User login
WASHINGTON, DC—Years of investigation into cellular mechanisms has produced a translational shift in the approach to diagnosing neurodegenerative diseases by proteinopathy, the abnormal proteins associated with their histopathology, rather than by clinical manifestations of each disease, according to a presentation at the 67th Annual Meeting of the American Academy of Neurology.
During the Frontiers in Neuroscience plenary session, Keith A. Josephs, Jr., MD, Professor of Neurology at the Mayo Clinic in Rochester, Minnesota, presented a summary of a decade of research that led to the proteinopathy classification. The clinical benefit to diagnosing patients according to proteinopathy is that the drug therapies target the specific proteins involved, Dr. Josephs explained. “Hence, trying to predict the specific pathological diagnosis is not as important—at least currently—as trying to predict the underlying protein.”
Why Reclassify?
Clinical diagnosis is currently useful predominantly for prognostic purposes, Dr. Josephs said, noting that when clinicians diagnose a neurodegenerative disease, they tend to segregate them into three categories: parkinsonian conditions, dementia, and motor neuron disease. This system of definition has not led to the development of highly effective drug therapies, he said.
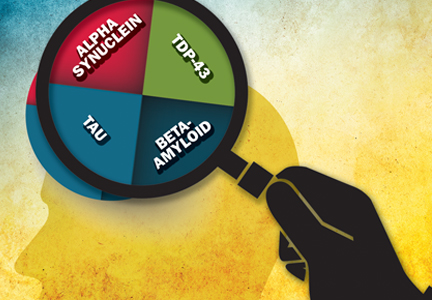
Diagnosis by pathology, the current gold standard, creates categories based on the abnormal protein associated with each disease: beta-amyloid, tau, TDP-43, alpha-synuclein, and others. “The translational part of this science is that we need to move away from one pathological diagnosis to characterizing patients based on the predominant protein that is identified histologically,” Dr. Josephs said.
Most importantly, diagnosis by proteinopathy creates many new avenues of drug development for interventions, and a large body of investigation is currently underway. “Alzheimer’s disease is considered by most a mixed proteinopathy, while progressive supranuclear palsy [PSP], corticobasal degeneration [CBD], Pick’s disease, and familial MAPT [microtubule-associated protein tau gene] disease are tauopathies, and diffuse Lewy body disease, brainstem/transitional Lewy body disease, and multiple system atrophy [MSA] are examples of synucleinopathies,” Dr. Josephs said. Parkinson’s disease is not a pathologic diagnosis but a clinical diagnosis that is associated with the presence of Lewy bodies. In addition, amyotrophic lateral sclerosis and some forms of frontotemporal lobar degeneration are examples of TDPopathies.
Identifying Signatures of Proteinopathies
Dr. Josephs began to wonder whether each of the proteinopathies had a unique neurodegenerative signature of atrophy that could be clinically useful. “If a signature exists, it should be common across all members of the same group,” he said.
So far, however, this has not turned out to be the case. Using statistical parametric mapping to analyze antemortem imaging patterns of atrophy in a database of more than 1,000 subjects who had MRI brain scans between 2002 and 2012, Dr. Josephs observed patterns associated with the proteinopathies. “With tauopathies, for example, you mainly see bilateral premotor atrophy,” he said. He then divided the tauopathies into four types: CBD, PSP, Pick’s disease, and familial MAPT. “But when you compare images of these four diseases, there is little evidence of a signature pattern across all four variants—the pattern seems to be quite different with each of the four tauopathies. This [finding] would argue against a signature for tauopathies,” he said.
Upon further examination of the scans and further refinement of the proteinopathy categories, Dr. Josephs found that for some proteinopathies, such as the 4-repeat tauopathies (CBD and PSP) and TDP-43, the signature appears to be a network of atrophy rather than a focal region of atrophy as he expected. In the case of mixed proteinopathies, in which multiple proteins may be involved (as with beta amyloid, tau, and TDP-43 in Alzheimer’s disease), the individual protein signatures may mask each other, he said.
Most significantly, no signature pattern for alpha-synuclein has emerged. “When you look at the synucleinopathies, there is very little atrophy. So, alpha-synuclein associated conditions—MSA and the Lewy body diseases—aren’t associated with a significant amount of brain atrophy,” Dr. Josephs said.
—Linda Peckel
Suggested Reading
Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280-289.
Josephs KA, Whitwell JL, Weigand SD, et al. Acta Neuropathol. 2014;127(6):811-824.
Whitwell JL, Josephs KA. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat Rev Neurol. 2012;8(3):131-142.
WASHINGTON, DC—Years of investigation into cellular mechanisms has produced a translational shift in the approach to diagnosing neurodegenerative diseases by proteinopathy, the abnormal proteins associated with their histopathology, rather than by clinical manifestations of each disease, according to a presentation at the 67th Annual Meeting of the American Academy of Neurology.
During the Frontiers in Neuroscience plenary session, Keith A. Josephs, Jr., MD, Professor of Neurology at the Mayo Clinic in Rochester, Minnesota, presented a summary of a decade of research that led to the proteinopathy classification. The clinical benefit to diagnosing patients according to proteinopathy is that the drug therapies target the specific proteins involved, Dr. Josephs explained. “Hence, trying to predict the specific pathological diagnosis is not as important—at least currently—as trying to predict the underlying protein.”
Why Reclassify?
Clinical diagnosis is currently useful predominantly for prognostic purposes, Dr. Josephs said, noting that when clinicians diagnose a neurodegenerative disease, they tend to segregate them into three categories: parkinsonian conditions, dementia, and motor neuron disease. This system of definition has not led to the development of highly effective drug therapies, he said.
Diagnosis by pathology, the current gold standard, creates categories based on the abnormal protein associated with each disease: beta-amyloid, tau, TDP-43, alpha-synuclein, and others. “The translational part of this science is that we need to move away from one pathological diagnosis to characterizing patients based on the predominant protein that is identified histologically,” Dr. Josephs said.
Most importantly, diagnosis by proteinopathy creates many new avenues of drug development for interventions, and a large body of investigation is currently underway. “Alzheimer’s disease is considered by most a mixed proteinopathy, while progressive supranuclear palsy [PSP], corticobasal degeneration [CBD], Pick’s disease, and familial MAPT [microtubule-associated protein tau gene] disease are tauopathies, and diffuse Lewy body disease, brainstem/transitional Lewy body disease, and multiple system atrophy [MSA] are examples of synucleinopathies,” Dr. Josephs said. Parkinson’s disease is not a pathologic diagnosis but a clinical diagnosis that is associated with the presence of Lewy bodies. In addition, amyotrophic lateral sclerosis and some forms of frontotemporal lobar degeneration are examples of TDPopathies.
Identifying Signatures of Proteinopathies
Dr. Josephs began to wonder whether each of the proteinopathies had a unique neurodegenerative signature of atrophy that could be clinically useful. “If a signature exists, it should be common across all members of the same group,” he said.
So far, however, this has not turned out to be the case. Using statistical parametric mapping to analyze antemortem imaging patterns of atrophy in a database of more than 1,000 subjects who had MRI brain scans between 2002 and 2012, Dr. Josephs observed patterns associated with the proteinopathies. “With tauopathies, for example, you mainly see bilateral premotor atrophy,” he said. He then divided the tauopathies into four types: CBD, PSP, Pick’s disease, and familial MAPT. “But when you compare images of these four diseases, there is little evidence of a signature pattern across all four variants—the pattern seems to be quite different with each of the four tauopathies. This [finding] would argue against a signature for tauopathies,” he said.
Upon further examination of the scans and further refinement of the proteinopathy categories, Dr. Josephs found that for some proteinopathies, such as the 4-repeat tauopathies (CBD and PSP) and TDP-43, the signature appears to be a network of atrophy rather than a focal region of atrophy as he expected. In the case of mixed proteinopathies, in which multiple proteins may be involved (as with beta amyloid, tau, and TDP-43 in Alzheimer’s disease), the individual protein signatures may mask each other, he said.
Most significantly, no signature pattern for alpha-synuclein has emerged. “When you look at the synucleinopathies, there is very little atrophy. So, alpha-synuclein associated conditions—MSA and the Lewy body diseases—aren’t associated with a significant amount of brain atrophy,” Dr. Josephs said.
—Linda Peckel
WASHINGTON, DC—Years of investigation into cellular mechanisms has produced a translational shift in the approach to diagnosing neurodegenerative diseases by proteinopathy, the abnormal proteins associated with their histopathology, rather than by clinical manifestations of each disease, according to a presentation at the 67th Annual Meeting of the American Academy of Neurology.
During the Frontiers in Neuroscience plenary session, Keith A. Josephs, Jr., MD, Professor of Neurology at the Mayo Clinic in Rochester, Minnesota, presented a summary of a decade of research that led to the proteinopathy classification. The clinical benefit to diagnosing patients according to proteinopathy is that the drug therapies target the specific proteins involved, Dr. Josephs explained. “Hence, trying to predict the specific pathological diagnosis is not as important—at least currently—as trying to predict the underlying protein.”
Why Reclassify?
Clinical diagnosis is currently useful predominantly for prognostic purposes, Dr. Josephs said, noting that when clinicians diagnose a neurodegenerative disease, they tend to segregate them into three categories: parkinsonian conditions, dementia, and motor neuron disease. This system of definition has not led to the development of highly effective drug therapies, he said.
Diagnosis by pathology, the current gold standard, creates categories based on the abnormal protein associated with each disease: beta-amyloid, tau, TDP-43, alpha-synuclein, and others. “The translational part of this science is that we need to move away from one pathological diagnosis to characterizing patients based on the predominant protein that is identified histologically,” Dr. Josephs said.
Most importantly, diagnosis by proteinopathy creates many new avenues of drug development for interventions, and a large body of investigation is currently underway. “Alzheimer’s disease is considered by most a mixed proteinopathy, while progressive supranuclear palsy [PSP], corticobasal degeneration [CBD], Pick’s disease, and familial MAPT [microtubule-associated protein tau gene] disease are tauopathies, and diffuse Lewy body disease, brainstem/transitional Lewy body disease, and multiple system atrophy [MSA] are examples of synucleinopathies,” Dr. Josephs said. Parkinson’s disease is not a pathologic diagnosis but a clinical diagnosis that is associated with the presence of Lewy bodies. In addition, amyotrophic lateral sclerosis and some forms of frontotemporal lobar degeneration are examples of TDPopathies.
Identifying Signatures of Proteinopathies
Dr. Josephs began to wonder whether each of the proteinopathies had a unique neurodegenerative signature of atrophy that could be clinically useful. “If a signature exists, it should be common across all members of the same group,” he said.
So far, however, this has not turned out to be the case. Using statistical parametric mapping to analyze antemortem imaging patterns of atrophy in a database of more than 1,000 subjects who had MRI brain scans between 2002 and 2012, Dr. Josephs observed patterns associated with the proteinopathies. “With tauopathies, for example, you mainly see bilateral premotor atrophy,” he said. He then divided the tauopathies into four types: CBD, PSP, Pick’s disease, and familial MAPT. “But when you compare images of these four diseases, there is little evidence of a signature pattern across all four variants—the pattern seems to be quite different with each of the four tauopathies. This [finding] would argue against a signature for tauopathies,” he said.
Upon further examination of the scans and further refinement of the proteinopathy categories, Dr. Josephs found that for some proteinopathies, such as the 4-repeat tauopathies (CBD and PSP) and TDP-43, the signature appears to be a network of atrophy rather than a focal region of atrophy as he expected. In the case of mixed proteinopathies, in which multiple proteins may be involved (as with beta amyloid, tau, and TDP-43 in Alzheimer’s disease), the individual protein signatures may mask each other, he said.
Most significantly, no signature pattern for alpha-synuclein has emerged. “When you look at the synucleinopathies, there is very little atrophy. So, alpha-synuclein associated conditions—MSA and the Lewy body diseases—aren’t associated with a significant amount of brain atrophy,” Dr. Josephs said.
—Linda Peckel
Suggested Reading
Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280-289.
Josephs KA, Whitwell JL, Weigand SD, et al. Acta Neuropathol. 2014;127(6):811-824.
Whitwell JL, Josephs KA. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat Rev Neurol. 2012;8(3):131-142.
Suggested Reading
Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280-289.
Josephs KA, Whitwell JL, Weigand SD, et al. Acta Neuropathol. 2014;127(6):811-824.
Whitwell JL, Josephs KA. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat Rev Neurol. 2012;8(3):131-142.