User login
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
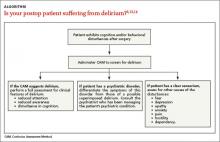
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.