User login
Mogamulizumab is ‘valuable’ option for CTCL
LA JOLLA, CA—Mogamulizumab is a valuable new therapeutic option for patients with cutaneous T-cell lymphoma (CTCL), according to researchers.
Results of the phase 3 MAVORIC study indicated that mogamulizumab is more effective than vorinostat in previously treated patients with CTCL.
Mogamulizumab produced a better overall response rate (ORR) and prolonged progression-free survival (PFS) in these patients.
Infusion-related reactions and drug eruptions were more common in patients who received mogamulizumab.
Youn H. Kim, MD, of the Stanford Cancer Institute in Palo Alto, California, and her colleagues presented these results in a poster at the 10th Annual T-cell Lymphoma Forum. The study was funded by Kyowa Kirin Pharmaceutical Development, Inc.
MAVORIC enrolled 372 adults with histologically confirmed mycosis fungoides (MF) or Sézary syndrome (SS) who had failed at least 1 systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily.
Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could crossover to mogamulizumab if they progressed or experienced intolerable toxicity.
Baseline characteristics were similar between the treatment arms. The median age was 64 (range, 54-73) in the mogamulizumab arm and 65 (range, 56-72) in the vorinostat arm. Ninety-nine percent and 100% of patients, respectively, had an ECOG performance status of 0 to 1.
A little more than half of patients in each arm had MF—57% in the mogamulizumab arm and 53% in the vorinostat arm.
The median number of prior systemic therapies was 3 in both arms (range, 1-18 in the mogamulizumab arm and 0-14 in the vorinostat arm).
Efficacy
The primary endpoint was PFS, and mogamulizumab provided a significant improvement there. The median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
The researchers also observed a significant improvement in global ORR with mogamulizumab. It was 28% (52/189) in that arm and 5% (9/186) in the vorinostat arm (P<0.0001).
For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat. For SS patients, the ORR was 37% and 2%, respectively.
Responses by disease compartment were superior with mogamulizumab as well.
“Especially in the blood compartment, mogamulizumab had very striking activity over vorinostat,” Dr Kim said.
The blood ORR was 68% with mogamulizumab and 19% with vorinostat. The skin ORR was 42% and 16%, respectively. The lymph node ORR was 17% and 4%, respectively. The viscera ORR was 0% in both arms.
After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm.
For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat. For SS patients, the median DOR was 17 months and 7 months, respectively.
Safety
“Side effects [of mogamulizumab] were very well tolerable,” Dr Kim said. “Most significant is rash and infusion reactions, but, in terms of severe adverse events, [they] were very minimal.”
The most common treatment-emergent adverse events (AEs), occurring in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), were:
- Infusion-related reactions (33.2% vs 0.5%)
- Drug eruptions (23.9% vs 0.5%)
- Diarrhea (23.4% vs 61.8%)
- Nausea (15.2% vs 42.5%)
- Thrombocytopenia (11.4% vs 30.6%)
- Dysgeusia (3.3% vs 28.0%)
- Increased blood creatinine (3.3% vs 28.0%)
- Decreased appetite (7.6% vs 24.7%).
There were no grade 4 AEs in the mogamulizumab arm and 2 cases of grade 4 thrombocytopenia in the vorinostat arm.
Grade 3 AEs in the mogamulizumab arm included drug eruptions (n=8), infusion-related reactions (n=3), fatigue (n=3), decreased appetite (n=2), nausea (n=1), pyrexia (n=1), and diarrhea (n=1).
Grade 3 AEs in the vorinostat arm included thrombocytopenia (n=11), fatigue (n=11), diarrhea (n=9), nausea (n=3), decreased appetite (n=2), and dysgeusia (n=1).
“So the results are, overall, positive,” Dr Kim said. “The data is submitted to the [US Food and Drug Administration]. We are really hoping that [mogamulizumab] will be approved so that we would have a new, exciting treatment for our patients with mycosis fungoides and Sézary syndrome.” ![]()
LA JOLLA, CA—Mogamulizumab is a valuable new therapeutic option for patients with cutaneous T-cell lymphoma (CTCL), according to researchers.
Results of the phase 3 MAVORIC study indicated that mogamulizumab is more effective than vorinostat in previously treated patients with CTCL.
Mogamulizumab produced a better overall response rate (ORR) and prolonged progression-free survival (PFS) in these patients.
Infusion-related reactions and drug eruptions were more common in patients who received mogamulizumab.
Youn H. Kim, MD, of the Stanford Cancer Institute in Palo Alto, California, and her colleagues presented these results in a poster at the 10th Annual T-cell Lymphoma Forum. The study was funded by Kyowa Kirin Pharmaceutical Development, Inc.
MAVORIC enrolled 372 adults with histologically confirmed mycosis fungoides (MF) or Sézary syndrome (SS) who had failed at least 1 systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily.
Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could crossover to mogamulizumab if they progressed or experienced intolerable toxicity.
Baseline characteristics were similar between the treatment arms. The median age was 64 (range, 54-73) in the mogamulizumab arm and 65 (range, 56-72) in the vorinostat arm. Ninety-nine percent and 100% of patients, respectively, had an ECOG performance status of 0 to 1.
A little more than half of patients in each arm had MF—57% in the mogamulizumab arm and 53% in the vorinostat arm.
The median number of prior systemic therapies was 3 in both arms (range, 1-18 in the mogamulizumab arm and 0-14 in the vorinostat arm).
Efficacy
The primary endpoint was PFS, and mogamulizumab provided a significant improvement there. The median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
The researchers also observed a significant improvement in global ORR with mogamulizumab. It was 28% (52/189) in that arm and 5% (9/186) in the vorinostat arm (P<0.0001).
For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat. For SS patients, the ORR was 37% and 2%, respectively.
Responses by disease compartment were superior with mogamulizumab as well.
“Especially in the blood compartment, mogamulizumab had very striking activity over vorinostat,” Dr Kim said.
The blood ORR was 68% with mogamulizumab and 19% with vorinostat. The skin ORR was 42% and 16%, respectively. The lymph node ORR was 17% and 4%, respectively. The viscera ORR was 0% in both arms.
After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm.
For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat. For SS patients, the median DOR was 17 months and 7 months, respectively.
Safety
“Side effects [of mogamulizumab] were very well tolerable,” Dr Kim said. “Most significant is rash and infusion reactions, but, in terms of severe adverse events, [they] were very minimal.”
The most common treatment-emergent adverse events (AEs), occurring in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), were:
- Infusion-related reactions (33.2% vs 0.5%)
- Drug eruptions (23.9% vs 0.5%)
- Diarrhea (23.4% vs 61.8%)
- Nausea (15.2% vs 42.5%)
- Thrombocytopenia (11.4% vs 30.6%)
- Dysgeusia (3.3% vs 28.0%)
- Increased blood creatinine (3.3% vs 28.0%)
- Decreased appetite (7.6% vs 24.7%).
There were no grade 4 AEs in the mogamulizumab arm and 2 cases of grade 4 thrombocytopenia in the vorinostat arm.
Grade 3 AEs in the mogamulizumab arm included drug eruptions (n=8), infusion-related reactions (n=3), fatigue (n=3), decreased appetite (n=2), nausea (n=1), pyrexia (n=1), and diarrhea (n=1).
Grade 3 AEs in the vorinostat arm included thrombocytopenia (n=11), fatigue (n=11), diarrhea (n=9), nausea (n=3), decreased appetite (n=2), and dysgeusia (n=1).
“So the results are, overall, positive,” Dr Kim said. “The data is submitted to the [US Food and Drug Administration]. We are really hoping that [mogamulizumab] will be approved so that we would have a new, exciting treatment for our patients with mycosis fungoides and Sézary syndrome.” ![]()
LA JOLLA, CA—Mogamulizumab is a valuable new therapeutic option for patients with cutaneous T-cell lymphoma (CTCL), according to researchers.
Results of the phase 3 MAVORIC study indicated that mogamulizumab is more effective than vorinostat in previously treated patients with CTCL.
Mogamulizumab produced a better overall response rate (ORR) and prolonged progression-free survival (PFS) in these patients.
Infusion-related reactions and drug eruptions were more common in patients who received mogamulizumab.
Youn H. Kim, MD, of the Stanford Cancer Institute in Palo Alto, California, and her colleagues presented these results in a poster at the 10th Annual T-cell Lymphoma Forum. The study was funded by Kyowa Kirin Pharmaceutical Development, Inc.
MAVORIC enrolled 372 adults with histologically confirmed mycosis fungoides (MF) or Sézary syndrome (SS) who had failed at least 1 systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily.
Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could crossover to mogamulizumab if they progressed or experienced intolerable toxicity.
Baseline characteristics were similar between the treatment arms. The median age was 64 (range, 54-73) in the mogamulizumab arm and 65 (range, 56-72) in the vorinostat arm. Ninety-nine percent and 100% of patients, respectively, had an ECOG performance status of 0 to 1.
A little more than half of patients in each arm had MF—57% in the mogamulizumab arm and 53% in the vorinostat arm.
The median number of prior systemic therapies was 3 in both arms (range, 1-18 in the mogamulizumab arm and 0-14 in the vorinostat arm).
Efficacy
The primary endpoint was PFS, and mogamulizumab provided a significant improvement there. The median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
The researchers also observed a significant improvement in global ORR with mogamulizumab. It was 28% (52/189) in that arm and 5% (9/186) in the vorinostat arm (P<0.0001).
For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat. For SS patients, the ORR was 37% and 2%, respectively.
Responses by disease compartment were superior with mogamulizumab as well.
“Especially in the blood compartment, mogamulizumab had very striking activity over vorinostat,” Dr Kim said.
The blood ORR was 68% with mogamulizumab and 19% with vorinostat. The skin ORR was 42% and 16%, respectively. The lymph node ORR was 17% and 4%, respectively. The viscera ORR was 0% in both arms.
After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm.
For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat. For SS patients, the median DOR was 17 months and 7 months, respectively.
Safety
“Side effects [of mogamulizumab] were very well tolerable,” Dr Kim said. “Most significant is rash and infusion reactions, but, in terms of severe adverse events, [they] were very minimal.”
The most common treatment-emergent adverse events (AEs), occurring in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), were:
- Infusion-related reactions (33.2% vs 0.5%)
- Drug eruptions (23.9% vs 0.5%)
- Diarrhea (23.4% vs 61.8%)
- Nausea (15.2% vs 42.5%)
- Thrombocytopenia (11.4% vs 30.6%)
- Dysgeusia (3.3% vs 28.0%)
- Increased blood creatinine (3.3% vs 28.0%)
- Decreased appetite (7.6% vs 24.7%).
There were no grade 4 AEs in the mogamulizumab arm and 2 cases of grade 4 thrombocytopenia in the vorinostat arm.
Grade 3 AEs in the mogamulizumab arm included drug eruptions (n=8), infusion-related reactions (n=3), fatigue (n=3), decreased appetite (n=2), nausea (n=1), pyrexia (n=1), and diarrhea (n=1).
Grade 3 AEs in the vorinostat arm included thrombocytopenia (n=11), fatigue (n=11), diarrhea (n=9), nausea (n=3), decreased appetite (n=2), and dysgeusia (n=1).
“So the results are, overall, positive,” Dr Kim said. “The data is submitted to the [US Food and Drug Administration]. We are really hoping that [mogamulizumab] will be approved so that we would have a new, exciting treatment for our patients with mycosis fungoides and Sézary syndrome.” ![]()
Socioeconomic deprivation tied to survival in ALL
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
In situ vaccination eradicates lymphoma, other cancers
Experiments in mice have shown that injecting immune-stimulating agents directly into a tumor can help the immune system eradicate tumors in other areas of the body.
The approach worked for several cancers, including lymphomas.
The researchers believe the local application of the agents could serve as a rapid and relatively inexpensive cancer therapy that is unlikely to cause the adverse effects often seen with more widespread immune stimulation.
“Our approach uses a one-time application of very small amounts of two agents to stimulate the immune cells only within the tumor itself,” said Ronald Levy, MD, of Stanford University Medical Center in California.
“In the mice, we saw amazing, body-wide effects, including the elimination of tumors all over the animal. This approach bypasses the need to identify tumor-specific immune targets and doesn’t require wholesale activation of the immune system or customization of a patient’s immune cells.”
Dr Levy and his colleagues described this approach in Science Translational Medicine.
The method involves reactivating cancer-specific T cells by injecting microgram amounts of two agents directly into the tumor site.
One of the agents is an unmethylated CG–enriched oligodeoxynucleotide (CpG)—a Toll-like receptor 9 (TLR9) ligand. It works with nearby immune cells to amplify the expression of OX40 on the surface of T cells.
The other agent is an antibody that binds to OX40. It activates the T cells to lead the charge against the cancer cells.
Because the agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Some of these tumor-specific, activated T cells then leave the original tumor to find and destroy other identical tumors throughout the body.
The researchers found this approach worked well in mice with A20 B-cell lymphoma tumors transplanted in two sites on their bodies.
Injecting one tumor site with the agents caused regression of the untreated tumor as well as the treated one. In this way, 87 of 90 mice were cured.
Although lymphoma recurred in 3 of the mice, the tumors again regressed after a second treatment with CpG and anti-OX40.
The researchers saw similar results in mice with melanoma as well as breast and colon cancer.
Mice genetically engineered to spontaneously develop breast cancers in all 10 of their mammary pads also responded to the treatment. Treating the first tumor that arose often prevented the occurrence of future tumors and significantly increased the animals’ life span, the researchers found.
Finally, the team explored the specificity of the T cells by transplanting two types of tumors into mice.
They transplanted A20 lymphoma cells in two locations and a colon cancer cell line in a third location. Treatment of one of the lymphoma sites caused the regression of both lymphoma tumors but did not affect the colon cancer cells.
“This is a very targeted approach,” Dr Levy said. “Only the tumor that shares the protein targets displayed by the treated site is affected. We’re attacking specific targets without having to identify exactly what proteins the T cells are recognizing.”
Dr Levy and his colleagues have launched a clinical trial (NCT03410901) to test this treatment approach. The researchers hope to determine the adverse effects and optimal dose of the TLR9 agonist SD-101, the anti-OX40 antibody BMS 986178, and radiation therapy in patients with low-grade B-cell non-Hodgkin lymphomas.
If the trial is successful, Dr Levy believes the treatment could be useful for many tumor types.
“I don’t think there’s a limit to the type of tumor we could potentially treat,” he said, “as long as it has been infiltrated by the immune system.” ![]()
Experiments in mice have shown that injecting immune-stimulating agents directly into a tumor can help the immune system eradicate tumors in other areas of the body.
The approach worked for several cancers, including lymphomas.
The researchers believe the local application of the agents could serve as a rapid and relatively inexpensive cancer therapy that is unlikely to cause the adverse effects often seen with more widespread immune stimulation.
“Our approach uses a one-time application of very small amounts of two agents to stimulate the immune cells only within the tumor itself,” said Ronald Levy, MD, of Stanford University Medical Center in California.
“In the mice, we saw amazing, body-wide effects, including the elimination of tumors all over the animal. This approach bypasses the need to identify tumor-specific immune targets and doesn’t require wholesale activation of the immune system or customization of a patient’s immune cells.”
Dr Levy and his colleagues described this approach in Science Translational Medicine.
The method involves reactivating cancer-specific T cells by injecting microgram amounts of two agents directly into the tumor site.
One of the agents is an unmethylated CG–enriched oligodeoxynucleotide (CpG)—a Toll-like receptor 9 (TLR9) ligand. It works with nearby immune cells to amplify the expression of OX40 on the surface of T cells.
The other agent is an antibody that binds to OX40. It activates the T cells to lead the charge against the cancer cells.
Because the agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Some of these tumor-specific, activated T cells then leave the original tumor to find and destroy other identical tumors throughout the body.
The researchers found this approach worked well in mice with A20 B-cell lymphoma tumors transplanted in two sites on their bodies.
Injecting one tumor site with the agents caused regression of the untreated tumor as well as the treated one. In this way, 87 of 90 mice were cured.
Although lymphoma recurred in 3 of the mice, the tumors again regressed after a second treatment with CpG and anti-OX40.
The researchers saw similar results in mice with melanoma as well as breast and colon cancer.
Mice genetically engineered to spontaneously develop breast cancers in all 10 of their mammary pads also responded to the treatment. Treating the first tumor that arose often prevented the occurrence of future tumors and significantly increased the animals’ life span, the researchers found.
Finally, the team explored the specificity of the T cells by transplanting two types of tumors into mice.
They transplanted A20 lymphoma cells in two locations and a colon cancer cell line in a third location. Treatment of one of the lymphoma sites caused the regression of both lymphoma tumors but did not affect the colon cancer cells.
“This is a very targeted approach,” Dr Levy said. “Only the tumor that shares the protein targets displayed by the treated site is affected. We’re attacking specific targets without having to identify exactly what proteins the T cells are recognizing.”
Dr Levy and his colleagues have launched a clinical trial (NCT03410901) to test this treatment approach. The researchers hope to determine the adverse effects and optimal dose of the TLR9 agonist SD-101, the anti-OX40 antibody BMS 986178, and radiation therapy in patients with low-grade B-cell non-Hodgkin lymphomas.
If the trial is successful, Dr Levy believes the treatment could be useful for many tumor types.
“I don’t think there’s a limit to the type of tumor we could potentially treat,” he said, “as long as it has been infiltrated by the immune system.” ![]()
Experiments in mice have shown that injecting immune-stimulating agents directly into a tumor can help the immune system eradicate tumors in other areas of the body.
The approach worked for several cancers, including lymphomas.
The researchers believe the local application of the agents could serve as a rapid and relatively inexpensive cancer therapy that is unlikely to cause the adverse effects often seen with more widespread immune stimulation.
“Our approach uses a one-time application of very small amounts of two agents to stimulate the immune cells only within the tumor itself,” said Ronald Levy, MD, of Stanford University Medical Center in California.
“In the mice, we saw amazing, body-wide effects, including the elimination of tumors all over the animal. This approach bypasses the need to identify tumor-specific immune targets and doesn’t require wholesale activation of the immune system or customization of a patient’s immune cells.”
Dr Levy and his colleagues described this approach in Science Translational Medicine.
The method involves reactivating cancer-specific T cells by injecting microgram amounts of two agents directly into the tumor site.
One of the agents is an unmethylated CG–enriched oligodeoxynucleotide (CpG)—a Toll-like receptor 9 (TLR9) ligand. It works with nearby immune cells to amplify the expression of OX40 on the surface of T cells.
The other agent is an antibody that binds to OX40. It activates the T cells to lead the charge against the cancer cells.
Because the agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Some of these tumor-specific, activated T cells then leave the original tumor to find and destroy other identical tumors throughout the body.
The researchers found this approach worked well in mice with A20 B-cell lymphoma tumors transplanted in two sites on their bodies.
Injecting one tumor site with the agents caused regression of the untreated tumor as well as the treated one. In this way, 87 of 90 mice were cured.
Although lymphoma recurred in 3 of the mice, the tumors again regressed after a second treatment with CpG and anti-OX40.
The researchers saw similar results in mice with melanoma as well as breast and colon cancer.
Mice genetically engineered to spontaneously develop breast cancers in all 10 of their mammary pads also responded to the treatment. Treating the first tumor that arose often prevented the occurrence of future tumors and significantly increased the animals’ life span, the researchers found.
Finally, the team explored the specificity of the T cells by transplanting two types of tumors into mice.
They transplanted A20 lymphoma cells in two locations and a colon cancer cell line in a third location. Treatment of one of the lymphoma sites caused the regression of both lymphoma tumors but did not affect the colon cancer cells.
“This is a very targeted approach,” Dr Levy said. “Only the tumor that shares the protein targets displayed by the treated site is affected. We’re attacking specific targets without having to identify exactly what proteins the T cells are recognizing.”
Dr Levy and his colleagues have launched a clinical trial (NCT03410901) to test this treatment approach. The researchers hope to determine the adverse effects and optimal dose of the TLR9 agonist SD-101, the anti-OX40 antibody BMS 986178, and radiation therapy in patients with low-grade B-cell non-Hodgkin lymphomas.
If the trial is successful, Dr Levy believes the treatment could be useful for many tumor types.
“I don’t think there’s a limit to the type of tumor we could potentially treat,” he said, “as long as it has been infiltrated by the immune system.” ![]()
Combo could treat double-hit lymphoma
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Interim trial results may be misleading
Interim results from randomized trials may be misleading at times, according to research published in JAMA.
Researchers compared interim and final publications on randomized trials and found that, 21% of the time, results changed significantly.
“Changes between interim and final publication matter because clinicians and the public could have been misled about whether an intervention was beneficial, harmful, or ineffective,” said study author Lisa Schwartz, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
She and her colleagues searched PubMed for randomized trials from 2006 to 2015 with “interim,” “not mature,” or “immature” in the title or abstract.
To identify final publications, they searched PubMed, ClinicalTrials.gov, and Web of Science through 2016. The team emailed authors of interim reports when no final publication was identified.
For interim and final publications reporting the same efficacy and or safety outcome, the researchers compared trial characteristics and prominence. They also categorized abstract conclusions (not different, beneficial, or harmful) and compared changes between interim and final publications.
Findings
Interim results were reported in 613 of 1267 screened publications.
Of those publications, 72% reported on trials stopped early (for benefit, harm, futility, or other problems). The remaining 171 ongoing trials (mostly in oncology, surgery, or cardiology) reported interim efficacy or safety results.
Forty-one percent of the publications stated that the interim analysis was specified in the protocol, but half provided no reason for the interim publication.
Final results were published for 98 of the 160 trials (61%) that were more than 1 year beyond the completion date.
The researchers compared 73 interim and final publications reporting the same efficacy or safety outcome. And they found that interim and final publications had similar prominence.
In most cases (79%), the abstract conclusions did not change from the interim publication to the final publication. However, for 21% of trials, there were significant changes.
For 4 trials, the conclusions changed from “no difference” between randomized to treatments to the study treatment being “beneficial.” In 3 trials, the conclusions changed from “not different” to “harmful or possibly harmful.”
In 6 trials, the conclusions changed from “beneficial” to “not different.” One trial changed from “beneficial” to “harmful,” and another changed from “inconclusive” to “noninferior.”
Dr Schwartz and her colleagues concluded that while most interim and final publications reached similar conclusions, frequent non-publication of final results can lead to confusion or unfounded assumptions with true treatment effects remaining unknown.
To safeguard against any such confusion, the researchers recommended routinely adding the word “interim” in the title and justifying the reason in the publication. (Many interim publications reported analyses without any justification.)
“Most importantly, journals, authors, and funders should commit to making final results accessible by linking interim publications to final reports whenever available,” said study author Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice. ![]()
Interim results from randomized trials may be misleading at times, according to research published in JAMA.
Researchers compared interim and final publications on randomized trials and found that, 21% of the time, results changed significantly.
“Changes between interim and final publication matter because clinicians and the public could have been misled about whether an intervention was beneficial, harmful, or ineffective,” said study author Lisa Schwartz, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
She and her colleagues searched PubMed for randomized trials from 2006 to 2015 with “interim,” “not mature,” or “immature” in the title or abstract.
To identify final publications, they searched PubMed, ClinicalTrials.gov, and Web of Science through 2016. The team emailed authors of interim reports when no final publication was identified.
For interim and final publications reporting the same efficacy and or safety outcome, the researchers compared trial characteristics and prominence. They also categorized abstract conclusions (not different, beneficial, or harmful) and compared changes between interim and final publications.
Findings
Interim results were reported in 613 of 1267 screened publications.
Of those publications, 72% reported on trials stopped early (for benefit, harm, futility, or other problems). The remaining 171 ongoing trials (mostly in oncology, surgery, or cardiology) reported interim efficacy or safety results.
Forty-one percent of the publications stated that the interim analysis was specified in the protocol, but half provided no reason for the interim publication.
Final results were published for 98 of the 160 trials (61%) that were more than 1 year beyond the completion date.
The researchers compared 73 interim and final publications reporting the same efficacy or safety outcome. And they found that interim and final publications had similar prominence.
In most cases (79%), the abstract conclusions did not change from the interim publication to the final publication. However, for 21% of trials, there were significant changes.
For 4 trials, the conclusions changed from “no difference” between randomized to treatments to the study treatment being “beneficial.” In 3 trials, the conclusions changed from “not different” to “harmful or possibly harmful.”
In 6 trials, the conclusions changed from “beneficial” to “not different.” One trial changed from “beneficial” to “harmful,” and another changed from “inconclusive” to “noninferior.”
Dr Schwartz and her colleagues concluded that while most interim and final publications reached similar conclusions, frequent non-publication of final results can lead to confusion or unfounded assumptions with true treatment effects remaining unknown.
To safeguard against any such confusion, the researchers recommended routinely adding the word “interim” in the title and justifying the reason in the publication. (Many interim publications reported analyses without any justification.)
“Most importantly, journals, authors, and funders should commit to making final results accessible by linking interim publications to final reports whenever available,” said study author Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice. ![]()
Interim results from randomized trials may be misleading at times, according to research published in JAMA.
Researchers compared interim and final publications on randomized trials and found that, 21% of the time, results changed significantly.
“Changes between interim and final publication matter because clinicians and the public could have been misled about whether an intervention was beneficial, harmful, or ineffective,” said study author Lisa Schwartz, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
She and her colleagues searched PubMed for randomized trials from 2006 to 2015 with “interim,” “not mature,” or “immature” in the title or abstract.
To identify final publications, they searched PubMed, ClinicalTrials.gov, and Web of Science through 2016. The team emailed authors of interim reports when no final publication was identified.
For interim and final publications reporting the same efficacy and or safety outcome, the researchers compared trial characteristics and prominence. They also categorized abstract conclusions (not different, beneficial, or harmful) and compared changes between interim and final publications.
Findings
Interim results were reported in 613 of 1267 screened publications.
Of those publications, 72% reported on trials stopped early (for benefit, harm, futility, or other problems). The remaining 171 ongoing trials (mostly in oncology, surgery, or cardiology) reported interim efficacy or safety results.
Forty-one percent of the publications stated that the interim analysis was specified in the protocol, but half provided no reason for the interim publication.
Final results were published for 98 of the 160 trials (61%) that were more than 1 year beyond the completion date.
The researchers compared 73 interim and final publications reporting the same efficacy or safety outcome. And they found that interim and final publications had similar prominence.
In most cases (79%), the abstract conclusions did not change from the interim publication to the final publication. However, for 21% of trials, there were significant changes.
For 4 trials, the conclusions changed from “no difference” between randomized to treatments to the study treatment being “beneficial.” In 3 trials, the conclusions changed from “not different” to “harmful or possibly harmful.”
In 6 trials, the conclusions changed from “beneficial” to “not different.” One trial changed from “beneficial” to “harmful,” and another changed from “inconclusive” to “noninferior.”
Dr Schwartz and her colleagues concluded that while most interim and final publications reached similar conclusions, frequent non-publication of final results can lead to confusion or unfounded assumptions with true treatment effects remaining unknown.
To safeguard against any such confusion, the researchers recommended routinely adding the word “interim” in the title and justifying the reason in the publication. (Many interim publications reported analyses without any justification.)
“Most importantly, journals, authors, and funders should commit to making final results accessible by linking interim publications to final reports whenever available,” said study author Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice. ![]()
CAR T-cell therapy produces durable CRs in ALL
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
FDA places T-cell therapy on clinical hold
The US Food and Drug Administration (FDA) has placed BPX-501, a T-cell therapy being evaluated in patients who undergo haploidentical hematopoietic stem cell transplants (HSCTs), on clinical hold.
Three cases of encephalopathy possibly related to BPX-501 prompted the agency to impose the hold.
Bellicum Pharmaceuticals is the developer of BPX-501, and the company was conducting 4 trials in the US in children and adults with hematologic disorders.
The BPX-501 registration trial in Europe is not affected by the clinical hold.
BPX-501 is designed to fight infection, support engraftment, prevent disease relapse, and potentially stop graft-versus-host disease (GVHD) should it occur.
BPX-501 contains a safety switch, CaspaCIDe®, that can be activated with the administration of rimiducid to kill the toxic T cells in the event of GVHD.
The 3 cases of encephalopathy are complex, according to a company press release, and have confounding factors. These include prior failed transplants, prior history of immunodeficiency, concurrent infection, and administration of rimiducid in combination with other medications.
Encephalopathy had not emerged as an adverse event in 240 patients treated with the cell therapy, until now.
BPX-501 had produced encouraging results, according to trial data presented at EHA 2017 and ASH 2017 (abstract 211*).
In this trial, 112 pediatric patients were transfused with BPX-501 cells about 2 weeks after transplant. Patients had acute leukemia (n=53), primary immune deficiencies (n=26), erythroid disorders (n=17), Fanconi anemia (n=7), and other diseases (n=9).
Investigators reported that infused cells expanded and persisted, with peak expansion reached at 9 months after infusion. Investigators continued to detect BPX-501 cells after 2 years.
The European Commission granted BPX-501 orphan drug designation for the agent for treatment in HSCT, and for the activator agent rimiducid for the treatment of GVHD.
And the FDA had granted the agents orphan drug status as a combination replacement T-cell therapy for the treatment of immunodeficiency and GVHD after HSCT.
Bellicum says it is working with the FDA to evaluate the risk of encephalopathy in patients receiving BPX-501. ![]()
* Data in the abstract were updated in the oral presentation and reported on the company’s website.
The US Food and Drug Administration (FDA) has placed BPX-501, a T-cell therapy being evaluated in patients who undergo haploidentical hematopoietic stem cell transplants (HSCTs), on clinical hold.
Three cases of encephalopathy possibly related to BPX-501 prompted the agency to impose the hold.
Bellicum Pharmaceuticals is the developer of BPX-501, and the company was conducting 4 trials in the US in children and adults with hematologic disorders.
The BPX-501 registration trial in Europe is not affected by the clinical hold.
BPX-501 is designed to fight infection, support engraftment, prevent disease relapse, and potentially stop graft-versus-host disease (GVHD) should it occur.
BPX-501 contains a safety switch, CaspaCIDe®, that can be activated with the administration of rimiducid to kill the toxic T cells in the event of GVHD.
The 3 cases of encephalopathy are complex, according to a company press release, and have confounding factors. These include prior failed transplants, prior history of immunodeficiency, concurrent infection, and administration of rimiducid in combination with other medications.
Encephalopathy had not emerged as an adverse event in 240 patients treated with the cell therapy, until now.
BPX-501 had produced encouraging results, according to trial data presented at EHA 2017 and ASH 2017 (abstract 211*).
In this trial, 112 pediatric patients were transfused with BPX-501 cells about 2 weeks after transplant. Patients had acute leukemia (n=53), primary immune deficiencies (n=26), erythroid disorders (n=17), Fanconi anemia (n=7), and other diseases (n=9).
Investigators reported that infused cells expanded and persisted, with peak expansion reached at 9 months after infusion. Investigators continued to detect BPX-501 cells after 2 years.
The European Commission granted BPX-501 orphan drug designation for the agent for treatment in HSCT, and for the activator agent rimiducid for the treatment of GVHD.
And the FDA had granted the agents orphan drug status as a combination replacement T-cell therapy for the treatment of immunodeficiency and GVHD after HSCT.
Bellicum says it is working with the FDA to evaluate the risk of encephalopathy in patients receiving BPX-501. ![]()
* Data in the abstract were updated in the oral presentation and reported on the company’s website.
The US Food and Drug Administration (FDA) has placed BPX-501, a T-cell therapy being evaluated in patients who undergo haploidentical hematopoietic stem cell transplants (HSCTs), on clinical hold.
Three cases of encephalopathy possibly related to BPX-501 prompted the agency to impose the hold.
Bellicum Pharmaceuticals is the developer of BPX-501, and the company was conducting 4 trials in the US in children and adults with hematologic disorders.
The BPX-501 registration trial in Europe is not affected by the clinical hold.
BPX-501 is designed to fight infection, support engraftment, prevent disease relapse, and potentially stop graft-versus-host disease (GVHD) should it occur.
BPX-501 contains a safety switch, CaspaCIDe®, that can be activated with the administration of rimiducid to kill the toxic T cells in the event of GVHD.
The 3 cases of encephalopathy are complex, according to a company press release, and have confounding factors. These include prior failed transplants, prior history of immunodeficiency, concurrent infection, and administration of rimiducid in combination with other medications.
Encephalopathy had not emerged as an adverse event in 240 patients treated with the cell therapy, until now.
BPX-501 had produced encouraging results, according to trial data presented at EHA 2017 and ASH 2017 (abstract 211*).
In this trial, 112 pediatric patients were transfused with BPX-501 cells about 2 weeks after transplant. Patients had acute leukemia (n=53), primary immune deficiencies (n=26), erythroid disorders (n=17), Fanconi anemia (n=7), and other diseases (n=9).
Investigators reported that infused cells expanded and persisted, with peak expansion reached at 9 months after infusion. Investigators continued to detect BPX-501 cells after 2 years.
The European Commission granted BPX-501 orphan drug designation for the agent for treatment in HSCT, and for the activator agent rimiducid for the treatment of GVHD.
And the FDA had granted the agents orphan drug status as a combination replacement T-cell therapy for the treatment of immunodeficiency and GVHD after HSCT.
Bellicum says it is working with the FDA to evaluate the risk of encephalopathy in patients receiving BPX-501.
* Data in the abstract were updated in the oral presentation and reported on the company’s website.
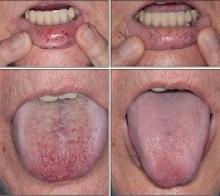
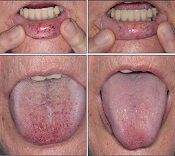
IV bevacizumab improves severe bleeding in HHT
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.”
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.”
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.”
Trial protocols redacted by industry sponsors
New research has revealed a lack of public information regarding protocols for industry-sponsored, randomized drug trials in Denmark.
First, researchers found it difficult to obtain protocols for commercially sponsored trials, with some sponsors taking legal action in an attempt to keep protocols private.
When the researchers did receive protocols, many had widespread redactions.
The researchers reported these findings in the Journal of the Royal Society of Medicine.
“We wished to compare the information in the protocols with the information provided to the patients in order to evaluate whether the trials were ethical and necessary and whether essential information about the benefits and the harms of the drugs had been hidden from the patients,” said study author Peter Gøtzsche, MD, director of the Nordic Cochrane Centre in Copenhagen, Denmark.
To that end, Dr Gøtzsche and his colleagues used the Danish Freedom of Information Act to request access to 78 protocols for randomized trials that were approved by a research ethics committee from October 2012 to March 2013.
The researchers said several companies refused to provide protocols and involved their lawyers. In fact, Sanofi-Aventis sued the National Committee on Health Research Ethics but lost.
Three years after this project was started, the researchers were able to obtain all the protocols they had requested. Eight protocols were excluded from analysis because they did not meet the research inclusion criteria.
Seventeen of 34 protocols for commercially sponsored trials were unredacted, compared to 34 of 36 non-commercially sponsored trials.
The researchers said the redactions “were most widespread in those sections of the protocol where there is empirical evidence of substantial problems with the trustworthiness of published drug trials.”
This includes data analysis, the handling of missing data, the detection/analysis of adverse events, the definition of patient outcomes, interim analyses and premature study termination, the sponsor’s access to incoming data while the study is ongoing, ownership of the data, and investigators’ publication rights.
“The amount of redactions in the protocols we received was so vast that it made them rather useless for assessing the ethical justification for the studies and to identify discrepancies with subsequent publications,” Dr Gøtzsche said.
“We could not identify any legitimate rationale for the redactions. The current mistrust in industry-sponsored drug trials can only change if the industry offers unconditional access to its trial protocols and other relevant documents and data.”
New research has revealed a lack of public information regarding protocols for industry-sponsored, randomized drug trials in Denmark.
First, researchers found it difficult to obtain protocols for commercially sponsored trials, with some sponsors taking legal action in an attempt to keep protocols private.
When the researchers did receive protocols, many had widespread redactions.
The researchers reported these findings in the Journal of the Royal Society of Medicine.
“We wished to compare the information in the protocols with the information provided to the patients in order to evaluate whether the trials were ethical and necessary and whether essential information about the benefits and the harms of the drugs had been hidden from the patients,” said study author Peter Gøtzsche, MD, director of the Nordic Cochrane Centre in Copenhagen, Denmark.
To that end, Dr Gøtzsche and his colleagues used the Danish Freedom of Information Act to request access to 78 protocols for randomized trials that were approved by a research ethics committee from October 2012 to March 2013.
The researchers said several companies refused to provide protocols and involved their lawyers. In fact, Sanofi-Aventis sued the National Committee on Health Research Ethics but lost.
Three years after this project was started, the researchers were able to obtain all the protocols they had requested. Eight protocols were excluded from analysis because they did not meet the research inclusion criteria.
Seventeen of 34 protocols for commercially sponsored trials were unredacted, compared to 34 of 36 non-commercially sponsored trials.
The researchers said the redactions “were most widespread in those sections of the protocol where there is empirical evidence of substantial problems with the trustworthiness of published drug trials.”
This includes data analysis, the handling of missing data, the detection/analysis of adverse events, the definition of patient outcomes, interim analyses and premature study termination, the sponsor’s access to incoming data while the study is ongoing, ownership of the data, and investigators’ publication rights.
“The amount of redactions in the protocols we received was so vast that it made them rather useless for assessing the ethical justification for the studies and to identify discrepancies with subsequent publications,” Dr Gøtzsche said.
“We could not identify any legitimate rationale for the redactions. The current mistrust in industry-sponsored drug trials can only change if the industry offers unconditional access to its trial protocols and other relevant documents and data.”
New research has revealed a lack of public information regarding protocols for industry-sponsored, randomized drug trials in Denmark.
First, researchers found it difficult to obtain protocols for commercially sponsored trials, with some sponsors taking legal action in an attempt to keep protocols private.
When the researchers did receive protocols, many had widespread redactions.
The researchers reported these findings in the Journal of the Royal Society of Medicine.
“We wished to compare the information in the protocols with the information provided to the patients in order to evaluate whether the trials were ethical and necessary and whether essential information about the benefits and the harms of the drugs had been hidden from the patients,” said study author Peter Gøtzsche, MD, director of the Nordic Cochrane Centre in Copenhagen, Denmark.
To that end, Dr Gøtzsche and his colleagues used the Danish Freedom of Information Act to request access to 78 protocols for randomized trials that were approved by a research ethics committee from October 2012 to March 2013.
The researchers said several companies refused to provide protocols and involved their lawyers. In fact, Sanofi-Aventis sued the National Committee on Health Research Ethics but lost.
Three years after this project was started, the researchers were able to obtain all the protocols they had requested. Eight protocols were excluded from analysis because they did not meet the research inclusion criteria.
Seventeen of 34 protocols for commercially sponsored trials were unredacted, compared to 34 of 36 non-commercially sponsored trials.
The researchers said the redactions “were most widespread in those sections of the protocol where there is empirical evidence of substantial problems with the trustworthiness of published drug trials.”
This includes data analysis, the handling of missing data, the detection/analysis of adverse events, the definition of patient outcomes, interim analyses and premature study termination, the sponsor’s access to incoming data while the study is ongoing, ownership of the data, and investigators’ publication rights.
“The amount of redactions in the protocols we received was so vast that it made them rather useless for assessing the ethical justification for the studies and to identify discrepancies with subsequent publications,” Dr Gøtzsche said.
“We could not identify any legitimate rationale for the redactions. The current mistrust in industry-sponsored drug trials can only change if the industry offers unconditional access to its trial protocols and other relevant documents and data.”
No need for structured PE assessment, team says
There is no need for a structured algorithm to rule out pulmonary embolism (PE) in patients who visit the emergency department (ED) for syncope, according to researchers.
The team analyzed data on nearly 1.7 million patients who presented to the ED for syncope in 4 different countries.
PE occurred in less than 1% of these patients and in less than 3% of patients who were hospitalized.
The researchers therefore concluded that PE should be considered in such patients, but a systematic protocol for ruling out PE is not necessary.
“Our results are saying that there is no need, so far, for using a structured clinical algorithm to assess pulmonary emboli in all the patients presenting [to the ED with syncope],” said Nicola Montano, MD, PhD, of the University of Milan in Italy.
Dr Montano and his colleagues described these results in JAMA Internal Medicine.
The team noted that, in the PESIT trial, researchers used a standardized algorithm to evaluate PE prevalence in patients hospitalized after a first syncope episode.
The algorithm was based on pretest clinical probability and results of the D-dimer assay. Any patient with positive D-dimer results or high pretest PE probability underwent computed tomography or ventilation perfusion lung scanning.
In this study, the prevalence of PE was 17.3%.
A subsequent study showed a much lower prevalence of PE and deep vein thrombosis in patients hospitalized for syncope. The prevalence of venous thromboembolism (VTE) in this study was 1.4%.
The differences between these studies and the fact that both studies included only hospitalized patients prompted Dr Montano and his colleagues to conduct the current study.
The team set out to determine PE/VTE prevalence in unselected patients with syncope presenting to the ED.
Results
The researchers analyzed administrative data from 5 databases in 4 countries—Canada, Denmark, Italy, and the US—collected from January 1, 2000, through September 30, 2016.
The data included 1,671,944 adults who presented to the ED for syncope. The prevalence of PE at ED or hospital discharge ranged from 0.06% to 0.55%. Among the hospitalized patients only, the prevalence of PE ranged from 0.15% to 2.10%.
At 90 days of follow-up, the prevalence of PE ranged from 0.14% to 0.83% for all patients and from 0.35% to 2.63% for hospitalized patients.
The prevalence of VTE at 90 days ranged from 0.30% to 1.37% for all patients and from 0.75% to 3.86% for hospitalized patients.
The researchers said the main limitation of this study is the use of administrative data because some patients with syncope, PE, or VTE may have been missed.
There is no need for a structured algorithm to rule out pulmonary embolism (PE) in patients who visit the emergency department (ED) for syncope, according to researchers.
The team analyzed data on nearly 1.7 million patients who presented to the ED for syncope in 4 different countries.
PE occurred in less than 1% of these patients and in less than 3% of patients who were hospitalized.
The researchers therefore concluded that PE should be considered in such patients, but a systematic protocol for ruling out PE is not necessary.
“Our results are saying that there is no need, so far, for using a structured clinical algorithm to assess pulmonary emboli in all the patients presenting [to the ED with syncope],” said Nicola Montano, MD, PhD, of the University of Milan in Italy.
Dr Montano and his colleagues described these results in JAMA Internal Medicine.
The team noted that, in the PESIT trial, researchers used a standardized algorithm to evaluate PE prevalence in patients hospitalized after a first syncope episode.
The algorithm was based on pretest clinical probability and results of the D-dimer assay. Any patient with positive D-dimer results or high pretest PE probability underwent computed tomography or ventilation perfusion lung scanning.
In this study, the prevalence of PE was 17.3%.
A subsequent study showed a much lower prevalence of PE and deep vein thrombosis in patients hospitalized for syncope. The prevalence of venous thromboembolism (VTE) in this study was 1.4%.
The differences between these studies and the fact that both studies included only hospitalized patients prompted Dr Montano and his colleagues to conduct the current study.
The team set out to determine PE/VTE prevalence in unselected patients with syncope presenting to the ED.
Results
The researchers analyzed administrative data from 5 databases in 4 countries—Canada, Denmark, Italy, and the US—collected from January 1, 2000, through September 30, 2016.
The data included 1,671,944 adults who presented to the ED for syncope. The prevalence of PE at ED or hospital discharge ranged from 0.06% to 0.55%. Among the hospitalized patients only, the prevalence of PE ranged from 0.15% to 2.10%.
At 90 days of follow-up, the prevalence of PE ranged from 0.14% to 0.83% for all patients and from 0.35% to 2.63% for hospitalized patients.
The prevalence of VTE at 90 days ranged from 0.30% to 1.37% for all patients and from 0.75% to 3.86% for hospitalized patients.
The researchers said the main limitation of this study is the use of administrative data because some patients with syncope, PE, or VTE may have been missed.
There is no need for a structured algorithm to rule out pulmonary embolism (PE) in patients who visit the emergency department (ED) for syncope, according to researchers.
The team analyzed data on nearly 1.7 million patients who presented to the ED for syncope in 4 different countries.
PE occurred in less than 1% of these patients and in less than 3% of patients who were hospitalized.
The researchers therefore concluded that PE should be considered in such patients, but a systematic protocol for ruling out PE is not necessary.
“Our results are saying that there is no need, so far, for using a structured clinical algorithm to assess pulmonary emboli in all the patients presenting [to the ED with syncope],” said Nicola Montano, MD, PhD, of the University of Milan in Italy.
Dr Montano and his colleagues described these results in JAMA Internal Medicine.
The team noted that, in the PESIT trial, researchers used a standardized algorithm to evaluate PE prevalence in patients hospitalized after a first syncope episode.
The algorithm was based on pretest clinical probability and results of the D-dimer assay. Any patient with positive D-dimer results or high pretest PE probability underwent computed tomography or ventilation perfusion lung scanning.
In this study, the prevalence of PE was 17.3%.
A subsequent study showed a much lower prevalence of PE and deep vein thrombosis in patients hospitalized for syncope. The prevalence of venous thromboembolism (VTE) in this study was 1.4%.
The differences between these studies and the fact that both studies included only hospitalized patients prompted Dr Montano and his colleagues to conduct the current study.
The team set out to determine PE/VTE prevalence in unselected patients with syncope presenting to the ED.
Results
The researchers analyzed administrative data from 5 databases in 4 countries—Canada, Denmark, Italy, and the US—collected from January 1, 2000, through September 30, 2016.
The data included 1,671,944 adults who presented to the ED for syncope. The prevalence of PE at ED or hospital discharge ranged from 0.06% to 0.55%. Among the hospitalized patients only, the prevalence of PE ranged from 0.15% to 2.10%.
At 90 days of follow-up, the prevalence of PE ranged from 0.14% to 0.83% for all patients and from 0.35% to 2.63% for hospitalized patients.
The prevalence of VTE at 90 days ranged from 0.30% to 1.37% for all patients and from 0.75% to 3.86% for hospitalized patients.
The researchers said the main limitation of this study is the use of administrative data because some patients with syncope, PE, or VTE may have been missed.