User login
Substances use during adolescence is common in the United States. Data from the 2014 Monitoring the Future Survey estimated that among 12th graders, 60.2% used alcohol, 35.1% used marijuana, and 13.9% used a prescription drug for nonmedical use within the previous year.1 An estimated 11.4% of adolescents meet DSM-IV threshold criteria for a substance use disorder (SUD).2 Substance use in adolescents often co-occurs with psychological distress and psychiatric illness. Adolescents with a psychiatric disorder are at increased risk for developing a SUD; conversely, high rates of psychiatric illness are seen in adolescents with a SUD.3,4 In one study, 82% of adolescents hospitalized for SUD treatment were found to have a co-occurring axis I disorder.5 Furthermore, co-occurring psychiatric illness and SUD complicates treatment course and prognosis. Adolescents with co-occurring psychiatric illness and SUD often benefit from an integrated, multimodal treatment approach that includes psychotherapy, pharmacologic interventions, family involvement, and collaboration with community supports.
In this article, we focus on pharmacologic management of non-nicotinic SUDs in adolescents, with an emphasis on those with comorbid psychiatric illness.
Screening and assessment of substance use
It is important to counsel children with a psychiatric illness and their parents about the increased risk for SUD before a patient transitions to adolescence. Discussions about substance abuse should begin during the 5th grade because data suggests that adolescent substance use often starts in middle school (6th to 9th grade). Clinicians should routinely screen adolescent patients for substance use. Nonproprietary screening tools available through the National Institute on Alcohol and Alcoholism and the National Institute on Drug Abuse are listed in Table 1.6-8 The Screening to Brief Intervention (S2BI) is a newer tool that has been shown to be highly effective in identifying adolescents at risk for substance abuse and differentiating severity of illness.8 The S2BI includes screening questions that assess for use of 8 substances in the past year.
Adolescents with psychiatric illness who are identified to be at risk for problems associated with substance use should be evaluated further for the presence or absence of a SUD. The number of criteria a patient endorses over the past year (Table 29) is used to assess SUD severity—mild, moderate, or severe. Additional considerations include substance use patterns such as type, site, quantity, frequency, context, and combinations of substances.
It is important to be curious and nonjudgmental when evaluating substance use patterns with adolescents to obtain a comprehensive assessment. Teenagers often are creative and inventive in their efforts to maximize intoxication, which can put them at risk for complications associated with acute intoxication. Rapidly evolving methods of ingesting highly concentrated forms of tetrahydrocannabinol (“wax,” “dabs”) are an example of use patterns that are ahead of what is reported in the literature.
Any substance use in an adolescent with a psychiatric illness is of concern and should be monitored closely because of the potential impact of substance use on the co-occurring psychiatric illness and possible interactions between the abused substance and prescribed medication.
Treatment interventions
Although this review will focus on pharmacotherapy, individual, group, and family psychotherapies are a critical part of a treatment plan for adolescents with comorbid psychiatric illness and SUD (Table 3). Collaboration with community supports, including school and legal officials, can help reinforce contingencies and assist with connecting a teen with positive prosocial activities. Involvement with mutual help organizations, such as Alcoholics Anonymous, can facilitate adolescent engagement with a positive sober network.10
Pharmacologic strategies for treating co-occurring psychiatric illness and SUD include medication to:
• decrease substance use and promote abstinence
• alleviate withdrawal symptoms (medication to treat withdrawal symptoms and agonist treatments)
• block the effect of substance use (antagonist agents)
• decrease likelihood of substance use with aversive agents
• target comorbid psychiatric illness.
Medication to decrease substance use and promote abstinence. One strategy is to target cravings and urges to use substances with medication. Naltrexone is an opiate antagonist FDA-approved for treating alcohol and opioid use disorders in adults and is available as a daily oral medication and a monthly injectable depot preparation (extended-release naltrexone). Two small open-label studies showed decreased alcohol use with naltrexone treatment in adolescents with alcohol use disorder.11,12 In a randomized double-blind placebo controlled (RCT) crossover study of 22 adolescent problem drinkers, naltrexone, 50 mg/d, reduced the likelihood of drinking and heavy drinking (P ≤ .03).13 Acamprosate, another anti-craving medication FDA-approved for treating alcohol use disorder in adults, has no data on the safety or efficacy for adolescent alcohol use disorder.
There is limited research on agents that decrease use and promote abstinence from non-nicotinic substances other than alcohol. There is one pilot RCT that evaluated N-acetylcysteine (NAC)—an over-the-counter supplement that modulates the glutamate system—for treating adolescent Cannabis dependence. Treatment with NAC, 2,400 mg/d, was well tolerated and had twice the odds of increasing negative urine cannabinoid tests during treatment than placebo.14 Although NAC treatment was associated with decreased Cannabis use, it did not significantly decrease cravings compared with placebo.15
Medication to alleviate withdrawal symptoms. Some patients may find the physical discomfort and psychological distress associated with substance withdrawal so intolerable that to avoid it they continue to use drugs or alcohol. Medication to treat withdrawal symptoms and agonist treatments can be used to alleviate discomfort and distress associated with withdrawal. Agonist treatments, such as methadone and buprenorphine, bind to the same receptors as the target substance, which allows the patient to shift to controlled use of a prescribed substitute. Agonist treatments are used for short detoxification and over longer periods of time for maintenance treatment. Methadone, which decreases craving and withdrawal symptoms from opiates by binding to the μ-opiate receptor and blocking other substances from binding, is frequently used for detoxification and maintenance treatment in adults. There is limited data on methadone substitution therapy for adolescents in the United States.16 Methadone maintenance for adolescents in the United States is restricted to severe cases of opioid use disorder. Federal guidelines specify that adolescents age <18 can only receive methadone if they have had 2 unsuccessful detoxification attempts or outpatient psychosocial treatments and have met DSM criteria for an opioid use disorder for 1 year.17
Buprenorphine is a partial μ-opiate receptor agonist that is FDA-approved for use in adolescents age ≥16 with opioid dependence. Although a waiver from the U.S. Drug Enforcement Administration is required to prescribe buprenorphine, it generally can be administered in outpatient settings with relative ease compared with methadone.
Marsch et al18 examined the efficacy of buprenorphine compared with clonidine for detoxification over 1 month in 36 adolescents with opioid dependence. Clonidine is an α-2 adrenergic agonist that often is used during detoxification from opioids.19 Although both buprenorphine and clonidine relieved withdrawal symptoms, a significantly higher percentage of patients receiving buprenorphine completed treatment (72%) compared with those taking clonidine (39%) (P < .05).18 Detoxification with buprenorphine also was associated with a higher percentage of negative urine drug screens (64% vs 32%, P = .01), and those receiving buprenorphine were more likely to continue on naltrexone maintenance for continued medication-assisted treatment after detoxification compared with those randomized to clonidine.
Woody et al20 compared use of buprenorphine/naloxone for opioid detoxification vs short-term maintenance. Patients age 16 to 21 were randomized to detoxification over 2 weeks vs stabilization and maintenance for 9 weeks and taper over 3 weeks. Maintenance treatment with buprenorphine/naloxone was associated with less opioid use, less injection drug use, and less need for addiction treatment outside of that received through the study compared with detoxification treatment. When buprenorphine/naloxone was discontinued both the detoxification and maintenance groups had high rates of positive urine toxicology screens at 1-year follow up (mean 48% to 72%). These data suggests maintenance with buprenorphine/ naloxone for adolescents and young adults is more effective than short-term detoxification for stabilizing opioid use disorders, although optimal treatment duration is unclear. Clinically, it is important to continue buprenorphine/naloxone maintenance until the patient has stabilized in recovery and has acquired coping skills to manage urges, cravings, and psychological distress (eg, anger, stress) that often arise during a slow taper of agonist treatment.
Antagonist treatment to block the effect of substance use
As an opioid receptor antagonist, naltrexone is effective for treating opioid use disorder because it blocks the action of opioids. Fishman et al21 published a descriptive series of 16 adolescents and young adults followed over 4 months who received the injectable depot preparation (extended-release) naltrexone while in residential treatment, and then discharged to outpatient care. Most patients who received extended-release naltrexone remained in outpatient treatment (63%) and reduced their opioid use or were abstinent at 4 months (56%). One barrier to naltrexone treatment is the need to be abstinent from opioids for 7 to 10 days to prevent precipitated opioid withdrawal. Therefore, naltrexone is a good option for adolescents who present for treatment early and are not physiologically dependent on opioids or are receiving treatment in a structured environment after detoxification, such as residential treatment or sober living.
Aversive agents to diminish substance use. Aversive agents produce an unpleasant reaction when a target substance is consumed. Disulfiram is prototypic aversive agent that prevents the breakdown of acetaldehyde, a toxic metabolite of alcohol. Patients who drink alcohol while taking disulfiram may experience adverse effects, including tachycardia, shortness of breath, nausea, dizziness, and confusion. There have been 2 studies examining the efficacy of disulfiram in adolescents with alcohol use disorder. Niederhofer et al22 found that disulfiram treatment significantly increased cumulative abstinence in a small RCT (P = .012). In another small randomized, open-label, 3-month study of adolescents who received disulfiram or naltrexone in addition to weekly psychotherapy, disulfiram was superior to naltrexone in mean days abstinent from alcohol, 84 days vs 51 days, respectively (P = .0001).23 Often adolescents are not willing to adhere to disulfiram because they are concerned about the aversive reaction when combined with alcohol use. Consider prescribing disulfiram for adolescents who are about to go “on pass” from a therapeutic school or residential SUD treatment center and will be returning to an environment where they may be tempted to use alcohol.
Pharmacotherapy to treat co-occurring psychiatric illness
Continued treatment of a psychiatric illness that co-occurs with SUD is important. As we recommended, consider psychosocial treatments for both the SUD and comorbid psychopathology. Several single-site RCTs have evaluated the efficacy of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline for depressive disorders in adolescents with a co-occurring SUD.24-28 Most studies have shown improvement in depressive symptoms and substance use in medication and placebo groups.24,25,27,28 However, treatment with fluoxetine, 20 mg/d, or sertraline, 100 mg/d, when compared with placebo was associated with improved depressive symptoms in 1 of 3 studies and had no significant difference in SUD outcome. The authors of these studies believe that the general improvement in depression and the SUD was related to use of cognitive-behavioral therapy (CBT) and/or motivational enhancement therapy.24,25,27,28
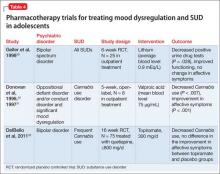
Research on the use of mood stabilizers for adolescents with mood dysregulation and a SUD is limited but has suggested benefit associated with pharmacotherapy (Table 4).29-32 Two RCTs and 1 open-label study demonstrated reductions in substance use with mood stabilizer treatment in adolescents with co-occurring SUD and mood dysregulation.29-32 The effect of pharmacotherapy on mood dysregulation ratings are less clear because there was no change in severity of affective symptoms observed in a small RCT of lithium (average blood level 0.9 mEq/L)29; and improvement in affective symptoms was noted in topiramate (300 mg/d) and placebo groups when both groups were treated with concurrent quetiapine.32 Because of the high risk of SUD and severe morbidity in juvenile bipolar disorder and severe mood dysregulation,33 larger RCTs are warranted.
Several studies have evaluated the impact of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder (ADHD) in adolescents with a co-occurring SUD.34-39 The largest and only multisite study evaluated the efficacy of osmotic (extended) release methylphenidate (OROS-MPH) vs placebo for adolescents who also were receiving CBT for SUD.36 In this 16-week RCT, the OROS-MPH and placebo groups showed improvement in self-reported ADHD symptoms with no difference between groups. Parent report of ADHD symptoms did indicate a greater reduction in symptoms in the OROS-MPH group compared with placebo. Both groups had a decrease in self-reported days of substance use over the past month with no differences between groups. Pharmacotherapy trials for ADHD that have included psychotherapy highlight the effectiveness of CBT for SUD and co-occurring psychiatric illness.36,39,40
Although conduct disorder and anxiety disorders commonly co-occur with SUD, there has been less research evaluating the impact of pharmacotherapy on treating these disorders. Riggs et al25,34,35,41 evaluated the impact of pharmacotherapy targeted to co-occurring ADHD and major depressive disorder in the context of conduct disorder and SUD. When evaluated in an outpatient setting, the presence of a treatment intervention to address the co-occurring SUD was an important component that led to a reduction in conduct symptoms.25,35 There have been no comprehensive studies on the impact of pharmacotherapy for treating anxiety and SUD in adolescents.
Recommendations for clinical management
Although more research is needed to evaluate the role of pharmacotherapy for adolescents with co-occurring psychiatric illness and a SUD, recommended practice is to continue pharmacotherapy and closely monitor response to treatment when at-risk substance use begins in patients with co-occurring psychiatric illness. In adolescents with a threshold SUD, continue pharmacotherapy for unstable mood disorders with first-line choices of SSRIs for unipolar depression and second-generation antipsychotics for bipolar spectrum illness. Suggested conservative pharmacological interventions for anxiety disorders include SSRIs and buspirone, which have been shown to be effective for treating anxiety in children and adolescents.42,43 For patients with comorbid ADHD and SUD, if possible, it is recommended to first stabilize substance use (low-level use or abstinence) and consider treating ADHD immediately thereafter with a nonstimulant such as atomoxetine, which has data on efficacy and safety in context to substance use; and/or an α-agonist or an extended-release stimulant. Because of the potential for misuse and toxicity associated with concurrent substance use, benzodiazepines should be considered a last treatment of choice for adolescents with anxiety disorders and a SUD. Similarly, the use of immediate-release stimulants should be avoided in patients with ADHD and a SUD. When prescribing medications that could be misused or toxic when combined with a substance, it is important to evaluate the risk and benefit of continued use of a particular medication and consider prescribing lower quantities to decrease risk for misuse (1- to 2-week supply). Adolescents often are reluctant to engage in SUD treatment and one strategy to consider is to make continued prescription of any medication contingent on engaging in SUD treatment. Enlist parents in helping to monitor, store, and administer their child’s medication to improve adherence and decrease the potential for misuse, diversion, and complications associated with substance intoxication.
Bottom Line
It is important to screen for substance use in adolescents with co-occurring
psychiatric illness and vice versa. When at-risk or hazardous substance use is
detected there are effective psychosocial and pharmacologic interventions that
can be used to treat adolescent substance use disorders alone and in combination
with certain psychiatric disorders.
Related Resources
• National Institute on Drug Abuse. www.drugabuse.gov.
• National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
• Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
Drug Brand Names
Acamprosate • Campral
Atomoxetine • Strattera
Buprenorphine• Subutex
Buprenorphine/naloxone • Suboxone
Buspirone • Buspar
Clonidine • Catapres
Disulfiram • Antabuse
Fluoxetine • Prozac
Lithium • Lithobid, Eskalith
Methadone • Dolophine
Naltrexone • ReVia, Vivitrol
Osmotic (extended) release methylphenidate • Concerta
Sertraline • Zoloft
Topiramate • Topamax
Quetiapine • Seroquel
Valproic acid • Depakote
Disclosures
Dr. Yule received grant support from the 2012 American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC, and receives grant support from the 2014 Louis V. Gerstner III Research Scholar Award. Dr. Wilens has received grant support from the National Institute on Drug Abuse (NIDA); has been a consultant for Euthymics/Neurovance, NIDA, Ironshore Pharmaceuticals and Development, Theravance Biopharma, Tris Pharma, the U.S. National Football League (ERM Associates), U.S. Minor/Major League Baseball, and Bay Cove Human Services (Clinical Services).
1. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future, Table 2: trends in annual prevalence of use of various drugs in grades 8, 10, and 12. http://www. monitoringthefuture.org/data/14data.html#2014data-drugs. Published December 16, 2014. Accessed January 6, 2015.
2. Merikangas KR, He JP, Burnstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-- Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
3. Kandel DB, Johnson JG, Bird HR, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25(2):122-132.
4. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(suppl 1):S4-S13.
5. Stowell R, Estroff TW. Psychiatric disorders in substance-abusing adolescent inpatients: a pilot study. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1036-1040.
6. National Institute of Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: a practitioner’s guide. http://www.niaaa.nih.gov/ Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. Accessed March 11, 2015.
7. Children’s Hospital Boston. The CRAFFT screening interview. http://www.integration.samhsa.gov/clinical-practice/sbirt/CRAFFT_Screening_interview.pdf. Published 2009. Accessed March 11, 2015.
8. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Kelly JF, Myers MG. Adolescents’ participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259-269.
11. Lifrak PD, Alterman AI, O’Brien CP, et al. Naltrexone for alcoholic adolescents. Am J Psychiatry. 1997;154(3):439-441.
12. Deas D, May MP, Randall C, et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723-728.
13. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941-954.
14. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
15. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
16. Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23(3):231-237.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. (SMA) 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
18. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62(10):1157-1164.
19. Gowing L, Farrell MF, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:CD002024.
20. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008; 300(17):2003-2011.
21. Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669-1676.
22. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295-297.
23. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. J Subst Abuse Treat. 2008;13(6):382-388.
24. Deas D, Randall CL, Roberts JS, et al. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461-469.
25. Riggs PD, Mikulich-Gilbertson SK, Davies RD, et al. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026-1034.
26. Findling RL, Pagano ME, McNamara NK, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial. Child Adolesc Psychiatry Ment Health. 2009;3(1):11.
27. Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39-45.
28. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
29. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
30. Donovan SJ, Susser ES, Nunes E. Divalproex sodium for use with conduct disordered adolescent marijuana users. Am J Addict. 1996;5(2):181.
31. Donovan SJ, Susser ES, Nunes EV, et al. Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry. 1997;58(1):12-15.
32. DelBello, M. Topiramate plus quetiapine cut Cannabis use in bipolar teens. Paper presented at: American Academy of Child and Adolescent Psychiatry’s Annual Meeting. November 2011; Toronto, Ontario, Canada.
33. Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008;95(3):188-198.
34. Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1271-1278.
35. Riggs PD, Hall SK, Mikulich-Gilbertson SK, et al. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(4):420-429.
36. Riggs PD, Winhusen T, Davies RD, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914.
37. Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate- SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41(3):250-257.
38. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005;15(5): 777-786.
39. Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573-582.
40. Zulauf CA, Sprich SE, Safren SA, et al. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436.
41. Riggs PD, Mikulich SK, Coffman LM, et al. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7(2):87-95.
42. Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171(7):741-748.
43. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012; 21(3):527-539.
Substances use during adolescence is common in the United States. Data from the 2014 Monitoring the Future Survey estimated that among 12th graders, 60.2% used alcohol, 35.1% used marijuana, and 13.9% used a prescription drug for nonmedical use within the previous year.1 An estimated 11.4% of adolescents meet DSM-IV threshold criteria for a substance use disorder (SUD).2 Substance use in adolescents often co-occurs with psychological distress and psychiatric illness. Adolescents with a psychiatric disorder are at increased risk for developing a SUD; conversely, high rates of psychiatric illness are seen in adolescents with a SUD.3,4 In one study, 82% of adolescents hospitalized for SUD treatment were found to have a co-occurring axis I disorder.5 Furthermore, co-occurring psychiatric illness and SUD complicates treatment course and prognosis. Adolescents with co-occurring psychiatric illness and SUD often benefit from an integrated, multimodal treatment approach that includes psychotherapy, pharmacologic interventions, family involvement, and collaboration with community supports.
In this article, we focus on pharmacologic management of non-nicotinic SUDs in adolescents, with an emphasis on those with comorbid psychiatric illness.
Screening and assessment of substance use
It is important to counsel children with a psychiatric illness and their parents about the increased risk for SUD before a patient transitions to adolescence. Discussions about substance abuse should begin during the 5th grade because data suggests that adolescent substance use often starts in middle school (6th to 9th grade). Clinicians should routinely screen adolescent patients for substance use. Nonproprietary screening tools available through the National Institute on Alcohol and Alcoholism and the National Institute on Drug Abuse are listed in Table 1.6-8 The Screening to Brief Intervention (S2BI) is a newer tool that has been shown to be highly effective in identifying adolescents at risk for substance abuse and differentiating severity of illness.8 The S2BI includes screening questions that assess for use of 8 substances in the past year.
Adolescents with psychiatric illness who are identified to be at risk for problems associated with substance use should be evaluated further for the presence or absence of a SUD. The number of criteria a patient endorses over the past year (Table 29) is used to assess SUD severity—mild, moderate, or severe. Additional considerations include substance use patterns such as type, site, quantity, frequency, context, and combinations of substances.
It is important to be curious and nonjudgmental when evaluating substance use patterns with adolescents to obtain a comprehensive assessment. Teenagers often are creative and inventive in their efforts to maximize intoxication, which can put them at risk for complications associated with acute intoxication. Rapidly evolving methods of ingesting highly concentrated forms of tetrahydrocannabinol (“wax,” “dabs”) are an example of use patterns that are ahead of what is reported in the literature.
Any substance use in an adolescent with a psychiatric illness is of concern and should be monitored closely because of the potential impact of substance use on the co-occurring psychiatric illness and possible interactions between the abused substance and prescribed medication.
Treatment interventions
Although this review will focus on pharmacotherapy, individual, group, and family psychotherapies are a critical part of a treatment plan for adolescents with comorbid psychiatric illness and SUD (Table 3). Collaboration with community supports, including school and legal officials, can help reinforce contingencies and assist with connecting a teen with positive prosocial activities. Involvement with mutual help organizations, such as Alcoholics Anonymous, can facilitate adolescent engagement with a positive sober network.10
Pharmacologic strategies for treating co-occurring psychiatric illness and SUD include medication to:
• decrease substance use and promote abstinence
• alleviate withdrawal symptoms (medication to treat withdrawal symptoms and agonist treatments)
• block the effect of substance use (antagonist agents)
• decrease likelihood of substance use with aversive agents
• target comorbid psychiatric illness.
Medication to decrease substance use and promote abstinence. One strategy is to target cravings and urges to use substances with medication. Naltrexone is an opiate antagonist FDA-approved for treating alcohol and opioid use disorders in adults and is available as a daily oral medication and a monthly injectable depot preparation (extended-release naltrexone). Two small open-label studies showed decreased alcohol use with naltrexone treatment in adolescents with alcohol use disorder.11,12 In a randomized double-blind placebo controlled (RCT) crossover study of 22 adolescent problem drinkers, naltrexone, 50 mg/d, reduced the likelihood of drinking and heavy drinking (P ≤ .03).13 Acamprosate, another anti-craving medication FDA-approved for treating alcohol use disorder in adults, has no data on the safety or efficacy for adolescent alcohol use disorder.
There is limited research on agents that decrease use and promote abstinence from non-nicotinic substances other than alcohol. There is one pilot RCT that evaluated N-acetylcysteine (NAC)—an over-the-counter supplement that modulates the glutamate system—for treating adolescent Cannabis dependence. Treatment with NAC, 2,400 mg/d, was well tolerated and had twice the odds of increasing negative urine cannabinoid tests during treatment than placebo.14 Although NAC treatment was associated with decreased Cannabis use, it did not significantly decrease cravings compared with placebo.15
Medication to alleviate withdrawal symptoms. Some patients may find the physical discomfort and psychological distress associated with substance withdrawal so intolerable that to avoid it they continue to use drugs or alcohol. Medication to treat withdrawal symptoms and agonist treatments can be used to alleviate discomfort and distress associated with withdrawal. Agonist treatments, such as methadone and buprenorphine, bind to the same receptors as the target substance, which allows the patient to shift to controlled use of a prescribed substitute. Agonist treatments are used for short detoxification and over longer periods of time for maintenance treatment. Methadone, which decreases craving and withdrawal symptoms from opiates by binding to the μ-opiate receptor and blocking other substances from binding, is frequently used for detoxification and maintenance treatment in adults. There is limited data on methadone substitution therapy for adolescents in the United States.16 Methadone maintenance for adolescents in the United States is restricted to severe cases of opioid use disorder. Federal guidelines specify that adolescents age <18 can only receive methadone if they have had 2 unsuccessful detoxification attempts or outpatient psychosocial treatments and have met DSM criteria for an opioid use disorder for 1 year.17
Buprenorphine is a partial μ-opiate receptor agonist that is FDA-approved for use in adolescents age ≥16 with opioid dependence. Although a waiver from the U.S. Drug Enforcement Administration is required to prescribe buprenorphine, it generally can be administered in outpatient settings with relative ease compared with methadone.
Marsch et al18 examined the efficacy of buprenorphine compared with clonidine for detoxification over 1 month in 36 adolescents with opioid dependence. Clonidine is an α-2 adrenergic agonist that often is used during detoxification from opioids.19 Although both buprenorphine and clonidine relieved withdrawal symptoms, a significantly higher percentage of patients receiving buprenorphine completed treatment (72%) compared with those taking clonidine (39%) (P < .05).18 Detoxification with buprenorphine also was associated with a higher percentage of negative urine drug screens (64% vs 32%, P = .01), and those receiving buprenorphine were more likely to continue on naltrexone maintenance for continued medication-assisted treatment after detoxification compared with those randomized to clonidine.
Woody et al20 compared use of buprenorphine/naloxone for opioid detoxification vs short-term maintenance. Patients age 16 to 21 were randomized to detoxification over 2 weeks vs stabilization and maintenance for 9 weeks and taper over 3 weeks. Maintenance treatment with buprenorphine/naloxone was associated with less opioid use, less injection drug use, and less need for addiction treatment outside of that received through the study compared with detoxification treatment. When buprenorphine/naloxone was discontinued both the detoxification and maintenance groups had high rates of positive urine toxicology screens at 1-year follow up (mean 48% to 72%). These data suggests maintenance with buprenorphine/ naloxone for adolescents and young adults is more effective than short-term detoxification for stabilizing opioid use disorders, although optimal treatment duration is unclear. Clinically, it is important to continue buprenorphine/naloxone maintenance until the patient has stabilized in recovery and has acquired coping skills to manage urges, cravings, and psychological distress (eg, anger, stress) that often arise during a slow taper of agonist treatment.
Antagonist treatment to block the effect of substance use
As an opioid receptor antagonist, naltrexone is effective for treating opioid use disorder because it blocks the action of opioids. Fishman et al21 published a descriptive series of 16 adolescents and young adults followed over 4 months who received the injectable depot preparation (extended-release) naltrexone while in residential treatment, and then discharged to outpatient care. Most patients who received extended-release naltrexone remained in outpatient treatment (63%) and reduced their opioid use or were abstinent at 4 months (56%). One barrier to naltrexone treatment is the need to be abstinent from opioids for 7 to 10 days to prevent precipitated opioid withdrawal. Therefore, naltrexone is a good option for adolescents who present for treatment early and are not physiologically dependent on opioids or are receiving treatment in a structured environment after detoxification, such as residential treatment or sober living.
Aversive agents to diminish substance use. Aversive agents produce an unpleasant reaction when a target substance is consumed. Disulfiram is prototypic aversive agent that prevents the breakdown of acetaldehyde, a toxic metabolite of alcohol. Patients who drink alcohol while taking disulfiram may experience adverse effects, including tachycardia, shortness of breath, nausea, dizziness, and confusion. There have been 2 studies examining the efficacy of disulfiram in adolescents with alcohol use disorder. Niederhofer et al22 found that disulfiram treatment significantly increased cumulative abstinence in a small RCT (P = .012). In another small randomized, open-label, 3-month study of adolescents who received disulfiram or naltrexone in addition to weekly psychotherapy, disulfiram was superior to naltrexone in mean days abstinent from alcohol, 84 days vs 51 days, respectively (P = .0001).23 Often adolescents are not willing to adhere to disulfiram because they are concerned about the aversive reaction when combined with alcohol use. Consider prescribing disulfiram for adolescents who are about to go “on pass” from a therapeutic school or residential SUD treatment center and will be returning to an environment where they may be tempted to use alcohol.
Pharmacotherapy to treat co-occurring psychiatric illness
Continued treatment of a psychiatric illness that co-occurs with SUD is important. As we recommended, consider psychosocial treatments for both the SUD and comorbid psychopathology. Several single-site RCTs have evaluated the efficacy of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline for depressive disorders in adolescents with a co-occurring SUD.24-28 Most studies have shown improvement in depressive symptoms and substance use in medication and placebo groups.24,25,27,28 However, treatment with fluoxetine, 20 mg/d, or sertraline, 100 mg/d, when compared with placebo was associated with improved depressive symptoms in 1 of 3 studies and had no significant difference in SUD outcome. The authors of these studies believe that the general improvement in depression and the SUD was related to use of cognitive-behavioral therapy (CBT) and/or motivational enhancement therapy.24,25,27,28
Research on the use of mood stabilizers for adolescents with mood dysregulation and a SUD is limited but has suggested benefit associated with pharmacotherapy (Table 4).29-32 Two RCTs and 1 open-label study demonstrated reductions in substance use with mood stabilizer treatment in adolescents with co-occurring SUD and mood dysregulation.29-32 The effect of pharmacotherapy on mood dysregulation ratings are less clear because there was no change in severity of affective symptoms observed in a small RCT of lithium (average blood level 0.9 mEq/L)29; and improvement in affective symptoms was noted in topiramate (300 mg/d) and placebo groups when both groups were treated with concurrent quetiapine.32 Because of the high risk of SUD and severe morbidity in juvenile bipolar disorder and severe mood dysregulation,33 larger RCTs are warranted.
Several studies have evaluated the impact of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder (ADHD) in adolescents with a co-occurring SUD.34-39 The largest and only multisite study evaluated the efficacy of osmotic (extended) release methylphenidate (OROS-MPH) vs placebo for adolescents who also were receiving CBT for SUD.36 In this 16-week RCT, the OROS-MPH and placebo groups showed improvement in self-reported ADHD symptoms with no difference between groups. Parent report of ADHD symptoms did indicate a greater reduction in symptoms in the OROS-MPH group compared with placebo. Both groups had a decrease in self-reported days of substance use over the past month with no differences between groups. Pharmacotherapy trials for ADHD that have included psychotherapy highlight the effectiveness of CBT for SUD and co-occurring psychiatric illness.36,39,40
Although conduct disorder and anxiety disorders commonly co-occur with SUD, there has been less research evaluating the impact of pharmacotherapy on treating these disorders. Riggs et al25,34,35,41 evaluated the impact of pharmacotherapy targeted to co-occurring ADHD and major depressive disorder in the context of conduct disorder and SUD. When evaluated in an outpatient setting, the presence of a treatment intervention to address the co-occurring SUD was an important component that led to a reduction in conduct symptoms.25,35 There have been no comprehensive studies on the impact of pharmacotherapy for treating anxiety and SUD in adolescents.
Recommendations for clinical management
Although more research is needed to evaluate the role of pharmacotherapy for adolescents with co-occurring psychiatric illness and a SUD, recommended practice is to continue pharmacotherapy and closely monitor response to treatment when at-risk substance use begins in patients with co-occurring psychiatric illness. In adolescents with a threshold SUD, continue pharmacotherapy for unstable mood disorders with first-line choices of SSRIs for unipolar depression and second-generation antipsychotics for bipolar spectrum illness. Suggested conservative pharmacological interventions for anxiety disorders include SSRIs and buspirone, which have been shown to be effective for treating anxiety in children and adolescents.42,43 For patients with comorbid ADHD and SUD, if possible, it is recommended to first stabilize substance use (low-level use or abstinence) and consider treating ADHD immediately thereafter with a nonstimulant such as atomoxetine, which has data on efficacy and safety in context to substance use; and/or an α-agonist or an extended-release stimulant. Because of the potential for misuse and toxicity associated with concurrent substance use, benzodiazepines should be considered a last treatment of choice for adolescents with anxiety disorders and a SUD. Similarly, the use of immediate-release stimulants should be avoided in patients with ADHD and a SUD. When prescribing medications that could be misused or toxic when combined with a substance, it is important to evaluate the risk and benefit of continued use of a particular medication and consider prescribing lower quantities to decrease risk for misuse (1- to 2-week supply). Adolescents often are reluctant to engage in SUD treatment and one strategy to consider is to make continued prescription of any medication contingent on engaging in SUD treatment. Enlist parents in helping to monitor, store, and administer their child’s medication to improve adherence and decrease the potential for misuse, diversion, and complications associated with substance intoxication.
Bottom Line
It is important to screen for substance use in adolescents with co-occurring
psychiatric illness and vice versa. When at-risk or hazardous substance use is
detected there are effective psychosocial and pharmacologic interventions that
can be used to treat adolescent substance use disorders alone and in combination
with certain psychiatric disorders.
Related Resources
• National Institute on Drug Abuse. www.drugabuse.gov.
• National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
• Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
Drug Brand Names
Acamprosate • Campral
Atomoxetine • Strattera
Buprenorphine• Subutex
Buprenorphine/naloxone • Suboxone
Buspirone • Buspar
Clonidine • Catapres
Disulfiram • Antabuse
Fluoxetine • Prozac
Lithium • Lithobid, Eskalith
Methadone • Dolophine
Naltrexone • ReVia, Vivitrol
Osmotic (extended) release methylphenidate • Concerta
Sertraline • Zoloft
Topiramate • Topamax
Quetiapine • Seroquel
Valproic acid • Depakote
Disclosures
Dr. Yule received grant support from the 2012 American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC, and receives grant support from the 2014 Louis V. Gerstner III Research Scholar Award. Dr. Wilens has received grant support from the National Institute on Drug Abuse (NIDA); has been a consultant for Euthymics/Neurovance, NIDA, Ironshore Pharmaceuticals and Development, Theravance Biopharma, Tris Pharma, the U.S. National Football League (ERM Associates), U.S. Minor/Major League Baseball, and Bay Cove Human Services (Clinical Services).
Substances use during adolescence is common in the United States. Data from the 2014 Monitoring the Future Survey estimated that among 12th graders, 60.2% used alcohol, 35.1% used marijuana, and 13.9% used a prescription drug for nonmedical use within the previous year.1 An estimated 11.4% of adolescents meet DSM-IV threshold criteria for a substance use disorder (SUD).2 Substance use in adolescents often co-occurs with psychological distress and psychiatric illness. Adolescents with a psychiatric disorder are at increased risk for developing a SUD; conversely, high rates of psychiatric illness are seen in adolescents with a SUD.3,4 In one study, 82% of adolescents hospitalized for SUD treatment were found to have a co-occurring axis I disorder.5 Furthermore, co-occurring psychiatric illness and SUD complicates treatment course and prognosis. Adolescents with co-occurring psychiatric illness and SUD often benefit from an integrated, multimodal treatment approach that includes psychotherapy, pharmacologic interventions, family involvement, and collaboration with community supports.
In this article, we focus on pharmacologic management of non-nicotinic SUDs in adolescents, with an emphasis on those with comorbid psychiatric illness.
Screening and assessment of substance use
It is important to counsel children with a psychiatric illness and their parents about the increased risk for SUD before a patient transitions to adolescence. Discussions about substance abuse should begin during the 5th grade because data suggests that adolescent substance use often starts in middle school (6th to 9th grade). Clinicians should routinely screen adolescent patients for substance use. Nonproprietary screening tools available through the National Institute on Alcohol and Alcoholism and the National Institute on Drug Abuse are listed in Table 1.6-8 The Screening to Brief Intervention (S2BI) is a newer tool that has been shown to be highly effective in identifying adolescents at risk for substance abuse and differentiating severity of illness.8 The S2BI includes screening questions that assess for use of 8 substances in the past year.
Adolescents with psychiatric illness who are identified to be at risk for problems associated with substance use should be evaluated further for the presence or absence of a SUD. The number of criteria a patient endorses over the past year (Table 29) is used to assess SUD severity—mild, moderate, or severe. Additional considerations include substance use patterns such as type, site, quantity, frequency, context, and combinations of substances.
It is important to be curious and nonjudgmental when evaluating substance use patterns with adolescents to obtain a comprehensive assessment. Teenagers often are creative and inventive in their efforts to maximize intoxication, which can put them at risk for complications associated with acute intoxication. Rapidly evolving methods of ingesting highly concentrated forms of tetrahydrocannabinol (“wax,” “dabs”) are an example of use patterns that are ahead of what is reported in the literature.
Any substance use in an adolescent with a psychiatric illness is of concern and should be monitored closely because of the potential impact of substance use on the co-occurring psychiatric illness and possible interactions between the abused substance and prescribed medication.
Treatment interventions
Although this review will focus on pharmacotherapy, individual, group, and family psychotherapies are a critical part of a treatment plan for adolescents with comorbid psychiatric illness and SUD (Table 3). Collaboration with community supports, including school and legal officials, can help reinforce contingencies and assist with connecting a teen with positive prosocial activities. Involvement with mutual help organizations, such as Alcoholics Anonymous, can facilitate adolescent engagement with a positive sober network.10
Pharmacologic strategies for treating co-occurring psychiatric illness and SUD include medication to:
• decrease substance use and promote abstinence
• alleviate withdrawal symptoms (medication to treat withdrawal symptoms and agonist treatments)
• block the effect of substance use (antagonist agents)
• decrease likelihood of substance use with aversive agents
• target comorbid psychiatric illness.
Medication to decrease substance use and promote abstinence. One strategy is to target cravings and urges to use substances with medication. Naltrexone is an opiate antagonist FDA-approved for treating alcohol and opioid use disorders in adults and is available as a daily oral medication and a monthly injectable depot preparation (extended-release naltrexone). Two small open-label studies showed decreased alcohol use with naltrexone treatment in adolescents with alcohol use disorder.11,12 In a randomized double-blind placebo controlled (RCT) crossover study of 22 adolescent problem drinkers, naltrexone, 50 mg/d, reduced the likelihood of drinking and heavy drinking (P ≤ .03).13 Acamprosate, another anti-craving medication FDA-approved for treating alcohol use disorder in adults, has no data on the safety or efficacy for adolescent alcohol use disorder.
There is limited research on agents that decrease use and promote abstinence from non-nicotinic substances other than alcohol. There is one pilot RCT that evaluated N-acetylcysteine (NAC)—an over-the-counter supplement that modulates the glutamate system—for treating adolescent Cannabis dependence. Treatment with NAC, 2,400 mg/d, was well tolerated and had twice the odds of increasing negative urine cannabinoid tests during treatment than placebo.14 Although NAC treatment was associated with decreased Cannabis use, it did not significantly decrease cravings compared with placebo.15
Medication to alleviate withdrawal symptoms. Some patients may find the physical discomfort and psychological distress associated with substance withdrawal so intolerable that to avoid it they continue to use drugs or alcohol. Medication to treat withdrawal symptoms and agonist treatments can be used to alleviate discomfort and distress associated with withdrawal. Agonist treatments, such as methadone and buprenorphine, bind to the same receptors as the target substance, which allows the patient to shift to controlled use of a prescribed substitute. Agonist treatments are used for short detoxification and over longer periods of time for maintenance treatment. Methadone, which decreases craving and withdrawal symptoms from opiates by binding to the μ-opiate receptor and blocking other substances from binding, is frequently used for detoxification and maintenance treatment in adults. There is limited data on methadone substitution therapy for adolescents in the United States.16 Methadone maintenance for adolescents in the United States is restricted to severe cases of opioid use disorder. Federal guidelines specify that adolescents age <18 can only receive methadone if they have had 2 unsuccessful detoxification attempts or outpatient psychosocial treatments and have met DSM criteria for an opioid use disorder for 1 year.17
Buprenorphine is a partial μ-opiate receptor agonist that is FDA-approved for use in adolescents age ≥16 with opioid dependence. Although a waiver from the U.S. Drug Enforcement Administration is required to prescribe buprenorphine, it generally can be administered in outpatient settings with relative ease compared with methadone.
Marsch et al18 examined the efficacy of buprenorphine compared with clonidine for detoxification over 1 month in 36 adolescents with opioid dependence. Clonidine is an α-2 adrenergic agonist that often is used during detoxification from opioids.19 Although both buprenorphine and clonidine relieved withdrawal symptoms, a significantly higher percentage of patients receiving buprenorphine completed treatment (72%) compared with those taking clonidine (39%) (P < .05).18 Detoxification with buprenorphine also was associated with a higher percentage of negative urine drug screens (64% vs 32%, P = .01), and those receiving buprenorphine were more likely to continue on naltrexone maintenance for continued medication-assisted treatment after detoxification compared with those randomized to clonidine.
Woody et al20 compared use of buprenorphine/naloxone for opioid detoxification vs short-term maintenance. Patients age 16 to 21 were randomized to detoxification over 2 weeks vs stabilization and maintenance for 9 weeks and taper over 3 weeks. Maintenance treatment with buprenorphine/naloxone was associated with less opioid use, less injection drug use, and less need for addiction treatment outside of that received through the study compared with detoxification treatment. When buprenorphine/naloxone was discontinued both the detoxification and maintenance groups had high rates of positive urine toxicology screens at 1-year follow up (mean 48% to 72%). These data suggests maintenance with buprenorphine/ naloxone for adolescents and young adults is more effective than short-term detoxification for stabilizing opioid use disorders, although optimal treatment duration is unclear. Clinically, it is important to continue buprenorphine/naloxone maintenance until the patient has stabilized in recovery and has acquired coping skills to manage urges, cravings, and psychological distress (eg, anger, stress) that often arise during a slow taper of agonist treatment.
Antagonist treatment to block the effect of substance use
As an opioid receptor antagonist, naltrexone is effective for treating opioid use disorder because it blocks the action of opioids. Fishman et al21 published a descriptive series of 16 adolescents and young adults followed over 4 months who received the injectable depot preparation (extended-release) naltrexone while in residential treatment, and then discharged to outpatient care. Most patients who received extended-release naltrexone remained in outpatient treatment (63%) and reduced their opioid use or were abstinent at 4 months (56%). One barrier to naltrexone treatment is the need to be abstinent from opioids for 7 to 10 days to prevent precipitated opioid withdrawal. Therefore, naltrexone is a good option for adolescents who present for treatment early and are not physiologically dependent on opioids or are receiving treatment in a structured environment after detoxification, such as residential treatment or sober living.
Aversive agents to diminish substance use. Aversive agents produce an unpleasant reaction when a target substance is consumed. Disulfiram is prototypic aversive agent that prevents the breakdown of acetaldehyde, a toxic metabolite of alcohol. Patients who drink alcohol while taking disulfiram may experience adverse effects, including tachycardia, shortness of breath, nausea, dizziness, and confusion. There have been 2 studies examining the efficacy of disulfiram in adolescents with alcohol use disorder. Niederhofer et al22 found that disulfiram treatment significantly increased cumulative abstinence in a small RCT (P = .012). In another small randomized, open-label, 3-month study of adolescents who received disulfiram or naltrexone in addition to weekly psychotherapy, disulfiram was superior to naltrexone in mean days abstinent from alcohol, 84 days vs 51 days, respectively (P = .0001).23 Often adolescents are not willing to adhere to disulfiram because they are concerned about the aversive reaction when combined with alcohol use. Consider prescribing disulfiram for adolescents who are about to go “on pass” from a therapeutic school or residential SUD treatment center and will be returning to an environment where they may be tempted to use alcohol.
Pharmacotherapy to treat co-occurring psychiatric illness
Continued treatment of a psychiatric illness that co-occurs with SUD is important. As we recommended, consider psychosocial treatments for both the SUD and comorbid psychopathology. Several single-site RCTs have evaluated the efficacy of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline for depressive disorders in adolescents with a co-occurring SUD.24-28 Most studies have shown improvement in depressive symptoms and substance use in medication and placebo groups.24,25,27,28 However, treatment with fluoxetine, 20 mg/d, or sertraline, 100 mg/d, when compared with placebo was associated with improved depressive symptoms in 1 of 3 studies and had no significant difference in SUD outcome. The authors of these studies believe that the general improvement in depression and the SUD was related to use of cognitive-behavioral therapy (CBT) and/or motivational enhancement therapy.24,25,27,28
Research on the use of mood stabilizers for adolescents with mood dysregulation and a SUD is limited but has suggested benefit associated with pharmacotherapy (Table 4).29-32 Two RCTs and 1 open-label study demonstrated reductions in substance use with mood stabilizer treatment in adolescents with co-occurring SUD and mood dysregulation.29-32 The effect of pharmacotherapy on mood dysregulation ratings are less clear because there was no change in severity of affective symptoms observed in a small RCT of lithium (average blood level 0.9 mEq/L)29; and improvement in affective symptoms was noted in topiramate (300 mg/d) and placebo groups when both groups were treated with concurrent quetiapine.32 Because of the high risk of SUD and severe morbidity in juvenile bipolar disorder and severe mood dysregulation,33 larger RCTs are warranted.
Several studies have evaluated the impact of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder (ADHD) in adolescents with a co-occurring SUD.34-39 The largest and only multisite study evaluated the efficacy of osmotic (extended) release methylphenidate (OROS-MPH) vs placebo for adolescents who also were receiving CBT for SUD.36 In this 16-week RCT, the OROS-MPH and placebo groups showed improvement in self-reported ADHD symptoms with no difference between groups. Parent report of ADHD symptoms did indicate a greater reduction in symptoms in the OROS-MPH group compared with placebo. Both groups had a decrease in self-reported days of substance use over the past month with no differences between groups. Pharmacotherapy trials for ADHD that have included psychotherapy highlight the effectiveness of CBT for SUD and co-occurring psychiatric illness.36,39,40
Although conduct disorder and anxiety disorders commonly co-occur with SUD, there has been less research evaluating the impact of pharmacotherapy on treating these disorders. Riggs et al25,34,35,41 evaluated the impact of pharmacotherapy targeted to co-occurring ADHD and major depressive disorder in the context of conduct disorder and SUD. When evaluated in an outpatient setting, the presence of a treatment intervention to address the co-occurring SUD was an important component that led to a reduction in conduct symptoms.25,35 There have been no comprehensive studies on the impact of pharmacotherapy for treating anxiety and SUD in adolescents.
Recommendations for clinical management
Although more research is needed to evaluate the role of pharmacotherapy for adolescents with co-occurring psychiatric illness and a SUD, recommended practice is to continue pharmacotherapy and closely monitor response to treatment when at-risk substance use begins in patients with co-occurring psychiatric illness. In adolescents with a threshold SUD, continue pharmacotherapy for unstable mood disorders with first-line choices of SSRIs for unipolar depression and second-generation antipsychotics for bipolar spectrum illness. Suggested conservative pharmacological interventions for anxiety disorders include SSRIs and buspirone, which have been shown to be effective for treating anxiety in children and adolescents.42,43 For patients with comorbid ADHD and SUD, if possible, it is recommended to first stabilize substance use (low-level use or abstinence) and consider treating ADHD immediately thereafter with a nonstimulant such as atomoxetine, which has data on efficacy and safety in context to substance use; and/or an α-agonist or an extended-release stimulant. Because of the potential for misuse and toxicity associated with concurrent substance use, benzodiazepines should be considered a last treatment of choice for adolescents with anxiety disorders and a SUD. Similarly, the use of immediate-release stimulants should be avoided in patients with ADHD and a SUD. When prescribing medications that could be misused or toxic when combined with a substance, it is important to evaluate the risk and benefit of continued use of a particular medication and consider prescribing lower quantities to decrease risk for misuse (1- to 2-week supply). Adolescents often are reluctant to engage in SUD treatment and one strategy to consider is to make continued prescription of any medication contingent on engaging in SUD treatment. Enlist parents in helping to monitor, store, and administer their child’s medication to improve adherence and decrease the potential for misuse, diversion, and complications associated with substance intoxication.
Bottom Line
It is important to screen for substance use in adolescents with co-occurring
psychiatric illness and vice versa. When at-risk or hazardous substance use is
detected there are effective psychosocial and pharmacologic interventions that
can be used to treat adolescent substance use disorders alone and in combination
with certain psychiatric disorders.
Related Resources
• National Institute on Drug Abuse. www.drugabuse.gov.
• National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
• Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
Drug Brand Names
Acamprosate • Campral
Atomoxetine • Strattera
Buprenorphine• Subutex
Buprenorphine/naloxone • Suboxone
Buspirone • Buspar
Clonidine • Catapres
Disulfiram • Antabuse
Fluoxetine • Prozac
Lithium • Lithobid, Eskalith
Methadone • Dolophine
Naltrexone • ReVia, Vivitrol
Osmotic (extended) release methylphenidate • Concerta
Sertraline • Zoloft
Topiramate • Topamax
Quetiapine • Seroquel
Valproic acid • Depakote
Disclosures
Dr. Yule received grant support from the 2012 American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC, and receives grant support from the 2014 Louis V. Gerstner III Research Scholar Award. Dr. Wilens has received grant support from the National Institute on Drug Abuse (NIDA); has been a consultant for Euthymics/Neurovance, NIDA, Ironshore Pharmaceuticals and Development, Theravance Biopharma, Tris Pharma, the U.S. National Football League (ERM Associates), U.S. Minor/Major League Baseball, and Bay Cove Human Services (Clinical Services).
1. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future, Table 2: trends in annual prevalence of use of various drugs in grades 8, 10, and 12. http://www. monitoringthefuture.org/data/14data.html#2014data-drugs. Published December 16, 2014. Accessed January 6, 2015.
2. Merikangas KR, He JP, Burnstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-- Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
3. Kandel DB, Johnson JG, Bird HR, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25(2):122-132.
4. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(suppl 1):S4-S13.
5. Stowell R, Estroff TW. Psychiatric disorders in substance-abusing adolescent inpatients: a pilot study. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1036-1040.
6. National Institute of Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: a practitioner’s guide. http://www.niaaa.nih.gov/ Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. Accessed March 11, 2015.
7. Children’s Hospital Boston. The CRAFFT screening interview. http://www.integration.samhsa.gov/clinical-practice/sbirt/CRAFFT_Screening_interview.pdf. Published 2009. Accessed March 11, 2015.
8. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Kelly JF, Myers MG. Adolescents’ participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259-269.
11. Lifrak PD, Alterman AI, O’Brien CP, et al. Naltrexone for alcoholic adolescents. Am J Psychiatry. 1997;154(3):439-441.
12. Deas D, May MP, Randall C, et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723-728.
13. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941-954.
14. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
15. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
16. Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23(3):231-237.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. (SMA) 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
18. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62(10):1157-1164.
19. Gowing L, Farrell MF, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:CD002024.
20. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008; 300(17):2003-2011.
21. Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669-1676.
22. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295-297.
23. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. J Subst Abuse Treat. 2008;13(6):382-388.
24. Deas D, Randall CL, Roberts JS, et al. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461-469.
25. Riggs PD, Mikulich-Gilbertson SK, Davies RD, et al. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026-1034.
26. Findling RL, Pagano ME, McNamara NK, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial. Child Adolesc Psychiatry Ment Health. 2009;3(1):11.
27. Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39-45.
28. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
29. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
30. Donovan SJ, Susser ES, Nunes E. Divalproex sodium for use with conduct disordered adolescent marijuana users. Am J Addict. 1996;5(2):181.
31. Donovan SJ, Susser ES, Nunes EV, et al. Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry. 1997;58(1):12-15.
32. DelBello, M. Topiramate plus quetiapine cut Cannabis use in bipolar teens. Paper presented at: American Academy of Child and Adolescent Psychiatry’s Annual Meeting. November 2011; Toronto, Ontario, Canada.
33. Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008;95(3):188-198.
34. Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1271-1278.
35. Riggs PD, Hall SK, Mikulich-Gilbertson SK, et al. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(4):420-429.
36. Riggs PD, Winhusen T, Davies RD, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914.
37. Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate- SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41(3):250-257.
38. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005;15(5): 777-786.
39. Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573-582.
40. Zulauf CA, Sprich SE, Safren SA, et al. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436.
41. Riggs PD, Mikulich SK, Coffman LM, et al. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7(2):87-95.
42. Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171(7):741-748.
43. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012; 21(3):527-539.
1. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future, Table 2: trends in annual prevalence of use of various drugs in grades 8, 10, and 12. http://www. monitoringthefuture.org/data/14data.html#2014data-drugs. Published December 16, 2014. Accessed January 6, 2015.
2. Merikangas KR, He JP, Burnstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-- Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
3. Kandel DB, Johnson JG, Bird HR, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25(2):122-132.
4. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(suppl 1):S4-S13.
5. Stowell R, Estroff TW. Psychiatric disorders in substance-abusing adolescent inpatients: a pilot study. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1036-1040.
6. National Institute of Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: a practitioner’s guide. http://www.niaaa.nih.gov/ Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. Accessed March 11, 2015.
7. Children’s Hospital Boston. The CRAFFT screening interview. http://www.integration.samhsa.gov/clinical-practice/sbirt/CRAFFT_Screening_interview.pdf. Published 2009. Accessed March 11, 2015.
8. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Kelly JF, Myers MG. Adolescents’ participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259-269.
11. Lifrak PD, Alterman AI, O’Brien CP, et al. Naltrexone for alcoholic adolescents. Am J Psychiatry. 1997;154(3):439-441.
12. Deas D, May MP, Randall C, et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723-728.
13. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941-954.
14. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
15. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
16. Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23(3):231-237.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. (SMA) 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
18. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62(10):1157-1164.
19. Gowing L, Farrell MF, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:CD002024.
20. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008; 300(17):2003-2011.
21. Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669-1676.
22. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295-297.
23. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. J Subst Abuse Treat. 2008;13(6):382-388.
24. Deas D, Randall CL, Roberts JS, et al. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461-469.
25. Riggs PD, Mikulich-Gilbertson SK, Davies RD, et al. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026-1034.
26. Findling RL, Pagano ME, McNamara NK, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial. Child Adolesc Psychiatry Ment Health. 2009;3(1):11.
27. Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39-45.
28. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
29. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
30. Donovan SJ, Susser ES, Nunes E. Divalproex sodium for use with conduct disordered adolescent marijuana users. Am J Addict. 1996;5(2):181.
31. Donovan SJ, Susser ES, Nunes EV, et al. Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry. 1997;58(1):12-15.
32. DelBello, M. Topiramate plus quetiapine cut Cannabis use in bipolar teens. Paper presented at: American Academy of Child and Adolescent Psychiatry’s Annual Meeting. November 2011; Toronto, Ontario, Canada.
33. Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008;95(3):188-198.
34. Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1271-1278.
35. Riggs PD, Hall SK, Mikulich-Gilbertson SK, et al. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(4):420-429.
36. Riggs PD, Winhusen T, Davies RD, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914.
37. Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate- SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41(3):250-257.
38. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005;15(5): 777-786.
39. Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573-582.
40. Zulauf CA, Sprich SE, Safren SA, et al. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436.
41. Riggs PD, Mikulich SK, Coffman LM, et al. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7(2):87-95.
42. Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171(7):741-748.
43. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012; 21(3):527-539.