User login
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
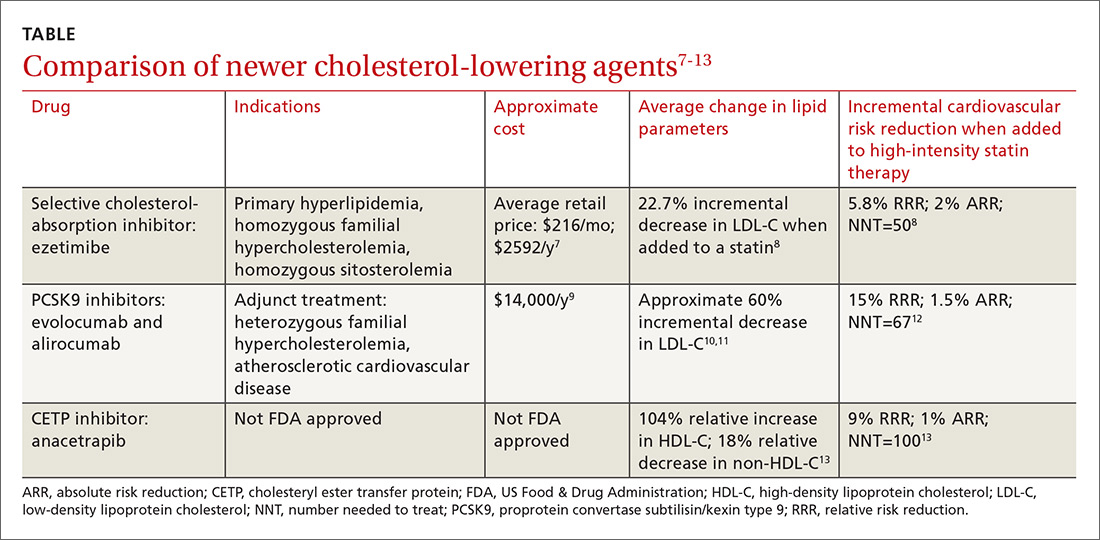
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.

Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.

Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
From The Journal of Family Practice | 2018;67(6):339-341,344-345.
PRACTICE RECOMMENDATIONS
› Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting low-density lipoprotein cholesterol (LDL-C) goals with a statin alone. B
› Limit consideration of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors to adults at highest risk: those with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who must further lower LDL-C levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series