User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


FDA approves first tx for rare, deadly clotting disorder
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Highlights in Metastatic Breast Cancer From ESMO 2023
Developments in metastatic breast cancer (MBC) were reported at the European Society for Medical Oncology (ESMO) 2023 Congress and are discussed by Dr Ann Partridge of Dana-Farber Cancer Institute.
To begin, Dr Partridge highlights a late-breaking abstract showing that use of the antibody-drug conjugate (ADC) datopotamab deruxtecan in hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) MBC results in improved progression-free survival (PFS) compared with chemotherapy.
Next, Dr Partridge turns to two studies on another ADC, trastuzumab deruxtecan (T-DXd), in MBC. The first study showed positive PFS and overall survival results among patients with either estrogen receptor–positive (ER+)/HER2-low or triple-negative/HER2-low breast cancer. The second T-DXd study examined the ADC's impact on brain metastases in patients with HER2+ disease and reported favorable results.
She then highlights promising phase 2 results for a novel agent called OP-1250, or palazestrant, studied in patients with ER+/HER2- MBC.
Finally, Dr Partridge points to a study of a supportive-care program called MOATT, designed for patients on oral MBC therapy, which aims to improve home management. Compared with local standard of care, patients in the program show higher rates of persistence in therapy management and, importantly, concomitant improvements in PFS.
--
Ann H. Partridge, MD, Professor of Medicine, Harvard Medical School; Vice Chair of Clinical Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Ann H. Partridge, MD, MPH, has disclosed no relevant financial relationships.
Developments in metastatic breast cancer (MBC) were reported at the European Society for Medical Oncology (ESMO) 2023 Congress and are discussed by Dr Ann Partridge of Dana-Farber Cancer Institute.
To begin, Dr Partridge highlights a late-breaking abstract showing that use of the antibody-drug conjugate (ADC) datopotamab deruxtecan in hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) MBC results in improved progression-free survival (PFS) compared with chemotherapy.
Next, Dr Partridge turns to two studies on another ADC, trastuzumab deruxtecan (T-DXd), in MBC. The first study showed positive PFS and overall survival results among patients with either estrogen receptor–positive (ER+)/HER2-low or triple-negative/HER2-low breast cancer. The second T-DXd study examined the ADC's impact on brain metastases in patients with HER2+ disease and reported favorable results.
She then highlights promising phase 2 results for a novel agent called OP-1250, or palazestrant, studied in patients with ER+/HER2- MBC.
Finally, Dr Partridge points to a study of a supportive-care program called MOATT, designed for patients on oral MBC therapy, which aims to improve home management. Compared with local standard of care, patients in the program show higher rates of persistence in therapy management and, importantly, concomitant improvements in PFS.
--
Ann H. Partridge, MD, Professor of Medicine, Harvard Medical School; Vice Chair of Clinical Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Ann H. Partridge, MD, MPH, has disclosed no relevant financial relationships.
Developments in metastatic breast cancer (MBC) were reported at the European Society for Medical Oncology (ESMO) 2023 Congress and are discussed by Dr Ann Partridge of Dana-Farber Cancer Institute.
To begin, Dr Partridge highlights a late-breaking abstract showing that use of the antibody-drug conjugate (ADC) datopotamab deruxtecan in hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) MBC results in improved progression-free survival (PFS) compared with chemotherapy.
Next, Dr Partridge turns to two studies on another ADC, trastuzumab deruxtecan (T-DXd), in MBC. The first study showed positive PFS and overall survival results among patients with either estrogen receptor–positive (ER+)/HER2-low or triple-negative/HER2-low breast cancer. The second T-DXd study examined the ADC's impact on brain metastases in patients with HER2+ disease and reported favorable results.
She then highlights promising phase 2 results for a novel agent called OP-1250, or palazestrant, studied in patients with ER+/HER2- MBC.
Finally, Dr Partridge points to a study of a supportive-care program called MOATT, designed for patients on oral MBC therapy, which aims to improve home management. Compared with local standard of care, patients in the program show higher rates of persistence in therapy management and, importantly, concomitant improvements in PFS.
--
Ann H. Partridge, MD, Professor of Medicine, Harvard Medical School; Vice Chair of Clinical Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Ann H. Partridge, MD, MPH, has disclosed no relevant financial relationships.

In MI with anemia, results may favor liberal transfusion: MINT
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).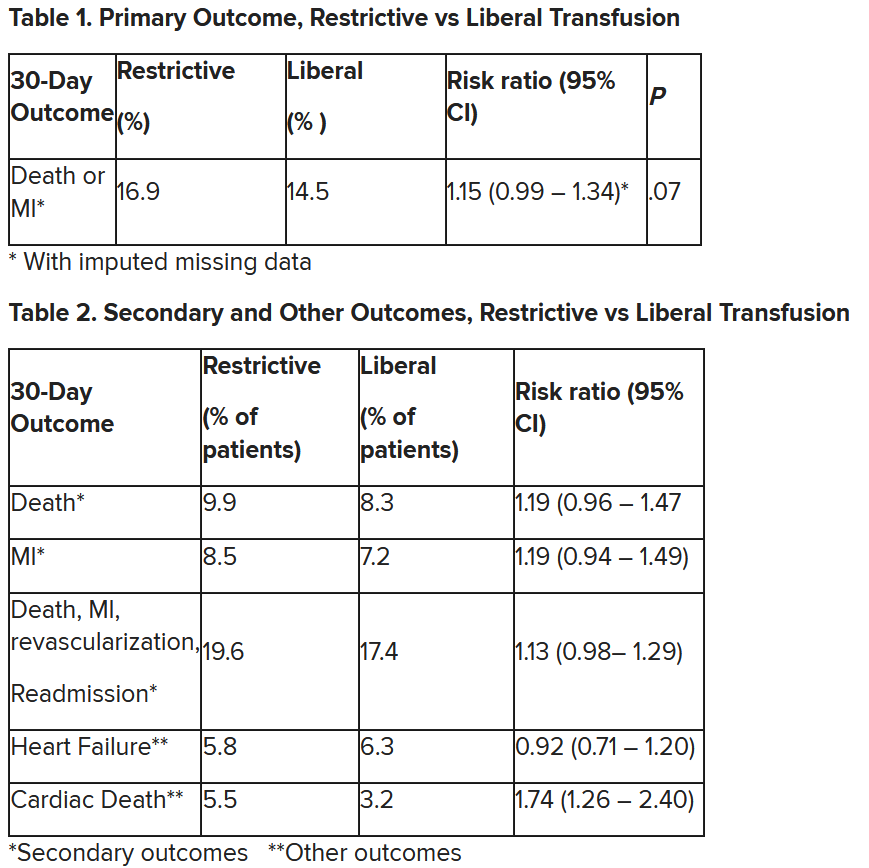
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Specialty-trained pathologists more likely to make higher-grade diagnoses for melanocytic lesions
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
FROM JAMA DERMATOLOGY
Meta-analysis of postcancer use of immunosuppressive therapies shows no increase in cancer recurrence risk
that covered approximately 24,000 patients and 86,000 person-years of follow-up.
The findings could “help guide clinical decision making,” providing “reassurance that it remains safe to use conventional immunomodulators, anti-TNF [tumor necrosis factor] agents, or newer biologics in individuals with [immune-mediated diseases] with a prior malignancy consistent with recent guidelines,” Akshita Gupta, MD, of Massachusetts General Hospital, Boston, and coinvestigators wrote in Clinical Gastroenterology and Hepatology.
And because a stratification of studies by the timing of immunosuppression therapy initiation found no increased risk when treatment was started within 5 years of a cancer diagnosis compared to later on, the meta-analysis could “potentially reduce the time to initiation of immunosuppressive treatment,” the authors wrote, noting a continued need for individualized decision-making.
Ustekinumab, a monoclonal antibody targeting interleukin-12 and IL-23, and vedolizumab, a monoclonal antibody that binds to alpha4beta7 integrin, were covered in the meta-analysis, but investigators found no studies on the use of upadacitinib or other Janus kinase (JAK) inhibitors, or the use of S1P modulators, in patients with prior malignancies.
The analysis included 31 observational studies, 17 of which involved patients with inflammatory bowel disease (IBD). (Of the other studies, 14 involved patients with rheumatoid arthritis, 2 covered psoriasis, and 1 covered ankylosing spondylitis.)
Similar levels of risk
The incidence rate of new or recurrent cancers among individuals not receiving any immunosuppressive therapy for IBD or other immune-mediated diseases after an index cancer was 35 per 1,000 patient-years (95% confidence interval, 27-43 per 1,000 patient-years; 1,627 incident cancers among 12,238 patients, 43,765 patient-years), and the rate among anti-TNF users was similar at 32 per 1,000 patient-years (95% CI, 25-38 per 1,000 patient-years; 571 cancers among 3,939 patients, 17,772 patient-years).
Among patients on conventional immunomodulator therapy (thiopurines, methotrexate), the incidence rate was numerically higher at 46 per 1,000 patient-years (95% CI, 31-61; 1,104 incident cancers among 5,930 patients; 17,018 patient-years), but was not statistically different from anti-TNF (P = .92) or no immunosuppression (P = .98).
Patients on combination immunosuppression also had numerically higher rates of new or recurrent cancers at 56 per 1,000 patient-years (95% CI, 31-81; 179 incident cancers, 2,659 patient-years), but these rates were not statistically different from immunomodulator use alone (P = .19), anti-TNF alone (P = .06) or no immunosuppressive therapy (P = .14).
Patients on ustekinumab and vedolizumab similarly had numerically lower rates of cancer recurrence, compared with other treatment groups: 21 per 1,000 patient-years (95% CI, 0-44; 5 cancers among 41 patients, 213 patient-years) and 16 per 1,000 patient-years (95% CI, 5-26; 37 cancers among 281 patients, 1,951 patient-years). However, the difference was statistically significant only for vedolizumab (P = .03 vs. immunomodulators and P = .04 vs. anti-TNF agents).
Subgroup analyses for new primary cancers, recurrence of a prior cancer, and type of index cancer (skin cancer vs. other cancers) similarly found no statistically significant differences between treatment arms. Results were similar in patients with IBD and RA.
Timing of therapy
The new meta-analysis confirms and expands a previous meta-analysis published in Gastroenterology in 2016 that showed no impact of treatment – primarily IMM or anti-TNF treatment – on cancer recurrence in patients with immune-mediated diseases, Dr. Gupta and coauthors wrote.
The 2016 meta-analysis reported similar cancer recurrence rates with IMMs and anti-TNFs when immunosuppression was introduced before or after 6 years of cancer diagnosis. In the new meta-analysis – with twice the number of patients, a longer duration of follow-up, and the inclusion of other biologic therapies – a stratification of results at the median interval of therapy initiation similarly found no increased risk before 5 years, compared with after 5 years.
“Although several existing guidelines recommend avoiding immunosuppression for 5 years after the index cancer, our results indicate that it may be safe to initiate these agents earlier than 5 years, at least in some patients,” Dr. Gupta and coauthors wrote, mentioning the possible impact of selection bias and surveillance bias in the study. Ongoing registries “may help answer this question more definitively with prospectively collected data, but inherently may suffer from this selection bias as well.”
Assessment of the newer biologics ustekinumab and vedolizumab is limited by the low number of studies (four and five, respectively) and by limited duration of follow-up. “Longer-term evaluation after these treatments is essential but it is reassuring that in the early analysis we did not observe an increase and in fact noted numerically lower rates of cancers,” they wrote.
It is also “critically important” to generate more data on JAK inhibitors, and to further study the safety of combining systemic chemotherapy and the continuation of IBD therapy in the setting of a new cancer diagnosis, they wrote.
The study was funded in part by grants from the Crohn’s and Colitis Foundation, and the Chleck Family Foundation. Dr. Gupta disclosed no conflicts. One coauthor disclosed consulting for Abbvie, Amgen, Biogen, and other companies, and receiving grants from several companies. Another coauthor disclosed serving on the scientific advisory boards for AbbVie and other companies, and receiving research support from Pfizer.
that covered approximately 24,000 patients and 86,000 person-years of follow-up.
The findings could “help guide clinical decision making,” providing “reassurance that it remains safe to use conventional immunomodulators, anti-TNF [tumor necrosis factor] agents, or newer biologics in individuals with [immune-mediated diseases] with a prior malignancy consistent with recent guidelines,” Akshita Gupta, MD, of Massachusetts General Hospital, Boston, and coinvestigators wrote in Clinical Gastroenterology and Hepatology.
And because a stratification of studies by the timing of immunosuppression therapy initiation found no increased risk when treatment was started within 5 years of a cancer diagnosis compared to later on, the meta-analysis could “potentially reduce the time to initiation of immunosuppressive treatment,” the authors wrote, noting a continued need for individualized decision-making.
Ustekinumab, a monoclonal antibody targeting interleukin-12 and IL-23, and vedolizumab, a monoclonal antibody that binds to alpha4beta7 integrin, were covered in the meta-analysis, but investigators found no studies on the use of upadacitinib or other Janus kinase (JAK) inhibitors, or the use of S1P modulators, in patients with prior malignancies.
The analysis included 31 observational studies, 17 of which involved patients with inflammatory bowel disease (IBD). (Of the other studies, 14 involved patients with rheumatoid arthritis, 2 covered psoriasis, and 1 covered ankylosing spondylitis.)
Similar levels of risk
The incidence rate of new or recurrent cancers among individuals not receiving any immunosuppressive therapy for IBD or other immune-mediated diseases after an index cancer was 35 per 1,000 patient-years (95% confidence interval, 27-43 per 1,000 patient-years; 1,627 incident cancers among 12,238 patients, 43,765 patient-years), and the rate among anti-TNF users was similar at 32 per 1,000 patient-years (95% CI, 25-38 per 1,000 patient-years; 571 cancers among 3,939 patients, 17,772 patient-years).
Among patients on conventional immunomodulator therapy (thiopurines, methotrexate), the incidence rate was numerically higher at 46 per 1,000 patient-years (95% CI, 31-61; 1,104 incident cancers among 5,930 patients; 17,018 patient-years), but was not statistically different from anti-TNF (P = .92) or no immunosuppression (P = .98).
Patients on combination immunosuppression also had numerically higher rates of new or recurrent cancers at 56 per 1,000 patient-years (95% CI, 31-81; 179 incident cancers, 2,659 patient-years), but these rates were not statistically different from immunomodulator use alone (P = .19), anti-TNF alone (P = .06) or no immunosuppressive therapy (P = .14).
Patients on ustekinumab and vedolizumab similarly had numerically lower rates of cancer recurrence, compared with other treatment groups: 21 per 1,000 patient-years (95% CI, 0-44; 5 cancers among 41 patients, 213 patient-years) and 16 per 1,000 patient-years (95% CI, 5-26; 37 cancers among 281 patients, 1,951 patient-years). However, the difference was statistically significant only for vedolizumab (P = .03 vs. immunomodulators and P = .04 vs. anti-TNF agents).
Subgroup analyses for new primary cancers, recurrence of a prior cancer, and type of index cancer (skin cancer vs. other cancers) similarly found no statistically significant differences between treatment arms. Results were similar in patients with IBD and RA.
Timing of therapy
The new meta-analysis confirms and expands a previous meta-analysis published in Gastroenterology in 2016 that showed no impact of treatment – primarily IMM or anti-TNF treatment – on cancer recurrence in patients with immune-mediated diseases, Dr. Gupta and coauthors wrote.
The 2016 meta-analysis reported similar cancer recurrence rates with IMMs and anti-TNFs when immunosuppression was introduced before or after 6 years of cancer diagnosis. In the new meta-analysis – with twice the number of patients, a longer duration of follow-up, and the inclusion of other biologic therapies – a stratification of results at the median interval of therapy initiation similarly found no increased risk before 5 years, compared with after 5 years.
“Although several existing guidelines recommend avoiding immunosuppression for 5 years after the index cancer, our results indicate that it may be safe to initiate these agents earlier than 5 years, at least in some patients,” Dr. Gupta and coauthors wrote, mentioning the possible impact of selection bias and surveillance bias in the study. Ongoing registries “may help answer this question more definitively with prospectively collected data, but inherently may suffer from this selection bias as well.”
Assessment of the newer biologics ustekinumab and vedolizumab is limited by the low number of studies (four and five, respectively) and by limited duration of follow-up. “Longer-term evaluation after these treatments is essential but it is reassuring that in the early analysis we did not observe an increase and in fact noted numerically lower rates of cancers,” they wrote.
It is also “critically important” to generate more data on JAK inhibitors, and to further study the safety of combining systemic chemotherapy and the continuation of IBD therapy in the setting of a new cancer diagnosis, they wrote.
The study was funded in part by grants from the Crohn’s and Colitis Foundation, and the Chleck Family Foundation. Dr. Gupta disclosed no conflicts. One coauthor disclosed consulting for Abbvie, Amgen, Biogen, and other companies, and receiving grants from several companies. Another coauthor disclosed serving on the scientific advisory boards for AbbVie and other companies, and receiving research support from Pfizer.
that covered approximately 24,000 patients and 86,000 person-years of follow-up.
The findings could “help guide clinical decision making,” providing “reassurance that it remains safe to use conventional immunomodulators, anti-TNF [tumor necrosis factor] agents, or newer biologics in individuals with [immune-mediated diseases] with a prior malignancy consistent with recent guidelines,” Akshita Gupta, MD, of Massachusetts General Hospital, Boston, and coinvestigators wrote in Clinical Gastroenterology and Hepatology.
And because a stratification of studies by the timing of immunosuppression therapy initiation found no increased risk when treatment was started within 5 years of a cancer diagnosis compared to later on, the meta-analysis could “potentially reduce the time to initiation of immunosuppressive treatment,” the authors wrote, noting a continued need for individualized decision-making.
Ustekinumab, a monoclonal antibody targeting interleukin-12 and IL-23, and vedolizumab, a monoclonal antibody that binds to alpha4beta7 integrin, were covered in the meta-analysis, but investigators found no studies on the use of upadacitinib or other Janus kinase (JAK) inhibitors, or the use of S1P modulators, in patients with prior malignancies.
The analysis included 31 observational studies, 17 of which involved patients with inflammatory bowel disease (IBD). (Of the other studies, 14 involved patients with rheumatoid arthritis, 2 covered psoriasis, and 1 covered ankylosing spondylitis.)
Similar levels of risk
The incidence rate of new or recurrent cancers among individuals not receiving any immunosuppressive therapy for IBD or other immune-mediated diseases after an index cancer was 35 per 1,000 patient-years (95% confidence interval, 27-43 per 1,000 patient-years; 1,627 incident cancers among 12,238 patients, 43,765 patient-years), and the rate among anti-TNF users was similar at 32 per 1,000 patient-years (95% CI, 25-38 per 1,000 patient-years; 571 cancers among 3,939 patients, 17,772 patient-years).
Among patients on conventional immunomodulator therapy (thiopurines, methotrexate), the incidence rate was numerically higher at 46 per 1,000 patient-years (95% CI, 31-61; 1,104 incident cancers among 5,930 patients; 17,018 patient-years), but was not statistically different from anti-TNF (P = .92) or no immunosuppression (P = .98).
Patients on combination immunosuppression also had numerically higher rates of new or recurrent cancers at 56 per 1,000 patient-years (95% CI, 31-81; 179 incident cancers, 2,659 patient-years), but these rates were not statistically different from immunomodulator use alone (P = .19), anti-TNF alone (P = .06) or no immunosuppressive therapy (P = .14).
Patients on ustekinumab and vedolizumab similarly had numerically lower rates of cancer recurrence, compared with other treatment groups: 21 per 1,000 patient-years (95% CI, 0-44; 5 cancers among 41 patients, 213 patient-years) and 16 per 1,000 patient-years (95% CI, 5-26; 37 cancers among 281 patients, 1,951 patient-years). However, the difference was statistically significant only for vedolizumab (P = .03 vs. immunomodulators and P = .04 vs. anti-TNF agents).
Subgroup analyses for new primary cancers, recurrence of a prior cancer, and type of index cancer (skin cancer vs. other cancers) similarly found no statistically significant differences between treatment arms. Results were similar in patients with IBD and RA.
Timing of therapy
The new meta-analysis confirms and expands a previous meta-analysis published in Gastroenterology in 2016 that showed no impact of treatment – primarily IMM or anti-TNF treatment – on cancer recurrence in patients with immune-mediated diseases, Dr. Gupta and coauthors wrote.
The 2016 meta-analysis reported similar cancer recurrence rates with IMMs and anti-TNFs when immunosuppression was introduced before or after 6 years of cancer diagnosis. In the new meta-analysis – with twice the number of patients, a longer duration of follow-up, and the inclusion of other biologic therapies – a stratification of results at the median interval of therapy initiation similarly found no increased risk before 5 years, compared with after 5 years.
“Although several existing guidelines recommend avoiding immunosuppression for 5 years after the index cancer, our results indicate that it may be safe to initiate these agents earlier than 5 years, at least in some patients,” Dr. Gupta and coauthors wrote, mentioning the possible impact of selection bias and surveillance bias in the study. Ongoing registries “may help answer this question more definitively with prospectively collected data, but inherently may suffer from this selection bias as well.”
Assessment of the newer biologics ustekinumab and vedolizumab is limited by the low number of studies (four and five, respectively) and by limited duration of follow-up. “Longer-term evaluation after these treatments is essential but it is reassuring that in the early analysis we did not observe an increase and in fact noted numerically lower rates of cancers,” they wrote.
It is also “critically important” to generate more data on JAK inhibitors, and to further study the safety of combining systemic chemotherapy and the continuation of IBD therapy in the setting of a new cancer diagnosis, they wrote.
The study was funded in part by grants from the Crohn’s and Colitis Foundation, and the Chleck Family Foundation. Dr. Gupta disclosed no conflicts. One coauthor disclosed consulting for Abbvie, Amgen, Biogen, and other companies, and receiving grants from several companies. Another coauthor disclosed serving on the scientific advisory boards for AbbVie and other companies, and receiving research support from Pfizer.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Particulate pollution increases the risk for breast cancer
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
AT ESMO 2023
Microsimulation model identifies 4-year window for pancreatic cancer screening
, based on a microsimulation model.
To seize this opportunity, however, a greater understanding of natural disease course is needed, along with more sensitive screening tools, reported Brechtje D. M. Koopmann, MD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues.
Previous studies have suggested that the window of opportunity for pancreatic cancer screening may span decades, with estimates ranging from 12 to 50 years, the investigators wrote. Their report was published in Gastroenterology.
“Unfortunately, the poor results of pancreatic cancer screening do not align with this assumption, leaving unanswered whether this large window of opportunity truly exists,” they noted. “Microsimulation modeling, combined with available, if limited data, can provide new information on the natural disease course.”
For the present study, the investigators used the Microsimulation Screening Analysis (MISCAN) model, which has guided development of screening programs around the world for cervical, breast, and colorectal cancer. The model incorporates natural disease course, screening, and demographic data, then uses observable inputs such as precursor lesion prevalence and cancer incidence to estimate unobservable outcomes like stage durations and precursor lesion onset.
Dr. Koopmann and colleagues programmed this model with Dutch pancreatic cancer incidence data and findings from Japanese autopsy cases without pancreatic cancer.
First, the model offered insights into precursor lesion prevalence.
The estimated prevalence of any cystic lesion in the pancreas was 6.1% for individuals 50 years of age and 29.6% for those 80 years of age. Solid precursor lesions (PanINs) were estimated to be mainly multifocal (three or more lesions) in individuals older than 80 years. By this age, almost 12% had at least two PanINs. For those lesions that eventually became cancerous, the mean time since cyst onset was estimated to be 8.8 years, and mean time since PanIN onset was 9.0 years.
However, less than 10% of cystic and PanIN lesions progress to become cancers. PanIN lesions are not visible on imaging, and therefore current screening focuses on finding cystic precursor lesions, although these represent only about 10% of pancreatic cancers.
“Given the low pancreatic cancer progression risk of cysts, evaluation of the efficiency of current surveillance guidelines is necessary,” the investigators noted.
Screening should instead focus on identifying high-grade dysplastic lesions, they suggested. While these lesions may have a very low estimated prevalence, at just 0.3% among individuals 90 years of age, they present the greatest risk of pancreatic cancer.
For precursor cysts exhibiting HGD that progressed to pancreatic cancer, the mean interval between dysplasia and cancer was just 4 years. Among 13.7% of individuals, the interval was less than 1 year, suggesting an even shorter window of opportunity for screening.
Beyond this brief timeframe, low test sensitivity explains why screening efforts to date have fallen short, the investigators wrote.
Better tests are “urgently needed,” they added, while acknowledging the challenges inherent to this endeavor. Previous research has shown that precursor lesions in the pancreas are often less than 5 mm in diameter, making them extremely challenging to detect. An effective tool would need to identify solid precursor lesions (PanINs), and also need to simultaneously determine grade of dysplasia.
“Biomarkers could be the future in this matter,” the investigators suggested.
Dr. Koopmann and colleagues concluded by noting that more research is needed to characterize the pathophysiology of pancreatic cancer. On their part, “the current model will be validated, adjusted, and improved whenever new data from autopsy or prospective surveillance studies become available.”
The study was funded in part by Maag Lever Darm Stichting. The investigators disclosed no conflicts of interest.
We continue to search for a way to effectively screen for and prevent pancreatic cancer. Most pancreatic cancers come from pancreatic intraepithelial neoplasms (PanINs), which are essentially invisible on imaging. Pancreatic cysts are relatively common, and only a small number will progress to cancer. Screening via MRI or EUS can look for high-risk features of visible cysts or find early-stage cancers, but whom to screen, how often, and what to do with the results remains unclear. Many of the steps from development of the initial cyst or PanIN to the transformation to cancer cannot be observed, and as such this is a perfect application for disease modeling that allows us to fill in the gaps of what can be observed and estimate what we cannot see.
Mary Linton B. Peters, MD, MS, is a medical oncologist specializing in hepatic and pancreatobiliary cancers at Beth Israel Deaconess Medical Center, Boston, an assistant professor at Harvard Medical School, and a senior scientist at the Institute for Technology Assessment of Massachusetts General Hospital. She reports unrelated institutional research funding from NuCana and Helsinn.
We continue to search for a way to effectively screen for and prevent pancreatic cancer. Most pancreatic cancers come from pancreatic intraepithelial neoplasms (PanINs), which are essentially invisible on imaging. Pancreatic cysts are relatively common, and only a small number will progress to cancer. Screening via MRI or EUS can look for high-risk features of visible cysts or find early-stage cancers, but whom to screen, how often, and what to do with the results remains unclear. Many of the steps from development of the initial cyst or PanIN to the transformation to cancer cannot be observed, and as such this is a perfect application for disease modeling that allows us to fill in the gaps of what can be observed and estimate what we cannot see.
Mary Linton B. Peters, MD, MS, is a medical oncologist specializing in hepatic and pancreatobiliary cancers at Beth Israel Deaconess Medical Center, Boston, an assistant professor at Harvard Medical School, and a senior scientist at the Institute for Technology Assessment of Massachusetts General Hospital. She reports unrelated institutional research funding from NuCana and Helsinn.
We continue to search for a way to effectively screen for and prevent pancreatic cancer. Most pancreatic cancers come from pancreatic intraepithelial neoplasms (PanINs), which are essentially invisible on imaging. Pancreatic cysts are relatively common, and only a small number will progress to cancer. Screening via MRI or EUS can look for high-risk features of visible cysts or find early-stage cancers, but whom to screen, how often, and what to do with the results remains unclear. Many of the steps from development of the initial cyst or PanIN to the transformation to cancer cannot be observed, and as such this is a perfect application for disease modeling that allows us to fill in the gaps of what can be observed and estimate what we cannot see.
Mary Linton B. Peters, MD, MS, is a medical oncologist specializing in hepatic and pancreatobiliary cancers at Beth Israel Deaconess Medical Center, Boston, an assistant professor at Harvard Medical School, and a senior scientist at the Institute for Technology Assessment of Massachusetts General Hospital. She reports unrelated institutional research funding from NuCana and Helsinn.
, based on a microsimulation model.
To seize this opportunity, however, a greater understanding of natural disease course is needed, along with more sensitive screening tools, reported Brechtje D. M. Koopmann, MD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues.
Previous studies have suggested that the window of opportunity for pancreatic cancer screening may span decades, with estimates ranging from 12 to 50 years, the investigators wrote. Their report was published in Gastroenterology.
“Unfortunately, the poor results of pancreatic cancer screening do not align with this assumption, leaving unanswered whether this large window of opportunity truly exists,” they noted. “Microsimulation modeling, combined with available, if limited data, can provide new information on the natural disease course.”
For the present study, the investigators used the Microsimulation Screening Analysis (MISCAN) model, which has guided development of screening programs around the world for cervical, breast, and colorectal cancer. The model incorporates natural disease course, screening, and demographic data, then uses observable inputs such as precursor lesion prevalence and cancer incidence to estimate unobservable outcomes like stage durations and precursor lesion onset.
Dr. Koopmann and colleagues programmed this model with Dutch pancreatic cancer incidence data and findings from Japanese autopsy cases without pancreatic cancer.
First, the model offered insights into precursor lesion prevalence.
The estimated prevalence of any cystic lesion in the pancreas was 6.1% for individuals 50 years of age and 29.6% for those 80 years of age. Solid precursor lesions (PanINs) were estimated to be mainly multifocal (three or more lesions) in individuals older than 80 years. By this age, almost 12% had at least two PanINs. For those lesions that eventually became cancerous, the mean time since cyst onset was estimated to be 8.8 years, and mean time since PanIN onset was 9.0 years.
However, less than 10% of cystic and PanIN lesions progress to become cancers. PanIN lesions are not visible on imaging, and therefore current screening focuses on finding cystic precursor lesions, although these represent only about 10% of pancreatic cancers.
“Given the low pancreatic cancer progression risk of cysts, evaluation of the efficiency of current surveillance guidelines is necessary,” the investigators noted.
Screening should instead focus on identifying high-grade dysplastic lesions, they suggested. While these lesions may have a very low estimated prevalence, at just 0.3% among individuals 90 years of age, they present the greatest risk of pancreatic cancer.
For precursor cysts exhibiting HGD that progressed to pancreatic cancer, the mean interval between dysplasia and cancer was just 4 years. Among 13.7% of individuals, the interval was less than 1 year, suggesting an even shorter window of opportunity for screening.
Beyond this brief timeframe, low test sensitivity explains why screening efforts to date have fallen short, the investigators wrote.
Better tests are “urgently needed,” they added, while acknowledging the challenges inherent to this endeavor. Previous research has shown that precursor lesions in the pancreas are often less than 5 mm in diameter, making them extremely challenging to detect. An effective tool would need to identify solid precursor lesions (PanINs), and also need to simultaneously determine grade of dysplasia.
“Biomarkers could be the future in this matter,” the investigators suggested.
Dr. Koopmann and colleagues concluded by noting that more research is needed to characterize the pathophysiology of pancreatic cancer. On their part, “the current model will be validated, adjusted, and improved whenever new data from autopsy or prospective surveillance studies become available.”
The study was funded in part by Maag Lever Darm Stichting. The investigators disclosed no conflicts of interest.
, based on a microsimulation model.
To seize this opportunity, however, a greater understanding of natural disease course is needed, along with more sensitive screening tools, reported Brechtje D. M. Koopmann, MD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues.
Previous studies have suggested that the window of opportunity for pancreatic cancer screening may span decades, with estimates ranging from 12 to 50 years, the investigators wrote. Their report was published in Gastroenterology.
“Unfortunately, the poor results of pancreatic cancer screening do not align with this assumption, leaving unanswered whether this large window of opportunity truly exists,” they noted. “Microsimulation modeling, combined with available, if limited data, can provide new information on the natural disease course.”
For the present study, the investigators used the Microsimulation Screening Analysis (MISCAN) model, which has guided development of screening programs around the world for cervical, breast, and colorectal cancer. The model incorporates natural disease course, screening, and demographic data, then uses observable inputs such as precursor lesion prevalence and cancer incidence to estimate unobservable outcomes like stage durations and precursor lesion onset.
Dr. Koopmann and colleagues programmed this model with Dutch pancreatic cancer incidence data and findings from Japanese autopsy cases without pancreatic cancer.
First, the model offered insights into precursor lesion prevalence.
The estimated prevalence of any cystic lesion in the pancreas was 6.1% for individuals 50 years of age and 29.6% for those 80 years of age. Solid precursor lesions (PanINs) were estimated to be mainly multifocal (three or more lesions) in individuals older than 80 years. By this age, almost 12% had at least two PanINs. For those lesions that eventually became cancerous, the mean time since cyst onset was estimated to be 8.8 years, and mean time since PanIN onset was 9.0 years.
However, less than 10% of cystic and PanIN lesions progress to become cancers. PanIN lesions are not visible on imaging, and therefore current screening focuses on finding cystic precursor lesions, although these represent only about 10% of pancreatic cancers.
“Given the low pancreatic cancer progression risk of cysts, evaluation of the efficiency of current surveillance guidelines is necessary,” the investigators noted.
Screening should instead focus on identifying high-grade dysplastic lesions, they suggested. While these lesions may have a very low estimated prevalence, at just 0.3% among individuals 90 years of age, they present the greatest risk of pancreatic cancer.
For precursor cysts exhibiting HGD that progressed to pancreatic cancer, the mean interval between dysplasia and cancer was just 4 years. Among 13.7% of individuals, the interval was less than 1 year, suggesting an even shorter window of opportunity for screening.
Beyond this brief timeframe, low test sensitivity explains why screening efforts to date have fallen short, the investigators wrote.
Better tests are “urgently needed,” they added, while acknowledging the challenges inherent to this endeavor. Previous research has shown that precursor lesions in the pancreas are often less than 5 mm in diameter, making them extremely challenging to detect. An effective tool would need to identify solid precursor lesions (PanINs), and also need to simultaneously determine grade of dysplasia.
“Biomarkers could be the future in this matter,” the investigators suggested.
Dr. Koopmann and colleagues concluded by noting that more research is needed to characterize the pathophysiology of pancreatic cancer. On their part, “the current model will be validated, adjusted, and improved whenever new data from autopsy or prospective surveillance studies become available.”
The study was funded in part by Maag Lever Darm Stichting. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
T-cell cancers: CAR T therapy to the rescue?
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
FROM SITC 2023
AHA joins new cardiovascular certification group ABCVM
T to be known as the American Board of Cardiovascular Medicine (ABCVM).
The ABCVM would be independent of the American Board of Internal Medicine (ABIM), the current organization providing maintenance of certification for cardiologists along with 20 other internal medicine subspecialties. The ABIM’s maintenance of certification process has been widely criticized for many years and has been described as “needlessly burdensome and expensive.”
The AHA will be joining the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography & Interventions (SCAI) in forming the ABCVM.
These four other societies issued a joint statement in September saying that they will apply to the American Board of Medical Specialties (ABMS) to request an independent cardiology board that follows a “new competency-based approach to continuous certification — one that harnesses the knowledge, skills, and attitudes required to sustain professional excellence and care for cardiovascular patients effectively.”
The new board requirements will “de-emphasize timed, high stakes performance exams in the continuous certification process and instead will focus on learning assessments to identify gaps in current knowledge or skills,” the statement noted.
At the time the September statement was issued, the AHA was said to be supportive of the move but was waiting for formal endorsement to join the effort by its board of directors.
That has now happened, with the AHA’s national board of directors voting to provide “full support” for the creation of the proposed ABCVM.
“We enthusiastically join with our colleagues in proposing a new professional certification body to accredit cardiovascular professionals called the American Board of Cardiovascular Medicine,” said the association’s volunteer president Joseph C. Wu, MD. “The new ABCVM will be independent of the ABIM and focus on the specific competency-based trainings and appropriate ongoing certifications that align with and strengthen skills for cardiovascular physicians and enhance quality of care for people with cardiovascular disease,” Wu said.
“The AHA joins the consortium to submit the application to the American Board of Medical Specialties (ABMS) requesting an independent medical board for cardiovascular medicine. The consortium’s robust proposal harnesses the knowledge, skills, and benchmarks appropriate for professional excellence and delivery of effective, high-quality cardiovascular care,” Wu added.
The leaders of the ABCVM will include professional representatives from the consortium of member organizations, with a specific focus on relevant education, trainings, and supports that recognize the increasing specialization in cardiology and the latest advances in the various subspecialties of cardiovascular medicine, the AHA notes in a statement.
Professional certification by ABIM is a condition of employment for physicians practicing in large hospitals or health systems. A dedicated certification board separate from ABIM will help to ensure that cardiovascular professionals are maintaining the expertise appropriate to high-quality care and improved outcomes for their patients, the AHA said.
A version of this article first appeared on Medscape.com.
T to be known as the American Board of Cardiovascular Medicine (ABCVM).
The ABCVM would be independent of the American Board of Internal Medicine (ABIM), the current organization providing maintenance of certification for cardiologists along with 20 other internal medicine subspecialties. The ABIM’s maintenance of certification process has been widely criticized for many years and has been described as “needlessly burdensome and expensive.”
The AHA will be joining the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography & Interventions (SCAI) in forming the ABCVM.
These four other societies issued a joint statement in September saying that they will apply to the American Board of Medical Specialties (ABMS) to request an independent cardiology board that follows a “new competency-based approach to continuous certification — one that harnesses the knowledge, skills, and attitudes required to sustain professional excellence and care for cardiovascular patients effectively.”
The new board requirements will “de-emphasize timed, high stakes performance exams in the continuous certification process and instead will focus on learning assessments to identify gaps in current knowledge or skills,” the statement noted.
At the time the September statement was issued, the AHA was said to be supportive of the move but was waiting for formal endorsement to join the effort by its board of directors.
That has now happened, with the AHA’s national board of directors voting to provide “full support” for the creation of the proposed ABCVM.
“We enthusiastically join with our colleagues in proposing a new professional certification body to accredit cardiovascular professionals called the American Board of Cardiovascular Medicine,” said the association’s volunteer president Joseph C. Wu, MD. “The new ABCVM will be independent of the ABIM and focus on the specific competency-based trainings and appropriate ongoing certifications that align with and strengthen skills for cardiovascular physicians and enhance quality of care for people with cardiovascular disease,” Wu said.
“The AHA joins the consortium to submit the application to the American Board of Medical Specialties (ABMS) requesting an independent medical board for cardiovascular medicine. The consortium’s robust proposal harnesses the knowledge, skills, and benchmarks appropriate for professional excellence and delivery of effective, high-quality cardiovascular care,” Wu added.
The leaders of the ABCVM will include professional representatives from the consortium of member organizations, with a specific focus on relevant education, trainings, and supports that recognize the increasing specialization in cardiology and the latest advances in the various subspecialties of cardiovascular medicine, the AHA notes in a statement.
Professional certification by ABIM is a condition of employment for physicians practicing in large hospitals or health systems. A dedicated certification board separate from ABIM will help to ensure that cardiovascular professionals are maintaining the expertise appropriate to high-quality care and improved outcomes for their patients, the AHA said.
A version of this article first appeared on Medscape.com.
T to be known as the American Board of Cardiovascular Medicine (ABCVM).
The ABCVM would be independent of the American Board of Internal Medicine (ABIM), the current organization providing maintenance of certification for cardiologists along with 20 other internal medicine subspecialties. The ABIM’s maintenance of certification process has been widely criticized for many years and has been described as “needlessly burdensome and expensive.”
The AHA will be joining the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography & Interventions (SCAI) in forming the ABCVM.
These four other societies issued a joint statement in September saying that they will apply to the American Board of Medical Specialties (ABMS) to request an independent cardiology board that follows a “new competency-based approach to continuous certification — one that harnesses the knowledge, skills, and attitudes required to sustain professional excellence and care for cardiovascular patients effectively.”
The new board requirements will “de-emphasize timed, high stakes performance exams in the continuous certification process and instead will focus on learning assessments to identify gaps in current knowledge or skills,” the statement noted.
At the time the September statement was issued, the AHA was said to be supportive of the move but was waiting for formal endorsement to join the effort by its board of directors.
That has now happened, with the AHA’s national board of directors voting to provide “full support” for the creation of the proposed ABCVM.
“We enthusiastically join with our colleagues in proposing a new professional certification body to accredit cardiovascular professionals called the American Board of Cardiovascular Medicine,” said the association’s volunteer president Joseph C. Wu, MD. “The new ABCVM will be independent of the ABIM and focus on the specific competency-based trainings and appropriate ongoing certifications that align with and strengthen skills for cardiovascular physicians and enhance quality of care for people with cardiovascular disease,” Wu said.
“The AHA joins the consortium to submit the application to the American Board of Medical Specialties (ABMS) requesting an independent medical board for cardiovascular medicine. The consortium’s robust proposal harnesses the knowledge, skills, and benchmarks appropriate for professional excellence and delivery of effective, high-quality cardiovascular care,” Wu added.
The leaders of the ABCVM will include professional representatives from the consortium of member organizations, with a specific focus on relevant education, trainings, and supports that recognize the increasing specialization in cardiology and the latest advances in the various subspecialties of cardiovascular medicine, the AHA notes in a statement.
Professional certification by ABIM is a condition of employment for physicians practicing in large hospitals or health systems. A dedicated certification board separate from ABIM will help to ensure that cardiovascular professionals are maintaining the expertise appropriate to high-quality care and improved outcomes for their patients, the AHA said.
A version of this article first appeared on Medscape.com.
Low vitamin D linked to paclitaxel-induced peripheral neuropathy
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.

