User login
The Case
A 24-year-old white man with no past medical history is admitted after sustaining bilateral, closed femur fractures in a motor vehicle accident. Within hours of the trauma, he is taken to the operating room for open reduction and internal fixation. Of note, preoperatively, his hematocrit is 40%. After surgery, he is easily extubated and transferred to an unmonitored bed for further care. Approximately 30 hours after admission, he develops tachypnea with a respiratory rate of 35 breaths per minute and hypoxia with an oxygen saturation of 86% on room air. He is tachycardic (120 beats per minute) and febrile to 39.0oC. His blood pressure remains stable. He is somnolent, and when awake, he is confused. Notably, his hematocrit is now 22%. An electrocardiogram shows sinus tachycardia, an initial chest X-ray is normal, and a high-resolution CT scan is negative for a pulmonary embolism (PE).
Is this clinical picture consistent with fat embolism syndrome and, if so, how should he be managed?
Overview
“Fat embolism” refers to the presence of fat globules that obstruct the lung parenchyma and peripheral circulation. Fat embolism syndrome, on the other hand, is a more serious manifestation involving multiple organ systems. Specifically, it is a clinical diagnosis presenting with the classic triad of hypoxemia, neurologic abnormalities, and a petechial rash.
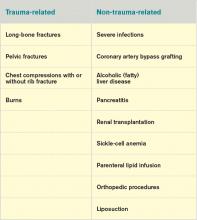
Fat embolism syndrome is usually associated with multiple traumas, including long-bone injuries and pelvic fractures. It is more frequently associated with closed fractures than open fractures, possibly due to the higher pressures associated with closed fractures. This syndrome has been less commonly associated with a variety of nontraumatic conditions (Table 1).
With an increased incidence of long-bone fractures in the younger demographic, fat embolism syndrome is most common in the second or third decade of life. While fat embolism occurs in up to 90% of patients with traumatic skeletal injuries, fat embolism syndrome occurs in 0.5% to 10% of patients following trauma, with a higher incidence in multiple fractures (5% to 10%) than in single long-bone fractures (0.5% to 2%).1-3
With the increasing role of hospitalists in assisting in the management of orthopedic patients, their knowledge of fat embolism syndrome is important so that it can be included in the differential diagnosis of acute respiratory failure in these orthopedic patients.
Review of the Data
Pathogenesis. Clinical manifestations of fat embolism syndrome have been acknowledged for more than 100 years. Since its first description in the 1860s, there has been speculation about the etiology of this condition. In the 1920s, two theories were proposed to explain the origin of the fat droplets: the mechanical and biochemical theories.2,4
Mechanical theory suggests that trauma to long bones disturbs fat cells within the bone marrow or adipose tissue, causing fat globules to mobilize.2,3 There is a rise in marrow pressure above venous pressure, which allows fat particles to enter the circulation through damaged venules surrounding the fracture site. Once lodged in the pulmonary microvasculature, embolized fat causes local ischemia and inflammation. Fat globules may pass into the arterial circulation either by paradoxical embolism through a patent foramen ovale, or by microemboli that pass through the lungs into the arterial circulation. This explains embolization to other organs, including the brain, retina, and skin.
Alternatively, biochemical theory hypothesizes that fat embolism syndrome is contingent on the production of toxic intermediaries from the breakdown of embolized fat.2,3 This theory suggests that the release of catecholamines after severe trauma can liberate free fatty acids from fat stores, or that acute-phase reactants at the trauma site affect fat solubility, causing agglutination and embolization. This theory helps to explain nontraumatic fat embolism syndrome, as well as the delay in development of the clinical syndrome after acute injury.
Clinical presentation. Most patients have a latent period after trauma of 12 to 72 hours before symptoms of fat embolism syndrome become apparent; however, clinical manifestations might occur immediately or up to one to two weeks following injury.2,4 As previously mentioned, the classic triad of symptoms includes respiratory compromise, neurological impairment, and a petechial rash.
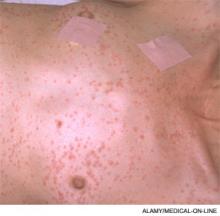
The most common and usually earliest manifestation is acute hypoxia, which must be distinguished from other treatable causes of hypoxia, including pneumothorax, hemothorax, PE, and pneumonia. Pulmonary changes might progress to respiratory failure similar to acute respiratory distress syndrome. Neurological manifestations are primarily nonspecific and include headache, irritability, delirium, seizures, and coma. Focal neurological deficits are rare but have been described.5 Almost all neurological symptoms are fully reversible. The petechial rash is distinctive and occurs on the chest, axilla, and subconjunctiva. Although the rash occurs in only 20% to 50% of patients and resolves fairly quickly, in the appropriate clinical setting, this rash is considered pathognomonic.1,2,4
A variety of other nonspecific signs and symptoms might also occur: pyrexia, tachycardia, fat in the urine or sputum, retinal changes, renal insufficiency, myocardial dysfunction, and an otherwise unexplained drop in hematocrit or platelet count.
Diagnosis. Fat embolism syndrome is a clinical diagnosis and a diagnosis of exclusion. There are no specific confirmatory tests. An arterial blood gas will usually reveal a PaO2 of <60 mmHg.3 Laboratory evaluation might also show fat globules in the urine or sputum on Sudan or Oil Red O staining, but these findings are nonspecific.3,4 Bronchoscopy with bronchial alveolar lavage (BAL) might similarly detect fat droplets in alveolar macrophages in the BAL fluid; however, the sensitivity and specificity for diagnosis of fat embolism syndrome are unknown.4 None of these tests can be used solely for the diagnosis of fat embolism syndrome.
Thrombocytopenia and anemia out of proportion to the expected drop from surgery are not uncommon in addition to other nonspecific laboratory findings, including hypocalcemia, elevated serum lipase level, and elevated erythrocyte sedimentation rate.4 Several radiological findings have been observed on lung and brain imaging, though the findings are nonspecific and none are diagnostic. A chest X-ray might be normal, but abnormalities are seen in 30% to 50% of cases.2 Typically, when abnormal, the chest X-ray shows diffuse interstitial and alveolar densities, as well as patchy perihilar and basilar infiltrates resembling pulmonary edema. These X-ray findings might not be seen for up to 12 to 24 hours following the onset of clinical symptoms.
The most commonly used diagnostic criteria for the diagnosis of fat embolism syndrome are published by Gurd et al.6 At least two major criteria or one major criterion and four minor criteria are required for the diagnosis of fat embolism syndrome. The major criteria are based on the three classic signs and symptoms of fat embolism syndrome; the minor criteria include the finding of fat globules in the urine and sputum as well as some of the previously mentioned nonspecific clinical signs and laboratory tests.
Other criteria for diagnosis have been suggested, including those published by Lindeque et al, which focuses primarily on the respiratory characteristics, and a more recent set of semiquantitative diagnostic criteria called the fat embolism index, published by Schonfeld et al.7,8 Schonfeld’s scoring index accounts for the major signs and symptoms of fat embolism syndrome and weighs them according to relative specificity. A score of 5 or more is required for diagnosis of fat embolism syndrome. Table 2 compares the three sets of criteria used for diagnosis of fat embolism syndrome.
Treatment. The treatment of fat embolism syndrome is supportive. Most often, this requires supplemental oxygen for hypoxia and, possibly, fluid resuscitation in the case of hypovolemia. Occasionally, though, these relatively minor supportive therapies need to be escalated to bipap or even full ventilatory support and vasopressors in the more severe cases.
Based on the premise that steroids will attenuate the inflammatory reaction to free fatty acids within the lung, steroids have been tried in the treatment of fat embolism syndrome. However, there are no studies that clearly show benefit with their use.
Prevention. Most of the methods of prevention involve surgical intervention rather than medical therapy. Because microscopic fat emboli are showered during manipulation of long-bone fragments, early immobilization of fractures is recommended, and operative correction rather than conservative management is the preferred method.2,3 One report estimates a 70% reduction in pulmonary complications from this intervention alone.9
Further, two surgical techniques are debated as possible means of preventing fat embolism syndrome. The first is “venting,” in which a hole is made distal to the site of intramedullary nail placement. This reduces intramedullary pressure elevation and, therefore, extravasation of fat into the circulation.10 The second technique is the use of a reamer, irrigator, aspirator (RIA) device. A reamer is a tool used to create an accurate-sized hole for an intramedullary nail. Reaming before intramedullary nail placement can release fat deposits into the circulation. The RIA device irrigates and aspirates resident fat deposits as it reams the canal, releasing fewer deposits into the circulation.11 At this time, these two techniques are considered but not used routinely by surgeons.
Corticosteroids remain a debated method of prevention of fat embolism syndrome. A number of smaller studies suggest steroid therapy might reduce the incidence of fat embolism syndrome and hypoxia; a 2009 meta-analysis pooling nearly 400 patients from these smaller studies found such results.12 Unfortunately, the included studies were noted to be of poor quality, and no change in mortality was found. These results, combined with the possibility of poor wound healing or infection as a complication of steroid use, keep steroids from being used routinely to prevent fat embolism syndrome.
Clinical course. The severity of fat embolism syndrome ranges from mild transient hypoxia with confusion to progressively worsening symptoms leading to acute respiratory distress syndrome and coma. Bulger et al found a 7% mortality rate in this population.1 Less commonly, patients have a fulminant presentation with symptom onset less than 12 hours after injury. With this presentation, patients have a higher rate of mortality—as high as 15%.13
Back to the Case
This young man with bilateral long-bone fractures was at high risk of developing fat embolism syndrome. As is recommended, he was quickly taken to the operating room for fracture stabilization with open reduction and internal fixation. In addition, a RIA device was used to decrease intramedullary pressure. Nonetheless, within the first two days of injury, he developed hypoxia and confusion. These clinical changes were associated with an unexpected drop in hematocrit.
Chest X-ray and high-resolution computed tomography did not reveal a cause of his hypoxia. Similarly, laboratory evaluation for a reversible cause of encephalopathy was negative. A Sudan stain of his urine revealed free fat globules. Though he did not develop axillary petechiae, this clinical picture is consistent with fat embolism syndrome based on Gurd’s criteria. He was supported with oxygen therapy, and he stabilized without further complications.
Drs. Smith and Rice are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn.
References
- Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132:435-439.
- Levy D. The fat embolism syndrome. Clin Orthop. 1990;261:281-286.
- Akhtar S. Fat embolism. Anes Clin. 2009;27:533-550.
- Gupta A, Reilly C. Fat embolism. Anaesth Crit Care Pain. 2007;7:148-151.
- Thomas JE, Ayyar DR. Systemic fat embolism. Arch Neurol. 1972;26:517-523.
- Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56B:408-416.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br. 1987;69:128-131.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99:438-443.
- Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83:781-791.
- Kim YH, Oh SW, Kim JS. Prevalence of fat embolism following bilateral simultaneous and unilateral total hip arthroplasty performed with or without cement: a prospective, randomized clinical study. J Bone Joint Surg Am. 2002;84A:1372-1379.
- Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41 Suppl 2:S90-S93.
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009;52:386-393.
- Bracco D, Favre JB, Joris F, Ravussin A. Fatal fat embolism syndrome: a case report. J Neurosurg Anesthesiol. 2000;12:221-224.
The Case
A 24-year-old white man with no past medical history is admitted after sustaining bilateral, closed femur fractures in a motor vehicle accident. Within hours of the trauma, he is taken to the operating room for open reduction and internal fixation. Of note, preoperatively, his hematocrit is 40%. After surgery, he is easily extubated and transferred to an unmonitored bed for further care. Approximately 30 hours after admission, he develops tachypnea with a respiratory rate of 35 breaths per minute and hypoxia with an oxygen saturation of 86% on room air. He is tachycardic (120 beats per minute) and febrile to 39.0oC. His blood pressure remains stable. He is somnolent, and when awake, he is confused. Notably, his hematocrit is now 22%. An electrocardiogram shows sinus tachycardia, an initial chest X-ray is normal, and a high-resolution CT scan is negative for a pulmonary embolism (PE).
Is this clinical picture consistent with fat embolism syndrome and, if so, how should he be managed?
Overview
“Fat embolism” refers to the presence of fat globules that obstruct the lung parenchyma and peripheral circulation. Fat embolism syndrome, on the other hand, is a more serious manifestation involving multiple organ systems. Specifically, it is a clinical diagnosis presenting with the classic triad of hypoxemia, neurologic abnormalities, and a petechial rash.
Fat embolism syndrome is usually associated with multiple traumas, including long-bone injuries and pelvic fractures. It is more frequently associated with closed fractures than open fractures, possibly due to the higher pressures associated with closed fractures. This syndrome has been less commonly associated with a variety of nontraumatic conditions (Table 1).
With an increased incidence of long-bone fractures in the younger demographic, fat embolism syndrome is most common in the second or third decade of life. While fat embolism occurs in up to 90% of patients with traumatic skeletal injuries, fat embolism syndrome occurs in 0.5% to 10% of patients following trauma, with a higher incidence in multiple fractures (5% to 10%) than in single long-bone fractures (0.5% to 2%).1-3
With the increasing role of hospitalists in assisting in the management of orthopedic patients, their knowledge of fat embolism syndrome is important so that it can be included in the differential diagnosis of acute respiratory failure in these orthopedic patients.
Review of the Data
Pathogenesis. Clinical manifestations of fat embolism syndrome have been acknowledged for more than 100 years. Since its first description in the 1860s, there has been speculation about the etiology of this condition. In the 1920s, two theories were proposed to explain the origin of the fat droplets: the mechanical and biochemical theories.2,4
Mechanical theory suggests that trauma to long bones disturbs fat cells within the bone marrow or adipose tissue, causing fat globules to mobilize.2,3 There is a rise in marrow pressure above venous pressure, which allows fat particles to enter the circulation through damaged venules surrounding the fracture site. Once lodged in the pulmonary microvasculature, embolized fat causes local ischemia and inflammation. Fat globules may pass into the arterial circulation either by paradoxical embolism through a patent foramen ovale, or by microemboli that pass through the lungs into the arterial circulation. This explains embolization to other organs, including the brain, retina, and skin.
Alternatively, biochemical theory hypothesizes that fat embolism syndrome is contingent on the production of toxic intermediaries from the breakdown of embolized fat.2,3 This theory suggests that the release of catecholamines after severe trauma can liberate free fatty acids from fat stores, or that acute-phase reactants at the trauma site affect fat solubility, causing agglutination and embolization. This theory helps to explain nontraumatic fat embolism syndrome, as well as the delay in development of the clinical syndrome after acute injury.
Clinical presentation. Most patients have a latent period after trauma of 12 to 72 hours before symptoms of fat embolism syndrome become apparent; however, clinical manifestations might occur immediately or up to one to two weeks following injury.2,4 As previously mentioned, the classic triad of symptoms includes respiratory compromise, neurological impairment, and a petechial rash.
The most common and usually earliest manifestation is acute hypoxia, which must be distinguished from other treatable causes of hypoxia, including pneumothorax, hemothorax, PE, and pneumonia. Pulmonary changes might progress to respiratory failure similar to acute respiratory distress syndrome. Neurological manifestations are primarily nonspecific and include headache, irritability, delirium, seizures, and coma. Focal neurological deficits are rare but have been described.5 Almost all neurological symptoms are fully reversible. The petechial rash is distinctive and occurs on the chest, axilla, and subconjunctiva. Although the rash occurs in only 20% to 50% of patients and resolves fairly quickly, in the appropriate clinical setting, this rash is considered pathognomonic.1,2,4
A variety of other nonspecific signs and symptoms might also occur: pyrexia, tachycardia, fat in the urine or sputum, retinal changes, renal insufficiency, myocardial dysfunction, and an otherwise unexplained drop in hematocrit or platelet count.
Diagnosis. Fat embolism syndrome is a clinical diagnosis and a diagnosis of exclusion. There are no specific confirmatory tests. An arterial blood gas will usually reveal a PaO2 of <60 mmHg.3 Laboratory evaluation might also show fat globules in the urine or sputum on Sudan or Oil Red O staining, but these findings are nonspecific.3,4 Bronchoscopy with bronchial alveolar lavage (BAL) might similarly detect fat droplets in alveolar macrophages in the BAL fluid; however, the sensitivity and specificity for diagnosis of fat embolism syndrome are unknown.4 None of these tests can be used solely for the diagnosis of fat embolism syndrome.
Thrombocytopenia and anemia out of proportion to the expected drop from surgery are not uncommon in addition to other nonspecific laboratory findings, including hypocalcemia, elevated serum lipase level, and elevated erythrocyte sedimentation rate.4 Several radiological findings have been observed on lung and brain imaging, though the findings are nonspecific and none are diagnostic. A chest X-ray might be normal, but abnormalities are seen in 30% to 50% of cases.2 Typically, when abnormal, the chest X-ray shows diffuse interstitial and alveolar densities, as well as patchy perihilar and basilar infiltrates resembling pulmonary edema. These X-ray findings might not be seen for up to 12 to 24 hours following the onset of clinical symptoms.
The most commonly used diagnostic criteria for the diagnosis of fat embolism syndrome are published by Gurd et al.6 At least two major criteria or one major criterion and four minor criteria are required for the diagnosis of fat embolism syndrome. The major criteria are based on the three classic signs and symptoms of fat embolism syndrome; the minor criteria include the finding of fat globules in the urine and sputum as well as some of the previously mentioned nonspecific clinical signs and laboratory tests.
Other criteria for diagnosis have been suggested, including those published by Lindeque et al, which focuses primarily on the respiratory characteristics, and a more recent set of semiquantitative diagnostic criteria called the fat embolism index, published by Schonfeld et al.7,8 Schonfeld’s scoring index accounts for the major signs and symptoms of fat embolism syndrome and weighs them according to relative specificity. A score of 5 or more is required for diagnosis of fat embolism syndrome. Table 2 compares the three sets of criteria used for diagnosis of fat embolism syndrome.
Treatment. The treatment of fat embolism syndrome is supportive. Most often, this requires supplemental oxygen for hypoxia and, possibly, fluid resuscitation in the case of hypovolemia. Occasionally, though, these relatively minor supportive therapies need to be escalated to bipap or even full ventilatory support and vasopressors in the more severe cases.
Based on the premise that steroids will attenuate the inflammatory reaction to free fatty acids within the lung, steroids have been tried in the treatment of fat embolism syndrome. However, there are no studies that clearly show benefit with their use.
Prevention. Most of the methods of prevention involve surgical intervention rather than medical therapy. Because microscopic fat emboli are showered during manipulation of long-bone fragments, early immobilization of fractures is recommended, and operative correction rather than conservative management is the preferred method.2,3 One report estimates a 70% reduction in pulmonary complications from this intervention alone.9
Further, two surgical techniques are debated as possible means of preventing fat embolism syndrome. The first is “venting,” in which a hole is made distal to the site of intramedullary nail placement. This reduces intramedullary pressure elevation and, therefore, extravasation of fat into the circulation.10 The second technique is the use of a reamer, irrigator, aspirator (RIA) device. A reamer is a tool used to create an accurate-sized hole for an intramedullary nail. Reaming before intramedullary nail placement can release fat deposits into the circulation. The RIA device irrigates and aspirates resident fat deposits as it reams the canal, releasing fewer deposits into the circulation.11 At this time, these two techniques are considered but not used routinely by surgeons.
Corticosteroids remain a debated method of prevention of fat embolism syndrome. A number of smaller studies suggest steroid therapy might reduce the incidence of fat embolism syndrome and hypoxia; a 2009 meta-analysis pooling nearly 400 patients from these smaller studies found such results.12 Unfortunately, the included studies were noted to be of poor quality, and no change in mortality was found. These results, combined with the possibility of poor wound healing or infection as a complication of steroid use, keep steroids from being used routinely to prevent fat embolism syndrome.
Clinical course. The severity of fat embolism syndrome ranges from mild transient hypoxia with confusion to progressively worsening symptoms leading to acute respiratory distress syndrome and coma. Bulger et al found a 7% mortality rate in this population.1 Less commonly, patients have a fulminant presentation with symptom onset less than 12 hours after injury. With this presentation, patients have a higher rate of mortality—as high as 15%.13
Back to the Case
This young man with bilateral long-bone fractures was at high risk of developing fat embolism syndrome. As is recommended, he was quickly taken to the operating room for fracture stabilization with open reduction and internal fixation. In addition, a RIA device was used to decrease intramedullary pressure. Nonetheless, within the first two days of injury, he developed hypoxia and confusion. These clinical changes were associated with an unexpected drop in hematocrit.
Chest X-ray and high-resolution computed tomography did not reveal a cause of his hypoxia. Similarly, laboratory evaluation for a reversible cause of encephalopathy was negative. A Sudan stain of his urine revealed free fat globules. Though he did not develop axillary petechiae, this clinical picture is consistent with fat embolism syndrome based on Gurd’s criteria. He was supported with oxygen therapy, and he stabilized without further complications.
Drs. Smith and Rice are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn.
References
- Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132:435-439.
- Levy D. The fat embolism syndrome. Clin Orthop. 1990;261:281-286.
- Akhtar S. Fat embolism. Anes Clin. 2009;27:533-550.
- Gupta A, Reilly C. Fat embolism. Anaesth Crit Care Pain. 2007;7:148-151.
- Thomas JE, Ayyar DR. Systemic fat embolism. Arch Neurol. 1972;26:517-523.
- Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56B:408-416.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br. 1987;69:128-131.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99:438-443.
- Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83:781-791.
- Kim YH, Oh SW, Kim JS. Prevalence of fat embolism following bilateral simultaneous and unilateral total hip arthroplasty performed with or without cement: a prospective, randomized clinical study. J Bone Joint Surg Am. 2002;84A:1372-1379.
- Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41 Suppl 2:S90-S93.
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009;52:386-393.
- Bracco D, Favre JB, Joris F, Ravussin A. Fatal fat embolism syndrome: a case report. J Neurosurg Anesthesiol. 2000;12:221-224.
The Case
A 24-year-old white man with no past medical history is admitted after sustaining bilateral, closed femur fractures in a motor vehicle accident. Within hours of the trauma, he is taken to the operating room for open reduction and internal fixation. Of note, preoperatively, his hematocrit is 40%. After surgery, he is easily extubated and transferred to an unmonitored bed for further care. Approximately 30 hours after admission, he develops tachypnea with a respiratory rate of 35 breaths per minute and hypoxia with an oxygen saturation of 86% on room air. He is tachycardic (120 beats per minute) and febrile to 39.0oC. His blood pressure remains stable. He is somnolent, and when awake, he is confused. Notably, his hematocrit is now 22%. An electrocardiogram shows sinus tachycardia, an initial chest X-ray is normal, and a high-resolution CT scan is negative for a pulmonary embolism (PE).
Is this clinical picture consistent with fat embolism syndrome and, if so, how should he be managed?
Overview
“Fat embolism” refers to the presence of fat globules that obstruct the lung parenchyma and peripheral circulation. Fat embolism syndrome, on the other hand, is a more serious manifestation involving multiple organ systems. Specifically, it is a clinical diagnosis presenting with the classic triad of hypoxemia, neurologic abnormalities, and a petechial rash.
Fat embolism syndrome is usually associated with multiple traumas, including long-bone injuries and pelvic fractures. It is more frequently associated with closed fractures than open fractures, possibly due to the higher pressures associated with closed fractures. This syndrome has been less commonly associated with a variety of nontraumatic conditions (Table 1).
With an increased incidence of long-bone fractures in the younger demographic, fat embolism syndrome is most common in the second or third decade of life. While fat embolism occurs in up to 90% of patients with traumatic skeletal injuries, fat embolism syndrome occurs in 0.5% to 10% of patients following trauma, with a higher incidence in multiple fractures (5% to 10%) than in single long-bone fractures (0.5% to 2%).1-3
With the increasing role of hospitalists in assisting in the management of orthopedic patients, their knowledge of fat embolism syndrome is important so that it can be included in the differential diagnosis of acute respiratory failure in these orthopedic patients.
Review of the Data
Pathogenesis. Clinical manifestations of fat embolism syndrome have been acknowledged for more than 100 years. Since its first description in the 1860s, there has been speculation about the etiology of this condition. In the 1920s, two theories were proposed to explain the origin of the fat droplets: the mechanical and biochemical theories.2,4
Mechanical theory suggests that trauma to long bones disturbs fat cells within the bone marrow or adipose tissue, causing fat globules to mobilize.2,3 There is a rise in marrow pressure above venous pressure, which allows fat particles to enter the circulation through damaged venules surrounding the fracture site. Once lodged in the pulmonary microvasculature, embolized fat causes local ischemia and inflammation. Fat globules may pass into the arterial circulation either by paradoxical embolism through a patent foramen ovale, or by microemboli that pass through the lungs into the arterial circulation. This explains embolization to other organs, including the brain, retina, and skin.
Alternatively, biochemical theory hypothesizes that fat embolism syndrome is contingent on the production of toxic intermediaries from the breakdown of embolized fat.2,3 This theory suggests that the release of catecholamines after severe trauma can liberate free fatty acids from fat stores, or that acute-phase reactants at the trauma site affect fat solubility, causing agglutination and embolization. This theory helps to explain nontraumatic fat embolism syndrome, as well as the delay in development of the clinical syndrome after acute injury.
Clinical presentation. Most patients have a latent period after trauma of 12 to 72 hours before symptoms of fat embolism syndrome become apparent; however, clinical manifestations might occur immediately or up to one to two weeks following injury.2,4 As previously mentioned, the classic triad of symptoms includes respiratory compromise, neurological impairment, and a petechial rash.
The most common and usually earliest manifestation is acute hypoxia, which must be distinguished from other treatable causes of hypoxia, including pneumothorax, hemothorax, PE, and pneumonia. Pulmonary changes might progress to respiratory failure similar to acute respiratory distress syndrome. Neurological manifestations are primarily nonspecific and include headache, irritability, delirium, seizures, and coma. Focal neurological deficits are rare but have been described.5 Almost all neurological symptoms are fully reversible. The petechial rash is distinctive and occurs on the chest, axilla, and subconjunctiva. Although the rash occurs in only 20% to 50% of patients and resolves fairly quickly, in the appropriate clinical setting, this rash is considered pathognomonic.1,2,4
A variety of other nonspecific signs and symptoms might also occur: pyrexia, tachycardia, fat in the urine or sputum, retinal changes, renal insufficiency, myocardial dysfunction, and an otherwise unexplained drop in hematocrit or platelet count.
Diagnosis. Fat embolism syndrome is a clinical diagnosis and a diagnosis of exclusion. There are no specific confirmatory tests. An arterial blood gas will usually reveal a PaO2 of <60 mmHg.3 Laboratory evaluation might also show fat globules in the urine or sputum on Sudan or Oil Red O staining, but these findings are nonspecific.3,4 Bronchoscopy with bronchial alveolar lavage (BAL) might similarly detect fat droplets in alveolar macrophages in the BAL fluid; however, the sensitivity and specificity for diagnosis of fat embolism syndrome are unknown.4 None of these tests can be used solely for the diagnosis of fat embolism syndrome.
Thrombocytopenia and anemia out of proportion to the expected drop from surgery are not uncommon in addition to other nonspecific laboratory findings, including hypocalcemia, elevated serum lipase level, and elevated erythrocyte sedimentation rate.4 Several radiological findings have been observed on lung and brain imaging, though the findings are nonspecific and none are diagnostic. A chest X-ray might be normal, but abnormalities are seen in 30% to 50% of cases.2 Typically, when abnormal, the chest X-ray shows diffuse interstitial and alveolar densities, as well as patchy perihilar and basilar infiltrates resembling pulmonary edema. These X-ray findings might not be seen for up to 12 to 24 hours following the onset of clinical symptoms.
The most commonly used diagnostic criteria for the diagnosis of fat embolism syndrome are published by Gurd et al.6 At least two major criteria or one major criterion and four minor criteria are required for the diagnosis of fat embolism syndrome. The major criteria are based on the three classic signs and symptoms of fat embolism syndrome; the minor criteria include the finding of fat globules in the urine and sputum as well as some of the previously mentioned nonspecific clinical signs and laboratory tests.
Other criteria for diagnosis have been suggested, including those published by Lindeque et al, which focuses primarily on the respiratory characteristics, and a more recent set of semiquantitative diagnostic criteria called the fat embolism index, published by Schonfeld et al.7,8 Schonfeld’s scoring index accounts for the major signs and symptoms of fat embolism syndrome and weighs them according to relative specificity. A score of 5 or more is required for diagnosis of fat embolism syndrome. Table 2 compares the three sets of criteria used for diagnosis of fat embolism syndrome.
Treatment. The treatment of fat embolism syndrome is supportive. Most often, this requires supplemental oxygen for hypoxia and, possibly, fluid resuscitation in the case of hypovolemia. Occasionally, though, these relatively minor supportive therapies need to be escalated to bipap or even full ventilatory support and vasopressors in the more severe cases.
Based on the premise that steroids will attenuate the inflammatory reaction to free fatty acids within the lung, steroids have been tried in the treatment of fat embolism syndrome. However, there are no studies that clearly show benefit with their use.
Prevention. Most of the methods of prevention involve surgical intervention rather than medical therapy. Because microscopic fat emboli are showered during manipulation of long-bone fragments, early immobilization of fractures is recommended, and operative correction rather than conservative management is the preferred method.2,3 One report estimates a 70% reduction in pulmonary complications from this intervention alone.9
Further, two surgical techniques are debated as possible means of preventing fat embolism syndrome. The first is “venting,” in which a hole is made distal to the site of intramedullary nail placement. This reduces intramedullary pressure elevation and, therefore, extravasation of fat into the circulation.10 The second technique is the use of a reamer, irrigator, aspirator (RIA) device. A reamer is a tool used to create an accurate-sized hole for an intramedullary nail. Reaming before intramedullary nail placement can release fat deposits into the circulation. The RIA device irrigates and aspirates resident fat deposits as it reams the canal, releasing fewer deposits into the circulation.11 At this time, these two techniques are considered but not used routinely by surgeons.
Corticosteroids remain a debated method of prevention of fat embolism syndrome. A number of smaller studies suggest steroid therapy might reduce the incidence of fat embolism syndrome and hypoxia; a 2009 meta-analysis pooling nearly 400 patients from these smaller studies found such results.12 Unfortunately, the included studies were noted to be of poor quality, and no change in mortality was found. These results, combined with the possibility of poor wound healing or infection as a complication of steroid use, keep steroids from being used routinely to prevent fat embolism syndrome.
Clinical course. The severity of fat embolism syndrome ranges from mild transient hypoxia with confusion to progressively worsening symptoms leading to acute respiratory distress syndrome and coma. Bulger et al found a 7% mortality rate in this population.1 Less commonly, patients have a fulminant presentation with symptom onset less than 12 hours after injury. With this presentation, patients have a higher rate of mortality—as high as 15%.13
Back to the Case
This young man with bilateral long-bone fractures was at high risk of developing fat embolism syndrome. As is recommended, he was quickly taken to the operating room for fracture stabilization with open reduction and internal fixation. In addition, a RIA device was used to decrease intramedullary pressure. Nonetheless, within the first two days of injury, he developed hypoxia and confusion. These clinical changes were associated with an unexpected drop in hematocrit.
Chest X-ray and high-resolution computed tomography did not reveal a cause of his hypoxia. Similarly, laboratory evaluation for a reversible cause of encephalopathy was negative. A Sudan stain of his urine revealed free fat globules. Though he did not develop axillary petechiae, this clinical picture is consistent with fat embolism syndrome based on Gurd’s criteria. He was supported with oxygen therapy, and he stabilized without further complications.
Drs. Smith and Rice are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn.
References
- Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132:435-439.
- Levy D. The fat embolism syndrome. Clin Orthop. 1990;261:281-286.
- Akhtar S. Fat embolism. Anes Clin. 2009;27:533-550.
- Gupta A, Reilly C. Fat embolism. Anaesth Crit Care Pain. 2007;7:148-151.
- Thomas JE, Ayyar DR. Systemic fat embolism. Arch Neurol. 1972;26:517-523.
- Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56B:408-416.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br. 1987;69:128-131.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99:438-443.
- Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83:781-791.
- Kim YH, Oh SW, Kim JS. Prevalence of fat embolism following bilateral simultaneous and unilateral total hip arthroplasty performed with or without cement: a prospective, randomized clinical study. J Bone Joint Surg Am. 2002;84A:1372-1379.
- Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41 Suppl 2:S90-S93.
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009;52:386-393.
- Bracco D, Favre JB, Joris F, Ravussin A. Fatal fat embolism syndrome: a case report. J Neurosurg Anesthesiol. 2000;12:221-224.