User login
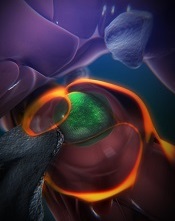
(green) in a zebrafish
Boston Children’s Hospital
Using a zebrafish model and enhanced imaging, a group of researchers discovered how hematopoietic stem and progenitor cells (HSPCs) interact with their niche.
Subsequent imaging in mice showed that HSPCs behaved the same way in mammals, which suggests similar results might be observed in humans.
In fact, the researchers are already using the results of this study to inform research on hematopoietic stem cell transplants.
“The same process occurs during a bone marrow transplant as occurs in the body naturally,” said senior study investigator Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“Our direct visualization gives us a series of steps to target, and, in theory, we can look for drugs that affect every step of that process.”
He and his colleagues described this research in a paper published in Cell, as well as in two animations on YouTube, one that’s general and one more technical.
“Stem cell and bone marrow transplants are still very much a black box,” said study author Owen Tamplin, PhD, also of Boston Children’s Hospital.
“Cells are introduced into a patient, and, later on, we can measure recovery of their blood system, but what happens in between can’t be seen. Now, we have a system where we can actually watch that middle step.”
The researchers already knew that HSPCs bud off from cells in the aorta, then circulate in the body until they find a niche where they’re prepped for creating blood.
With the current study, the team observed how this niche forms, using time-lapse imaging of naturally transparent zebrafish embryos and a genetic modification that tagged the HSPCs green.
On arrival in its niche (in the tail in zebrafish), the newborn HSPC attaches itself to the blood vessel wall. There, chemical signals prompt it to squeeze itself through the wall and into a space just outside the blood vessel. Other cells begin to interact with the HSPC, and nearby endothelial cells wrap themselves around it.
“We think that is the beginning of making a stem cell happy in its niche, like a mother cuddling a baby,” Dr Zon said.
As the HSPC is being “cuddled,” it’s brought into contact with a nearby stromal cell that helps keep it attached.
The “cuddling” was reconstructed from confocal and electron microscopy images of the zebrafish taken during this stage. Through a series of image slices, the researchers were able to reassemble the whole 3D structure—HSPC, cuddling endothelial cells, and stromal cells.
“Nobody’s ever visualized live how a stem cell interacts with its niche,” Dr Zon said. “This is the first time we get a very high-resolution view of the process.”
Next, the cuddled HSPC begins dividing. One daughter cell leaves the niche, while the other stays. Eventually, all the HSPCs leave and begin colonizing their future site of blood production. (In zebrafish, this is in the kidney, which is similar to mammalian bone marrow.)
Additional imaging in mice revealed evidence that HSPCs go through much the same process in mammals, which makes it likely in humans too.
These detailed observations are already informing the Zon lab’s attempt to improve stem cell transplants. By conducting a chemical screen in large numbers of zebrafish embryos, the researchers found that the compound lycorine promotes interaction between the HSPC and its niche, leading to greater numbers of HSPCs in the adult fish. ![]()

(green) in a zebrafish
Boston Children’s Hospital
Using a zebrafish model and enhanced imaging, a group of researchers discovered how hematopoietic stem and progenitor cells (HSPCs) interact with their niche.
Subsequent imaging in mice showed that HSPCs behaved the same way in mammals, which suggests similar results might be observed in humans.
In fact, the researchers are already using the results of this study to inform research on hematopoietic stem cell transplants.
“The same process occurs during a bone marrow transplant as occurs in the body naturally,” said senior study investigator Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“Our direct visualization gives us a series of steps to target, and, in theory, we can look for drugs that affect every step of that process.”
He and his colleagues described this research in a paper published in Cell, as well as in two animations on YouTube, one that’s general and one more technical.
“Stem cell and bone marrow transplants are still very much a black box,” said study author Owen Tamplin, PhD, also of Boston Children’s Hospital.
“Cells are introduced into a patient, and, later on, we can measure recovery of their blood system, but what happens in between can’t be seen. Now, we have a system where we can actually watch that middle step.”
The researchers already knew that HSPCs bud off from cells in the aorta, then circulate in the body until they find a niche where they’re prepped for creating blood.
With the current study, the team observed how this niche forms, using time-lapse imaging of naturally transparent zebrafish embryos and a genetic modification that tagged the HSPCs green.
On arrival in its niche (in the tail in zebrafish), the newborn HSPC attaches itself to the blood vessel wall. There, chemical signals prompt it to squeeze itself through the wall and into a space just outside the blood vessel. Other cells begin to interact with the HSPC, and nearby endothelial cells wrap themselves around it.
“We think that is the beginning of making a stem cell happy in its niche, like a mother cuddling a baby,” Dr Zon said.
As the HSPC is being “cuddled,” it’s brought into contact with a nearby stromal cell that helps keep it attached.
The “cuddling” was reconstructed from confocal and electron microscopy images of the zebrafish taken during this stage. Through a series of image slices, the researchers were able to reassemble the whole 3D structure—HSPC, cuddling endothelial cells, and stromal cells.
“Nobody’s ever visualized live how a stem cell interacts with its niche,” Dr Zon said. “This is the first time we get a very high-resolution view of the process.”
Next, the cuddled HSPC begins dividing. One daughter cell leaves the niche, while the other stays. Eventually, all the HSPCs leave and begin colonizing their future site of blood production. (In zebrafish, this is in the kidney, which is similar to mammalian bone marrow.)
Additional imaging in mice revealed evidence that HSPCs go through much the same process in mammals, which makes it likely in humans too.
These detailed observations are already informing the Zon lab’s attempt to improve stem cell transplants. By conducting a chemical screen in large numbers of zebrafish embryos, the researchers found that the compound lycorine promotes interaction between the HSPC and its niche, leading to greater numbers of HSPCs in the adult fish. ![]()

(green) in a zebrafish
Boston Children’s Hospital
Using a zebrafish model and enhanced imaging, a group of researchers discovered how hematopoietic stem and progenitor cells (HSPCs) interact with their niche.
Subsequent imaging in mice showed that HSPCs behaved the same way in mammals, which suggests similar results might be observed in humans.
In fact, the researchers are already using the results of this study to inform research on hematopoietic stem cell transplants.
“The same process occurs during a bone marrow transplant as occurs in the body naturally,” said senior study investigator Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“Our direct visualization gives us a series of steps to target, and, in theory, we can look for drugs that affect every step of that process.”
He and his colleagues described this research in a paper published in Cell, as well as in two animations on YouTube, one that’s general and one more technical.
“Stem cell and bone marrow transplants are still very much a black box,” said study author Owen Tamplin, PhD, also of Boston Children’s Hospital.
“Cells are introduced into a patient, and, later on, we can measure recovery of their blood system, but what happens in between can’t be seen. Now, we have a system where we can actually watch that middle step.”
The researchers already knew that HSPCs bud off from cells in the aorta, then circulate in the body until they find a niche where they’re prepped for creating blood.
With the current study, the team observed how this niche forms, using time-lapse imaging of naturally transparent zebrafish embryos and a genetic modification that tagged the HSPCs green.
On arrival in its niche (in the tail in zebrafish), the newborn HSPC attaches itself to the blood vessel wall. There, chemical signals prompt it to squeeze itself through the wall and into a space just outside the blood vessel. Other cells begin to interact with the HSPC, and nearby endothelial cells wrap themselves around it.
“We think that is the beginning of making a stem cell happy in its niche, like a mother cuddling a baby,” Dr Zon said.
As the HSPC is being “cuddled,” it’s brought into contact with a nearby stromal cell that helps keep it attached.
The “cuddling” was reconstructed from confocal and electron microscopy images of the zebrafish taken during this stage. Through a series of image slices, the researchers were able to reassemble the whole 3D structure—HSPC, cuddling endothelial cells, and stromal cells.
“Nobody’s ever visualized live how a stem cell interacts with its niche,” Dr Zon said. “This is the first time we get a very high-resolution view of the process.”
Next, the cuddled HSPC begins dividing. One daughter cell leaves the niche, while the other stays. Eventually, all the HSPCs leave and begin colonizing their future site of blood production. (In zebrafish, this is in the kidney, which is similar to mammalian bone marrow.)
Additional imaging in mice revealed evidence that HSPCs go through much the same process in mammals, which makes it likely in humans too.
These detailed observations are already informing the Zon lab’s attempt to improve stem cell transplants. By conducting a chemical screen in large numbers of zebrafish embryos, the researchers found that the compound lycorine promotes interaction between the HSPC and its niche, leading to greater numbers of HSPCs in the adult fish. ![]()