User login
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Paranoid and scared
Police bring Mr. C, age 42, to a local crisis center after he is found masturbating in public the same day he was released from jail after serving time for the same behavior. Previously, Mr. C was diagnosed with schizophrenia, paranoid type, and alcohol dependence. He is single, unemployed, and lives with his parents. He has had 3 previous admissions to a psychiatric hospital, but no preexisting medical illness. A judge involuntarily commits Mr. C to our psychiatric facility.
Mr. C looks older than his age and has poor hygiene. He appears bizarre, makes poor eye contact, and speaks slowly but with normal volume. His speech is not coherent, relevant, or goal-directed. He is not able to answer questions properly, chanting “it’s eternity, eternity, eternity.” He shows no tremors, repetitive motor behavior, or muscle rigidity. His affect is flat and he has no suicidal or homicidal ideations. Based on Mr. C’s history, we diagnose him with schizophrenia, paranoid type and alcohol dependence.
Over the next 9 days, Mr. C receives trials of haloperidol, lorazepam, diphenhydramine, ziprasidone, olanzapine, hydroxyzine, trazodone, and benztropine to treat his schizophrenia. From days 1 to 3, all medications are given on an as-needed basis. On day 1, Mr. C receives haloperidol, 20 mg, lorazepam, 9 mg, diphenhydramine, 150 mg, and ziprasidone, 20 mg. On day 2, he receives haloperidol, 15 mg, lorazepam, 10 mg, olanzapine, 20 mg, hydroxyzine, 100 mg, and trazodone, 50 mg. On day 3, he receives haloperidol, 20 mg, lorazepam, 6 mg, and trazodone, 100 mg. On days 4 to 8, in addition to scheduled haloperidol, 30 mg/d, benztropine, 1 mg/d, and trazodone, 100 mg/d, he receives haloperidol, 5 mg, and lorazepam, 2 mg, as needed. On day 9, he receives the scheduled haloperidol, 30 mg/d, benztropine, 1 mg/d, and trazodone, 100 mg/d.
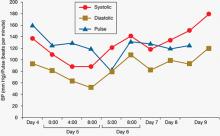
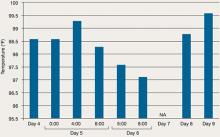
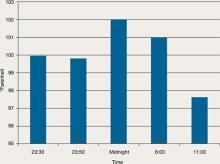
During his stay, Mr. C is incoherent and disorganized. On day 9, he eats all of his lunch, none of his dinner, but sips milk and juice and eats snacks. He drinks 2 small cups of water with medication and 2 small cups of water during oral care. His mucosa and tongue are dry. At 11:30 pm, while lying in bed mumbling “scared, scared,” he experiences shortness of breath. His temperature is 99.6°F, blood pressure is 151/93 mm Hg, pulse is 125 beats per minute, respiratory rate is 40 breaths per minute, and oxygen saturation is 91% on ambient air. Twenty minutes later, his blood pressure increases to 180/120 mm Hg. On physical examination, he has “lead pipe” rigidity of both arms. He is awake, confused, and not able to communicate, still mumbling “scared, scared.” Changes in his blood pressure, pulse, and temperature during his stay in the psychiatric hospital are depicted in Figures 1 and 2, respectively.
Figure 1: Mr. C’s blood pressure and pulse changes from day 4 to day 9 in the psychiatric hospital
BP: blood pressure
Figure 2: Mr. C’s temperature changes from day 4 to day 9 in the psychiatric hospital
The authors’ observations
NMS is a life-threatening, iatrogenic neurologic emergency associated with antipsychotic use. Early incidence rate estimates ran as high as 3% of patients treated with antipsychotics; however, more recent data suggest an incidence of 0.01% to 0.02%.1 This decrease in frequency likely reflects increased awareness of the disorder, more conservative prescribing patterns, and a shift to using atypical antipsychotics.2 In the mid 1980s and early 1990s the mortality rate was 25% to 30% if NMS was not promptly recognized and treated3; however, progression to more fulminant, lethal NMS episodes now occurs less often and the mortality rate ranges from 10% to 20%.4
If NMS is suspected, immediate transfer to an emergency department (ED) is necessary. Even with early diagnosis, however, complications of NMS are still likely, including:
- rhabdomyolysis
- renal failure
- seizures
- respiratory failure
- aspiration pneumonia
- disseminated intravascular coagulation
- venous thromboembolism.5-9
Caroff et al reported observing a residual catatonic state after acute NMS symptoms subsided.10
Although the pathophysiology of NMS is complex—involving a cascade of dysregulation in multiple neurochemical and neuroendocrine systems—dopamine blockade likely plays a pivotal role in triggering the condition.2 In addition, evidence supports the hypothesis that dysregulated sympathetic nervous system hyperactivity is responsible for most NMS features.11
TREATMENT: Arrival in the ED
Based on his elevated blood pressure (151/93 mm Hg), “lead pipe” rigidity, and increased body temperature associated with Mr. C’s history of haloperidol use for 9 days, the treatment team suspects NMS. Labile blood pressure, which changed from 151/93 to 180/120 mm Hg in 20 minutes, reinforces the NMS diagnosis. Approximately 30 minutes after Mr. C shows signs of NMS, he is transferred to a local ED. He is awake, alert, and communicative after he arrives in the ED, but becomes confused and noncommunicative the next morning. When he arrives in the ED, he is found to have tachycardia (114 beats per minute), tachypnea (26 breaths per minute), blood pressure of 132/84 mm Hg, and temperature of 102°F. In the ED, he is given IV normal saline, diphenhydramine, 25 mg, and IV lorazepam, 1 mg. His rigidity slightly improves.
Early the next morning, his blood pressure is 182/89 mm Hg, respirations are 30 to 40 breaths per minute, and heart rate is 120 beats per minute. He then receives IV lorazepam, 2 mg, after which his tachypnea, tachycardia, and elevated blood pressure improve.
The authors’ observations
A case-control study by Keck et al12 comparing 18 patients with NMS and 36 matched neuroleptic-treated patients with no history of the syndrome identified greater psychomotor agitation, significantly higher doses of neuroleptics, greater rates of dosage increase, and a greater number of IM injections as potential risk factors. Other potential risk factors include use of restraints, pre-existing CNS dopamine activity or receptor function abnormalities, and iron deficiency.2 Agitation, dehydration, and exhaustion were found to be the most consistent systemic factors predisposing patients taking antipsychotics to NMS in small case-control studies.13,14 Well-supported risk factors also include use of high-potency antipsychotics, prior episodes of NMS, age <40, male sex, malnutrition, organic brain syndromes, and lithium use.3,5,15
There is no way to predict the risk of NMS for an individual patient. Usually, symptoms develop within 4 weeks of starting an antipsychotic, but can occur after taking the same dose for many months. The onset may be within hours, but on average it is 4 to 14 days after initiating therapy. Among patients who develop NMS, 90% do so within 10 days.3,5
Mr. C’s risk factors include high-potency antipsychotic use, male sex, relatively high dose (haloperidol, 30 to 35 mg/d), agitation, dehydration, and exhaustion.
Managing NMS
The standard approaches for managing patients with NMS include discontinuing suspected triggering drugs and providing supportive care. Beyond supportive care, oral or IV benzodiazepines may relieve symptoms and speed recovery.2 Dopaminergic drugs, such as bromocriptine or amantadine, used alone or with other treatments, can reduce parkinsonism and disease duration and mortality.2 Dantrolene may be useful only for NMS patients who exhibit extreme temperature elevations, rigidity, and true hypermetabolism.16 Electroconvulsive therapy may be effective for NMS patients whose symptoms do not respond to supportive care and drug therapy or those with residual catatonic or parkinsonian symptoms.2
OUTCOME: Improvement, discharge
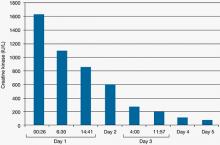
Mr. C is admitted to the hospital with the diagnosis of NMS and transferred to the intensive care unit (ICU) for treatment. After Mr. C is admitted to the ICU, apart from continuing the medication given in the ED, he also receives dantrolene, 2 mg/kg, then 1 mg/kg, 4 times a day, as well as IV lorazepam, 1 mg every 6 hours. His other medications include IV pantoprazole, 40 mg/d, for prophylaxis of stress ulcer. Diphenhydramine administration is changed to as needed. On the second day in the ICU, he has only mild upper extremity rigidity but no lower extremity rigidity. However, he suffers 1 seizure, which is treated with IV fosphenytoin at the loading dose, 18 mg/kg, then a maintaining dose of 5 mg phenytoin equivalent/kg/d.
Figure 3: Mr. C’s creatine kinase level (IU/L) during the first 5 days in the intensive care unit
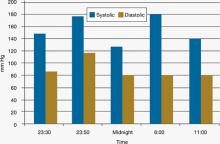
Figure 4: Mr. C’s blood pressure before and after admission
Figure 5: Mr. C’s temperature before and after admissionMr. C remains in the ICU for 7 days. There he receives valproic acid, titrated to 500 mg in the morning and 1,000 mg at bedtime, for agitation. He also receives olanzapine, 5 mg/d, for psychotic symptoms. He develops deep vein thrombosis in the right cephalic vein, which is treated with subcutaneous enoxaparin, 1 mg/kg, and warfarin, 5 mg/d.
He is discharged from the hospital after 2 weeks and returns to the psychiatric facility. He continues to be treated for paranoid schizophrenia with olanzapine, 5 mg/d.
The authors’ observations
High-potency, typical antipsychotics can cause NMS, as shown in Mr. C’s case. It also can be caused by typical low-potency antipsychotics,3 atypical antipsychotics,17 antiemetic drugs,18 and lithium,19,20 and can occur after the withdrawal of levodopa and similar dopaminergic agents during Parkinson’s disease treatment.21 Atypical antipsychotics reported to be associated with NMS include clozapine, risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, and paliperidone.22-27 Atypical antipsychotic-induced NMS also has been reported in children and adolescents.22,28-30
With the broad application of atypical antipsychotics, physicians should be aware of atypical NMS presentation. Although NMS diagnosis commonly requires core symptoms of hyperthermia and muscle rigidity (Table 1 and 2),31 atypical presentations may not demonstrate temperature changes and/or muscle rigidity or may progress slowly over several days, leading to a delay in diagnosis and treatment.28,30,32,33 Therefore, clinicians should evaluate any patient taking antipsychotics for features of NMS and not prematurely exclude a NMS diagnosis in cases where severe rigidity or hyperthermia is not initially apparent.33
Table 1
DSM-IV-TR criteria for neuroleptic malignant syndrome
| A. The development of severe muscle rigidity and elevated temperature associated with the use of neuroleptic medication |
| B. 2 (or more) of the following: |
|
| Source: Reference 31 |
Table 2
Diagnostic features of neuroleptic malignant syndrome
| Essential features: severe muscle rigidity and elevated temperature in an individual using neuroleptic medication |
| Elevated temperature: from mild (eg, 99º to 100ºF) to markedly hyperthermic states (eg, 106ºF) |
| Creatine kinase: typically elevated, ranging from minor elevations to extremely high levels (exceeding 16,000 IU) |
| Other features: mental status changes, unstable blood pressure, diaphoresis, other signs of autonomic dysfunction |
| Source: Reference 31 |
Related Resource
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
Drug Brand Names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Diphenhydramine • Benadryl
- Enoxaparin • Lovenox
- Fosphenytoin • Cerebyx
- Haloperidol • Haldol
- Hydroxyzine • Vistaril
- Levodopa • Sinemet
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Pantoprazole • Protonix
- Phenytoin • Dilantin
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Trazodone • Desyrel, Oleptro
- Valproic acid • Depakote
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors are very grateful for the critical reviews by James R. Allen, MD, MPH, professor of Child and Adolescent Psychiatry Fellowship Program at the University of Oklahoma and Lori Hake, DO, director of Psychiatry Residency Training Program at Griffin Memorial Hospital in Norman, OK.
1. Stubner S, Rustenbeck E, Grohmann R, et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry. 2004;37:S54-S64.
2. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164:870-876.
3. Ropper AH, Brown RH. Adams and Victor’s principles of neurology. 8th ed. New York, NY: McGraw Hill; 2005;1025-1026.
4. Sheil AT, Collins KA, Schandl CA, et al. Fetal neurotoxic response to neuroleptic medications: case report and review of the literature. Am J Forensic Med Pathol. 2007;28:116-120.
5. Balzan MV. The neuroleptic malignant syndrome: a logical approach to the patient with temperature and rigidity. Postgrad Med J. 1998;74:72-76.
6. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77:185-202.
7. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the critical care unit. Crit Care Med. 2002;30:2609-2610.
8. Caroff SN, Campbell EC, Sullivan KA. Neuroleptic malignant syndrome in elderly patients. Expert Rev Neurother. 2007;7:423-431.
9. Gurrera RJ, Simpson JC, Tsuang MT. Meta-analytic evidence of systematic bias in estimates of neuroleptic malignant syndrome incidence. Compr Psychiatry. 2007;48:205-211.
10. Caroff SN, Mann SC, Keck PE, Jr, et al. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol. 2001;21:121-122.
11. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
12. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry. 1989;46:914-918.
13. Berardi D, Amore M, Keck PE, Jr, et al. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome: a case-control study. Biol Psychiatry. 1998;44:748-754.
14. Rosebush PI, Stewart TD. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146:717-725.
15. Martinez M, Marangell LB, Martinez JM. Psychopharmacology. In: Hales RE, Yudofsky SC, Gabbard GO, eds. American Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2008:1059-1132.
16. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, et al, eds. Neuroleptic malignant syndrome and related conditions. Washington, DC: American Psychiatric Publishing; 2003:1-44.
17. Hammerman S, Lam C, Caroff SN. Neuroleptic malignant syndrome and aripiprazole. J Am Acad Child Adolesc Psychiatry. 2006;45:639-641.
18. Stein MH, Sorscher M, Caroff SN. Neuroleptic malignant syndrome induced by metoclopramide in an infant with Freeman-Sheldon syndrome. Anesth Analg. 2006;103:786-787.
19. Borovicka MC, Bond LC, Gaughan KM. Ziprasidone- and lithium-induced neuroleptic malignant syndrome. Ann Pharmacother. 2006;40:139-142.
20. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy. 2003;23:811-815.
21. Ward C. Neuroleptic malignant syndrome in a patient with Parkinson’s disease: a case study. J Neurosci Nurs. 2005;37:160-162.
22. Leibold J, Patel V, Hasan RA. Neuroleptic malignant syndrome associated with ziprasidone in an adolescent. Clin Ther. 2004;26:1105-1108.
23. Corallo CE, Ernest D. Atypical neuroleptic malignant syndrome with long-term clozapine. Crit Care Resusc. 2007;9:338-340.
24. Molina D, Tingle LE, Lu X. Aripiprazole as the causative agent of neuroleptic malignant syndrome: a case report. Prim Care Companion J Clin Psychiatry. 2007;9:148-150.
25. Trollor JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23:477-492.
26. Gortney JS, Fagan A, Kissack JC. Neuroleptic malignant syndrome secondary to quetiapine. Ann Pharmacother. 2009;43:785-791.
27. Han C, Lee SJ, Pae CU. Paliperidone-associated atypical neuroleptic malignant syndrome: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:650-651.
28. Hanft A, Eggleston CF, Bourgeois JA. Neuroleptic malignant syndrome in an adolescent after brief exposure to olanzapine. J Child Adolesc Psychopharmacol. 2004;14:481-487.
29. Abu-Kishk I, Toledano M, Reis A, et al. Neuroleptic malignant syndrome in a child treated with an atypical antipsychotic. J Toxicol Clin Toxicol. 2004;42:921-925.
30. Neuhut R, Lindenmayer JP, Silva R. Neuroleptic malignant syndrome in children and adolescents on atypical antipsychotic medication: a review. J Child Adolesc Psychopharmacol. 2009;19:415-422.
31. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association: 2000.
32. Carroll BT, Surber SA. The problem of atypical neuroleptic malignant syndrome: a case report. Psychiatry (Edgmont). 2009;6:45-47.
33. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28:530-535.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Paranoid and scared
Police bring Mr. C, age 42, to a local crisis center after he is found masturbating in public the same day he was released from jail after serving time for the same behavior. Previously, Mr. C was diagnosed with schizophrenia, paranoid type, and alcohol dependence. He is single, unemployed, and lives with his parents. He has had 3 previous admissions to a psychiatric hospital, but no preexisting medical illness. A judge involuntarily commits Mr. C to our psychiatric facility.
Mr. C looks older than his age and has poor hygiene. He appears bizarre, makes poor eye contact, and speaks slowly but with normal volume. His speech is not coherent, relevant, or goal-directed. He is not able to answer questions properly, chanting “it’s eternity, eternity, eternity.” He shows no tremors, repetitive motor behavior, or muscle rigidity. His affect is flat and he has no suicidal or homicidal ideations. Based on Mr. C’s history, we diagnose him with schizophrenia, paranoid type and alcohol dependence.
Over the next 9 days, Mr. C receives trials of haloperidol, lorazepam, diphenhydramine, ziprasidone, olanzapine, hydroxyzine, trazodone, and benztropine to treat his schizophrenia. From days 1 to 3, all medications are given on an as-needed basis. On day 1, Mr. C receives haloperidol, 20 mg, lorazepam, 9 mg, diphenhydramine, 150 mg, and ziprasidone, 20 mg. On day 2, he receives haloperidol, 15 mg, lorazepam, 10 mg, olanzapine, 20 mg, hydroxyzine, 100 mg, and trazodone, 50 mg. On day 3, he receives haloperidol, 20 mg, lorazepam, 6 mg, and trazodone, 100 mg. On days 4 to 8, in addition to scheduled haloperidol, 30 mg/d, benztropine, 1 mg/d, and trazodone, 100 mg/d, he receives haloperidol, 5 mg, and lorazepam, 2 mg, as needed. On day 9, he receives the scheduled haloperidol, 30 mg/d, benztropine, 1 mg/d, and trazodone, 100 mg/d.
During his stay, Mr. C is incoherent and disorganized. On day 9, he eats all of his lunch, none of his dinner, but sips milk and juice and eats snacks. He drinks 2 small cups of water with medication and 2 small cups of water during oral care. His mucosa and tongue are dry. At 11:30 pm, while lying in bed mumbling “scared, scared,” he experiences shortness of breath. His temperature is 99.6°F, blood pressure is 151/93 mm Hg, pulse is 125 beats per minute, respiratory rate is 40 breaths per minute, and oxygen saturation is 91% on ambient air. Twenty minutes later, his blood pressure increases to 180/120 mm Hg. On physical examination, he has “lead pipe” rigidity of both arms. He is awake, confused, and not able to communicate, still mumbling “scared, scared.” Changes in his blood pressure, pulse, and temperature during his stay in the psychiatric hospital are depicted in Figures 1 and 2, respectively.
Figure 1: Mr. C’s blood pressure and pulse changes from day 4 to day 9 in the psychiatric hospital
BP: blood pressure
Figure 2: Mr. C’s temperature changes from day 4 to day 9 in the psychiatric hospital
The authors’ observations
NMS is a life-threatening, iatrogenic neurologic emergency associated with antipsychotic use. Early incidence rate estimates ran as high as 3% of patients treated with antipsychotics; however, more recent data suggest an incidence of 0.01% to 0.02%.1 This decrease in frequency likely reflects increased awareness of the disorder, more conservative prescribing patterns, and a shift to using atypical antipsychotics.2 In the mid 1980s and early 1990s the mortality rate was 25% to 30% if NMS was not promptly recognized and treated3; however, progression to more fulminant, lethal NMS episodes now occurs less often and the mortality rate ranges from 10% to 20%.4
If NMS is suspected, immediate transfer to an emergency department (ED) is necessary. Even with early diagnosis, however, complications of NMS are still likely, including:
- rhabdomyolysis
- renal failure
- seizures
- respiratory failure
- aspiration pneumonia
- disseminated intravascular coagulation
- venous thromboembolism.5-9
Caroff et al reported observing a residual catatonic state after acute NMS symptoms subsided.10
Although the pathophysiology of NMS is complex—involving a cascade of dysregulation in multiple neurochemical and neuroendocrine systems—dopamine blockade likely plays a pivotal role in triggering the condition.2 In addition, evidence supports the hypothesis that dysregulated sympathetic nervous system hyperactivity is responsible for most NMS features.11
TREATMENT: Arrival in the ED
Based on his elevated blood pressure (151/93 mm Hg), “lead pipe” rigidity, and increased body temperature associated with Mr. C’s history of haloperidol use for 9 days, the treatment team suspects NMS. Labile blood pressure, which changed from 151/93 to 180/120 mm Hg in 20 minutes, reinforces the NMS diagnosis. Approximately 30 minutes after Mr. C shows signs of NMS, he is transferred to a local ED. He is awake, alert, and communicative after he arrives in the ED, but becomes confused and noncommunicative the next morning. When he arrives in the ED, he is found to have tachycardia (114 beats per minute), tachypnea (26 breaths per minute), blood pressure of 132/84 mm Hg, and temperature of 102°F. In the ED, he is given IV normal saline, diphenhydramine, 25 mg, and IV lorazepam, 1 mg. His rigidity slightly improves.
Early the next morning, his blood pressure is 182/89 mm Hg, respirations are 30 to 40 breaths per minute, and heart rate is 120 beats per minute. He then receives IV lorazepam, 2 mg, after which his tachypnea, tachycardia, and elevated blood pressure improve.
The authors’ observations
A case-control study by Keck et al12 comparing 18 patients with NMS and 36 matched neuroleptic-treated patients with no history of the syndrome identified greater psychomotor agitation, significantly higher doses of neuroleptics, greater rates of dosage increase, and a greater number of IM injections as potential risk factors. Other potential risk factors include use of restraints, pre-existing CNS dopamine activity or receptor function abnormalities, and iron deficiency.2 Agitation, dehydration, and exhaustion were found to be the most consistent systemic factors predisposing patients taking antipsychotics to NMS in small case-control studies.13,14 Well-supported risk factors also include use of high-potency antipsychotics, prior episodes of NMS, age <40, male sex, malnutrition, organic brain syndromes, and lithium use.3,5,15
There is no way to predict the risk of NMS for an individual patient. Usually, symptoms develop within 4 weeks of starting an antipsychotic, but can occur after taking the same dose for many months. The onset may be within hours, but on average it is 4 to 14 days after initiating therapy. Among patients who develop NMS, 90% do so within 10 days.3,5
Mr. C’s risk factors include high-potency antipsychotic use, male sex, relatively high dose (haloperidol, 30 to 35 mg/d), agitation, dehydration, and exhaustion.
Managing NMS
The standard approaches for managing patients with NMS include discontinuing suspected triggering drugs and providing supportive care. Beyond supportive care, oral or IV benzodiazepines may relieve symptoms and speed recovery.2 Dopaminergic drugs, such as bromocriptine or amantadine, used alone or with other treatments, can reduce parkinsonism and disease duration and mortality.2 Dantrolene may be useful only for NMS patients who exhibit extreme temperature elevations, rigidity, and true hypermetabolism.16 Electroconvulsive therapy may be effective for NMS patients whose symptoms do not respond to supportive care and drug therapy or those with residual catatonic or parkinsonian symptoms.2
OUTCOME: Improvement, discharge
Mr. C is admitted to the hospital with the diagnosis of NMS and transferred to the intensive care unit (ICU) for treatment. After Mr. C is admitted to the ICU, apart from continuing the medication given in the ED, he also receives dantrolene, 2 mg/kg, then 1 mg/kg, 4 times a day, as well as IV lorazepam, 1 mg every 6 hours. His other medications include IV pantoprazole, 40 mg/d, for prophylaxis of stress ulcer. Diphenhydramine administration is changed to as needed. On the second day in the ICU, he has only mild upper extremity rigidity but no lower extremity rigidity. However, he suffers 1 seizure, which is treated with IV fosphenytoin at the loading dose, 18 mg/kg, then a maintaining dose of 5 mg phenytoin equivalent/kg/d.
Figure 3: Mr. C’s creatine kinase level (IU/L) during the first 5 days in the intensive care unit
Figure 4: Mr. C’s blood pressure before and after admission
Figure 5: Mr. C’s temperature before and after admissionMr. C remains in the ICU for 7 days. There he receives valproic acid, titrated to 500 mg in the morning and 1,000 mg at bedtime, for agitation. He also receives olanzapine, 5 mg/d, for psychotic symptoms. He develops deep vein thrombosis in the right cephalic vein, which is treated with subcutaneous enoxaparin, 1 mg/kg, and warfarin, 5 mg/d.
He is discharged from the hospital after 2 weeks and returns to the psychiatric facility. He continues to be treated for paranoid schizophrenia with olanzapine, 5 mg/d.
The authors’ observations
High-potency, typical antipsychotics can cause NMS, as shown in Mr. C’s case. It also can be caused by typical low-potency antipsychotics,3 atypical antipsychotics,17 antiemetic drugs,18 and lithium,19,20 and can occur after the withdrawal of levodopa and similar dopaminergic agents during Parkinson’s disease treatment.21 Atypical antipsychotics reported to be associated with NMS include clozapine, risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, and paliperidone.22-27 Atypical antipsychotic-induced NMS also has been reported in children and adolescents.22,28-30
With the broad application of atypical antipsychotics, physicians should be aware of atypical NMS presentation. Although NMS diagnosis commonly requires core symptoms of hyperthermia and muscle rigidity (Table 1 and 2),31 atypical presentations may not demonstrate temperature changes and/or muscle rigidity or may progress slowly over several days, leading to a delay in diagnosis and treatment.28,30,32,33 Therefore, clinicians should evaluate any patient taking antipsychotics for features of NMS and not prematurely exclude a NMS diagnosis in cases where severe rigidity or hyperthermia is not initially apparent.33
Table 1
DSM-IV-TR criteria for neuroleptic malignant syndrome
| A. The development of severe muscle rigidity and elevated temperature associated with the use of neuroleptic medication |
| B. 2 (or more) of the following: |
|
| Source: Reference 31 |
Table 2
Diagnostic features of neuroleptic malignant syndrome
| Essential features: severe muscle rigidity and elevated temperature in an individual using neuroleptic medication |
| Elevated temperature: from mild (eg, 99º to 100ºF) to markedly hyperthermic states (eg, 106ºF) |
| Creatine kinase: typically elevated, ranging from minor elevations to extremely high levels (exceeding 16,000 IU) |
| Other features: mental status changes, unstable blood pressure, diaphoresis, other signs of autonomic dysfunction |
| Source: Reference 31 |
Related Resource
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
Drug Brand Names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Diphenhydramine • Benadryl
- Enoxaparin • Lovenox
- Fosphenytoin • Cerebyx
- Haloperidol • Haldol
- Hydroxyzine • Vistaril
- Levodopa • Sinemet
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Pantoprazole • Protonix
- Phenytoin • Dilantin
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Trazodone • Desyrel, Oleptro
- Valproic acid • Depakote
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors are very grateful for the critical reviews by James R. Allen, MD, MPH, professor of Child and Adolescent Psychiatry Fellowship Program at the University of Oklahoma and Lori Hake, DO, director of Psychiatry Residency Training Program at Griffin Memorial Hospital in Norman, OK.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Paranoid and scared
Police bring Mr. C, age 42, to a local crisis center after he is found masturbating in public the same day he was released from jail after serving time for the same behavior. Previously, Mr. C was diagnosed with schizophrenia, paranoid type, and alcohol dependence. He is single, unemployed, and lives with his parents. He has had 3 previous admissions to a psychiatric hospital, but no preexisting medical illness. A judge involuntarily commits Mr. C to our psychiatric facility.
Mr. C looks older than his age and has poor hygiene. He appears bizarre, makes poor eye contact, and speaks slowly but with normal volume. His speech is not coherent, relevant, or goal-directed. He is not able to answer questions properly, chanting “it’s eternity, eternity, eternity.” He shows no tremors, repetitive motor behavior, or muscle rigidity. His affect is flat and he has no suicidal or homicidal ideations. Based on Mr. C’s history, we diagnose him with schizophrenia, paranoid type and alcohol dependence.
Over the next 9 days, Mr. C receives trials of haloperidol, lorazepam, diphenhydramine, ziprasidone, olanzapine, hydroxyzine, trazodone, and benztropine to treat his schizophrenia. From days 1 to 3, all medications are given on an as-needed basis. On day 1, Mr. C receives haloperidol, 20 mg, lorazepam, 9 mg, diphenhydramine, 150 mg, and ziprasidone, 20 mg. On day 2, he receives haloperidol, 15 mg, lorazepam, 10 mg, olanzapine, 20 mg, hydroxyzine, 100 mg, and trazodone, 50 mg. On day 3, he receives haloperidol, 20 mg, lorazepam, 6 mg, and trazodone, 100 mg. On days 4 to 8, in addition to scheduled haloperidol, 30 mg/d, benztropine, 1 mg/d, and trazodone, 100 mg/d, he receives haloperidol, 5 mg, and lorazepam, 2 mg, as needed. On day 9, he receives the scheduled haloperidol, 30 mg/d, benztropine, 1 mg/d, and trazodone, 100 mg/d.
During his stay, Mr. C is incoherent and disorganized. On day 9, he eats all of his lunch, none of his dinner, but sips milk and juice and eats snacks. He drinks 2 small cups of water with medication and 2 small cups of water during oral care. His mucosa and tongue are dry. At 11:30 pm, while lying in bed mumbling “scared, scared,” he experiences shortness of breath. His temperature is 99.6°F, blood pressure is 151/93 mm Hg, pulse is 125 beats per minute, respiratory rate is 40 breaths per minute, and oxygen saturation is 91% on ambient air. Twenty minutes later, his blood pressure increases to 180/120 mm Hg. On physical examination, he has “lead pipe” rigidity of both arms. He is awake, confused, and not able to communicate, still mumbling “scared, scared.” Changes in his blood pressure, pulse, and temperature during his stay in the psychiatric hospital are depicted in Figures 1 and 2, respectively.
Figure 1: Mr. C’s blood pressure and pulse changes from day 4 to day 9 in the psychiatric hospital
BP: blood pressure
Figure 2: Mr. C’s temperature changes from day 4 to day 9 in the psychiatric hospital
The authors’ observations
NMS is a life-threatening, iatrogenic neurologic emergency associated with antipsychotic use. Early incidence rate estimates ran as high as 3% of patients treated with antipsychotics; however, more recent data suggest an incidence of 0.01% to 0.02%.1 This decrease in frequency likely reflects increased awareness of the disorder, more conservative prescribing patterns, and a shift to using atypical antipsychotics.2 In the mid 1980s and early 1990s the mortality rate was 25% to 30% if NMS was not promptly recognized and treated3; however, progression to more fulminant, lethal NMS episodes now occurs less often and the mortality rate ranges from 10% to 20%.4
If NMS is suspected, immediate transfer to an emergency department (ED) is necessary. Even with early diagnosis, however, complications of NMS are still likely, including:
- rhabdomyolysis
- renal failure
- seizures
- respiratory failure
- aspiration pneumonia
- disseminated intravascular coagulation
- venous thromboembolism.5-9
Caroff et al reported observing a residual catatonic state after acute NMS symptoms subsided.10
Although the pathophysiology of NMS is complex—involving a cascade of dysregulation in multiple neurochemical and neuroendocrine systems—dopamine blockade likely plays a pivotal role in triggering the condition.2 In addition, evidence supports the hypothesis that dysregulated sympathetic nervous system hyperactivity is responsible for most NMS features.11
TREATMENT: Arrival in the ED
Based on his elevated blood pressure (151/93 mm Hg), “lead pipe” rigidity, and increased body temperature associated with Mr. C’s history of haloperidol use for 9 days, the treatment team suspects NMS. Labile blood pressure, which changed from 151/93 to 180/120 mm Hg in 20 minutes, reinforces the NMS diagnosis. Approximately 30 minutes after Mr. C shows signs of NMS, he is transferred to a local ED. He is awake, alert, and communicative after he arrives in the ED, but becomes confused and noncommunicative the next morning. When he arrives in the ED, he is found to have tachycardia (114 beats per minute), tachypnea (26 breaths per minute), blood pressure of 132/84 mm Hg, and temperature of 102°F. In the ED, he is given IV normal saline, diphenhydramine, 25 mg, and IV lorazepam, 1 mg. His rigidity slightly improves.
Early the next morning, his blood pressure is 182/89 mm Hg, respirations are 30 to 40 breaths per minute, and heart rate is 120 beats per minute. He then receives IV lorazepam, 2 mg, after which his tachypnea, tachycardia, and elevated blood pressure improve.
The authors’ observations
A case-control study by Keck et al12 comparing 18 patients with NMS and 36 matched neuroleptic-treated patients with no history of the syndrome identified greater psychomotor agitation, significantly higher doses of neuroleptics, greater rates of dosage increase, and a greater number of IM injections as potential risk factors. Other potential risk factors include use of restraints, pre-existing CNS dopamine activity or receptor function abnormalities, and iron deficiency.2 Agitation, dehydration, and exhaustion were found to be the most consistent systemic factors predisposing patients taking antipsychotics to NMS in small case-control studies.13,14 Well-supported risk factors also include use of high-potency antipsychotics, prior episodes of NMS, age <40, male sex, malnutrition, organic brain syndromes, and lithium use.3,5,15
There is no way to predict the risk of NMS for an individual patient. Usually, symptoms develop within 4 weeks of starting an antipsychotic, but can occur after taking the same dose for many months. The onset may be within hours, but on average it is 4 to 14 days after initiating therapy. Among patients who develop NMS, 90% do so within 10 days.3,5
Mr. C’s risk factors include high-potency antipsychotic use, male sex, relatively high dose (haloperidol, 30 to 35 mg/d), agitation, dehydration, and exhaustion.
Managing NMS
The standard approaches for managing patients with NMS include discontinuing suspected triggering drugs and providing supportive care. Beyond supportive care, oral or IV benzodiazepines may relieve symptoms and speed recovery.2 Dopaminergic drugs, such as bromocriptine or amantadine, used alone or with other treatments, can reduce parkinsonism and disease duration and mortality.2 Dantrolene may be useful only for NMS patients who exhibit extreme temperature elevations, rigidity, and true hypermetabolism.16 Electroconvulsive therapy may be effective for NMS patients whose symptoms do not respond to supportive care and drug therapy or those with residual catatonic or parkinsonian symptoms.2
OUTCOME: Improvement, discharge
Mr. C is admitted to the hospital with the diagnosis of NMS and transferred to the intensive care unit (ICU) for treatment. After Mr. C is admitted to the ICU, apart from continuing the medication given in the ED, he also receives dantrolene, 2 mg/kg, then 1 mg/kg, 4 times a day, as well as IV lorazepam, 1 mg every 6 hours. His other medications include IV pantoprazole, 40 mg/d, for prophylaxis of stress ulcer. Diphenhydramine administration is changed to as needed. On the second day in the ICU, he has only mild upper extremity rigidity but no lower extremity rigidity. However, he suffers 1 seizure, which is treated with IV fosphenytoin at the loading dose, 18 mg/kg, then a maintaining dose of 5 mg phenytoin equivalent/kg/d.
Figure 3: Mr. C’s creatine kinase level (IU/L) during the first 5 days in the intensive care unit
Figure 4: Mr. C’s blood pressure before and after admission
Figure 5: Mr. C’s temperature before and after admissionMr. C remains in the ICU for 7 days. There he receives valproic acid, titrated to 500 mg in the morning and 1,000 mg at bedtime, for agitation. He also receives olanzapine, 5 mg/d, for psychotic symptoms. He develops deep vein thrombosis in the right cephalic vein, which is treated with subcutaneous enoxaparin, 1 mg/kg, and warfarin, 5 mg/d.
He is discharged from the hospital after 2 weeks and returns to the psychiatric facility. He continues to be treated for paranoid schizophrenia with olanzapine, 5 mg/d.
The authors’ observations
High-potency, typical antipsychotics can cause NMS, as shown in Mr. C’s case. It also can be caused by typical low-potency antipsychotics,3 atypical antipsychotics,17 antiemetic drugs,18 and lithium,19,20 and can occur after the withdrawal of levodopa and similar dopaminergic agents during Parkinson’s disease treatment.21 Atypical antipsychotics reported to be associated with NMS include clozapine, risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, and paliperidone.22-27 Atypical antipsychotic-induced NMS also has been reported in children and adolescents.22,28-30
With the broad application of atypical antipsychotics, physicians should be aware of atypical NMS presentation. Although NMS diagnosis commonly requires core symptoms of hyperthermia and muscle rigidity (Table 1 and 2),31 atypical presentations may not demonstrate temperature changes and/or muscle rigidity or may progress slowly over several days, leading to a delay in diagnosis and treatment.28,30,32,33 Therefore, clinicians should evaluate any patient taking antipsychotics for features of NMS and not prematurely exclude a NMS diagnosis in cases where severe rigidity or hyperthermia is not initially apparent.33
Table 1
DSM-IV-TR criteria for neuroleptic malignant syndrome
| A. The development of severe muscle rigidity and elevated temperature associated with the use of neuroleptic medication |
| B. 2 (or more) of the following: |
|
| Source: Reference 31 |
Table 2
Diagnostic features of neuroleptic malignant syndrome
| Essential features: severe muscle rigidity and elevated temperature in an individual using neuroleptic medication |
| Elevated temperature: from mild (eg, 99º to 100ºF) to markedly hyperthermic states (eg, 106ºF) |
| Creatine kinase: typically elevated, ranging from minor elevations to extremely high levels (exceeding 16,000 IU) |
| Other features: mental status changes, unstable blood pressure, diaphoresis, other signs of autonomic dysfunction |
| Source: Reference 31 |
Related Resource
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
Drug Brand Names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Diphenhydramine • Benadryl
- Enoxaparin • Lovenox
- Fosphenytoin • Cerebyx
- Haloperidol • Haldol
- Hydroxyzine • Vistaril
- Levodopa • Sinemet
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Pantoprazole • Protonix
- Phenytoin • Dilantin
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Trazodone • Desyrel, Oleptro
- Valproic acid • Depakote
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors are very grateful for the critical reviews by James R. Allen, MD, MPH, professor of Child and Adolescent Psychiatry Fellowship Program at the University of Oklahoma and Lori Hake, DO, director of Psychiatry Residency Training Program at Griffin Memorial Hospital in Norman, OK.
1. Stubner S, Rustenbeck E, Grohmann R, et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry. 2004;37:S54-S64.
2. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164:870-876.
3. Ropper AH, Brown RH. Adams and Victor’s principles of neurology. 8th ed. New York, NY: McGraw Hill; 2005;1025-1026.
4. Sheil AT, Collins KA, Schandl CA, et al. Fetal neurotoxic response to neuroleptic medications: case report and review of the literature. Am J Forensic Med Pathol. 2007;28:116-120.
5. Balzan MV. The neuroleptic malignant syndrome: a logical approach to the patient with temperature and rigidity. Postgrad Med J. 1998;74:72-76.
6. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77:185-202.
7. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the critical care unit. Crit Care Med. 2002;30:2609-2610.
8. Caroff SN, Campbell EC, Sullivan KA. Neuroleptic malignant syndrome in elderly patients. Expert Rev Neurother. 2007;7:423-431.
9. Gurrera RJ, Simpson JC, Tsuang MT. Meta-analytic evidence of systematic bias in estimates of neuroleptic malignant syndrome incidence. Compr Psychiatry. 2007;48:205-211.
10. Caroff SN, Mann SC, Keck PE, Jr, et al. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol. 2001;21:121-122.
11. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
12. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry. 1989;46:914-918.
13. Berardi D, Amore M, Keck PE, Jr, et al. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome: a case-control study. Biol Psychiatry. 1998;44:748-754.
14. Rosebush PI, Stewart TD. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146:717-725.
15. Martinez M, Marangell LB, Martinez JM. Psychopharmacology. In: Hales RE, Yudofsky SC, Gabbard GO, eds. American Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2008:1059-1132.
16. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, et al, eds. Neuroleptic malignant syndrome and related conditions. Washington, DC: American Psychiatric Publishing; 2003:1-44.
17. Hammerman S, Lam C, Caroff SN. Neuroleptic malignant syndrome and aripiprazole. J Am Acad Child Adolesc Psychiatry. 2006;45:639-641.
18. Stein MH, Sorscher M, Caroff SN. Neuroleptic malignant syndrome induced by metoclopramide in an infant with Freeman-Sheldon syndrome. Anesth Analg. 2006;103:786-787.
19. Borovicka MC, Bond LC, Gaughan KM. Ziprasidone- and lithium-induced neuroleptic malignant syndrome. Ann Pharmacother. 2006;40:139-142.
20. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy. 2003;23:811-815.
21. Ward C. Neuroleptic malignant syndrome in a patient with Parkinson’s disease: a case study. J Neurosci Nurs. 2005;37:160-162.
22. Leibold J, Patel V, Hasan RA. Neuroleptic malignant syndrome associated with ziprasidone in an adolescent. Clin Ther. 2004;26:1105-1108.
23. Corallo CE, Ernest D. Atypical neuroleptic malignant syndrome with long-term clozapine. Crit Care Resusc. 2007;9:338-340.
24. Molina D, Tingle LE, Lu X. Aripiprazole as the causative agent of neuroleptic malignant syndrome: a case report. Prim Care Companion J Clin Psychiatry. 2007;9:148-150.
25. Trollor JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23:477-492.
26. Gortney JS, Fagan A, Kissack JC. Neuroleptic malignant syndrome secondary to quetiapine. Ann Pharmacother. 2009;43:785-791.
27. Han C, Lee SJ, Pae CU. Paliperidone-associated atypical neuroleptic malignant syndrome: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:650-651.
28. Hanft A, Eggleston CF, Bourgeois JA. Neuroleptic malignant syndrome in an adolescent after brief exposure to olanzapine. J Child Adolesc Psychopharmacol. 2004;14:481-487.
29. Abu-Kishk I, Toledano M, Reis A, et al. Neuroleptic malignant syndrome in a child treated with an atypical antipsychotic. J Toxicol Clin Toxicol. 2004;42:921-925.
30. Neuhut R, Lindenmayer JP, Silva R. Neuroleptic malignant syndrome in children and adolescents on atypical antipsychotic medication: a review. J Child Adolesc Psychopharmacol. 2009;19:415-422.
31. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association: 2000.
32. Carroll BT, Surber SA. The problem of atypical neuroleptic malignant syndrome: a case report. Psychiatry (Edgmont). 2009;6:45-47.
33. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28:530-535.
1. Stubner S, Rustenbeck E, Grohmann R, et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry. 2004;37:S54-S64.
2. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164:870-876.
3. Ropper AH, Brown RH. Adams and Victor’s principles of neurology. 8th ed. New York, NY: McGraw Hill; 2005;1025-1026.
4. Sheil AT, Collins KA, Schandl CA, et al. Fetal neurotoxic response to neuroleptic medications: case report and review of the literature. Am J Forensic Med Pathol. 2007;28:116-120.
5. Balzan MV. The neuroleptic malignant syndrome: a logical approach to the patient with temperature and rigidity. Postgrad Med J. 1998;74:72-76.
6. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77:185-202.
7. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the critical care unit. Crit Care Med. 2002;30:2609-2610.
8. Caroff SN, Campbell EC, Sullivan KA. Neuroleptic malignant syndrome in elderly patients. Expert Rev Neurother. 2007;7:423-431.
9. Gurrera RJ, Simpson JC, Tsuang MT. Meta-analytic evidence of systematic bias in estimates of neuroleptic malignant syndrome incidence. Compr Psychiatry. 2007;48:205-211.
10. Caroff SN, Mann SC, Keck PE, Jr, et al. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol. 2001;21:121-122.
11. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
12. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry. 1989;46:914-918.
13. Berardi D, Amore M, Keck PE, Jr, et al. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome: a case-control study. Biol Psychiatry. 1998;44:748-754.
14. Rosebush PI, Stewart TD. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146:717-725.
15. Martinez M, Marangell LB, Martinez JM. Psychopharmacology. In: Hales RE, Yudofsky SC, Gabbard GO, eds. American Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2008:1059-1132.
16. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, et al, eds. Neuroleptic malignant syndrome and related conditions. Washington, DC: American Psychiatric Publishing; 2003:1-44.
17. Hammerman S, Lam C, Caroff SN. Neuroleptic malignant syndrome and aripiprazole. J Am Acad Child Adolesc Psychiatry. 2006;45:639-641.
18. Stein MH, Sorscher M, Caroff SN. Neuroleptic malignant syndrome induced by metoclopramide in an infant with Freeman-Sheldon syndrome. Anesth Analg. 2006;103:786-787.
19. Borovicka MC, Bond LC, Gaughan KM. Ziprasidone- and lithium-induced neuroleptic malignant syndrome. Ann Pharmacother. 2006;40:139-142.
20. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy. 2003;23:811-815.
21. Ward C. Neuroleptic malignant syndrome in a patient with Parkinson’s disease: a case study. J Neurosci Nurs. 2005;37:160-162.
22. Leibold J, Patel V, Hasan RA. Neuroleptic malignant syndrome associated with ziprasidone in an adolescent. Clin Ther. 2004;26:1105-1108.
23. Corallo CE, Ernest D. Atypical neuroleptic malignant syndrome with long-term clozapine. Crit Care Resusc. 2007;9:338-340.
24. Molina D, Tingle LE, Lu X. Aripiprazole as the causative agent of neuroleptic malignant syndrome: a case report. Prim Care Companion J Clin Psychiatry. 2007;9:148-150.
25. Trollor JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23:477-492.
26. Gortney JS, Fagan A, Kissack JC. Neuroleptic malignant syndrome secondary to quetiapine. Ann Pharmacother. 2009;43:785-791.
27. Han C, Lee SJ, Pae CU. Paliperidone-associated atypical neuroleptic malignant syndrome: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:650-651.
28. Hanft A, Eggleston CF, Bourgeois JA. Neuroleptic malignant syndrome in an adolescent after brief exposure to olanzapine. J Child Adolesc Psychopharmacol. 2004;14:481-487.
29. Abu-Kishk I, Toledano M, Reis A, et al. Neuroleptic malignant syndrome in a child treated with an atypical antipsychotic. J Toxicol Clin Toxicol. 2004;42:921-925.
30. Neuhut R, Lindenmayer JP, Silva R. Neuroleptic malignant syndrome in children and adolescents on atypical antipsychotic medication: a review. J Child Adolesc Psychopharmacol. 2009;19:415-422.
31. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association: 2000.
32. Carroll BT, Surber SA. The problem of atypical neuroleptic malignant syndrome: a case report. Psychiatry (Edgmont). 2009;6:45-47.
33. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28:530-535.