risk of developing certain cancers. ![]()
Reversible FXIa inhibitor may reduce bleeding risk

Photo by Sakurai Midori
Researchers say they have developed an antibody that inhibits factor XIa (FXIa) without increasing the risk of bleeding, as well as an antibody that can reverse the inhibitor’s effects.
The inhibitor—known as DEF—staved off clotting in human blood and animal models.
Even when it was given at extremely high doses, DEF did not induce bleeding in the animals.
Still, the researchers developed an antibody—revC4—that can reverse DEF’s activity.
Tovo David, PhD, of University of California, San Francisco, and his colleagues described this work in Science Translational Medicine.
Initially, the researchers generated a series of human immunoglobulin Gs (IgGs) that blocked FXIa active-site function but did not bind FXI zymogen or other coagulation proteases.
The most potent of these was C24. This IgG inhibited FXIIa-induced thrombin generation and intrinsic pathway–triggered clot formation in human plasma and whole blood. C24 also inhibited FeCl3-induced arterial thrombosis in an FXI-humanized mouse.
Dr David and his colleagues then set out to improve upon C24, rendering it unable to activate complement, engage Fc receptors, or activate platelets and other cells. The resulting molecule was DEF.
The researchers tested DEF in rabbits and cynomolgus macaques. At doses much higher than those required to inhibit thrombus formation, DEF did not increase cuticle bleeding in the rabbits or cause spontaneous bleeding in the monkeys.
Despite this lack of bleeding, Dr David and his colleagues generated a human IgG that can reverse DEF activity because FXI deficiency can be associated with bleeding in humans.
This reversal agent—revC4—proved effective in human plasma (ex vivo) and in rabbits. revC4 reversed the anticoagulant effect of DEF within 30 minutes of dosing.
Based on these results, the researchers concluded that, with further development, their reversible FXIa-specific antibody might provide a new—and potentially safer—type of anticoagulant. ![]()

Photo by Sakurai Midori
Researchers say they have developed an antibody that inhibits factor XIa (FXIa) without increasing the risk of bleeding, as well as an antibody that can reverse the inhibitor’s effects.
The inhibitor—known as DEF—staved off clotting in human blood and animal models.
Even when it was given at extremely high doses, DEF did not induce bleeding in the animals.
Still, the researchers developed an antibody—revC4—that can reverse DEF’s activity.
Tovo David, PhD, of University of California, San Francisco, and his colleagues described this work in Science Translational Medicine.
Initially, the researchers generated a series of human immunoglobulin Gs (IgGs) that blocked FXIa active-site function but did not bind FXI zymogen or other coagulation proteases.
The most potent of these was C24. This IgG inhibited FXIIa-induced thrombin generation and intrinsic pathway–triggered clot formation in human plasma and whole blood. C24 also inhibited FeCl3-induced arterial thrombosis in an FXI-humanized mouse.
Dr David and his colleagues then set out to improve upon C24, rendering it unable to activate complement, engage Fc receptors, or activate platelets and other cells. The resulting molecule was DEF.
The researchers tested DEF in rabbits and cynomolgus macaques. At doses much higher than those required to inhibit thrombus formation, DEF did not increase cuticle bleeding in the rabbits or cause spontaneous bleeding in the monkeys.
Despite this lack of bleeding, Dr David and his colleagues generated a human IgG that can reverse DEF activity because FXI deficiency can be associated with bleeding in humans.
This reversal agent—revC4—proved effective in human plasma (ex vivo) and in rabbits. revC4 reversed the anticoagulant effect of DEF within 30 minutes of dosing.
Based on these results, the researchers concluded that, with further development, their reversible FXIa-specific antibody might provide a new—and potentially safer—type of anticoagulant. ![]()

Photo by Sakurai Midori
Researchers say they have developed an antibody that inhibits factor XIa (FXIa) without increasing the risk of bleeding, as well as an antibody that can reverse the inhibitor’s effects.
The inhibitor—known as DEF—staved off clotting in human blood and animal models.
Even when it was given at extremely high doses, DEF did not induce bleeding in the animals.
Still, the researchers developed an antibody—revC4—that can reverse DEF’s activity.
Tovo David, PhD, of University of California, San Francisco, and his colleagues described this work in Science Translational Medicine.
Initially, the researchers generated a series of human immunoglobulin Gs (IgGs) that blocked FXIa active-site function but did not bind FXI zymogen or other coagulation proteases.
The most potent of these was C24. This IgG inhibited FXIIa-induced thrombin generation and intrinsic pathway–triggered clot formation in human plasma and whole blood. C24 also inhibited FeCl3-induced arterial thrombosis in an FXI-humanized mouse.
Dr David and his colleagues then set out to improve upon C24, rendering it unable to activate complement, engage Fc receptors, or activate platelets and other cells. The resulting molecule was DEF.
The researchers tested DEF in rabbits and cynomolgus macaques. At doses much higher than those required to inhibit thrombus formation, DEF did not increase cuticle bleeding in the rabbits or cause spontaneous bleeding in the monkeys.
Despite this lack of bleeding, Dr David and his colleagues generated a human IgG that can reverse DEF activity because FXI deficiency can be associated with bleeding in humans.
This reversal agent—revC4—proved effective in human plasma (ex vivo) and in rabbits. revC4 reversed the anticoagulant effect of DEF within 30 minutes of dosing.
Based on these results, the researchers concluded that, with further development, their reversible FXIa-specific antibody might provide a new—and potentially safer—type of anticoagulant. ![]()
‘Barcoding’ reveals insights regarding HSCs

in the bone marrow
By assigning a “barcode” to hematopoietic stem cells (HSCs), researchers have found they can monitor the cells and study changes that occur over
time.
In tracking the barcoded HSCs, the team discovered why B-1a cells develop primarily during fetal and neonatal life, while adult bone marrow HSCs preferentially give rise to B-2 cells.
Joan Yuan, PhD, of Lund University in Sweden, and her colleagues described this discovery in Immunity.
“By assigning a barcode to the stem cells, we were able to track their performance over long periods of time and see which cells in the blood and the immune system they can induce,” Dr Yuan explained.
“Without the barcode, we only see a bunch of red and white blood cells, without knowing how they are related. This allows us to track which stem cell has given rise to which subsidiary cells and thereby distinguish the ‘family tree’ in the blood.”
In this way, the researchers found that B-1a cells and B-2 cells have a shared precursor in the fetal liver. And definitive fetal liver HSCs gave rise to both B-1a and B-2 cells. However, over time, the HSCs were not able to maintain B-1a output.
“The same stem cells exist within adults [and fetuses], but they have lost their ability to regenerate the entire immune system [in adulthood],” said study author Trine Kristiansen, a doctoral student at Lund University.
“By adding a protein normally only found in the stem cells of a fetus, we were able to reconstruct [the HSCs’] capacity to produce white blood cells.”
The researchers restored the HSC’s ability to produce B-1a cells by inducing expression of the RNA binding protein LIN28B, which regulates fetal hematopoiesis.
The team said these results suggest the decline in regenerative potential is a reversible state for HSCs. The researchers believe this finding could have implications for the treatment of blood disorders and particularly for HSC transplant.
“In this treatment, the patient’s blood system is replaced with that of an adult donor, which could mean losing the B cells that are only produced in fetuses,” Kristiansen said.
Without these cells, a person is at risk of developing immune system disorders that can lead to severe infections and autoimmune diseases.
“Every day, millions of blood cells die, and they can emit DNA and other debris that cause inflammation if not taken care of by the white blood cells,” said study author Elin Jaensson Gyllenbäck, PhD, of Lund University.
“The discovery is a step towards understanding which processes create a proper immune system for those who suffer from blood diseases.” ![]()

in the bone marrow
By assigning a “barcode” to hematopoietic stem cells (HSCs), researchers have found they can monitor the cells and study changes that occur over
time.
In tracking the barcoded HSCs, the team discovered why B-1a cells develop primarily during fetal and neonatal life, while adult bone marrow HSCs preferentially give rise to B-2 cells.
Joan Yuan, PhD, of Lund University in Sweden, and her colleagues described this discovery in Immunity.
“By assigning a barcode to the stem cells, we were able to track their performance over long periods of time and see which cells in the blood and the immune system they can induce,” Dr Yuan explained.
“Without the barcode, we only see a bunch of red and white blood cells, without knowing how they are related. This allows us to track which stem cell has given rise to which subsidiary cells and thereby distinguish the ‘family tree’ in the blood.”
In this way, the researchers found that B-1a cells and B-2 cells have a shared precursor in the fetal liver. And definitive fetal liver HSCs gave rise to both B-1a and B-2 cells. However, over time, the HSCs were not able to maintain B-1a output.
“The same stem cells exist within adults [and fetuses], but they have lost their ability to regenerate the entire immune system [in adulthood],” said study author Trine Kristiansen, a doctoral student at Lund University.
“By adding a protein normally only found in the stem cells of a fetus, we were able to reconstruct [the HSCs’] capacity to produce white blood cells.”
The researchers restored the HSC’s ability to produce B-1a cells by inducing expression of the RNA binding protein LIN28B, which regulates fetal hematopoiesis.
The team said these results suggest the decline in regenerative potential is a reversible state for HSCs. The researchers believe this finding could have implications for the treatment of blood disorders and particularly for HSC transplant.
“In this treatment, the patient’s blood system is replaced with that of an adult donor, which could mean losing the B cells that are only produced in fetuses,” Kristiansen said.
Without these cells, a person is at risk of developing immune system disorders that can lead to severe infections and autoimmune diseases.
“Every day, millions of blood cells die, and they can emit DNA and other debris that cause inflammation if not taken care of by the white blood cells,” said study author Elin Jaensson Gyllenbäck, PhD, of Lund University.
“The discovery is a step towards understanding which processes create a proper immune system for those who suffer from blood diseases.” ![]()

in the bone marrow
By assigning a “barcode” to hematopoietic stem cells (HSCs), researchers have found they can monitor the cells and study changes that occur over
time.
In tracking the barcoded HSCs, the team discovered why B-1a cells develop primarily during fetal and neonatal life, while adult bone marrow HSCs preferentially give rise to B-2 cells.
Joan Yuan, PhD, of Lund University in Sweden, and her colleagues described this discovery in Immunity.
“By assigning a barcode to the stem cells, we were able to track their performance over long periods of time and see which cells in the blood and the immune system they can induce,” Dr Yuan explained.
“Without the barcode, we only see a bunch of red and white blood cells, without knowing how they are related. This allows us to track which stem cell has given rise to which subsidiary cells and thereby distinguish the ‘family tree’ in the blood.”
In this way, the researchers found that B-1a cells and B-2 cells have a shared precursor in the fetal liver. And definitive fetal liver HSCs gave rise to both B-1a and B-2 cells. However, over time, the HSCs were not able to maintain B-1a output.
“The same stem cells exist within adults [and fetuses], but they have lost their ability to regenerate the entire immune system [in adulthood],” said study author Trine Kristiansen, a doctoral student at Lund University.
“By adding a protein normally only found in the stem cells of a fetus, we were able to reconstruct [the HSCs’] capacity to produce white blood cells.”
The researchers restored the HSC’s ability to produce B-1a cells by inducing expression of the RNA binding protein LIN28B, which regulates fetal hematopoiesis.
The team said these results suggest the decline in regenerative potential is a reversible state for HSCs. The researchers believe this finding could have implications for the treatment of blood disorders and particularly for HSC transplant.
“In this treatment, the patient’s blood system is replaced with that of an adult donor, which could mean losing the B cells that are only produced in fetuses,” Kristiansen said.
Without these cells, a person is at risk of developing immune system disorders that can lead to severe infections and autoimmune diseases.
“Every day, millions of blood cells die, and they can emit DNA and other debris that cause inflammation if not taken care of by the white blood cells,” said study author Elin Jaensson Gyllenbäck, PhD, of Lund University.
“The discovery is a step towards understanding which processes create a proper immune system for those who suffer from blood diseases.” ![]()
Nanoparticles speed blood clotting in multiple models
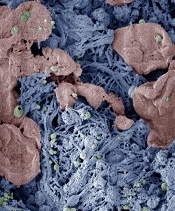
form clots in injured liver
Image courtesy of Erin Lavik
PHILADELPHIA—Researchers say they have developed nanoparticles that can congregate at sites of injury and speed blood clotting in vitro and in vivo.
The nanoparticles are designed to bind with activated platelets and help them join together to form clots faster.
The nanoparticles have helped stop bleeding and improved animals’ survival in different models of injury, without producing off-target clotting.
“When you have uncontrolled internal bleeding, that’s when these particles could really make a difference,” said Erin B. Lavik, ScD, of the University of Maryland, Baltimore County (UMBC) in Baltimore, Maryland.
“Compared to injuries that aren’t treated with the nanoparticles, we can cut bleeding time in half and reduce total blood loss.”
Dr Lavik discussed this work at the 252nd National Meeting & Exposition of the American Chemical Society.
She and her colleagues have been testing different versions of these nanoparticles for years. They previously reported results with the particles in 2012 and 2014.
The current nanoparticles are made from polylactic acid or polylactic co-glycolic acid. Dr Lavik said that by using the 2 different materials, the researchers can change the temperature at which the nanoparticles melt. Thus far, the team has developed nanoparticles that are stable up to 50° C (122° F).
The nanoparticles have an outer coating of polyethylene glycol and an attached peptide—RGD—that enables them to bind to natural platelets. Dr Lavik said the researchers chose RGD because platelets have receptors for that peptide, it’s ubiquitous in the body, and it wouldn’t alter the chemistry of the nanoparticles.
The researchers have tested the nanoparticles in a femoral injury model, a liver injury model, and blast injury model. The team has also started testing in models of brain and spinal cord injury, but the results are “very preliminary,” according to Dr Lavik.
In the femoral injury model, the nanoparticles halved bleeding time when compared to no treatment. In both the liver injury model and the blast injury model, the nanoparticles stopped bleeding and improved survival.
Dr Lavik said these experiments have shown that the nanoparticles successfully speed clotting, and the clots formed are mechanically robust.
The nanoparticles also clear the body easily. When the particles are bound into a clot, they stay as long as the clot remains. They begin to degrade once the clot does and clear out of the bloodstream quickly.
Dr Lavik said the researchers haven’t seen non-specific clotting with the nanoparticles yet. However, this side effect remains a possibility because the particles are designed to bind to activated platelets, which may not be confined to the site of injury.
One unfortunate side effect occurred with an earlier version of the nanoparticles. They triggered an immune response, activating complement in pig’s blood. However, the researchers were able to modify the particles to reduce complement activation and the accompanying complications.
In future studies, Dr Lavik and her colleagues plan to test whether the new nanoparticles activate complement in human blood. The team also aims to verify that the nanoparticles don’t cause non-specific clotting. ![]()

form clots in injured liver
Image courtesy of Erin Lavik
PHILADELPHIA—Researchers say they have developed nanoparticles that can congregate at sites of injury and speed blood clotting in vitro and in vivo.
The nanoparticles are designed to bind with activated platelets and help them join together to form clots faster.
The nanoparticles have helped stop bleeding and improved animals’ survival in different models of injury, without producing off-target clotting.
“When you have uncontrolled internal bleeding, that’s when these particles could really make a difference,” said Erin B. Lavik, ScD, of the University of Maryland, Baltimore County (UMBC) in Baltimore, Maryland.
“Compared to injuries that aren’t treated with the nanoparticles, we can cut bleeding time in half and reduce total blood loss.”
Dr Lavik discussed this work at the 252nd National Meeting & Exposition of the American Chemical Society.
She and her colleagues have been testing different versions of these nanoparticles for years. They previously reported results with the particles in 2012 and 2014.
The current nanoparticles are made from polylactic acid or polylactic co-glycolic acid. Dr Lavik said that by using the 2 different materials, the researchers can change the temperature at which the nanoparticles melt. Thus far, the team has developed nanoparticles that are stable up to 50° C (122° F).
The nanoparticles have an outer coating of polyethylene glycol and an attached peptide—RGD—that enables them to bind to natural platelets. Dr Lavik said the researchers chose RGD because platelets have receptors for that peptide, it’s ubiquitous in the body, and it wouldn’t alter the chemistry of the nanoparticles.
The researchers have tested the nanoparticles in a femoral injury model, a liver injury model, and blast injury model. The team has also started testing in models of brain and spinal cord injury, but the results are “very preliminary,” according to Dr Lavik.
In the femoral injury model, the nanoparticles halved bleeding time when compared to no treatment. In both the liver injury model and the blast injury model, the nanoparticles stopped bleeding and improved survival.
Dr Lavik said these experiments have shown that the nanoparticles successfully speed clotting, and the clots formed are mechanically robust.
The nanoparticles also clear the body easily. When the particles are bound into a clot, they stay as long as the clot remains. They begin to degrade once the clot does and clear out of the bloodstream quickly.
Dr Lavik said the researchers haven’t seen non-specific clotting with the nanoparticles yet. However, this side effect remains a possibility because the particles are designed to bind to activated platelets, which may not be confined to the site of injury.
One unfortunate side effect occurred with an earlier version of the nanoparticles. They triggered an immune response, activating complement in pig’s blood. However, the researchers were able to modify the particles to reduce complement activation and the accompanying complications.
In future studies, Dr Lavik and her colleagues plan to test whether the new nanoparticles activate complement in human blood. The team also aims to verify that the nanoparticles don’t cause non-specific clotting. ![]()

form clots in injured liver
Image courtesy of Erin Lavik
PHILADELPHIA—Researchers say they have developed nanoparticles that can congregate at sites of injury and speed blood clotting in vitro and in vivo.
The nanoparticles are designed to bind with activated platelets and help them join together to form clots faster.
The nanoparticles have helped stop bleeding and improved animals’ survival in different models of injury, without producing off-target clotting.
“When you have uncontrolled internal bleeding, that’s when these particles could really make a difference,” said Erin B. Lavik, ScD, of the University of Maryland, Baltimore County (UMBC) in Baltimore, Maryland.
“Compared to injuries that aren’t treated with the nanoparticles, we can cut bleeding time in half and reduce total blood loss.”
Dr Lavik discussed this work at the 252nd National Meeting & Exposition of the American Chemical Society.
She and her colleagues have been testing different versions of these nanoparticles for years. They previously reported results with the particles in 2012 and 2014.
The current nanoparticles are made from polylactic acid or polylactic co-glycolic acid. Dr Lavik said that by using the 2 different materials, the researchers can change the temperature at which the nanoparticles melt. Thus far, the team has developed nanoparticles that are stable up to 50° C (122° F).
The nanoparticles have an outer coating of polyethylene glycol and an attached peptide—RGD—that enables them to bind to natural platelets. Dr Lavik said the researchers chose RGD because platelets have receptors for that peptide, it’s ubiquitous in the body, and it wouldn’t alter the chemistry of the nanoparticles.
The researchers have tested the nanoparticles in a femoral injury model, a liver injury model, and blast injury model. The team has also started testing in models of brain and spinal cord injury, but the results are “very preliminary,” according to Dr Lavik.
In the femoral injury model, the nanoparticles halved bleeding time when compared to no treatment. In both the liver injury model and the blast injury model, the nanoparticles stopped bleeding and improved survival.
Dr Lavik said these experiments have shown that the nanoparticles successfully speed clotting, and the clots formed are mechanically robust.
The nanoparticles also clear the body easily. When the particles are bound into a clot, they stay as long as the clot remains. They begin to degrade once the clot does and clear out of the bloodstream quickly.
Dr Lavik said the researchers haven’t seen non-specific clotting with the nanoparticles yet. However, this side effect remains a possibility because the particles are designed to bind to activated platelets, which may not be confined to the site of injury.
One unfortunate side effect occurred with an earlier version of the nanoparticles. They triggered an immune response, activating complement in pig’s blood. However, the researchers were able to modify the particles to reduce complement activation and the accompanying complications.
In future studies, Dr Lavik and her colleagues plan to test whether the new nanoparticles activate complement in human blood. The team also aims to verify that the nanoparticles don’t cause non-specific clotting.
Massage therapy seems to benefit cancer patients

Photo courtesy of Barbara
E. Carver/New York College
of Health Professions
Massage therapy can reduce pain, fatigue, and anxiety in cancer patients, according to a review and meta-analysis published in Pain Medicine.
Massage therapy is commonly used among people seeking pain management, and research has generally supported its use.
However, there has been no published, rigorous review of the available research and evidence for massage therapy’s efficacy for pain populations, especially for cancer populations.
So Courtney Boyd, of the Samueli Institute in Alexandria, Virginia, and her colleagues set out to conduct just such a review.
The team assessed the quality of massage therapy research and evidence for its efficacy in treating pain, function-related quality of life, and health-related quality of life in cancer patients.
The researchers reviewed data from 12 high-quality studies and 4 low-quality studies.
The team said they could not assess health-related quality of life, emotional stress, or activity outcomes because too few of the studies examined these outcomes.
However, the data suggested that massage therapy can effectively reduce pain intensity or severity when compared to no treatment (standardized mean difference [SMD]=-0.20) or active comparators (SMD=-0.55).
Massage therapy also proved effective for reducing fatigue (SMD=-1.06) and anxiety (SMD=-1.24) when compared to active comparators.
Based on these results, the researchers concluded that massage therapy may have beneficial effects in cancer patients, so they should consider it as an option.
However, before definitive clinical conclusions and recommendations can be made at a policy level, specific factors surrounding the massage protocol, as well as selection of appropriate controls and standard outcomes, need to be well-understood.

Photo courtesy of Barbara
E. Carver/New York College
of Health Professions
Massage therapy can reduce pain, fatigue, and anxiety in cancer patients, according to a review and meta-analysis published in Pain Medicine.
Massage therapy is commonly used among people seeking pain management, and research has generally supported its use.
However, there has been no published, rigorous review of the available research and evidence for massage therapy’s efficacy for pain populations, especially for cancer populations.
So Courtney Boyd, of the Samueli Institute in Alexandria, Virginia, and her colleagues set out to conduct just such a review.
The team assessed the quality of massage therapy research and evidence for its efficacy in treating pain, function-related quality of life, and health-related quality of life in cancer patients.
The researchers reviewed data from 12 high-quality studies and 4 low-quality studies.
The team said they could not assess health-related quality of life, emotional stress, or activity outcomes because too few of the studies examined these outcomes.
However, the data suggested that massage therapy can effectively reduce pain intensity or severity when compared to no treatment (standardized mean difference [SMD]=-0.20) or active comparators (SMD=-0.55).
Massage therapy also proved effective for reducing fatigue (SMD=-1.06) and anxiety (SMD=-1.24) when compared to active comparators.
Based on these results, the researchers concluded that massage therapy may have beneficial effects in cancer patients, so they should consider it as an option.
However, before definitive clinical conclusions and recommendations can be made at a policy level, specific factors surrounding the massage protocol, as well as selection of appropriate controls and standard outcomes, need to be well-understood.

Photo courtesy of Barbara
E. Carver/New York College
of Health Professions
Massage therapy can reduce pain, fatigue, and anxiety in cancer patients, according to a review and meta-analysis published in Pain Medicine.
Massage therapy is commonly used among people seeking pain management, and research has generally supported its use.
However, there has been no published, rigorous review of the available research and evidence for massage therapy’s efficacy for pain populations, especially for cancer populations.
So Courtney Boyd, of the Samueli Institute in Alexandria, Virginia, and her colleagues set out to conduct just such a review.
The team assessed the quality of massage therapy research and evidence for its efficacy in treating pain, function-related quality of life, and health-related quality of life in cancer patients.
The researchers reviewed data from 12 high-quality studies and 4 low-quality studies.
The team said they could not assess health-related quality of life, emotional stress, or activity outcomes because too few of the studies examined these outcomes.
However, the data suggested that massage therapy can effectively reduce pain intensity or severity when compared to no treatment (standardized mean difference [SMD]=-0.20) or active comparators (SMD=-0.55).
Massage therapy also proved effective for reducing fatigue (SMD=-1.06) and anxiety (SMD=-1.24) when compared to active comparators.
Based on these results, the researchers concluded that massage therapy may have beneficial effects in cancer patients, so they should consider it as an option.
However, before definitive clinical conclusions and recommendations can be made at a policy level, specific factors surrounding the massage protocol, as well as selection of appropriate controls and standard outcomes, need to be well-understood.
Drug granted breakthrough designation for BPDCN

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for SL-401 in the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies.
SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
The drug is under development by Stemline Therapeutics, Inc.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 2 trial of SL-401
The breakthrough designation for SL-401 was supported by data from a phase 2 trial of patients with BPDCN or acute myeloid leukemia. Results observed in the BPDCN patients were presented at the 2016 EHA Congress (abstract S812).
The study consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
At the data cut-off, 18 BPDCN patients had received SL-401 at 7 μg/kg (n=3, stage 1) or 12 μg/kg (n=15, 6 in stage 1, 9 in stage 2).
Fifteen of these patients were evaluable for response. The overall response rate was 87% (13/15). All 10 previously untreated BPDCN patients achieved a response, including 7 complete responses (CRs).
All 8 previously untreated BPDCN patients treated at the optimal dose (12 μg/kg) achieved a response, including 6 CRs. Four of these patients were still on SL-401 and in CR at the data cutoff, and 2 had gone on to stem cell transplant.
The most common treatment-related adverse events in the BPDCN patients were transient transaminase elevation (57%), hypoalbuminemia (40%), and transient thrombocytopenia (15%).
In stage 1, two patients had capillary leak syndrome (CLS)—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS. There have been no cases of severe CLS since then.

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for SL-401 in the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies.
SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
The drug is under development by Stemline Therapeutics, Inc.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 2 trial of SL-401
The breakthrough designation for SL-401 was supported by data from a phase 2 trial of patients with BPDCN or acute myeloid leukemia. Results observed in the BPDCN patients were presented at the 2016 EHA Congress (abstract S812).
The study consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
At the data cut-off, 18 BPDCN patients had received SL-401 at 7 μg/kg (n=3, stage 1) or 12 μg/kg (n=15, 6 in stage 1, 9 in stage 2).
Fifteen of these patients were evaluable for response. The overall response rate was 87% (13/15). All 10 previously untreated BPDCN patients achieved a response, including 7 complete responses (CRs).
All 8 previously untreated BPDCN patients treated at the optimal dose (12 μg/kg) achieved a response, including 6 CRs. Four of these patients were still on SL-401 and in CR at the data cutoff, and 2 had gone on to stem cell transplant.
The most common treatment-related adverse events in the BPDCN patients were transient transaminase elevation (57%), hypoalbuminemia (40%), and transient thrombocytopenia (15%).
In stage 1, two patients had capillary leak syndrome (CLS)—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS. There have been no cases of severe CLS since then.

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for SL-401 in the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies.
SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
The drug is under development by Stemline Therapeutics, Inc.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 2 trial of SL-401
The breakthrough designation for SL-401 was supported by data from a phase 2 trial of patients with BPDCN or acute myeloid leukemia. Results observed in the BPDCN patients were presented at the 2016 EHA Congress (abstract S812).
The study consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
At the data cut-off, 18 BPDCN patients had received SL-401 at 7 μg/kg (n=3, stage 1) or 12 μg/kg (n=15, 6 in stage 1, 9 in stage 2).
Fifteen of these patients were evaluable for response. The overall response rate was 87% (13/15). All 10 previously untreated BPDCN patients achieved a response, including 7 complete responses (CRs).
All 8 previously untreated BPDCN patients treated at the optimal dose (12 μg/kg) achieved a response, including 6 CRs. Four of these patients were still on SL-401 and in CR at the data cutoff, and 2 had gone on to stem cell transplant.
The most common treatment-related adverse events in the BPDCN patients were transient transaminase elevation (57%), hypoalbuminemia (40%), and transient thrombocytopenia (15%).
In stage 1, two patients had capillary leak syndrome (CLS)—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS. There have been no cases of severe CLS since then.
Reasons for high cost of prescription drugs in US

Photo courtesy of the CDC
High prescription drug prices in the US have a few causes, according to researchers.
They said causes include the approach the US has taken to granting government-protected monopolies to drug manufacturers, strategies that delay access to generic drugs, and the restriction of price negotiation at a level not observed in other industrialized nations.
The researchers outlined these issues in JAMA.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted this research.
The team reviewed the medical and health policy literature from January 2005 to July 2016, looking for articles addressing the sources of drug prices in the US, the justifications and consequences of high prices, and possible solutions.
The researchers found that per-capita prescription drug spending is higher in the US than in all other countries. In 2013, per-capita spending on prescription drugs was $858 in the US, compared with an average of $400 for 19 other industrialized nations.
Dr Kesselheim and his colleagues said prescription drug spending in the US is largely driven by brand-name drug prices that have been increasing in recent years. And drug prices are higher in the US because the US healthcare system allows manufacturers to set their own price for a given product.
In countries with national health insurance systems, on the other hand, a delegated body negotiates drug prices or rejects coverage of products if the price demanded by the manufacturer is excessive in light of the benefit provided. Manufacturers may then decide to offer the drug at a lower price.
The researchers said the most important factor that allows US manufacturers to set high drug prices is market exclusivity. And although cheaper generic drugs can be made available after an exclusivity period has passed, there are strategies for keeping these drugs off the market.
Furthermore, the negotiating power of the payer is constrained by several factors, including the requirement that most government drug payment plans cover nearly all products.
Considering these findings together, Dr Kesselheim and his colleagues said the most realistic short-term strategies to address high drug prices in the US include:
- Enforcing more stringent requirements for market exclusivity rights
- Ensuring timely generic drug availability
- Providing greater opportunities for price negotiation by governmental payers
- Generating more evidence about comparative cost-effectiveness of therapeutic alternatives
- Educating patients, prescribers, payers, and policy makers about these choices.
The researchers said there is little evidence that such policies would hamper innovation. In fact, they might even drive the development of more valuable new therapies rather than rewarding the persistence of older ones.

Photo courtesy of the CDC
High prescription drug prices in the US have a few causes, according to researchers.
They said causes include the approach the US has taken to granting government-protected monopolies to drug manufacturers, strategies that delay access to generic drugs, and the restriction of price negotiation at a level not observed in other industrialized nations.
The researchers outlined these issues in JAMA.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted this research.
The team reviewed the medical and health policy literature from January 2005 to July 2016, looking for articles addressing the sources of drug prices in the US, the justifications and consequences of high prices, and possible solutions.
The researchers found that per-capita prescription drug spending is higher in the US than in all other countries. In 2013, per-capita spending on prescription drugs was $858 in the US, compared with an average of $400 for 19 other industrialized nations.
Dr Kesselheim and his colleagues said prescription drug spending in the US is largely driven by brand-name drug prices that have been increasing in recent years. And drug prices are higher in the US because the US healthcare system allows manufacturers to set their own price for a given product.
In countries with national health insurance systems, on the other hand, a delegated body negotiates drug prices or rejects coverage of products if the price demanded by the manufacturer is excessive in light of the benefit provided. Manufacturers may then decide to offer the drug at a lower price.
The researchers said the most important factor that allows US manufacturers to set high drug prices is market exclusivity. And although cheaper generic drugs can be made available after an exclusivity period has passed, there are strategies for keeping these drugs off the market.
Furthermore, the negotiating power of the payer is constrained by several factors, including the requirement that most government drug payment plans cover nearly all products.
Considering these findings together, Dr Kesselheim and his colleagues said the most realistic short-term strategies to address high drug prices in the US include:
- Enforcing more stringent requirements for market exclusivity rights
- Ensuring timely generic drug availability
- Providing greater opportunities for price negotiation by governmental payers
- Generating more evidence about comparative cost-effectiveness of therapeutic alternatives
- Educating patients, prescribers, payers, and policy makers about these choices.
The researchers said there is little evidence that such policies would hamper innovation. In fact, they might even drive the development of more valuable new therapies rather than rewarding the persistence of older ones.

Photo courtesy of the CDC
High prescription drug prices in the US have a few causes, according to researchers.
They said causes include the approach the US has taken to granting government-protected monopolies to drug manufacturers, strategies that delay access to generic drugs, and the restriction of price negotiation at a level not observed in other industrialized nations.
The researchers outlined these issues in JAMA.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted this research.
The team reviewed the medical and health policy literature from January 2005 to July 2016, looking for articles addressing the sources of drug prices in the US, the justifications and consequences of high prices, and possible solutions.
The researchers found that per-capita prescription drug spending is higher in the US than in all other countries. In 2013, per-capita spending on prescription drugs was $858 in the US, compared with an average of $400 for 19 other industrialized nations.
Dr Kesselheim and his colleagues said prescription drug spending in the US is largely driven by brand-name drug prices that have been increasing in recent years. And drug prices are higher in the US because the US healthcare system allows manufacturers to set their own price for a given product.
In countries with national health insurance systems, on the other hand, a delegated body negotiates drug prices or rejects coverage of products if the price demanded by the manufacturer is excessive in light of the benefit provided. Manufacturers may then decide to offer the drug at a lower price.
The researchers said the most important factor that allows US manufacturers to set high drug prices is market exclusivity. And although cheaper generic drugs can be made available after an exclusivity period has passed, there are strategies for keeping these drugs off the market.
Furthermore, the negotiating power of the payer is constrained by several factors, including the requirement that most government drug payment plans cover nearly all products.
Considering these findings together, Dr Kesselheim and his colleagues said the most realistic short-term strategies to address high drug prices in the US include:
- Enforcing more stringent requirements for market exclusivity rights
- Ensuring timely generic drug availability
- Providing greater opportunities for price negotiation by governmental payers
- Generating more evidence about comparative cost-effectiveness of therapeutic alternatives
- Educating patients, prescribers, payers, and policy makers about these choices.
The researchers said there is little evidence that such policies would hamper innovation. In fact, they might even drive the development of more valuable new therapies rather than rewarding the persistence of older ones.