User login
Procedure deemed unnecessary in most DVT patients
Researchers have found evidence to suggest that pharmacomechanical catheter-directed thrombolysis is largely inappropriate as first-line treatment for patients with acute proximal deep vein thrombosis (DVT).
The team found that anticoagulation plus pharmacomechanical thrombolysis was no more effective than anticoagulation alone for preventing post-thrombotic syndrome (PTS).
Additionally, patients who received pharmacomechanical thrombolysis had a higher risk of major bleeding.
The researchers reported these findings in NEJM.
The trial, known as the ATTRACT study, was designed to determine whether performing pharmacomechanical thrombolysis as part of initial treatment for patients with DVT would reduce the number of patients who later develop PTS.
“The clinical research in deep vein thrombosis and post-thrombotic syndrome is very important to the clinical community and of interest to the National Heart, Lung, and Blood Institute [NHLBI],” said Andrei Kindzelski, MD, PhD, the NHLBI program officer for the ATTRACT trial.
“This landmark study, conducted at 56 clinical sites, demonstrated, in an unbiased manner, no benefits of catheter-directed thrombolysis as a first-line deep vein thrombosis treatment, enabling patients to avoid an unnecessary medical procedure. At the same time, ATTRACT identified a potential future research need in more targeted use of catheter-directed thrombolysis in specific patient groups.”
The study included 692 patients who were randomized to receive anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis.
Anticoagulation consisted of unfractionated heparin, enoxaparin, dalteparin, or tinzaparin for at least 5 days, overlapped with long-term warfarin, as well as the prescription of elastic compression stockings.
Pharmacomechanical thrombolysis consisted of catheter-mediated or device-mediated intrathrombus delivery of recombinant tissue plasminogen activator and thrombus aspiration or maceration, with or without stenting.
Results
The study’s primary outcome was the development of PTS between 6 and 24 months of follow-up.
There was no significant difference in PTS incidence between the treatment arms. PTS occurred in 47% (157/336) of patients in the pharmacomechanical-thrombolysis group and 48% (171/355) of patients in the control group (risk ratio=0.96, P=0.56).
Pharmacomechanical thrombolysis did reduce the severity of PTS, however. The incidence of moderate-to-severe PTS was 24% in the control group and 18% in the pharmacomechanical-thrombolysis group (risk ratio=0.73; P=0.04).
Severity scores for PTS were significantly lower in the pharmacomechanical-thrombolysis group than the control group at 6, 12, 18, and 24 months of follow-up (P<0.01 for each time point).
There was no significant difference between the treatment arms in the incidence of recurrent venous thromboembolism (VTE) over the 24-month follow-up period. Recurrent VTE was observed in 12% of patients in the pharmacomechanical-thrombolysis group and 8% of controls (P=0.09).
On the other hand, there was a significant increase in the incidence of major bleeding within 10 days in the pharmacomechanical-thrombolysis group. The incidence was 1.7% (n=6) in the pharmacomechanical thrombolysis group and 0.3% (n=1) in the control group (P=0.049).
“None of us was surprised to find that this treatment [pharmacomechanical thrombolysis] is riskier than blood-thinning drugs alone,” said study author Suresh Vedantham, MD, of Washington University School of Medicine in St. Louis, Missouri.
“To justify that extra risk, we would have had to show a dramatic improvement in long-term outcomes, and the study didn’t show that. We saw some improvement in disease severity but not enough to justify the risks for most patients.”
While the results indicate that most patients should not undergo pharmacomechanical thrombolysis, the data also hint that the benefits may outweigh the risks in some patients, such as those with exceptionally large clots.
“This is the first large, rigorous study to examine the ability of imaging-guided treatment to address post-thrombotic syndrome,” Dr Vedantham said. “This study will advance patient care by helping many people avoid an unnecessary procedure.”
“The findings are also interesting because there is the suggestion that at least some patients may have benefited. Sorting that out is going to be very important.”
For now, pharmacomechanical thrombolysis should be reserved for use as a second-line treatment for some carefully selected patients who are experiencing particularly severe limitations of leg function from DVT and who are not responding to anticoagulation alone, Dr Vedantham added.
This research was sponsored by NHLBI. Additional funding was provided by Boston Scientific, Covidien (now Medtronic), and Genentech, which provided recombinant tissue plasminogen activator for the study. Compression stockings were donated by BSN Medical. ![]()
Researchers have found evidence to suggest that pharmacomechanical catheter-directed thrombolysis is largely inappropriate as first-line treatment for patients with acute proximal deep vein thrombosis (DVT).
The team found that anticoagulation plus pharmacomechanical thrombolysis was no more effective than anticoagulation alone for preventing post-thrombotic syndrome (PTS).
Additionally, patients who received pharmacomechanical thrombolysis had a higher risk of major bleeding.
The researchers reported these findings in NEJM.
The trial, known as the ATTRACT study, was designed to determine whether performing pharmacomechanical thrombolysis as part of initial treatment for patients with DVT would reduce the number of patients who later develop PTS.
“The clinical research in deep vein thrombosis and post-thrombotic syndrome is very important to the clinical community and of interest to the National Heart, Lung, and Blood Institute [NHLBI],” said Andrei Kindzelski, MD, PhD, the NHLBI program officer for the ATTRACT trial.
“This landmark study, conducted at 56 clinical sites, demonstrated, in an unbiased manner, no benefits of catheter-directed thrombolysis as a first-line deep vein thrombosis treatment, enabling patients to avoid an unnecessary medical procedure. At the same time, ATTRACT identified a potential future research need in more targeted use of catheter-directed thrombolysis in specific patient groups.”
The study included 692 patients who were randomized to receive anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis.
Anticoagulation consisted of unfractionated heparin, enoxaparin, dalteparin, or tinzaparin for at least 5 days, overlapped with long-term warfarin, as well as the prescription of elastic compression stockings.
Pharmacomechanical thrombolysis consisted of catheter-mediated or device-mediated intrathrombus delivery of recombinant tissue plasminogen activator and thrombus aspiration or maceration, with or without stenting.
Results
The study’s primary outcome was the development of PTS between 6 and 24 months of follow-up.
There was no significant difference in PTS incidence between the treatment arms. PTS occurred in 47% (157/336) of patients in the pharmacomechanical-thrombolysis group and 48% (171/355) of patients in the control group (risk ratio=0.96, P=0.56).
Pharmacomechanical thrombolysis did reduce the severity of PTS, however. The incidence of moderate-to-severe PTS was 24% in the control group and 18% in the pharmacomechanical-thrombolysis group (risk ratio=0.73; P=0.04).
Severity scores for PTS were significantly lower in the pharmacomechanical-thrombolysis group than the control group at 6, 12, 18, and 24 months of follow-up (P<0.01 for each time point).
There was no significant difference between the treatment arms in the incidence of recurrent venous thromboembolism (VTE) over the 24-month follow-up period. Recurrent VTE was observed in 12% of patients in the pharmacomechanical-thrombolysis group and 8% of controls (P=0.09).
On the other hand, there was a significant increase in the incidence of major bleeding within 10 days in the pharmacomechanical-thrombolysis group. The incidence was 1.7% (n=6) in the pharmacomechanical thrombolysis group and 0.3% (n=1) in the control group (P=0.049).
“None of us was surprised to find that this treatment [pharmacomechanical thrombolysis] is riskier than blood-thinning drugs alone,” said study author Suresh Vedantham, MD, of Washington University School of Medicine in St. Louis, Missouri.
“To justify that extra risk, we would have had to show a dramatic improvement in long-term outcomes, and the study didn’t show that. We saw some improvement in disease severity but not enough to justify the risks for most patients.”
While the results indicate that most patients should not undergo pharmacomechanical thrombolysis, the data also hint that the benefits may outweigh the risks in some patients, such as those with exceptionally large clots.
“This is the first large, rigorous study to examine the ability of imaging-guided treatment to address post-thrombotic syndrome,” Dr Vedantham said. “This study will advance patient care by helping many people avoid an unnecessary procedure.”
“The findings are also interesting because there is the suggestion that at least some patients may have benefited. Sorting that out is going to be very important.”
For now, pharmacomechanical thrombolysis should be reserved for use as a second-line treatment for some carefully selected patients who are experiencing particularly severe limitations of leg function from DVT and who are not responding to anticoagulation alone, Dr Vedantham added.
This research was sponsored by NHLBI. Additional funding was provided by Boston Scientific, Covidien (now Medtronic), and Genentech, which provided recombinant tissue plasminogen activator for the study. Compression stockings were donated by BSN Medical. ![]()
Researchers have found evidence to suggest that pharmacomechanical catheter-directed thrombolysis is largely inappropriate as first-line treatment for patients with acute proximal deep vein thrombosis (DVT).
The team found that anticoagulation plus pharmacomechanical thrombolysis was no more effective than anticoagulation alone for preventing post-thrombotic syndrome (PTS).
Additionally, patients who received pharmacomechanical thrombolysis had a higher risk of major bleeding.
The researchers reported these findings in NEJM.
The trial, known as the ATTRACT study, was designed to determine whether performing pharmacomechanical thrombolysis as part of initial treatment for patients with DVT would reduce the number of patients who later develop PTS.
“The clinical research in deep vein thrombosis and post-thrombotic syndrome is very important to the clinical community and of interest to the National Heart, Lung, and Blood Institute [NHLBI],” said Andrei Kindzelski, MD, PhD, the NHLBI program officer for the ATTRACT trial.
“This landmark study, conducted at 56 clinical sites, demonstrated, in an unbiased manner, no benefits of catheter-directed thrombolysis as a first-line deep vein thrombosis treatment, enabling patients to avoid an unnecessary medical procedure. At the same time, ATTRACT identified a potential future research need in more targeted use of catheter-directed thrombolysis in specific patient groups.”
The study included 692 patients who were randomized to receive anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis.
Anticoagulation consisted of unfractionated heparin, enoxaparin, dalteparin, or tinzaparin for at least 5 days, overlapped with long-term warfarin, as well as the prescription of elastic compression stockings.
Pharmacomechanical thrombolysis consisted of catheter-mediated or device-mediated intrathrombus delivery of recombinant tissue plasminogen activator and thrombus aspiration or maceration, with or without stenting.
Results
The study’s primary outcome was the development of PTS between 6 and 24 months of follow-up.
There was no significant difference in PTS incidence between the treatment arms. PTS occurred in 47% (157/336) of patients in the pharmacomechanical-thrombolysis group and 48% (171/355) of patients in the control group (risk ratio=0.96, P=0.56).
Pharmacomechanical thrombolysis did reduce the severity of PTS, however. The incidence of moderate-to-severe PTS was 24% in the control group and 18% in the pharmacomechanical-thrombolysis group (risk ratio=0.73; P=0.04).
Severity scores for PTS were significantly lower in the pharmacomechanical-thrombolysis group than the control group at 6, 12, 18, and 24 months of follow-up (P<0.01 for each time point).
There was no significant difference between the treatment arms in the incidence of recurrent venous thromboembolism (VTE) over the 24-month follow-up period. Recurrent VTE was observed in 12% of patients in the pharmacomechanical-thrombolysis group and 8% of controls (P=0.09).
On the other hand, there was a significant increase in the incidence of major bleeding within 10 days in the pharmacomechanical-thrombolysis group. The incidence was 1.7% (n=6) in the pharmacomechanical thrombolysis group and 0.3% (n=1) in the control group (P=0.049).
“None of us was surprised to find that this treatment [pharmacomechanical thrombolysis] is riskier than blood-thinning drugs alone,” said study author Suresh Vedantham, MD, of Washington University School of Medicine in St. Louis, Missouri.
“To justify that extra risk, we would have had to show a dramatic improvement in long-term outcomes, and the study didn’t show that. We saw some improvement in disease severity but not enough to justify the risks for most patients.”
While the results indicate that most patients should not undergo pharmacomechanical thrombolysis, the data also hint that the benefits may outweigh the risks in some patients, such as those with exceptionally large clots.
“This is the first large, rigorous study to examine the ability of imaging-guided treatment to address post-thrombotic syndrome,” Dr Vedantham said. “This study will advance patient care by helping many people avoid an unnecessary procedure.”
“The findings are also interesting because there is the suggestion that at least some patients may have benefited. Sorting that out is going to be very important.”
For now, pharmacomechanical thrombolysis should be reserved for use as a second-line treatment for some carefully selected patients who are experiencing particularly severe limitations of leg function from DVT and who are not responding to anticoagulation alone, Dr Vedantham added.
This research was sponsored by NHLBI. Additional funding was provided by Boston Scientific, Covidien (now Medtronic), and Genentech, which provided recombinant tissue plasminogen activator for the study. Compression stockings were donated by BSN Medical. ![]()
Gene-based Zika vaccine proves immunogenic in healthy adults
An experimental Zika vaccine is safe and induces an immune response in healthy adults, according to research published in The Lancet.
Investigators tested 2 potential Zika vaccines, VRC5288 and VRC5283, in a pair of phase 1 trials.
Both vaccines were considered well-tolerated, but one of them, VRC5283, induced a greater immune response than the other.
Both vaccines were developed by investigators at the National Institute of Allergy and Infectious Diseases (NIAID).
Now, NIAID is leading an international effort to evaluate VRC5283 in a phase 2/2b trial.
“Following early reports that Zika infection during pregnancy can lead to birth defects, NIAID scientists rapidly created one of the first investigational Zika vaccines using a DNA-based platform and began initial studies in healthy adults less than 1 year later,” said NIAID Director Anthony S. Fauci, MD.
To create their vaccines, the investigators inserted into plasmids genes that encode proteins found on the surface of the Zika virus.
The team developed 2 different plasmids for clinical testing: VRC5288 (Zika virus and Japanese encephalitis virus chimera) and VRC5283 (wild-type Zika virus).
In August 2016, NIAID initiated a phase 1 trial of the VRC5288 plasmid in 80 healthy volunteers ages 18 to 35. Subjects received a 4 mg dose via a needle and syringe injection in the arm muscle.
They received doses of VRC5288 at 0 and 8 weeks; 0 and 12 weeks; 0, 4, and 8 weeks; or 0, 4, and 20 weeks.
In December 2016, NIAID initiated a separate trial testing the VRC5283 plasmid. This study enrolled 45 healthy volunteers ages 18 to 50.
Subjects in this trial received 4 mg doses of VRC5283 at 0, 4, and 8 weeks. They were vaccinated in 3 different ways—via single-dose needle and syringe injection in 1 arm, via split-dose needle and syringe injection in each arm, or via needle-free injection in each arm.
Safety
The vaccinations were considered safe and well-tolerated in both trials, although some participants experienced mild to moderate reactions.
Local reactions (with VRC5288 and VRC5283, respectively) included pain/tenderness (mild—46% and 73%, moderate—0% and 7%), mild swelling (1% and 7%), and mild redness (6% and 2%).
Systemic reactions (with VRC5288 and VRC5283, respectively) included malaise (mild—25% and 33%, moderate—3% and 4%), myalgia (mild—18% and 13%, moderate—4% and 7%), headache (mild—19% and 29%, moderate—4% and 4%), chills (mild—6% and 2%, moderate—1% and 2%), nausea (mild—8% and 4%, moderate—1% and 0%), and joint pain (mild—5% and 16%, moderate—0% and 2%).
Immunogenicity
The investigators analyzed blood samples from all subjects 4 weeks after their final vaccinations.
The team found that 60% to 89% of subjects generated a neutralizing antibody response to VRC5288, and 77% to 100% of subjects generated a neutralizing antibody response to VRC5283.
Subjects who received VRC5283 via the needle-free injector all generated a neutralizing antibody response and had the highest levels of neutralizing antibodies.
Subjects who received VRC5283 in a split-dose administered to both arms had more robust immune responses than those receiving the full dose in 1 arm.
“NIAID has begun phase 2 testing of this candidate to determine if it can prevent Zika virus infection, and the promising phase 1 data . . . support its continued development,” Dr Fauci said. ![]()
An experimental Zika vaccine is safe and induces an immune response in healthy adults, according to research published in The Lancet.
Investigators tested 2 potential Zika vaccines, VRC5288 and VRC5283, in a pair of phase 1 trials.
Both vaccines were considered well-tolerated, but one of them, VRC5283, induced a greater immune response than the other.
Both vaccines were developed by investigators at the National Institute of Allergy and Infectious Diseases (NIAID).
Now, NIAID is leading an international effort to evaluate VRC5283 in a phase 2/2b trial.
“Following early reports that Zika infection during pregnancy can lead to birth defects, NIAID scientists rapidly created one of the first investigational Zika vaccines using a DNA-based platform and began initial studies in healthy adults less than 1 year later,” said NIAID Director Anthony S. Fauci, MD.
To create their vaccines, the investigators inserted into plasmids genes that encode proteins found on the surface of the Zika virus.
The team developed 2 different plasmids for clinical testing: VRC5288 (Zika virus and Japanese encephalitis virus chimera) and VRC5283 (wild-type Zika virus).
In August 2016, NIAID initiated a phase 1 trial of the VRC5288 plasmid in 80 healthy volunteers ages 18 to 35. Subjects received a 4 mg dose via a needle and syringe injection in the arm muscle.
They received doses of VRC5288 at 0 and 8 weeks; 0 and 12 weeks; 0, 4, and 8 weeks; or 0, 4, and 20 weeks.
In December 2016, NIAID initiated a separate trial testing the VRC5283 plasmid. This study enrolled 45 healthy volunteers ages 18 to 50.
Subjects in this trial received 4 mg doses of VRC5283 at 0, 4, and 8 weeks. They were vaccinated in 3 different ways—via single-dose needle and syringe injection in 1 arm, via split-dose needle and syringe injection in each arm, or via needle-free injection in each arm.
Safety
The vaccinations were considered safe and well-tolerated in both trials, although some participants experienced mild to moderate reactions.
Local reactions (with VRC5288 and VRC5283, respectively) included pain/tenderness (mild—46% and 73%, moderate—0% and 7%), mild swelling (1% and 7%), and mild redness (6% and 2%).
Systemic reactions (with VRC5288 and VRC5283, respectively) included malaise (mild—25% and 33%, moderate—3% and 4%), myalgia (mild—18% and 13%, moderate—4% and 7%), headache (mild—19% and 29%, moderate—4% and 4%), chills (mild—6% and 2%, moderate—1% and 2%), nausea (mild—8% and 4%, moderate—1% and 0%), and joint pain (mild—5% and 16%, moderate—0% and 2%).
Immunogenicity
The investigators analyzed blood samples from all subjects 4 weeks after their final vaccinations.
The team found that 60% to 89% of subjects generated a neutralizing antibody response to VRC5288, and 77% to 100% of subjects generated a neutralizing antibody response to VRC5283.
Subjects who received VRC5283 via the needle-free injector all generated a neutralizing antibody response and had the highest levels of neutralizing antibodies.
Subjects who received VRC5283 in a split-dose administered to both arms had more robust immune responses than those receiving the full dose in 1 arm.
“NIAID has begun phase 2 testing of this candidate to determine if it can prevent Zika virus infection, and the promising phase 1 data . . . support its continued development,” Dr Fauci said. ![]()
An experimental Zika vaccine is safe and induces an immune response in healthy adults, according to research published in The Lancet.
Investigators tested 2 potential Zika vaccines, VRC5288 and VRC5283, in a pair of phase 1 trials.
Both vaccines were considered well-tolerated, but one of them, VRC5283, induced a greater immune response than the other.
Both vaccines were developed by investigators at the National Institute of Allergy and Infectious Diseases (NIAID).
Now, NIAID is leading an international effort to evaluate VRC5283 in a phase 2/2b trial.
“Following early reports that Zika infection during pregnancy can lead to birth defects, NIAID scientists rapidly created one of the first investigational Zika vaccines using a DNA-based platform and began initial studies in healthy adults less than 1 year later,” said NIAID Director Anthony S. Fauci, MD.
To create their vaccines, the investigators inserted into plasmids genes that encode proteins found on the surface of the Zika virus.
The team developed 2 different plasmids for clinical testing: VRC5288 (Zika virus and Japanese encephalitis virus chimera) and VRC5283 (wild-type Zika virus).
In August 2016, NIAID initiated a phase 1 trial of the VRC5288 plasmid in 80 healthy volunteers ages 18 to 35. Subjects received a 4 mg dose via a needle and syringe injection in the arm muscle.
They received doses of VRC5288 at 0 and 8 weeks; 0 and 12 weeks; 0, 4, and 8 weeks; or 0, 4, and 20 weeks.
In December 2016, NIAID initiated a separate trial testing the VRC5283 plasmid. This study enrolled 45 healthy volunteers ages 18 to 50.
Subjects in this trial received 4 mg doses of VRC5283 at 0, 4, and 8 weeks. They were vaccinated in 3 different ways—via single-dose needle and syringe injection in 1 arm, via split-dose needle and syringe injection in each arm, or via needle-free injection in each arm.
Safety
The vaccinations were considered safe and well-tolerated in both trials, although some participants experienced mild to moderate reactions.
Local reactions (with VRC5288 and VRC5283, respectively) included pain/tenderness (mild—46% and 73%, moderate—0% and 7%), mild swelling (1% and 7%), and mild redness (6% and 2%).
Systemic reactions (with VRC5288 and VRC5283, respectively) included malaise (mild—25% and 33%, moderate—3% and 4%), myalgia (mild—18% and 13%, moderate—4% and 7%), headache (mild—19% and 29%, moderate—4% and 4%), chills (mild—6% and 2%, moderate—1% and 2%), nausea (mild—8% and 4%, moderate—1% and 0%), and joint pain (mild—5% and 16%, moderate—0% and 2%).
Immunogenicity
The investigators analyzed blood samples from all subjects 4 weeks after their final vaccinations.
The team found that 60% to 89% of subjects generated a neutralizing antibody response to VRC5288, and 77% to 100% of subjects generated a neutralizing antibody response to VRC5283.
Subjects who received VRC5283 via the needle-free injector all generated a neutralizing antibody response and had the highest levels of neutralizing antibodies.
Subjects who received VRC5283 in a split-dose administered to both arms had more robust immune responses than those receiving the full dose in 1 arm.
“NIAID has begun phase 2 testing of this candidate to determine if it can prevent Zika virus infection, and the promising phase 1 data . . . support its continued development,” Dr Fauci said. ![]()
Gene therapy seems safe, effective in hemophilia B
The gene therapy SPK-9001 has produced positive results in a phase 1/2 trial of adults with hemophilia B, according to researchers.
SPK-9001 produced sustained factor IX (FIX) activity and allowed all 10 patients in this study to stop FIX prophylaxis.
Eight patients had no further FIX treatment after SPK-9001, and 9 patients had 0 bleeds.
There were no serious adverse events, and none of the patients had thrombotic events or developed FIX inhibitors.
“A one-time therapy sufficient to prevent bleeding without further medical intervention is the ideal treatment goal for patients with hemophilia,” said Lindsey A. George, MD, a hematologist at Children’s Hospital of Philadelphia in Pennsylvania.
“This cohort of 10 patients all safely experienced sustained clinical benefit after one infusion.”
Dr George and her colleagues reported these results in NEJM. The trial was sponsored by Spark Therapeutics and Pfizer.
SPK-9001 is an investigational vector that contains a bio-engineered adeno-associated virus capsid and a codon-optimized, high-activity human FIX gene enabling endogenous production of FIX.
The researchers tested SPK-9001 in 10 males with hemophilia B.
With a cumulative follow-up of 492 weeks, the mean steady-state FIX activity was 34% of normal (range, 14% to 81%) after a single administration of SPK-9001.
The annualized bleeding rate was reduced 97%, from a mean rate of 11.1 events per year before SPK-9001 administration to 0.4 events per year after (P=0.02).
Nine patients had 0 bleeds after SPK-9001, and 1 patient had 4 bleeds.
Use of FIX treatment was reduced 99% during this study, from a mean dose of 2908 IU/kg (range, 0 to 8090) before SPK-9001 to a mean dose of 49.3 IU/kg (range, 0 to 376) after SPK-9001 (P=0.004).
Two patients did receive FIX treatment after SPK-9001, but none of the patients received FIX prophylaxis.
The researchers noted that the patient who required FIX treatment for bleeding (4 events) had significant baseline joint damage. However, this patient still used 91% less FIX than before he received SPK-9001.
There were a total of 40 adverse events in this study, but none were serious. There were no deaths, no thrombotic events, and no cases of inhibitor development.
The only adverse events thought to be related to SPK-9001 were asymptomatic and transient increases in liver enzymes in 2 patients. These events resolved with a tapering dose of oral corticosteroids.
“People who live with hemophilia face a life-long need for vigilant monitoring and recurrent factor concentrate infusions to prevent spontaneous, potentially life-threatening bleeds and to protect their joints,” said study author Katherine High, MD, president and head of Research and Development at Spark Therapeutics.
“The data suggest a one-time infusion of SPK-9001 has the potential to safely sustain factor IX coagulant activity level that may result in the termination of baseline prophylaxis factor infusions, significantly reduce bleeding, and nearly eliminate the need for exogenous factor IX concentrate infusions.” ![]()
The gene therapy SPK-9001 has produced positive results in a phase 1/2 trial of adults with hemophilia B, according to researchers.
SPK-9001 produced sustained factor IX (FIX) activity and allowed all 10 patients in this study to stop FIX prophylaxis.
Eight patients had no further FIX treatment after SPK-9001, and 9 patients had 0 bleeds.
There were no serious adverse events, and none of the patients had thrombotic events or developed FIX inhibitors.
“A one-time therapy sufficient to prevent bleeding without further medical intervention is the ideal treatment goal for patients with hemophilia,” said Lindsey A. George, MD, a hematologist at Children’s Hospital of Philadelphia in Pennsylvania.
“This cohort of 10 patients all safely experienced sustained clinical benefit after one infusion.”
Dr George and her colleagues reported these results in NEJM. The trial was sponsored by Spark Therapeutics and Pfizer.
SPK-9001 is an investigational vector that contains a bio-engineered adeno-associated virus capsid and a codon-optimized, high-activity human FIX gene enabling endogenous production of FIX.
The researchers tested SPK-9001 in 10 males with hemophilia B.
With a cumulative follow-up of 492 weeks, the mean steady-state FIX activity was 34% of normal (range, 14% to 81%) after a single administration of SPK-9001.
The annualized bleeding rate was reduced 97%, from a mean rate of 11.1 events per year before SPK-9001 administration to 0.4 events per year after (P=0.02).
Nine patients had 0 bleeds after SPK-9001, and 1 patient had 4 bleeds.
Use of FIX treatment was reduced 99% during this study, from a mean dose of 2908 IU/kg (range, 0 to 8090) before SPK-9001 to a mean dose of 49.3 IU/kg (range, 0 to 376) after SPK-9001 (P=0.004).
Two patients did receive FIX treatment after SPK-9001, but none of the patients received FIX prophylaxis.
The researchers noted that the patient who required FIX treatment for bleeding (4 events) had significant baseline joint damage. However, this patient still used 91% less FIX than before he received SPK-9001.
There were a total of 40 adverse events in this study, but none were serious. There were no deaths, no thrombotic events, and no cases of inhibitor development.
The only adverse events thought to be related to SPK-9001 were asymptomatic and transient increases in liver enzymes in 2 patients. These events resolved with a tapering dose of oral corticosteroids.
“People who live with hemophilia face a life-long need for vigilant monitoring and recurrent factor concentrate infusions to prevent spontaneous, potentially life-threatening bleeds and to protect their joints,” said study author Katherine High, MD, president and head of Research and Development at Spark Therapeutics.
“The data suggest a one-time infusion of SPK-9001 has the potential to safely sustain factor IX coagulant activity level that may result in the termination of baseline prophylaxis factor infusions, significantly reduce bleeding, and nearly eliminate the need for exogenous factor IX concentrate infusions.” ![]()
The gene therapy SPK-9001 has produced positive results in a phase 1/2 trial of adults with hemophilia B, according to researchers.
SPK-9001 produced sustained factor IX (FIX) activity and allowed all 10 patients in this study to stop FIX prophylaxis.
Eight patients had no further FIX treatment after SPK-9001, and 9 patients had 0 bleeds.
There were no serious adverse events, and none of the patients had thrombotic events or developed FIX inhibitors.
“A one-time therapy sufficient to prevent bleeding without further medical intervention is the ideal treatment goal for patients with hemophilia,” said Lindsey A. George, MD, a hematologist at Children’s Hospital of Philadelphia in Pennsylvania.
“This cohort of 10 patients all safely experienced sustained clinical benefit after one infusion.”
Dr George and her colleagues reported these results in NEJM. The trial was sponsored by Spark Therapeutics and Pfizer.
SPK-9001 is an investigational vector that contains a bio-engineered adeno-associated virus capsid and a codon-optimized, high-activity human FIX gene enabling endogenous production of FIX.
The researchers tested SPK-9001 in 10 males with hemophilia B.
With a cumulative follow-up of 492 weeks, the mean steady-state FIX activity was 34% of normal (range, 14% to 81%) after a single administration of SPK-9001.
The annualized bleeding rate was reduced 97%, from a mean rate of 11.1 events per year before SPK-9001 administration to 0.4 events per year after (P=0.02).
Nine patients had 0 bleeds after SPK-9001, and 1 patient had 4 bleeds.
Use of FIX treatment was reduced 99% during this study, from a mean dose of 2908 IU/kg (range, 0 to 8090) before SPK-9001 to a mean dose of 49.3 IU/kg (range, 0 to 376) after SPK-9001 (P=0.004).
Two patients did receive FIX treatment after SPK-9001, but none of the patients received FIX prophylaxis.
The researchers noted that the patient who required FIX treatment for bleeding (4 events) had significant baseline joint damage. However, this patient still used 91% less FIX than before he received SPK-9001.
There were a total of 40 adverse events in this study, but none were serious. There were no deaths, no thrombotic events, and no cases of inhibitor development.
The only adverse events thought to be related to SPK-9001 were asymptomatic and transient increases in liver enzymes in 2 patients. These events resolved with a tapering dose of oral corticosteroids.
“People who live with hemophilia face a life-long need for vigilant monitoring and recurrent factor concentrate infusions to prevent spontaneous, potentially life-threatening bleeds and to protect their joints,” said study author Katherine High, MD, president and head of Research and Development at Spark Therapeutics.
“The data suggest a one-time infusion of SPK-9001 has the potential to safely sustain factor IX coagulant activity level that may result in the termination of baseline prophylaxis factor infusions, significantly reduce bleeding, and nearly eliminate the need for exogenous factor IX concentrate infusions.” ![]()
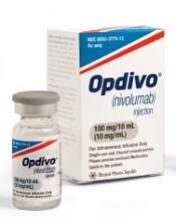
FDA lifts partial hold on nivolumab trials
The US Food and Drug Administration (FDA) has lifted the partial clinical hold placed on 2 trials of the PD-1 immune checkpoint inhibitor nivolumab (Opdivo).
In these trials, CheckMate-039 (CA209 -039) and CA204142, researchers are investigating nivolumab-based combinations in patients with relapsed or refractory multiple myeloma (MM).
The partial clinical hold on these trials was a result of risks identified in studies involving another anti-PD-1 agent, pembrolizumab.
Data from the pembrolizumab trials suggested that risks outweigh benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there could be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
Therefore, the FDA placed a partial hold on the following nivolumab trials:
- CheckMate-602: A randomized, phase 3 trial of combinations of nivolumab, elotuzumab, pomalidomide, and dexamethasone in relapsed and refractory MM
- CheckMate-039: A phase 1 study intended to establish the tolerability of nivolumab and the combination of nivolumab and daratumumab, with or without pomalidomide and dexamethasone, in patients with relapsed or refractory MM
- CA204142: A phase 2 study of elotuzumab in combination with pomalidomide and low-dose dexamethasone, and in combination with nivolumab, in patients with MM who relapsed after or were refractory to prior treatment with lenalidomide.
After consulting with Bristol-Myers Squibb, the FDA decided to lift the hold on CheckMate-039 (CA209 -039) and CA204142. These trials will continue with amended protocols.
The third trial, CheckMate-602, remains on partial clinical hold. Bristol-Myers Squibb said it is continuing to work with the FDA to determine next steps for this trial.
CheckMate-602 is not enrolling new patients. However, patients who are experiencing clinical benefit are continuing to receive treatment. ![]()
The US Food and Drug Administration (FDA) has lifted the partial clinical hold placed on 2 trials of the PD-1 immune checkpoint inhibitor nivolumab (Opdivo).
In these trials, CheckMate-039 (CA209 -039) and CA204142, researchers are investigating nivolumab-based combinations in patients with relapsed or refractory multiple myeloma (MM).
The partial clinical hold on these trials was a result of risks identified in studies involving another anti-PD-1 agent, pembrolizumab.
Data from the pembrolizumab trials suggested that risks outweigh benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there could be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
Therefore, the FDA placed a partial hold on the following nivolumab trials:
- CheckMate-602: A randomized, phase 3 trial of combinations of nivolumab, elotuzumab, pomalidomide, and dexamethasone in relapsed and refractory MM
- CheckMate-039: A phase 1 study intended to establish the tolerability of nivolumab and the combination of nivolumab and daratumumab, with or without pomalidomide and dexamethasone, in patients with relapsed or refractory MM
- CA204142: A phase 2 study of elotuzumab in combination with pomalidomide and low-dose dexamethasone, and in combination with nivolumab, in patients with MM who relapsed after or were refractory to prior treatment with lenalidomide.
After consulting with Bristol-Myers Squibb, the FDA decided to lift the hold on CheckMate-039 (CA209 -039) and CA204142. These trials will continue with amended protocols.
The third trial, CheckMate-602, remains on partial clinical hold. Bristol-Myers Squibb said it is continuing to work with the FDA to determine next steps for this trial.
CheckMate-602 is not enrolling new patients. However, patients who are experiencing clinical benefit are continuing to receive treatment. ![]()
The US Food and Drug Administration (FDA) has lifted the partial clinical hold placed on 2 trials of the PD-1 immune checkpoint inhibitor nivolumab (Opdivo).
In these trials, CheckMate-039 (CA209 -039) and CA204142, researchers are investigating nivolumab-based combinations in patients with relapsed or refractory multiple myeloma (MM).
The partial clinical hold on these trials was a result of risks identified in studies involving another anti-PD-1 agent, pembrolizumab.
Data from the pembrolizumab trials suggested that risks outweigh benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there could be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
Therefore, the FDA placed a partial hold on the following nivolumab trials:
- CheckMate-602: A randomized, phase 3 trial of combinations of nivolumab, elotuzumab, pomalidomide, and dexamethasone in relapsed and refractory MM
- CheckMate-039: A phase 1 study intended to establish the tolerability of nivolumab and the combination of nivolumab and daratumumab, with or without pomalidomide and dexamethasone, in patients with relapsed or refractory MM
- CA204142: A phase 2 study of elotuzumab in combination with pomalidomide and low-dose dexamethasone, and in combination with nivolumab, in patients with MM who relapsed after or were refractory to prior treatment with lenalidomide.
After consulting with Bristol-Myers Squibb, the FDA decided to lift the hold on CheckMate-039 (CA209 -039) and CA204142. These trials will continue with amended protocols.
The third trial, CheckMate-602, remains on partial clinical hold. Bristol-Myers Squibb said it is continuing to work with the FDA to determine next steps for this trial.
CheckMate-602 is not enrolling new patients. However, patients who are experiencing clinical benefit are continuing to receive treatment. ![]()
Company launches digital PCR test for monitoring CML
Bio-Rad Laboratories, Inc., has launched the QXDx BCR-ABL %IS Kit, a digital polymerase chain reaction (PCR) test that can monitor molecular response to therapy in patients with chronic myeloid leukemia (CML).
The kit has a CE-IVD mark and is available for in vitro diagnostic use in Europe, Hong Kong, and New Zealand.
The QXDx BCR-ABL %IS Kit uses Bio-Rad’s Droplet Digital PCR (ddPCR) technology to provide an absolute measure of BCR-ABL transcripts.
The kit measures BCR-ABL1 and ABL1 chromosomal transcripts in total RNA from whole blood of t(9;22)-positive CML patients expressing BCR-ABL1 fusion transcripts type e13a2 and/or e14a2.
The QXDx BCR-ABL %IS Kit measures the e13a2 and/or e14a2 transcripts of BCR-ABL1, normalized to the ABL1 endogenous control. The kit does not differentiate between e13a2 and e14a2 transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
The kit’s results are reported as percent reduction from a baseline of 100% on the International Scale (%IS) and on a log molecular reduction (MR) scale.
The kit is able to detect deep molecular response values of MR 4.7 (%IS 0.002) or MR 5.0 (%IS 0.001) in 2- or 4-well formats. This exceeds the typical limitations of reverse transcription quantitative PCR-based tests that are reliable down to MR 4.5 (%IS 0.0032).
Bio-Rad says the QXDx BCR-ABL %IS Kit delivers absolute quantitation, which eliminates the need for standard curves and minimizes variation between samples. The kit also provides scalable throughput, allowing for testing of 8 to 48 samples per run.
The QXDx BCR-ABL %IS Kit can be used with Bio-Rad’s QX200 AutoDG ddPCR Dx System or with the QX200 ddPCR Dx System. The QXDx BCR-ABL %IS Kit uses QuantaSoft Software v1.7 for data acquisition and output. ![]()
Bio-Rad Laboratories, Inc., has launched the QXDx BCR-ABL %IS Kit, a digital polymerase chain reaction (PCR) test that can monitor molecular response to therapy in patients with chronic myeloid leukemia (CML).
The kit has a CE-IVD mark and is available for in vitro diagnostic use in Europe, Hong Kong, and New Zealand.
The QXDx BCR-ABL %IS Kit uses Bio-Rad’s Droplet Digital PCR (ddPCR) technology to provide an absolute measure of BCR-ABL transcripts.
The kit measures BCR-ABL1 and ABL1 chromosomal transcripts in total RNA from whole blood of t(9;22)-positive CML patients expressing BCR-ABL1 fusion transcripts type e13a2 and/or e14a2.
The QXDx BCR-ABL %IS Kit measures the e13a2 and/or e14a2 transcripts of BCR-ABL1, normalized to the ABL1 endogenous control. The kit does not differentiate between e13a2 and e14a2 transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
The kit’s results are reported as percent reduction from a baseline of 100% on the International Scale (%IS) and on a log molecular reduction (MR) scale.
The kit is able to detect deep molecular response values of MR 4.7 (%IS 0.002) or MR 5.0 (%IS 0.001) in 2- or 4-well formats. This exceeds the typical limitations of reverse transcription quantitative PCR-based tests that are reliable down to MR 4.5 (%IS 0.0032).
Bio-Rad says the QXDx BCR-ABL %IS Kit delivers absolute quantitation, which eliminates the need for standard curves and minimizes variation between samples. The kit also provides scalable throughput, allowing for testing of 8 to 48 samples per run.
The QXDx BCR-ABL %IS Kit can be used with Bio-Rad’s QX200 AutoDG ddPCR Dx System or with the QX200 ddPCR Dx System. The QXDx BCR-ABL %IS Kit uses QuantaSoft Software v1.7 for data acquisition and output. ![]()
Bio-Rad Laboratories, Inc., has launched the QXDx BCR-ABL %IS Kit, a digital polymerase chain reaction (PCR) test that can monitor molecular response to therapy in patients with chronic myeloid leukemia (CML).
The kit has a CE-IVD mark and is available for in vitro diagnostic use in Europe, Hong Kong, and New Zealand.
The QXDx BCR-ABL %IS Kit uses Bio-Rad’s Droplet Digital PCR (ddPCR) technology to provide an absolute measure of BCR-ABL transcripts.
The kit measures BCR-ABL1 and ABL1 chromosomal transcripts in total RNA from whole blood of t(9;22)-positive CML patients expressing BCR-ABL1 fusion transcripts type e13a2 and/or e14a2.
The QXDx BCR-ABL %IS Kit measures the e13a2 and/or e14a2 transcripts of BCR-ABL1, normalized to the ABL1 endogenous control. The kit does not differentiate between e13a2 and e14a2 transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
The kit’s results are reported as percent reduction from a baseline of 100% on the International Scale (%IS) and on a log molecular reduction (MR) scale.
The kit is able to detect deep molecular response values of MR 4.7 (%IS 0.002) or MR 5.0 (%IS 0.001) in 2- or 4-well formats. This exceeds the typical limitations of reverse transcription quantitative PCR-based tests that are reliable down to MR 4.5 (%IS 0.0032).
Bio-Rad says the QXDx BCR-ABL %IS Kit delivers absolute quantitation, which eliminates the need for standard curves and minimizes variation between samples. The kit also provides scalable throughput, allowing for testing of 8 to 48 samples per run.
The QXDx BCR-ABL %IS Kit can be used with Bio-Rad’s QX200 AutoDG ddPCR Dx System or with the QX200 ddPCR Dx System. The QXDx BCR-ABL %IS Kit uses QuantaSoft Software v1.7 for data acquisition and output. ![]()
Enzyme may be target for MM treatment
The enzyme ADAR1 may be a therapeutic target for multiple myeloma (MM), according to research published in Nature Communications.
Investigators found that high ADAR1 levels correlate with reduced survival rates in MM patients.
The team also discovered that blocking ADAR1 reduced regeneration of high-risk MM in serially transplantable patient-derived xenografts.
The investigators believe that JAK2 inhibitors could be used to dampen ADAR1 activity and ultimately prevent progression or relapse in patients with MM.
“Despite new therapies, it’s virtually inevitable that a patient with multiple myeloma will experience relapse of the disease at some point,” said study author Catriona Jamieson, MD, PhD, of University of California, San Diego, in La Jolla.
“That’s why it’s exciting that this discovery may allow us to detect the disease earlier and address the root cause.”
Dr Jamieson and her colleagues knew that ADAR1 is normally expressed during fetal development to help blood cells form. The enzyme edits the sequence of RNA and is known to promote cancer progression and resistance to therapy.
With previous work, Dr Jamieson’s team described ADAR1’s contributions to chronic myeloid leukemia (CML). The enzyme’s RNA-editing activity boosts leukemic stem cells, giving rise to CML, increasing disease recurrence, and allowing CML to resist treatment.
For their current study, Dr Jamieson and her colleagues investigated ADAR1’s role in MM, first analyzing a database of nearly 800 MM patient samples.
The investigators found that patients with low ADAR1 levels in their tumor cells survived longer than patients with high ADAR1 levels.
While more than 90% of patients with low ADAR1 levels survived longer than 2 years after their initial diagnosis, fewer than 70% of patients with high ADAR1 levels did the same.
The investigators also created a humanized mouse model of MM and found that silencing the ADAR1 gene reduced the engraftment of MM.
“This is a difficult disease to model in animals; there isn’t a single gene we can manipulate to mimic multiple myeloma,” said study author Leslie A. Crews, PhD, of University of California, San Diego.
“This study is important, in part because we now have a new xenograft model that will, for the first time, allow us to apply new biomarkers to better predict disease progression and test new therapeutics.”
To advance their findings from this study, the investigators are exploring ways to leverage ADAR1 to detect MM progression as early as possible.
They are also testing inhibitors of JAK2, a molecule that influences ADAR1 activity, for their ability to eliminate cancer stem cells in MM models.
“Several major advances in recent years have been good news for multiple myeloma patients, but those new drugs only target terminally differentiated cancer cells and, thus, can only reduce the bulk of the tumor,” Dr Jamieson said.
“They don’t get to the root cause of disease development, progression, and relapse—cancer stem cells—the way inhibiting ADAR1 does. I like to call our approach ‘precision regenerative medicine.’” ![]()
The enzyme ADAR1 may be a therapeutic target for multiple myeloma (MM), according to research published in Nature Communications.
Investigators found that high ADAR1 levels correlate with reduced survival rates in MM patients.
The team also discovered that blocking ADAR1 reduced regeneration of high-risk MM in serially transplantable patient-derived xenografts.
The investigators believe that JAK2 inhibitors could be used to dampen ADAR1 activity and ultimately prevent progression or relapse in patients with MM.
“Despite new therapies, it’s virtually inevitable that a patient with multiple myeloma will experience relapse of the disease at some point,” said study author Catriona Jamieson, MD, PhD, of University of California, San Diego, in La Jolla.
“That’s why it’s exciting that this discovery may allow us to detect the disease earlier and address the root cause.”
Dr Jamieson and her colleagues knew that ADAR1 is normally expressed during fetal development to help blood cells form. The enzyme edits the sequence of RNA and is known to promote cancer progression and resistance to therapy.
With previous work, Dr Jamieson’s team described ADAR1’s contributions to chronic myeloid leukemia (CML). The enzyme’s RNA-editing activity boosts leukemic stem cells, giving rise to CML, increasing disease recurrence, and allowing CML to resist treatment.
For their current study, Dr Jamieson and her colleagues investigated ADAR1’s role in MM, first analyzing a database of nearly 800 MM patient samples.
The investigators found that patients with low ADAR1 levels in their tumor cells survived longer than patients with high ADAR1 levels.
While more than 90% of patients with low ADAR1 levels survived longer than 2 years after their initial diagnosis, fewer than 70% of patients with high ADAR1 levels did the same.
The investigators also created a humanized mouse model of MM and found that silencing the ADAR1 gene reduced the engraftment of MM.
“This is a difficult disease to model in animals; there isn’t a single gene we can manipulate to mimic multiple myeloma,” said study author Leslie A. Crews, PhD, of University of California, San Diego.
“This study is important, in part because we now have a new xenograft model that will, for the first time, allow us to apply new biomarkers to better predict disease progression and test new therapeutics.”
To advance their findings from this study, the investigators are exploring ways to leverage ADAR1 to detect MM progression as early as possible.
They are also testing inhibitors of JAK2, a molecule that influences ADAR1 activity, for their ability to eliminate cancer stem cells in MM models.
“Several major advances in recent years have been good news for multiple myeloma patients, but those new drugs only target terminally differentiated cancer cells and, thus, can only reduce the bulk of the tumor,” Dr Jamieson said.
“They don’t get to the root cause of disease development, progression, and relapse—cancer stem cells—the way inhibiting ADAR1 does. I like to call our approach ‘precision regenerative medicine.’” ![]()
The enzyme ADAR1 may be a therapeutic target for multiple myeloma (MM), according to research published in Nature Communications.
Investigators found that high ADAR1 levels correlate with reduced survival rates in MM patients.
The team also discovered that blocking ADAR1 reduced regeneration of high-risk MM in serially transplantable patient-derived xenografts.
The investigators believe that JAK2 inhibitors could be used to dampen ADAR1 activity and ultimately prevent progression or relapse in patients with MM.
“Despite new therapies, it’s virtually inevitable that a patient with multiple myeloma will experience relapse of the disease at some point,” said study author Catriona Jamieson, MD, PhD, of University of California, San Diego, in La Jolla.
“That’s why it’s exciting that this discovery may allow us to detect the disease earlier and address the root cause.”
Dr Jamieson and her colleagues knew that ADAR1 is normally expressed during fetal development to help blood cells form. The enzyme edits the sequence of RNA and is known to promote cancer progression and resistance to therapy.
With previous work, Dr Jamieson’s team described ADAR1’s contributions to chronic myeloid leukemia (CML). The enzyme’s RNA-editing activity boosts leukemic stem cells, giving rise to CML, increasing disease recurrence, and allowing CML to resist treatment.
For their current study, Dr Jamieson and her colleagues investigated ADAR1’s role in MM, first analyzing a database of nearly 800 MM patient samples.
The investigators found that patients with low ADAR1 levels in their tumor cells survived longer than patients with high ADAR1 levels.
While more than 90% of patients with low ADAR1 levels survived longer than 2 years after their initial diagnosis, fewer than 70% of patients with high ADAR1 levels did the same.
The investigators also created a humanized mouse model of MM and found that silencing the ADAR1 gene reduced the engraftment of MM.
“This is a difficult disease to model in animals; there isn’t a single gene we can manipulate to mimic multiple myeloma,” said study author Leslie A. Crews, PhD, of University of California, San Diego.
“This study is important, in part because we now have a new xenograft model that will, for the first time, allow us to apply new biomarkers to better predict disease progression and test new therapeutics.”
To advance their findings from this study, the investigators are exploring ways to leverage ADAR1 to detect MM progression as early as possible.
They are also testing inhibitors of JAK2, a molecule that influences ADAR1 activity, for their ability to eliminate cancer stem cells in MM models.
“Several major advances in recent years have been good news for multiple myeloma patients, but those new drugs only target terminally differentiated cancer cells and, thus, can only reduce the bulk of the tumor,” Dr Jamieson said.
“They don’t get to the root cause of disease development, progression, and relapse—cancer stem cells—the way inhibiting ADAR1 does. I like to call our approach ‘precision regenerative medicine.’” ![]()
Health Canada approves midostaurin for AML
Health Canada has approved use of the multi-targeted kinase inhibitor midostaurin (Rydapt™).
This makes midostaurin the first targeted therapy approved to treat FLT3-mutated acute myeloid leukemia (AML) in Canada.
Midostaurin is approved for use with standard cytarabine and daunorubicin induction and cytarabine consolidation for the treatment of adults with newly diagnosed FLT3-mutated AML.
Health Canada’s approval of midostaurin is based on results from the phase 3 RATIFY trial, which were published in NEJM in August.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
The median overall survival was significantly longer in the midostaurin arm than the placebo arm—74.7 months and 25.6 months, respectively (hazard ratio=0.77, P=0.016).
And the median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infection.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis.
Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm. ![]()
Health Canada has approved use of the multi-targeted kinase inhibitor midostaurin (Rydapt™).
This makes midostaurin the first targeted therapy approved to treat FLT3-mutated acute myeloid leukemia (AML) in Canada.
Midostaurin is approved for use with standard cytarabine and daunorubicin induction and cytarabine consolidation for the treatment of adults with newly diagnosed FLT3-mutated AML.
Health Canada’s approval of midostaurin is based on results from the phase 3 RATIFY trial, which were published in NEJM in August.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
The median overall survival was significantly longer in the midostaurin arm than the placebo arm—74.7 months and 25.6 months, respectively (hazard ratio=0.77, P=0.016).
And the median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infection.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis.
Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm. ![]()
Health Canada has approved use of the multi-targeted kinase inhibitor midostaurin (Rydapt™).
This makes midostaurin the first targeted therapy approved to treat FLT3-mutated acute myeloid leukemia (AML) in Canada.
Midostaurin is approved for use with standard cytarabine and daunorubicin induction and cytarabine consolidation for the treatment of adults with newly diagnosed FLT3-mutated AML.
Health Canada’s approval of midostaurin is based on results from the phase 3 RATIFY trial, which were published in NEJM in August.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
The median overall survival was significantly longer in the midostaurin arm than the placebo arm—74.7 months and 25.6 months, respectively (hazard ratio=0.77, P=0.016).
And the median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infection.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis.
Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Companies launch generic busulfan in US
Two companies have announced the US launch of a generic busulfan product, Myleran Injection.
Mylan NV and Aspen have partnered to develop Myleran (busulfan) Injection, 60 mg/10 mL (6 mg/mL) Single-dose Vial, a generic version of Otsuka Pharmaceutical’s Busulfex® Injection.
The US Food and Drug Administration approved Myleran Injection for use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic stem cell transplant in patients with chronic myeloid leukemia.
Two companies have announced the US launch of a generic busulfan product, Myleran Injection.
Mylan NV and Aspen have partnered to develop Myleran (busulfan) Injection, 60 mg/10 mL (6 mg/mL) Single-dose Vial, a generic version of Otsuka Pharmaceutical’s Busulfex® Injection.
The US Food and Drug Administration approved Myleran Injection for use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic stem cell transplant in patients with chronic myeloid leukemia.
Two companies have announced the US launch of a generic busulfan product, Myleran Injection.
Mylan NV and Aspen have partnered to develop Myleran (busulfan) Injection, 60 mg/10 mL (6 mg/mL) Single-dose Vial, a generic version of Otsuka Pharmaceutical’s Busulfex® Injection.
The US Food and Drug Administration approved Myleran Injection for use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic stem cell transplant in patients with chronic myeloid leukemia.
System helps predict RFS, OS in BCP-ALL
Researchers say they have developed a more accurate risk scoring system for children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who are typically thought to have standard- or medium-risk disease.
The scoring system includes 3 factors associated with higher-risk BCP-ALL—the presence of high-risk ALL gene microdeletions, having minimal residual disease (MRD) greater than 5 x 10-5 at day 33, and being high-risk according to National Cancer Institute (NCI) classification.
The researchers found that children with 2 or more of these characteristics were most likely to relapse or die within 7 years of treatment initiation.
On the other hand, children without any of the 3 characteristics had high rates of relapse-free survival (RFS) and overall survival (OS).
Rosemary Sutton, PhD, of Children’s Cancer Institute in Sydney, New South Wales, Australia, and her colleagues devised this risk scoring system and described it in the British Journal of Haematology.
The researchers created their system with the help of data from 475 patients (ages 1 to 18) who had BCP-ALL and were considered non-high-risk. The patients were enrolled on the ANZCHOG ALL8 trial.
Dr Sutton and her colleagues noted that children with standard- or medium-risk BCP-ALL typically receive less intensive treatment than children with high-risk BCP-ALL. However, some of the standard- and medium-risk patients do relapse.
“For the standard- to medium-risk group, we needed more information to get a better handle on the biology of the child’s cancer to better determine their risk,” Dr Sutton said. “So we supplemented MRD results with 2 other pieces of patient information—the presence or absence of specific gene microdeletions and a score called the NCI risk, based on age and white blood cell count.”
“We tested for microdeletions in 9 genes involved in leukemia and found that 2 of the genes—IKZF1 and P2RY8-CRLF2—were important predictors of relapse.”
The researchers combined patients with IKZF1 intragenic deletions, P2RY8-CRLF2 gene fusion, or both into a “high-risk deletion group.”
And the team based the scoring system on 3 factors—the high-risk deletion group, MRD >5 x 10-5 at day 33, and high risk according to NCI risk classification. Patients received 1 point for each of these factors.
The RFS rate was 93% for patients with a score of 0, 78% for those with a score of 1, and 49% for patients with a score of 2 or 3. The OS rate was 99%, 91%, and 71%, respectively.
The researchers said their scoring system provided greater discrimination than MRD-based risk stratification into a standard-risk group—which had an RFS of 89% and an OS of 96%—and a medium-risk group—which had an RFS of 79% and an OS of 91%.
Study author Toby Trahair, MBBS, PhD, of Sydney Children’s Hospital in Randwick, New South Wales, said this scoring system could make a big difference to the success of BCP-ALL treatment.
“We are always trying to improve how we diagnose and treat children with this most common childhood cancer,” Dr Trahair said. “This risk score will mean doctors can fine tune a child’s risk category and so fine tune their treatment. It will mean more kids can conquer this horrible disease, which, only 50 years ago, had survival rates of close to 0.”
Researchers say they have developed a more accurate risk scoring system for children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who are typically thought to have standard- or medium-risk disease.
The scoring system includes 3 factors associated with higher-risk BCP-ALL—the presence of high-risk ALL gene microdeletions, having minimal residual disease (MRD) greater than 5 x 10-5 at day 33, and being high-risk according to National Cancer Institute (NCI) classification.
The researchers found that children with 2 or more of these characteristics were most likely to relapse or die within 7 years of treatment initiation.
On the other hand, children without any of the 3 characteristics had high rates of relapse-free survival (RFS) and overall survival (OS).
Rosemary Sutton, PhD, of Children’s Cancer Institute in Sydney, New South Wales, Australia, and her colleagues devised this risk scoring system and described it in the British Journal of Haematology.
The researchers created their system with the help of data from 475 patients (ages 1 to 18) who had BCP-ALL and were considered non-high-risk. The patients were enrolled on the ANZCHOG ALL8 trial.
Dr Sutton and her colleagues noted that children with standard- or medium-risk BCP-ALL typically receive less intensive treatment than children with high-risk BCP-ALL. However, some of the standard- and medium-risk patients do relapse.
“For the standard- to medium-risk group, we needed more information to get a better handle on the biology of the child’s cancer to better determine their risk,” Dr Sutton said. “So we supplemented MRD results with 2 other pieces of patient information—the presence or absence of specific gene microdeletions and a score called the NCI risk, based on age and white blood cell count.”
“We tested for microdeletions in 9 genes involved in leukemia and found that 2 of the genes—IKZF1 and P2RY8-CRLF2—were important predictors of relapse.”
The researchers combined patients with IKZF1 intragenic deletions, P2RY8-CRLF2 gene fusion, or both into a “high-risk deletion group.”
And the team based the scoring system on 3 factors—the high-risk deletion group, MRD >5 x 10-5 at day 33, and high risk according to NCI risk classification. Patients received 1 point for each of these factors.
The RFS rate was 93% for patients with a score of 0, 78% for those with a score of 1, and 49% for patients with a score of 2 or 3. The OS rate was 99%, 91%, and 71%, respectively.
The researchers said their scoring system provided greater discrimination than MRD-based risk stratification into a standard-risk group—which had an RFS of 89% and an OS of 96%—and a medium-risk group—which had an RFS of 79% and an OS of 91%.
Study author Toby Trahair, MBBS, PhD, of Sydney Children’s Hospital in Randwick, New South Wales, said this scoring system could make a big difference to the success of BCP-ALL treatment.
“We are always trying to improve how we diagnose and treat children with this most common childhood cancer,” Dr Trahair said. “This risk score will mean doctors can fine tune a child’s risk category and so fine tune their treatment. It will mean more kids can conquer this horrible disease, which, only 50 years ago, had survival rates of close to 0.”
Researchers say they have developed a more accurate risk scoring system for children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who are typically thought to have standard- or medium-risk disease.
The scoring system includes 3 factors associated with higher-risk BCP-ALL—the presence of high-risk ALL gene microdeletions, having minimal residual disease (MRD) greater than 5 x 10-5 at day 33, and being high-risk according to National Cancer Institute (NCI) classification.
The researchers found that children with 2 or more of these characteristics were most likely to relapse or die within 7 years of treatment initiation.
On the other hand, children without any of the 3 characteristics had high rates of relapse-free survival (RFS) and overall survival (OS).
Rosemary Sutton, PhD, of Children’s Cancer Institute in Sydney, New South Wales, Australia, and her colleagues devised this risk scoring system and described it in the British Journal of Haematology.
The researchers created their system with the help of data from 475 patients (ages 1 to 18) who had BCP-ALL and were considered non-high-risk. The patients were enrolled on the ANZCHOG ALL8 trial.
Dr Sutton and her colleagues noted that children with standard- or medium-risk BCP-ALL typically receive less intensive treatment than children with high-risk BCP-ALL. However, some of the standard- and medium-risk patients do relapse.
“For the standard- to medium-risk group, we needed more information to get a better handle on the biology of the child’s cancer to better determine their risk,” Dr Sutton said. “So we supplemented MRD results with 2 other pieces of patient information—the presence or absence of specific gene microdeletions and a score called the NCI risk, based on age and white blood cell count.”
“We tested for microdeletions in 9 genes involved in leukemia and found that 2 of the genes—IKZF1 and P2RY8-CRLF2—were important predictors of relapse.”
The researchers combined patients with IKZF1 intragenic deletions, P2RY8-CRLF2 gene fusion, or both into a “high-risk deletion group.”
And the team based the scoring system on 3 factors—the high-risk deletion group, MRD >5 x 10-5 at day 33, and high risk according to NCI risk classification. Patients received 1 point for each of these factors.
The RFS rate was 93% for patients with a score of 0, 78% for those with a score of 1, and 49% for patients with a score of 2 or 3. The OS rate was 99%, 91%, and 71%, respectively.
The researchers said their scoring system provided greater discrimination than MRD-based risk stratification into a standard-risk group—which had an RFS of 89% and an OS of 96%—and a medium-risk group—which had an RFS of 79% and an OS of 91%.
Study author Toby Trahair, MBBS, PhD, of Sydney Children’s Hospital in Randwick, New South Wales, said this scoring system could make a big difference to the success of BCP-ALL treatment.
“We are always trying to improve how we diagnose and treat children with this most common childhood cancer,” Dr Trahair said. “This risk score will mean doctors can fine tune a child’s risk category and so fine tune their treatment. It will mean more kids can conquer this horrible disease, which, only 50 years ago, had survival rates of close to 0.”
FDA approves use of heparin products
The US Food and Drug Administration (FDA) has approved use of Mylan NV’s heparin products.
This includes Heparin Sodium Injection at 1000 USP/mL, 5000 USP/mL, 10,000 USP/mL, and 20,000 USP/mL, all of which are packaged in multi-dose vials.
These products should be available in the coming weeks, according to Rajiv Malik, the president of Mylan.
Mylan’s heparin products are approved for the same uses as other heparin products approved in the US.
This includes as prophylaxis and treatment for venous thrombosis and its extension, pulmonary embolism, and peripheral arterial embolism.
The heparin products can also be used to treat atrial fibrillation with embolization, prevent clotting in arterial and cardiac surgery, and diagnose/treat acute and chronic consumptive coagulopathies (disseminated intravascular coagulation).
Low-dose heparin can be used to prevent post-operative deep vein thrombosis and pulmonary embolism in patients undergoing major abdominothoracic surgery or who, for other reasons, are at risk of developing thromboembolic disease.
Heparin can also be used as an anticoagulant in blood transfusions, extracorporeal circulation, and dialysis procedures, as well as in blood samples for laboratory purposes.
“We are very proud of today’s FDA approval of Heparin Sodium Injection, as this approval adds yet another highly complex and difficult-to-manufacture product to our portfolio,” Malik said.
“We expect to make our heparin products available to US hospitals in the coming weeks, further supporting our institutional customers in meeting the needs of their patients who depend on high-quality anticoagulants.”
The US Food and Drug Administration (FDA) has approved use of Mylan NV’s heparin products.
This includes Heparin Sodium Injection at 1000 USP/mL, 5000 USP/mL, 10,000 USP/mL, and 20,000 USP/mL, all of which are packaged in multi-dose vials.
These products should be available in the coming weeks, according to Rajiv Malik, the president of Mylan.
Mylan’s heparin products are approved for the same uses as other heparin products approved in the US.
This includes as prophylaxis and treatment for venous thrombosis and its extension, pulmonary embolism, and peripheral arterial embolism.
The heparin products can also be used to treat atrial fibrillation with embolization, prevent clotting in arterial and cardiac surgery, and diagnose/treat acute and chronic consumptive coagulopathies (disseminated intravascular coagulation).
Low-dose heparin can be used to prevent post-operative deep vein thrombosis and pulmonary embolism in patients undergoing major abdominothoracic surgery or who, for other reasons, are at risk of developing thromboembolic disease.
Heparin can also be used as an anticoagulant in blood transfusions, extracorporeal circulation, and dialysis procedures, as well as in blood samples for laboratory purposes.
“We are very proud of today’s FDA approval of Heparin Sodium Injection, as this approval adds yet another highly complex and difficult-to-manufacture product to our portfolio,” Malik said.
“We expect to make our heparin products available to US hospitals in the coming weeks, further supporting our institutional customers in meeting the needs of their patients who depend on high-quality anticoagulants.”
The US Food and Drug Administration (FDA) has approved use of Mylan NV’s heparin products.
This includes Heparin Sodium Injection at 1000 USP/mL, 5000 USP/mL, 10,000 USP/mL, and 20,000 USP/mL, all of which are packaged in multi-dose vials.
These products should be available in the coming weeks, according to Rajiv Malik, the president of Mylan.
Mylan’s heparin products are approved for the same uses as other heparin products approved in the US.
This includes as prophylaxis and treatment for venous thrombosis and its extension, pulmonary embolism, and peripheral arterial embolism.
The heparin products can also be used to treat atrial fibrillation with embolization, prevent clotting in arterial and cardiac surgery, and diagnose/treat acute and chronic consumptive coagulopathies (disseminated intravascular coagulation).
Low-dose heparin can be used to prevent post-operative deep vein thrombosis and pulmonary embolism in patients undergoing major abdominothoracic surgery or who, for other reasons, are at risk of developing thromboembolic disease.
Heparin can also be used as an anticoagulant in blood transfusions, extracorporeal circulation, and dialysis procedures, as well as in blood samples for laboratory purposes.
“We are very proud of today’s FDA approval of Heparin Sodium Injection, as this approval adds yet another highly complex and difficult-to-manufacture product to our portfolio,” Malik said.
“We expect to make our heparin products available to US hospitals in the coming weeks, further supporting our institutional customers in meeting the needs of their patients who depend on high-quality anticoagulants.”