User login
A 44-year-old African American man went to his primary care provider complaining of 5/10 dull lower back pain, which he attributed to a recent fall. He reported no radiation of the pain, lower extremity weakness or numbness, or any bowel/ bladder incontinence. Further review of systems and past medical history was unremarkable. He was initially treated with nonsteroidal anti-inflammatory agents and physical therapy. Approximately 2 months later he returned with similar persistent lower back pain complaints; an opiate was added to his pain control regimen. The following week he went to the emergency department (ED), where he reported worsening pain.
In the ED, he was afebrile with normal vital signs. He had a benign musculoskeletal and abdominal exam, and his neurological exam was without any focal deficits. Blood tests revealed a hemoglobin level of 9.8 g/dL with a hematocrit of 27.9%, serum blood urea nitrogen/creatinine ratio of 143/11.0 mg/dL, potassium of 6.5 mmol/L, calcium of 11.8 mg/dL, and phosphorus of 8.5 mg/dL. Urine protein was elevated at 88 mg/dL.
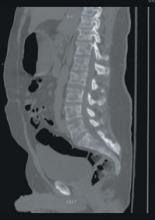
A stat CT of the patient’s abdomen and pelvis revealed no signs of obstructive uropathy to explain his acute renal failure. A sagittal CT reconstruction in bone windows of the initial ED radiological workup was revealing (FIGURE 1).
FIGURE 1
Sagittal CT reconstruction of thoracolumbar spine
What is your diagnosis?
How would you manage this condition?
Dx: Multiple myeloma
The sagittal CT reconstruction in bone windows of the thoracolumbar spine (FIGURE 1) revealed multiple lucent foci throughout the osseous structures, with an anterior compression deformity of the L2 vertebral body. A subsequent skeletal survey showed a diffuse salt and pepper pattern affecting most of the osseous structures, with additional lytic lesions in the calvarium and extremities.
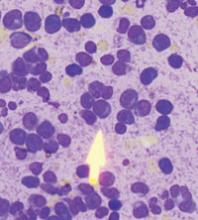
Following inpatient admission, the patient underwent a bone marrow biopsy that showed 90% marrow plasma cells (FIGURE 2). Serum protein electrophoresis (SPEP) and immunofixation electrophoresis revealed an elevated monoclonal protein of 7.52 g/dL IgG kappa, prompting us to diagnose multiple myeloma.
FIGURE 2
Bone marrow smear reveals 90% marrow plasma cells
Patients are typically much older
Multiple myeloma is a malignant proliferation of plasma cells derived from a single clone and accounts for 13% of all hematologic malignancies in Caucasians and 33% in African Americans. As with most hematopoeitic malignancies, the incidence of multiple myeloma increases with age, with the median age at diagnosis estimated at 69 years. In addition, 47% of multiple myeloma patients are >70 years of age, and 75% are >60.1
Clinical findings in multiple myeloma vary from asymptomatic patients whose disease is discovered incidentally to patients presenting with life-threatening symptoms. In a recent study of more than 1000 newly diagnosed patients, the most common presenting symptoms were bone pain (58%), fatigue (32%), and weight loss (24%).2 Tumor cells, tumor products (ie, monoclonal immunoglobulin protein), and host response to both elements account for other focal and systemic symptoms, including bone fracture, anemia, renal failure, vascular manifestations of hyperviscosity, hypercalcemia, and increased susceptibility to infection.
This presentation, in conjunction with radiological evidence of lytic bone lesions, increased total serum protein concentration, and a monoclonal protein in the urine or serum, constitutes the hallmark findings of multiple myeloma.
Working group keys in on 3 criteria
The International Myeloma Working Group has agreed on 3 simplified criteria for diagnosis of symptomatic multiple myeloma.3 These include the presence of (1) clonal bone marrow plasma cells or plasmacytoma, (2) an M-protein in serum of unspecified concentration, and (3) tissue- or organ-related impairment, such as renal insufficiency or anemia.
Our patient easily met all 3. His bone marrow biopsy displayed 90% plasma cell cellularity. Additionally, his SPEP exhibited markedly increased IgG immunoglobulin, and immunofixation data suggested IgG type kappa monoclonal gammopathy. These findings, in combination with our patient’s blood chemistry abnormalities, marked proteinuria, and imaging findings, confirmed the diagnosis of multiple myeloma.
Rule out MGUS
The differential diagnosis for multiple myeloma includes monoclonal gammopathy of undetermined significance (MGUS). The characteristic findings of MGUS include:
- absence of symptoms,
- M-protein component (either IgG, IgA, or IgM) <3 g/dL,
- <10% plasma cells in the marrow,
- absence of lytic lesions, and
- no signs of anemia, hypercalcemia, or renal insufficiency.
Essentially, MGUS is a milder form of myeloma with a more indolent course. However, MGUS does carry a 1% annual risk of progression to frank myeloma. An additional concern for patients with multiple myeloma is progression to plasma cell leukemia (PCL). The prognosis for PCL is poor, and the diagnosis is made when the absolute plasma cell count exceeds 2000/mcL. The rate of occurrence of PCL as a progression of multiple myeloma is 1% to 4%.4
In addition to MGUS, the differential diagnosis for multiple myeloma includes tuberculosis, sarcoidosis, and metastatic disease.
A 2-pronged Tx approach
There has been a paradigm shift in the treatment of multiple myeloma in the past decade, and while it appears to be incurable with current approaches, considerable progress has been made.5 Median survival prior to 1997 was nearly 2.5 years; it is now nearly 4 years for patients diagnosed in the last decade.5
Treatment of multiple myeloma generally consists of systemic chemotherapy to control progression and supportive care to prevent serious complications. The standard treatment has traditionally consisted of intermittent pulses of an alkylating agent and prednisone administered for 4 to 7 days every 4 to 6 weeks.
Complications requiring supportive therapy include infections (eg, in the urinary tract), pneumonia, hypercalcemia, and renal failure. Of note, hypercalcemia and renal failure may be alleviated with adequate hydration. If necessary, more aggressive management with dialysis may be initiated.
Other considerations include the administration of allopurinol during chemotherapy, which may help control hyperuricemia from tumor lysis. Transfusions may be required for anemic patients, and plasmapheresis may be indicated to treat hyperviscosity syndrome.
Our patient improves
In the hospital our patient began 4 days on dexamethasone, a 7-day regimen of plasmapheresis, and dialysis. He was also started on a proteasome inhibitor (bortezomib), a newer antineoplastic agent approved for the treatment of multiple myeloma.6 The patient responded well and was discharged home with hematology-oncology follow-up, where he remains clinically improved after treatment with a combination of bortezomib and dexamethasone.
CORRESPONDENCE
Vincent Timpone, MD, Department of Radiology, David Grant United States Air Force Medical Center, Travis Air Force Base, CA 94535; [email protected]
1. Zulian GB, Babare R, Zagonel V. Multiple myeloma. Crit Rev Oncol Hematol. 1998;27:165-167.
2. Kyle RA, Gertz MA. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
3. International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757.
4. Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13:1259-1272.
5. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-2520.
6. Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776-783.
A 44-year-old African American man went to his primary care provider complaining of 5/10 dull lower back pain, which he attributed to a recent fall. He reported no radiation of the pain, lower extremity weakness or numbness, or any bowel/ bladder incontinence. Further review of systems and past medical history was unremarkable. He was initially treated with nonsteroidal anti-inflammatory agents and physical therapy. Approximately 2 months later he returned with similar persistent lower back pain complaints; an opiate was added to his pain control regimen. The following week he went to the emergency department (ED), where he reported worsening pain.
In the ED, he was afebrile with normal vital signs. He had a benign musculoskeletal and abdominal exam, and his neurological exam was without any focal deficits. Blood tests revealed a hemoglobin level of 9.8 g/dL with a hematocrit of 27.9%, serum blood urea nitrogen/creatinine ratio of 143/11.0 mg/dL, potassium of 6.5 mmol/L, calcium of 11.8 mg/dL, and phosphorus of 8.5 mg/dL. Urine protein was elevated at 88 mg/dL.
A stat CT of the patient’s abdomen and pelvis revealed no signs of obstructive uropathy to explain his acute renal failure. A sagittal CT reconstruction in bone windows of the initial ED radiological workup was revealing (FIGURE 1).
FIGURE 1
Sagittal CT reconstruction of thoracolumbar spine
What is your diagnosis?
How would you manage this condition?
Dx: Multiple myeloma
The sagittal CT reconstruction in bone windows of the thoracolumbar spine (FIGURE 1) revealed multiple lucent foci throughout the osseous structures, with an anterior compression deformity of the L2 vertebral body. A subsequent skeletal survey showed a diffuse salt and pepper pattern affecting most of the osseous structures, with additional lytic lesions in the calvarium and extremities.
Following inpatient admission, the patient underwent a bone marrow biopsy that showed 90% marrow plasma cells (FIGURE 2). Serum protein electrophoresis (SPEP) and immunofixation electrophoresis revealed an elevated monoclonal protein of 7.52 g/dL IgG kappa, prompting us to diagnose multiple myeloma.
FIGURE 2
Bone marrow smear reveals 90% marrow plasma cells
Patients are typically much older
Multiple myeloma is a malignant proliferation of plasma cells derived from a single clone and accounts for 13% of all hematologic malignancies in Caucasians and 33% in African Americans. As with most hematopoeitic malignancies, the incidence of multiple myeloma increases with age, with the median age at diagnosis estimated at 69 years. In addition, 47% of multiple myeloma patients are >70 years of age, and 75% are >60.1
Clinical findings in multiple myeloma vary from asymptomatic patients whose disease is discovered incidentally to patients presenting with life-threatening symptoms. In a recent study of more than 1000 newly diagnosed patients, the most common presenting symptoms were bone pain (58%), fatigue (32%), and weight loss (24%).2 Tumor cells, tumor products (ie, monoclonal immunoglobulin protein), and host response to both elements account for other focal and systemic symptoms, including bone fracture, anemia, renal failure, vascular manifestations of hyperviscosity, hypercalcemia, and increased susceptibility to infection.
This presentation, in conjunction with radiological evidence of lytic bone lesions, increased total serum protein concentration, and a monoclonal protein in the urine or serum, constitutes the hallmark findings of multiple myeloma.
Working group keys in on 3 criteria
The International Myeloma Working Group has agreed on 3 simplified criteria for diagnosis of symptomatic multiple myeloma.3 These include the presence of (1) clonal bone marrow plasma cells or plasmacytoma, (2) an M-protein in serum of unspecified concentration, and (3) tissue- or organ-related impairment, such as renal insufficiency or anemia.
Our patient easily met all 3. His bone marrow biopsy displayed 90% plasma cell cellularity. Additionally, his SPEP exhibited markedly increased IgG immunoglobulin, and immunofixation data suggested IgG type kappa monoclonal gammopathy. These findings, in combination with our patient’s blood chemistry abnormalities, marked proteinuria, and imaging findings, confirmed the diagnosis of multiple myeloma.
Rule out MGUS
The differential diagnosis for multiple myeloma includes monoclonal gammopathy of undetermined significance (MGUS). The characteristic findings of MGUS include:
- absence of symptoms,
- M-protein component (either IgG, IgA, or IgM) <3 g/dL,
- <10% plasma cells in the marrow,
- absence of lytic lesions, and
- no signs of anemia, hypercalcemia, or renal insufficiency.
Essentially, MGUS is a milder form of myeloma with a more indolent course. However, MGUS does carry a 1% annual risk of progression to frank myeloma. An additional concern for patients with multiple myeloma is progression to plasma cell leukemia (PCL). The prognosis for PCL is poor, and the diagnosis is made when the absolute plasma cell count exceeds 2000/mcL. The rate of occurrence of PCL as a progression of multiple myeloma is 1% to 4%.4
In addition to MGUS, the differential diagnosis for multiple myeloma includes tuberculosis, sarcoidosis, and metastatic disease.
A 2-pronged Tx approach
There has been a paradigm shift in the treatment of multiple myeloma in the past decade, and while it appears to be incurable with current approaches, considerable progress has been made.5 Median survival prior to 1997 was nearly 2.5 years; it is now nearly 4 years for patients diagnosed in the last decade.5
Treatment of multiple myeloma generally consists of systemic chemotherapy to control progression and supportive care to prevent serious complications. The standard treatment has traditionally consisted of intermittent pulses of an alkylating agent and prednisone administered for 4 to 7 days every 4 to 6 weeks.
Complications requiring supportive therapy include infections (eg, in the urinary tract), pneumonia, hypercalcemia, and renal failure. Of note, hypercalcemia and renal failure may be alleviated with adequate hydration. If necessary, more aggressive management with dialysis may be initiated.
Other considerations include the administration of allopurinol during chemotherapy, which may help control hyperuricemia from tumor lysis. Transfusions may be required for anemic patients, and plasmapheresis may be indicated to treat hyperviscosity syndrome.
Our patient improves
In the hospital our patient began 4 days on dexamethasone, a 7-day regimen of plasmapheresis, and dialysis. He was also started on a proteasome inhibitor (bortezomib), a newer antineoplastic agent approved for the treatment of multiple myeloma.6 The patient responded well and was discharged home with hematology-oncology follow-up, where he remains clinically improved after treatment with a combination of bortezomib and dexamethasone.
CORRESPONDENCE
Vincent Timpone, MD, Department of Radiology, David Grant United States Air Force Medical Center, Travis Air Force Base, CA 94535; [email protected]
A 44-year-old African American man went to his primary care provider complaining of 5/10 dull lower back pain, which he attributed to a recent fall. He reported no radiation of the pain, lower extremity weakness or numbness, or any bowel/ bladder incontinence. Further review of systems and past medical history was unremarkable. He was initially treated with nonsteroidal anti-inflammatory agents and physical therapy. Approximately 2 months later he returned with similar persistent lower back pain complaints; an opiate was added to his pain control regimen. The following week he went to the emergency department (ED), where he reported worsening pain.
In the ED, he was afebrile with normal vital signs. He had a benign musculoskeletal and abdominal exam, and his neurological exam was without any focal deficits. Blood tests revealed a hemoglobin level of 9.8 g/dL with a hematocrit of 27.9%, serum blood urea nitrogen/creatinine ratio of 143/11.0 mg/dL, potassium of 6.5 mmol/L, calcium of 11.8 mg/dL, and phosphorus of 8.5 mg/dL. Urine protein was elevated at 88 mg/dL.
A stat CT of the patient’s abdomen and pelvis revealed no signs of obstructive uropathy to explain his acute renal failure. A sagittal CT reconstruction in bone windows of the initial ED radiological workup was revealing (FIGURE 1).
FIGURE 1
Sagittal CT reconstruction of thoracolumbar spine
What is your diagnosis?
How would you manage this condition?
Dx: Multiple myeloma
The sagittal CT reconstruction in bone windows of the thoracolumbar spine (FIGURE 1) revealed multiple lucent foci throughout the osseous structures, with an anterior compression deformity of the L2 vertebral body. A subsequent skeletal survey showed a diffuse salt and pepper pattern affecting most of the osseous structures, with additional lytic lesions in the calvarium and extremities.
Following inpatient admission, the patient underwent a bone marrow biopsy that showed 90% marrow plasma cells (FIGURE 2). Serum protein electrophoresis (SPEP) and immunofixation electrophoresis revealed an elevated monoclonal protein of 7.52 g/dL IgG kappa, prompting us to diagnose multiple myeloma.
FIGURE 2
Bone marrow smear reveals 90% marrow plasma cells
Patients are typically much older
Multiple myeloma is a malignant proliferation of plasma cells derived from a single clone and accounts for 13% of all hematologic malignancies in Caucasians and 33% in African Americans. As with most hematopoeitic malignancies, the incidence of multiple myeloma increases with age, with the median age at diagnosis estimated at 69 years. In addition, 47% of multiple myeloma patients are >70 years of age, and 75% are >60.1
Clinical findings in multiple myeloma vary from asymptomatic patients whose disease is discovered incidentally to patients presenting with life-threatening symptoms. In a recent study of more than 1000 newly diagnosed patients, the most common presenting symptoms were bone pain (58%), fatigue (32%), and weight loss (24%).2 Tumor cells, tumor products (ie, monoclonal immunoglobulin protein), and host response to both elements account for other focal and systemic symptoms, including bone fracture, anemia, renal failure, vascular manifestations of hyperviscosity, hypercalcemia, and increased susceptibility to infection.
This presentation, in conjunction with radiological evidence of lytic bone lesions, increased total serum protein concentration, and a monoclonal protein in the urine or serum, constitutes the hallmark findings of multiple myeloma.
Working group keys in on 3 criteria
The International Myeloma Working Group has agreed on 3 simplified criteria for diagnosis of symptomatic multiple myeloma.3 These include the presence of (1) clonal bone marrow plasma cells or plasmacytoma, (2) an M-protein in serum of unspecified concentration, and (3) tissue- or organ-related impairment, such as renal insufficiency or anemia.
Our patient easily met all 3. His bone marrow biopsy displayed 90% plasma cell cellularity. Additionally, his SPEP exhibited markedly increased IgG immunoglobulin, and immunofixation data suggested IgG type kappa monoclonal gammopathy. These findings, in combination with our patient’s blood chemistry abnormalities, marked proteinuria, and imaging findings, confirmed the diagnosis of multiple myeloma.
Rule out MGUS
The differential diagnosis for multiple myeloma includes monoclonal gammopathy of undetermined significance (MGUS). The characteristic findings of MGUS include:
- absence of symptoms,
- M-protein component (either IgG, IgA, or IgM) <3 g/dL,
- <10% plasma cells in the marrow,
- absence of lytic lesions, and
- no signs of anemia, hypercalcemia, or renal insufficiency.
Essentially, MGUS is a milder form of myeloma with a more indolent course. However, MGUS does carry a 1% annual risk of progression to frank myeloma. An additional concern for patients with multiple myeloma is progression to plasma cell leukemia (PCL). The prognosis for PCL is poor, and the diagnosis is made when the absolute plasma cell count exceeds 2000/mcL. The rate of occurrence of PCL as a progression of multiple myeloma is 1% to 4%.4
In addition to MGUS, the differential diagnosis for multiple myeloma includes tuberculosis, sarcoidosis, and metastatic disease.
A 2-pronged Tx approach
There has been a paradigm shift in the treatment of multiple myeloma in the past decade, and while it appears to be incurable with current approaches, considerable progress has been made.5 Median survival prior to 1997 was nearly 2.5 years; it is now nearly 4 years for patients diagnosed in the last decade.5
Treatment of multiple myeloma generally consists of systemic chemotherapy to control progression and supportive care to prevent serious complications. The standard treatment has traditionally consisted of intermittent pulses of an alkylating agent and prednisone administered for 4 to 7 days every 4 to 6 weeks.
Complications requiring supportive therapy include infections (eg, in the urinary tract), pneumonia, hypercalcemia, and renal failure. Of note, hypercalcemia and renal failure may be alleviated with adequate hydration. If necessary, more aggressive management with dialysis may be initiated.
Other considerations include the administration of allopurinol during chemotherapy, which may help control hyperuricemia from tumor lysis. Transfusions may be required for anemic patients, and plasmapheresis may be indicated to treat hyperviscosity syndrome.
Our patient improves
In the hospital our patient began 4 days on dexamethasone, a 7-day regimen of plasmapheresis, and dialysis. He was also started on a proteasome inhibitor (bortezomib), a newer antineoplastic agent approved for the treatment of multiple myeloma.6 The patient responded well and was discharged home with hematology-oncology follow-up, where he remains clinically improved after treatment with a combination of bortezomib and dexamethasone.
CORRESPONDENCE
Vincent Timpone, MD, Department of Radiology, David Grant United States Air Force Medical Center, Travis Air Force Base, CA 94535; [email protected]
1. Zulian GB, Babare R, Zagonel V. Multiple myeloma. Crit Rev Oncol Hematol. 1998;27:165-167.
2. Kyle RA, Gertz MA. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
3. International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757.
4. Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13:1259-1272.
5. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-2520.
6. Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776-783.
1. Zulian GB, Babare R, Zagonel V. Multiple myeloma. Crit Rev Oncol Hematol. 1998;27:165-167.
2. Kyle RA, Gertz MA. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
3. International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757.
4. Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13:1259-1272.
5. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-2520.
6. Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776-783.