User login
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
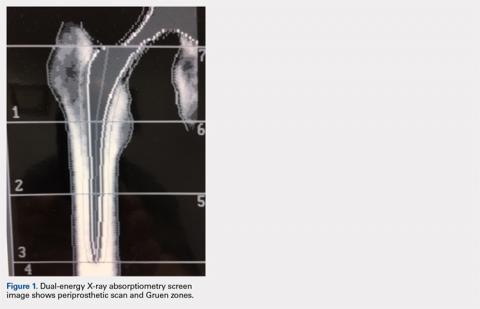
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
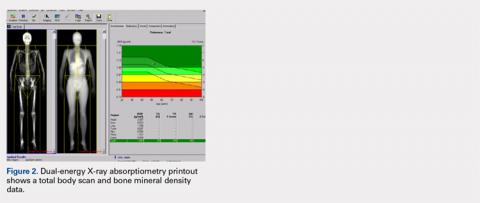
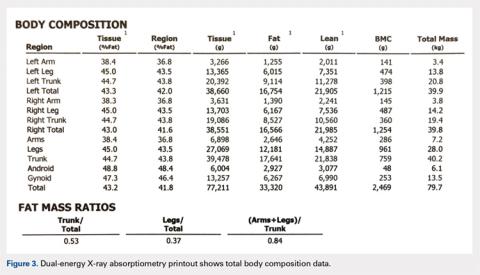
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
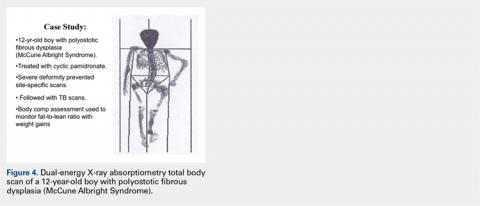
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
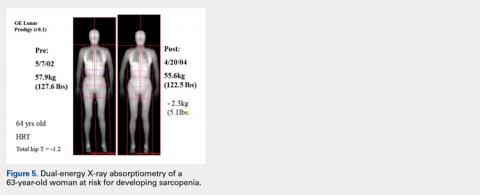
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
TAKE-HOME POINTS
- DXA is underutilized technology in orthopedics.
- More data ("ancillary data") are often available from a DXA scan then typically included in a standard report from a referral center.
- Most orthopedists are likely unaware of the detailed body composition data available with a total body scan.
- Preoperative DXA scans and knowledge of BMD may be informative when planning the type of fixation and implant metal to used.
- Serial follow-up body composition scans can be useful in monitoring the course of bone healing (mineralization) and soft tissue changes (fat and lean mass).