User login
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.
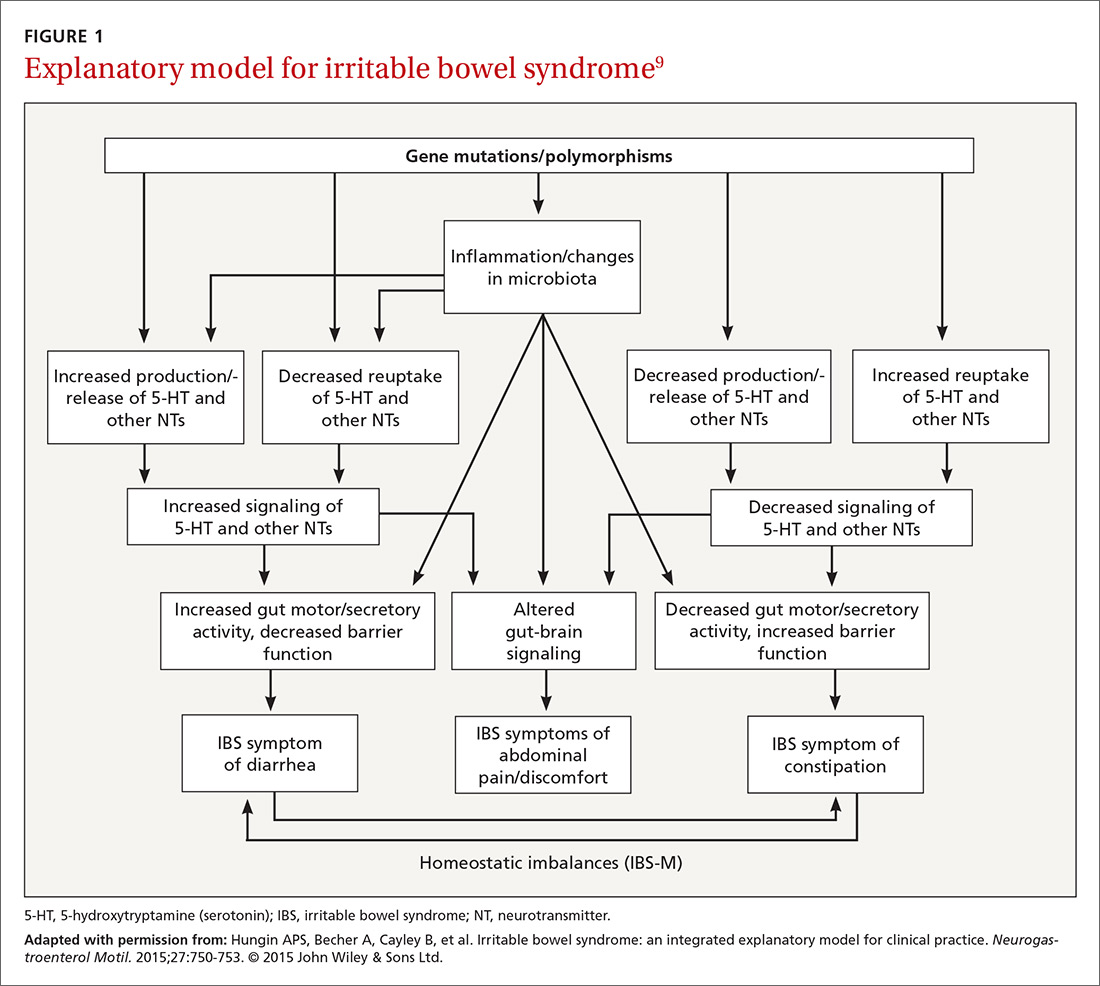
It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3
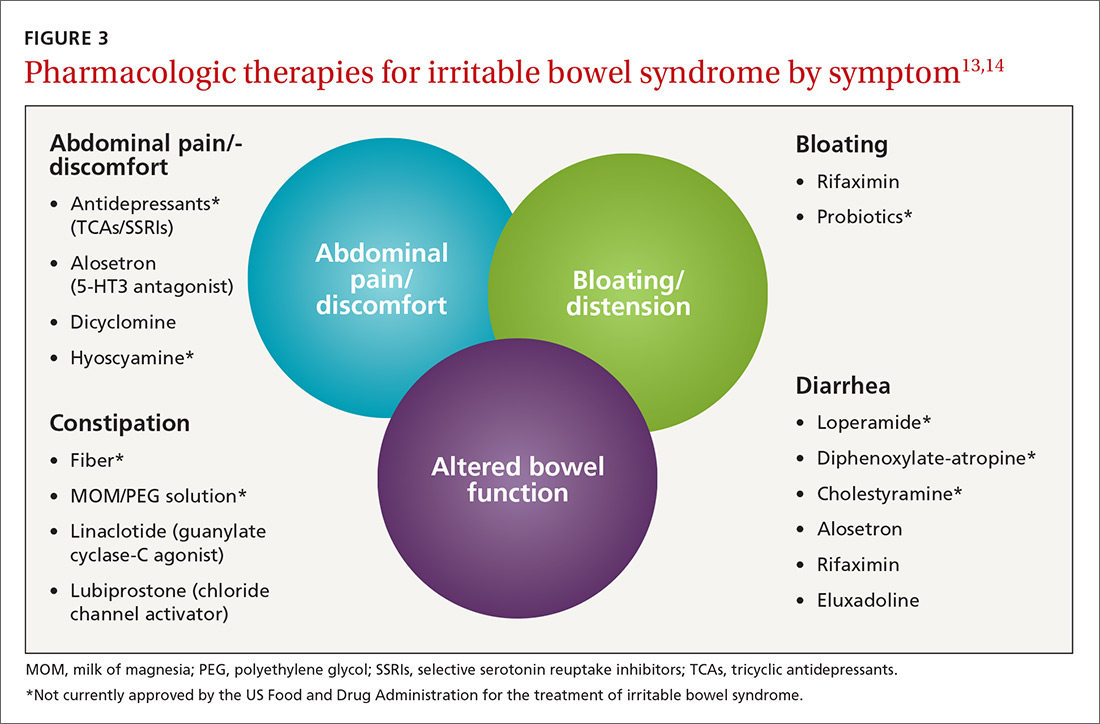
Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.

It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3

Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.

It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3

Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
PRACTICE RECOMMENDATIONS
› Prescribe eluxadoline, rifaximin, or alosetron for diarrhea-predominant IBS because all 3 have proven efficacy with this diagnosis. A
› Prescribe linaclotide or lubiprostone for constipation-predominant IBS, as both have proven efficacy with this condition. A
› Suggest that patients with IBS follow a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet; probiotics, prunes, and peppermint oil may also offer some improvement of IBS symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series