User login
Role of Computerized Physician Order Entry Systems in Facilitating Medication Errors
Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293:1197-1203.
Computerized Physician Order Entry (CPOE) has been touted as an effective means to reduce medical errors, especially medication errors. There have been preliminary studies that showed both potential and actual error reductions with CPOE. More recent data suggested that there may be potential for facilitating errors as well.
Koppel et al. aimed to study CPOE system-related factors that may actually increase risk of medication errors. The authors conducted structured interviews with end users (housestaff, pharmacists, nurses, nurse managers, and attending physicians), real-time observations of end users interfacing with the system, entering orders, charting medications, and reviewing orders, and focus groups with housestaff. These qualitative data were used to help generate a 71-question structured survey subsequently given to the housestaff. These questions pertain to working conditions, sources of stress, and errors. There were 261 responses representing an 88% response rate.
Twenty-two previously unexplored potential medication error sources abstracted from the survey were grouped into the 2 categories: 1) information errors, and 2) human-machine interface flaws. The first category refers to fragmented data and the disparate information systems within hospitals. The latter category includes rigid machine programming that does not correspond to or facilitate workflow. Only 10 survey elements with sufficiently robust results were reported. About 40% of respondents used CPOE to determine dosage of infrequently prescribed medications at least once a week or more. Incorrect doses may be ordered if users follow the dosage information in the system that is based on drug inventory rather than clinical recommendations. Twenty-two percent of respondents noted that more than once a week duplicate or conflicting medications were ordered and not detected for several hours. Disorganized display of patient medications was believed to be partly responsible. More than 80% of respondents noted unintended delay in renewing antibiotics at least once. Such gaps were possible partially because the reminder system occurred in the paper chart while order entry was done with the computer. With respect to the human-machine interface, 55% reported difficulty identifying the correct patient because of poor or fragmented displays, and 23% reported this occurring more than a few times per week. System downtime leading to delay in order entry was reported by 47% to occur more than once a week. System inflexibility also led to difficulties in specifying medications and ordering nonformulary medications. This was reported by 31% to occur at least several times a week, and 24% reported this daily or more frequently.
This was a survey of end users of a CPOE system in a single institution, and the survey elements were mainly estimates of error risks. Nevertheless, it appropriately draws attention to the importance of the unique culture of each institution, efficient workflow, and coherent human-machine interface. The anticipated error reductions may not materialize if these issues are neglected. Hospitalists can serve a critical role in implementation and customization of CPOE systems that allow clinicians to do the right thing more timely and efficiently.
Risk Stratification for In-hospital Mortality in Acutely Decompensated Heart Failure: Classification and Regression Tree Analysis
Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572-80.
Heart failure is an important and growing cause of hospitalization in this country, and it is one of the most common clinical entities encountered by hospitalists. While there are some risk assessment tools available for outpatients with heart failure, there has not been a risk stratification tool published for inpatients. In this study by Fonarow et al. in JAMA, the authors describe a simple risk-stratification formula for in-hospital mortality in patients with acutely decompensated heart failure. Data from the ADHERE registry (Acute Decompensated Heart Failure National Registry, which is industry sponsored, as was this study) were used to model the risk of in-hospital death using a classification and regression tree (CART) analysis. This was done in a 2-stage process. First, investigators established a derivation cohort of approximately 33,000 patients (sequential hospital admissions from October 2001 to February 2003) from the ADHERE registry, and used the CART method to analyze 39 clinical variables to determine which were the best predictors of in-hospital mortality. This analysis was used to derive a risk tree to partition patients into low-, intermediate-, and high-risk groups. Second, the validity of this method was tested by applying the prediction tool to a cohort of the subsequent 32,229 patients hospitalized in the ADHERE registry, from March 2003 to July 2003. The results were striking. Baseline characteristics and clinical outcomes between the derivation and validation cohorts were similar across the wide range of parameters examined. The difference in mortality between the low-, intermediate-, and high-risk groups was 23.6% in the highest-risk category and 1.8% in the low-risk category, while the intermediate group was stratified into 3 levels, with 20.0%, 5.0%, and 5.1% mortality risk in intermediate group levels 1, 2, and 3, respectively. Aside from the more than 10-fold range in mortality risk across the various groups, the outstanding feature of the authors’ findings was that 3 simple parameters were the most significant predictors of in-hospital mortality risk: BUN, SBP, and serum creatinine. Specifically, combinations of a serum BUN of 43 or greater, a serum creatinine of 2.75 or greater, and a systolic blood pressure of less than 115 were associated with higher mortality. They note that adding other predictors did not meaningfully increase the model’s accuracy. The authors comment that unlike other predictive models based on multivariate analyses (which are often complex, and therefore difficult to employ at bedside), this simple tool is easy to use. An additional advantage is that the data needed are typically available at time of admission and can therefore be used to make a timely clinical decision in terms of triage into an appropriate level of care. Similar risk assessment tools exist for the risk stratification of patients with the acute coronary syndrome, and given the frequency with which patients are admitted with acutely decompensated heart failure, this new tool should prove a welcome addition to the clinical decision-making abilities of hospitalists.
Risk of Endocarditis among Patients with Prosthetic Valves and Staphylococcus Aureus Bacteremia
El-Ahdab F, Benjamin DK, Wang A, , et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225-9.
The risk of developing endocarditis in patients with Staphylococcus aureus bacteremia and prosthetic valves increases as more than 600,000 prosthetic valves are implanted annually in the United States. A prospective study at Duke University identified 51 patients with prosthetic valves or mitral ring who developed S. aureus bacteremia. The modified Duke criteria were used for the diagnosis of endocarditis. The onset and sources of bacteremia, locations of acquiring bacteremia, as well as clinical outcome were analyzed. The overall incidence of definite prosthetic valve endocarditis was as high as 51%, with the remaining 49% patients meeting Duke criteria for possible endocarditis. The results showed that endocarditis occurred more frequently in mitral (62%) and aortic positions (48%), and with mitral ring the rate of endocarditis was slightly lower (33%). Among prostheses, mechanical and bioprosthetic valves had endocarditis rates of 62% and 44%, respectively. About 63% of patients had early onset of bacteremia (<1 year after valve placement), and 37% had late onset of bacteremia (>1 year after valve placement). Overall, the most common source of bacteremia was from infected surgical wound sites (33%). Early bacteremia was more likely to result from infected surgical wound sites (59%), while late bacteremia was more likely to have an unidentified source (48%). The majority of episodes of bacteremia (47%) were hospital-acquired (i.e., a positive blood culture occurred >72 hours after admission). The frequency of healthcare-associated bacteremia and community-acquired bacteremia was about 26%–27%.
In terms of mortality, there was no difference for a patient with early and late S. aureus bacteremia, bioprosthetic and mechanical valves, and infection due to methicillin-resistant or methicillin-susceptible S. aureus. However, mortality was higher among patients with definite endocarditis (62%) vs. possible endocarditis (28%). Patients with endocarditis who underwent valve surgery had lower mortality than those who did not undergo valve surgery due to inoperable comorbid conditions, such as stroke, multiorgan system failure, and mediastinitis. Persistent fever (≥ 38°C after 72 hours of adequate parenteral antibiotics) and persistent bacteremia (positive blood culture within 2–4 days of the initial positive blood culture) were independently associated with definite endocarditis with odds ratio of 4.4 and 11.7, respectively. Overall, 96% of patients underwent echocardiography (55% with both transesophageal and transthoracic echo, 14% with only transesophageal echo, 27% with only transthoracic echo). However, 10% patients with definite endocarditis had no diagnostic finding on either transthoracic or transesophageal echocardiography.
S. aureus bacteremia is a common phenomenon in inpatient settings. This study demonstrated an approximately 50% rate of definite prosthetic valve endocarditis in patients with S. aureus bacteremia. The risks of endocarditis were independent of valve type, location, and duration of implantation. This study highlights the need for aggressive treatment and evaluation of S. aureus bacteremia in patients with prosthetic valves. Clinically, persistent fever and bacteremia were independently associated with definite endocarditis in this study population. Clinicians cannot over-rely on transesophageal echocardiogram to identify occult endocarditis in high-risk patients.
Optimizing the Prediction of Perioperative Mortality in Vascular Surgery by Using a Customized Probability Model
Kertai MD, Boersma E, Klein J, van Urk H, Poldermans D. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med. 2005;165:898-904.
Traditional perioperative risk-assessment models and indexes have focused primarily on cardiac outcomes and involved mainly clinical risk factors. The model proposed in this paper focused instead on overall mortality and incorporated not only clinical risk factors but also more precise surgery-specific risks and the use of beta-blocker and statin agents.
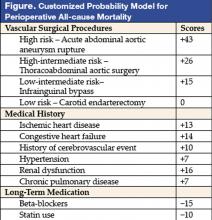
Investigators in the Netherlands targeted only vascular surgery patients identified from a computerized hospital information system. From a system, 2,310 patients who underwent 2,758 noncardiac vascular surgeries during a 10-year period in the 1990s were selected. Clinical risk factors, data on noninvasive stress testing, and long-term medication use, including statin agents, beta-blockers, calcium channel blockers, diuretics, insulin, nitrate, and aspirin, were abstracted. Outcome measures were all-cause mortality before discharge or within 30 days after surgery. The proposed model (see Figure) was based on modifications of the original Goldman Index, with the addition of more precise surgery risk stratification and statin and beta-blocker use. The specific types of vascular surgeries: carotid endarterectomy, infrainguinal bypass, abdominal aortic surgery, thoracoabdominal surgery, and acute abdominal aortic aneurysm rupture repair, carried systematically increased risk in expected fashion. Upon univariate analysis in the derivation cohort (n = 1,537), most of the clinical predictors from the Goldman Index were associated with increased perioperative mortality. Similar conclusions persisted in multivariate logistic regression analysis. Risk of surgical procedures, cardiovascular morbidity (ischemic heart disease, congestive heart failure, history of cerebrovascular event, and hypertension), renal dysfunction, and chronic pulmonary disease are independent predictors of increased all-cause perioperative mortality. In contrast, use of beta-blockers and statins were associated with reduced incidence of perioperative mortality. The final model included a scoring system with points assigned according to risk estimates of individual predictors. Beta-blocker and statin use in this model are assigned negative scores as their use lowers risk. For example, a patient with ischemic heart disease and hypertension undergoing abdominal aortic surgery would have a score of 46, corresponding to a 14% probability of mortality. That risk would be reduced to about 4% (score of 31) by use of beta-blockers (−15). In the same database, with 773 patients as the validation cohort, this prediction model performed nearly as well as the derivation model. Hypertension was not found to be an independent predictor in this validation cohort.
This tool appears provide robust risk assessment for vascular surgery patients. The inclusion of estimated benefit-of-statin and beta-blocker use may allow a more accurate “net” risk assessment. Those patients who are already on these 2 agents but still deemed at higher risk can be informed and may benefit from close monitoring. Additional preoperative interventions may include revascularization, if these high-risk patients have a decompensated cardiac status.
Role of Computerized Physician Order Entry Systems in Facilitating Medication Errors
Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293:1197-1203.
Computerized Physician Order Entry (CPOE) has been touted as an effective means to reduce medical errors, especially medication errors. There have been preliminary studies that showed both potential and actual error reductions with CPOE. More recent data suggested that there may be potential for facilitating errors as well.
Koppel et al. aimed to study CPOE system-related factors that may actually increase risk of medication errors. The authors conducted structured interviews with end users (housestaff, pharmacists, nurses, nurse managers, and attending physicians), real-time observations of end users interfacing with the system, entering orders, charting medications, and reviewing orders, and focus groups with housestaff. These qualitative data were used to help generate a 71-question structured survey subsequently given to the housestaff. These questions pertain to working conditions, sources of stress, and errors. There were 261 responses representing an 88% response rate.
Twenty-two previously unexplored potential medication error sources abstracted from the survey were grouped into the 2 categories: 1) information errors, and 2) human-machine interface flaws. The first category refers to fragmented data and the disparate information systems within hospitals. The latter category includes rigid machine programming that does not correspond to or facilitate workflow. Only 10 survey elements with sufficiently robust results were reported. About 40% of respondents used CPOE to determine dosage of infrequently prescribed medications at least once a week or more. Incorrect doses may be ordered if users follow the dosage information in the system that is based on drug inventory rather than clinical recommendations. Twenty-two percent of respondents noted that more than once a week duplicate or conflicting medications were ordered and not detected for several hours. Disorganized display of patient medications was believed to be partly responsible. More than 80% of respondents noted unintended delay in renewing antibiotics at least once. Such gaps were possible partially because the reminder system occurred in the paper chart while order entry was done with the computer. With respect to the human-machine interface, 55% reported difficulty identifying the correct patient because of poor or fragmented displays, and 23% reported this occurring more than a few times per week. System downtime leading to delay in order entry was reported by 47% to occur more than once a week. System inflexibility also led to difficulties in specifying medications and ordering nonformulary medications. This was reported by 31% to occur at least several times a week, and 24% reported this daily or more frequently.
This was a survey of end users of a CPOE system in a single institution, and the survey elements were mainly estimates of error risks. Nevertheless, it appropriately draws attention to the importance of the unique culture of each institution, efficient workflow, and coherent human-machine interface. The anticipated error reductions may not materialize if these issues are neglected. Hospitalists can serve a critical role in implementation and customization of CPOE systems that allow clinicians to do the right thing more timely and efficiently.
Risk Stratification for In-hospital Mortality in Acutely Decompensated Heart Failure: Classification and Regression Tree Analysis
Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572-80.
Heart failure is an important and growing cause of hospitalization in this country, and it is one of the most common clinical entities encountered by hospitalists. While there are some risk assessment tools available for outpatients with heart failure, there has not been a risk stratification tool published for inpatients. In this study by Fonarow et al. in JAMA, the authors describe a simple risk-stratification formula for in-hospital mortality in patients with acutely decompensated heart failure. Data from the ADHERE registry (Acute Decompensated Heart Failure National Registry, which is industry sponsored, as was this study) were used to model the risk of in-hospital death using a classification and regression tree (CART) analysis. This was done in a 2-stage process. First, investigators established a derivation cohort of approximately 33,000 patients (sequential hospital admissions from October 2001 to February 2003) from the ADHERE registry, and used the CART method to analyze 39 clinical variables to determine which were the best predictors of in-hospital mortality. This analysis was used to derive a risk tree to partition patients into low-, intermediate-, and high-risk groups. Second, the validity of this method was tested by applying the prediction tool to a cohort of the subsequent 32,229 patients hospitalized in the ADHERE registry, from March 2003 to July 2003. The results were striking. Baseline characteristics and clinical outcomes between the derivation and validation cohorts were similar across the wide range of parameters examined. The difference in mortality between the low-, intermediate-, and high-risk groups was 23.6% in the highest-risk category and 1.8% in the low-risk category, while the intermediate group was stratified into 3 levels, with 20.0%, 5.0%, and 5.1% mortality risk in intermediate group levels 1, 2, and 3, respectively. Aside from the more than 10-fold range in mortality risk across the various groups, the outstanding feature of the authors’ findings was that 3 simple parameters were the most significant predictors of in-hospital mortality risk: BUN, SBP, and serum creatinine. Specifically, combinations of a serum BUN of 43 or greater, a serum creatinine of 2.75 or greater, and a systolic blood pressure of less than 115 were associated with higher mortality. They note that adding other predictors did not meaningfully increase the model’s accuracy. The authors comment that unlike other predictive models based on multivariate analyses (which are often complex, and therefore difficult to employ at bedside), this simple tool is easy to use. An additional advantage is that the data needed are typically available at time of admission and can therefore be used to make a timely clinical decision in terms of triage into an appropriate level of care. Similar risk assessment tools exist for the risk stratification of patients with the acute coronary syndrome, and given the frequency with which patients are admitted with acutely decompensated heart failure, this new tool should prove a welcome addition to the clinical decision-making abilities of hospitalists.
Risk of Endocarditis among Patients with Prosthetic Valves and Staphylococcus Aureus Bacteremia
El-Ahdab F, Benjamin DK, Wang A, , et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225-9.
The risk of developing endocarditis in patients with Staphylococcus aureus bacteremia and prosthetic valves increases as more than 600,000 prosthetic valves are implanted annually in the United States. A prospective study at Duke University identified 51 patients with prosthetic valves or mitral ring who developed S. aureus bacteremia. The modified Duke criteria were used for the diagnosis of endocarditis. The onset and sources of bacteremia, locations of acquiring bacteremia, as well as clinical outcome were analyzed. The overall incidence of definite prosthetic valve endocarditis was as high as 51%, with the remaining 49% patients meeting Duke criteria for possible endocarditis. The results showed that endocarditis occurred more frequently in mitral (62%) and aortic positions (48%), and with mitral ring the rate of endocarditis was slightly lower (33%). Among prostheses, mechanical and bioprosthetic valves had endocarditis rates of 62% and 44%, respectively. About 63% of patients had early onset of bacteremia (<1 year after valve placement), and 37% had late onset of bacteremia (>1 year after valve placement). Overall, the most common source of bacteremia was from infected surgical wound sites (33%). Early bacteremia was more likely to result from infected surgical wound sites (59%), while late bacteremia was more likely to have an unidentified source (48%). The majority of episodes of bacteremia (47%) were hospital-acquired (i.e., a positive blood culture occurred >72 hours after admission). The frequency of healthcare-associated bacteremia and community-acquired bacteremia was about 26%–27%.
In terms of mortality, there was no difference for a patient with early and late S. aureus bacteremia, bioprosthetic and mechanical valves, and infection due to methicillin-resistant or methicillin-susceptible S. aureus. However, mortality was higher among patients with definite endocarditis (62%) vs. possible endocarditis (28%). Patients with endocarditis who underwent valve surgery had lower mortality than those who did not undergo valve surgery due to inoperable comorbid conditions, such as stroke, multiorgan system failure, and mediastinitis. Persistent fever (≥ 38°C after 72 hours of adequate parenteral antibiotics) and persistent bacteremia (positive blood culture within 2–4 days of the initial positive blood culture) were independently associated with definite endocarditis with odds ratio of 4.4 and 11.7, respectively. Overall, 96% of patients underwent echocardiography (55% with both transesophageal and transthoracic echo, 14% with only transesophageal echo, 27% with only transthoracic echo). However, 10% patients with definite endocarditis had no diagnostic finding on either transthoracic or transesophageal echocardiography.
S. aureus bacteremia is a common phenomenon in inpatient settings. This study demonstrated an approximately 50% rate of definite prosthetic valve endocarditis in patients with S. aureus bacteremia. The risks of endocarditis were independent of valve type, location, and duration of implantation. This study highlights the need for aggressive treatment and evaluation of S. aureus bacteremia in patients with prosthetic valves. Clinically, persistent fever and bacteremia were independently associated with definite endocarditis in this study population. Clinicians cannot over-rely on transesophageal echocardiogram to identify occult endocarditis in high-risk patients.
Optimizing the Prediction of Perioperative Mortality in Vascular Surgery by Using a Customized Probability Model
Kertai MD, Boersma E, Klein J, van Urk H, Poldermans D. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med. 2005;165:898-904.
Traditional perioperative risk-assessment models and indexes have focused primarily on cardiac outcomes and involved mainly clinical risk factors. The model proposed in this paper focused instead on overall mortality and incorporated not only clinical risk factors but also more precise surgery-specific risks and the use of beta-blocker and statin agents.
Investigators in the Netherlands targeted only vascular surgery patients identified from a computerized hospital information system. From a system, 2,310 patients who underwent 2,758 noncardiac vascular surgeries during a 10-year period in the 1990s were selected. Clinical risk factors, data on noninvasive stress testing, and long-term medication use, including statin agents, beta-blockers, calcium channel blockers, diuretics, insulin, nitrate, and aspirin, were abstracted. Outcome measures were all-cause mortality before discharge or within 30 days after surgery. The proposed model (see Figure) was based on modifications of the original Goldman Index, with the addition of more precise surgery risk stratification and statin and beta-blocker use. The specific types of vascular surgeries: carotid endarterectomy, infrainguinal bypass, abdominal aortic surgery, thoracoabdominal surgery, and acute abdominal aortic aneurysm rupture repair, carried systematically increased risk in expected fashion. Upon univariate analysis in the derivation cohort (n = 1,537), most of the clinical predictors from the Goldman Index were associated with increased perioperative mortality. Similar conclusions persisted in multivariate logistic regression analysis. Risk of surgical procedures, cardiovascular morbidity (ischemic heart disease, congestive heart failure, history of cerebrovascular event, and hypertension), renal dysfunction, and chronic pulmonary disease are independent predictors of increased all-cause perioperative mortality. In contrast, use of beta-blockers and statins were associated with reduced incidence of perioperative mortality. The final model included a scoring system with points assigned according to risk estimates of individual predictors. Beta-blocker and statin use in this model are assigned negative scores as their use lowers risk. For example, a patient with ischemic heart disease and hypertension undergoing abdominal aortic surgery would have a score of 46, corresponding to a 14% probability of mortality. That risk would be reduced to about 4% (score of 31) by use of beta-blockers (−15). In the same database, with 773 patients as the validation cohort, this prediction model performed nearly as well as the derivation model. Hypertension was not found to be an independent predictor in this validation cohort.
This tool appears provide robust risk assessment for vascular surgery patients. The inclusion of estimated benefit-of-statin and beta-blocker use may allow a more accurate “net” risk assessment. Those patients who are already on these 2 agents but still deemed at higher risk can be informed and may benefit from close monitoring. Additional preoperative interventions may include revascularization, if these high-risk patients have a decompensated cardiac status.
Role of Computerized Physician Order Entry Systems in Facilitating Medication Errors
Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293:1197-1203.
Computerized Physician Order Entry (CPOE) has been touted as an effective means to reduce medical errors, especially medication errors. There have been preliminary studies that showed both potential and actual error reductions with CPOE. More recent data suggested that there may be potential for facilitating errors as well.
Koppel et al. aimed to study CPOE system-related factors that may actually increase risk of medication errors. The authors conducted structured interviews with end users (housestaff, pharmacists, nurses, nurse managers, and attending physicians), real-time observations of end users interfacing with the system, entering orders, charting medications, and reviewing orders, and focus groups with housestaff. These qualitative data were used to help generate a 71-question structured survey subsequently given to the housestaff. These questions pertain to working conditions, sources of stress, and errors. There were 261 responses representing an 88% response rate.
Twenty-two previously unexplored potential medication error sources abstracted from the survey were grouped into the 2 categories: 1) information errors, and 2) human-machine interface flaws. The first category refers to fragmented data and the disparate information systems within hospitals. The latter category includes rigid machine programming that does not correspond to or facilitate workflow. Only 10 survey elements with sufficiently robust results were reported. About 40% of respondents used CPOE to determine dosage of infrequently prescribed medications at least once a week or more. Incorrect doses may be ordered if users follow the dosage information in the system that is based on drug inventory rather than clinical recommendations. Twenty-two percent of respondents noted that more than once a week duplicate or conflicting medications were ordered and not detected for several hours. Disorganized display of patient medications was believed to be partly responsible. More than 80% of respondents noted unintended delay in renewing antibiotics at least once. Such gaps were possible partially because the reminder system occurred in the paper chart while order entry was done with the computer. With respect to the human-machine interface, 55% reported difficulty identifying the correct patient because of poor or fragmented displays, and 23% reported this occurring more than a few times per week. System downtime leading to delay in order entry was reported by 47% to occur more than once a week. System inflexibility also led to difficulties in specifying medications and ordering nonformulary medications. This was reported by 31% to occur at least several times a week, and 24% reported this daily or more frequently.
This was a survey of end users of a CPOE system in a single institution, and the survey elements were mainly estimates of error risks. Nevertheless, it appropriately draws attention to the importance of the unique culture of each institution, efficient workflow, and coherent human-machine interface. The anticipated error reductions may not materialize if these issues are neglected. Hospitalists can serve a critical role in implementation and customization of CPOE systems that allow clinicians to do the right thing more timely and efficiently.
Risk Stratification for In-hospital Mortality in Acutely Decompensated Heart Failure: Classification and Regression Tree Analysis
Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572-80.
Heart failure is an important and growing cause of hospitalization in this country, and it is one of the most common clinical entities encountered by hospitalists. While there are some risk assessment tools available for outpatients with heart failure, there has not been a risk stratification tool published for inpatients. In this study by Fonarow et al. in JAMA, the authors describe a simple risk-stratification formula for in-hospital mortality in patients with acutely decompensated heart failure. Data from the ADHERE registry (Acute Decompensated Heart Failure National Registry, which is industry sponsored, as was this study) were used to model the risk of in-hospital death using a classification and regression tree (CART) analysis. This was done in a 2-stage process. First, investigators established a derivation cohort of approximately 33,000 patients (sequential hospital admissions from October 2001 to February 2003) from the ADHERE registry, and used the CART method to analyze 39 clinical variables to determine which were the best predictors of in-hospital mortality. This analysis was used to derive a risk tree to partition patients into low-, intermediate-, and high-risk groups. Second, the validity of this method was tested by applying the prediction tool to a cohort of the subsequent 32,229 patients hospitalized in the ADHERE registry, from March 2003 to July 2003. The results were striking. Baseline characteristics and clinical outcomes between the derivation and validation cohorts were similar across the wide range of parameters examined. The difference in mortality between the low-, intermediate-, and high-risk groups was 23.6% in the highest-risk category and 1.8% in the low-risk category, while the intermediate group was stratified into 3 levels, with 20.0%, 5.0%, and 5.1% mortality risk in intermediate group levels 1, 2, and 3, respectively. Aside from the more than 10-fold range in mortality risk across the various groups, the outstanding feature of the authors’ findings was that 3 simple parameters were the most significant predictors of in-hospital mortality risk: BUN, SBP, and serum creatinine. Specifically, combinations of a serum BUN of 43 or greater, a serum creatinine of 2.75 or greater, and a systolic blood pressure of less than 115 were associated with higher mortality. They note that adding other predictors did not meaningfully increase the model’s accuracy. The authors comment that unlike other predictive models based on multivariate analyses (which are often complex, and therefore difficult to employ at bedside), this simple tool is easy to use. An additional advantage is that the data needed are typically available at time of admission and can therefore be used to make a timely clinical decision in terms of triage into an appropriate level of care. Similar risk assessment tools exist for the risk stratification of patients with the acute coronary syndrome, and given the frequency with which patients are admitted with acutely decompensated heart failure, this new tool should prove a welcome addition to the clinical decision-making abilities of hospitalists.
Risk of Endocarditis among Patients with Prosthetic Valves and Staphylococcus Aureus Bacteremia
El-Ahdab F, Benjamin DK, Wang A, , et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225-9.
The risk of developing endocarditis in patients with Staphylococcus aureus bacteremia and prosthetic valves increases as more than 600,000 prosthetic valves are implanted annually in the United States. A prospective study at Duke University identified 51 patients with prosthetic valves or mitral ring who developed S. aureus bacteremia. The modified Duke criteria were used for the diagnosis of endocarditis. The onset and sources of bacteremia, locations of acquiring bacteremia, as well as clinical outcome were analyzed. The overall incidence of definite prosthetic valve endocarditis was as high as 51%, with the remaining 49% patients meeting Duke criteria for possible endocarditis. The results showed that endocarditis occurred more frequently in mitral (62%) and aortic positions (48%), and with mitral ring the rate of endocarditis was slightly lower (33%). Among prostheses, mechanical and bioprosthetic valves had endocarditis rates of 62% and 44%, respectively. About 63% of patients had early onset of bacteremia (<1 year after valve placement), and 37% had late onset of bacteremia (>1 year after valve placement). Overall, the most common source of bacteremia was from infected surgical wound sites (33%). Early bacteremia was more likely to result from infected surgical wound sites (59%), while late bacteremia was more likely to have an unidentified source (48%). The majority of episodes of bacteremia (47%) were hospital-acquired (i.e., a positive blood culture occurred >72 hours after admission). The frequency of healthcare-associated bacteremia and community-acquired bacteremia was about 26%–27%.
In terms of mortality, there was no difference for a patient with early and late S. aureus bacteremia, bioprosthetic and mechanical valves, and infection due to methicillin-resistant or methicillin-susceptible S. aureus. However, mortality was higher among patients with definite endocarditis (62%) vs. possible endocarditis (28%). Patients with endocarditis who underwent valve surgery had lower mortality than those who did not undergo valve surgery due to inoperable comorbid conditions, such as stroke, multiorgan system failure, and mediastinitis. Persistent fever (≥ 38°C after 72 hours of adequate parenteral antibiotics) and persistent bacteremia (positive blood culture within 2–4 days of the initial positive blood culture) were independently associated with definite endocarditis with odds ratio of 4.4 and 11.7, respectively. Overall, 96% of patients underwent echocardiography (55% with both transesophageal and transthoracic echo, 14% with only transesophageal echo, 27% with only transthoracic echo). However, 10% patients with definite endocarditis had no diagnostic finding on either transthoracic or transesophageal echocardiography.
S. aureus bacteremia is a common phenomenon in inpatient settings. This study demonstrated an approximately 50% rate of definite prosthetic valve endocarditis in patients with S. aureus bacteremia. The risks of endocarditis were independent of valve type, location, and duration of implantation. This study highlights the need for aggressive treatment and evaluation of S. aureus bacteremia in patients with prosthetic valves. Clinically, persistent fever and bacteremia were independently associated with definite endocarditis in this study population. Clinicians cannot over-rely on transesophageal echocardiogram to identify occult endocarditis in high-risk patients.
Optimizing the Prediction of Perioperative Mortality in Vascular Surgery by Using a Customized Probability Model
Kertai MD, Boersma E, Klein J, van Urk H, Poldermans D. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med. 2005;165:898-904.
Traditional perioperative risk-assessment models and indexes have focused primarily on cardiac outcomes and involved mainly clinical risk factors. The model proposed in this paper focused instead on overall mortality and incorporated not only clinical risk factors but also more precise surgery-specific risks and the use of beta-blocker and statin agents.
Investigators in the Netherlands targeted only vascular surgery patients identified from a computerized hospital information system. From a system, 2,310 patients who underwent 2,758 noncardiac vascular surgeries during a 10-year period in the 1990s were selected. Clinical risk factors, data on noninvasive stress testing, and long-term medication use, including statin agents, beta-blockers, calcium channel blockers, diuretics, insulin, nitrate, and aspirin, were abstracted. Outcome measures were all-cause mortality before discharge or within 30 days after surgery. The proposed model (see Figure) was based on modifications of the original Goldman Index, with the addition of more precise surgery risk stratification and statin and beta-blocker use. The specific types of vascular surgeries: carotid endarterectomy, infrainguinal bypass, abdominal aortic surgery, thoracoabdominal surgery, and acute abdominal aortic aneurysm rupture repair, carried systematically increased risk in expected fashion. Upon univariate analysis in the derivation cohort (n = 1,537), most of the clinical predictors from the Goldman Index were associated with increased perioperative mortality. Similar conclusions persisted in multivariate logistic regression analysis. Risk of surgical procedures, cardiovascular morbidity (ischemic heart disease, congestive heart failure, history of cerebrovascular event, and hypertension), renal dysfunction, and chronic pulmonary disease are independent predictors of increased all-cause perioperative mortality. In contrast, use of beta-blockers and statins were associated with reduced incidence of perioperative mortality. The final model included a scoring system with points assigned according to risk estimates of individual predictors. Beta-blocker and statin use in this model are assigned negative scores as their use lowers risk. For example, a patient with ischemic heart disease and hypertension undergoing abdominal aortic surgery would have a score of 46, corresponding to a 14% probability of mortality. That risk would be reduced to about 4% (score of 31) by use of beta-blockers (−15). In the same database, with 773 patients as the validation cohort, this prediction model performed nearly as well as the derivation model. Hypertension was not found to be an independent predictor in this validation cohort.
This tool appears provide robust risk assessment for vascular surgery patients. The inclusion of estimated benefit-of-statin and beta-blocker use may allow a more accurate “net” risk assessment. Those patients who are already on these 2 agents but still deemed at higher risk can be informed and may benefit from close monitoring. Additional preoperative interventions may include revascularization, if these high-risk patients have a decompensated cardiac status.