User login
More than one-third of American adults and approximately 17% of children and adolescents between the ages of 2 and 19 years are obese.1,2 Poor diet coupled with a sedentary lifestyle is the highest ranked cause of non-communicable disease and a leading cause of preventable death, according to the National Research Council.3
Bariatric surgery (BS) is a viable therapeutic option for obese patients who do not respond to conventional lifestyle interventions for losing weight. There are multiple gastrointestinal (GI) procedures available that are classified as either malabsorptive (Roux-en-Y gastric bypass [RYGB] and biliopancreatic diversion [BPD] with or without duodenal switch) or restrictive (laparoscopic adjustable gastric banding [LAGB] and vertical sleeve gastrectomy [VSG]).
Approximately half of the 196,000 bariatric procedures performed in the United States in 2015 were of the sleeve variety, another 23% were RYGB, and the remaining percentage was divided among the other types.4 Postoperative risks include nutritional deficiencies, decreased bone mineral density (BMD), dumping syndrome (when food rapidly dumps from the stomach to the intestine), and gastroesophageal reflux disease (GERD) with possible ulceration.
Despite these potential complications, a systematic review and meta-analysis found that obese people who underwent BS (gastric banding or gastric bypass) had significantly reduced risks of global, non-cardiovascular (CV), and CV mortality compared with obese controls.5 Helping patients to realize these benefits requires that the entire health care team—especially the family physician—is aware of the special considerations for this population.
To that end, this article reviews the details of diagnosing and managing post-surgical complications. It also addresses issues unique to managing certain subpopulations, such as post-BS patients who require revision surgery or who want to pursue body contouring surgery; adolescents who undergo BS surgery; and women who want to get pregnant postoperatively.
Monitor patients for these post-surgery complications
Postoperative BS follow-up varies depending on location, surgeon preference, and availability of multidisciplinary resources. At our institution, patients have a minimum of 3 follow-up visits with their surgeon (during hospitalization and 2 weeks and 2 months postoperatively). This is followed by visits with Endocrinology 6 months after surgery and annually thereafter. Given the variability of follow-up, family physicians should coordinate with specialists where appropriate and be aware of postoperative complications and monitoring since it is likely they will have the most frequent contact with these patients.
Nutritional deficiencies are common and require lifelong screening
Nutritional deficiencies are the most common complications of malabsorptive BS. Guidelines from the Endocrine Society, as well as guidelines from the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS), recommend routine lifetime screening for deficiencies after surgery.6,7 Complete blood cell count, electrolytes, glucose, creatinine, and liver function tests should be obtained at one, 3, 6, 12, 18, and 24 months following surgery and annually thereafter.6
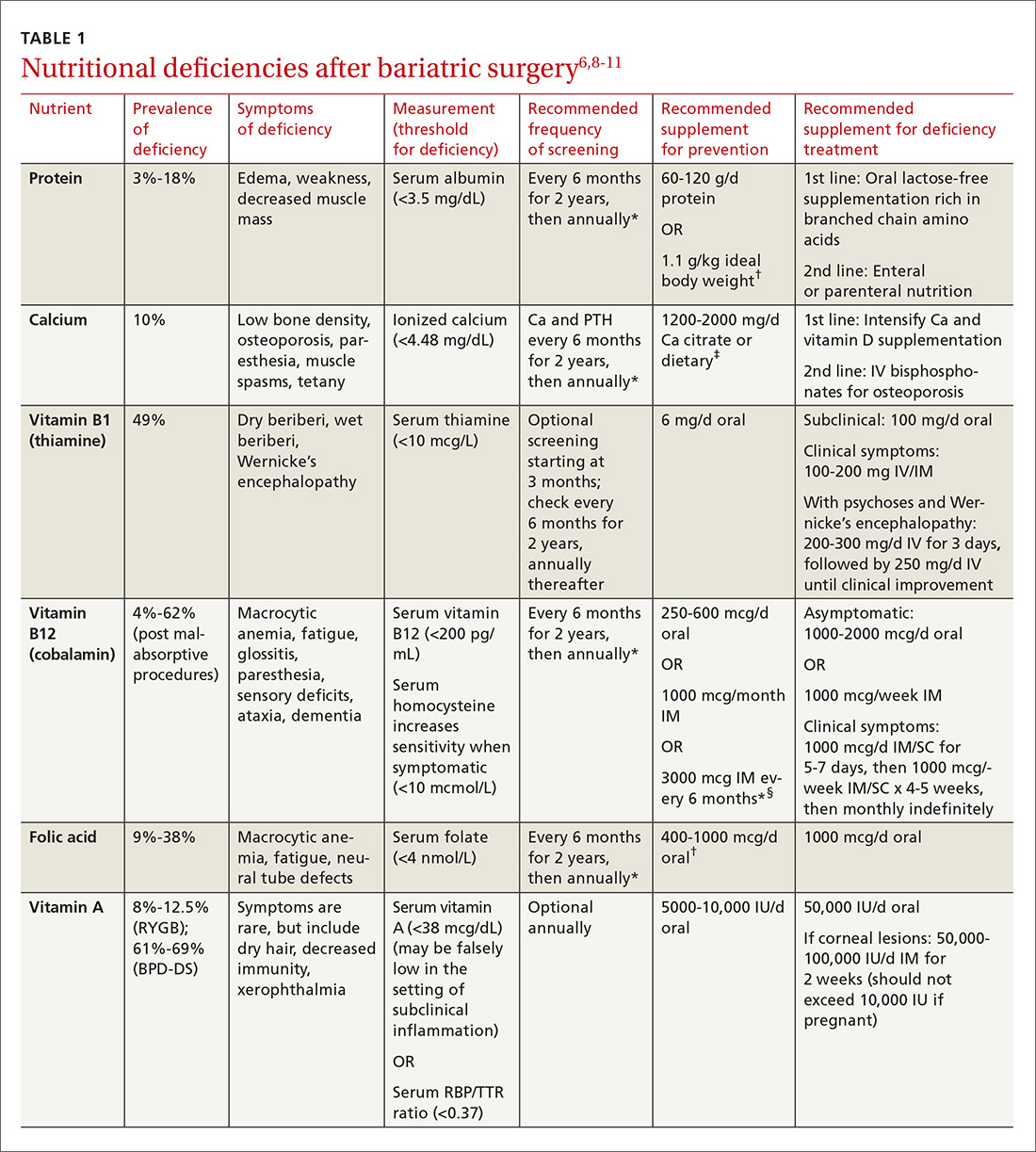
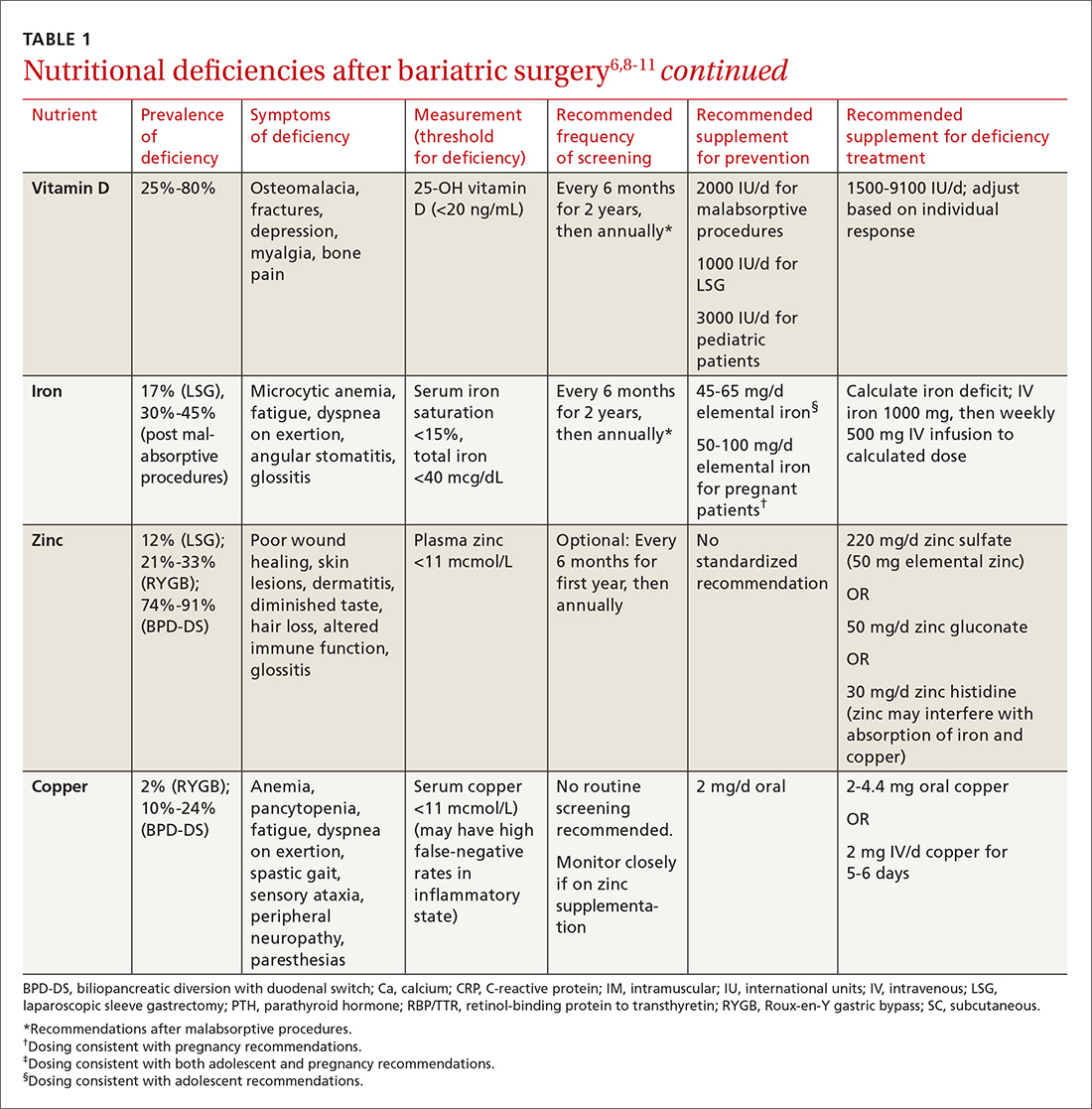
Multiple factors contribute to nutritional and micronutrient deficiencies, including reduced oral intake of food, decreased GI absorption, food intolerance, nausea/vomiting, and nonadherence with dietary supplements.8 Oral supplementation should be in chewable, powder, or liquid form because pill and capsule absorption may be altered.8,9 Over-the-counter multivitamins may not contain the requisite daily doses recommended after BS.9 Patients and physicians should evaluate supplements together to ensure appropriate nutritional and micronutrient supplementation (TABLE 16,8-11).
Bone mineral density can start to decrease soon after surgery
Studies evaluating BMD after BS have produced variable findings. In obese patients, dual-energy x-ray absorptiometry (DEXA) measurements may not be accurate due to adipose tissue artifact and table weight limits. In addition, limited data exist on the incidence of fractures after BS. Of 2 notable studies, only one, a population-based study involving 258 Minnesota residents who underwent a first bariatric surgery between 1985 and 2004, demonstrated a significantly increased incidence of fractures.12,13
In addition, studies show bone turnover markers, including C-terminal telopeptide, increase as early as 3 months after BS.14 Several guidelines recommend routine BMD screening after BS (TABLE 2).6,7 The mechanism of bone demineralization is likely multifactorial—a function of the magnitude of the weight loss and skeletal unloading, calcium and vitamin D deficiencies, and associated secondary hyperparathyroidism.15 Treatment for secondary hyperparathyroidism is adequate supplementation with vitamin D and calcium.

Optimal dosing for vitamin D has not been determined. One recent systematic review suggests routine prophylaxis with at least 2000 international units (IU)/d and found the greatest improvement for known deficiency with doses of 1500-9100 IU/d following malabsorptive surgeries.11 After laparoscopic sleeve gastrectomy, at least 1000 IU/d vitamin D is recommended.11
Overall, high variability exists among patients, and an individualized approach for dosing is recommended.11 Vitamin D levels should be monitored 2 and 4 weeks after initiation of treatment and every 3 months thereafter.11 Normal levels of serum calcium, 25-OH vitamin D, bone-specific alkaline phosphatase, and 24-hour urinary calcium excretion indicate adequate calcium and vitamin D supplementation.6
Dumping syndrome can lead to hypoglycemia
Dumping syndrome is a common complication following BS, with prevalence ranging from 25% to 75%, depending upon the type of procedure performed.16,17 There are 2 types: early and late. Early dumping syndrome occurs within 30 minutes of eating. Symptoms are related to the robust release of gastrointestinal hormones caused by rapid gastric emptying. Symptoms include nausea, abdominal pain, diarrhea, flushing, hypotension, and tachycardia.
Late dumping is characterized as postprandial hypoglycemia occurring one to 3 hours after eating. Late dumping is likely caused by a combination of changes within the pancreatic beta cells and abnormal insulin response to glucose.16-18 Rapid gastric emptying leads to rapid release of glucose in the gut, which, in turn, leads to brisk insulin secretion. Since glucose is absorbed faster than insulin’s half-life, the resulting (relatively) high levels of insulin may cause hypoglycemia.16-18
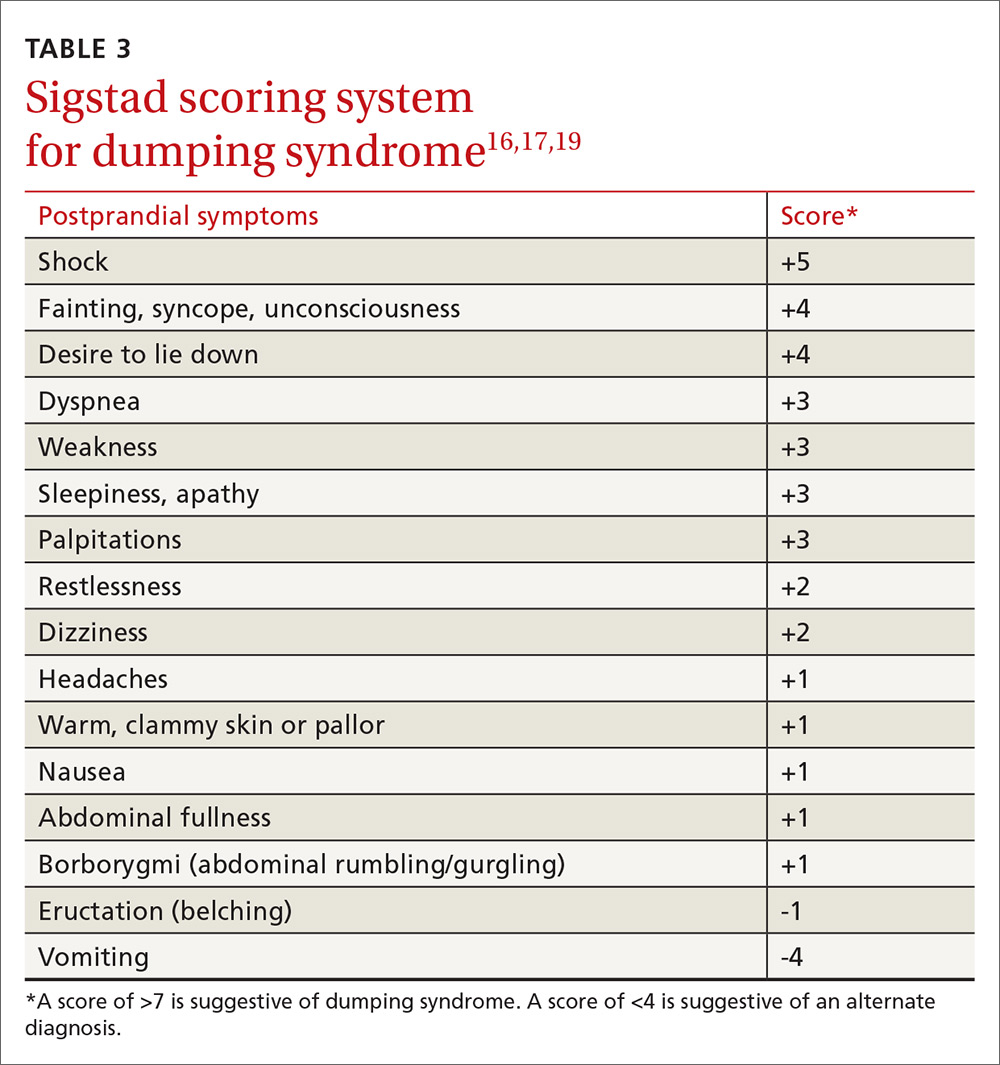
Sigstad’s scoring system can be used to confirm suspected cases of dumping syndrome (TABLE 316,17,19). A diagnosis can also be made with an oral glucose challenge in which pulse, blood pressure, glucose, and hematocrit are measured after ingestion of 50 g glucose. The test is positive if heart rate increases by 10 beats per minute, hematocrit increases by 3% 30 minutes after ingestion, or glucose falls below 60 mg/dL 2 to 3 hours after ingestion.17
First-line treatment of dumping syndrome consists of dietary modifications. The goal is to slow the rate of gastric emptying by eating smaller, more frequent meals; separating beverages from food; decreasing carbohydrates; and increasing fiber and protein content.
If results are suboptimal after dietary changes, medications can be prescribed including acarbose to prevent postprandial hypoglycemia; anticholinergics such as dicyclomine to slow gastric emptying; and somatostatin to decrease gastric emptying and inhibit GI hormone release.17 Lastly, for resistant and severe postprandial hypoglycemia, a few patients have undergone pancreatectomy, but only about 65% experienced improvement in symptoms and 12% developed diabetes post-surgically.20
Gout attacks may initially increase, but then decrease
BS affects the incidence of gout attacks in patients with a history of gout. One comparative study of approximately 150 patients demonstrated that those with a history of gout had significantly more gout attacks in the first month after BS compared with obese patients with a history of gout undergoing other upper GI surgeries.21 There was no difference between malabsorptive and restrictive procedures. But after the first month, BS patients had significantly fewer gout attacks and lower uric acid levels than their obese counterparts.21
Protein rich diets, catabolism potentiated by aggressive caloric restriction following BS, and dehydration contribute to the initial increase. Therefore, patients who have had at least one gout attack in the year prior to surgery or who are on hypouricemic medication may benefit from at least one month of prophylactic therapy (eg, allopurinol and colchicine) after surgery.
GERD and ulceration: How to respond
Obesity is a known risk factor for GERD, but the effect of BS on GERD is uncertain and seems to vary with the procedure performed. RYGB decreases GERD and is, therefore, used as both a secondary treatment in those not responding to medications and a revision treatment for fundoplication and other types of BSs. Sleeve gastrectomy and adjustable gastric banding have mixed effects on GERD. A systematic review by de Jong et al revealed a decreased prevalence of reflux symptoms and GERD medication use after LAGB; however, during longer follow-up, 15% of previously unaffected patients reported experiencing GERD.22 The 2011 International Sleeve Gastrectomy Expert Panel Consensus Statement retrospectively noted a postoperative incidence of GERD as high as 31%.23
BS patients with GERD should be treated with a proton pump inhibitor. If this fails, refer patients to a gastroenterologist for further evaluation.24
Ulcers after BS may be an indication for revision surgery. Data are mixed regarding increased risk of marginal ulceration from nonsteroidal anti-inflammatory drug (NSAID) use, but NSAIDs have been linked to an increased risk of anastomotic leakage.25-28 Thus, it seems prudent to avoid NSAIDs in people who have undergone BS.
Keeping watch over psychiatric comorbidities
A recent meta-analysis by Dawes et al29 showed that about 23% of patients pursuing BS have a comorbid mood disorder. Specifically, the preoperative prevalence of depression (19%) and binge-eating disorder (17%) were found to be higher than rates in the general population.29 The meta-analysis found improvement in the prevalence of depression with fewer symptoms and less antidepressant medication use in the first 3 years after surgery and a decrease in the rate of binge-eating disorder, although there were fewer supporting data for the latter. These findings were observed with both restrictive and malabsorptive procedures.
The data are mixed regarding rates of alcohol abuse and suicide. Further research is necessary in this field. Patients who have had BS should receive ongoing psychiatric and psychological care from a multidisciplinary team as a matter of course.
Will a second surgery be needed?
Revision surgery. In 2015, about 14% of the almost 200,000 BSs performed were revisions.4 Revision surgery is indicated in BS patients with weight regain, recurrent comorbid diseases (eg, diabetes, hypertension), or complications of primary BS. Restrictive procedures have a higher revision rate than malabsorptive procedures, primarily due to a higher rate of weight regain.6,30
Because revision surgery is associated with more complications and possibly longer hospital stays than primary BS, it should be performed by a bariatric surgeon with extensive experience.30,31 Restrictive revisions are typically converted to malabsorptive procedures. Cost is a limiting factor as many patients’ insurance coverage is limited to one BS per lifetime.
Body contouring. Body contouring surgery (BCS) can improve physical and mental well-being and may be a protective factor for weight regain after bariatric surgery.32 Despite its desirability—particularly to women, adolescents, and those with large decreases in body mass index (BMI)—few patients can afford BCS since it is rarely covered by insurance.
Complications of BCS vary, but are most commonly infection and wound dehiscence. This is, in part, due to poorer wound healing in BS patients compared to those with nonsurgical massive weight loss. The cause of poor wound healing is thought to be secondary to nutritional deficiencies and the catabolic state induced by post-surgical weight loss. Recommendations for BCS include weight stability for more than one year after BS, age >16 years, excess skin causing significant functional impairment, non-smoking status, and presence of good social support.33
Bariatric surgery in adolescents is on the rise
Children in the highest body mass index quartile have more than twice the death rate of those in the lowest BMI quartile.34 Thus, it is not surprising that the rate of BS in adolescents is increasing.7 BS in this age group is successful for weight loss and improvement of comorbid conditions, with relatively low complication rates.35 Options include malabsorptive and restrictive procedures, although gastric banding has not been approved by the US Food and Drug Administration for patients under the age of 18 years.
After BS, adolescent girls should be counseled regarding the possibility of pregnancy (restoration of fertility) and appropriate contraception. Adolescent patients require nutritional supplementation after BS as indicated in TABLE 1.6,8-11
When determining which adolescents to refer for BS, we recommend the following criteria: 35-38
- failure of a minimum 6-month trial of a staged treatment approach, as recommended by Barlow et al,36 including diet, exercise, and pharmacologic treatment
- BMI ≥35 with type 2 diabetes or severe sleep apnea (apnea hypopnea index [AHI] >15)37
- BMI ≥40 with mild sleep apnea (AHI >5), hypertension, or pre-diabetes37
- Tanner stage IV or V
- at least 95% skeletal growth (for malabsorptive surgery).37 This can be determined using an estimated adult height from mid-parental height formula and assessing growth plate closure with hand radiographs for bone age
- appropriate maturity level permitting adherence
- good psychological support
- a multidisciplinary team for postoperative and long-term follow-up care.
Planning for the future: Exploring the possibility of pregnancy
Obesity is the primary cause of maternal and fetal morbidity during pregnancy. It is associated with increased rates of early miscarriage, congenital defects, macrosomia, and fetal death. Maternal risks of obesity include: gestational hypertension, gestational diabetes mellitus (GDM), and pre-eclampsia. Obese mothers also have a higher incidence of failed induction, caesarean section, and breastfeeding failure.10,39 Given that half of all BSs are performed in women of reproductive age, this population deserves special consideration.10
A recent meta-analysis by Galazis et al40 concluded that BS performed prior to pregnancy led to decreased rates of preeclampsia, GDM, large neonates, preterm birth, and neonatal intensive care unit admission. Perinatal mortality did not increase after BS. However, BS led to higher rates of maternal anemia. There was no significant difference between groups in incidence of cesarean section.
The post BS female patient should be advised to use a reliable form of contraception for a minimum of 12 to 18 months after surgery.6,10,39 Involve high-risk obstetric specialists during pregnancies. Diet should be supplemented as indicated in TABLE 1.6,8-11
CORRESPONDENCE
Amy Rothberg, MD, PhD, Domino’s Farms, Lobby G, Suite 1500, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; [email protected].
1. Centers for Disease Control and Prevention. Overweight and obesity. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed April 5, 2017.
2. Centers for Disease Control and Prevention. Overweight and obesity. Childhood obesity facts. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed April 5, 2017.
3. McGinnis JM. Actual causes of death, 1990-2010. Workshop on Determinants of Premature Mortality, September 18, 2013, National Research Council, Washington, DC.
4. American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2015. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 5, 2017.
5. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487.
6. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823-4843.
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1-S27.
8. Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609.
9. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2016;26:966-971.
10. Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18:1023-1027.
11. Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65:574-585.
12. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2014;25:151-158.
13. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population-based, retrospective cohort study. BMJ. 2012;345:e5085.
14. Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061-1065.
15. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2:165-174.
16. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28:741-749.
17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016;61:11-18.
18. Ritz P, Vaurs C, Barigou M, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18:217-223.
19. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479-486.
20. Mala T. Postprandial hyperinsulinemic hypoglycaemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220-1225.
21. Romero-Talamás H, Daigle CR, Aminian A. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161-1165.
22. de Jong JR, Besselink MG, van Ramshorst B, et al. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11:297-305.
23. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
24. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579-591.
25. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150:223-228.
26. El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26:2789-2796.
27. Sverdén E, Mattsson F, Sondén AM, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263:733-737.
28. Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954.
29. Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150-163.
30. Ferrer-Márquez MP, Belda-Lozano R, Solvas-Salmerón MJ, et al. Revisional surgery after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:6-9.
31. Sanchez H, Cabrera A, Cabrera K, et al. Laparoscopic Roux-en-Y gastric bypass as a revision procedure after restrictive bariatric surgery. Obes Surg. 2008;18:1539-1543.
32. van der Beek ES, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20:36-41.
33. Ellison JM, Steffen KJ, Sarwer DB. Body contouring after bariatric surgery. Eur Eat Disord Rev. 2015;23:479-487.
34. Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. NEJM. 2010;362:485-489.
35. Gravelle BL, Broyles M. Interventions of weight reduction and prevention in children and adolescents: update. Am J Ther. 2015;22:159-166.
36. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164-S192.
37. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901-910.
38. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740-746.
39. Nicklas JM, Barbour LA. Optimizing weight for maternal and infant health: tenable, or too late? Expert Rev Endocrinol Metab. 2015;10:227-242.
40. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45-53.
More than one-third of American adults and approximately 17% of children and adolescents between the ages of 2 and 19 years are obese.1,2 Poor diet coupled with a sedentary lifestyle is the highest ranked cause of non-communicable disease and a leading cause of preventable death, according to the National Research Council.3
Bariatric surgery (BS) is a viable therapeutic option for obese patients who do not respond to conventional lifestyle interventions for losing weight. There are multiple gastrointestinal (GI) procedures available that are classified as either malabsorptive (Roux-en-Y gastric bypass [RYGB] and biliopancreatic diversion [BPD] with or without duodenal switch) or restrictive (laparoscopic adjustable gastric banding [LAGB] and vertical sleeve gastrectomy [VSG]).
Approximately half of the 196,000 bariatric procedures performed in the United States in 2015 were of the sleeve variety, another 23% were RYGB, and the remaining percentage was divided among the other types.4 Postoperative risks include nutritional deficiencies, decreased bone mineral density (BMD), dumping syndrome (when food rapidly dumps from the stomach to the intestine), and gastroesophageal reflux disease (GERD) with possible ulceration.
Despite these potential complications, a systematic review and meta-analysis found that obese people who underwent BS (gastric banding or gastric bypass) had significantly reduced risks of global, non-cardiovascular (CV), and CV mortality compared with obese controls.5 Helping patients to realize these benefits requires that the entire health care team—especially the family physician—is aware of the special considerations for this population.
To that end, this article reviews the details of diagnosing and managing post-surgical complications. It also addresses issues unique to managing certain subpopulations, such as post-BS patients who require revision surgery or who want to pursue body contouring surgery; adolescents who undergo BS surgery; and women who want to get pregnant postoperatively.
Monitor patients for these post-surgery complications
Postoperative BS follow-up varies depending on location, surgeon preference, and availability of multidisciplinary resources. At our institution, patients have a minimum of 3 follow-up visits with their surgeon (during hospitalization and 2 weeks and 2 months postoperatively). This is followed by visits with Endocrinology 6 months after surgery and annually thereafter. Given the variability of follow-up, family physicians should coordinate with specialists where appropriate and be aware of postoperative complications and monitoring since it is likely they will have the most frequent contact with these patients.
Nutritional deficiencies are common and require lifelong screening
Nutritional deficiencies are the most common complications of malabsorptive BS. Guidelines from the Endocrine Society, as well as guidelines from the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS), recommend routine lifetime screening for deficiencies after surgery.6,7 Complete blood cell count, electrolytes, glucose, creatinine, and liver function tests should be obtained at one, 3, 6, 12, 18, and 24 months following surgery and annually thereafter.6

Multiple factors contribute to nutritional and micronutrient deficiencies, including reduced oral intake of food, decreased GI absorption, food intolerance, nausea/vomiting, and nonadherence with dietary supplements.8 Oral supplementation should be in chewable, powder, or liquid form because pill and capsule absorption may be altered.8,9 Over-the-counter multivitamins may not contain the requisite daily doses recommended after BS.9 Patients and physicians should evaluate supplements together to ensure appropriate nutritional and micronutrient supplementation (TABLE 16,8-11).

Bone mineral density can start to decrease soon after surgery
Studies evaluating BMD after BS have produced variable findings. In obese patients, dual-energy x-ray absorptiometry (DEXA) measurements may not be accurate due to adipose tissue artifact and table weight limits. In addition, limited data exist on the incidence of fractures after BS. Of 2 notable studies, only one, a population-based study involving 258 Minnesota residents who underwent a first bariatric surgery between 1985 and 2004, demonstrated a significantly increased incidence of fractures.12,13
In addition, studies show bone turnover markers, including C-terminal telopeptide, increase as early as 3 months after BS.14 Several guidelines recommend routine BMD screening after BS (TABLE 2).6,7 The mechanism of bone demineralization is likely multifactorial—a function of the magnitude of the weight loss and skeletal unloading, calcium and vitamin D deficiencies, and associated secondary hyperparathyroidism.15 Treatment for secondary hyperparathyroidism is adequate supplementation with vitamin D and calcium.

Optimal dosing for vitamin D has not been determined. One recent systematic review suggests routine prophylaxis with at least 2000 international units (IU)/d and found the greatest improvement for known deficiency with doses of 1500-9100 IU/d following malabsorptive surgeries.11 After laparoscopic sleeve gastrectomy, at least 1000 IU/d vitamin D is recommended.11
Overall, high variability exists among patients, and an individualized approach for dosing is recommended.11 Vitamin D levels should be monitored 2 and 4 weeks after initiation of treatment and every 3 months thereafter.11 Normal levels of serum calcium, 25-OH vitamin D, bone-specific alkaline phosphatase, and 24-hour urinary calcium excretion indicate adequate calcium and vitamin D supplementation.6
Dumping syndrome can lead to hypoglycemia
Dumping syndrome is a common complication following BS, with prevalence ranging from 25% to 75%, depending upon the type of procedure performed.16,17 There are 2 types: early and late. Early dumping syndrome occurs within 30 minutes of eating. Symptoms are related to the robust release of gastrointestinal hormones caused by rapid gastric emptying. Symptoms include nausea, abdominal pain, diarrhea, flushing, hypotension, and tachycardia.
Late dumping is characterized as postprandial hypoglycemia occurring one to 3 hours after eating. Late dumping is likely caused by a combination of changes within the pancreatic beta cells and abnormal insulin response to glucose.16-18 Rapid gastric emptying leads to rapid release of glucose in the gut, which, in turn, leads to brisk insulin secretion. Since glucose is absorbed faster than insulin’s half-life, the resulting (relatively) high levels of insulin may cause hypoglycemia.16-18
Sigstad’s scoring system can be used to confirm suspected cases of dumping syndrome (TABLE 316,17,19). A diagnosis can also be made with an oral glucose challenge in which pulse, blood pressure, glucose, and hematocrit are measured after ingestion of 50 g glucose. The test is positive if heart rate increases by 10 beats per minute, hematocrit increases by 3% 30 minutes after ingestion, or glucose falls below 60 mg/dL 2 to 3 hours after ingestion.17

First-line treatment of dumping syndrome consists of dietary modifications. The goal is to slow the rate of gastric emptying by eating smaller, more frequent meals; separating beverages from food; decreasing carbohydrates; and increasing fiber and protein content.
If results are suboptimal after dietary changes, medications can be prescribed including acarbose to prevent postprandial hypoglycemia; anticholinergics such as dicyclomine to slow gastric emptying; and somatostatin to decrease gastric emptying and inhibit GI hormone release.17 Lastly, for resistant and severe postprandial hypoglycemia, a few patients have undergone pancreatectomy, but only about 65% experienced improvement in symptoms and 12% developed diabetes post-surgically.20
Gout attacks may initially increase, but then decrease
BS affects the incidence of gout attacks in patients with a history of gout. One comparative study of approximately 150 patients demonstrated that those with a history of gout had significantly more gout attacks in the first month after BS compared with obese patients with a history of gout undergoing other upper GI surgeries.21 There was no difference between malabsorptive and restrictive procedures. But after the first month, BS patients had significantly fewer gout attacks and lower uric acid levels than their obese counterparts.21
Protein rich diets, catabolism potentiated by aggressive caloric restriction following BS, and dehydration contribute to the initial increase. Therefore, patients who have had at least one gout attack in the year prior to surgery or who are on hypouricemic medication may benefit from at least one month of prophylactic therapy (eg, allopurinol and colchicine) after surgery.
GERD and ulceration: How to respond
Obesity is a known risk factor for GERD, but the effect of BS on GERD is uncertain and seems to vary with the procedure performed. RYGB decreases GERD and is, therefore, used as both a secondary treatment in those not responding to medications and a revision treatment for fundoplication and other types of BSs. Sleeve gastrectomy and adjustable gastric banding have mixed effects on GERD. A systematic review by de Jong et al revealed a decreased prevalence of reflux symptoms and GERD medication use after LAGB; however, during longer follow-up, 15% of previously unaffected patients reported experiencing GERD.22 The 2011 International Sleeve Gastrectomy Expert Panel Consensus Statement retrospectively noted a postoperative incidence of GERD as high as 31%.23
BS patients with GERD should be treated with a proton pump inhibitor. If this fails, refer patients to a gastroenterologist for further evaluation.24
Ulcers after BS may be an indication for revision surgery. Data are mixed regarding increased risk of marginal ulceration from nonsteroidal anti-inflammatory drug (NSAID) use, but NSAIDs have been linked to an increased risk of anastomotic leakage.25-28 Thus, it seems prudent to avoid NSAIDs in people who have undergone BS.
Keeping watch over psychiatric comorbidities
A recent meta-analysis by Dawes et al29 showed that about 23% of patients pursuing BS have a comorbid mood disorder. Specifically, the preoperative prevalence of depression (19%) and binge-eating disorder (17%) were found to be higher than rates in the general population.29 The meta-analysis found improvement in the prevalence of depression with fewer symptoms and less antidepressant medication use in the first 3 years after surgery and a decrease in the rate of binge-eating disorder, although there were fewer supporting data for the latter. These findings were observed with both restrictive and malabsorptive procedures.
The data are mixed regarding rates of alcohol abuse and suicide. Further research is necessary in this field. Patients who have had BS should receive ongoing psychiatric and psychological care from a multidisciplinary team as a matter of course.
Will a second surgery be needed?
Revision surgery. In 2015, about 14% of the almost 200,000 BSs performed were revisions.4 Revision surgery is indicated in BS patients with weight regain, recurrent comorbid diseases (eg, diabetes, hypertension), or complications of primary BS. Restrictive procedures have a higher revision rate than malabsorptive procedures, primarily due to a higher rate of weight regain.6,30
Because revision surgery is associated with more complications and possibly longer hospital stays than primary BS, it should be performed by a bariatric surgeon with extensive experience.30,31 Restrictive revisions are typically converted to malabsorptive procedures. Cost is a limiting factor as many patients’ insurance coverage is limited to one BS per lifetime.
Body contouring. Body contouring surgery (BCS) can improve physical and mental well-being and may be a protective factor for weight regain after bariatric surgery.32 Despite its desirability—particularly to women, adolescents, and those with large decreases in body mass index (BMI)—few patients can afford BCS since it is rarely covered by insurance.
Complications of BCS vary, but are most commonly infection and wound dehiscence. This is, in part, due to poorer wound healing in BS patients compared to those with nonsurgical massive weight loss. The cause of poor wound healing is thought to be secondary to nutritional deficiencies and the catabolic state induced by post-surgical weight loss. Recommendations for BCS include weight stability for more than one year after BS, age >16 years, excess skin causing significant functional impairment, non-smoking status, and presence of good social support.33
Bariatric surgery in adolescents is on the rise
Children in the highest body mass index quartile have more than twice the death rate of those in the lowest BMI quartile.34 Thus, it is not surprising that the rate of BS in adolescents is increasing.7 BS in this age group is successful for weight loss and improvement of comorbid conditions, with relatively low complication rates.35 Options include malabsorptive and restrictive procedures, although gastric banding has not been approved by the US Food and Drug Administration for patients under the age of 18 years.
After BS, adolescent girls should be counseled regarding the possibility of pregnancy (restoration of fertility) and appropriate contraception. Adolescent patients require nutritional supplementation after BS as indicated in TABLE 1.6,8-11
When determining which adolescents to refer for BS, we recommend the following criteria: 35-38
- failure of a minimum 6-month trial of a staged treatment approach, as recommended by Barlow et al,36 including diet, exercise, and pharmacologic treatment
- BMI ≥35 with type 2 diabetes or severe sleep apnea (apnea hypopnea index [AHI] >15)37
- BMI ≥40 with mild sleep apnea (AHI >5), hypertension, or pre-diabetes37
- Tanner stage IV or V
- at least 95% skeletal growth (for malabsorptive surgery).37 This can be determined using an estimated adult height from mid-parental height formula and assessing growth plate closure with hand radiographs for bone age
- appropriate maturity level permitting adherence
- good psychological support
- a multidisciplinary team for postoperative and long-term follow-up care.
Planning for the future: Exploring the possibility of pregnancy
Obesity is the primary cause of maternal and fetal morbidity during pregnancy. It is associated with increased rates of early miscarriage, congenital defects, macrosomia, and fetal death. Maternal risks of obesity include: gestational hypertension, gestational diabetes mellitus (GDM), and pre-eclampsia. Obese mothers also have a higher incidence of failed induction, caesarean section, and breastfeeding failure.10,39 Given that half of all BSs are performed in women of reproductive age, this population deserves special consideration.10
A recent meta-analysis by Galazis et al40 concluded that BS performed prior to pregnancy led to decreased rates of preeclampsia, GDM, large neonates, preterm birth, and neonatal intensive care unit admission. Perinatal mortality did not increase after BS. However, BS led to higher rates of maternal anemia. There was no significant difference between groups in incidence of cesarean section.
The post BS female patient should be advised to use a reliable form of contraception for a minimum of 12 to 18 months after surgery.6,10,39 Involve high-risk obstetric specialists during pregnancies. Diet should be supplemented as indicated in TABLE 1.6,8-11
CORRESPONDENCE
Amy Rothberg, MD, PhD, Domino’s Farms, Lobby G, Suite 1500, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; [email protected].
More than one-third of American adults and approximately 17% of children and adolescents between the ages of 2 and 19 years are obese.1,2 Poor diet coupled with a sedentary lifestyle is the highest ranked cause of non-communicable disease and a leading cause of preventable death, according to the National Research Council.3
Bariatric surgery (BS) is a viable therapeutic option for obese patients who do not respond to conventional lifestyle interventions for losing weight. There are multiple gastrointestinal (GI) procedures available that are classified as either malabsorptive (Roux-en-Y gastric bypass [RYGB] and biliopancreatic diversion [BPD] with or without duodenal switch) or restrictive (laparoscopic adjustable gastric banding [LAGB] and vertical sleeve gastrectomy [VSG]).
Approximately half of the 196,000 bariatric procedures performed in the United States in 2015 were of the sleeve variety, another 23% were RYGB, and the remaining percentage was divided among the other types.4 Postoperative risks include nutritional deficiencies, decreased bone mineral density (BMD), dumping syndrome (when food rapidly dumps from the stomach to the intestine), and gastroesophageal reflux disease (GERD) with possible ulceration.
Despite these potential complications, a systematic review and meta-analysis found that obese people who underwent BS (gastric banding or gastric bypass) had significantly reduced risks of global, non-cardiovascular (CV), and CV mortality compared with obese controls.5 Helping patients to realize these benefits requires that the entire health care team—especially the family physician—is aware of the special considerations for this population.
To that end, this article reviews the details of diagnosing and managing post-surgical complications. It also addresses issues unique to managing certain subpopulations, such as post-BS patients who require revision surgery or who want to pursue body contouring surgery; adolescents who undergo BS surgery; and women who want to get pregnant postoperatively.
Monitor patients for these post-surgery complications
Postoperative BS follow-up varies depending on location, surgeon preference, and availability of multidisciplinary resources. At our institution, patients have a minimum of 3 follow-up visits with their surgeon (during hospitalization and 2 weeks and 2 months postoperatively). This is followed by visits with Endocrinology 6 months after surgery and annually thereafter. Given the variability of follow-up, family physicians should coordinate with specialists where appropriate and be aware of postoperative complications and monitoring since it is likely they will have the most frequent contact with these patients.
Nutritional deficiencies are common and require lifelong screening
Nutritional deficiencies are the most common complications of malabsorptive BS. Guidelines from the Endocrine Society, as well as guidelines from the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS), recommend routine lifetime screening for deficiencies after surgery.6,7 Complete blood cell count, electrolytes, glucose, creatinine, and liver function tests should be obtained at one, 3, 6, 12, 18, and 24 months following surgery and annually thereafter.6

Multiple factors contribute to nutritional and micronutrient deficiencies, including reduced oral intake of food, decreased GI absorption, food intolerance, nausea/vomiting, and nonadherence with dietary supplements.8 Oral supplementation should be in chewable, powder, or liquid form because pill and capsule absorption may be altered.8,9 Over-the-counter multivitamins may not contain the requisite daily doses recommended after BS.9 Patients and physicians should evaluate supplements together to ensure appropriate nutritional and micronutrient supplementation (TABLE 16,8-11).

Bone mineral density can start to decrease soon after surgery
Studies evaluating BMD after BS have produced variable findings. In obese patients, dual-energy x-ray absorptiometry (DEXA) measurements may not be accurate due to adipose tissue artifact and table weight limits. In addition, limited data exist on the incidence of fractures after BS. Of 2 notable studies, only one, a population-based study involving 258 Minnesota residents who underwent a first bariatric surgery between 1985 and 2004, demonstrated a significantly increased incidence of fractures.12,13
In addition, studies show bone turnover markers, including C-terminal telopeptide, increase as early as 3 months after BS.14 Several guidelines recommend routine BMD screening after BS (TABLE 2).6,7 The mechanism of bone demineralization is likely multifactorial—a function of the magnitude of the weight loss and skeletal unloading, calcium and vitamin D deficiencies, and associated secondary hyperparathyroidism.15 Treatment for secondary hyperparathyroidism is adequate supplementation with vitamin D and calcium.

Optimal dosing for vitamin D has not been determined. One recent systematic review suggests routine prophylaxis with at least 2000 international units (IU)/d and found the greatest improvement for known deficiency with doses of 1500-9100 IU/d following malabsorptive surgeries.11 After laparoscopic sleeve gastrectomy, at least 1000 IU/d vitamin D is recommended.11
Overall, high variability exists among patients, and an individualized approach for dosing is recommended.11 Vitamin D levels should be monitored 2 and 4 weeks after initiation of treatment and every 3 months thereafter.11 Normal levels of serum calcium, 25-OH vitamin D, bone-specific alkaline phosphatase, and 24-hour urinary calcium excretion indicate adequate calcium and vitamin D supplementation.6
Dumping syndrome can lead to hypoglycemia
Dumping syndrome is a common complication following BS, with prevalence ranging from 25% to 75%, depending upon the type of procedure performed.16,17 There are 2 types: early and late. Early dumping syndrome occurs within 30 minutes of eating. Symptoms are related to the robust release of gastrointestinal hormones caused by rapid gastric emptying. Symptoms include nausea, abdominal pain, diarrhea, flushing, hypotension, and tachycardia.
Late dumping is characterized as postprandial hypoglycemia occurring one to 3 hours after eating. Late dumping is likely caused by a combination of changes within the pancreatic beta cells and abnormal insulin response to glucose.16-18 Rapid gastric emptying leads to rapid release of glucose in the gut, which, in turn, leads to brisk insulin secretion. Since glucose is absorbed faster than insulin’s half-life, the resulting (relatively) high levels of insulin may cause hypoglycemia.16-18
Sigstad’s scoring system can be used to confirm suspected cases of dumping syndrome (TABLE 316,17,19). A diagnosis can also be made with an oral glucose challenge in which pulse, blood pressure, glucose, and hematocrit are measured after ingestion of 50 g glucose. The test is positive if heart rate increases by 10 beats per minute, hematocrit increases by 3% 30 minutes after ingestion, or glucose falls below 60 mg/dL 2 to 3 hours after ingestion.17

First-line treatment of dumping syndrome consists of dietary modifications. The goal is to slow the rate of gastric emptying by eating smaller, more frequent meals; separating beverages from food; decreasing carbohydrates; and increasing fiber and protein content.
If results are suboptimal after dietary changes, medications can be prescribed including acarbose to prevent postprandial hypoglycemia; anticholinergics such as dicyclomine to slow gastric emptying; and somatostatin to decrease gastric emptying and inhibit GI hormone release.17 Lastly, for resistant and severe postprandial hypoglycemia, a few patients have undergone pancreatectomy, but only about 65% experienced improvement in symptoms and 12% developed diabetes post-surgically.20
Gout attacks may initially increase, but then decrease
BS affects the incidence of gout attacks in patients with a history of gout. One comparative study of approximately 150 patients demonstrated that those with a history of gout had significantly more gout attacks in the first month after BS compared with obese patients with a history of gout undergoing other upper GI surgeries.21 There was no difference between malabsorptive and restrictive procedures. But after the first month, BS patients had significantly fewer gout attacks and lower uric acid levels than their obese counterparts.21
Protein rich diets, catabolism potentiated by aggressive caloric restriction following BS, and dehydration contribute to the initial increase. Therefore, patients who have had at least one gout attack in the year prior to surgery or who are on hypouricemic medication may benefit from at least one month of prophylactic therapy (eg, allopurinol and colchicine) after surgery.
GERD and ulceration: How to respond
Obesity is a known risk factor for GERD, but the effect of BS on GERD is uncertain and seems to vary with the procedure performed. RYGB decreases GERD and is, therefore, used as both a secondary treatment in those not responding to medications and a revision treatment for fundoplication and other types of BSs. Sleeve gastrectomy and adjustable gastric banding have mixed effects on GERD. A systematic review by de Jong et al revealed a decreased prevalence of reflux symptoms and GERD medication use after LAGB; however, during longer follow-up, 15% of previously unaffected patients reported experiencing GERD.22 The 2011 International Sleeve Gastrectomy Expert Panel Consensus Statement retrospectively noted a postoperative incidence of GERD as high as 31%.23
BS patients with GERD should be treated with a proton pump inhibitor. If this fails, refer patients to a gastroenterologist for further evaluation.24
Ulcers after BS may be an indication for revision surgery. Data are mixed regarding increased risk of marginal ulceration from nonsteroidal anti-inflammatory drug (NSAID) use, but NSAIDs have been linked to an increased risk of anastomotic leakage.25-28 Thus, it seems prudent to avoid NSAIDs in people who have undergone BS.
Keeping watch over psychiatric comorbidities
A recent meta-analysis by Dawes et al29 showed that about 23% of patients pursuing BS have a comorbid mood disorder. Specifically, the preoperative prevalence of depression (19%) and binge-eating disorder (17%) were found to be higher than rates in the general population.29 The meta-analysis found improvement in the prevalence of depression with fewer symptoms and less antidepressant medication use in the first 3 years after surgery and a decrease in the rate of binge-eating disorder, although there were fewer supporting data for the latter. These findings were observed with both restrictive and malabsorptive procedures.
The data are mixed regarding rates of alcohol abuse and suicide. Further research is necessary in this field. Patients who have had BS should receive ongoing psychiatric and psychological care from a multidisciplinary team as a matter of course.
Will a second surgery be needed?
Revision surgery. In 2015, about 14% of the almost 200,000 BSs performed were revisions.4 Revision surgery is indicated in BS patients with weight regain, recurrent comorbid diseases (eg, diabetes, hypertension), or complications of primary BS. Restrictive procedures have a higher revision rate than malabsorptive procedures, primarily due to a higher rate of weight regain.6,30
Because revision surgery is associated with more complications and possibly longer hospital stays than primary BS, it should be performed by a bariatric surgeon with extensive experience.30,31 Restrictive revisions are typically converted to malabsorptive procedures. Cost is a limiting factor as many patients’ insurance coverage is limited to one BS per lifetime.
Body contouring. Body contouring surgery (BCS) can improve physical and mental well-being and may be a protective factor for weight regain after bariatric surgery.32 Despite its desirability—particularly to women, adolescents, and those with large decreases in body mass index (BMI)—few patients can afford BCS since it is rarely covered by insurance.
Complications of BCS vary, but are most commonly infection and wound dehiscence. This is, in part, due to poorer wound healing in BS patients compared to those with nonsurgical massive weight loss. The cause of poor wound healing is thought to be secondary to nutritional deficiencies and the catabolic state induced by post-surgical weight loss. Recommendations for BCS include weight stability for more than one year after BS, age >16 years, excess skin causing significant functional impairment, non-smoking status, and presence of good social support.33
Bariatric surgery in adolescents is on the rise
Children in the highest body mass index quartile have more than twice the death rate of those in the lowest BMI quartile.34 Thus, it is not surprising that the rate of BS in adolescents is increasing.7 BS in this age group is successful for weight loss and improvement of comorbid conditions, with relatively low complication rates.35 Options include malabsorptive and restrictive procedures, although gastric banding has not been approved by the US Food and Drug Administration for patients under the age of 18 years.
After BS, adolescent girls should be counseled regarding the possibility of pregnancy (restoration of fertility) and appropriate contraception. Adolescent patients require nutritional supplementation after BS as indicated in TABLE 1.6,8-11
When determining which adolescents to refer for BS, we recommend the following criteria: 35-38
- failure of a minimum 6-month trial of a staged treatment approach, as recommended by Barlow et al,36 including diet, exercise, and pharmacologic treatment
- BMI ≥35 with type 2 diabetes or severe sleep apnea (apnea hypopnea index [AHI] >15)37
- BMI ≥40 with mild sleep apnea (AHI >5), hypertension, or pre-diabetes37
- Tanner stage IV or V
- at least 95% skeletal growth (for malabsorptive surgery).37 This can be determined using an estimated adult height from mid-parental height formula and assessing growth plate closure with hand radiographs for bone age
- appropriate maturity level permitting adherence
- good psychological support
- a multidisciplinary team for postoperative and long-term follow-up care.
Planning for the future: Exploring the possibility of pregnancy
Obesity is the primary cause of maternal and fetal morbidity during pregnancy. It is associated with increased rates of early miscarriage, congenital defects, macrosomia, and fetal death. Maternal risks of obesity include: gestational hypertension, gestational diabetes mellitus (GDM), and pre-eclampsia. Obese mothers also have a higher incidence of failed induction, caesarean section, and breastfeeding failure.10,39 Given that half of all BSs are performed in women of reproductive age, this population deserves special consideration.10
A recent meta-analysis by Galazis et al40 concluded that BS performed prior to pregnancy led to decreased rates of preeclampsia, GDM, large neonates, preterm birth, and neonatal intensive care unit admission. Perinatal mortality did not increase after BS. However, BS led to higher rates of maternal anemia. There was no significant difference between groups in incidence of cesarean section.
The post BS female patient should be advised to use a reliable form of contraception for a minimum of 12 to 18 months after surgery.6,10,39 Involve high-risk obstetric specialists during pregnancies. Diet should be supplemented as indicated in TABLE 1.6,8-11
CORRESPONDENCE
Amy Rothberg, MD, PhD, Domino’s Farms, Lobby G, Suite 1500, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; [email protected].
1. Centers for Disease Control and Prevention. Overweight and obesity. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed April 5, 2017.
2. Centers for Disease Control and Prevention. Overweight and obesity. Childhood obesity facts. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed April 5, 2017.
3. McGinnis JM. Actual causes of death, 1990-2010. Workshop on Determinants of Premature Mortality, September 18, 2013, National Research Council, Washington, DC.
4. American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2015. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 5, 2017.
5. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487.
6. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823-4843.
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1-S27.
8. Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609.
9. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2016;26:966-971.
10. Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18:1023-1027.
11. Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65:574-585.
12. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2014;25:151-158.
13. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population-based, retrospective cohort study. BMJ. 2012;345:e5085.
14. Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061-1065.
15. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2:165-174.
16. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28:741-749.
17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016;61:11-18.
18. Ritz P, Vaurs C, Barigou M, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18:217-223.
19. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479-486.
20. Mala T. Postprandial hyperinsulinemic hypoglycaemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220-1225.
21. Romero-Talamás H, Daigle CR, Aminian A. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161-1165.
22. de Jong JR, Besselink MG, van Ramshorst B, et al. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11:297-305.
23. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
24. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579-591.
25. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150:223-228.
26. El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26:2789-2796.
27. Sverdén E, Mattsson F, Sondén AM, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263:733-737.
28. Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954.
29. Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150-163.
30. Ferrer-Márquez MP, Belda-Lozano R, Solvas-Salmerón MJ, et al. Revisional surgery after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:6-9.
31. Sanchez H, Cabrera A, Cabrera K, et al. Laparoscopic Roux-en-Y gastric bypass as a revision procedure after restrictive bariatric surgery. Obes Surg. 2008;18:1539-1543.
32. van der Beek ES, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20:36-41.
33. Ellison JM, Steffen KJ, Sarwer DB. Body contouring after bariatric surgery. Eur Eat Disord Rev. 2015;23:479-487.
34. Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. NEJM. 2010;362:485-489.
35. Gravelle BL, Broyles M. Interventions of weight reduction and prevention in children and adolescents: update. Am J Ther. 2015;22:159-166.
36. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164-S192.
37. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901-910.
38. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740-746.
39. Nicklas JM, Barbour LA. Optimizing weight for maternal and infant health: tenable, or too late? Expert Rev Endocrinol Metab. 2015;10:227-242.
40. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45-53.
1. Centers for Disease Control and Prevention. Overweight and obesity. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed April 5, 2017.
2. Centers for Disease Control and Prevention. Overweight and obesity. Childhood obesity facts. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed April 5, 2017.
3. McGinnis JM. Actual causes of death, 1990-2010. Workshop on Determinants of Premature Mortality, September 18, 2013, National Research Council, Washington, DC.
4. American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2015. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 5, 2017.
5. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487.
6. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823-4843.
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1-S27.
8. Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609.
9. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2016;26:966-971.
10. Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18:1023-1027.
11. Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65:574-585.
12. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2014;25:151-158.
13. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population-based, retrospective cohort study. BMJ. 2012;345:e5085.
14. Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061-1065.
15. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2:165-174.
16. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28:741-749.
17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016;61:11-18.
18. Ritz P, Vaurs C, Barigou M, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18:217-223.
19. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479-486.
20. Mala T. Postprandial hyperinsulinemic hypoglycaemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220-1225.
21. Romero-Talamás H, Daigle CR, Aminian A. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161-1165.
22. de Jong JR, Besselink MG, van Ramshorst B, et al. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11:297-305.
23. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
24. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579-591.
25. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150:223-228.
26. El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26:2789-2796.
27. Sverdén E, Mattsson F, Sondén AM, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263:733-737.
28. Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954.
29. Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150-163.
30. Ferrer-Márquez MP, Belda-Lozano R, Solvas-Salmerón MJ, et al. Revisional surgery after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:6-9.
31. Sanchez H, Cabrera A, Cabrera K, et al. Laparoscopic Roux-en-Y gastric bypass as a revision procedure after restrictive bariatric surgery. Obes Surg. 2008;18:1539-1543.
32. van der Beek ES, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20:36-41.
33. Ellison JM, Steffen KJ, Sarwer DB. Body contouring after bariatric surgery. Eur Eat Disord Rev. 2015;23:479-487.
34. Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. NEJM. 2010;362:485-489.
35. Gravelle BL, Broyles M. Interventions of weight reduction and prevention in children and adolescents: update. Am J Ther. 2015;22:159-166.
36. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164-S192.
37. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901-910.
38. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740-746.
39. Nicklas JM, Barbour LA. Optimizing weight for maternal and infant health: tenable, or too late? Expert Rev Endocrinol Metab. 2015;10:227-242.
40. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45-53.
From The Journal of Family Practice | 2017;66(6):356-363.
PRACTICE RECOMMENDATIONS
› Routinely screen bariatric surgery patients for nutritional deficiencies throughout their life. A
› Avoid the use of nonsteroidal anti-inflammatory medications in patients who have had bariatric surgery becauseof the risk of anastomotic ulceration and leakage. A
› Consider revision surgery for bariatric surgery patients with weight regain, recurrent comorbid diseases, or surgical complications. B
› Counsel obese women who want to become pregnant that bariatric surgery decreases rates of future pregnancy complications. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
