User login
A 68-year-old Filipino man with a history of hypertension, type 2 diabetes, and osteoarthritis presented to the emergency department with a one-week history of increasing pain, swelling, erythema, and seepage of his right great toe. The patient denied paresthesias, fever, chills, night sweats, cough, dyspnea, or any change in his diet, medications (which included lisinopril, metformin, and acetaminophen as needed), or routine. Social history was negative for alcohol use and cigarette smoking.
He previously had similar symptoms in his right fourth toe that resulted in amputation. The patient was told at the time that he had a “bone infection” and amputation was necessary.
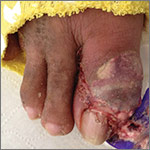
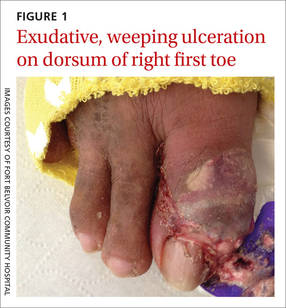
The patient was thin, alert, oriented, and in no acute distress. His vital signs and a cardiopulmonary exam were normal. The patient’s right great toe was tender to touch, with ulceration of the skin dorsally at the proximal nail fold. In addition, his toe was oozing a purulent, non-foul smelling discharge (FIGURE 1). Other pertinent findings included multiple enlarged joints on both hands with visible yellow-white subcutaneous nodules on the hands and dorsum of the forearm (FIGURE 2).
The patient’s white blood cell count was 10,800/mcL, C-reactive protein (CRP) was 18 mg/L, erythrocyte sedimentation rate (ESR) was 80 mm/hr, and uric acid was 12.5 mg/dL. His blood urea nitrogen was 52 mg/dL and creatinine was 2.5 mg/dL. A glycated hemoglobin test was 7.2%. A wound culture, aspirate from the dorsum of the toe, and x-ray were obtained.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tophaceous gouty arthritis
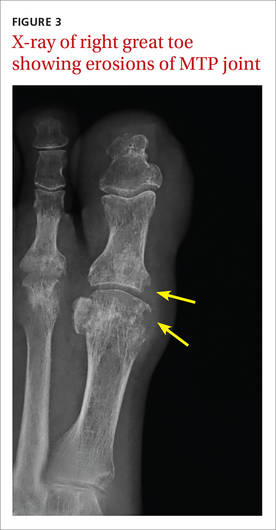
The x-ray of the right great toe showed erosions of the metatarsophalangeal (MTP) joint (FIGURE 3). Given the patient’s age, underlying diabetes, skin ulceration, and elevation of CRP and ESR, the initial concern was for septic arthritis and osteomyelitis. However, the absence of leukocytosis and hyperglycemia argued against an infectious process.
The diagnosis of tophaceous gouty arthritis was confirmed by aspiration from the tophus, which demonstrated monosodium urate (MSU) crystals on polarized light microscopy. The patient presented with acute gout on the first right toe overlaying chronic tophaceous gout, complicated by renal failure.
Four phases. Gout progresses through 4 phases: asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout (intervals between acute attacks), and chronic tophaceous gout.1 Chronic tophaceous gout is characterized by tophi—collections of solid urate in connective tissues (from bone to bursa, tendons, ligaments, and entheses).2 There are often multiple tophi and they may be calcified. An acute gouty attack is marked by a relatively sudden increase in pain and swelling, and may improve spontaneously over the course of 7 to 10 days.3
Gout is the most common form of inflammatory arthritis, with a prevalence in the United States of 3.9%.4The findings of several studies suggest that the prevalence and incidence of gout have risen in recent decades, which may be attributable to a growing aging population, the rise in obesity, increasing numbers of people who have other conditions such as heart disease, kidney disease, and/or diabetes, and the use of diuretics by individuals with cardiovascular disease.5
In a meta-analysis on gouty involvement of the first MTP joint, the occurrence of acute first MTP arthritis has been reported to be an independent predictor of MSU crystal presence in patients with gout.6 The presence of first MTP arthritis and the predilection for MSU deposition in the medial and dorsal aspects of the joint suggested an association, but no causation, between the 2 disease processes. The authors concluded that the distinction between osteoarthritis and gout as the cause of the joint damage is often difficult.
The diagnosis of gout may be made clinically based on established clinical criteria. The most commonly used are the 1977 American College of Rheumatology (ACR) criteria for the classification of acute arthritis of primary gout. (See “The 12 diagnostic criteria for gout.”7) However, in 2015, the ACR/European League Against Rheumatism (EULAR) published a new set of criteria that include the signs and symptoms of chronic gout, as well.8 (The ACR-EULAR Gout Classification Criteria Calculator may be accessed at http://goutclassificationcalculator.auckland.ac.nz/.)
The 12 diagnostic criteria for gout7
1. Recurrent arthritic attack
2. Joint redness
3. Pain or swelling in the first metatarsophalangeal joint
4. Unilateral attack involving the first metatarsophalangeal joint
5. Unilateral attack involving the tarsal joint
6. Suspected tophus
7. Hyperuricemia
8. Radiographic evidence of asymmetric swelling within a joint
9. Attack of monoarticular arthritis
10. Development of maximal inflammation within one day
11. Negative culture of joint fluid for microorganisms during joint inflammation attack
12. Radiographic evidence of subcortical cyst without erosions
Differential diagnosis includes trauma, septic and reactive arthritis
The differential diagnosis of acute gouty arthritis includes trauma, pseudogout (arthritis involving calcium pyrophosphate dehydrate), septic arthritis, reactive arthritis, post-streptococcal arthritis, and Lyme disease.
Trauma with a resulting acute or stress fracture can be determined by x-ray or magnetic resonance imaging (MRI).
Pseudogout requires aspiration of fluid and examination for calcium pyrophosphate crystals under polarizing microscopy.9
Septic arthritis may present in a similar manner to other causes of acute arthritis. Therefore, arthrocentesis is needed to identify the causative infectious agent.10 Septic arthritis was considered in our patient, given his age, history of diabetes mellitus, and finding of skin ulceration over the toe. However, our patient did not have systemic symptoms, and the joint aspiration did not show the presence of bacteria.
Reactive arthritis typically presents with inflammation of the ligaments and tendons at their sites of insertion into the bone, and can affect other areas of the body, such as the genitourinary and ocular systems.11
Post-streptococcal arthritis (aka acute rheumatic fever [ARF]) and Lyme disease can also present with joint complaints. The arthritis in ARF is usually migratory and involves several joints. Fever, rash, and a history of group A streptococcal infection are also diagnostic features.12 History of travel to an endemic area or seasonal exposure would further differentiate Lyme disease from other causes of arthritis.
Deciding on a course of treatment
Initial treatment choices for acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.13 Various NSAIDs have been studied in the treatment of acute gout, but none showed absolute superiority over others. One option is naproxen 500 mg twice daily, but the choice of NSAID is mainly based on the adverse reaction profile and the physician’s preference.
Due to frequent gastrointestinal adverse effects and the concern for toxicity and drug interactions, colchicine and corticosteroids are not typically the first-line agents for acute gout treatment. Patients with frequent recurrent gouty attacks require urate-lowering therapies, such as allopurinol or probenecid. Other indications for urate-lowering therapies include evidence of tophaceous deposits in joints and soft tissues and gouty arthropathy.
Our patient’s renal failure was likely chronic, secondary to his untreated tophaceous gouty disease. Because his creatinine clearance was between 30 and 60 mL/min per 1.73 m2, he was not treated with NSAIDs, but with colchicine 1.2 mg for his initial flare, followed with a single dose of 0.6 mg in one hour. The patient had dramatic improvement of his pain the following day.
Patient education on chronic gout was also provided. We specifically discussed the patient’s dietary habits with him, advising him to minimize his intake of seafood, animal organs, and red meat products, which are high in purines. The patient was also told that he would need to start urate-lowering therapy to prevent recurrent gouty attacks and further complications from gout.
CORRESPONDENCE
Joseph Huang, MD, Fort Belvoir Community Hospital, Family Medicine Clinic, 1st Floor Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925-934.
2. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis. 2013;72:1545-1548.
3. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;CD006190.
4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136-3141.
5. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
6. Stewart S, Dalbeth N, Vandal AC, et al. The first metatarsophalangeal joint in gout: a systemic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:69.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
9. Rosenthal AK. Pseudogout: Presentation, natural history, and associated conditions. In: Wortmann RL, Schumacher HR Jr, Becker MA, et al, eds. Crystal-Induced Arthropathies: Gout, Pseudogout, and Apatite-Associated Syndromes. New York: Taylor and Francis Group;2006:99.
10. Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84:653-660.
11. Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Physician. 1999;60:499-507.
12. Beaudoin A, Edison L, Introcaso CE, et al; Centers for Disease Control and Prevention (CDC). Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64:555-558.
13. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
A 68-year-old Filipino man with a history of hypertension, type 2 diabetes, and osteoarthritis presented to the emergency department with a one-week history of increasing pain, swelling, erythema, and seepage of his right great toe. The patient denied paresthesias, fever, chills, night sweats, cough, dyspnea, or any change in his diet, medications (which included lisinopril, metformin, and acetaminophen as needed), or routine. Social history was negative for alcohol use and cigarette smoking.
He previously had similar symptoms in his right fourth toe that resulted in amputation. The patient was told at the time that he had a “bone infection” and amputation was necessary.
The patient was thin, alert, oriented, and in no acute distress. His vital signs and a cardiopulmonary exam were normal. The patient’s right great toe was tender to touch, with ulceration of the skin dorsally at the proximal nail fold. In addition, his toe was oozing a purulent, non-foul smelling discharge (FIGURE 1). Other pertinent findings included multiple enlarged joints on both hands with visible yellow-white subcutaneous nodules on the hands and dorsum of the forearm (FIGURE 2).
The patient’s white blood cell count was 10,800/mcL, C-reactive protein (CRP) was 18 mg/L, erythrocyte sedimentation rate (ESR) was 80 mm/hr, and uric acid was 12.5 mg/dL. His blood urea nitrogen was 52 mg/dL and creatinine was 2.5 mg/dL. A glycated hemoglobin test was 7.2%. A wound culture, aspirate from the dorsum of the toe, and x-ray were obtained.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tophaceous gouty arthritis
The x-ray of the right great toe showed erosions of the metatarsophalangeal (MTP) joint (FIGURE 3). Given the patient’s age, underlying diabetes, skin ulceration, and elevation of CRP and ESR, the initial concern was for septic arthritis and osteomyelitis. However, the absence of leukocytosis and hyperglycemia argued against an infectious process.

The diagnosis of tophaceous gouty arthritis was confirmed by aspiration from the tophus, which demonstrated monosodium urate (MSU) crystals on polarized light microscopy. The patient presented with acute gout on the first right toe overlaying chronic tophaceous gout, complicated by renal failure.
Four phases. Gout progresses through 4 phases: asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout (intervals between acute attacks), and chronic tophaceous gout.1 Chronic tophaceous gout is characterized by tophi—collections of solid urate in connective tissues (from bone to bursa, tendons, ligaments, and entheses).2 There are often multiple tophi and they may be calcified. An acute gouty attack is marked by a relatively sudden increase in pain and swelling, and may improve spontaneously over the course of 7 to 10 days.3
Gout is the most common form of inflammatory arthritis, with a prevalence in the United States of 3.9%.4The findings of several studies suggest that the prevalence and incidence of gout have risen in recent decades, which may be attributable to a growing aging population, the rise in obesity, increasing numbers of people who have other conditions such as heart disease, kidney disease, and/or diabetes, and the use of diuretics by individuals with cardiovascular disease.5
In a meta-analysis on gouty involvement of the first MTP joint, the occurrence of acute first MTP arthritis has been reported to be an independent predictor of MSU crystal presence in patients with gout.6 The presence of first MTP arthritis and the predilection for MSU deposition in the medial and dorsal aspects of the joint suggested an association, but no causation, between the 2 disease processes. The authors concluded that the distinction between osteoarthritis and gout as the cause of the joint damage is often difficult.
The diagnosis of gout may be made clinically based on established clinical criteria. The most commonly used are the 1977 American College of Rheumatology (ACR) criteria for the classification of acute arthritis of primary gout. (See “The 12 diagnostic criteria for gout.”7) However, in 2015, the ACR/European League Against Rheumatism (EULAR) published a new set of criteria that include the signs and symptoms of chronic gout, as well.8 (The ACR-EULAR Gout Classification Criteria Calculator may be accessed at http://goutclassificationcalculator.auckland.ac.nz/.)
The 12 diagnostic criteria for gout7
1. Recurrent arthritic attack
2. Joint redness
3. Pain or swelling in the first metatarsophalangeal joint
4. Unilateral attack involving the first metatarsophalangeal joint
5. Unilateral attack involving the tarsal joint
6. Suspected tophus
7. Hyperuricemia
8. Radiographic evidence of asymmetric swelling within a joint
9. Attack of monoarticular arthritis
10. Development of maximal inflammation within one day
11. Negative culture of joint fluid for microorganisms during joint inflammation attack
12. Radiographic evidence of subcortical cyst without erosions
Differential diagnosis includes trauma, septic and reactive arthritis
The differential diagnosis of acute gouty arthritis includes trauma, pseudogout (arthritis involving calcium pyrophosphate dehydrate), septic arthritis, reactive arthritis, post-streptococcal arthritis, and Lyme disease.
Trauma with a resulting acute or stress fracture can be determined by x-ray or magnetic resonance imaging (MRI).
Pseudogout requires aspiration of fluid and examination for calcium pyrophosphate crystals under polarizing microscopy.9
Septic arthritis may present in a similar manner to other causes of acute arthritis. Therefore, arthrocentesis is needed to identify the causative infectious agent.10 Septic arthritis was considered in our patient, given his age, history of diabetes mellitus, and finding of skin ulceration over the toe. However, our patient did not have systemic symptoms, and the joint aspiration did not show the presence of bacteria.
Reactive arthritis typically presents with inflammation of the ligaments and tendons at their sites of insertion into the bone, and can affect other areas of the body, such as the genitourinary and ocular systems.11
Post-streptococcal arthritis (aka acute rheumatic fever [ARF]) and Lyme disease can also present with joint complaints. The arthritis in ARF is usually migratory and involves several joints. Fever, rash, and a history of group A streptococcal infection are also diagnostic features.12 History of travel to an endemic area or seasonal exposure would further differentiate Lyme disease from other causes of arthritis.
Deciding on a course of treatment
Initial treatment choices for acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.13 Various NSAIDs have been studied in the treatment of acute gout, but none showed absolute superiority over others. One option is naproxen 500 mg twice daily, but the choice of NSAID is mainly based on the adverse reaction profile and the physician’s preference.
Due to frequent gastrointestinal adverse effects and the concern for toxicity and drug interactions, colchicine and corticosteroids are not typically the first-line agents for acute gout treatment. Patients with frequent recurrent gouty attacks require urate-lowering therapies, such as allopurinol or probenecid. Other indications for urate-lowering therapies include evidence of tophaceous deposits in joints and soft tissues and gouty arthropathy.
Our patient’s renal failure was likely chronic, secondary to his untreated tophaceous gouty disease. Because his creatinine clearance was between 30 and 60 mL/min per 1.73 m2, he was not treated with NSAIDs, but with colchicine 1.2 mg for his initial flare, followed with a single dose of 0.6 mg in one hour. The patient had dramatic improvement of his pain the following day.
Patient education on chronic gout was also provided. We specifically discussed the patient’s dietary habits with him, advising him to minimize his intake of seafood, animal organs, and red meat products, which are high in purines. The patient was also told that he would need to start urate-lowering therapy to prevent recurrent gouty attacks and further complications from gout.
CORRESPONDENCE
Joseph Huang, MD, Fort Belvoir Community Hospital, Family Medicine Clinic, 1st Floor Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
A 68-year-old Filipino man with a history of hypertension, type 2 diabetes, and osteoarthritis presented to the emergency department with a one-week history of increasing pain, swelling, erythema, and seepage of his right great toe. The patient denied paresthesias, fever, chills, night sweats, cough, dyspnea, or any change in his diet, medications (which included lisinopril, metformin, and acetaminophen as needed), or routine. Social history was negative for alcohol use and cigarette smoking.
He previously had similar symptoms in his right fourth toe that resulted in amputation. The patient was told at the time that he had a “bone infection” and amputation was necessary.
The patient was thin, alert, oriented, and in no acute distress. His vital signs and a cardiopulmonary exam were normal. The patient’s right great toe was tender to touch, with ulceration of the skin dorsally at the proximal nail fold. In addition, his toe was oozing a purulent, non-foul smelling discharge (FIGURE 1). Other pertinent findings included multiple enlarged joints on both hands with visible yellow-white subcutaneous nodules on the hands and dorsum of the forearm (FIGURE 2).
The patient’s white blood cell count was 10,800/mcL, C-reactive protein (CRP) was 18 mg/L, erythrocyte sedimentation rate (ESR) was 80 mm/hr, and uric acid was 12.5 mg/dL. His blood urea nitrogen was 52 mg/dL and creatinine was 2.5 mg/dL. A glycated hemoglobin test was 7.2%. A wound culture, aspirate from the dorsum of the toe, and x-ray were obtained.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tophaceous gouty arthritis
The x-ray of the right great toe showed erosions of the metatarsophalangeal (MTP) joint (FIGURE 3). Given the patient’s age, underlying diabetes, skin ulceration, and elevation of CRP and ESR, the initial concern was for septic arthritis and osteomyelitis. However, the absence of leukocytosis and hyperglycemia argued against an infectious process.

The diagnosis of tophaceous gouty arthritis was confirmed by aspiration from the tophus, which demonstrated monosodium urate (MSU) crystals on polarized light microscopy. The patient presented with acute gout on the first right toe overlaying chronic tophaceous gout, complicated by renal failure.
Four phases. Gout progresses through 4 phases: asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout (intervals between acute attacks), and chronic tophaceous gout.1 Chronic tophaceous gout is characterized by tophi—collections of solid urate in connective tissues (from bone to bursa, tendons, ligaments, and entheses).2 There are often multiple tophi and they may be calcified. An acute gouty attack is marked by a relatively sudden increase in pain and swelling, and may improve spontaneously over the course of 7 to 10 days.3
Gout is the most common form of inflammatory arthritis, with a prevalence in the United States of 3.9%.4The findings of several studies suggest that the prevalence and incidence of gout have risen in recent decades, which may be attributable to a growing aging population, the rise in obesity, increasing numbers of people who have other conditions such as heart disease, kidney disease, and/or diabetes, and the use of diuretics by individuals with cardiovascular disease.5
In a meta-analysis on gouty involvement of the first MTP joint, the occurrence of acute first MTP arthritis has been reported to be an independent predictor of MSU crystal presence in patients with gout.6 The presence of first MTP arthritis and the predilection for MSU deposition in the medial and dorsal aspects of the joint suggested an association, but no causation, between the 2 disease processes. The authors concluded that the distinction between osteoarthritis and gout as the cause of the joint damage is often difficult.
The diagnosis of gout may be made clinically based on established clinical criteria. The most commonly used are the 1977 American College of Rheumatology (ACR) criteria for the classification of acute arthritis of primary gout. (See “The 12 diagnostic criteria for gout.”7) However, in 2015, the ACR/European League Against Rheumatism (EULAR) published a new set of criteria that include the signs and symptoms of chronic gout, as well.8 (The ACR-EULAR Gout Classification Criteria Calculator may be accessed at http://goutclassificationcalculator.auckland.ac.nz/.)
The 12 diagnostic criteria for gout7
1. Recurrent arthritic attack
2. Joint redness
3. Pain or swelling in the first metatarsophalangeal joint
4. Unilateral attack involving the first metatarsophalangeal joint
5. Unilateral attack involving the tarsal joint
6. Suspected tophus
7. Hyperuricemia
8. Radiographic evidence of asymmetric swelling within a joint
9. Attack of monoarticular arthritis
10. Development of maximal inflammation within one day
11. Negative culture of joint fluid for microorganisms during joint inflammation attack
12. Radiographic evidence of subcortical cyst without erosions
Differential diagnosis includes trauma, septic and reactive arthritis
The differential diagnosis of acute gouty arthritis includes trauma, pseudogout (arthritis involving calcium pyrophosphate dehydrate), septic arthritis, reactive arthritis, post-streptococcal arthritis, and Lyme disease.
Trauma with a resulting acute or stress fracture can be determined by x-ray or magnetic resonance imaging (MRI).
Pseudogout requires aspiration of fluid and examination for calcium pyrophosphate crystals under polarizing microscopy.9
Septic arthritis may present in a similar manner to other causes of acute arthritis. Therefore, arthrocentesis is needed to identify the causative infectious agent.10 Septic arthritis was considered in our patient, given his age, history of diabetes mellitus, and finding of skin ulceration over the toe. However, our patient did not have systemic symptoms, and the joint aspiration did not show the presence of bacteria.
Reactive arthritis typically presents with inflammation of the ligaments and tendons at their sites of insertion into the bone, and can affect other areas of the body, such as the genitourinary and ocular systems.11
Post-streptococcal arthritis (aka acute rheumatic fever [ARF]) and Lyme disease can also present with joint complaints. The arthritis in ARF is usually migratory and involves several joints. Fever, rash, and a history of group A streptococcal infection are also diagnostic features.12 History of travel to an endemic area or seasonal exposure would further differentiate Lyme disease from other causes of arthritis.
Deciding on a course of treatment
Initial treatment choices for acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.13 Various NSAIDs have been studied in the treatment of acute gout, but none showed absolute superiority over others. One option is naproxen 500 mg twice daily, but the choice of NSAID is mainly based on the adverse reaction profile and the physician’s preference.
Due to frequent gastrointestinal adverse effects and the concern for toxicity and drug interactions, colchicine and corticosteroids are not typically the first-line agents for acute gout treatment. Patients with frequent recurrent gouty attacks require urate-lowering therapies, such as allopurinol or probenecid. Other indications for urate-lowering therapies include evidence of tophaceous deposits in joints and soft tissues and gouty arthropathy.
Our patient’s renal failure was likely chronic, secondary to his untreated tophaceous gouty disease. Because his creatinine clearance was between 30 and 60 mL/min per 1.73 m2, he was not treated with NSAIDs, but with colchicine 1.2 mg for his initial flare, followed with a single dose of 0.6 mg in one hour. The patient had dramatic improvement of his pain the following day.
Patient education on chronic gout was also provided. We specifically discussed the patient’s dietary habits with him, advising him to minimize his intake of seafood, animal organs, and red meat products, which are high in purines. The patient was also told that he would need to start urate-lowering therapy to prevent recurrent gouty attacks and further complications from gout.
CORRESPONDENCE
Joseph Huang, MD, Fort Belvoir Community Hospital, Family Medicine Clinic, 1st Floor Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925-934.
2. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis. 2013;72:1545-1548.
3. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;CD006190.
4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136-3141.
5. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
6. Stewart S, Dalbeth N, Vandal AC, et al. The first metatarsophalangeal joint in gout: a systemic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:69.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
9. Rosenthal AK. Pseudogout: Presentation, natural history, and associated conditions. In: Wortmann RL, Schumacher HR Jr, Becker MA, et al, eds. Crystal-Induced Arthropathies: Gout, Pseudogout, and Apatite-Associated Syndromes. New York: Taylor and Francis Group;2006:99.
10. Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84:653-660.
11. Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Physician. 1999;60:499-507.
12. Beaudoin A, Edison L, Introcaso CE, et al; Centers for Disease Control and Prevention (CDC). Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64:555-558.
13. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
1. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925-934.
2. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis. 2013;72:1545-1548.
3. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;CD006190.
4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136-3141.
5. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
6. Stewart S, Dalbeth N, Vandal AC, et al. The first metatarsophalangeal joint in gout: a systemic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:69.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
9. Rosenthal AK. Pseudogout: Presentation, natural history, and associated conditions. In: Wortmann RL, Schumacher HR Jr, Becker MA, et al, eds. Crystal-Induced Arthropathies: Gout, Pseudogout, and Apatite-Associated Syndromes. New York: Taylor and Francis Group;2006:99.
10. Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84:653-660.
11. Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Physician. 1999;60:499-507.
12. Beaudoin A, Edison L, Introcaso CE, et al; Centers for Disease Control and Prevention (CDC). Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64:555-558.
13. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.