User login
Janssen has applied to the Food and Drug Administration and the European Medicines Agency to allow splitting of the first infusion of daratumumab (Darzalex) in multiple myeloma patients over 2 consecutive days.
The goal is to improve the treatment experience for patients and physicians, according to the announcement from Janssen.
The regulatory submissions are based on the global, multi-arm, phase 1b MMY1001 study (NCT01998971). The study evaluated daratumumab in combination with various other treatments in 240 patients with multiple myeloma. It found that both the safety profile and the pharmacokinetics concentrations seen with either single dosing or split dosing were similar.
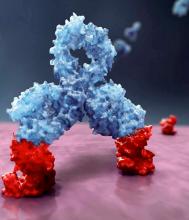
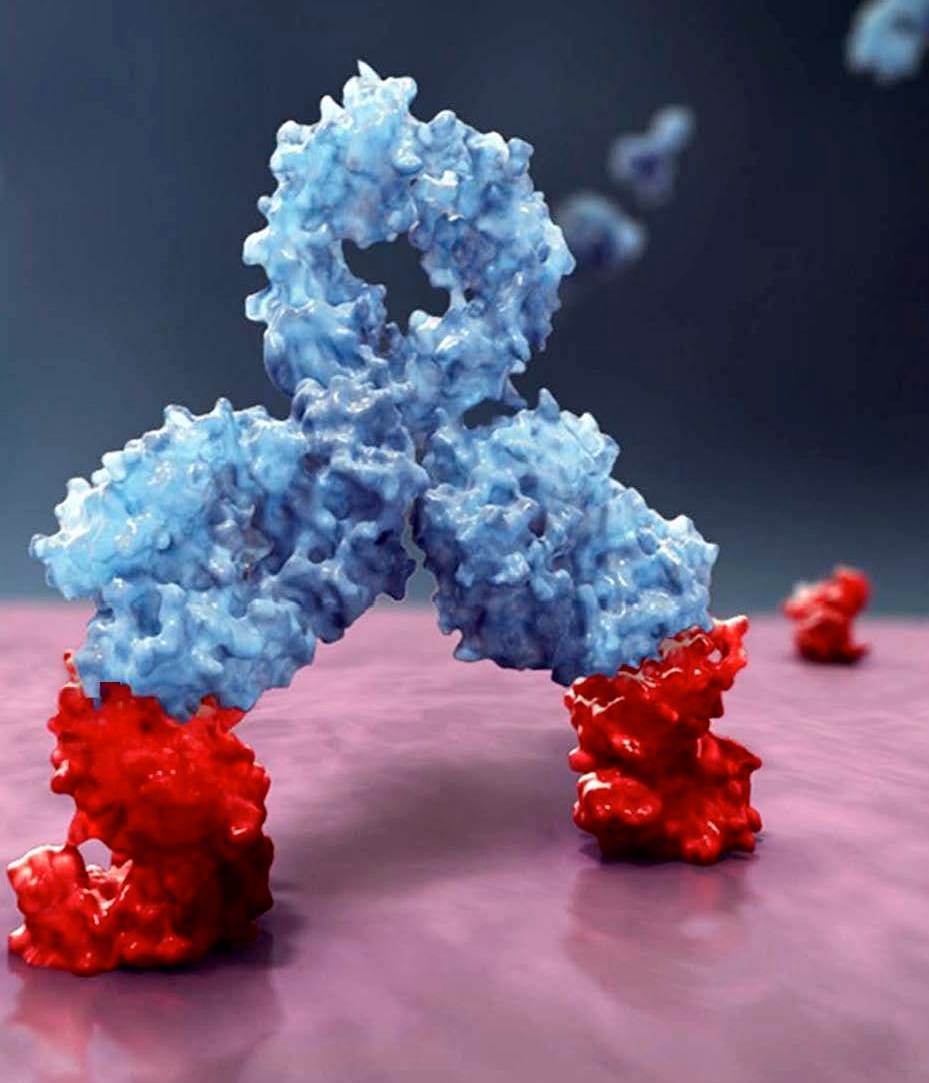
Daratumumab is the first approved monoclonal antibody that targets CD38, which is expressed across multiple myeloma cells regardless of disease stage. Daratumumab is currently approved for treatment of multiple myeloma in both the United States and the European Union either as monotherapy or in conjunction with other treatments.
Daratumumab is known to sometimes cause severe/serious infusion reactions, such as anaphylactic reactions; interfere with serological testing; and cause neutropenia or thrombocytopenia.
Janssen has applied to the Food and Drug Administration and the European Medicines Agency to allow splitting of the first infusion of daratumumab (Darzalex) in multiple myeloma patients over 2 consecutive days.
The goal is to improve the treatment experience for patients and physicians, according to the announcement from Janssen.
The regulatory submissions are based on the global, multi-arm, phase 1b MMY1001 study (NCT01998971). The study evaluated daratumumab in combination with various other treatments in 240 patients with multiple myeloma. It found that both the safety profile and the pharmacokinetics concentrations seen with either single dosing or split dosing were similar.
Daratumumab is the first approved monoclonal antibody that targets CD38, which is expressed across multiple myeloma cells regardless of disease stage. Daratumumab is currently approved for treatment of multiple myeloma in both the United States and the European Union either as monotherapy or in conjunction with other treatments.
Daratumumab is known to sometimes cause severe/serious infusion reactions, such as anaphylactic reactions; interfere with serological testing; and cause neutropenia or thrombocytopenia.
Janssen has applied to the Food and Drug Administration and the European Medicines Agency to allow splitting of the first infusion of daratumumab (Darzalex) in multiple myeloma patients over 2 consecutive days.
The goal is to improve the treatment experience for patients and physicians, according to the announcement from Janssen.
The regulatory submissions are based on the global, multi-arm, phase 1b MMY1001 study (NCT01998971). The study evaluated daratumumab in combination with various other treatments in 240 patients with multiple myeloma. It found that both the safety profile and the pharmacokinetics concentrations seen with either single dosing or split dosing were similar.
Daratumumab is the first approved monoclonal antibody that targets CD38, which is expressed across multiple myeloma cells regardless of disease stage. Daratumumab is currently approved for treatment of multiple myeloma in both the United States and the European Union either as monotherapy or in conjunction with other treatments.
Daratumumab is known to sometimes cause severe/serious infusion reactions, such as anaphylactic reactions; interfere with serological testing; and cause neutropenia or thrombocytopenia.