User login
The medical management of non-ST-elevation acute coronary syndromes focuses on blocking the coagulation cascade and inhibiting platelets. This—plus diagnostic angiography followed, if needed, by revascularization—has reduced the rates of death and recurrent ischemic events.1 However, the combination of potent antithrombotic drugs and invasive procedures also increases the risk of bleeding.
This review discusses the incidence and complications associated with bleeding during the treatment of acute coronary syndromes and summarizes recommendations for preventing and managing bleeding in this setting.
THE TRUE INCIDENCE OF BLEEDING IS HARD TO DETERMINE
The optimal way to detect and analyze bleeding events in clinical trials and registries is highly debated. The reported incidences of bleeding during antithrombotic and antiplatelet therapy for non-ST-elevation acute coronary syndromes depend on how bleeding was defined, how the acute coronary syndromes were treated, and on other factors such as how the study was designed.
How was bleeding defined?
Since these classification schemes are based on different types of data, they yield different numbers when applied to the same study population. For instance, Rao et al4 pooled the data from the PURSUIT and PARAGON B trials (15,454 patients in all) and found that the incidence of severe bleeding (by the GUSTO criteria) was 1.2%, while the rate of major bleeding (by the TIMI criteria) was 8.2%.
What was the treatment strategy?
Another reason that the true incidence of bleeding is hard to determine is that different studies used treatment strategies that differed in the type, timing, and dose of antithrombotic agents and whether invasive procedures were used early. For example, if unfractionated heparin is used aggressively in regimens that are not adjusted for weight and with a higher target for the activated clotting time, the risk of bleeding is higher than with conservative dosing.5–7
Subherwal et al8 evaluated the effect of treatment strategy on the incidence of bleeding in patients with non-ST-elevation acute coronary syndromes who received two or more antithrombotic drugs in the CRUSADE Quality Improvement Initiative. The risk of bleeding was higher with an invasive approach (catheterization) than with a conservative approach (no catheterization), regardless of baseline bleeding risk.
What type of study was it?
Another source of variation is the design of the study. Registries differ from clinical trials in patient characteristics and in the way data are gathered (prospectively vs retrospectively).
In registries, data are often collected retrospectively, whereas in clinical trials the data are prospectively collected. For this reason, the definition of bleeding in registries is often based on events that are easily identified through chart review, such as transfusion. This may lead to a lower reported rate of bleeding, since other, less serious bleeding events such as access-site hematomas and epistaxis may not be documented in the medical record.
On the other hand, registries often include older and sicker patients, who may be more prone to bleeding and who are often excluded from clinical trials. This may lead to a higher rate of reported bleeding.9
Where the study was conducted makes a difference as well, owing to regional practice differences. For example, Moscucci et al10 reported that the incidence of major bleeding in 24,045 patients with non-ST-elevation acute coronary syndromes in the GRACE registry (in 14 countries worldwide) was 3.9%. In contrast, Yang et al11 reported that the rate of bleeding in the CRUSADE registry (in the United States) was 10.3%.
This difference was partly influenced by different definitions of bleeding. The GRACE registry defined major bleeding as life-threatening events requiring transfusion of two or more units of packed red blood cells, or resulting in an absolute decrease in the hematocrit of 10% or more or death, or hemorrhagic subdural hematoma. In contrast, the CRUSADE data reflect bleeding requiring transfusion. However, practice patterns such as greater use of invasive procedures in the United States may also be responsible.
Rao and colleagues12 examined international variation in blood transfusion rates among patients with acute coronary syndromes. Patients outside the United States were significantly less likely to receive transfusions, even after adjusting for patient and practice differences.
Taking these confounders into account, it is reasonable to estimate that the frequency of bleeding in patients with non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%.13

BLEEDING IS ASSOCIATED WITH POOR OUTCOMES
Regardless of the definition or the data source, hemorrhagic complications are associated with a higher risk of death and nonfatal adverse events, both in the short term and in the long term.
Short-term outcomes
A higher risk of death. In the GRACE registry study by Moscucci et al10 discussed above, patients who had major bleeding were significantly more likely to die during their hospitalization than those who did not (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.18–2.28).
Rao et al14 evaluated pooled data from the multicenter international GUSTO IIb, PURSUIT, and PARAGON A and B trials and found that the effects of bleeding in non-ST-elevation acute coronary syndromes extended beyond the hospital stay. The more severe the bleeding (by the GUSTO criteria), the greater the adjusted hazard ratio (HR) for death within 30 days:
- With mild bleeding—HR 1.6, 95% CI 1.3–1.9
- With moderate bleeding—HR 2.7, 95% CI 2.3–3.4
- With severe bleeding—HR 10.6, 95% CI 8.3–13.6.
The pattern was the same for death within 6 months:
- With mild bleeding—HR 1.4, 95% CI 1.2–1.6
- With moderate bleeding—HR 2.1, 95% CI 1.8–2.4
- With severe bleeding, HR 7.5, 95% CI 6.1–9.3.
These findings were confirmed by Eikelboom et al15 in 34,146 patients with acute coronary syndromes in the OASIS registry, the OASIS-2 trial, and the CURE randomized trial. In the first 30 days, five times as many patients died (12.8% vs 2.5%; P < .0009) among those who developed major bleeding compared with those who did not. These investigators defined major bleeding as bleeding that was life-threatening or significantly disabling or that required transfusion of two or more units of packed red blood cells.
A higher risk of nonfatal adverse events. Bleeding after antithrombotic therapy for non-ST-elevation acute coronary syndromes has also been associated with nonfatal adverse events such as stroke and stent thrombosis.
For example, in the study by Eikelboom et al,15 major bleeding was associated with a higher risk of recurrent ischemic events. Approximately 1 in 5 patients in the OASIS trials who developed major bleeding during the first 30 days died or had a myocardial infarction or stroke by 30 days, compared with 1 in 20 of those who did not develop major bleeding during the first 30 days. However, after events that occurred during the first 30 days were excluded, the association between major bleeding and both myocardial infarction and stroke was no longer evident between 30 days and 6 months.
Manoukian et al16 evaluated the impact of major bleeding in 13,819 patients with highrisk acute coronary syndromes undergoing treatment with an early invasive strategy in the ACUITY trial. At 30 days, patients with major bleeding had higher rates of the composite end point of death, myocardial infarction, or unplanned revascularization for ischemia (23.1% vs 6.8%, P < .0001) and of stent thrombosis (3.4% vs 0.6%, P < .0001).
Long-term outcomes
The association between bleeding and adverse outcomes persists in the long term as well, although the mechanisms underlying this association are not well studied.
Kinnaird et al17 examined the data from 10,974 unselected patients who underwent percutaneous coronary intervention. At 1 year, the following percentages of patients had died:
- After TIMI major bleeding—17.2%
- After TIMI minor bleeding—9.1%
- After no bleeding—5.5%.
However, after adjustment for potential confounders, only transfusion remained a significant predictor of 1-year mortality.
Mehran et al18 evaluated 1-year mortality data from the ACUITY trial. Compared with the rate in patients who had no major bleeding and no myocardial infarction, the hazard ratios for death were:
- After major bleeding—HR 3.5, 95% CI 2.7–4.4
- After myocardial infarction—HR 3.1, 95% CI 2.4–3.9.
Interestingly, the risk of death associated with myocardial infarction abated after 7 days, while the risk associated with bleeding persisted beyond 30 days and remained constant throughout the first year following the bleeding event.
Similarly, Ndrepepa and colleagues19 examined pooled data from four ISAR trials using the TIMI bleeding scale and found that myocardial infarction, target vessel revascularization, and major bleeding all had similar discriminatory ability at predicting 1-year mortality.
In patients undergoing elective or urgent percutaneous coronary intervention in the REPLACE-2 trial,20 independent predictors of death by 1 year were21:
- Major hemorrhage (OR 2.66, 95% CI 1.44–4.92)
- Periprocedural myocardial infarction (OR 2.46, 95% CI 1.44–4.20).
THEORIES OF HOW BLEEDING MAY CAUSE ADVERSE OUTCOMES
Several mechanisms have been proposed to explain the association between bleeding during treatment for acute coronary syndromes and adverse clinical outcomes.13,22
The immediate effects of bleeding are thought to be hypotension and a reflex hyperadrenergic state to compensate for the loss of intravascular volume.23 This physiologic response is believed to contribute to myocardial ischemia by further decreasing myocardial oxygen supply in obstructive coronary disease.
Trying to minimize blood loss, physicians may withhold anticoagulation and antiplatelet therapy, which in turn may lead to further ischemia.24 To compensate for blood loss, physicians may also resort to blood transfusion. However, depletion of 2,3-diphosphoglycerate and nitric oxide in stored donor red blood cells is postulated to reduce oxygen delivery by increasing hemoglobin’s affinity for oxygen, leading to induced microvascular obstruction and adverse inflammatory reactions.15,25
Recent data have also begun to elucidate the long-term effects of bleeding during acute coronary syndrome management. Patients with anemia during the acute phase of infarction have greater neurohormonal activation.26 These adaptive responses to anemia may lead to eccentric left ventricular remodeling that may lead to higher oxygen consumption, increased diastolic wall stress, interstitial fibrosis, and accelerated myocyte loss.27–30
Nevertheless, we must point out that although strong associations between bleeding and adverse outcomes have been established, direct causality has not.
TO PREVENT BLEEDING, START BY ASSESSING RISK
The CRUSADE bleeding risk score
The CRUSADE bleeding score (calculator available at http://www.crusadebleedingscore.org/) was developed and validated in more than 89,000 community-treated patients with non-ST-elevation acute coronary syndromes.8 It is based on eight variables:
- Sex (higher risk in women)
- History of diabetes (higher risk)
- Prior vascular disease (higher risk)
- Heart rate (the higher the rate, the higher the risk)
- Systolic blood pressure (higher risk with pressures above or below the 121–180 mm Hg range)
- Signs of congestive heart failure (higher risk)
- Baseline hematocrit (the lower the hematocrit, the higher the risk)
- Creatinine clearance (by the Cockcroft-Gault formula; the lower the creatinine clearance, the higher the risk).
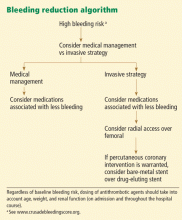
Patients who are found to have bleeding scores suggesting a moderate or higher risk of bleeding should be considered for medications associated with a favorable bleeding profile, and for radial access at the time of coronary angiography. Scores are graded as follows8:
- < 21: Very low risk
- 21–30: Low risk
- 31–40: Moderate risk
- 41–50: High risk
- > 50: Very high risk.
The CRUSADE bleeding score is unique in that, unlike earlier risk stratification tools, it was developed in a “real world” population, not in subgroups or in a clinical trial. It can be calculated at baseline to help guide the selection of treatment.8
Adjusting the heparin regimen in patients at risk of bleeding
Both the joint American College of Cardiology/American Heart Association1 and the European Society of Cardiology guidelines31 for the treatment of non-ST-elevation acute coronary syndromes recommend taking steps to prevent bleeding, such as adjusting the dosage of unfractionated heparin, using safer drugs, reducing the duration of antithrombotic treatment, and using combinations of antithrombotic and antiplatelet agents according to proven indications.31
In the CRUSADE registry, 42% of patients with non-ST-elevation acute coronary syndromes received at least one initial dose of antithrombotic drug outside the recommended range, resulting in an estimated 15% excess of bleeding events.32 Thus, proper dosing is a target for prevention.
Appropriate antithrombotic dosing takes into account the patient’s age, weight, and renal function. However, heparin dosage in the current guidelines1 is based on weight only: a loading dose of 60 U/kg (maximum 4,000 U) by intravenous bolus, then 12 U/kg/hour (maximum 1,000 U/hour) to maintain an activated partial thromboplastin time of 50 to 70 seconds.1
Renal dysfunction is particularly worrisome in patients with non-ST-elevation acute coronary syndromes because it is associated with higher rates of major bleeding and death. In the OASIS-5 trial,33 the overall risk of death was approximately five times higher in patients in the lowest quartile of renal function (glomerular filtration rate < 58 mL/min/1.73 m2) than in the highest quartile (glomerular filtration rate ≥ 86 mL/min/1.73 m2).
Renal function must be evaluated not only on admission but also throughout the hospital stay. Patients presenting with acute coronary syndromes often experience fluctuations in renal function that would call for adjustment of heparin dosing, either increasing the dose to maximize the drug’s efficacy if renal function is recovering or decreasing the dose to prevent bleeding if renal function is deteriorating.
Clopidogrel vs prasugrel
Certain medications should be avoided when the risk of bleeding outweighs any potential benefit in terms of ischemia.
For example, in a randomized trial,34 prasugrel (Effient), a potent thienopyridine, was associated with a significantly lower rate of the composite end point of stroke, myocardial infarction, or death than clopidogrel (Plavix) in patients with acute coronary syndromes undergoing percutaneous coronary interventions. However, it did not seem to offer any advantage in patients 75 years old and older, those with prior stroke or transient ischemic attack, or those weighing less than 60 kg, and it posed a substantially higher risk of bleeding.
With clopidogrel, the risk of acute bleeding is primarily in patients who undergo coronary artery bypass grafting within 5 days of receiving a dose.35,36 Therefore, clopidogrel should be stopped 5 to 7 days before bypass surgery.1 Importantly, there is no increased risk of recurrent ischemic events during this 5-day waiting period in patients who receive clopidogrel early. Therefore, the recommendation to stop clopidogrel before surgery does not negate the benefits of early treatment.36
Lower-risk drugs: Fondaparinux and bivalirudin
At this time, only two agents have been studied in clinical trials that have specifically focused on reducing bleeding risk: fondaparinux (Arixtra) and bivalirudin (Angiomax).20,37–39
Fondaparinux
OASIS-5 was a randomized, double-blind trial that compared fondaparinux and enoxaparin (Lovenox) in patients with acute coronary syndromes.38 Fondaparinux was similar to enoxaparin in terms of the combined end point of death, myocardial infarction, or refractory ischemia at 9 days, and fewer patients on fondaparinux developed bleeding (2.2% vs 4.1%, HR 0.52; 95% CI 0.44–0.61). This difference persisted during long-term follow-up.
Importantly, fewer patients died in the fondaparinux group. At 180 days, 638 (6.5%) of the patients in the enoxaparin group had died, compared with 574 (5.8%) in the fondaparinux group, a difference of 64 deaths (P = .05). The authors found that 41 fewer patients in the fondaparinux group than in the enoxaparin group died after major bleeding, and 20 fewer patients in the fondaparinux group died after minor bleeding.38 Thus, most of the difference in mortality rates between the two groups was attributed to a lower incidence of bleeding with fondaparinux.
Unfortunately, despite its safe bleeding profile, fondaparinux has fallen out of favor for use in acute coronary syndromes, owing to a higher risk of catheter thrombosis in the fondaparinux group (0.9%) than in those undergoing percutaneous coronary interventions with enoxaparin alone (0.4%) in the OASIS-5 trial.40
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in three large randomized trials in patients undergoing percutaneous coronary interventions.20,37,41
The ACUITY trial37 was a prospective, open-label, randomized, multicenter trial that compared three regimens in patients with moderate or high-risk non-ST-elevation acute coronary syndromes:
- Heparin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin alone.
Bivalirudin alone was as effective as heparin plus a glycoprotein IIb/IIIa inhibitor with respect to the composite ischemia end point, which at 30 days had occurred in 7.8% vs 7.3% of the patients in these treatment groups (P = .32, RR 1.08; 95% CI 0.93–1.24), and it was superior with respect to major bleeding (3.0% vs 5.7%, P < .001, RR 0.53; 95% CI 0.43–0.65).
The HORIZONS-AMI study41 was a prospective, open-label, randomized, multicenter trial that compared bivalirudin alone vs heparin plus a glycoprotein IIb/IIIa inhibitor in patients with ST-elevation acute coronary syndromes who were undergoing primary percutaneous coronary interventions. The two primary end points were major bleeding and net adverse events.
At 1 year, patients assigned to bivalirudin had a lower rate of major bleeding than did controls (5.8% vs 9.2%, HR 0.61, 95% CI 0.48–0.78, P < .0001), with similar rates of major adverse cardiac events in both groups (11.9% vs 11.9%, HR 1.00, 95% CI 0.82– 1.21, P = .98).41
Both OASIS 5 and HORIZONS-AMI are examples of clinical trials in which strategies that reduced bleeding risk were also associated with improved survival.
For cardiac catheterization, inserting the catheter in the wrist poses less risk
Bleeding is currently the most common noncardiac complication in patients undergoing percutaneous coronary interventions, and it most often occurs at the vascular access site.17
Rao et al12 evaluated data from 593,094 procedures in the National Cardiovascular Data Registry and found that, compared with the femoral approach, the use of transradial percutaneous coronary intervention was associated with a similar rate of procedural success (OR 1.02, 95% CI 0.93–1.12) but a significantly lower risk of bleeding complications (OR 0.42, 95% CI 0.31–0.56) after multivariable adjustment.
The use of smaller sheath sizes (4F–6F) and preferential use of bivalirudin over unfractionated heparin and glycoprotein IIb/IIIa inhibitor therapy are other methods described to decrease the risk of bleeding after percutaneous coronary interventions.20,41–49
IF BLEEDING OCCURS
Once a bleeding complication occurs, cessation of therapy is a potential option. Stopping or reversing antithrombotic and antiplatelet therapy is warranted in the event of major bleeding (eg, gastrointestinal, retroperitoneal, intracranial).31
Stopping antithrombotic and antiplatelet therapy
Whether bleeding is minor or major, the risk of a recurrent thrombotic event must be considered, especially in patients who have undergone revascularization, stent implantation, or both. The risk of acute thrombotic events after interrupting antithrombotic or antiplatelet agents is considered greatest 4 to 5 days following revascularization or percutaneous coronary intervention.15 If bleeding can be controlled with local treatment such as pressure, packing, or dressing, antithrombotic and antiplatelet therapy need not be interrupted.50
Current guidelines recommend strict control of hemorrhage for at least 24 hours before reintroducing antiplatelet or antithrombotic agents.
It is also important to remember that in the setting of gastrointestinal bleeding due to peptic ulcer disease, adjunctive proton pump inhibitors are recommended after restarting antiplatelet or antithrombotic therapy or both.
Importantly, evidence-based antithrombotic medications (especially dual antiplatelet therapy) should be restarted once the acute bleeding event has resolved.31
Reversal of anticoagulant and antiplatelet therapies
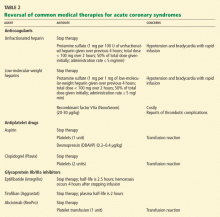
Unfractionated heparin is reversed with infusion of protamine sulfate at a dose of 1 mg per 100 U of unfractionated heparin given over the previous 4 hours.51,52 The rate of protamine sulfate infusion should be less than 100 mg over 2 hours, with 50% of the dose given initially and subsequent doses titrated according to bleeding response.52,53 Protamine sulfate is associated with a risk of hypotension and bradycardia, and for this reason it should be given no faster than 5 mg/min.
Low-molecular-weight heparin (LMWH) can be inhibited by 1 mg of protamine sulfate for each 1 mg of LMWH given over the previous 4 hours.51,52
However, protamine sulfate only partially neutralizes the anticoagulant effect of LMWH. In cases in which protamine sulfate is unsuccessful in abating bleeding associated with LMWH use, guidelines allow for the use of recombinant factor VIIa (NovoSeven).31 In healthy volunteers given fondaparinux, recombinant factor VIIa normalized coagulation times and thrombin generation within 1.5 hours, with a sustained effect for 6 hours.52
It is important to note that the use of this agent has not been fully studied, it is very costly (a single dose of 40 μg/kg costs from $3,000 to $4,000), and it is linked to reports of increased risk of thrombotic complications.54,55
Antiplatelet agents are more complex to reverse. The antiplatelet actions of aspirin and clopidogrel wear off as new platelets are produced. Approximately 10% of a patient’s platelet count is produced daily; thus, the antiplatelet effects of aspirin and clopidogrel can persist for 5 to 10 days.31,56
If these agents need to be reversed quickly to stop bleeding, according to expert consensus the aspirin effect can be reversed by transfusion of one unit of platelets. The antiplatelet effect of clopidogrel is more significant than that of aspirin; thus, two units of platelets are recommended.56
Glycoprotein IIb/IIIa inhibitors. If a major bleeding event requires the reversal of glycoprotein IIb/IIIa inhibitor therapy, the treatment must take into consideration the pharmacodynamics of the target drug. Both eptifibatide (Integrilin) and tirofiban (Aggrastat) competitively inhibit glycoprotein IIb/IIIa receptors; thus, their effects depend on dosing, elimination, and time. Due to the stoichiometry of both drugs, transfusion of platelets is ineffective. Both eptifibatide and tirofiban are eliminated by the kidney; thus, normal renal function is key to the amount of time it takes for platelet function to return to baseline.57 Evidence suggests that fibrinogen-rich plasma can be administered to restore platelet function.31,58,59
Abciximab (ReoPro). Whereas reversal of eptifibatide and tirofiban focuses on overcoming competitive inhibition, neutralization of abciximab involves overcoming its high receptor affinity. At 24 hours after abciximab infusion is stopped, platelet aggregation may still be inhibited by up to 50%. Fortunately, owing to abciximab’s short plasma half-life and its dilution in serum, platelet transfusion is effective in reversing its antiplatelet effects.31,57
Blood transfusion
Long considered beneficial to critically ill patients, blood transfusion to maintain hematocrit levels during acute coronary syndromes has come under intense scrutiny. Randomized trials have shown that transfusion should not be given aggressively to critically ill patients.60 In acute coronary syndromes, there are only observational data.
Rao et al61 used detailed clinical data from 24,112 patients with acute coronary syndromes in the GUSTO IIb, PURSUIT, and PARAGON B trials to determine the association between blood transfusion and outcomes in patients who developed moderate to severe bleeding, anemia, or both during their hospitalization. The rates of death in the hospital and at 30 days were significantly higher in patients who received a transfusion (30-day mortality HR 3.94; 95% CI 3.36–4.75). However, there was no significant association between transfusion and the 30-day mortality rate if the nadir hematocrit was 25% or less.
Of note: no randomized clinical trial has evaluated transfusion strategies in acute coronary syndromes at this time. Until such data are available, it is reasonable to follow published guidelines and to avoid transfusion in stable patients with ischemic heart disease unless the hematocrit is 25% or less.31 The risks and benefits of blood transfusion should be carefully weighed. Routine use of transfusion to maintain predefined hemoglobin levels is not recommended in stable patients.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154.
- Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47:809–816.
- Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996; 93:870–878.
- Gilchrist IC, Berkowitz SD, Thompson TD, Califf RM, Granger CB. Heparin dosing and outcome in acute coronary syndromes: the GUSTO-IIb experience. Global Use of Strategies to Open Occluded Coronary Arteries. Am Heart J 2002; 144:73–80.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol 2003; 41:386–393.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119:1873–1882.
- Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart 2008; 94:661–666.
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24:1815–1823.
- Yang X, Alexander KP, Chen AY, et al; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005; 46:1490–1495.
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008; 1:379–386.
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1193–1204.
- Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96:1200–1206.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92:930–935.
- Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009; 30:1457–1466.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51:690–697.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100:1364–1369.
- Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol 2007; 23:663–671.
- Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J 2007; 28:1273–1274.
- Yan AT, Yan RT, Huynh T, et al; INTERACT Investigators. Bleeding and outcome in acute coronary syndrome: insights from continuous electrocardiogram monitoring in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial. Am Heart J 2008; 156:769–775.
- Jolicoeur EM, O’Neill WW, Hellkamp A, et al; APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2009; 30:2575–2583.
- Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol 2005; 95:1474–1477.
- Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149–154.
- O’Riordan E, Foley RN. Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 2000; 15(suppl 3):19–22.
- Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol 1992; 141:227–239.
- Aronson D, Suleiman M, Agmon Y, et al. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J 2007; 28:1289–1296.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Alexander KP, Chen AY, Roe MT, et al; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 2005; 294:3108–3116.
- Fox KA, Bassand JP, Mehta SR, et al; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med 2007; 147:304–310.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008; 52:1693–1701.
- Fox KA, Mehta SR, Peters R, et al; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Potsis TZ, Katsouras C, Goudevenos JA. Avoiding and managing bleeding complications in patients with non-ST-segment elevation acute coronary syndromes. Angiology 2009; 60:148–158.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Stone GW, Ware JH, Bertrand ME, et al; ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA 2007; 298:2497–2506.
- Cantor WJ, Mahaffey KW, Huang Z, et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv 2007; 69:73–83.
- Büchler JR, Ribeiro EE, Falcão JL, et al. A randomized trial of 5 versus 7 French guiding catheters for transfemoral percutaneous coronary stent implantation. J Interv Cardiol 2008; 21:50–55.
- Shammas NW, Allie D, Hall P, et al; APPROVE Investigators. Predictors of in-hospital and 30-day complications of peripheral vascular interventions using bivalirudin as the primary anticoagulant: results from the APPROVE Registry. J Invasive Cardiol 2005; 17:356–359.
- Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1:202–209.
- Stone GW, White HD, Ohman EM, et al; Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial investigators. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007; 369:907–919.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004; 93:1092–1096.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth 2002; 49:S11–S25.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008; 111:4871–4879.
- Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol 2004; 41(suppl 1):44–50.
- Ganguly S, Spengel K, Tilzer LL, O’Neal B, Simpson SQ. Recombinant factor VIIa: unregulated continuous use in patients with bleeding and coagulopathy does not alter mortality and outcome. Clin Lab Haematol 2006; 28:309–312.
- O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006; 295:293–298.
- Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg 2010; 112:307–318.
- Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139:S38–S45.
- Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J 2002; 143:725–732.
- Schroeder WS, Gandhi PJ. Emergency management of hemorrhagic complications in the era of glycoprotein IIb/IIIa receptor antagonists, clopidogrel, low molecular weight heparin, and third-generation fibrinolytic agents. Curr Cardiol Rep 2003; 5:310–317.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
The medical management of non-ST-elevation acute coronary syndromes focuses on blocking the coagulation cascade and inhibiting platelets. This—plus diagnostic angiography followed, if needed, by revascularization—has reduced the rates of death and recurrent ischemic events.1 However, the combination of potent antithrombotic drugs and invasive procedures also increases the risk of bleeding.
This review discusses the incidence and complications associated with bleeding during the treatment of acute coronary syndromes and summarizes recommendations for preventing and managing bleeding in this setting.
THE TRUE INCIDENCE OF BLEEDING IS HARD TO DETERMINE
The optimal way to detect and analyze bleeding events in clinical trials and registries is highly debated. The reported incidences of bleeding during antithrombotic and antiplatelet therapy for non-ST-elevation acute coronary syndromes depend on how bleeding was defined, how the acute coronary syndromes were treated, and on other factors such as how the study was designed.
How was bleeding defined?
Since these classification schemes are based on different types of data, they yield different numbers when applied to the same study population. For instance, Rao et al4 pooled the data from the PURSUIT and PARAGON B trials (15,454 patients in all) and found that the incidence of severe bleeding (by the GUSTO criteria) was 1.2%, while the rate of major bleeding (by the TIMI criteria) was 8.2%.
What was the treatment strategy?
Another reason that the true incidence of bleeding is hard to determine is that different studies used treatment strategies that differed in the type, timing, and dose of antithrombotic agents and whether invasive procedures were used early. For example, if unfractionated heparin is used aggressively in regimens that are not adjusted for weight and with a higher target for the activated clotting time, the risk of bleeding is higher than with conservative dosing.5–7
Subherwal et al8 evaluated the effect of treatment strategy on the incidence of bleeding in patients with non-ST-elevation acute coronary syndromes who received two or more antithrombotic drugs in the CRUSADE Quality Improvement Initiative. The risk of bleeding was higher with an invasive approach (catheterization) than with a conservative approach (no catheterization), regardless of baseline bleeding risk.
What type of study was it?
Another source of variation is the design of the study. Registries differ from clinical trials in patient characteristics and in the way data are gathered (prospectively vs retrospectively).
In registries, data are often collected retrospectively, whereas in clinical trials the data are prospectively collected. For this reason, the definition of bleeding in registries is often based on events that are easily identified through chart review, such as transfusion. This may lead to a lower reported rate of bleeding, since other, less serious bleeding events such as access-site hematomas and epistaxis may not be documented in the medical record.
On the other hand, registries often include older and sicker patients, who may be more prone to bleeding and who are often excluded from clinical trials. This may lead to a higher rate of reported bleeding.9
Where the study was conducted makes a difference as well, owing to regional practice differences. For example, Moscucci et al10 reported that the incidence of major bleeding in 24,045 patients with non-ST-elevation acute coronary syndromes in the GRACE registry (in 14 countries worldwide) was 3.9%. In contrast, Yang et al11 reported that the rate of bleeding in the CRUSADE registry (in the United States) was 10.3%.
This difference was partly influenced by different definitions of bleeding. The GRACE registry defined major bleeding as life-threatening events requiring transfusion of two or more units of packed red blood cells, or resulting in an absolute decrease in the hematocrit of 10% or more or death, or hemorrhagic subdural hematoma. In contrast, the CRUSADE data reflect bleeding requiring transfusion. However, practice patterns such as greater use of invasive procedures in the United States may also be responsible.
Rao and colleagues12 examined international variation in blood transfusion rates among patients with acute coronary syndromes. Patients outside the United States were significantly less likely to receive transfusions, even after adjusting for patient and practice differences.
Taking these confounders into account, it is reasonable to estimate that the frequency of bleeding in patients with non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%.13

BLEEDING IS ASSOCIATED WITH POOR OUTCOMES
Regardless of the definition or the data source, hemorrhagic complications are associated with a higher risk of death and nonfatal adverse events, both in the short term and in the long term.
Short-term outcomes
A higher risk of death. In the GRACE registry study by Moscucci et al10 discussed above, patients who had major bleeding were significantly more likely to die during their hospitalization than those who did not (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.18–2.28).
Rao et al14 evaluated pooled data from the multicenter international GUSTO IIb, PURSUIT, and PARAGON A and B trials and found that the effects of bleeding in non-ST-elevation acute coronary syndromes extended beyond the hospital stay. The more severe the bleeding (by the GUSTO criteria), the greater the adjusted hazard ratio (HR) for death within 30 days:
- With mild bleeding—HR 1.6, 95% CI 1.3–1.9
- With moderate bleeding—HR 2.7, 95% CI 2.3–3.4
- With severe bleeding—HR 10.6, 95% CI 8.3–13.6.
The pattern was the same for death within 6 months:
- With mild bleeding—HR 1.4, 95% CI 1.2–1.6
- With moderate bleeding—HR 2.1, 95% CI 1.8–2.4
- With severe bleeding, HR 7.5, 95% CI 6.1–9.3.
These findings were confirmed by Eikelboom et al15 in 34,146 patients with acute coronary syndromes in the OASIS registry, the OASIS-2 trial, and the CURE randomized trial. In the first 30 days, five times as many patients died (12.8% vs 2.5%; P < .0009) among those who developed major bleeding compared with those who did not. These investigators defined major bleeding as bleeding that was life-threatening or significantly disabling or that required transfusion of two or more units of packed red blood cells.
A higher risk of nonfatal adverse events. Bleeding after antithrombotic therapy for non-ST-elevation acute coronary syndromes has also been associated with nonfatal adverse events such as stroke and stent thrombosis.
For example, in the study by Eikelboom et al,15 major bleeding was associated with a higher risk of recurrent ischemic events. Approximately 1 in 5 patients in the OASIS trials who developed major bleeding during the first 30 days died or had a myocardial infarction or stroke by 30 days, compared with 1 in 20 of those who did not develop major bleeding during the first 30 days. However, after events that occurred during the first 30 days were excluded, the association between major bleeding and both myocardial infarction and stroke was no longer evident between 30 days and 6 months.
Manoukian et al16 evaluated the impact of major bleeding in 13,819 patients with highrisk acute coronary syndromes undergoing treatment with an early invasive strategy in the ACUITY trial. At 30 days, patients with major bleeding had higher rates of the composite end point of death, myocardial infarction, or unplanned revascularization for ischemia (23.1% vs 6.8%, P < .0001) and of stent thrombosis (3.4% vs 0.6%, P < .0001).
Long-term outcomes
The association between bleeding and adverse outcomes persists in the long term as well, although the mechanisms underlying this association are not well studied.
Kinnaird et al17 examined the data from 10,974 unselected patients who underwent percutaneous coronary intervention. At 1 year, the following percentages of patients had died:
- After TIMI major bleeding—17.2%
- After TIMI minor bleeding—9.1%
- After no bleeding—5.5%.
However, after adjustment for potential confounders, only transfusion remained a significant predictor of 1-year mortality.
Mehran et al18 evaluated 1-year mortality data from the ACUITY trial. Compared with the rate in patients who had no major bleeding and no myocardial infarction, the hazard ratios for death were:
- After major bleeding—HR 3.5, 95% CI 2.7–4.4
- After myocardial infarction—HR 3.1, 95% CI 2.4–3.9.
Interestingly, the risk of death associated with myocardial infarction abated after 7 days, while the risk associated with bleeding persisted beyond 30 days and remained constant throughout the first year following the bleeding event.
Similarly, Ndrepepa and colleagues19 examined pooled data from four ISAR trials using the TIMI bleeding scale and found that myocardial infarction, target vessel revascularization, and major bleeding all had similar discriminatory ability at predicting 1-year mortality.
In patients undergoing elective or urgent percutaneous coronary intervention in the REPLACE-2 trial,20 independent predictors of death by 1 year were21:
- Major hemorrhage (OR 2.66, 95% CI 1.44–4.92)
- Periprocedural myocardial infarction (OR 2.46, 95% CI 1.44–4.20).
THEORIES OF HOW BLEEDING MAY CAUSE ADVERSE OUTCOMES
Several mechanisms have been proposed to explain the association between bleeding during treatment for acute coronary syndromes and adverse clinical outcomes.13,22
The immediate effects of bleeding are thought to be hypotension and a reflex hyperadrenergic state to compensate for the loss of intravascular volume.23 This physiologic response is believed to contribute to myocardial ischemia by further decreasing myocardial oxygen supply in obstructive coronary disease.
Trying to minimize blood loss, physicians may withhold anticoagulation and antiplatelet therapy, which in turn may lead to further ischemia.24 To compensate for blood loss, physicians may also resort to blood transfusion. However, depletion of 2,3-diphosphoglycerate and nitric oxide in stored donor red blood cells is postulated to reduce oxygen delivery by increasing hemoglobin’s affinity for oxygen, leading to induced microvascular obstruction and adverse inflammatory reactions.15,25
Recent data have also begun to elucidate the long-term effects of bleeding during acute coronary syndrome management. Patients with anemia during the acute phase of infarction have greater neurohormonal activation.26 These adaptive responses to anemia may lead to eccentric left ventricular remodeling that may lead to higher oxygen consumption, increased diastolic wall stress, interstitial fibrosis, and accelerated myocyte loss.27–30
Nevertheless, we must point out that although strong associations between bleeding and adverse outcomes have been established, direct causality has not.
TO PREVENT BLEEDING, START BY ASSESSING RISK
The CRUSADE bleeding risk score
The CRUSADE bleeding score (calculator available at http://www.crusadebleedingscore.org/) was developed and validated in more than 89,000 community-treated patients with non-ST-elevation acute coronary syndromes.8 It is based on eight variables:
- Sex (higher risk in women)
- History of diabetes (higher risk)
- Prior vascular disease (higher risk)
- Heart rate (the higher the rate, the higher the risk)
- Systolic blood pressure (higher risk with pressures above or below the 121–180 mm Hg range)
- Signs of congestive heart failure (higher risk)
- Baseline hematocrit (the lower the hematocrit, the higher the risk)
- Creatinine clearance (by the Cockcroft-Gault formula; the lower the creatinine clearance, the higher the risk).
Patients who are found to have bleeding scores suggesting a moderate or higher risk of bleeding should be considered for medications associated with a favorable bleeding profile, and for radial access at the time of coronary angiography. Scores are graded as follows8:
- < 21: Very low risk
- 21–30: Low risk
- 31–40: Moderate risk
- 41–50: High risk
- > 50: Very high risk.
The CRUSADE bleeding score is unique in that, unlike earlier risk stratification tools, it was developed in a “real world” population, not in subgroups or in a clinical trial. It can be calculated at baseline to help guide the selection of treatment.8
Adjusting the heparin regimen in patients at risk of bleeding
Both the joint American College of Cardiology/American Heart Association1 and the European Society of Cardiology guidelines31 for the treatment of non-ST-elevation acute coronary syndromes recommend taking steps to prevent bleeding, such as adjusting the dosage of unfractionated heparin, using safer drugs, reducing the duration of antithrombotic treatment, and using combinations of antithrombotic and antiplatelet agents according to proven indications.31
In the CRUSADE registry, 42% of patients with non-ST-elevation acute coronary syndromes received at least one initial dose of antithrombotic drug outside the recommended range, resulting in an estimated 15% excess of bleeding events.32 Thus, proper dosing is a target for prevention.
Appropriate antithrombotic dosing takes into account the patient’s age, weight, and renal function. However, heparin dosage in the current guidelines1 is based on weight only: a loading dose of 60 U/kg (maximum 4,000 U) by intravenous bolus, then 12 U/kg/hour (maximum 1,000 U/hour) to maintain an activated partial thromboplastin time of 50 to 70 seconds.1
Renal dysfunction is particularly worrisome in patients with non-ST-elevation acute coronary syndromes because it is associated with higher rates of major bleeding and death. In the OASIS-5 trial,33 the overall risk of death was approximately five times higher in patients in the lowest quartile of renal function (glomerular filtration rate < 58 mL/min/1.73 m2) than in the highest quartile (glomerular filtration rate ≥ 86 mL/min/1.73 m2).
Renal function must be evaluated not only on admission but also throughout the hospital stay. Patients presenting with acute coronary syndromes often experience fluctuations in renal function that would call for adjustment of heparin dosing, either increasing the dose to maximize the drug’s efficacy if renal function is recovering or decreasing the dose to prevent bleeding if renal function is deteriorating.
Clopidogrel vs prasugrel
Certain medications should be avoided when the risk of bleeding outweighs any potential benefit in terms of ischemia.
For example, in a randomized trial,34 prasugrel (Effient), a potent thienopyridine, was associated with a significantly lower rate of the composite end point of stroke, myocardial infarction, or death than clopidogrel (Plavix) in patients with acute coronary syndromes undergoing percutaneous coronary interventions. However, it did not seem to offer any advantage in patients 75 years old and older, those with prior stroke or transient ischemic attack, or those weighing less than 60 kg, and it posed a substantially higher risk of bleeding.
With clopidogrel, the risk of acute bleeding is primarily in patients who undergo coronary artery bypass grafting within 5 days of receiving a dose.35,36 Therefore, clopidogrel should be stopped 5 to 7 days before bypass surgery.1 Importantly, there is no increased risk of recurrent ischemic events during this 5-day waiting period in patients who receive clopidogrel early. Therefore, the recommendation to stop clopidogrel before surgery does not negate the benefits of early treatment.36
Lower-risk drugs: Fondaparinux and bivalirudin
At this time, only two agents have been studied in clinical trials that have specifically focused on reducing bleeding risk: fondaparinux (Arixtra) and bivalirudin (Angiomax).20,37–39
Fondaparinux
OASIS-5 was a randomized, double-blind trial that compared fondaparinux and enoxaparin (Lovenox) in patients with acute coronary syndromes.38 Fondaparinux was similar to enoxaparin in terms of the combined end point of death, myocardial infarction, or refractory ischemia at 9 days, and fewer patients on fondaparinux developed bleeding (2.2% vs 4.1%, HR 0.52; 95% CI 0.44–0.61). This difference persisted during long-term follow-up.
Importantly, fewer patients died in the fondaparinux group. At 180 days, 638 (6.5%) of the patients in the enoxaparin group had died, compared with 574 (5.8%) in the fondaparinux group, a difference of 64 deaths (P = .05). The authors found that 41 fewer patients in the fondaparinux group than in the enoxaparin group died after major bleeding, and 20 fewer patients in the fondaparinux group died after minor bleeding.38 Thus, most of the difference in mortality rates between the two groups was attributed to a lower incidence of bleeding with fondaparinux.
Unfortunately, despite its safe bleeding profile, fondaparinux has fallen out of favor for use in acute coronary syndromes, owing to a higher risk of catheter thrombosis in the fondaparinux group (0.9%) than in those undergoing percutaneous coronary interventions with enoxaparin alone (0.4%) in the OASIS-5 trial.40
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in three large randomized trials in patients undergoing percutaneous coronary interventions.20,37,41
The ACUITY trial37 was a prospective, open-label, randomized, multicenter trial that compared three regimens in patients with moderate or high-risk non-ST-elevation acute coronary syndromes:
- Heparin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin alone.
Bivalirudin alone was as effective as heparin plus a glycoprotein IIb/IIIa inhibitor with respect to the composite ischemia end point, which at 30 days had occurred in 7.8% vs 7.3% of the patients in these treatment groups (P = .32, RR 1.08; 95% CI 0.93–1.24), and it was superior with respect to major bleeding (3.0% vs 5.7%, P < .001, RR 0.53; 95% CI 0.43–0.65).
The HORIZONS-AMI study41 was a prospective, open-label, randomized, multicenter trial that compared bivalirudin alone vs heparin plus a glycoprotein IIb/IIIa inhibitor in patients with ST-elevation acute coronary syndromes who were undergoing primary percutaneous coronary interventions. The two primary end points were major bleeding and net adverse events.
At 1 year, patients assigned to bivalirudin had a lower rate of major bleeding than did controls (5.8% vs 9.2%, HR 0.61, 95% CI 0.48–0.78, P < .0001), with similar rates of major adverse cardiac events in both groups (11.9% vs 11.9%, HR 1.00, 95% CI 0.82– 1.21, P = .98).41
Both OASIS 5 and HORIZONS-AMI are examples of clinical trials in which strategies that reduced bleeding risk were also associated with improved survival.
For cardiac catheterization, inserting the catheter in the wrist poses less risk
Bleeding is currently the most common noncardiac complication in patients undergoing percutaneous coronary interventions, and it most often occurs at the vascular access site.17
Rao et al12 evaluated data from 593,094 procedures in the National Cardiovascular Data Registry and found that, compared with the femoral approach, the use of transradial percutaneous coronary intervention was associated with a similar rate of procedural success (OR 1.02, 95% CI 0.93–1.12) but a significantly lower risk of bleeding complications (OR 0.42, 95% CI 0.31–0.56) after multivariable adjustment.
The use of smaller sheath sizes (4F–6F) and preferential use of bivalirudin over unfractionated heparin and glycoprotein IIb/IIIa inhibitor therapy are other methods described to decrease the risk of bleeding after percutaneous coronary interventions.20,41–49
IF BLEEDING OCCURS
Once a bleeding complication occurs, cessation of therapy is a potential option. Stopping or reversing antithrombotic and antiplatelet therapy is warranted in the event of major bleeding (eg, gastrointestinal, retroperitoneal, intracranial).31
Stopping antithrombotic and antiplatelet therapy
Whether bleeding is minor or major, the risk of a recurrent thrombotic event must be considered, especially in patients who have undergone revascularization, stent implantation, or both. The risk of acute thrombotic events after interrupting antithrombotic or antiplatelet agents is considered greatest 4 to 5 days following revascularization or percutaneous coronary intervention.15 If bleeding can be controlled with local treatment such as pressure, packing, or dressing, antithrombotic and antiplatelet therapy need not be interrupted.50
Current guidelines recommend strict control of hemorrhage for at least 24 hours before reintroducing antiplatelet or antithrombotic agents.
It is also important to remember that in the setting of gastrointestinal bleeding due to peptic ulcer disease, adjunctive proton pump inhibitors are recommended after restarting antiplatelet or antithrombotic therapy or both.
Importantly, evidence-based antithrombotic medications (especially dual antiplatelet therapy) should be restarted once the acute bleeding event has resolved.31
Reversal of anticoagulant and antiplatelet therapies
Unfractionated heparin is reversed with infusion of protamine sulfate at a dose of 1 mg per 100 U of unfractionated heparin given over the previous 4 hours.51,52 The rate of protamine sulfate infusion should be less than 100 mg over 2 hours, with 50% of the dose given initially and subsequent doses titrated according to bleeding response.52,53 Protamine sulfate is associated with a risk of hypotension and bradycardia, and for this reason it should be given no faster than 5 mg/min.
Low-molecular-weight heparin (LMWH) can be inhibited by 1 mg of protamine sulfate for each 1 mg of LMWH given over the previous 4 hours.51,52
However, protamine sulfate only partially neutralizes the anticoagulant effect of LMWH. In cases in which protamine sulfate is unsuccessful in abating bleeding associated with LMWH use, guidelines allow for the use of recombinant factor VIIa (NovoSeven).31 In healthy volunteers given fondaparinux, recombinant factor VIIa normalized coagulation times and thrombin generation within 1.5 hours, with a sustained effect for 6 hours.52
It is important to note that the use of this agent has not been fully studied, it is very costly (a single dose of 40 μg/kg costs from $3,000 to $4,000), and it is linked to reports of increased risk of thrombotic complications.54,55
Antiplatelet agents are more complex to reverse. The antiplatelet actions of aspirin and clopidogrel wear off as new platelets are produced. Approximately 10% of a patient’s platelet count is produced daily; thus, the antiplatelet effects of aspirin and clopidogrel can persist for 5 to 10 days.31,56
If these agents need to be reversed quickly to stop bleeding, according to expert consensus the aspirin effect can be reversed by transfusion of one unit of platelets. The antiplatelet effect of clopidogrel is more significant than that of aspirin; thus, two units of platelets are recommended.56
Glycoprotein IIb/IIIa inhibitors. If a major bleeding event requires the reversal of glycoprotein IIb/IIIa inhibitor therapy, the treatment must take into consideration the pharmacodynamics of the target drug. Both eptifibatide (Integrilin) and tirofiban (Aggrastat) competitively inhibit glycoprotein IIb/IIIa receptors; thus, their effects depend on dosing, elimination, and time. Due to the stoichiometry of both drugs, transfusion of platelets is ineffective. Both eptifibatide and tirofiban are eliminated by the kidney; thus, normal renal function is key to the amount of time it takes for platelet function to return to baseline.57 Evidence suggests that fibrinogen-rich plasma can be administered to restore platelet function.31,58,59
Abciximab (ReoPro). Whereas reversal of eptifibatide and tirofiban focuses on overcoming competitive inhibition, neutralization of abciximab involves overcoming its high receptor affinity. At 24 hours after abciximab infusion is stopped, platelet aggregation may still be inhibited by up to 50%. Fortunately, owing to abciximab’s short plasma half-life and its dilution in serum, platelet transfusion is effective in reversing its antiplatelet effects.31,57
Blood transfusion
Long considered beneficial to critically ill patients, blood transfusion to maintain hematocrit levels during acute coronary syndromes has come under intense scrutiny. Randomized trials have shown that transfusion should not be given aggressively to critically ill patients.60 In acute coronary syndromes, there are only observational data.
Rao et al61 used detailed clinical data from 24,112 patients with acute coronary syndromes in the GUSTO IIb, PURSUIT, and PARAGON B trials to determine the association between blood transfusion and outcomes in patients who developed moderate to severe bleeding, anemia, or both during their hospitalization. The rates of death in the hospital and at 30 days were significantly higher in patients who received a transfusion (30-day mortality HR 3.94; 95% CI 3.36–4.75). However, there was no significant association between transfusion and the 30-day mortality rate if the nadir hematocrit was 25% or less.
Of note: no randomized clinical trial has evaluated transfusion strategies in acute coronary syndromes at this time. Until such data are available, it is reasonable to follow published guidelines and to avoid transfusion in stable patients with ischemic heart disease unless the hematocrit is 25% or less.31 The risks and benefits of blood transfusion should be carefully weighed. Routine use of transfusion to maintain predefined hemoglobin levels is not recommended in stable patients.
The medical management of non-ST-elevation acute coronary syndromes focuses on blocking the coagulation cascade and inhibiting platelets. This—plus diagnostic angiography followed, if needed, by revascularization—has reduced the rates of death and recurrent ischemic events.1 However, the combination of potent antithrombotic drugs and invasive procedures also increases the risk of bleeding.
This review discusses the incidence and complications associated with bleeding during the treatment of acute coronary syndromes and summarizes recommendations for preventing and managing bleeding in this setting.
THE TRUE INCIDENCE OF BLEEDING IS HARD TO DETERMINE
The optimal way to detect and analyze bleeding events in clinical trials and registries is highly debated. The reported incidences of bleeding during antithrombotic and antiplatelet therapy for non-ST-elevation acute coronary syndromes depend on how bleeding was defined, how the acute coronary syndromes were treated, and on other factors such as how the study was designed.
How was bleeding defined?
Since these classification schemes are based on different types of data, they yield different numbers when applied to the same study population. For instance, Rao et al4 pooled the data from the PURSUIT and PARAGON B trials (15,454 patients in all) and found that the incidence of severe bleeding (by the GUSTO criteria) was 1.2%, while the rate of major bleeding (by the TIMI criteria) was 8.2%.
What was the treatment strategy?
Another reason that the true incidence of bleeding is hard to determine is that different studies used treatment strategies that differed in the type, timing, and dose of antithrombotic agents and whether invasive procedures were used early. For example, if unfractionated heparin is used aggressively in regimens that are not adjusted for weight and with a higher target for the activated clotting time, the risk of bleeding is higher than with conservative dosing.5–7
Subherwal et al8 evaluated the effect of treatment strategy on the incidence of bleeding in patients with non-ST-elevation acute coronary syndromes who received two or more antithrombotic drugs in the CRUSADE Quality Improvement Initiative. The risk of bleeding was higher with an invasive approach (catheterization) than with a conservative approach (no catheterization), regardless of baseline bleeding risk.
What type of study was it?
Another source of variation is the design of the study. Registries differ from clinical trials in patient characteristics and in the way data are gathered (prospectively vs retrospectively).
In registries, data are often collected retrospectively, whereas in clinical trials the data are prospectively collected. For this reason, the definition of bleeding in registries is often based on events that are easily identified through chart review, such as transfusion. This may lead to a lower reported rate of bleeding, since other, less serious bleeding events such as access-site hematomas and epistaxis may not be documented in the medical record.
On the other hand, registries often include older and sicker patients, who may be more prone to bleeding and who are often excluded from clinical trials. This may lead to a higher rate of reported bleeding.9
Where the study was conducted makes a difference as well, owing to regional practice differences. For example, Moscucci et al10 reported that the incidence of major bleeding in 24,045 patients with non-ST-elevation acute coronary syndromes in the GRACE registry (in 14 countries worldwide) was 3.9%. In contrast, Yang et al11 reported that the rate of bleeding in the CRUSADE registry (in the United States) was 10.3%.
This difference was partly influenced by different definitions of bleeding. The GRACE registry defined major bleeding as life-threatening events requiring transfusion of two or more units of packed red blood cells, or resulting in an absolute decrease in the hematocrit of 10% or more or death, or hemorrhagic subdural hematoma. In contrast, the CRUSADE data reflect bleeding requiring transfusion. However, practice patterns such as greater use of invasive procedures in the United States may also be responsible.
Rao and colleagues12 examined international variation in blood transfusion rates among patients with acute coronary syndromes. Patients outside the United States were significantly less likely to receive transfusions, even after adjusting for patient and practice differences.
Taking these confounders into account, it is reasonable to estimate that the frequency of bleeding in patients with non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%.13

BLEEDING IS ASSOCIATED WITH POOR OUTCOMES
Regardless of the definition or the data source, hemorrhagic complications are associated with a higher risk of death and nonfatal adverse events, both in the short term and in the long term.
Short-term outcomes
A higher risk of death. In the GRACE registry study by Moscucci et al10 discussed above, patients who had major bleeding were significantly more likely to die during their hospitalization than those who did not (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.18–2.28).
Rao et al14 evaluated pooled data from the multicenter international GUSTO IIb, PURSUIT, and PARAGON A and B trials and found that the effects of bleeding in non-ST-elevation acute coronary syndromes extended beyond the hospital stay. The more severe the bleeding (by the GUSTO criteria), the greater the adjusted hazard ratio (HR) for death within 30 days:
- With mild bleeding—HR 1.6, 95% CI 1.3–1.9
- With moderate bleeding—HR 2.7, 95% CI 2.3–3.4
- With severe bleeding—HR 10.6, 95% CI 8.3–13.6.
The pattern was the same for death within 6 months:
- With mild bleeding—HR 1.4, 95% CI 1.2–1.6
- With moderate bleeding—HR 2.1, 95% CI 1.8–2.4
- With severe bleeding, HR 7.5, 95% CI 6.1–9.3.
These findings were confirmed by Eikelboom et al15 in 34,146 patients with acute coronary syndromes in the OASIS registry, the OASIS-2 trial, and the CURE randomized trial. In the first 30 days, five times as many patients died (12.8% vs 2.5%; P < .0009) among those who developed major bleeding compared with those who did not. These investigators defined major bleeding as bleeding that was life-threatening or significantly disabling or that required transfusion of two or more units of packed red blood cells.
A higher risk of nonfatal adverse events. Bleeding after antithrombotic therapy for non-ST-elevation acute coronary syndromes has also been associated with nonfatal adverse events such as stroke and stent thrombosis.
For example, in the study by Eikelboom et al,15 major bleeding was associated with a higher risk of recurrent ischemic events. Approximately 1 in 5 patients in the OASIS trials who developed major bleeding during the first 30 days died or had a myocardial infarction or stroke by 30 days, compared with 1 in 20 of those who did not develop major bleeding during the first 30 days. However, after events that occurred during the first 30 days were excluded, the association between major bleeding and both myocardial infarction and stroke was no longer evident between 30 days and 6 months.
Manoukian et al16 evaluated the impact of major bleeding in 13,819 patients with highrisk acute coronary syndromes undergoing treatment with an early invasive strategy in the ACUITY trial. At 30 days, patients with major bleeding had higher rates of the composite end point of death, myocardial infarction, or unplanned revascularization for ischemia (23.1% vs 6.8%, P < .0001) and of stent thrombosis (3.4% vs 0.6%, P < .0001).
Long-term outcomes
The association between bleeding and adverse outcomes persists in the long term as well, although the mechanisms underlying this association are not well studied.
Kinnaird et al17 examined the data from 10,974 unselected patients who underwent percutaneous coronary intervention. At 1 year, the following percentages of patients had died:
- After TIMI major bleeding—17.2%
- After TIMI minor bleeding—9.1%
- After no bleeding—5.5%.
However, after adjustment for potential confounders, only transfusion remained a significant predictor of 1-year mortality.
Mehran et al18 evaluated 1-year mortality data from the ACUITY trial. Compared with the rate in patients who had no major bleeding and no myocardial infarction, the hazard ratios for death were:
- After major bleeding—HR 3.5, 95% CI 2.7–4.4
- After myocardial infarction—HR 3.1, 95% CI 2.4–3.9.
Interestingly, the risk of death associated with myocardial infarction abated after 7 days, while the risk associated with bleeding persisted beyond 30 days and remained constant throughout the first year following the bleeding event.
Similarly, Ndrepepa and colleagues19 examined pooled data from four ISAR trials using the TIMI bleeding scale and found that myocardial infarction, target vessel revascularization, and major bleeding all had similar discriminatory ability at predicting 1-year mortality.
In patients undergoing elective or urgent percutaneous coronary intervention in the REPLACE-2 trial,20 independent predictors of death by 1 year were21:
- Major hemorrhage (OR 2.66, 95% CI 1.44–4.92)
- Periprocedural myocardial infarction (OR 2.46, 95% CI 1.44–4.20).
THEORIES OF HOW BLEEDING MAY CAUSE ADVERSE OUTCOMES
Several mechanisms have been proposed to explain the association between bleeding during treatment for acute coronary syndromes and adverse clinical outcomes.13,22
The immediate effects of bleeding are thought to be hypotension and a reflex hyperadrenergic state to compensate for the loss of intravascular volume.23 This physiologic response is believed to contribute to myocardial ischemia by further decreasing myocardial oxygen supply in obstructive coronary disease.
Trying to minimize blood loss, physicians may withhold anticoagulation and antiplatelet therapy, which in turn may lead to further ischemia.24 To compensate for blood loss, physicians may also resort to blood transfusion. However, depletion of 2,3-diphosphoglycerate and nitric oxide in stored donor red blood cells is postulated to reduce oxygen delivery by increasing hemoglobin’s affinity for oxygen, leading to induced microvascular obstruction and adverse inflammatory reactions.15,25
Recent data have also begun to elucidate the long-term effects of bleeding during acute coronary syndrome management. Patients with anemia during the acute phase of infarction have greater neurohormonal activation.26 These adaptive responses to anemia may lead to eccentric left ventricular remodeling that may lead to higher oxygen consumption, increased diastolic wall stress, interstitial fibrosis, and accelerated myocyte loss.27–30
Nevertheless, we must point out that although strong associations between bleeding and adverse outcomes have been established, direct causality has not.
TO PREVENT BLEEDING, START BY ASSESSING RISK
The CRUSADE bleeding risk score
The CRUSADE bleeding score (calculator available at http://www.crusadebleedingscore.org/) was developed and validated in more than 89,000 community-treated patients with non-ST-elevation acute coronary syndromes.8 It is based on eight variables:
- Sex (higher risk in women)
- History of diabetes (higher risk)
- Prior vascular disease (higher risk)
- Heart rate (the higher the rate, the higher the risk)
- Systolic blood pressure (higher risk with pressures above or below the 121–180 mm Hg range)
- Signs of congestive heart failure (higher risk)
- Baseline hematocrit (the lower the hematocrit, the higher the risk)
- Creatinine clearance (by the Cockcroft-Gault formula; the lower the creatinine clearance, the higher the risk).
Patients who are found to have bleeding scores suggesting a moderate or higher risk of bleeding should be considered for medications associated with a favorable bleeding profile, and for radial access at the time of coronary angiography. Scores are graded as follows8:
- < 21: Very low risk
- 21–30: Low risk
- 31–40: Moderate risk
- 41–50: High risk
- > 50: Very high risk.
The CRUSADE bleeding score is unique in that, unlike earlier risk stratification tools, it was developed in a “real world” population, not in subgroups or in a clinical trial. It can be calculated at baseline to help guide the selection of treatment.8
Adjusting the heparin regimen in patients at risk of bleeding
Both the joint American College of Cardiology/American Heart Association1 and the European Society of Cardiology guidelines31 for the treatment of non-ST-elevation acute coronary syndromes recommend taking steps to prevent bleeding, such as adjusting the dosage of unfractionated heparin, using safer drugs, reducing the duration of antithrombotic treatment, and using combinations of antithrombotic and antiplatelet agents according to proven indications.31
In the CRUSADE registry, 42% of patients with non-ST-elevation acute coronary syndromes received at least one initial dose of antithrombotic drug outside the recommended range, resulting in an estimated 15% excess of bleeding events.32 Thus, proper dosing is a target for prevention.
Appropriate antithrombotic dosing takes into account the patient’s age, weight, and renal function. However, heparin dosage in the current guidelines1 is based on weight only: a loading dose of 60 U/kg (maximum 4,000 U) by intravenous bolus, then 12 U/kg/hour (maximum 1,000 U/hour) to maintain an activated partial thromboplastin time of 50 to 70 seconds.1
Renal dysfunction is particularly worrisome in patients with non-ST-elevation acute coronary syndromes because it is associated with higher rates of major bleeding and death. In the OASIS-5 trial,33 the overall risk of death was approximately five times higher in patients in the lowest quartile of renal function (glomerular filtration rate < 58 mL/min/1.73 m2) than in the highest quartile (glomerular filtration rate ≥ 86 mL/min/1.73 m2).
Renal function must be evaluated not only on admission but also throughout the hospital stay. Patients presenting with acute coronary syndromes often experience fluctuations in renal function that would call for adjustment of heparin dosing, either increasing the dose to maximize the drug’s efficacy if renal function is recovering or decreasing the dose to prevent bleeding if renal function is deteriorating.
Clopidogrel vs prasugrel
Certain medications should be avoided when the risk of bleeding outweighs any potential benefit in terms of ischemia.
For example, in a randomized trial,34 prasugrel (Effient), a potent thienopyridine, was associated with a significantly lower rate of the composite end point of stroke, myocardial infarction, or death than clopidogrel (Plavix) in patients with acute coronary syndromes undergoing percutaneous coronary interventions. However, it did not seem to offer any advantage in patients 75 years old and older, those with prior stroke or transient ischemic attack, or those weighing less than 60 kg, and it posed a substantially higher risk of bleeding.
With clopidogrel, the risk of acute bleeding is primarily in patients who undergo coronary artery bypass grafting within 5 days of receiving a dose.35,36 Therefore, clopidogrel should be stopped 5 to 7 days before bypass surgery.1 Importantly, there is no increased risk of recurrent ischemic events during this 5-day waiting period in patients who receive clopidogrel early. Therefore, the recommendation to stop clopidogrel before surgery does not negate the benefits of early treatment.36
Lower-risk drugs: Fondaparinux and bivalirudin
At this time, only two agents have been studied in clinical trials that have specifically focused on reducing bleeding risk: fondaparinux (Arixtra) and bivalirudin (Angiomax).20,37–39
Fondaparinux
OASIS-5 was a randomized, double-blind trial that compared fondaparinux and enoxaparin (Lovenox) in patients with acute coronary syndromes.38 Fondaparinux was similar to enoxaparin in terms of the combined end point of death, myocardial infarction, or refractory ischemia at 9 days, and fewer patients on fondaparinux developed bleeding (2.2% vs 4.1%, HR 0.52; 95% CI 0.44–0.61). This difference persisted during long-term follow-up.
Importantly, fewer patients died in the fondaparinux group. At 180 days, 638 (6.5%) of the patients in the enoxaparin group had died, compared with 574 (5.8%) in the fondaparinux group, a difference of 64 deaths (P = .05). The authors found that 41 fewer patients in the fondaparinux group than in the enoxaparin group died after major bleeding, and 20 fewer patients in the fondaparinux group died after minor bleeding.38 Thus, most of the difference in mortality rates between the two groups was attributed to a lower incidence of bleeding with fondaparinux.
Unfortunately, despite its safe bleeding profile, fondaparinux has fallen out of favor for use in acute coronary syndromes, owing to a higher risk of catheter thrombosis in the fondaparinux group (0.9%) than in those undergoing percutaneous coronary interventions with enoxaparin alone (0.4%) in the OASIS-5 trial.40
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in three large randomized trials in patients undergoing percutaneous coronary interventions.20,37,41
The ACUITY trial37 was a prospective, open-label, randomized, multicenter trial that compared three regimens in patients with moderate or high-risk non-ST-elevation acute coronary syndromes:
- Heparin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin alone.
Bivalirudin alone was as effective as heparin plus a glycoprotein IIb/IIIa inhibitor with respect to the composite ischemia end point, which at 30 days had occurred in 7.8% vs 7.3% of the patients in these treatment groups (P = .32, RR 1.08; 95% CI 0.93–1.24), and it was superior with respect to major bleeding (3.0% vs 5.7%, P < .001, RR 0.53; 95% CI 0.43–0.65).
The HORIZONS-AMI study41 was a prospective, open-label, randomized, multicenter trial that compared bivalirudin alone vs heparin plus a glycoprotein IIb/IIIa inhibitor in patients with ST-elevation acute coronary syndromes who were undergoing primary percutaneous coronary interventions. The two primary end points were major bleeding and net adverse events.
At 1 year, patients assigned to bivalirudin had a lower rate of major bleeding than did controls (5.8% vs 9.2%, HR 0.61, 95% CI 0.48–0.78, P < .0001), with similar rates of major adverse cardiac events in both groups (11.9% vs 11.9%, HR 1.00, 95% CI 0.82– 1.21, P = .98).41
Both OASIS 5 and HORIZONS-AMI are examples of clinical trials in which strategies that reduced bleeding risk were also associated with improved survival.
For cardiac catheterization, inserting the catheter in the wrist poses less risk
Bleeding is currently the most common noncardiac complication in patients undergoing percutaneous coronary interventions, and it most often occurs at the vascular access site.17
Rao et al12 evaluated data from 593,094 procedures in the National Cardiovascular Data Registry and found that, compared with the femoral approach, the use of transradial percutaneous coronary intervention was associated with a similar rate of procedural success (OR 1.02, 95% CI 0.93–1.12) but a significantly lower risk of bleeding complications (OR 0.42, 95% CI 0.31–0.56) after multivariable adjustment.
The use of smaller sheath sizes (4F–6F) and preferential use of bivalirudin over unfractionated heparin and glycoprotein IIb/IIIa inhibitor therapy are other methods described to decrease the risk of bleeding after percutaneous coronary interventions.20,41–49
IF BLEEDING OCCURS
Once a bleeding complication occurs, cessation of therapy is a potential option. Stopping or reversing antithrombotic and antiplatelet therapy is warranted in the event of major bleeding (eg, gastrointestinal, retroperitoneal, intracranial).31
Stopping antithrombotic and antiplatelet therapy
Whether bleeding is minor or major, the risk of a recurrent thrombotic event must be considered, especially in patients who have undergone revascularization, stent implantation, or both. The risk of acute thrombotic events after interrupting antithrombotic or antiplatelet agents is considered greatest 4 to 5 days following revascularization or percutaneous coronary intervention.15 If bleeding can be controlled with local treatment such as pressure, packing, or dressing, antithrombotic and antiplatelet therapy need not be interrupted.50
Current guidelines recommend strict control of hemorrhage for at least 24 hours before reintroducing antiplatelet or antithrombotic agents.
It is also important to remember that in the setting of gastrointestinal bleeding due to peptic ulcer disease, adjunctive proton pump inhibitors are recommended after restarting antiplatelet or antithrombotic therapy or both.
Importantly, evidence-based antithrombotic medications (especially dual antiplatelet therapy) should be restarted once the acute bleeding event has resolved.31
Reversal of anticoagulant and antiplatelet therapies
Unfractionated heparin is reversed with infusion of protamine sulfate at a dose of 1 mg per 100 U of unfractionated heparin given over the previous 4 hours.51,52 The rate of protamine sulfate infusion should be less than 100 mg over 2 hours, with 50% of the dose given initially and subsequent doses titrated according to bleeding response.52,53 Protamine sulfate is associated with a risk of hypotension and bradycardia, and for this reason it should be given no faster than 5 mg/min.
Low-molecular-weight heparin (LMWH) can be inhibited by 1 mg of protamine sulfate for each 1 mg of LMWH given over the previous 4 hours.51,52
However, protamine sulfate only partially neutralizes the anticoagulant effect of LMWH. In cases in which protamine sulfate is unsuccessful in abating bleeding associated with LMWH use, guidelines allow for the use of recombinant factor VIIa (NovoSeven).31 In healthy volunteers given fondaparinux, recombinant factor VIIa normalized coagulation times and thrombin generation within 1.5 hours, with a sustained effect for 6 hours.52
It is important to note that the use of this agent has not been fully studied, it is very costly (a single dose of 40 μg/kg costs from $3,000 to $4,000), and it is linked to reports of increased risk of thrombotic complications.54,55
Antiplatelet agents are more complex to reverse. The antiplatelet actions of aspirin and clopidogrel wear off as new platelets are produced. Approximately 10% of a patient’s platelet count is produced daily; thus, the antiplatelet effects of aspirin and clopidogrel can persist for 5 to 10 days.31,56
If these agents need to be reversed quickly to stop bleeding, according to expert consensus the aspirin effect can be reversed by transfusion of one unit of platelets. The antiplatelet effect of clopidogrel is more significant than that of aspirin; thus, two units of platelets are recommended.56
Glycoprotein IIb/IIIa inhibitors. If a major bleeding event requires the reversal of glycoprotein IIb/IIIa inhibitor therapy, the treatment must take into consideration the pharmacodynamics of the target drug. Both eptifibatide (Integrilin) and tirofiban (Aggrastat) competitively inhibit glycoprotein IIb/IIIa receptors; thus, their effects depend on dosing, elimination, and time. Due to the stoichiometry of both drugs, transfusion of platelets is ineffective. Both eptifibatide and tirofiban are eliminated by the kidney; thus, normal renal function is key to the amount of time it takes for platelet function to return to baseline.57 Evidence suggests that fibrinogen-rich plasma can be administered to restore platelet function.31,58,59
Abciximab (ReoPro). Whereas reversal of eptifibatide and tirofiban focuses on overcoming competitive inhibition, neutralization of abciximab involves overcoming its high receptor affinity. At 24 hours after abciximab infusion is stopped, platelet aggregation may still be inhibited by up to 50%. Fortunately, owing to abciximab’s short plasma half-life and its dilution in serum, platelet transfusion is effective in reversing its antiplatelet effects.31,57
Blood transfusion
Long considered beneficial to critically ill patients, blood transfusion to maintain hematocrit levels during acute coronary syndromes has come under intense scrutiny. Randomized trials have shown that transfusion should not be given aggressively to critically ill patients.60 In acute coronary syndromes, there are only observational data.
Rao et al61 used detailed clinical data from 24,112 patients with acute coronary syndromes in the GUSTO IIb, PURSUIT, and PARAGON B trials to determine the association between blood transfusion and outcomes in patients who developed moderate to severe bleeding, anemia, or both during their hospitalization. The rates of death in the hospital and at 30 days were significantly higher in patients who received a transfusion (30-day mortality HR 3.94; 95% CI 3.36–4.75). However, there was no significant association between transfusion and the 30-day mortality rate if the nadir hematocrit was 25% or less.
Of note: no randomized clinical trial has evaluated transfusion strategies in acute coronary syndromes at this time. Until such data are available, it is reasonable to follow published guidelines and to avoid transfusion in stable patients with ischemic heart disease unless the hematocrit is 25% or less.31 The risks and benefits of blood transfusion should be carefully weighed. Routine use of transfusion to maintain predefined hemoglobin levels is not recommended in stable patients.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154.
- Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47:809–816.
- Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996; 93:870–878.
- Gilchrist IC, Berkowitz SD, Thompson TD, Califf RM, Granger CB. Heparin dosing and outcome in acute coronary syndromes: the GUSTO-IIb experience. Global Use of Strategies to Open Occluded Coronary Arteries. Am Heart J 2002; 144:73–80.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol 2003; 41:386–393.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119:1873–1882.
- Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart 2008; 94:661–666.
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24:1815–1823.
- Yang X, Alexander KP, Chen AY, et al; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005; 46:1490–1495.
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008; 1:379–386.
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1193–1204.
- Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96:1200–1206.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92:930–935.
- Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009; 30:1457–1466.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51:690–697.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100:1364–1369.
- Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol 2007; 23:663–671.
- Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J 2007; 28:1273–1274.
- Yan AT, Yan RT, Huynh T, et al; INTERACT Investigators. Bleeding and outcome in acute coronary syndrome: insights from continuous electrocardiogram monitoring in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial. Am Heart J 2008; 156:769–775.
- Jolicoeur EM, O’Neill WW, Hellkamp A, et al; APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2009; 30:2575–2583.
- Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol 2005; 95:1474–1477.
- Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149–154.
- O’Riordan E, Foley RN. Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 2000; 15(suppl 3):19–22.
- Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol 1992; 141:227–239.
- Aronson D, Suleiman M, Agmon Y, et al. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J 2007; 28:1289–1296.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Alexander KP, Chen AY, Roe MT, et al; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 2005; 294:3108–3116.
- Fox KA, Bassand JP, Mehta SR, et al; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med 2007; 147:304–310.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008; 52:1693–1701.
- Fox KA, Mehta SR, Peters R, et al; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Potsis TZ, Katsouras C, Goudevenos JA. Avoiding and managing bleeding complications in patients with non-ST-segment elevation acute coronary syndromes. Angiology 2009; 60:148–158.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Stone GW, Ware JH, Bertrand ME, et al; ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA 2007; 298:2497–2506.
- Cantor WJ, Mahaffey KW, Huang Z, et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv 2007; 69:73–83.
- Büchler JR, Ribeiro EE, Falcão JL, et al. A randomized trial of 5 versus 7 French guiding catheters for transfemoral percutaneous coronary stent implantation. J Interv Cardiol 2008; 21:50–55.
- Shammas NW, Allie D, Hall P, et al; APPROVE Investigators. Predictors of in-hospital and 30-day complications of peripheral vascular interventions using bivalirudin as the primary anticoagulant: results from the APPROVE Registry. J Invasive Cardiol 2005; 17:356–359.
- Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1:202–209.
- Stone GW, White HD, Ohman EM, et al; Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial investigators. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007; 369:907–919.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004; 93:1092–1096.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth 2002; 49:S11–S25.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008; 111:4871–4879.
- Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol 2004; 41(suppl 1):44–50.
- Ganguly S, Spengel K, Tilzer LL, O’Neal B, Simpson SQ. Recombinant factor VIIa: unregulated continuous use in patients with bleeding and coagulopathy does not alter mortality and outcome. Clin Lab Haematol 2006; 28:309–312.
- O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006; 295:293–298.
- Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg 2010; 112:307–318.
- Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139:S38–S45.
- Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J 2002; 143:725–732.
- Schroeder WS, Gandhi PJ. Emergency management of hemorrhagic complications in the era of glycoprotein IIb/IIIa receptor antagonists, clopidogrel, low molecular weight heparin, and third-generation fibrinolytic agents. Curr Cardiol Rep 2003; 5:310–317.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154.
- Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47:809–816.
- Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996; 93:870–878.
- Gilchrist IC, Berkowitz SD, Thompson TD, Califf RM, Granger CB. Heparin dosing and outcome in acute coronary syndromes: the GUSTO-IIb experience. Global Use of Strategies to Open Occluded Coronary Arteries. Am Heart J 2002; 144:73–80.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol 2003; 41:386–393.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119:1873–1882.
- Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart 2008; 94:661–666.
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24:1815–1823.
- Yang X, Alexander KP, Chen AY, et al; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005; 46:1490–1495.
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008; 1:379–386.
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1193–1204.
- Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96:1200–1206.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92:930–935.
- Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009; 30:1457–1466.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51:690–697.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100:1364–1369.
- Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol 2007; 23:663–671.
- Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J 2007; 28:1273–1274.
- Yan AT, Yan RT, Huynh T, et al; INTERACT Investigators. Bleeding and outcome in acute coronary syndrome: insights from continuous electrocardiogram monitoring in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial. Am Heart J 2008; 156:769–775.
- Jolicoeur EM, O’Neill WW, Hellkamp A, et al; APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2009; 30:2575–2583.
- Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol 2005; 95:1474–1477.
- Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149–154.
- O’Riordan E, Foley RN. Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 2000; 15(suppl 3):19–22.
- Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol 1992; 141:227–239.
- Aronson D, Suleiman M, Agmon Y, et al. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J 2007; 28:1289–1296.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Alexander KP, Chen AY, Roe MT, et al; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 2005; 294:3108–3116.
- Fox KA, Bassand JP, Mehta SR, et al; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med 2007; 147:304–310.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008; 52:1693–1701.
- Fox KA, Mehta SR, Peters R, et al; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Potsis TZ, Katsouras C, Goudevenos JA. Avoiding and managing bleeding complications in patients with non-ST-segment elevation acute coronary syndromes. Angiology 2009; 60:148–158.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Stone GW, Ware JH, Bertrand ME, et al; ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA 2007; 298:2497–2506.
- Cantor WJ, Mahaffey KW, Huang Z, et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv 2007; 69:73–83.
- Büchler JR, Ribeiro EE, Falcão JL, et al. A randomized trial of 5 versus 7 French guiding catheters for transfemoral percutaneous coronary stent implantation. J Interv Cardiol 2008; 21:50–55.
- Shammas NW, Allie D, Hall P, et al; APPROVE Investigators. Predictors of in-hospital and 30-day complications of peripheral vascular interventions using bivalirudin as the primary anticoagulant: results from the APPROVE Registry. J Invasive Cardiol 2005; 17:356–359.
- Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1:202–209.
- Stone GW, White HD, Ohman EM, et al; Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial investigators. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007; 369:907–919.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004; 93:1092–1096.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth 2002; 49:S11–S25.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008; 111:4871–4879.
- Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol 2004; 41(suppl 1):44–50.
- Ganguly S, Spengel K, Tilzer LL, O’Neal B, Simpson SQ. Recombinant factor VIIa: unregulated continuous use in patients with bleeding and coagulopathy does not alter mortality and outcome. Clin Lab Haematol 2006; 28:309–312.
- O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006; 295:293–298.
- Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg 2010; 112:307–318.
- Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139:S38–S45.
- Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J 2002; 143:725–732.
- Schroeder WS, Gandhi PJ. Emergency management of hemorrhagic complications in the era of glycoprotein IIb/IIIa receptor antagonists, clopidogrel, low molecular weight heparin, and third-generation fibrinolytic agents. Curr Cardiol Rep 2003; 5:310–317.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
KEY POINTS
- The reported incidence of bleeding after treatment for non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%, depending on a number of factors.
- Bleeding is strongly associated with adverse outcomes, although a causal relationship has not been established.
- Patients should be assessed for risk of bleeding so that the antithrombotic and antiplatelet regimen can be adjusted, safer alternatives can be considered, and percutaneous interventions can be used less aggressively for those at high risk.
- If bleeding develops and the risk of continued bleeding outweighs the risk of recurrent ischemia, antithrombotic and antiplatelet drug therapy can be interrupted and other agents given to reverse the effects of these drugs.