User login
Our patient, a Hispanic baby boy, was born at 37 weeks’ gestation after induction of labor. He was delivered vaginally without complication. The newborn’s Apgar scores were 8 and 9, and he weighed 2.24 kg.
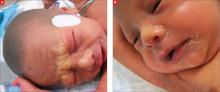
The child was born with cracked and peeling skin on his face, chest, hands, and feet. The skin on his face and chest had a taut, cellophane-like appearance. He had fine stubble on his scalp and no eyebrows or eyelashes (FIGURE 1A AND 1B).
The mother’s medical history and serology were unremarkable. Her prior obstetric history included a female infant who had died at 3 months of age of pneumonia, a skin infection, and dehydration. The mother indicated that the deceased child had “fish scale disease.”
FIGURE 1
Cracked skin, absence of eyebrows and eyelashes
What is your diagnosis?
How would you manage this condition?
Diagnosis: Congenital ichthyosis
Our patient had the congenital form of ichthyosis—a disorder that is sometimes referred to as fish scale disease. Congenital ichthyosis is a phenotypic expression of several different genotypes, and presents with varying degrees of severity. It is fairly common, occurring in 1 in 250 to 300 people.1,2 An extremely rare acquired form of ichthyosis may appear in adults, usually as a result of systemic disease or a medication reaction.
The diagnosis of congenital ichthyosis is made based on skin findings. Skin biopsy and genetic testing are typically not necessary for diagnosis.
Congenital ichthyosis is suspected in newborns who are either collodion babies (as was our patient) or who have harlequin ichthyosis.3
Collodion babies appear to be encased in a cellophane-like membrane at birth. The surface of the skin of the collodion baby usually appears taut and shiny. An absence of eyebrows, eyelashes, and scalp hair is common in these newborns; scarring alopecia can occur.4
The skin undergoes a variable degree of cracking and fissuring. Affected newborns may demonstrate ectropion (everted eyelids), eclabium (mouth held open by taut skin), and contracture of the fingers and joints.4,5 Degree of skin involvement at birth does not necessarily correlate with later disease severity.6
The collodion baby usually has a transglutaminase 1 gene mutation, which can cause either lamellar ichthyosis or congenital ichthyosiform erythroderma.7 (Absence of transglutaminase causes failed cross-linking of the proteins in the keratinocyte cellular envelope.)
Harlequin ichthyosis is a severe and usually lethal form of congenital ichthyosis, caused by a mutation in the ABCA12 gene. The product of this gene acts as a lipid transporter in epidermal keratinocytes. It is crucial for the correct formation of intercellular lipid layers in the stratum corneum, and its absence causes defective lipid transport and a loss of the skin lipid barrier.8
Affected infants have thick, armorlike plates of skin with deep moist fissures, along with severe ectropion and eclabium.4,5 Scaling and fissuring occur on the scalp, but hair is usually present.5
Differential diagnosis includes scalded skin syndrome
The differential for an infant with peeling skin includes staphylococcal scalded skin syndrome, physiologic desquamation, and infantile seborrheic dermatitis.
Staphylococcal scalded skin syndrome is a blistering skin disease induced by exfoliative toxins of Staphylococcus aureus. Toxins enter the skin, most often from the circulation, and disrupt intercellular linkages in the epidermis.9 Patients have generalized erythema, fever, and skin tenderness followed by the formation of large bullae, which rupture with slight pressure (Nikolsky sign). Rupture of these bullae results in extensive areas of denuded skin. These lesions do not scar because epidermal disruption occurs superficially.10 There is no hair loss.
Diagnosis is primarily clinical, but is supported by bacterial culture results. Sepsis can be a comorbid condition.
Physiologic desquamation is a common benign condition of full-term and post-date neonatal skin. Fine, diffuse scaling and peeling typically begin on the second day of life and last a few days. There is no hair loss or shiny membrane formation.11
Infantile seborrheic dermatitis, or “cradle cap,” is a common condition characterized by erythema and greasy white-to-yellowish scales on the scalp, forehead, eyebrows, cheeks, paranasal and nasolabial folds, retroauricular area, chest, and axillae.12 This condition usually develops in the first 3 to 4 weeks of life, but immunocompromised neonates often have generalized scaling and desquamation at birth.13 While hair loss is not caused by the primary process, aggressive scale removal during treatment can cause secondary hair loss. The etiology is unknown, but some evidence points to an abnormal host response to the yeast Malassezia.12
Management focuses on fluids and emollients
Management of congenital ichthyosis involves adequate pulmonary support, as the scaling and/or plate formation may cause a restrictive ventilatory defect; the use of humidified incubators; fluid and electrolyte replacement; emollients and wet compresses to maintain skin moisture and prevent further scaling or tautness of the membrane; ophthalmologic consultation to manage ectropion; and the treatment of infection.4,5 Oral retinoids are used in select cases.14
The prognosis varies with disease severity, but complications include infection, temperature instability, and dehydration secondary to skin barrier dysfunction. Most collodion babies survive to adulthood while few, if any, babies affected with harlequin ichthyosis survive the neonatal period. Ongoing research in corrective gene transfer provides hope for future therapy.15
Our patient required a humidified incubator
Following a consult with our hospital neonatologist, we closely monitored our patient’s volume status and electrolytes, placed him in a humidified incubator, and ensured that he was treated with emollient creams. Our patient was also treated for neonatal jaundice.
We obtained a consultation with a genetics counselor, who agreed with our diagnosis and ordered appropriate genetic testing. The patient has since been lost to follow-up.
Correspondence
T. Aaron Zeller, MD, 155 Academy Avenue, Greenwood, SC 29646; [email protected]
1. Schwartz RA. Ichthyosis vulgaris, hereditary and acquired. Available at: http://emedicine.medscape.com/article/1112753-overview. Accessed May 2, 2009.
2. Foundation for Ichthyosis & Related Skin Types FIRST). About ichthyosis. Available at: http://www.scalyskin.org/column.cfm?ColumnID=13. Accessed May 8, 2009.
3. Bale SJ, Richard G. Autosomal recessive congenital ichthyosis. Gene Reviews. December 11, 2008. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=li-ar. Accessed April 29, 2009.
4. Shwayder T, Akland T. Neonatal skin barrier: structure, function, and disorders. Dermatol Ther. 2005;18:87-103.
5. Akiyama M. Severe congenital ichthyosis of the neonate. Int J Dermatol. 1998;37:722-728.
6. Moss C. Genetic skin disorders. Semin Neonatol. 2000;5:311-320.
7. Parmentier L, Blanchet-Bardon C, Nguyen S, et al. Autosomal recessive lamellar ichthyosis: identification of a new mutation in transglutaminase 1 and evidence for genetic heterogeneity. Hum Mol Genet. 1995;4:1391-1395.
8. Akiyama M. Pathomechanisms of harlequin ichthyosis and ABCA transporters in human diseases. Arch Dermatol. 2006;142:914-918.
9. Ladhani S, Evans RW. Staphylococcal scalded skin syndrome. Arch Dis Child. 1998;78:85-88.
10. Farrell AM. Staphylococcal scalded-skin syndrome. Lancet. 1999;354:880-881.
11. Wallach D. Diagnosis of common, benign neonatal dermatoses. Clin Dermatol. 2003;21:264-268.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Kim HJ, Lim YS, Choi HY, et al. Generalized seborrheic dermatitis in an immunodeficient newborn. Cutis. 2001;67:52-54.
14. Lacour M, Mehta-Nikhar B, Atherton DJ, et al. An appraisal of acitretin therapy in children with inherited disorders of keratinization. Br J Dermatol. 1996;134:1023-1029.
15. Akiyama M, Sugiyama-Nakagirl Y, Sakai K. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777-1784.
Our patient, a Hispanic baby boy, was born at 37 weeks’ gestation after induction of labor. He was delivered vaginally without complication. The newborn’s Apgar scores were 8 and 9, and he weighed 2.24 kg.
The child was born with cracked and peeling skin on his face, chest, hands, and feet. The skin on his face and chest had a taut, cellophane-like appearance. He had fine stubble on his scalp and no eyebrows or eyelashes (FIGURE 1A AND 1B).
The mother’s medical history and serology were unremarkable. Her prior obstetric history included a female infant who had died at 3 months of age of pneumonia, a skin infection, and dehydration. The mother indicated that the deceased child had “fish scale disease.”
FIGURE 1
Cracked skin, absence of eyebrows and eyelashes
What is your diagnosis?
How would you manage this condition?
Diagnosis: Congenital ichthyosis
Our patient had the congenital form of ichthyosis—a disorder that is sometimes referred to as fish scale disease. Congenital ichthyosis is a phenotypic expression of several different genotypes, and presents with varying degrees of severity. It is fairly common, occurring in 1 in 250 to 300 people.1,2 An extremely rare acquired form of ichthyosis may appear in adults, usually as a result of systemic disease or a medication reaction.
The diagnosis of congenital ichthyosis is made based on skin findings. Skin biopsy and genetic testing are typically not necessary for diagnosis.
Congenital ichthyosis is suspected in newborns who are either collodion babies (as was our patient) or who have harlequin ichthyosis.3
Collodion babies appear to be encased in a cellophane-like membrane at birth. The surface of the skin of the collodion baby usually appears taut and shiny. An absence of eyebrows, eyelashes, and scalp hair is common in these newborns; scarring alopecia can occur.4
The skin undergoes a variable degree of cracking and fissuring. Affected newborns may demonstrate ectropion (everted eyelids), eclabium (mouth held open by taut skin), and contracture of the fingers and joints.4,5 Degree of skin involvement at birth does not necessarily correlate with later disease severity.6
The collodion baby usually has a transglutaminase 1 gene mutation, which can cause either lamellar ichthyosis or congenital ichthyosiform erythroderma.7 (Absence of transglutaminase causes failed cross-linking of the proteins in the keratinocyte cellular envelope.)
Harlequin ichthyosis is a severe and usually lethal form of congenital ichthyosis, caused by a mutation in the ABCA12 gene. The product of this gene acts as a lipid transporter in epidermal keratinocytes. It is crucial for the correct formation of intercellular lipid layers in the stratum corneum, and its absence causes defective lipid transport and a loss of the skin lipid barrier.8
Affected infants have thick, armorlike plates of skin with deep moist fissures, along with severe ectropion and eclabium.4,5 Scaling and fissuring occur on the scalp, but hair is usually present.5
Differential diagnosis includes scalded skin syndrome
The differential for an infant with peeling skin includes staphylococcal scalded skin syndrome, physiologic desquamation, and infantile seborrheic dermatitis.
Staphylococcal scalded skin syndrome is a blistering skin disease induced by exfoliative toxins of Staphylococcus aureus. Toxins enter the skin, most often from the circulation, and disrupt intercellular linkages in the epidermis.9 Patients have generalized erythema, fever, and skin tenderness followed by the formation of large bullae, which rupture with slight pressure (Nikolsky sign). Rupture of these bullae results in extensive areas of denuded skin. These lesions do not scar because epidermal disruption occurs superficially.10 There is no hair loss.
Diagnosis is primarily clinical, but is supported by bacterial culture results. Sepsis can be a comorbid condition.
Physiologic desquamation is a common benign condition of full-term and post-date neonatal skin. Fine, diffuse scaling and peeling typically begin on the second day of life and last a few days. There is no hair loss or shiny membrane formation.11
Infantile seborrheic dermatitis, or “cradle cap,” is a common condition characterized by erythema and greasy white-to-yellowish scales on the scalp, forehead, eyebrows, cheeks, paranasal and nasolabial folds, retroauricular area, chest, and axillae.12 This condition usually develops in the first 3 to 4 weeks of life, but immunocompromised neonates often have generalized scaling and desquamation at birth.13 While hair loss is not caused by the primary process, aggressive scale removal during treatment can cause secondary hair loss. The etiology is unknown, but some evidence points to an abnormal host response to the yeast Malassezia.12
Management focuses on fluids and emollients
Management of congenital ichthyosis involves adequate pulmonary support, as the scaling and/or plate formation may cause a restrictive ventilatory defect; the use of humidified incubators; fluid and electrolyte replacement; emollients and wet compresses to maintain skin moisture and prevent further scaling or tautness of the membrane; ophthalmologic consultation to manage ectropion; and the treatment of infection.4,5 Oral retinoids are used in select cases.14
The prognosis varies with disease severity, but complications include infection, temperature instability, and dehydration secondary to skin barrier dysfunction. Most collodion babies survive to adulthood while few, if any, babies affected with harlequin ichthyosis survive the neonatal period. Ongoing research in corrective gene transfer provides hope for future therapy.15
Our patient required a humidified incubator
Following a consult with our hospital neonatologist, we closely monitored our patient’s volume status and electrolytes, placed him in a humidified incubator, and ensured that he was treated with emollient creams. Our patient was also treated for neonatal jaundice.
We obtained a consultation with a genetics counselor, who agreed with our diagnosis and ordered appropriate genetic testing. The patient has since been lost to follow-up.
Correspondence
T. Aaron Zeller, MD, 155 Academy Avenue, Greenwood, SC 29646; [email protected]
Our patient, a Hispanic baby boy, was born at 37 weeks’ gestation after induction of labor. He was delivered vaginally without complication. The newborn’s Apgar scores were 8 and 9, and he weighed 2.24 kg.
The child was born with cracked and peeling skin on his face, chest, hands, and feet. The skin on his face and chest had a taut, cellophane-like appearance. He had fine stubble on his scalp and no eyebrows or eyelashes (FIGURE 1A AND 1B).
The mother’s medical history and serology were unremarkable. Her prior obstetric history included a female infant who had died at 3 months of age of pneumonia, a skin infection, and dehydration. The mother indicated that the deceased child had “fish scale disease.”
FIGURE 1
Cracked skin, absence of eyebrows and eyelashes
What is your diagnosis?
How would you manage this condition?
Diagnosis: Congenital ichthyosis
Our patient had the congenital form of ichthyosis—a disorder that is sometimes referred to as fish scale disease. Congenital ichthyosis is a phenotypic expression of several different genotypes, and presents with varying degrees of severity. It is fairly common, occurring in 1 in 250 to 300 people.1,2 An extremely rare acquired form of ichthyosis may appear in adults, usually as a result of systemic disease or a medication reaction.
The diagnosis of congenital ichthyosis is made based on skin findings. Skin biopsy and genetic testing are typically not necessary for diagnosis.
Congenital ichthyosis is suspected in newborns who are either collodion babies (as was our patient) or who have harlequin ichthyosis.3
Collodion babies appear to be encased in a cellophane-like membrane at birth. The surface of the skin of the collodion baby usually appears taut and shiny. An absence of eyebrows, eyelashes, and scalp hair is common in these newborns; scarring alopecia can occur.4
The skin undergoes a variable degree of cracking and fissuring. Affected newborns may demonstrate ectropion (everted eyelids), eclabium (mouth held open by taut skin), and contracture of the fingers and joints.4,5 Degree of skin involvement at birth does not necessarily correlate with later disease severity.6
The collodion baby usually has a transglutaminase 1 gene mutation, which can cause either lamellar ichthyosis or congenital ichthyosiform erythroderma.7 (Absence of transglutaminase causes failed cross-linking of the proteins in the keratinocyte cellular envelope.)
Harlequin ichthyosis is a severe and usually lethal form of congenital ichthyosis, caused by a mutation in the ABCA12 gene. The product of this gene acts as a lipid transporter in epidermal keratinocytes. It is crucial for the correct formation of intercellular lipid layers in the stratum corneum, and its absence causes defective lipid transport and a loss of the skin lipid barrier.8
Affected infants have thick, armorlike plates of skin with deep moist fissures, along with severe ectropion and eclabium.4,5 Scaling and fissuring occur on the scalp, but hair is usually present.5
Differential diagnosis includes scalded skin syndrome
The differential for an infant with peeling skin includes staphylococcal scalded skin syndrome, physiologic desquamation, and infantile seborrheic dermatitis.
Staphylococcal scalded skin syndrome is a blistering skin disease induced by exfoliative toxins of Staphylococcus aureus. Toxins enter the skin, most often from the circulation, and disrupt intercellular linkages in the epidermis.9 Patients have generalized erythema, fever, and skin tenderness followed by the formation of large bullae, which rupture with slight pressure (Nikolsky sign). Rupture of these bullae results in extensive areas of denuded skin. These lesions do not scar because epidermal disruption occurs superficially.10 There is no hair loss.
Diagnosis is primarily clinical, but is supported by bacterial culture results. Sepsis can be a comorbid condition.
Physiologic desquamation is a common benign condition of full-term and post-date neonatal skin. Fine, diffuse scaling and peeling typically begin on the second day of life and last a few days. There is no hair loss or shiny membrane formation.11
Infantile seborrheic dermatitis, or “cradle cap,” is a common condition characterized by erythema and greasy white-to-yellowish scales on the scalp, forehead, eyebrows, cheeks, paranasal and nasolabial folds, retroauricular area, chest, and axillae.12 This condition usually develops in the first 3 to 4 weeks of life, but immunocompromised neonates often have generalized scaling and desquamation at birth.13 While hair loss is not caused by the primary process, aggressive scale removal during treatment can cause secondary hair loss. The etiology is unknown, but some evidence points to an abnormal host response to the yeast Malassezia.12
Management focuses on fluids and emollients
Management of congenital ichthyosis involves adequate pulmonary support, as the scaling and/or plate formation may cause a restrictive ventilatory defect; the use of humidified incubators; fluid and electrolyte replacement; emollients and wet compresses to maintain skin moisture and prevent further scaling or tautness of the membrane; ophthalmologic consultation to manage ectropion; and the treatment of infection.4,5 Oral retinoids are used in select cases.14
The prognosis varies with disease severity, but complications include infection, temperature instability, and dehydration secondary to skin barrier dysfunction. Most collodion babies survive to adulthood while few, if any, babies affected with harlequin ichthyosis survive the neonatal period. Ongoing research in corrective gene transfer provides hope for future therapy.15
Our patient required a humidified incubator
Following a consult with our hospital neonatologist, we closely monitored our patient’s volume status and electrolytes, placed him in a humidified incubator, and ensured that he was treated with emollient creams. Our patient was also treated for neonatal jaundice.
We obtained a consultation with a genetics counselor, who agreed with our diagnosis and ordered appropriate genetic testing. The patient has since been lost to follow-up.
Correspondence
T. Aaron Zeller, MD, 155 Academy Avenue, Greenwood, SC 29646; [email protected]
1. Schwartz RA. Ichthyosis vulgaris, hereditary and acquired. Available at: http://emedicine.medscape.com/article/1112753-overview. Accessed May 2, 2009.
2. Foundation for Ichthyosis & Related Skin Types FIRST). About ichthyosis. Available at: http://www.scalyskin.org/column.cfm?ColumnID=13. Accessed May 8, 2009.
3. Bale SJ, Richard G. Autosomal recessive congenital ichthyosis. Gene Reviews. December 11, 2008. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=li-ar. Accessed April 29, 2009.
4. Shwayder T, Akland T. Neonatal skin barrier: structure, function, and disorders. Dermatol Ther. 2005;18:87-103.
5. Akiyama M. Severe congenital ichthyosis of the neonate. Int J Dermatol. 1998;37:722-728.
6. Moss C. Genetic skin disorders. Semin Neonatol. 2000;5:311-320.
7. Parmentier L, Blanchet-Bardon C, Nguyen S, et al. Autosomal recessive lamellar ichthyosis: identification of a new mutation in transglutaminase 1 and evidence for genetic heterogeneity. Hum Mol Genet. 1995;4:1391-1395.
8. Akiyama M. Pathomechanisms of harlequin ichthyosis and ABCA transporters in human diseases. Arch Dermatol. 2006;142:914-918.
9. Ladhani S, Evans RW. Staphylococcal scalded skin syndrome. Arch Dis Child. 1998;78:85-88.
10. Farrell AM. Staphylococcal scalded-skin syndrome. Lancet. 1999;354:880-881.
11. Wallach D. Diagnosis of common, benign neonatal dermatoses. Clin Dermatol. 2003;21:264-268.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Kim HJ, Lim YS, Choi HY, et al. Generalized seborrheic dermatitis in an immunodeficient newborn. Cutis. 2001;67:52-54.
14. Lacour M, Mehta-Nikhar B, Atherton DJ, et al. An appraisal of acitretin therapy in children with inherited disorders of keratinization. Br J Dermatol. 1996;134:1023-1029.
15. Akiyama M, Sugiyama-Nakagirl Y, Sakai K. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777-1784.
1. Schwartz RA. Ichthyosis vulgaris, hereditary and acquired. Available at: http://emedicine.medscape.com/article/1112753-overview. Accessed May 2, 2009.
2. Foundation for Ichthyosis & Related Skin Types FIRST). About ichthyosis. Available at: http://www.scalyskin.org/column.cfm?ColumnID=13. Accessed May 8, 2009.
3. Bale SJ, Richard G. Autosomal recessive congenital ichthyosis. Gene Reviews. December 11, 2008. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=li-ar. Accessed April 29, 2009.
4. Shwayder T, Akland T. Neonatal skin barrier: structure, function, and disorders. Dermatol Ther. 2005;18:87-103.
5. Akiyama M. Severe congenital ichthyosis of the neonate. Int J Dermatol. 1998;37:722-728.
6. Moss C. Genetic skin disorders. Semin Neonatol. 2000;5:311-320.
7. Parmentier L, Blanchet-Bardon C, Nguyen S, et al. Autosomal recessive lamellar ichthyosis: identification of a new mutation in transglutaminase 1 and evidence for genetic heterogeneity. Hum Mol Genet. 1995;4:1391-1395.
8. Akiyama M. Pathomechanisms of harlequin ichthyosis and ABCA transporters in human diseases. Arch Dermatol. 2006;142:914-918.
9. Ladhani S, Evans RW. Staphylococcal scalded skin syndrome. Arch Dis Child. 1998;78:85-88.
10. Farrell AM. Staphylococcal scalded-skin syndrome. Lancet. 1999;354:880-881.
11. Wallach D. Diagnosis of common, benign neonatal dermatoses. Clin Dermatol. 2003;21:264-268.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Kim HJ, Lim YS, Choi HY, et al. Generalized seborrheic dermatitis in an immunodeficient newborn. Cutis. 2001;67:52-54.
14. Lacour M, Mehta-Nikhar B, Atherton DJ, et al. An appraisal of acitretin therapy in children with inherited disorders of keratinization. Br J Dermatol. 1996;134:1023-1029.
15. Akiyama M, Sugiyama-Nakagirl Y, Sakai K. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777-1784.