User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Cre8 EVO stent loses sweet spot in diabetes at 2 years: SUGAR
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Uncontrolled BP linked to one-third of ED visits for CVD
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Add PCSK9 inhibitor to high-intensity statin at primary PCI, proposes sham-controlled EPIC-STEMI
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Yes, we should talk politics and religion with patients
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
Legacy of neutral renal denervation trial recast by long-term outcomes: SYMPLICITY HTN-3
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.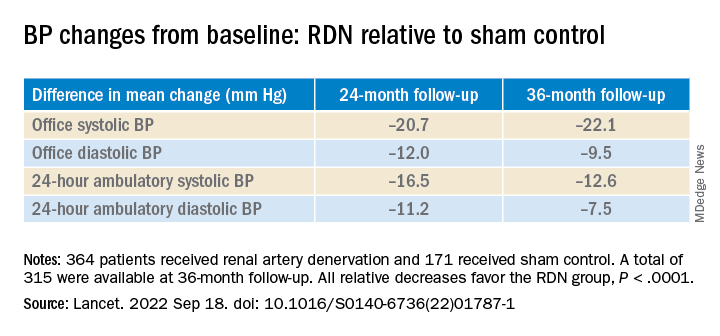
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Post-PCI FFR in multivessel disease predicts target vessel failure: FAME 3 analysis
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
Risk by FFR is continuous variable
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
FROM TCT 2022
Unsure on the best T2D drug choice? Let patients decide
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
AT EASD 2022
Desperate long COVID patients turn to unproven alternative therapies
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Early age at hysterectomy ups type 2 diabetes risk
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
FROM EASD 2022














