User login
Chest Infections
Antibiotics that "mist the target"
The increase in multi-drug resistance (MDR) and the dearth of new antibiotics in the "pipeline" has prompted interest in aerosolized antibiotics (AA) for treating ventilator-associated pneumonia (VAP). Toxic antibiotics like colistin may be aerosolized, reaching high concentrations in distal airways with minimal systemic absorption.
Aerosolized antibiotics have mostly gained traction in the "adjunctive" role (added to systemic antibiotics). Advances in nebulizer technology, and adjustments in ventilator settings and the breathing circuit to optimize drug delivery, have paved the way for clinical application.
Rattanaumpawan and colleagues randomized 100 patients with VAP due to MDR gram-negative bacilli (GNB) to aerosolized colistin vs placebo in addition to IV antibiotics, without benefit in clinical outcomes (J Antimicrob Chemother. 2010;65[12]: 2645).
Three recent studies used advanced vibrating plate aerosolization: Lu and colleagues compared aerosolized colistin +/- IV aminoglycoside in 43 patients with VAP with MDR GNB vs IV antibiotics alone in 122 others with sensitive GNB (Anesthesiology. 2012;117[6]:1335); outcomes were similar.
In another study, 40 patients with drug-susceptible GNB received inhaled-only ceftazidime plus amikacin, vs systemic antibiotics. Treatment success was nonsignificantly higher in the aerosol-only group; antibiotic resistance developed only in the IV group (Am J Respir Crit Care Med. 2011;184[1]: 106). Niederman and colleagues, in a randomized VAP trial (n=69), found adjunctive inhaled amikacin reduced overall exposure to antibiotics with less clinical failure compared with control (Intensive Care Med. 2012;38[2]:263).
The advantage of AA may be in avoiding systemic antibiotic overexposure. Further studies will investigate adjunctive vs stand-alone AA, optimal dosing strategies, and agents for gram-positives.
Dr. Paul Richman, FCCP
Steering Committee Member
Dr. Michael Niederman, FCCP
Chair
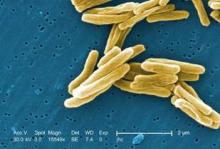
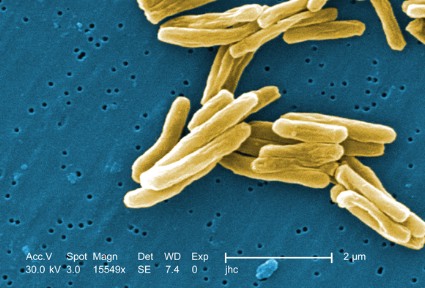
BCG vaccination for TB: Time for a reexamination?
Bacillus Calmette-Guerin (BCG) is the single most widely used human vaccine in history, with over 3 billion individuals vaccinated in total and 100 million annually(Liu et al. Hum Vaccine. 2009;5[2]:70).
The vaccine was developed in the early 20th century from a strain of Mycobacterium bovis. The original virulent strain had become attenuated by numerous subcultures in vitro over 13 years by Calmette and Guerin(McShane et al. Tuberculosis [Edinb]. 2012;92[3]:283).
Over the past 100 years, BCG has been disseminated to many laboratories and countries for use and has required frequent subculturing. As a result, the strains have diverged and do not have the same virulence properties as the original, and BCG should not be viewed as a single organism(Liu et al. Hum Vaccine. 2009;5[2]:70). Strain divergence has been reduced due to lyophilization of cultures over the past 47 years. Naturally occurring mutants of BCG have deletions of major virulence factors that affect the ESX-1 protein secretion system, one of several secretion systems found in the TB genome. Absence of these proteins results in impaired growth of TB in macrophages, modulates phagolysosomal fusion, and reduces bacterial virulence. The ESX-1 secretion system plays a major role in virulence of TB, and loss of the system accounts for much of the loss of virulence of BCG (Liu et al. Hum Vaccine. 2009;5[2]:70). These mutations may contribute to differences in side effects and efficacy of the vaccine utilized in different locales.
Other virulence factors of TB and BCG relate to the lipid content/composition of the cell wall of mycobacteria. These lipids are also integrally involved in pathogenicity(Liu et al. Hum Vaccine. 2009;5[2]:70). Absence or mutations in these lipids result in attenuation of infection in both mouse and guinea pig models.
Human trials and decades of clinical experience with BCG have shown that it is useful for prevention of TB and particularly for interdiction of major complications of TB (dissemination, meningitis, and mortality) in children(Checkley et al. Trends Pharmacol Sciences. 2011;32[10]:601; Trunz et al. Lancet. 2006;367[9517]:1173).
The responses for pulmonary TB in younger and older adults are not as robust (Colditz et al. JAMA. 1994;271[9]:698). For decades, the World Health Organization (WHO) has recommended BCG in high-risk endemic areas and for children. BCG is not recommended in general in developed countries where the endemic rate of TB is low; the utility of the skin test for diagnosis of latent TB would be compromised and the false-positivity rate of the test would be high.
Recognizing that BCG is not an ideal vaccine despite its widespread utility and experience over decades, further research has used the results of BCG attenuation to pursue avenues to improve BCG or to advance other vaccine candidates(Liu et al. Hum Vaccine. 2009;5[2]:70; McShane et al. Tuberculosis [Edinb]. 2012;92[3]: 283; Checkley et al. Trends Pharmacol Sciences. 2011;32[10]:601; Jeyanathan et al. J Immunol. 2008;181[8]:5618; Hokey et al. Tuberculosis [Edinb]. 2011;91[1]:82; Morais et al. Tuberculosis [Edinb]. 2010;90[2]:135; McShane et al. Philos Trans R Soc Lond B Biol Sci. 2011;366[1579]:2782; Rowland et al. Expert Rev Vaccines.2011;10[5]:645).
The newer vaccines in development against TB will have to be at least as good as the current BCG vaccine. Global challenges to the successful control of TB include the development of newer treatment drugs due to the presence of multi-drug (MDR) and extensively drug resistant (XDR) strains, efforts to halt the progression of HIV, improvement of hygiene and environmental factors of developing countries, and continued research into refinements of BCG, as well as newer vaccines against TB. Only by these combined efforts will the burden of TB be reduced by 50% by 2015 and have ultimate eradication by 2050(World Health Organization. 2011; http:// www.stoptb.org/assets/documents/global/plan.TB).
Dr. Richard Winn, FCCP
Vice-Chair
Cardiovascular Medicine and Surgery
ACC/AHA lipid guidelines spark controversy
Years in the making, the new lipid guidelines1,2 released by the American Heart Association (AHA) and the American College of Cardiology (ACC) to coincide with the AHA annual meeting in November ignited a firestorm of controversy.
The guidelines resulted from a complex process that stretched out 9 years from the publication of the consensus lipid guidelines in 2004. Convened and funded by the National Institutes of Health, the guidelines were eventually put out under the aegis of the AHA and ACC. The National Lipid Association, originally included in the process, ultimately declined to endorse them.
What was most controversial about the new guidelines was the move away from treating lipids to a specific target, instead focusing on which patients have been shown in randomized clinical trials to benefit from lipid-lowering therapy. These patients fall into 4 general categories:
1. Secondary prevention in patients with previous coronary or cerebrovascular events
2. With LDL cholesterol >190 mg/dL
3. Type II diabetics aged 40-75
4. Patients aged 40-75 with a 10-year risk of cardiovascular disease exceeding 7.5% according to a new algorithm
It was this last group that caused the most controversy. The classification has the potential to greatly increase the number of patients considered for lipid-lowering therapy, by as many as 45 million Americans.3 Drs. Paul Ridker and Nancy Cook published data that challenged the accuracy of the proposed algorithm, showing that when calibrated against patients in large randomized trials, it may overestimate the risk by as much as 75%-150%.3
Defenders of the algorithm pointed out that patients in clinical trials may be at lower risk than those in the general population, and that the algorithm, despite its flaws, is most likely more accurate than the previous algorithms that were derived from the Framingham population more than 30 years ago.
Other experts felt that the new guidelines overstressed randomized clinical trials to the exclusion of epidemiologic and population-based observational data, data they felt demonstrate convincingly that treatment to lower targets produces better outcomes even if clinical trials were not designed to not show statistically significant differences between patients who meet targets and those who have even more substantial lipid-lowering.
What remains unclear is whether the controversy about the guidelines will introduce unwanted uncertainty into the field, leading clinicians and patients to question the value of lipid-lowering as a preventive therapy, or whether disagreement will spur healthy discussions that will ultimately lead to more clarity and improved outcomes. Time will tell.
Dr. Steven M. Hollenberg, FCCP
Steering Committee Member
References
1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Nov 7. doi:pii: S0735-1097(13)06028-2. 10.1016/j.jacc.2013.11.002. [Epub ahead of print].
2. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
3. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013; 382:1762-1765.
Clinical Pulmonary Medicine
e-Cigarettes: Promise or peril?
e-Cigarettes are battery-powered devices that convert nicotine and other ingredients into vapor, simulating the visual, sensory, and behavioral aspects of smoking without the combustion products accountable for smoking’s damaging effects. An ever-growing number of companies around the world manufacture a wide variety of e-cigarette brands, despite scant information on the safety of the ingredients for human inhalation. The electronic cigarette is an emerging phenomenon that is becoming increasingly popular with smokers worldwide. Users report buying them to help quit smoking, to reduce cigarette consumption, to relieve tobacco withdrawal symptoms due to workplace smoking restrictions, and to continue to have a "smoking" experience but with reduced health risks. Electronic cigarette sales increased from 50,000 in 2008 to 3.5 million in 2012.
As of 2011, in the United States, one in five adults who smoke has tried electronic cigarettes. Among grade 6 to 12 students in the United States, those who have ever used the product increased from 3.3% in 2011 to 6.8% in 2012.
Tobacco-industry scientists argue that e-cigarettes deliver lower amounts of nicotine than regular cigarettes, are less toxic, and don’t expose others to second-hand smoke. One recent study showed that e-cigarettes, with or without nicotine, were modestly effective at helping smokers to quit, with similar achievement of abstinence as with nicotine patches and few adverse events. A recent study from France’s National Consumers Institute, however, concluded that e-cigarettes are "potentially carcinogenic" because some brands contain levels of formaldehyde that approach those of conventional cigarettes.
Uncertainty exists about the place of e-cigarettes in tobacco control, and more research is urgently needed to clearly establish their overall benefits and harms at both individual and population levels. Until then, we should not assume they are safe simply because they appear to be less harmful than traditional cigarettes. FDA is refusing to let them into the country and may soon ban their sale, as major US medical associations have strongly urged against the e-tobacco products.
Dr. Sat Sharma, FCCP
Steering Committee Member
Allied Health
Implementing mechanical ventilation orders by "Doing the Math"
Many RCPs (myself included) prefer mechanical ventilation (MV) orders that specify a target arterial pH (pHa), in lieu of listing a respiratory rate (RR) and tidal volume (Vt). If a baseline arterial blood gas (ABG) report is in hand, it’s easy to identify the target arterial carbon dioxide tension (Paco2), which will elicit a homeostatic pHa: target Paco2 = (5/3) • [HCO3–].
For example, suppose that a patient exhibits the following ABGs following an overdose of barbiturates: pHa = 7.20; Paco2 = 68 mm Hg; and [HCO3 -] = 26 mEq/L. If 7.40 is the pHa that we wish to impose: target Paco2 = (5/3) • 26 = 43 mm Hg.
Suppose further that our hypothetical patient initially displayed an RR of 10 breaths/min. We can reach the target Paco2 by applying the following expression: RRfinal = RRinitial • (Paco2initial / Paco2final).
For our hypothetical patient, this expression reverts to: RRfinal = 10 breaths/min • (68 mm Hg/43 mm Hg) = 16 breaths/min! On the other hand, if the attending physician or house officer indicates that s/he wishes to elicit a pHa that’s near the lower limit of the homeostatic range, we can simply select a target Paco2 that’s a few mm Hg higher than that shown above. This strategy is usually employed when the patient is known to be a CO2-retainer.
Want to "drill down" on this material? A video, handout, script, and posttest are accessible at: ambulatorypractice.org/education-research/respiratorytherapy-education/ventilator-targets. Enjoy!
Bob Demers, RRT
Chair
Chest Infections
Antibiotics that "mist the target"
The increase in multi-drug resistance (MDR) and the dearth of new antibiotics in the "pipeline" has prompted interest in aerosolized antibiotics (AA) for treating ventilator-associated pneumonia (VAP). Toxic antibiotics like colistin may be aerosolized, reaching high concentrations in distal airways with minimal systemic absorption.
Aerosolized antibiotics have mostly gained traction in the "adjunctive" role (added to systemic antibiotics). Advances in nebulizer technology, and adjustments in ventilator settings and the breathing circuit to optimize drug delivery, have paved the way for clinical application.
Rattanaumpawan and colleagues randomized 100 patients with VAP due to MDR gram-negative bacilli (GNB) to aerosolized colistin vs placebo in addition to IV antibiotics, without benefit in clinical outcomes (J Antimicrob Chemother. 2010;65[12]: 2645).
Three recent studies used advanced vibrating plate aerosolization: Lu and colleagues compared aerosolized colistin +/- IV aminoglycoside in 43 patients with VAP with MDR GNB vs IV antibiotics alone in 122 others with sensitive GNB (Anesthesiology. 2012;117[6]:1335); outcomes were similar.
In another study, 40 patients with drug-susceptible GNB received inhaled-only ceftazidime plus amikacin, vs systemic antibiotics. Treatment success was nonsignificantly higher in the aerosol-only group; antibiotic resistance developed only in the IV group (Am J Respir Crit Care Med. 2011;184[1]: 106). Niederman and colleagues, in a randomized VAP trial (n=69), found adjunctive inhaled amikacin reduced overall exposure to antibiotics with less clinical failure compared with control (Intensive Care Med. 2012;38[2]:263).
The advantage of AA may be in avoiding systemic antibiotic overexposure. Further studies will investigate adjunctive vs stand-alone AA, optimal dosing strategies, and agents for gram-positives.
Dr. Paul Richman, FCCP
Steering Committee Member
Dr. Michael Niederman, FCCP
Chair
BCG vaccination for TB: Time for a reexamination?
Bacillus Calmette-Guerin (BCG) is the single most widely used human vaccine in history, with over 3 billion individuals vaccinated in total and 100 million annually(Liu et al. Hum Vaccine. 2009;5[2]:70).
The vaccine was developed in the early 20th century from a strain of Mycobacterium bovis. The original virulent strain had become attenuated by numerous subcultures in vitro over 13 years by Calmette and Guerin(McShane et al. Tuberculosis [Edinb]. 2012;92[3]:283).
Over the past 100 years, BCG has been disseminated to many laboratories and countries for use and has required frequent subculturing. As a result, the strains have diverged and do not have the same virulence properties as the original, and BCG should not be viewed as a single organism(Liu et al. Hum Vaccine. 2009;5[2]:70). Strain divergence has been reduced due to lyophilization of cultures over the past 47 years. Naturally occurring mutants of BCG have deletions of major virulence factors that affect the ESX-1 protein secretion system, one of several secretion systems found in the TB genome. Absence of these proteins results in impaired growth of TB in macrophages, modulates phagolysosomal fusion, and reduces bacterial virulence. The ESX-1 secretion system plays a major role in virulence of TB, and loss of the system accounts for much of the loss of virulence of BCG (Liu et al. Hum Vaccine. 2009;5[2]:70). These mutations may contribute to differences in side effects and efficacy of the vaccine utilized in different locales.
Other virulence factors of TB and BCG relate to the lipid content/composition of the cell wall of mycobacteria. These lipids are also integrally involved in pathogenicity(Liu et al. Hum Vaccine. 2009;5[2]:70). Absence or mutations in these lipids result in attenuation of infection in both mouse and guinea pig models.
Human trials and decades of clinical experience with BCG have shown that it is useful for prevention of TB and particularly for interdiction of major complications of TB (dissemination, meningitis, and mortality) in children(Checkley et al. Trends Pharmacol Sciences. 2011;32[10]:601; Trunz et al. Lancet. 2006;367[9517]:1173).
The responses for pulmonary TB in younger and older adults are not as robust (Colditz et al. JAMA. 1994;271[9]:698). For decades, the World Health Organization (WHO) has recommended BCG in high-risk endemic areas and for children. BCG is not recommended in general in developed countries where the endemic rate of TB is low; the utility of the skin test for diagnosis of latent TB would be compromised and the false-positivity rate of the test would be high.
Recognizing that BCG is not an ideal vaccine despite its widespread utility and experience over decades, further research has used the results of BCG attenuation to pursue avenues to improve BCG or to advance other vaccine candidates(Liu et al. Hum Vaccine. 2009;5[2]:70; McShane et al. Tuberculosis [Edinb]. 2012;92[3]: 283; Checkley et al. Trends Pharmacol Sciences. 2011;32[10]:601; Jeyanathan et al. J Immunol. 2008;181[8]:5618; Hokey et al. Tuberculosis [Edinb]. 2011;91[1]:82; Morais et al. Tuberculosis [Edinb]. 2010;90[2]:135; McShane et al. Philos Trans R Soc Lond B Biol Sci. 2011;366[1579]:2782; Rowland et al. Expert Rev Vaccines.2011;10[5]:645).
The newer vaccines in development against TB will have to be at least as good as the current BCG vaccine. Global challenges to the successful control of TB include the development of newer treatment drugs due to the presence of multi-drug (MDR) and extensively drug resistant (XDR) strains, efforts to halt the progression of HIV, improvement of hygiene and environmental factors of developing countries, and continued research into refinements of BCG, as well as newer vaccines against TB. Only by these combined efforts will the burden of TB be reduced by 50% by 2015 and have ultimate eradication by 2050(World Health Organization. 2011; http:// www.stoptb.org/assets/documents/global/plan.TB).
Dr. Richard Winn, FCCP
Vice-Chair
Cardiovascular Medicine and Surgery
ACC/AHA lipid guidelines spark controversy
Years in the making, the new lipid guidelines1,2 released by the American Heart Association (AHA) and the American College of Cardiology (ACC) to coincide with the AHA annual meeting in November ignited a firestorm of controversy.
The guidelines resulted from a complex process that stretched out 9 years from the publication of the consensus lipid guidelines in 2004. Convened and funded by the National Institutes of Health, the guidelines were eventually put out under the aegis of the AHA and ACC. The National Lipid Association, originally included in the process, ultimately declined to endorse them.
What was most controversial about the new guidelines was the move away from treating lipids to a specific target, instead focusing on which patients have been shown in randomized clinical trials to benefit from lipid-lowering therapy. These patients fall into 4 general categories:
1. Secondary prevention in patients with previous coronary or cerebrovascular events
2. With LDL cholesterol >190 mg/dL
3. Type II diabetics aged 40-75
4. Patients aged 40-75 with a 10-year risk of cardiovascular disease exceeding 7.5% according to a new algorithm
It was this last group that caused the most controversy. The classification has the potential to greatly increase the number of patients considered for lipid-lowering therapy, by as many as 45 million Americans.3 Drs. Paul Ridker and Nancy Cook published data that challenged the accuracy of the proposed algorithm, showing that when calibrated against patients in large randomized trials, it may overestimate the risk by as much as 75%-150%.3
Defenders of the algorithm pointed out that patients in clinical trials may be at lower risk than those in the general population, and that the algorithm, despite its flaws, is most likely more accurate than the previous algorithms that were derived from the Framingham population more than 30 years ago.
Other experts felt that the new guidelines overstressed randomized clinical trials to the exclusion of epidemiologic and population-based observational data, data they felt demonstrate convincingly that treatment to lower targets produces better outcomes even if clinical trials were not designed to not show statistically significant differences between patients who meet targets and those who have even more substantial lipid-lowering.
What remains unclear is whether the controversy about the guidelines will introduce unwanted uncertainty into the field, leading clinicians and patients to question the value of lipid-lowering as a preventive therapy, or whether disagreement will spur healthy discussions that will ultimately lead to more clarity and improved outcomes. Time will tell.
Dr. Steven M. Hollenberg, FCCP
Steering Committee Member
References
1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Nov 7. doi:pii: S0735-1097(13)06028-2. 10.1016/j.jacc.2013.11.002. [Epub ahead of print].
2. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
3. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013; 382:1762-1765.
Clinical Pulmonary Medicine
e-Cigarettes: Promise or peril?
e-Cigarettes are battery-powered devices that convert nicotine and other ingredients into vapor, simulating the visual, sensory, and behavioral aspects of smoking without the combustion products accountable for smoking’s damaging effects. An ever-growing number of companies around the world manufacture a wide variety of e-cigarette brands, despite scant information on the safety of the ingredients for human inhalation. The electronic cigarette is an emerging phenomenon that is becoming increasingly popular with smokers worldwide. Users report buying them to help quit smoking, to reduce cigarette consumption, to relieve tobacco withdrawal symptoms due to workplace smoking restrictions, and to continue to have a "smoking" experience but with reduced health risks. Electronic cigarette sales increased from 50,000 in 2008 to 3.5 million in 2012.
As of 2011, in the United States, one in five adults who smoke has tried electronic cigarettes. Among grade 6 to 12 students in the United States, those who have ever used the product increased from 3.3% in 2011 to 6.8% in 2012.
Tobacco-industry scientists argue that e-cigarettes deliver lower amounts of nicotine than regular cigarettes, are less toxic, and don’t expose others to second-hand smoke. One recent study showed that e-cigarettes, with or without nicotine, were modestly effective at helping smokers to quit, with similar achievement of abstinence as with nicotine patches and few adverse events. A recent study from France’s National Consumers Institute, however, concluded that e-cigarettes are "potentially carcinogenic" because some brands contain levels of formaldehyde that approach those of conventional cigarettes.
Uncertainty exists about the place of e-cigarettes in tobacco control, and more research is urgently needed to clearly establish their overall benefits and harms at both individual and population levels. Until then, we should not assume they are safe simply because they appear to be less harmful than traditional cigarettes. FDA is refusing to let them into the country and may soon ban their sale, as major US medical associations have strongly urged against the e-tobacco products.
Dr. Sat Sharma, FCCP
Steering Committee Member
Allied Health
Implementing mechanical ventilation orders by "Doing the Math"
Many RCPs (myself included) prefer mechanical ventilation (MV) orders that specify a target arterial pH (pHa), in lieu of listing a respiratory rate (RR) and tidal volume (Vt). If a baseline arterial blood gas (ABG) report is in hand, it’s easy to identify the target arterial carbon dioxide tension (Paco2), which will elicit a homeostatic pHa: target Paco2 = (5/3) • [HCO3–].
For example, suppose that a patient exhibits the following ABGs following an overdose of barbiturates: pHa = 7.20; Paco2 = 68 mm Hg; and [HCO3 -] = 26 mEq/L. If 7.40 is the pHa that we wish to impose: target Paco2 = (5/3) • 26 = 43 mm Hg.
Suppose further that our hypothetical patient initially displayed an RR of 10 breaths/min. We can reach the target Paco2 by applying the following expression: RRfinal = RRinitial • (Paco2initial / Paco2final).
For our hypothetical patient, this expression reverts to: RRfinal = 10 breaths/min • (68 mm Hg/43 mm Hg) = 16 breaths/min! On the other hand, if the attending physician or house officer indicates that s/he wishes to elicit a pHa that’s near the lower limit of the homeostatic range, we can simply select a target Paco2 that’s a few mm Hg higher than that shown above. This strategy is usually employed when the patient is known to be a CO2-retainer.
Want to "drill down" on this material? A video, handout, script, and posttest are accessible at: ambulatorypractice.org/education-research/respiratorytherapy-education/ventilator-targets. Enjoy!
Bob Demers, RRT
Chair
Chest Infections
Antibiotics that "mist the target"
The increase in multi-drug resistance (MDR) and the dearth of new antibiotics in the "pipeline" has prompted interest in aerosolized antibiotics (AA) for treating ventilator-associated pneumonia (VAP). Toxic antibiotics like colistin may be aerosolized, reaching high concentrations in distal airways with minimal systemic absorption.
Aerosolized antibiotics have mostly gained traction in the "adjunctive" role (added to systemic antibiotics). Advances in nebulizer technology, and adjustments in ventilator settings and the breathing circuit to optimize drug delivery, have paved the way for clinical application.
Rattanaumpawan and colleagues randomized 100 patients with VAP due to MDR gram-negative bacilli (GNB) to aerosolized colistin vs placebo in addition to IV antibiotics, without benefit in clinical outcomes (J Antimicrob Chemother. 2010;65[12]: 2645).
Three recent studies used advanced vibrating plate aerosolization: Lu and colleagues compared aerosolized colistin +/- IV aminoglycoside in 43 patients with VAP with MDR GNB vs IV antibiotics alone in 122 others with sensitive GNB (Anesthesiology. 2012;117[6]:1335); outcomes were similar.
In another study, 40 patients with drug-susceptible GNB received inhaled-only ceftazidime plus amikacin, vs systemic antibiotics. Treatment success was nonsignificantly higher in the aerosol-only group; antibiotic resistance developed only in the IV group (Am J Respir Crit Care Med. 2011;184[1]: 106). Niederman and colleagues, in a randomized VAP trial (n=69), found adjunctive inhaled amikacin reduced overall exposure to antibiotics with less clinical failure compared with control (Intensive Care Med. 2012;38[2]:263).
The advantage of AA may be in avoiding systemic antibiotic overexposure. Further studies will investigate adjunctive vs stand-alone AA, optimal dosing strategies, and agents for gram-positives.
Dr. Paul Richman, FCCP
Steering Committee Member
Dr. Michael Niederman, FCCP
Chair
BCG vaccination for TB: Time for a reexamination?
Bacillus Calmette-Guerin (BCG) is the single most widely used human vaccine in history, with over 3 billion individuals vaccinated in total and 100 million annually(Liu et al. Hum Vaccine. 2009;5[2]:70).
The vaccine was developed in the early 20th century from a strain of Mycobacterium bovis. The original virulent strain had become attenuated by numerous subcultures in vitro over 13 years by Calmette and Guerin(McShane et al. Tuberculosis [Edinb]. 2012;92[3]:283).
Over the past 100 years, BCG has been disseminated to many laboratories and countries for use and has required frequent subculturing. As a result, the strains have diverged and do not have the same virulence properties as the original, and BCG should not be viewed as a single organism(Liu et al. Hum Vaccine. 2009;5[2]:70). Strain divergence has been reduced due to lyophilization of cultures over the past 47 years. Naturally occurring mutants of BCG have deletions of major virulence factors that affect the ESX-1 protein secretion system, one of several secretion systems found in the TB genome. Absence of these proteins results in impaired growth of TB in macrophages, modulates phagolysosomal fusion, and reduces bacterial virulence. The ESX-1 secretion system plays a major role in virulence of TB, and loss of the system accounts for much of the loss of virulence of BCG (Liu et al. Hum Vaccine. 2009;5[2]:70). These mutations may contribute to differences in side effects and efficacy of the vaccine utilized in different locales.
Other virulence factors of TB and BCG relate to the lipid content/composition of the cell wall of mycobacteria. These lipids are also integrally involved in pathogenicity(Liu et al. Hum Vaccine. 2009;5[2]:70). Absence or mutations in these lipids result in attenuation of infection in both mouse and guinea pig models.
Human trials and decades of clinical experience with BCG have shown that it is useful for prevention of TB and particularly for interdiction of major complications of TB (dissemination, meningitis, and mortality) in children(Checkley et al. Trends Pharmacol Sciences. 2011;32[10]:601; Trunz et al. Lancet. 2006;367[9517]:1173).
The responses for pulmonary TB in younger and older adults are not as robust (Colditz et al. JAMA. 1994;271[9]:698). For decades, the World Health Organization (WHO) has recommended BCG in high-risk endemic areas and for children. BCG is not recommended in general in developed countries where the endemic rate of TB is low; the utility of the skin test for diagnosis of latent TB would be compromised and the false-positivity rate of the test would be high.
Recognizing that BCG is not an ideal vaccine despite its widespread utility and experience over decades, further research has used the results of BCG attenuation to pursue avenues to improve BCG or to advance other vaccine candidates(Liu et al. Hum Vaccine. 2009;5[2]:70; McShane et al. Tuberculosis [Edinb]. 2012;92[3]: 283; Checkley et al. Trends Pharmacol Sciences. 2011;32[10]:601; Jeyanathan et al. J Immunol. 2008;181[8]:5618; Hokey et al. Tuberculosis [Edinb]. 2011;91[1]:82; Morais et al. Tuberculosis [Edinb]. 2010;90[2]:135; McShane et al. Philos Trans R Soc Lond B Biol Sci. 2011;366[1579]:2782; Rowland et al. Expert Rev Vaccines.2011;10[5]:645).
The newer vaccines in development against TB will have to be at least as good as the current BCG vaccine. Global challenges to the successful control of TB include the development of newer treatment drugs due to the presence of multi-drug (MDR) and extensively drug resistant (XDR) strains, efforts to halt the progression of HIV, improvement of hygiene and environmental factors of developing countries, and continued research into refinements of BCG, as well as newer vaccines against TB. Only by these combined efforts will the burden of TB be reduced by 50% by 2015 and have ultimate eradication by 2050(World Health Organization. 2011; http:// www.stoptb.org/assets/documents/global/plan.TB).
Dr. Richard Winn, FCCP
Vice-Chair
Cardiovascular Medicine and Surgery
ACC/AHA lipid guidelines spark controversy
Years in the making, the new lipid guidelines1,2 released by the American Heart Association (AHA) and the American College of Cardiology (ACC) to coincide with the AHA annual meeting in November ignited a firestorm of controversy.
The guidelines resulted from a complex process that stretched out 9 years from the publication of the consensus lipid guidelines in 2004. Convened and funded by the National Institutes of Health, the guidelines were eventually put out under the aegis of the AHA and ACC. The National Lipid Association, originally included in the process, ultimately declined to endorse them.
What was most controversial about the new guidelines was the move away from treating lipids to a specific target, instead focusing on which patients have been shown in randomized clinical trials to benefit from lipid-lowering therapy. These patients fall into 4 general categories:
1. Secondary prevention in patients with previous coronary or cerebrovascular events
2. With LDL cholesterol >190 mg/dL
3. Type II diabetics aged 40-75
4. Patients aged 40-75 with a 10-year risk of cardiovascular disease exceeding 7.5% according to a new algorithm
It was this last group that caused the most controversy. The classification has the potential to greatly increase the number of patients considered for lipid-lowering therapy, by as many as 45 million Americans.3 Drs. Paul Ridker and Nancy Cook published data that challenged the accuracy of the proposed algorithm, showing that when calibrated against patients in large randomized trials, it may overestimate the risk by as much as 75%-150%.3
Defenders of the algorithm pointed out that patients in clinical trials may be at lower risk than those in the general population, and that the algorithm, despite its flaws, is most likely more accurate than the previous algorithms that were derived from the Framingham population more than 30 years ago.
Other experts felt that the new guidelines overstressed randomized clinical trials to the exclusion of epidemiologic and population-based observational data, data they felt demonstrate convincingly that treatment to lower targets produces better outcomes even if clinical trials were not designed to not show statistically significant differences between patients who meet targets and those who have even more substantial lipid-lowering.
What remains unclear is whether the controversy about the guidelines will introduce unwanted uncertainty into the field, leading clinicians and patients to question the value of lipid-lowering as a preventive therapy, or whether disagreement will spur healthy discussions that will ultimately lead to more clarity and improved outcomes. Time will tell.
Dr. Steven M. Hollenberg, FCCP
Steering Committee Member
References
1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Nov 7. doi:pii: S0735-1097(13)06028-2. 10.1016/j.jacc.2013.11.002. [Epub ahead of print].
2. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
3. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013; 382:1762-1765.
Clinical Pulmonary Medicine
e-Cigarettes: Promise or peril?
e-Cigarettes are battery-powered devices that convert nicotine and other ingredients into vapor, simulating the visual, sensory, and behavioral aspects of smoking without the combustion products accountable for smoking’s damaging effects. An ever-growing number of companies around the world manufacture a wide variety of e-cigarette brands, despite scant information on the safety of the ingredients for human inhalation. The electronic cigarette is an emerging phenomenon that is becoming increasingly popular with smokers worldwide. Users report buying them to help quit smoking, to reduce cigarette consumption, to relieve tobacco withdrawal symptoms due to workplace smoking restrictions, and to continue to have a "smoking" experience but with reduced health risks. Electronic cigarette sales increased from 50,000 in 2008 to 3.5 million in 2012.
As of 2011, in the United States, one in five adults who smoke has tried electronic cigarettes. Among grade 6 to 12 students in the United States, those who have ever used the product increased from 3.3% in 2011 to 6.8% in 2012.
Tobacco-industry scientists argue that e-cigarettes deliver lower amounts of nicotine than regular cigarettes, are less toxic, and don’t expose others to second-hand smoke. One recent study showed that e-cigarettes, with or without nicotine, were modestly effective at helping smokers to quit, with similar achievement of abstinence as with nicotine patches and few adverse events. A recent study from France’s National Consumers Institute, however, concluded that e-cigarettes are "potentially carcinogenic" because some brands contain levels of formaldehyde that approach those of conventional cigarettes.
Uncertainty exists about the place of e-cigarettes in tobacco control, and more research is urgently needed to clearly establish their overall benefits and harms at both individual and population levels. Until then, we should not assume they are safe simply because they appear to be less harmful than traditional cigarettes. FDA is refusing to let them into the country and may soon ban their sale, as major US medical associations have strongly urged against the e-tobacco products.
Dr. Sat Sharma, FCCP
Steering Committee Member
Allied Health
Implementing mechanical ventilation orders by "Doing the Math"
Many RCPs (myself included) prefer mechanical ventilation (MV) orders that specify a target arterial pH (pHa), in lieu of listing a respiratory rate (RR) and tidal volume (Vt). If a baseline arterial blood gas (ABG) report is in hand, it’s easy to identify the target arterial carbon dioxide tension (Paco2), which will elicit a homeostatic pHa: target Paco2 = (5/3) • [HCO3–].
For example, suppose that a patient exhibits the following ABGs following an overdose of barbiturates: pHa = 7.20; Paco2 = 68 mm Hg; and [HCO3 -] = 26 mEq/L. If 7.40 is the pHa that we wish to impose: target Paco2 = (5/3) • 26 = 43 mm Hg.
Suppose further that our hypothetical patient initially displayed an RR of 10 breaths/min. We can reach the target Paco2 by applying the following expression: RRfinal = RRinitial • (Paco2initial / Paco2final).
For our hypothetical patient, this expression reverts to: RRfinal = 10 breaths/min • (68 mm Hg/43 mm Hg) = 16 breaths/min! On the other hand, if the attending physician or house officer indicates that s/he wishes to elicit a pHa that’s near the lower limit of the homeostatic range, we can simply select a target Paco2 that’s a few mm Hg higher than that shown above. This strategy is usually employed when the patient is known to be a CO2-retainer.
Want to "drill down" on this material? A video, handout, script, and posttest are accessible at: ambulatorypractice.org/education-research/respiratorytherapy-education/ventilator-targets. Enjoy!
Bob Demers, RRT
Chair