User login
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
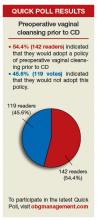
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.