User login
Although the cause of severe headaches in patients presenting to the ED are typically of benign etiology, there are serious and even life-threatening causes for which the EP should retain a high index of suspicion. In the physician’s thoughts lurk the small, but real, possibility that a missed diagnosis might lead to harm or even death of the patient. The cause of the headache may not be detectable during the ED visit—even after expensive, time-consuming, and, sometimes painful, studies. Fears of being charged with negligence or malpractice may be hovering somewhere in the back of an EP’s mind. Additionally, the goals of the physician and the patient may not be aligned (eg, the patient is often more concerned with symptom relief, while the physician is typically more concerned with rapid diagnosis and diagnostic accuracy).
The patient with chronic or recurrent headache presents a special management challenge. The EP may encounter the same patient at repeat ED visits. Assessment of pain—the “fifth vital sign”—and response of interventions are frequently the subjects of review by accreditation and regulatory organizations.1 A patient with pain from recurrent headache may not be completely satisfied during a visit, and this may be reflected in the ED’s patient satisfaction scores. On the other hand, the epidemic of prescription pain medication misuse has become a nationally recognized problem, and EPs in particular are being tasked to carefully assess opioid administration and prescriptions.2 In addition, chronic opioid administration may interfere with patient response to some headache-specific medications such as triptans.3 Cautious prescribing with screening before utilizing controlled substances in patients at risk is recommended to prevent misuse and abuse.4
Literature History
Much of the literature on headaches comes from pain and headache clinics and reflects the experience from that patient population. Patients referred to such clinics often have chronic, recurrent headaches that are difficult to manage. The consultation and referral processes at these centers exclude many time-sensitive causes of headache, and extensive neuroimaging and other studies likely will have detected patients with tumors and vascular lesions. In the nomenclature of headaches, these clinics specialize in treating patients with primary headache syndromes (eg, migraine, cluster, chronic daily headache). Conversely, secondary causes of headache are infrequent in these clinics. Studies based on these patient populations conclude that serious secondary causes of headaches are uncommon, reflecting ascertainment bias in these settings.
Secondary Causes of Headache
Diagnostically, the goal of the EP must be to detect or exclude serious secondary causes of headaches (sometimes referred to as headaches with organic causes), including headaches from tumors, vascular lesions, infections, and causes of increased intracranial pressure (ICP) such as:
- Pseudotumor cerebri (benign increased ICP)
- Meningitis
- Brain abscesses
- Dissections of the cranial and cervical vessels
- Aneurysms (both ruptured and unruptured)
- Arteriovenous malformations
- Dural sinus thromboses
- Inflammatory processes
- Vasculitis
- Traumatic hematomas (both acute and chronic).
This long list represents just some of the serious etiologies of headaches. While these processes are uncommon, they are not rare in the ED population, and the EP will encounter patients with each of these conditions during his or her career—along with the challenge to rapidly detect these potential life threats against the background of the very common primary or functional headaches. Thus, the EP is clearly placed in a dilemma: He or she needs to be thorough in diagnosing and treating the headache patient quickly without missing a serious etiology. History and physical examination can never be perfect in detecting all serious headaches, and even a lengthy, thorough, and time-consuming neurological examination will not detect all serious causes.
Neuroimaging
Neuroimaging is used liberally because computed tomography (CT) and magnetic resonance imaging (MRI) continue to detect findings not expected from the sometimes insensitivity of history-taking and physical examination. However, balanced against the need for thorough evaluation are concerns for expense and unnecessary radiation exposure.
Several recent high-visibility media posts note the overutilization of neuroimaging and the lack of physician adherence to guidelines.5-7 Yet, without imaging studies proving the case prior to admission, when patients present with high-risk symptoms and abnormal physical-examination findings, reimbursement for further investigation and hospitalization may be retrospectively denied. The author recently was involved in the care of a patient who arrived by aeromedical transport in a stroke-alert scenario with abrupt onset of headache, physical findings of left-sided weakness, and medical conditions that placed the patient at high-risk for stroke. After the hospitalization was over and investigations were completed, the insurance carrier concluded:
The information received does not show that the member’s headaches were due to suspected organic causes or findings requiring hospitalization and therapeutic intervention…. Treatment of this member could be provided at a less intensive level of care or in another setting, such as a nursing facility, outpatient setting or home.... This plan does not cover services that are not medically necessary.” (Personal written communication with Aetna Health Inc, January 22, 2014.)
A recent editorial in the Annals of Emergency Medicine summarizes this issue, concluding that though it appears many CTs are being ordered unnecessarily, a low yield is necessary to avoid misdiagnoses.8 This is particularly true in the case of older adults and patients with new or severe headache. Increasingly, the EP is placed in a bind with the implication being that clinical choices will never be “just right.” He or she will continue to be retrospectively studied for performing workups without yield but criticized for failure to do extensive workups when a serious cause of headache is later found.
Approach to Patient
The tools used to evaluate a patient with headache in the ED are the same as those employed for other patient encounters. Each patient evaluation begins with an appropriate history and physical examination. Arguably, history is the most important to determine whether the patient’s headache is recurrent, a first-time headache, or the worst headache in his or her life.
Obtaining an accurate patient history can be a challenge. One provocative article noted that the order in which a patient was asked about his or her headache seemed to change responses to key questions.9 Even the time-honored “worst headache of my life” should be viewed in context of other factors. One Internet search turned up a migraine blog where the comment was made by a patient who experienced severe migraines, “I have the worst headache of my life every month.”10
Does the headache exist in isolation? The concept of the lone acute severe headache (LASH) was developed as part of a clinical decision-making study, but the acronym helps to remind the EP that headache may or may not exist in isolation.11 Altered mental status not only makes a fine neurological examination impossible but suggests possible serious etiologies of headache. For example, a headache following a new seizure would divert diagnostic attention to the cause of seizure, and the presence of fever would of course suggest a possible infectious cause.
The initial diagnostic sorting of headaches must be either a likely primary headache (migraine, tension-type, etc) or so-called secondary headache. The concept of “red flags” has been developed to alert the practitioner to historical or physical examination findings that drive decisions for additional testing.12-17 Although widely advocated in the literature, the sensitivity of a red flag or flags have never been studied in a prospective manner. While different red flags are listed in various publications, no weighting or comparison has been published to date. A guideline that used red-flag features to drive additional investigations listed the evidentiary support for the concept of red flags at the lowest level of evidence.17
Notably, some of the teachings repeated in medical training have no evidential basis and simply do not stand up when studied. An example is the so-called “neurosurgical headache,” in which the headache is worse in the morning but improves throughout the day following upright position that is thought to reduce ICP. This makes sense—until someone actually studies patients with intracranial tumors and finds that their headache characteristics most typically resemble tension-type headaches.18 Even though this study was published more than 20 years ago, the traditional teaching that the “neurosurgical headaches are worse in the morning” continues.
The Table lists some of the red-flag features that may suggest the presence of a secondary headache and could identify patients who could benefit from additional investigations or neuroimaging. This listing is provided as a summary of current recommendations and though many of these seem to be consistent with anecdotal experiences, these red flags have not been studied in an organized manner. The use of any simple list is only an aid to clinical judgment. For example, every migraine has a first onset. The diagnosis of a first hemiplegic migraine can confidently be made only with additional historical information, additional testing, or evolution of a recurrent pattern, and probably with imaging studies to rule-out other possible causes. Another example is that of benign coital headache, a diagnosis of exclusion since subarachnoid hemorrhage certainly may occur with exertion.
A careful physical examination always should be performed. Some key signs are noted in the Table. The presence or absence of papilledema has been traditionally considered a red flag. Certainly the presence of papilledema warrants evaluation, but it may be quite difficult to detect by simple inspection—even by neurologists or ophthalmologists. Other funduscopic findings may mimic papilledema, and it may not be present with acute increased ICP since it may take some time to develop. The role of bedside ultrasound in detecting papilledema in the patient with acute headache is unclear, though a few small case series suggests its utility.19
Conclusion
In spite of being diligent, performing a good history and physical examination, and providing appropriate follow-up, EPs will sometimes “miss” serious and unusual causes of headaches. Current topics in headache diagnosis and management of migraine, thunderclap, and unusual causes of headache are examined in this special feature to help the EP in the diagnostic decision-making process.
Dr Huff is a professor of emergency medicine and neurology, University of Virginia, Charlottesville.
Migraine: An Evidence-Based Update
Rebecca H. Nerenberg, MD; Benjamin W. Friedman, MD
Multiple regimens have been shown effective in treating migraine with and without aura in both the acute-care and outpatient setting.
Recurrent episodic primary headache disorders such as migraine, tension-type, and cluster headache are a common presentation to the ED, with an estimated number of 5 million patients in the United States presenting annually.1 For these headaches, it is essential that the emergency physician (EP) focus on rapid and effective treatment, while minimizing side effects and expediting a return to work and usual activities.
Emergency physicians are adept at ruling-out serious secondary headaches (eg, bacterial meningitis, aneurysmal subarachnoid hemorrhage) and identifying benign secondary causes (eg, acute rhinosinusitis, cervicogenic headache). Migraine, the focus of this review, is the primary headache disorder that most commonly results in an ED visit.
Symptoms
Though presentations may be quite varied, migraine typically presents as a pulsating, unilateral headache, and is associated with nausea, vomiting, photophobia, phonophobia, and osmophobia.2 The condition is often described as severe in intensity and functionally impairing—characteristics that contribute to so many ED visits. Migraine, however, also may present bilaterally and be associated with muscle pain or spasms.
It is the constellation of symptoms—rather than any one symptom in particular—that leads to the correct diagnosis. Classically, the prodromal aura, which is reported by fewer than 20% of patients, precedes the onset of headache pain by no more than 1 hour and resolves by the time the headache begins. Many migraine patients, however, report visual or sensory disturbances during the headache phase itself. Other prodromal symptoms such as change in mood (eg, depression, sense of well-being, euphoria) and appetite commonly precede the acute headache by several days. Table 1 provides diagnostic criteria and characteristics of migraine with aura and without aura.
Pathophysiology
Although the mechanism of migraine is not completely understood, it is clear that vascular dysfunction alone does not adequately explain its pathophysiology. Migraine is not primarily a vascular headache, but rather it is fundamentally a brain disorder. The condition is best explained as a dysfunctional pain response to an as yet unidentified trigger that does not appear to cause tissue damage or otherwise threaten the body or brain.
In migraine, normally nonnoxious stimulation, such as light, sound, and touch, is perceived as painful. The trigeminal nerve is activated inappropriately and is a central component of activated pain pathways. Cortical spreading depression, a slow gap-junction mediated wave of depolarization causing changes in vascular and neural function, is associated with migraine aura. It is not yet understood why migraine attacks begin, and research to understand the migraine brain is ongoing.3
Diagnosis
Given the high prevalence of patients with migraine presenting to EDs and the relatively uncommon occurrence of malignant secondary headaches, migraine can often be correctly diagnosed based solely on specific historical features of the headache and/or the answers to a simple questionnaire. One of the best simple predictors of migraine is the POUNDing mnemonic:
- Pulsating headache quality;
- Duration of 4 to 72 hours;
- Unilateral pain;
- Nausea; and
- Disabling pain.
Patients with three or four of the above features can be diagnosed as having a migraine headache with high sensitivity and specificity.4 The combination of functional disability, nausea, and sensitivity to light has a high positive predictive value for a diagnosis of migraine among patients with recurrent episodes of headache.5
Having a short list of specific symptoms consistent with a diagnosis of migraine is helpful in a busy ED. These symptom checklists allow the health care provider to make a more specific diagnosis in a patient with a recurrent headache disorder. However, distinguishing among the various types of primary headache disorders prior to treatment is often unnecessary for the EP since acute migraine and tension-type headaches are likely to respond to similar treatments such as sumatriptan,6 the antiemetic dopamine antagonists,7 and parenteral ketorolac.
Treatment
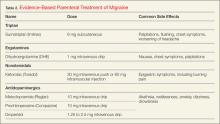
The EP has a large and varied armamentarium for treating acute migraine. First-line parenteral choices include migraine-specific medications (eg, sumatriptan, dihydroergotamine), nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ketorolac), and the various antiemetic dopamine antagonists (Table 2).
Sumatriptan
Subcutaneous sumatriptan is highly effective in treating migraine quickly, with a median time to relief of 34 minutes.8 The triptans, however, are not appropriate for all patients. Due to the high rate of side effects associated with sumatriptan, including palpitations, flushing, and chest pressure, it is contraindicated in those with cardiovascular (CV) risk factors.
Although effective in relieving migraine, there is a 67% rate of headache recurrence after successful initial treatment with subcutaneous sumatriptan. Therefore, along with counseling patients about side effects of the drug, EPs should also advise them of the high probability of recurrence and discharge patients with medication in anticipation of rebound headache. Table 3 lists the currently available triptans, along with routes of administration and recommended doses.
Ergotamines and Antiemetics
In addition to sumatriptan, dihydroergotamine, administered with an antimigraine antiemetic such as prochlorperazine, is another highly effective treatment option. Since the ergotamines have vasoconstrictive and oxytocic effects on the placenta and may cause harm to the fetus, they are rated Category X. As with sumatriptan, these agents are appropriate for use in nonpregnant patients and patients who have no CV risk factors.
Nonsteroidal Anti-inflammatory Drugs
Parenteral NSAIDs may also be considered to treat acute migraine. A recent meta-analysis of ketorolac for acute migraine showed it to be as effective as meperidine and the phenothiazines and more effective than intranasal sumatriptan.9 Side-effect profiles among the drugs were similar; however, it was common for patients receiving ketorolac to require rescue medications more frequently than patients receiving alternative medications for migraine. Given these findings, it is more appropriate to use ketorolac as a second-line—rather than first-line—agent for the treatment of acute migraine.
Antiemetic Dopamine Antagonists
Antiemetic dopamine antagonists such as metoclopramide, prochlorperazine, and droperidol are effective antimigraine agents. Intravenous metoclopramide and prochlorperazine have outperformed subcutaneous sumatriptan in head-to-head trials.10-12 Each of these medications has demonstrated superiority to placebo. Hyperkinetic motor side effects, such as akathisia or abrupt onset restlessness, are common but can be prevented with anticholinergics such as diphenhydramine. Irreversible motor disturbances after one dose of these medications have never been reported and fear of this occurrence should not dissuade the EP from their use.
Occipital Nerve Block
Regional nerve blocks may be effective for some patients. Performing a greater occipital block using a combination of a long-acting local anesthetic and a corticosteroid may provide rapid and lasting relief for some migraineurs. This strategy has many proponents, though data supporting or refuting its efficacy do not exist.13
Opioids
Opioids are the class of medication used most commonly to treat migraine in US and Canadian EDs.14,15 Though highly effective for acute pain, opioids are less desirable treatment for acute migraine for the following reasons: (1) Opioids are less effective than other treatment regimens such as the antiemetic dopamine antagonists and dihydroergotamine combinations; (2) they are associated with an increased number of repeat ED visits; (3) it is difficult to send patients back to work or allow them to drive home after treatment with an opioid; and (4) opioids are associated with worsening of the underlying migraine disorder. In outpatient studies, opioids were thought to cause transformation of episodic migraine into chronic daily headaches.17
Therefore, based on the above concerns, a patient who presents with a migraine to an ED for the first time should never be administered opioids unless contraindications or lack of response to other medications leave no alternative. A patient presenting frequently and insisting on opioid treatment represents a difficult patient population—one that is often characterized by psychiatric comorbidities and concomitant medication-overuse headache. Ideally, these patients are managed not during a busy shift but by a “difficult patient” committee that can create an appropriate interdisciplinary treatment plan for the patient and enforce that plan with a patient contract.
Other Treatment Options
For patients refractory to the treatments listed above, other options with potential benefit include propofol, haloperidol, valproic acid, and magnesium—the latter being particularly effective in treating migraine with aura.
Postdischarge Treatment
Regardless of the type of treatment, most patients who present to an ED with acute migraine have a recurrence of pain within 48 hours, thus requiring outpatient therapy.18 Parenteral or oral corticosteroids decrease the frequency of headache recurrence, though the optimal dose and route of administration is not known.19 Oral naproxen sodium, sumatriptan, or a combination of both (eg, combination oral tablet or a triptan taken along with naproxen sodium) are comparably effective in treating headache recurrence postdischarge. Because the two medications performed equally well in treating headache recurrence, physicians can choose between the two based on issues related to medication contraindications, cost, and patient preference.20
Conclusion
Headache is a common presenting complaint in the ED. Once it has been determined that a patient suffers from a primary headache disorder, it is not always relevant or necessary to determine from which headache subtype a patient suffers prior to treatment because most types respond to acute treatment. Multiple regimens have been shown effective for the treatment of acute migraine. Emergency physicians can choose a therapy based on medication availability, provider comfort with the medications, and patient comorbidities.
Opioids should almost never be used as initial treatment in patients presenting with migraine to the ED for the first time because they are less effective than other medications and may worsen the underlying migraine disorder. Once the acute pain is resolved, the EP should administer corticosteroids and discharge the patient with naproxen or a triptan (or a combination therapy) in the event of rebound headache.
Dr Nerenberg is an assistant professor, department of emergency medicine, Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York. Dr Friedman is an associate professor, department of emergency medicine, Albert Einstein College of Medicine Montefiore Medical Center, Bronx, New York.
Thunderclap Headache
Jonathan A. Edlow, MD
All patients presenting with thunderclap headache, including the neurologically intact, require a thorough evaluation to distinguish between benign and serious causes.
Among the 4% of ED patients presenting with headache, only a small percentage has serious “cannot miss” causes defined as treatable problems that are life, limb, brain, or vision threatening1,2 (Table 1). Some of these patients have abrupt, severe, and unique headaches referred to as a thunderclap headache. Thunderclap headaches begin suddenly, peak within 60 seconds and can last minutes to days.3 Any patient with a new-onset headache associated with new neurological deficits should be worked up sufficiently to explain the deficit. However, many patients—even those with headaches caused by serious secondary causes—have normal neurological examinations. This article reviews the diagnosis of neurologically intact patients with thunderclap headache.
Most of the “cannot miss” conditions can present with a thunderclap headache, though some are far more common than others. In an unselected ED population of 100 patients with thunderclap headache, approximately 88% to 89% will have nonserious causes; 10% will have subarachnoid hemorrhage (SAH); and the remaining 1% to 2% of patients will have one of several rare but important conditions.
The 88% to 89% comprises primary headache syndromes—first migraine or tension-type headache or “benign thunderclap headache,” which means that the workup for serious secondary causes was unrevealing. Because history and physical examination alone cannot distinguish between patients with benign causes of thunderclap headache from those with serious causes such as SAH, all thunderclap headache patients—even those who are entirely intact neurologically—need a thorough evaluation for SAH.1-4 This is consistent with the recent clinical decision rule to identify headache patients with SAH, in which thunderclap presentation mandates a workup for SAH.5
Despite the simplicity of a workup for SAH, studies reveal that emergency physicians (EPs) miss approximately 5% of cases.6,7 For a life-threatening and highly treatable condition, missing one in 20 is problematic.
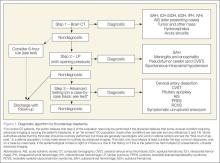
Evaluation of SAH
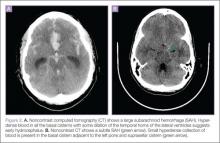
The time-honored evaluation for possible SAH is noncontrast brain computerized tomography (CT) followed by a lumbar puncture (LP) if the CT is normal or nondiagnostic (Figure 1). The accuracy of this paradigm approaches 100%. Computed tomography scans in patients with SAH show blood, which appears white in the acute phase, in the subarachnoid spaces (Figure 2). Sensitivity of CT decreases with time from headache onset and with size of the bleed. The brisk flow of cerebrospinal fluid (CSF), which is replaced several times per day, dilutes the blood. Modern CT scanners are only about 90% sensitive in neurologically intact patients, although it is very important to note that this study did not report the time from onset of headache.8
There are two typical locations for SAH to occur: the basal cisterns (usually aneurysmal) and over the high convexities (rarely, if ever, caused by aneurysms). The two common causes of convexal SAH are amyloid angiopathy in older patients and reversible cerebral vasoconstriction syndrome (RCVS) in younger patients.9 In some cases, CT will suggest another (non-SAH) cause of thunderclap headache. For example, although CT is not 100% sensitive for a small tumor or absces
s, it will nearly always show some abnormality (eg, edema, hydrocephalus, displacement of tissue) in a mass large enough to cause a severe headache. Computed tomography may also show nonspecific changes that warrant further imaging—eg, some cases of cerebral venous sinus thrombosis (CVST) and other conditions discussed below.
Shortly after the bleed, red blood cells appear in the CSF, but rapidly diminish in numbers over time due to CSF flow. In the hours to days that follow, xanthochromia, a yellowish discoloration, appears. This is due to degradation of hemoglobin from lysed red blood cells into bilirubin, oxyhemoglobin, and methemoglobin. Xanthochromia can be measured visually or by spectrophotometry, each of which has advantages and disadvantages.1
Almost every North American hospital laboratory uses visual inspection.10 Measuring opening pressure helps to diagnose the occasional case of pseudotumor cerebri, CVST (elevated pressure), or spontaneous intracranial hypotension (low pressure). It can also help to distinguish a traumatic LP (pressure is low) versus SAH (pressure is elevated in approximately 60%).
Is Lumbar Puncture Required After a Negative CT Within 6 Hours of Headache Onset?
It has long been recognized that CT sensitivity for SAH decreases over time and is most sensitive in the first 24 hours. Over the last few years, increasing data and expert opinion suggest that CTs performed within 6 hours of onset of classic thunderclap headache in neurologically normal patients are nearly 100% sensitive, obviating the need for LP.11-13 Although another study called these data into question, the methodology did not allow distinction between traumatic taps in patients with incidental aneurysms and true SAH and not all of their “negative” CT scans were truly negative.14
The author’s practice no longer uses LP in patients with classic thunderclap headache who are neurologically normal and whose CT scans are performed within 6 hours of headache onset and are read as negative by an attending-level radiologist. Physicians who follow this method should strictly adhere to each component of the preceding sentence. It is estimated that such an approach will miss as many as one in 600 to 1,000 patients. Because this represents a change in practice that has not yet been incorporated into any published guidelines, if a physician chooses to skip the LP in this circumstance, a discussion with the patient is warranted, the nature of which should focus on the balance of benefits (eg, LP picking up the very rare SAH in this group of patients) versus the harm (eg, side effects of the LP, which the author believes is mostly the increased number of advanced imaging due to traumatic taps and from incidental findings). This introduces the concept of “testing threshold,” an interesting and increasingly important concept for EPs to consider in diagnosing any low-frequency but high-stakes condition.15,16
Newer Diagnostic Strategies
Over the last few years, various alternative workups, including LP-first, magnetic resonance imaging (MRI) only and CT/CT angiography (CTA), have been proposed to replace the standard workup. Various authors have analyzed both the clinical and economic advantages and disadvantages of each modality.17,18
LP-First. The logic for the LP-first approach is because physicians often do not perform the LP in routine practice. A careful neurological examination is critical to ensure that this method is restricted to neurologically normal patients. Some neurologically intact patients with a lesion on CT that does not affect the CSF will be missed with this approach. In addition, if this approach is used, the opening pressure of the CSF must be measured in every case. The advantage is that it spares radiation exposure and forces an LP to be performed.
Primary MRI. The advantage of primary MRI is that current MRI sequences are as sensitive as CT for acute blood, and more sensitive for subacute and chronic blood. There is also no radiation exposure. Furthermore, depending on the sequences, which may include cerebrovascular imaging, one may be able to diagnose other rare causes of thunderclap headache beside SAH or unruptured aneurysm. It is important to note, however, that as with CT, smaller bleeds may appear negative on MRI—thus, spectrum bias exists for both modalities.1 The obvious disadvantage is cost and more importantly, availability in real time. As MRI technology and penetration into routine practice advance, this would become a very reasonable approach.
CT AND CTA. Finally, some have suggested that CT followed by CTA should be the new paradigm in order to avoid LP. These tests are easy for the EP to do and less painful to the patient, but there are unintended consequences to this strategy, including missing CSF-diagnosable conditions, diagnosing incidental aneurysms and other findings (all of which invariably lead to more imaging), and increased radiation and contrast dye exposure.19 The CT/CTA strategy does make sense in patients who cannot undergo LP (eg, patient refusal, unfavorable body habitus, anticoagulation use).
Beyond SAH
For most ED patients with thunderclap headache, CT and LP—the first two steps in the diagnostic workup—are sufficient to diagnose SAH. A meta-analysis of seven studies of neurologically normal subjects with thunderclap headache and normal CT and CSF results (813 patients) found no cases of SAH or occurrence of sudden death during at least 3 months of follow-up.20 Using the statistical worst-case scenario (upper bound of the 95% confidence interval) would be that four of 1,000 patients could have an SAH.
Dissections and CVST
Computed tomography and LP may miss some uncommon conditions associated with thunderclap headache that require some form of advanced imaging (Table 2).3,21 The more common of these include dissections and CVST, which appear to be diagnosed with increasing frequency. Both carotid and vertebral artery dissections can present with isolated head or neck pain without any neurological symptoms or signs during the highly variable phase after the intimal tear has occurred, but before downstream ischemia or infarction occurs.22 Approximately 15% of patients with CVST present with thunderclap headache, and roughly half of patients with CVST will show some abnormality on CT; however, CT venography or MR venography is necessary to confirm the diagnosis.23 These two modalities are probably equivalent in sensitivity.
Other Uncommon Conditions
There is a short list of other uncommon conditions: pituitary apoplexy, cerebellar infarction, and some vascular disorders. Patients with pituitary apoplexy (infarction of the gland usually due to bleeding into a previously undiagnosed adenoma) present with headache, symptoms of endocrine insufficiency, and visual field cuts—classically the bitemporal hemianopia due to the tumor pushing upwards on the optic chiasm.24 Some of these patients will have blood in the CSF, simulating an SAH. Dedicated CT or, preferably, MRI of the sella turcica is diagnostic. Cerebellar infarction can cause thunderclap headache and is generally accompanied by nonspecific symptoms such as vomiting and dizziness.
RCVS AND PRES
Emergency physicians should be aware of two other conditions associated with thunderclap headache: RCVS and posterior reversible encephalopathy syndrome (PRES). Reversible cerebral vasoconstriction syndrome is associated with reversible cerebral arterial spasm.25 Patients often have multiple thunderclap headaches over days to weeks, a pattern which is almost pathognomonic of RCVS. Risk factors include postpartum state, exposure to vasoactive drugs and immunosuppressive agents, catecholamine secreting tumors and others.3,25
In PRES, patients generally present with headache (thunderclap or otherwise), visual symptoms, and seizures.26 Blood pressure is usually, but not invariably, elevated in PRES, which is strongly related to hypertensive encephalopathy. There is also overlap between RCVS and PRES.27
Pregnant and Postpartum Patients
Lastly, one special circumstance merits discussion. Most headaches in pregnant and postpartum women are migraine and tension-related headaches. However EPs should have a very low threshold for advanced imaging in these patients with severe headache, who are at risk for RCVS, PRES, CVST, stroke, and low-pressure headaches.27 Some, but not all, of these conditions are eclampsia-related, and the risk if highest in late pregnancy or in the weeks afterward.
Conclusion
Despite the long differential diagnosis for thunderclap headache, most patients have primary headache disorders. As with many high-risk but low-frequency problems in EM, one must develop an organized diagnostic approach. Ideally, EPs should communicate the clinical situation to their radiology consultants to maximize the information to be acquired by imaging.28 Assuming a normal physical examination, one has to use clues in the history and epidemiological context to decide which patients to work up beyond the standard SAH evaluation.
Dr Edlow is a professor, department of medicine, Harvard Medical School; and vice-chair of emergency medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Unusual Causes of Headache
Allison Tadros MD; Joseph Minardi, MD
In evaluating patients presenting with severe headache, there are unusual and life-threatening etiologies EPs should include in the differential diagnosis.
Providers of acute care have been well educated on the red flags, work up, and treatment of life-threatening causes of headache such as intracranial bleeding and meningitis. However, there are other unusual but important headache etiologies and syndromes of which they also should be aware. For example, one of these conditions, cerebral venous sinus thrombosis (CVST), may have serious morbidity and mortality if not diagnosed and treated promptly. Another, giant cell arteritis (GCA), may lead to permanent blindness if not recognized. Other etiologies, while not quite as serious in nature, are still important to be acquainted with in order to provide relief of patient’s symptoms and appropriate referral. This article discusses the signs, symptoms, workup, and treatment of CVST; occipital neuralgia; short-lasting, unilateral, neuralgiform headache attacks with conjunctival injection and tearing (SUNCT); idiopathic intracranial hypertension (IIH); GCA; and indomethacin-responsive headache syndromes (IRHS).
Cerebral Venous Sinus Thrombosis
Cerebral venous sinus thrombosis (also referred to as cavernous venous thrombosis) represents about 1% of all strokes.1,2 While this condition is thought to be uncommon, no epidemiologic studies are currently available.2 In contrast to other forms of stroke, women and children are more commonly affected, with most patients presenting younger than the age 50 years.3,4 Thrombosis may occur in the cerebral veins, the major sinuses, or both, and lead to brain edema, venous infarction, and intracranial hypertension.1
The majority of patients with CVST have an underlying risk factor for thrombosis (eg, injury, infection). In addition, hypercoagulable states seem to be more commonly found in adults with CVST, whereas infection to the head and neck is a more common risk factor in children.1 Women in the peripartum period also seem to be particularly vulnerable.3,5
Symptoms and Diagnosis
Headache is the most common presenting symptom in patients with CVST, although focal neurologic deficits, seizures, and altered mental status may also be seen.3 The headache is usually described as worsening over days to weeks, but thunderclap headache may also be reported.2 In CVST, headache may be the only presenting symptom, making diagnosis difficult.6 A diagnosis of CVST should be considered in any patient presenting with headache in conjunction with cranial nerve 6 palsy or papilledema.
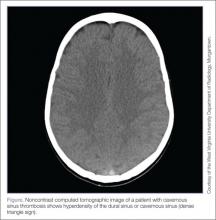
Workup of a suspected diagnosis of CVST can be challenging. A computed tomography (CT) scan of the brain with contrast may only diagnose 30% of cases.6 A noncontrast CT may be normal, but can show a hyperdensity of the dural sinus or cavernous sinus (dense triangle sign; Figure). Computed tomographic venography and magnetic resonance venography have much higher sensitivities in making the diagnosis.6 In addition to imaging studies, D-dimer elevation will be seen in most patients with this diagnosis, but a normal D-dimer may not exclude the presence of disease.4,7 Anticoagulation is considered first-line treatment even if an intracranial hemorrhage is present as a complication of the CVST.2,7 There may be a role for thrombolytic therapies in patients that clinically deteriorate despite anticoagulation.7
Occipital Neuralgia
Occipital neuralgia is characterized by a paroxysmal, lancinating pain over the area of the greater occipital nerve. It is typically associated with tenderness to palpation and may be described as a dysethesia or hypoesthesia.8,9 In occipital neuralgia, the pain originates in the suboccipital area of the head and radiates to the vertex. Although the exact etiology of the nerve irritation may be unknown, it is hypothesized that the cause could be vascular, muscular, osteogenic, or neurogenic.10 In addition to headache, patients may report blurry vision, orbital pain, nausea, dizziness, tinnitus, and nasal congestion.10 The true epidemiology of occipital neuralgia is unknown, as there are no clear diagnostic criteria or consensus on its definition.11 The pain may be spontaneous or provoked by exposure to cold or certain movements of the neck; it may be elicited by tapping on the occipital nerves (Tinel’s sign). The diagnosis is confirmed when the patient reports transient relief of pain after an occipital anesthetic block.11,12
Short-Lasting Unilateral Neuralgiform Headache Attacks With Conjunctival Injection and Tearing
Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing is a syndrome characterized by brief frequent attacks of stabbing pain in and around the eye, and associated tearing. Attacks may occur a few times a day, but often hundreds of times a day, lasting anywhere from seconds to several minutes.13 The short duration and higher frequency of attacks are useful criteria for differentiating SUNCT from cluster headache. Since SUNCT has some association with pituitary tumors, referral for outpatient magnetic resonance imaging is a reasonable recommendation.14 Small studies have shown some promise in improving symptoms with treatment with carbamazepine, gabapentin, and lamotrigine15,16 Steroids have been used with some success in temporarily aborting symptoms of this syndrome, which may be useful in treating patients presenting to the ED.17,18
Giant Cell Arteritis
Giant cell arteritis, also known as temporal arteritis or cranial arteritis, can be a cause for significant morbidity in those affected, with permanent visual loss being the most important complication. The typical presentation is new-onset focal headache in a patient older than age 50 years. The headache may be accompanied by a wide range of visual symptoms. In fact, visual symptoms with or without headache may be the presenting complaint, requiring clinician vigilance to seek out this diagnosis. Other historical features may include jaw claudication, fever, and symptoms of polymyalgia rheumatica.
The clinical presentation of polymyalgia rheumatica, which is present in up to half of patients, is one of proximal symmetric muscle and joint aches, accompanied by constitutional symptoms. Physical examination may be very nonspecific, but the temporal arteries may be tender with diminished pulses. The most useful laboratory finding is an elevated erythrocyte sedimentation rate, which is classically >50, though there have been documented cases with lower levels. An elevated C-reactive protein may be more predictive of the presence of GCA, but more research is needed in this area.19 A complete blood count may reveal a normochromic, microcytic anemia.
The pathophysiology of GCA involves inflammation of larger arteries, usually of the upper body and cranium. A wide range of vascular complications are possible, including ischemia, aneurysm, and dissection to affected vascular territories. The most common serious complication of GCA is permanent visual loss due to ischemic retinopathy. Ophthalmic involvement occurs in 26% of patients with permanent visual loss in 7% to 14%.
With respect to imaging studies, recent research has suggested that ultrasound with color Doppler techniques may be useful in diagnosing GCA.20,21 Other imaging studies may be needed based on the entire clinical presentation, specifically if involvement of other vascular territories is suspected.
Historically, the most definitive diagnostic test is temporal artery biopsy. It should be emphasized that the diagnosis of GCA is a clinical one in which biopsy, laboratory, and other clinical findings all play a supportive role in the diagnosis.
Treatment consists of high-dose corticosteroids, usually prednisone 60 to 80 mg/d initially. Treatment should not be withheld pending temporal artery biopsy as the findings remain positive for weeks. There is ongoing research into low-dose aspirin as well as other immune-modulating drugs. Ophthalmologic consultation should be sought for patients with visual symptoms and surgical follow-up is necessary for biopsy.22
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension, also known as pseudotumor cerebri or benign IIH, is a disease of abnormal elevated intracranial pressure (ICP) that typically affects overweight women of child-bearing age. The etiology is unclear. Patients present with nonspecific headaches and possible visual complaints that can range from blurring to sudden visual loss. Although IIH is rare in healthy men, those who do develop the condition have a higher risk of permanent vision loss.
The most important clinical finding is papilledema, which should be sought on all patients with a new headache as IIH may lead to progressive blindness.23 A detailed history and physical examination should be performed as well as imaging to investigate for intracranial masses and alternate etiologies of increased ICP. Once other causes are excluded, the diagnosis is established by measuring an elevated opening pressure during lumbar puncture performed with the patient in the lateral position.
Pharmacologic therapy is aimed at decreasing ICP. Acetazolamide is a mainstay of medical therapy. Surgical treatments such as optic nerve sheath fenestration and cerebrospinal fluid diversion procedures may be performed to limit visual loss. Ophthalmologic consultation should always be obtained.24
Indomethacin-Responsive Headache Syndromes
While indomethacin may be effective for many types of headaches, the hemicrania syndromes of paroxysmal hemicrania and hemicrania continua are defined by an absolute, invariable response to indomethacin therapy. In IRHS, headaches are typically unilateral, moderate to severe, last longer than other headache syndromes, and may occur in conjunction with autonomic symptoms.
Paroxysmal hemicrania consists of 5- to 30-minute episodes with pain-free periods. This syndrome, however, may progress to hemicrania continua, which is characterized by longer lasting, chronic headaches with less prominent autonomic symptoms. When considering either of these syndromes, a careful history and physical examination should be performed and other etiologies considered.24-26
Cluster Headache
Cluster headache is characterized clinically by unilateral paroxysmal pain, usually involving the upper half of the face and head. Episodes typically last between 15 to 180 minutes and can recur multiple times per day. To make the diagnosis, at least one local autonomic symptom must be present, such as forehead sweating or redness, conjunctival injection, lacrimation, or nasal congestion or drainage. Other ocular symptoms, such as miosis, ptosis, or lid swelling, may occur. Cluster headache is more common in men, and patients typically are restless or agitated. Laboratory studies and imaging are not typically necessary unless evaluation for other problems is warranted. Abortive treatment should include 100% oxygen by non-rebreather facemask, as well as a 5-HT1 receptor agonist, including metoclopramide, the triptan drugs, or ergotamine alkaloids. Corticosteroids may be effective in terminating a cluster headache cycle, and multiple medications have been used for prophylactic management. There are multiple surgical options available for refractory symptoms. Prevention should focus on a headache diary to identify triggers. Use of alcohol and tobacco products have also been shown to worsen symptoms.24
Conclusion
As patients present to the ED with a wide variety of headaches, EPs should include dangerous and secondary causes highest in the differential diagnosis. However, with a careful history and physical examination, other headache syndromes may be diagnosed with implications that can improve immediate and follow-up treatment, and, in some cases, prevent serious complications—particularly blindness and, though rare, death.
Dr Tadros is an associate professor, department of emergency medicine, West Virginia University, Morgantown. Dr Minardi is an associate professor, department of emergency medicine and medical education, West Virginia University, Morgantown.
- Headache
- Fernandes CL. The fifth vital sign. Fed Pract. 2010;27(10):26-28.
- Cantrill SV, Brown MD, Carlisle RJ et al; American College of Emergency Physicians Opioid Guideline Writing Panel. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525.
- Ho TW, Rodgers A, Bigal ME. Impact of recent prior opioid use on rizatriptan efficacy. A post hoc pooled analysis. Headache. 2009;49(3):395-403.
- Pylkas AM, Bart G. Prescribing controlled substances during a prescription drug epidemic. Neurol Clin Pract. 2014;4(2):99-105.
- Emergency Medicine Today. March 18, 2014. Vast majority of brain scans for headache patients may be unnecessary. http://acep.bulletinhealthcare.com/story.aspx?dt=140318. Accessed June 24, 2014.
- Shute N. Yes, It’s a headache. No, you don’t need a brain scan. NPR Shots. Your Health Web site. March 18, 2014. http://www.npr.org/blogs/health/2014/03/18/291044766/yes-its-a-headache-no-you-dont-need-a-brain-scan. Accessed June 18, 2014.
- Callaghan BC, Kerber KA, Pace RJ, Skolarus LE, Burke JF. Headaches and neuroimaging: high utilization and costs despite guidelines. JAMA Intern Med. 2014;174(5):819-821.
- Schwartz DT. US emergency physicians order too many computed tomography scans-or do they? Ann Emerg Med. 2013;62(5):495-497.
- Diaz M, Braude D, Skipper B. Concordance of historical questions used in risk-stratifying patients with headache. Am J Emerg Med. 2007;25(8):
907-910. - Worst headache of my life [List-serve comment]. mymigraineconnection.com. [currently inactive]
- Schull MJ. Lumbar puncture first: an alternative model for the investigation of lone acute sudden headache. Acad Emerg Med.1999;6(2):131-136.
- Clinch CR. Evaluation of acute headaches in adults. Am Fam Physician. 2001;63(4):685-692.
- Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol. 2003;76(908):532-535.
- Parizel PM, Voormolen M, Van Goethem JW, van den Hauwe L. Headache: when is neuroimaging needed? JBR-BTR. 2007;90(4):268-271.
- De Luca GC, Bartleson JD. When and how to investigate the patient with headache. Semin Neurol. 2010;30(2):131-144.
- Cady RK. Red flags and comfort signs for ominous secondary headaches. Otolaryngol Clin North Am. 2014;47(2):289-299.
- Duncan CW, Watson DP, Stein A; Guideline Development Group. Diagnosis and management of headache in adults: summary of SIGN guideline. BMJ. 2008;a2329.
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43(9):1678-1683.
- Daulaire S, Fine L, Salmon M, et al. Ultrasound assessment of optic disc edema in patients with headache. Am J Emerg Med. 2012;30(8):1654.e1-4.
- Migraine: An Evidence-Based Update
- Vinson DR. Treatment patterns of isolated benign headache in US emergency departments. Ann Emerg Med. 2002;39(3):215-222.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
- Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161(2):327-341.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296(10):1274-1283.
- Lipton RB, Dodick D, Sadovsky R, et al; ID Migraine Validation Study. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61(3):375-382.
- Miner JR, Smith SW, Moore J, Biros M. Sumatriptan for the treatment of undifferentiated primary headaches in the ED. Am J Emerg Med. 2007;25(1):60-64.
- Weinman D, Nicastro O, Akala O, Friedman BW. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
- Akpunonu BE, Mutgi AB, Federman DJ, et al. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: a multicenter study. Ann Emerg Med. 1995;25(4):464-269.
- Taggart E, Doran S, Kokotillo A, Campbell S, Villa-Roel C, Rowe BH. Ketorolac in the treatment of acute migraine: a systematic review. Headache. 2013;53(2):277-287.
- Friedman BW, Corbo J, Lipton RB, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology. 2005;64(3):463-468.
- Talabi S, Masoumi B, Azizkhani R, Esmailian M. Metoclopramide versus sumatriptan for treatment of migraine headache: A randomized clinical trial. J Res Med Sci. 2013;18(8):695-698.
- Kostic MA, Gutierrez FJ, Rieg TS, Moore TS, Gendron RT. A prospective, randomized trial of intravenous prochlorperazine versus subcutaneous sumatriptan in acute migraine therapy in the emergency department. Ann Emerg Med. 2010;56(1):1-6.
- Ashkenazi A, Levin M. Greater occipital nerve block for migraine and other headaches: is it useful? Curr Pain Headache Rep. 2007;11(3):231-235.
- Colman I, Rothney A, Wright SC, Zilkalns B, Rowe BH. Use of narcotic analgesics in the emergency department treatment of migraine headache. Neurology 2004;62(10):1695-1700.
- Vinson DR, Hurtado TR, Vandenberg JT, Banwart L. Variations among emergency departments in the treatment of benign headache. Ann Emerg Med. 2003;41(1):90-97.
- Friedman BW, Kapoor A, Friedman MS, Hochberg ML, Rowe BH. The relative efficacy of meperidine for the treatment of acute migraine: a meta-analysis of randomized controlled trials. Ann Emerg Med. 2008;52(6):705-713.
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168.
- Friedman BW, Hochberg ML, Esses D, et al. Recurrence of primary headache disorders after emergency department discharge: frequency and predictors of poor pain and functional outcomes. Ann Emerg Med. 2008;52(6):696-704.
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ. 2008;336(7657):1359-1361.
- Friedman BW, Solorzano C, Esses D, et al. Treating headache recurrence after emergency department discharge: a randomized controlled trial of naproxen versus sumatriptan. Ann Emerg Med. 2010;56(1):7-17.
: Thunderclap Headache
- Edlow JA, Malek AM, Ogilvy CS. Aneurysmal subarachnoid hemorrhage: update for emergency physicians. J Emerg Med. 2008;34(3):237-251.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med. 2008;52(4):407-436.
- Ducros A, Bousser MG. Thunderclap headache. BMJ. 2013;346:e8557.
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage [see comments]. N Engl J Med. 2000;342(1):29-36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA. 2013;310(12):1248-1255.
- Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291(7):866-869.
- Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke. 2007;38(4):1216-1221.
- Byyny RL, Mower WR, Shum N, Gabayan GZ, Fang S, Baraff LJ. Sensitivity of noncontrast cranial computed tomography for the emergency department diagnosis of subarachnoid hemorrhage. Ann Emerg Med. 2008;51(6) 697-703.
- Kumar S, Goddeau RP, Jr, Selim MH, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology 2010;74(11):893-899.
- Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Arch Pathol Lab Med. 2002;126(4):413-415.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke. 2012;43(8):2115-2119.
- Edlow JA, Fisher J. Diagnosis of subarachnoid hemorrhage: time to change the guidelines? Stroke. 2012;43(8):2031,2032.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
- Mark DG, Hung YY, Offerman SR, et al; Kaiser Permanente CREST Network Investigators. Nontraumatic subarachnoid hemorrhage in the setting of negative cranial computed tomography results: external validation of a clinical and imaging prediction rule. Ann Emerg Med. 2013;62(1):1-10e1.
- Mark DG, Pines JM. The detection of nontraumatic subarachnoid hemorrhage: still a diagnostic challenge. Am J Emerg Med. 2006;24(7):859-863.
- Pines JM, Szyld D. Risk tolerance for the exclusion of potentially life-threatening diseases in the ED. Am J Emerg Med. 2007;25(5):540-544.
- Farzad A, Radin B, Oh JS, et al. Emergency diagnosis of subarachnoid hemorrhage: an evidence-based debate. J Emerg Med. 2013;44(5):1045-1053.
- Ward MJ, Bonomo JB, Adeoye O, Raja AS, Pines JM. Cost-effectiveness of diagnostic strategies for evaluation of suspected subarachnoid hemorrhage in the emergency department. Acad Emerg Med. 2012;19(10):1134-1144.
- Edlow JA. What are the unintended consequences of changing the diagnostic paradigm for subarachnoid hemorrhage after brain computed tomography to computed tomographic angiography in place of lumbar puncture? Acad Emerg Med. 2010;17(9):991-995; discussion 996,997.
- Savitz SI, Levitan EB, Wears R, Edlow JA. Pooled analysis of patients with thunderclap headache evaluated by CT and LP: is angiography necessary in patients with negative evaluations? J Neurol Sci. 2009;276(1-2):123-125.
- Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol. 2006;5(7):621-631.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
- Rajasekaran S, Vanderpump M, Baldeweg S, et al. UK guidelines for the management of pituitary apoplexy. Clinical endocrinology (Oxf). 2011;74(1):9-20.
- Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005-1012.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
- Edlow JA, Caplan LR, O’Brien K, Tibbles CD. Diagnosis of acute neurological emergencies in pregnant and post-partum women. Lancet Neurol 2013;12(2):175-185.
- Mortimer AM, Bradley MD, Stoodley NG, Renowden SA. Thunderclap headache: diagnostic considerations and neuroimaging features. Clin Radiol. 2013;68(3):e101-e113.
: Unusual Causes of Headache
- Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158.
- Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791-1798.
- Sidhom Y, Mansour M, Messelmani M, et al. Cerebral venous thrombosis: clinical features, risk factors, and long-term outcome in a Tunisian cohort. J Stroke Cerebrovasc Dis. 2014;23(6):1291-1295.
- Kosinski CM, Mull M, Schwarz M, et al. Do normal D-dimer levels reliably exclude cerebral sinus thrombosis? Stroke. 2004;35(12):2820-2825.
- Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274-1282.
- Wasay M, Azeemuddin M. Neuroimaging of cerebral venous thrombosis. J Neuroimaging. 005;15(2):118-128.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
- Vincent MB. Headache and neck. Curr Pain Headache Rep. 2011;15(4):324-331.
- Vanelderen P, Lataster A, Levy R, Mekhail N, van Kleef M, Van Zundert J. Occipital neuralgia. Pain Pract. 2010;10(2):137-144.
- Bogduk N. The neck and headaches. Neurol Clin. 2004;22(1):151-171, vii.
- Vanderhoek MD, Hoang HT, Goff B. Ultrasound-guided greater occipital nerve blocks and pulsed radiofrequency ablation for diagnosis and treatment of occipital neuralgia. Anesth Pain Med. 2013;3(2):256-259.
- Young WB. Blocking the greater occipital nerve: utility in headache management. Curr Pain Headache Rep. 2010;14(5):404-408.
- Cohen AS, Matharu MS, Goadsby PJ. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)—a prospective clinical study of SUNCT and SUNA. Brain. 2006;129(Pt 10):2746-2760.
- Chitsantikul P, Becker WJ. SUNCT, SUNA and pituitary tumors: clinical characteristics and treatment. Cephalalgia. 2013;33(3):160-170.
- Williams MH, Broadley SA. SUNCT and SUNA: clinical features and medical treatment. J Clin Neurosci. 2008;15(5):526-534.
- Etemadifar M, Maghzi AH, Ghasemi M, Chitsaz A, Kaji Esfahani M. Efficacy of gabapentin in the treatment of SUNCT syndrome. Cephalalgia. 2008;28(12):1339-1342.
- Trauninger A, Alkonyi B, Kovács N, Komoly S, Pfund Z. Methylprednisolone therapy for short-term prevention of SUNCT syndrome. Cephalalgia. 2010;30(6):735-739.
- de Lourdes Figuerola M, Bruera O, Pozzo MJ, Leston J. SUNCT syndrome responding absolutely to steroids in two cases with different etiologies. J Headache Pain. 2009;10(1):55-57.
- Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012;41(6):866-871.
- Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336-1342.
- Stammler F, Grau C, Schnabel A. Value of colour doppler ultrasonography in relation to clinical pretest probability in giant cell (temporal) arteritis. Dtsch Med Wochenschr. 2009;134(42):2109-2115.
- Hellmann DB. Giant cell arteritis, polymyalgia rheumatica, and Takayasu’s arteritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, eds. Kelley’s Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier Saunders; 2012:1461-1472.
- Pham L, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri). University of Iowa Healthcare Ophthalmology and Visual Sciences Web site. http://webeye.ophth.uiowa.edu/eyeforum/cases/99-pseudotumor-cerebri.htm. Accessed July 1, 2014.
- Garza I, Swanson, JW, Chesire WP Jr, et al. Headache and other craniofacial pain. In: Daroff R, Fenichel GM, Jankovic J, Mazziotta J, eds. Bradley’s Neurology in Clinical Practice. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1732,1736.
- Rosenberg GA. Brain edema and disorders of cerebrospinal fluid circulation. In: Daroff R, Fenichel GM, Jankovic J, Mazziotta J, eds. Bradley’s Neurology in Clinical Practice. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1389-1391.
- Dodick DW. Indomethacin responsive headache syndromes. Curr Pain Headache Rep. 2004;8(1):19-26.
Although the cause of severe headaches in patients presenting to the ED are typically of benign etiology, there are serious and even life-threatening causes for which the EP should retain a high index of suspicion. In the physician’s thoughts lurk the small, but real, possibility that a missed diagnosis might lead to harm or even death of the patient. The cause of the headache may not be detectable during the ED visit—even after expensive, time-consuming, and, sometimes painful, studies. Fears of being charged with negligence or malpractice may be hovering somewhere in the back of an EP’s mind. Additionally, the goals of the physician and the patient may not be aligned (eg, the patient is often more concerned with symptom relief, while the physician is typically more concerned with rapid diagnosis and diagnostic accuracy).
The patient with chronic or recurrent headache presents a special management challenge. The EP may encounter the same patient at repeat ED visits. Assessment of pain—the “fifth vital sign”—and response of interventions are frequently the subjects of review by accreditation and regulatory organizations.1 A patient with pain from recurrent headache may not be completely satisfied during a visit, and this may be reflected in the ED’s patient satisfaction scores. On the other hand, the epidemic of prescription pain medication misuse has become a nationally recognized problem, and EPs in particular are being tasked to carefully assess opioid administration and prescriptions.2 In addition, chronic opioid administration may interfere with patient response to some headache-specific medications such as triptans.3 Cautious prescribing with screening before utilizing controlled substances in patients at risk is recommended to prevent misuse and abuse.4
Literature History
Much of the literature on headaches comes from pain and headache clinics and reflects the experience from that patient population. Patients referred to such clinics often have chronic, recurrent headaches that are difficult to manage. The consultation and referral processes at these centers exclude many time-sensitive causes of headache, and extensive neuroimaging and other studies likely will have detected patients with tumors and vascular lesions. In the nomenclature of headaches, these clinics specialize in treating patients with primary headache syndromes (eg, migraine, cluster, chronic daily headache). Conversely, secondary causes of headache are infrequent in these clinics. Studies based on these patient populations conclude that serious secondary causes of headaches are uncommon, reflecting ascertainment bias in these settings.
Secondary Causes of Headache
Diagnostically, the goal of the EP must be to detect or exclude serious secondary causes of headaches (sometimes referred to as headaches with organic causes), including headaches from tumors, vascular lesions, infections, and causes of increased intracranial pressure (ICP) such as:
- Pseudotumor cerebri (benign increased ICP)
- Meningitis
- Brain abscesses
- Dissections of the cranial and cervical vessels
- Aneurysms (both ruptured and unruptured)
- Arteriovenous malformations
- Dural sinus thromboses
- Inflammatory processes
- Vasculitis
- Traumatic hematomas (both acute and chronic).
This long list represents just some of the serious etiologies of headaches. While these processes are uncommon, they are not rare in the ED population, and the EP will encounter patients with each of these conditions during his or her career—along with the challenge to rapidly detect these potential life threats against the background of the very common primary or functional headaches. Thus, the EP is clearly placed in a dilemma: He or she needs to be thorough in diagnosing and treating the headache patient quickly without missing a serious etiology. History and physical examination can never be perfect in detecting all serious headaches, and even a lengthy, thorough, and time-consuming neurological examination will not detect all serious causes.
Neuroimaging
Neuroimaging is used liberally because computed tomography (CT) and magnetic resonance imaging (MRI) continue to detect findings not expected from the sometimes insensitivity of history-taking and physical examination. However, balanced against the need for thorough evaluation are concerns for expense and unnecessary radiation exposure.
Several recent high-visibility media posts note the overutilization of neuroimaging and the lack of physician adherence to guidelines.5-7 Yet, without imaging studies proving the case prior to admission, when patients present with high-risk symptoms and abnormal physical-examination findings, reimbursement for further investigation and hospitalization may be retrospectively denied. The author recently was involved in the care of a patient who arrived by aeromedical transport in a stroke-alert scenario with abrupt onset of headache, physical findings of left-sided weakness, and medical conditions that placed the patient at high-risk for stroke. After the hospitalization was over and investigations were completed, the insurance carrier concluded:
The information received does not show that the member’s headaches were due to suspected organic causes or findings requiring hospitalization and therapeutic intervention…. Treatment of this member could be provided at a less intensive level of care or in another setting, such as a nursing facility, outpatient setting or home.... This plan does not cover services that are not medically necessary.” (Personal written communication with Aetna Health Inc, January 22, 2014.)
A recent editorial in the Annals of Emergency Medicine summarizes this issue, concluding that though it appears many CTs are being ordered unnecessarily, a low yield is necessary to avoid misdiagnoses.8 This is particularly true in the case of older adults and patients with new or severe headache. Increasingly, the EP is placed in a bind with the implication being that clinical choices will never be “just right.” He or she will continue to be retrospectively studied for performing workups without yield but criticized for failure to do extensive workups when a serious cause of headache is later found.
Approach to Patient
The tools used to evaluate a patient with headache in the ED are the same as those employed for other patient encounters. Each patient evaluation begins with an appropriate history and physical examination. Arguably, history is the most important to determine whether the patient’s headache is recurrent, a first-time headache, or the worst headache in his or her life.
Obtaining an accurate patient history can be a challenge. One provocative article noted that the order in which a patient was asked about his or her headache seemed to change responses to key questions.9 Even the time-honored “worst headache of my life” should be viewed in context of other factors. One Internet search turned up a migraine blog where the comment was made by a patient who experienced severe migraines, “I have the worst headache of my life every month.”10
Does the headache exist in isolation? The concept of the lone acute severe headache (LASH) was developed as part of a clinical decision-making study, but the acronym helps to remind the EP that headache may or may not exist in isolation.11 Altered mental status not only makes a fine neurological examination impossible but suggests possible serious etiologies of headache. For example, a headache following a new seizure would divert diagnostic attention to the cause of seizure, and the presence of fever would of course suggest a possible infectious cause.
The initial diagnostic sorting of headaches must be either a likely primary headache (migraine, tension-type, etc) or so-called secondary headache. The concept of “red flags” has been developed to alert the practitioner to historical or physical examination findings that drive decisions for additional testing.12-17 Although widely advocated in the literature, the sensitivity of a red flag or flags have never been studied in a prospective manner. While different red flags are listed in various publications, no weighting or comparison has been published to date. A guideline that used red-flag features to drive additional investigations listed the evidentiary support for the concept of red flags at the lowest level of evidence.17
Notably, some of the teachings repeated in medical training have no evidential basis and simply do not stand up when studied. An example is the so-called “neurosurgical headache,” in which the headache is worse in the morning but improves throughout the day following upright position that is thought to reduce ICP. This makes sense—until someone actually studies patients with intracranial tumors and finds that their headache characteristics most typically resemble tension-type headaches.18 Even though this study was published more than 20 years ago, the traditional teaching that the “neurosurgical headaches are worse in the morning” continues.
The Table lists some of the red-flag features that may suggest the presence of a secondary headache and could identify patients who could benefit from additional investigations or neuroimaging. This listing is provided as a summary of current recommendations and though many of these seem to be consistent with anecdotal experiences, these red flags have not been studied in an organized manner. The use of any simple list is only an aid to clinical judgment. For example, every migraine has a first onset. The diagnosis of a first hemiplegic migraine can confidently be made only with additional historical information, additional testing, or evolution of a recurrent pattern, and probably with imaging studies to rule-out other possible causes. Another example is that of benign coital headache, a diagnosis of exclusion since subarachnoid hemorrhage certainly may occur with exertion.
A careful physical examination always should be performed. Some key signs are noted in the Table. The presence or absence of papilledema has been traditionally considered a red flag. Certainly the presence of papilledema warrants evaluation, but it may be quite difficult to detect by simple inspection—even by neurologists or ophthalmologists. Other funduscopic findings may mimic papilledema, and it may not be present with acute increased ICP since it may take some time to develop. The role of bedside ultrasound in detecting papilledema in the patient with acute headache is unclear, though a few small case series suggests its utility.19
Conclusion
In spite of being diligent, performing a good history and physical examination, and providing appropriate follow-up, EPs will sometimes “miss” serious and unusual causes of headaches. Current topics in headache diagnosis and management of migraine, thunderclap, and unusual causes of headache are examined in this special feature to help the EP in the diagnostic decision-making process.
Dr Huff is a professor of emergency medicine and neurology, University of Virginia, Charlottesville.
Migraine: An Evidence-Based Update
Rebecca H. Nerenberg, MD; Benjamin W. Friedman, MD
Multiple regimens have been shown effective in treating migraine with and without aura in both the acute-care and outpatient setting.
Recurrent episodic primary headache disorders such as migraine, tension-type, and cluster headache are a common presentation to the ED, with an estimated number of 5 million patients in the United States presenting annually.1 For these headaches, it is essential that the emergency physician (EP) focus on rapid and effective treatment, while minimizing side effects and expediting a return to work and usual activities.
Emergency physicians are adept at ruling-out serious secondary headaches (eg, bacterial meningitis, aneurysmal subarachnoid hemorrhage) and identifying benign secondary causes (eg, acute rhinosinusitis, cervicogenic headache). Migraine, the focus of this review, is the primary headache disorder that most commonly results in an ED visit.
Symptoms
Though presentations may be quite varied, migraine typically presents as a pulsating, unilateral headache, and is associated with nausea, vomiting, photophobia, phonophobia, and osmophobia.2 The condition is often described as severe in intensity and functionally impairing—characteristics that contribute to so many ED visits. Migraine, however, also may present bilaterally and be associated with muscle pain or spasms.
It is the constellation of symptoms—rather than any one symptom in particular—that leads to the correct diagnosis. Classically, the prodromal aura, which is reported by fewer than 20% of patients, precedes the onset of headache pain by no more than 1 hour and resolves by the time the headache begins. Many migraine patients, however, report visual or sensory disturbances during the headache phase itself. Other prodromal symptoms such as change in mood (eg, depression, sense of well-being, euphoria) and appetite commonly precede the acute headache by several days. Table 1 provides diagnostic criteria and characteristics of migraine with aura and without aura.
Pathophysiology
Although the mechanism of migraine is not completely understood, it is clear that vascular dysfunction alone does not adequately explain its pathophysiology. Migraine is not primarily a vascular headache, but rather it is fundamentally a brain disorder. The condition is best explained as a dysfunctional pain response to an as yet unidentified trigger that does not appear to cause tissue damage or otherwise threaten the body or brain.
In migraine, normally nonnoxious stimulation, such as light, sound, and touch, is perceived as painful. The trigeminal nerve is activated inappropriately and is a central component of activated pain pathways. Cortical spreading depression, a slow gap-junction mediated wave of depolarization causing changes in vascular and neural function, is associated with migraine aura. It is not yet understood why migraine attacks begin, and research to understand the migraine brain is ongoing.3
Diagnosis
Given the high prevalence of patients with migraine presenting to EDs and the relatively uncommon occurrence of malignant secondary headaches, migraine can often be correctly diagnosed based solely on specific historical features of the headache and/or the answers to a simple questionnaire. One of the best simple predictors of migraine is the POUNDing mnemonic:
- Pulsating headache quality;
- Duration of 4 to 72 hours;
- Unilateral pain;
- Nausea; and
- Disabling pain.
Patients with three or four of the above features can be diagnosed as having a migraine headache with high sensitivity and specificity.4 The combination of functional disability, nausea, and sensitivity to light has a high positive predictive value for a diagnosis of migraine among patients with recurrent episodes of headache.5
Having a short list of specific symptoms consistent with a diagnosis of migraine is helpful in a busy ED. These symptom checklists allow the health care provider to make a more specific diagnosis in a patient with a recurrent headache disorder. However, distinguishing among the various types of primary headache disorders prior to treatment is often unnecessary for the EP since acute migraine and tension-type headaches are likely to respond to similar treatments such as sumatriptan,6 the antiemetic dopamine antagonists,7 and parenteral ketorolac.
Treatment
The EP has a large and varied armamentarium for treating acute migraine. First-line parenteral choices include migraine-specific medications (eg, sumatriptan, dihydroergotamine), nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ketorolac), and the various antiemetic dopamine antagonists (Table 2).
Sumatriptan
Subcutaneous sumatriptan is highly effective in treating migraine quickly, with a median time to relief of 34 minutes.8 The triptans, however, are not appropriate for all patients. Due to the high rate of side effects associated with sumatriptan, including palpitations, flushing, and chest pressure, it is contraindicated in those with cardiovascular (CV) risk factors.
Although effective in relieving migraine, there is a 67% rate of headache recurrence after successful initial treatment with subcutaneous sumatriptan. Therefore, along with counseling patients about side effects of the drug, EPs should also advise them of the high probability of recurrence and discharge patients with medication in anticipation of rebound headache. Table 3 lists the currently available triptans, along with routes of administration and recommended doses.
Ergotamines and Antiemetics
In addition to sumatriptan, dihydroergotamine, administered with an antimigraine antiemetic such as prochlorperazine, is another highly effective treatment option. Since the ergotamines have vasoconstrictive and oxytocic effects on the placenta and may cause harm to the fetus, they are rated Category X. As with sumatriptan, these agents are appropriate for use in nonpregnant patients and patients who have no CV risk factors.
Nonsteroidal Anti-inflammatory Drugs
Parenteral NSAIDs may also be considered to treat acute migraine. A recent meta-analysis of ketorolac for acute migraine showed it to be as effective as meperidine and the phenothiazines and more effective than intranasal sumatriptan.9 Side-effect profiles among the drugs were similar; however, it was common for patients receiving ketorolac to require rescue medications more frequently than patients receiving alternative medications for migraine. Given these findings, it is more appropriate to use ketorolac as a second-line—rather than first-line—agent for the treatment of acute migraine.
Antiemetic Dopamine Antagonists
Antiemetic dopamine antagonists such as metoclopramide, prochlorperazine, and droperidol are effective antimigraine agents. Intravenous metoclopramide and prochlorperazine have outperformed subcutaneous sumatriptan in head-to-head trials.10-12 Each of these medications has demonstrated superiority to placebo. Hyperkinetic motor side effects, such as akathisia or abrupt onset restlessness, are common but can be prevented with anticholinergics such as diphenhydramine. Irreversible motor disturbances after one dose of these medications have never been reported and fear of this occurrence should not dissuade the EP from their use.
Occipital Nerve Block
Regional nerve blocks may be effective for some patients. Performing a greater occipital block using a combination of a long-acting local anesthetic and a corticosteroid may provide rapid and lasting relief for some migraineurs. This strategy has many proponents, though data supporting or refuting its efficacy do not exist.13
Opioids
Opioids are the class of medication used most commonly to treat migraine in US and Canadian EDs.14,15 Though highly effective for acute pain, opioids are less desirable treatment for acute migraine for the following reasons: (1) Opioids are less effective than other treatment regimens such as the antiemetic dopamine antagonists and dihydroergotamine combinations; (2) they are associated with an increased number of repeat ED visits; (3) it is difficult to send patients back to work or allow them to drive home after treatment with an opioid; and (4) opioids are associated with worsening of the underlying migraine disorder. In outpatient studies, opioids were thought to cause transformation of episodic migraine into chronic daily headaches.17
Therefore, based on the above concerns, a patient who presents with a migraine to an ED for the first time should never be administered opioids unless contraindications or lack of response to other medications leave no alternative. A patient presenting frequently and insisting on opioid treatment represents a difficult patient population—one that is often characterized by psychiatric comorbidities and concomitant medication-overuse headache. Ideally, these patients are managed not during a busy shift but by a “difficult patient” committee that can create an appropriate interdisciplinary treatment plan for the patient and enforce that plan with a patient contract.
Other Treatment Options
For patients refractory to the treatments listed above, other options with potential benefit include propofol, haloperidol, valproic acid, and magnesium—the latter being particularly effective in treating migraine with aura.
Postdischarge Treatment
Regardless of the type of treatment, most patients who present to an ED with acute migraine have a recurrence of pain within 48 hours, thus requiring outpatient therapy.18 Parenteral or oral corticosteroids decrease the frequency of headache recurrence, though the optimal dose and route of administration is not known.19 Oral naproxen sodium, sumatriptan, or a combination of both (eg, combination oral tablet or a triptan taken along with naproxen sodium) are comparably effective in treating headache recurrence postdischarge. Because the two medications performed equally well in treating headache recurrence, physicians can choose between the two based on issues related to medication contraindications, cost, and patient preference.20
Conclusion
Headache is a common presenting complaint in the ED. Once it has been determined that a patient suffers from a primary headache disorder, it is not always relevant or necessary to determine from which headache subtype a patient suffers prior to treatment because most types respond to acute treatment. Multiple regimens have been shown effective for the treatment of acute migraine. Emergency physicians can choose a therapy based on medication availability, provider comfort with the medications, and patient comorbidities.
Opioids should almost never be used as initial treatment in patients presenting with migraine to the ED for the first time because they are less effective than other medications and may worsen the underlying migraine disorder. Once the acute pain is resolved, the EP should administer corticosteroids and discharge the patient with naproxen or a triptan (or a combination therapy) in the event of rebound headache.
Dr Nerenberg is an assistant professor, department of emergency medicine, Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York. Dr Friedman is an associate professor, department of emergency medicine, Albert Einstein College of Medicine Montefiore Medical Center, Bronx, New York.
Thunderclap Headache
Jonathan A. Edlow, MD
All patients presenting with thunderclap headache, including the neurologically intact, require a thorough evaluation to distinguish between benign and serious causes.
Among the 4% of ED patients presenting with headache, only a small percentage has serious “cannot miss” causes defined as treatable problems that are life, limb, brain, or vision threatening1,2 (Table 1). Some of these patients have abrupt, severe, and unique headaches referred to as a thunderclap headache. Thunderclap headaches begin suddenly, peak within 60 seconds and can last minutes to days.3 Any patient with a new-onset headache associated with new neurological deficits should be worked up sufficiently to explain the deficit. However, many patients—even those with headaches caused by serious secondary causes—have normal neurological examinations. This article reviews the diagnosis of neurologically intact patients with thunderclap headache.
Most of the “cannot miss” conditions can present with a thunderclap headache, though some are far more common than others. In an unselected ED population of 100 patients with thunderclap headache, approximately 88% to 89% will have nonserious causes; 10% will have subarachnoid hemorrhage (SAH); and the remaining 1% to 2% of patients will have one of several rare but important conditions.
The 88% to 89% comprises primary headache syndromes—first migraine or tension-type headache or “benign thunderclap headache,” which means that the workup for serious secondary causes was unrevealing. Because history and physical examination alone cannot distinguish between patients with benign causes of thunderclap headache from those with serious causes such as SAH, all thunderclap headache patients—even those who are entirely intact neurologically—need a thorough evaluation for SAH.1-4 This is consistent with the recent clinical decision rule to identify headache patients with SAH, in which thunderclap presentation mandates a workup for SAH.5
Despite the simplicity of a workup for SAH, studies reveal that emergency physicians (EPs) miss approximately 5% of cases.6,7 For a life-threatening and highly treatable condition, missing one in 20 is problematic.
Evaluation of SAH
The time-honored evaluation for possible SAH is noncontrast brain computerized tomography (CT) followed by a lumbar puncture (LP) if the CT is normal or nondiagnostic (Figure 1). The accuracy of this paradigm approaches 100%. Computed tomography scans in patients with SAH show blood, which appears white in the acute phase, in the subarachnoid spaces (Figure 2). Sensitivity of CT decreases with time from headache onset and with size of the bleed. The brisk flow of cerebrospinal fluid (CSF), which is replaced several times per day, dilutes the blood. Modern CT scanners are only about 90% sensitive in neurologically intact patients, although it is very important to note that this study did not report the time from onset of headache.8
There are two typical locations for SAH to occur: the basal cisterns (usually aneurysmal) and over the high convexities (rarely, if ever, caused by aneurysms). The two common causes of convexal SAH are amyloid angiopathy in older patients and reversible cerebral vasoconstriction syndrome (RCVS) in younger patients.9 In some cases, CT will suggest another (non-SAH) cause of thunderclap headache. For example, although CT is not 100% sensitive for a small tumor or absces
s, it will nearly always show some abnormality (eg, edema, hydrocephalus, displacement of tissue) in a mass large enough to cause a severe headache. Computed tomography may also show nonspecific changes that warrant further imaging—eg, some cases of cerebral venous sinus thrombosis (CVST) and other conditions discussed below.
Shortly after the bleed, red blood cells appear in the CSF, but rapidly diminish in numbers over time due to CSF flow. In the hours to days that follow, xanthochromia, a yellowish discoloration, appears. This is due to degradation of hemoglobin from lysed red blood cells into bilirubin, oxyhemoglobin, and methemoglobin. Xanthochromia can be measured visually or by spectrophotometry, each of which has advantages and disadvantages.1
Almost every North American hospital laboratory uses visual inspection.10 Measuring opening pressure helps to diagnose the occasional case of pseudotumor cerebri, CVST (elevated pressure), or spontaneous intracranial hypotension (low pressure). It can also help to distinguish a traumatic LP (pressure is low) versus SAH (pressure is elevated in approximately 60%).
Is Lumbar Puncture Required After a Negative CT Within 6 Hours of Headache Onset?
It has long been recognized that CT sensitivity for SAH decreases over time and is most sensitive in the first 24 hours. Over the last few years, increasing data and expert opinion suggest that CTs performed within 6 hours of onset of classic thunderclap headache in neurologically normal patients are nearly 100% sensitive, obviating the need for LP.11-13 Although another study called these data into question, the methodology did not allow distinction between traumatic taps in patients with incidental aneurysms and true SAH and not all of their “negative” CT scans were truly negative.14
The author’s practice no longer uses LP in patients with classic thunderclap headache who are neurologically normal and whose CT scans are performed within 6 hours of headache onset and are read as negative by an attending-level radiologist. Physicians who follow this method should strictly adhere to each component of the preceding sentence. It is estimated that such an approach will miss as many as one in 600 to 1,000 patients. Because this represents a change in practice that has not yet been incorporated into any published guidelines, if a physician chooses to skip the LP in this circumstance, a discussion with the patient is warranted, the nature of which should focus on the balance of benefits (eg, LP picking up the very rare SAH in this group of patients) versus the harm (eg, side effects of the LP, which the author believes is mostly the increased number of advanced imaging due to traumatic taps and from incidental findings). This introduces the concept of “testing threshold,” an interesting and increasingly important concept for EPs to consider in diagnosing any low-frequency but high-stakes condition.15,16
Newer Diagnostic Strategies
Over the last few years, various alternative workups, including LP-first, magnetic resonance imaging (MRI) only and CT/CT angiography (CTA), have been proposed to replace the standard workup. Various authors have analyzed both the clinical and economic advantages and disadvantages of each modality.17,18
LP-First. The logic for the LP-first approach is because physicians often do not perform the LP in routine practice. A careful neurological examination is critical to ensure that this method is restricted to neurologically normal patients. Some neurologically intact patients with a lesion on CT that does not affect the CSF will be missed with this approach. In addition, if this approach is used, the opening pressure of the CSF must be measured in every case. The advantage is that it spares radiation exposure and forces an LP to be performed.
Primary MRI. The advantage of primary MRI is that current MRI sequences are as sensitive as CT for acute blood, and more sensitive for subacute and chronic blood. There is also no radiation exposure. Furthermore, depending on the sequences, which may include cerebrovascular imaging, one may be able to diagnose other rare causes of thunderclap headache beside SAH or unruptured aneurysm. It is important to note, however, that as with CT, smaller bleeds may appear negative on MRI—thus, spectrum bias exists for both modalities.1 The obvious disadvantage is cost and more importantly, availability in real time. As MRI technology and penetration into routine practice advance, this would become a very reasonable approach.
CT AND CTA. Finally, some have suggested that CT followed by CTA should be the new paradigm in order to avoid LP. These tests are easy for the EP to do and less painful to the patient, but there are unintended consequences to this strategy, including missing CSF-diagnosable conditions, diagnosing incidental aneurysms and other findings (all of which invariably lead to more imaging), and increased radiation and contrast dye exposure.19 The CT/CTA strategy does make sense in patients who cannot undergo LP (eg, patient refusal, unfavorable body habitus, anticoagulation use).
Beyond SAH
For most ED patients with thunderclap headache, CT and LP—the first two steps in the diagnostic workup—are sufficient to diagnose SAH. A meta-analysis of seven studies of neurologically normal subjects with thunderclap headache and normal CT and CSF results (813 patients) found no cases of SAH or occurrence of sudden death during at least 3 months of follow-up.20 Using the statistical worst-case scenario (upper bound of the 95% confidence interval) would be that four of 1,000 patients could have an SAH.
Dissections and CVST
Computed tomography and LP may miss some uncommon conditions associated with thunderclap headache that require some form of advanced imaging (Table 2).3,21 The more common of these include dissections and CVST, which appear to be diagnosed with increasing frequency. Both carotid and vertebral artery dissections can present with isolated head or neck pain without any neurological symptoms or signs during the highly variable phase after the intimal tear has occurred, but before downstream ischemia or infarction occurs.22 Approximately 15% of patients with CVST present with thunderclap headache, and roughly half of patients with CVST will show some abnormality on CT; however, CT venography or MR venography is necessary to confirm the diagnosis.23 These two modalities are probably equivalent in sensitivity.
Other Uncommon Conditions
There is a short list of other uncommon conditions: pituitary apoplexy, cerebellar infarction, and some vascular disorders. Patients with pituitary apoplexy (infarction of the gland usually due to bleeding into a previously undiagnosed adenoma) present with headache, symptoms of endocrine insufficiency, and visual field cuts—classically the bitemporal hemianopia due to the tumor pushing upwards on the optic chiasm.24 Some of these patients will have blood in the CSF, simulating an SAH. Dedicated CT or, preferably, MRI of the sella turcica is diagnostic. Cerebellar infarction can cause thunderclap headache and is generally accompanied by nonspecific symptoms such as vomiting and dizziness.
RCVS AND PRES
Emergency physicians should be aware of two other conditions associated with thunderclap headache: RCVS and posterior reversible encephalopathy syndrome (PRES). Reversible cerebral vasoconstriction syndrome is associated with reversible cerebral arterial spasm.25 Patients often have multiple thunderclap headaches over days to weeks, a pattern which is almost pathognomonic of RCVS. Risk factors include postpartum state, exposure to vasoactive drugs and immunosuppressive agents, catecholamine secreting tumors and others.3,25
In PRES, patients generally present with headache (thunderclap or otherwise), visual symptoms, and seizures.26 Blood pressure is usually, but not invariably, elevated in PRES, which is strongly related to hypertensive encephalopathy. There is also overlap between RCVS and PRES.27
Pregnant and Postpartum Patients
Lastly, one special circumstance merits discussion. Most headaches in pregnant and postpartum women are migraine and tension-related headaches. However EPs should have a very low threshold for advanced imaging in these patients with severe headache, who are at risk for RCVS, PRES, CVST, stroke, and low-pressure headaches.27 Some, but not all, of these conditions are eclampsia-related, and the risk if highest in late pregnancy or in the weeks afterward.
Conclusion
Despite the long differential diagnosis for thunderclap headache, most patients have primary headache disorders. As with many high-risk but low-frequency problems in EM, one must develop an organized diagnostic approach. Ideally, EPs should communicate the clinical situation to their radiology consultants to maximize the information to be acquired by imaging.28 Assuming a normal physical examination, one has to use clues in the history and epidemiological context to decide which patients to work up beyond the standard SAH evaluation.
Dr Edlow is a professor, department of medicine, Harvard Medical School; and vice-chair of emergency medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Unusual Causes of Headache
Allison Tadros MD; Joseph Minardi, MD
In evaluating patients presenting with severe headache, there are unusual and life-threatening etiologies EPs should include in the differential diagnosis.
Providers of acute care have been well educated on the red flags, work up, and treatment of life-threatening causes of headache such as intracranial bleeding and meningitis. However, there are other unusual but important headache etiologies and syndromes of which they also should be aware. For example, one of these conditions, cerebral venous sinus thrombosis (CVST), may have serious morbidity and mortality if not diagnosed and treated promptly. Another, giant cell arteritis (GCA), may lead to permanent blindness if not recognized. Other etiologies, while not quite as serious in nature, are still important to be acquainted with in order to provide relief of patient’s symptoms and appropriate referral. This article discusses the signs, symptoms, workup, and treatment of CVST; occipital neuralgia; short-lasting, unilateral, neuralgiform headache attacks with conjunctival injection and tearing (SUNCT); idiopathic intracranial hypertension (IIH); GCA; and indomethacin-responsive headache syndromes (IRHS).
Cerebral Venous Sinus Thrombosis
Cerebral venous sinus thrombosis (also referred to as cavernous venous thrombosis) represents about 1% of all strokes.1,2 While this condition is thought to be uncommon, no epidemiologic studies are currently available.2 In contrast to other forms of stroke, women and children are more commonly affected, with most patients presenting younger than the age 50 years.3,4 Thrombosis may occur in the cerebral veins, the major sinuses, or both, and lead to brain edema, venous infarction, and intracranial hypertension.1
The majority of patients with CVST have an underlying risk factor for thrombosis (eg, injury, infection). In addition, hypercoagulable states seem to be more commonly found in adults with CVST, whereas infection to the head and neck is a more common risk factor in children.1 Women in the peripartum period also seem to be particularly vulnerable.3,5
Symptoms and Diagnosis
Headache is the most common presenting symptom in patients with CVST, although focal neurologic deficits, seizures, and altered mental status may also be seen.3 The headache is usually described as worsening over days to weeks, but thunderclap headache may also be reported.2 In CVST, headache may be the only presenting symptom, making diagnosis difficult.6 A diagnosis of CVST should be considered in any patient presenting with headache in conjunction with cranial nerve 6 palsy or papilledema.
Workup of a suspected diagnosis of CVST can be challenging. A computed tomography (CT) scan of the brain with contrast may only diagnose 30% of cases.6 A noncontrast CT may be normal, but can show a hyperdensity of the dural sinus or cavernous sinus (dense triangle sign; Figure). Computed tomographic venography and magnetic resonance venography have much higher sensitivities in making the diagnosis.6 In addition to imaging studies, D-dimer elevation will be seen in most patients with this diagnosis, but a normal D-dimer may not exclude the presence of disease.4,7 Anticoagulation is considered first-line treatment even if an intracranial hemorrhage is present as a complication of the CVST.2,7 There may be a role for thrombolytic therapies in patients that clinically deteriorate despite anticoagulation.7
Occipital Neuralgia
Occipital neuralgia is characterized by a paroxysmal, lancinating pain over the area of the greater occipital nerve. It is typically associated with tenderness to palpation and may be described as a dysethesia or hypoesthesia.8,9 In occipital neuralgia, the pain originates in the suboccipital area of the head and radiates to the vertex. Although the exact etiology of the nerve irritation may be unknown, it is hypothesized that the cause could be vascular, muscular, osteogenic, or neurogenic.10 In addition to headache, patients may report blurry vision, orbital pain, nausea, dizziness, tinnitus, and nasal congestion.10 The true epidemiology of occipital neuralgia is unknown, as there are no clear diagnostic criteria or consensus on its definition.11 The pain may be spontaneous or provoked by exposure to cold or certain movements of the neck; it may be elicited by tapping on the occipital nerves (Tinel’s sign). The diagnosis is confirmed when the patient reports transient relief of pain after an occipital anesthetic block.11,12
Short-Lasting Unilateral Neuralgiform Headache Attacks With Conjunctival Injection and Tearing
Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing is a syndrome characterized by brief frequent attacks of stabbing pain in and around the eye, and associated tearing. Attacks may occur a few times a day, but often hundreds of times a day, lasting anywhere from seconds to several minutes.13 The short duration and higher frequency of attacks are useful criteria for differentiating SUNCT from cluster headache. Since SUNCT has some association with pituitary tumors, referral for outpatient magnetic resonance imaging is a reasonable recommendation.14 Small studies have shown some promise in improving symptoms with treatment with carbamazepine, gabapentin, and lamotrigine15,16 Steroids have been used with some success in temporarily aborting symptoms of this syndrome, which may be useful in treating patients presenting to the ED.17,18
Giant Cell Arteritis
Giant cell arteritis, also known as temporal arteritis or cranial arteritis, can be a cause for significant morbidity in those affected, with permanent visual loss being the most important complication. The typical presentation is new-onset focal headache in a patient older than age 50 years. The headache may be accompanied by a wide range of visual symptoms. In fact, visual symptoms with or without headache may be the presenting complaint, requiring clinician vigilance to seek out this diagnosis. Other historical features may include jaw claudication, fever, and symptoms of polymyalgia rheumatica.
The clinical presentation of polymyalgia rheumatica, which is present in up to half of patients, is one of proximal symmetric muscle and joint aches, accompanied by constitutional symptoms. Physical examination may be very nonspecific, but the temporal arteries may be tender with diminished pulses. The most useful laboratory finding is an elevated erythrocyte sedimentation rate, which is classically >50, though there have been documented cases with lower levels. An elevated C-reactive protein may be more predictive of the presence of GCA, but more research is needed in this area.19 A complete blood count may reveal a normochromic, microcytic anemia.
The pathophysiology of GCA involves inflammation of larger arteries, usually of the upper body and cranium. A wide range of vascular complications are possible, including ischemia, aneurysm, and dissection to affected vascular territories. The most common serious complication of GCA is permanent visual loss due to ischemic retinopathy. Ophthalmic involvement occurs in 26% of patients with permanent visual loss in 7% to 14%.
With respect to imaging studies, recent research has suggested that ultrasound with color Doppler techniques may be useful in diagnosing GCA.20,21 Other imaging studies may be needed based on the entire clinical presentation, specifically if involvement of other vascular territories is suspected.
Historically, the most definitive diagnostic test is temporal artery biopsy. It should be emphasized that the diagnosis of GCA is a clinical one in which biopsy, laboratory, and other clinical findings all play a supportive role in the diagnosis.
Treatment consists of high-dose corticosteroids, usually prednisone 60 to 80 mg/d initially. Treatment should not be withheld pending temporal artery biopsy as the findings remain positive for weeks. There is ongoing research into low-dose aspirin as well as other immune-modulating drugs. Ophthalmologic consultation should be sought for patients with visual symptoms and surgical follow-up is necessary for biopsy.22
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension, also known as pseudotumor cerebri or benign IIH, is a disease of abnormal elevated intracranial pressure (ICP) that typically affects overweight women of child-bearing age. The etiology is unclear. Patients present with nonspecific headaches and possible visual complaints that can range from blurring to sudden visual loss. Although IIH is rare in healthy men, those who do develop the condition have a higher risk of permanent vision loss.
The most important clinical finding is papilledema, which should be sought on all patients with a new headache as IIH may lead to progressive blindness.23 A detailed history and physical examination should be performed as well as imaging to investigate for intracranial masses and alternate etiologies of increased ICP. Once other causes are excluded, the diagnosis is established by measuring an elevated opening pressure during lumbar puncture performed with the patient in the lateral position.
Pharmacologic therapy is aimed at decreasing ICP. Acetazolamide is a mainstay of medical therapy. Surgical treatments such as optic nerve sheath fenestration and cerebrospinal fluid diversion procedures may be performed to limit visual loss. Ophthalmologic consultation should always be obtained.24
Indomethacin-Responsive Headache Syndromes
While indomethacin may be effective for many types of headaches, the hemicrania syndromes of paroxysmal hemicrania and hemicrania continua are defined by an absolute, invariable response to indomethacin therapy. In IRHS, headaches are typically unilateral, moderate to severe, last longer than other headache syndromes, and may occur in conjunction with autonomic symptoms.
Paroxysmal hemicrania consists of 5- to 30-minute episodes with pain-free periods. This syndrome, however, may progress to hemicrania continua, which is characterized by longer lasting, chronic headaches with less prominent autonomic symptoms. When considering either of these syndromes, a careful history and physical examination should be performed and other etiologies considered.24-26
Cluster Headache
Cluster headache is characterized clinically by unilateral paroxysmal pain, usually involving the upper half of the face and head. Episodes typically last between 15 to 180 minutes and can recur multiple times per day. To make the diagnosis, at least one local autonomic symptom must be present, such as forehead sweating or redness, conjunctival injection, lacrimation, or nasal congestion or drainage. Other ocular symptoms, such as miosis, ptosis, or lid swelling, may occur. Cluster headache is more common in men, and patients typically are restless or agitated. Laboratory studies and imaging are not typically necessary unless evaluation for other problems is warranted. Abortive treatment should include 100% oxygen by non-rebreather facemask, as well as a 5-HT1 receptor agonist, including metoclopramide, the triptan drugs, or ergotamine alkaloids. Corticosteroids may be effective in terminating a cluster headache cycle, and multiple medications have been used for prophylactic management. There are multiple surgical options available for refractory symptoms. Prevention should focus on a headache diary to identify triggers. Use of alcohol and tobacco products have also been shown to worsen symptoms.24
Conclusion
As patients present to the ED with a wide variety of headaches, EPs should include dangerous and secondary causes highest in the differential diagnosis. However, with a careful history and physical examination, other headache syndromes may be diagnosed with implications that can improve immediate and follow-up treatment, and, in some cases, prevent serious complications—particularly blindness and, though rare, death.
Dr Tadros is an associate professor, department of emergency medicine, West Virginia University, Morgantown. Dr Minardi is an associate professor, department of emergency medicine and medical education, West Virginia University, Morgantown.
Although the cause of severe headaches in patients presenting to the ED are typically of benign etiology, there are serious and even life-threatening causes for which the EP should retain a high index of suspicion. In the physician’s thoughts lurk the small, but real, possibility that a missed diagnosis might lead to harm or even death of the patient. The cause of the headache may not be detectable during the ED visit—even after expensive, time-consuming, and, sometimes painful, studies. Fears of being charged with negligence or malpractice may be hovering somewhere in the back of an EP’s mind. Additionally, the goals of the physician and the patient may not be aligned (eg, the patient is often more concerned with symptom relief, while the physician is typically more concerned with rapid diagnosis and diagnostic accuracy).
The patient with chronic or recurrent headache presents a special management challenge. The EP may encounter the same patient at repeat ED visits. Assessment of pain—the “fifth vital sign”—and response of interventions are frequently the subjects of review by accreditation and regulatory organizations.1 A patient with pain from recurrent headache may not be completely satisfied during a visit, and this may be reflected in the ED’s patient satisfaction scores. On the other hand, the epidemic of prescription pain medication misuse has become a nationally recognized problem, and EPs in particular are being tasked to carefully assess opioid administration and prescriptions.2 In addition, chronic opioid administration may interfere with patient response to some headache-specific medications such as triptans.3 Cautious prescribing with screening before utilizing controlled substances in patients at risk is recommended to prevent misuse and abuse.4
Literature History
Much of the literature on headaches comes from pain and headache clinics and reflects the experience from that patient population. Patients referred to such clinics often have chronic, recurrent headaches that are difficult to manage. The consultation and referral processes at these centers exclude many time-sensitive causes of headache, and extensive neuroimaging and other studies likely will have detected patients with tumors and vascular lesions. In the nomenclature of headaches, these clinics specialize in treating patients with primary headache syndromes (eg, migraine, cluster, chronic daily headache). Conversely, secondary causes of headache are infrequent in these clinics. Studies based on these patient populations conclude that serious secondary causes of headaches are uncommon, reflecting ascertainment bias in these settings.
Secondary Causes of Headache
Diagnostically, the goal of the EP must be to detect or exclude serious secondary causes of headaches (sometimes referred to as headaches with organic causes), including headaches from tumors, vascular lesions, infections, and causes of increased intracranial pressure (ICP) such as:
- Pseudotumor cerebri (benign increased ICP)
- Meningitis
- Brain abscesses
- Dissections of the cranial and cervical vessels
- Aneurysms (both ruptured and unruptured)
- Arteriovenous malformations
- Dural sinus thromboses
- Inflammatory processes
- Vasculitis
- Traumatic hematomas (both acute and chronic).
This long list represents just some of the serious etiologies of headaches. While these processes are uncommon, they are not rare in the ED population, and the EP will encounter patients with each of these conditions during his or her career—along with the challenge to rapidly detect these potential life threats against the background of the very common primary or functional headaches. Thus, the EP is clearly placed in a dilemma: He or she needs to be thorough in diagnosing and treating the headache patient quickly without missing a serious etiology. History and physical examination can never be perfect in detecting all serious headaches, and even a lengthy, thorough, and time-consuming neurological examination will not detect all serious causes.
Neuroimaging
Neuroimaging is used liberally because computed tomography (CT) and magnetic resonance imaging (MRI) continue to detect findings not expected from the sometimes insensitivity of history-taking and physical examination. However, balanced against the need for thorough evaluation are concerns for expense and unnecessary radiation exposure.
Several recent high-visibility media posts note the overutilization of neuroimaging and the lack of physician adherence to guidelines.5-7 Yet, without imaging studies proving the case prior to admission, when patients present with high-risk symptoms and abnormal physical-examination findings, reimbursement for further investigation and hospitalization may be retrospectively denied. The author recently was involved in the care of a patient who arrived by aeromedical transport in a stroke-alert scenario with abrupt onset of headache, physical findings of left-sided weakness, and medical conditions that placed the patient at high-risk for stroke. After the hospitalization was over and investigations were completed, the insurance carrier concluded:
The information received does not show that the member’s headaches were due to suspected organic causes or findings requiring hospitalization and therapeutic intervention…. Treatment of this member could be provided at a less intensive level of care or in another setting, such as a nursing facility, outpatient setting or home.... This plan does not cover services that are not medically necessary.” (Personal written communication with Aetna Health Inc, January 22, 2014.)
A recent editorial in the Annals of Emergency Medicine summarizes this issue, concluding that though it appears many CTs are being ordered unnecessarily, a low yield is necessary to avoid misdiagnoses.8 This is particularly true in the case of older adults and patients with new or severe headache. Increasingly, the EP is placed in a bind with the implication being that clinical choices will never be “just right.” He or she will continue to be retrospectively studied for performing workups without yield but criticized for failure to do extensive workups when a serious cause of headache is later found.
Approach to Patient
The tools used to evaluate a patient with headache in the ED are the same as those employed for other patient encounters. Each patient evaluation begins with an appropriate history and physical examination. Arguably, history is the most important to determine whether the patient’s headache is recurrent, a first-time headache, or the worst headache in his or her life.
Obtaining an accurate patient history can be a challenge. One provocative article noted that the order in which a patient was asked about his or her headache seemed to change responses to key questions.9 Even the time-honored “worst headache of my life” should be viewed in context of other factors. One Internet search turned up a migraine blog where the comment was made by a patient who experienced severe migraines, “I have the worst headache of my life every month.”10
Does the headache exist in isolation? The concept of the lone acute severe headache (LASH) was developed as part of a clinical decision-making study, but the acronym helps to remind the EP that headache may or may not exist in isolation.11 Altered mental status not only makes a fine neurological examination impossible but suggests possible serious etiologies of headache. For example, a headache following a new seizure would divert diagnostic attention to the cause of seizure, and the presence of fever would of course suggest a possible infectious cause.
The initial diagnostic sorting of headaches must be either a likely primary headache (migraine, tension-type, etc) or so-called secondary headache. The concept of “red flags” has been developed to alert the practitioner to historical or physical examination findings that drive decisions for additional testing.12-17 Although widely advocated in the literature, the sensitivity of a red flag or flags have never been studied in a prospective manner. While different red flags are listed in various publications, no weighting or comparison has been published to date. A guideline that used red-flag features to drive additional investigations listed the evidentiary support for the concept of red flags at the lowest level of evidence.17
Notably, some of the teachings repeated in medical training have no evidential basis and simply do not stand up when studied. An example is the so-called “neurosurgical headache,” in which the headache is worse in the morning but improves throughout the day following upright position that is thought to reduce ICP. This makes sense—until someone actually studies patients with intracranial tumors and finds that their headache characteristics most typically resemble tension-type headaches.18 Even though this study was published more than 20 years ago, the traditional teaching that the “neurosurgical headaches are worse in the morning” continues.
The Table lists some of the red-flag features that may suggest the presence of a secondary headache and could identify patients who could benefit from additional investigations or neuroimaging. This listing is provided as a summary of current recommendations and though many of these seem to be consistent with anecdotal experiences, these red flags have not been studied in an organized manner. The use of any simple list is only an aid to clinical judgment. For example, every migraine has a first onset. The diagnosis of a first hemiplegic migraine can confidently be made only with additional historical information, additional testing, or evolution of a recurrent pattern, and probably with imaging studies to rule-out other possible causes. Another example is that of benign coital headache, a diagnosis of exclusion since subarachnoid hemorrhage certainly may occur with exertion.
A careful physical examination always should be performed. Some key signs are noted in the Table. The presence or absence of papilledema has been traditionally considered a red flag. Certainly the presence of papilledema warrants evaluation, but it may be quite difficult to detect by simple inspection—even by neurologists or ophthalmologists. Other funduscopic findings may mimic papilledema, and it may not be present with acute increased ICP since it may take some time to develop. The role of bedside ultrasound in detecting papilledema in the patient with acute headache is unclear, though a few small case series suggests its utility.19
Conclusion
In spite of being diligent, performing a good history and physical examination, and providing appropriate follow-up, EPs will sometimes “miss” serious and unusual causes of headaches. Current topics in headache diagnosis and management of migraine, thunderclap, and unusual causes of headache are examined in this special feature to help the EP in the diagnostic decision-making process.
Dr Huff is a professor of emergency medicine and neurology, University of Virginia, Charlottesville.
Migraine: An Evidence-Based Update
Rebecca H. Nerenberg, MD; Benjamin W. Friedman, MD
Multiple regimens have been shown effective in treating migraine with and without aura in both the acute-care and outpatient setting.
Recurrent episodic primary headache disorders such as migraine, tension-type, and cluster headache are a common presentation to the ED, with an estimated number of 5 million patients in the United States presenting annually.1 For these headaches, it is essential that the emergency physician (EP) focus on rapid and effective treatment, while minimizing side effects and expediting a return to work and usual activities.
Emergency physicians are adept at ruling-out serious secondary headaches (eg, bacterial meningitis, aneurysmal subarachnoid hemorrhage) and identifying benign secondary causes (eg, acute rhinosinusitis, cervicogenic headache). Migraine, the focus of this review, is the primary headache disorder that most commonly results in an ED visit.
Symptoms
Though presentations may be quite varied, migraine typically presents as a pulsating, unilateral headache, and is associated with nausea, vomiting, photophobia, phonophobia, and osmophobia.2 The condition is often described as severe in intensity and functionally impairing—characteristics that contribute to so many ED visits. Migraine, however, also may present bilaterally and be associated with muscle pain or spasms.
It is the constellation of symptoms—rather than any one symptom in particular—that leads to the correct diagnosis. Classically, the prodromal aura, which is reported by fewer than 20% of patients, precedes the onset of headache pain by no more than 1 hour and resolves by the time the headache begins. Many migraine patients, however, report visual or sensory disturbances during the headache phase itself. Other prodromal symptoms such as change in mood (eg, depression, sense of well-being, euphoria) and appetite commonly precede the acute headache by several days. Table 1 provides diagnostic criteria and characteristics of migraine with aura and without aura.
Pathophysiology
Although the mechanism of migraine is not completely understood, it is clear that vascular dysfunction alone does not adequately explain its pathophysiology. Migraine is not primarily a vascular headache, but rather it is fundamentally a brain disorder. The condition is best explained as a dysfunctional pain response to an as yet unidentified trigger that does not appear to cause tissue damage or otherwise threaten the body or brain.
In migraine, normally nonnoxious stimulation, such as light, sound, and touch, is perceived as painful. The trigeminal nerve is activated inappropriately and is a central component of activated pain pathways. Cortical spreading depression, a slow gap-junction mediated wave of depolarization causing changes in vascular and neural function, is associated with migraine aura. It is not yet understood why migraine attacks begin, and research to understand the migraine brain is ongoing.3
Diagnosis
Given the high prevalence of patients with migraine presenting to EDs and the relatively uncommon occurrence of malignant secondary headaches, migraine can often be correctly diagnosed based solely on specific historical features of the headache and/or the answers to a simple questionnaire. One of the best simple predictors of migraine is the POUNDing mnemonic:
- Pulsating headache quality;
- Duration of 4 to 72 hours;
- Unilateral pain;
- Nausea; and
- Disabling pain.
Patients with three or four of the above features can be diagnosed as having a migraine headache with high sensitivity and specificity.4 The combination of functional disability, nausea, and sensitivity to light has a high positive predictive value for a diagnosis of migraine among patients with recurrent episodes of headache.5
Having a short list of specific symptoms consistent with a diagnosis of migraine is helpful in a busy ED. These symptom checklists allow the health care provider to make a more specific diagnosis in a patient with a recurrent headache disorder. However, distinguishing among the various types of primary headache disorders prior to treatment is often unnecessary for the EP since acute migraine and tension-type headaches are likely to respond to similar treatments such as sumatriptan,6 the antiemetic dopamine antagonists,7 and parenteral ketorolac.
Treatment
The EP has a large and varied armamentarium for treating acute migraine. First-line parenteral choices include migraine-specific medications (eg, sumatriptan, dihydroergotamine), nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ketorolac), and the various antiemetic dopamine antagonists (Table 2).
Sumatriptan
Subcutaneous sumatriptan is highly effective in treating migraine quickly, with a median time to relief of 34 minutes.8 The triptans, however, are not appropriate for all patients. Due to the high rate of side effects associated with sumatriptan, including palpitations, flushing, and chest pressure, it is contraindicated in those with cardiovascular (CV) risk factors.
Although effective in relieving migraine, there is a 67% rate of headache recurrence after successful initial treatment with subcutaneous sumatriptan. Therefore, along with counseling patients about side effects of the drug, EPs should also advise them of the high probability of recurrence and discharge patients with medication in anticipation of rebound headache. Table 3 lists the currently available triptans, along with routes of administration and recommended doses.
Ergotamines and Antiemetics
In addition to sumatriptan, dihydroergotamine, administered with an antimigraine antiemetic such as prochlorperazine, is another highly effective treatment option. Since the ergotamines have vasoconstrictive and oxytocic effects on the placenta and may cause harm to the fetus, they are rated Category X. As with sumatriptan, these agents are appropriate for use in nonpregnant patients and patients who have no CV risk factors.
Nonsteroidal Anti-inflammatory Drugs
Parenteral NSAIDs may also be considered to treat acute migraine. A recent meta-analysis of ketorolac for acute migraine showed it to be as effective as meperidine and the phenothiazines and more effective than intranasal sumatriptan.9 Side-effect profiles among the drugs were similar; however, it was common for patients receiving ketorolac to require rescue medications more frequently than patients receiving alternative medications for migraine. Given these findings, it is more appropriate to use ketorolac as a second-line—rather than first-line—agent for the treatment of acute migraine.
Antiemetic Dopamine Antagonists
Antiemetic dopamine antagonists such as metoclopramide, prochlorperazine, and droperidol are effective antimigraine agents. Intravenous metoclopramide and prochlorperazine have outperformed subcutaneous sumatriptan in head-to-head trials.10-12 Each of these medications has demonstrated superiority to placebo. Hyperkinetic motor side effects, such as akathisia or abrupt onset restlessness, are common but can be prevented with anticholinergics such as diphenhydramine. Irreversible motor disturbances after one dose of these medications have never been reported and fear of this occurrence should not dissuade the EP from their use.
Occipital Nerve Block
Regional nerve blocks may be effective for some patients. Performing a greater occipital block using a combination of a long-acting local anesthetic and a corticosteroid may provide rapid and lasting relief for some migraineurs. This strategy has many proponents, though data supporting or refuting its efficacy do not exist.13
Opioids
Opioids are the class of medication used most commonly to treat migraine in US and Canadian EDs.14,15 Though highly effective for acute pain, opioids are less desirable treatment for acute migraine for the following reasons: (1) Opioids are less effective than other treatment regimens such as the antiemetic dopamine antagonists and dihydroergotamine combinations; (2) they are associated with an increased number of repeat ED visits; (3) it is difficult to send patients back to work or allow them to drive home after treatment with an opioid; and (4) opioids are associated with worsening of the underlying migraine disorder. In outpatient studies, opioids were thought to cause transformation of episodic migraine into chronic daily headaches.17
Therefore, based on the above concerns, a patient who presents with a migraine to an ED for the first time should never be administered opioids unless contraindications or lack of response to other medications leave no alternative. A patient presenting frequently and insisting on opioid treatment represents a difficult patient population—one that is often characterized by psychiatric comorbidities and concomitant medication-overuse headache. Ideally, these patients are managed not during a busy shift but by a “difficult patient” committee that can create an appropriate interdisciplinary treatment plan for the patient and enforce that plan with a patient contract.
Other Treatment Options
For patients refractory to the treatments listed above, other options with potential benefit include propofol, haloperidol, valproic acid, and magnesium—the latter being particularly effective in treating migraine with aura.
Postdischarge Treatment
Regardless of the type of treatment, most patients who present to an ED with acute migraine have a recurrence of pain within 48 hours, thus requiring outpatient therapy.18 Parenteral or oral corticosteroids decrease the frequency of headache recurrence, though the optimal dose and route of administration is not known.19 Oral naproxen sodium, sumatriptan, or a combination of both (eg, combination oral tablet or a triptan taken along with naproxen sodium) are comparably effective in treating headache recurrence postdischarge. Because the two medications performed equally well in treating headache recurrence, physicians can choose between the two based on issues related to medication contraindications, cost, and patient preference.20
Conclusion
Headache is a common presenting complaint in the ED. Once it has been determined that a patient suffers from a primary headache disorder, it is not always relevant or necessary to determine from which headache subtype a patient suffers prior to treatment because most types respond to acute treatment. Multiple regimens have been shown effective for the treatment of acute migraine. Emergency physicians can choose a therapy based on medication availability, provider comfort with the medications, and patient comorbidities.
Opioids should almost never be used as initial treatment in patients presenting with migraine to the ED for the first time because they are less effective than other medications and may worsen the underlying migraine disorder. Once the acute pain is resolved, the EP should administer corticosteroids and discharge the patient with naproxen or a triptan (or a combination therapy) in the event of rebound headache.
Dr Nerenberg is an assistant professor, department of emergency medicine, Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York. Dr Friedman is an associate professor, department of emergency medicine, Albert Einstein College of Medicine Montefiore Medical Center, Bronx, New York.
Thunderclap Headache
Jonathan A. Edlow, MD
All patients presenting with thunderclap headache, including the neurologically intact, require a thorough evaluation to distinguish between benign and serious causes.
Among the 4% of ED patients presenting with headache, only a small percentage has serious “cannot miss” causes defined as treatable problems that are life, limb, brain, or vision threatening1,2 (Table 1). Some of these patients have abrupt, severe, and unique headaches referred to as a thunderclap headache. Thunderclap headaches begin suddenly, peak within 60 seconds and can last minutes to days.3 Any patient with a new-onset headache associated with new neurological deficits should be worked up sufficiently to explain the deficit. However, many patients—even those with headaches caused by serious secondary causes—have normal neurological examinations. This article reviews the diagnosis of neurologically intact patients with thunderclap headache.
Most of the “cannot miss” conditions can present with a thunderclap headache, though some are far more common than others. In an unselected ED population of 100 patients with thunderclap headache, approximately 88% to 89% will have nonserious causes; 10% will have subarachnoid hemorrhage (SAH); and the remaining 1% to 2% of patients will have one of several rare but important conditions.
The 88% to 89% comprises primary headache syndromes—first migraine or tension-type headache or “benign thunderclap headache,” which means that the workup for serious secondary causes was unrevealing. Because history and physical examination alone cannot distinguish between patients with benign causes of thunderclap headache from those with serious causes such as SAH, all thunderclap headache patients—even those who are entirely intact neurologically—need a thorough evaluation for SAH.1-4 This is consistent with the recent clinical decision rule to identify headache patients with SAH, in which thunderclap presentation mandates a workup for SAH.5
Despite the simplicity of a workup for SAH, studies reveal that emergency physicians (EPs) miss approximately 5% of cases.6,7 For a life-threatening and highly treatable condition, missing one in 20 is problematic.
Evaluation of SAH
The time-honored evaluation for possible SAH is noncontrast brain computerized tomography (CT) followed by a lumbar puncture (LP) if the CT is normal or nondiagnostic (Figure 1). The accuracy of this paradigm approaches 100%. Computed tomography scans in patients with SAH show blood, which appears white in the acute phase, in the subarachnoid spaces (Figure 2). Sensitivity of CT decreases with time from headache onset and with size of the bleed. The brisk flow of cerebrospinal fluid (CSF), which is replaced several times per day, dilutes the blood. Modern CT scanners are only about 90% sensitive in neurologically intact patients, although it is very important to note that this study did not report the time from onset of headache.8
There are two typical locations for SAH to occur: the basal cisterns (usually aneurysmal) and over the high convexities (rarely, if ever, caused by aneurysms). The two common causes of convexal SAH are amyloid angiopathy in older patients and reversible cerebral vasoconstriction syndrome (RCVS) in younger patients.9 In some cases, CT will suggest another (non-SAH) cause of thunderclap headache. For example, although CT is not 100% sensitive for a small tumor or absces
s, it will nearly always show some abnormality (eg, edema, hydrocephalus, displacement of tissue) in a mass large enough to cause a severe headache. Computed tomography may also show nonspecific changes that warrant further imaging—eg, some cases of cerebral venous sinus thrombosis (CVST) and other conditions discussed below.
Shortly after the bleed, red blood cells appear in the CSF, but rapidly diminish in numbers over time due to CSF flow. In the hours to days that follow, xanthochromia, a yellowish discoloration, appears. This is due to degradation of hemoglobin from lysed red blood cells into bilirubin, oxyhemoglobin, and methemoglobin. Xanthochromia can be measured visually or by spectrophotometry, each of which has advantages and disadvantages.1
Almost every North American hospital laboratory uses visual inspection.10 Measuring opening pressure helps to diagnose the occasional case of pseudotumor cerebri, CVST (elevated pressure), or spontaneous intracranial hypotension (low pressure). It can also help to distinguish a traumatic LP (pressure is low) versus SAH (pressure is elevated in approximately 60%).
Is Lumbar Puncture Required After a Negative CT Within 6 Hours of Headache Onset?
It has long been recognized that CT sensitivity for SAH decreases over time and is most sensitive in the first 24 hours. Over the last few years, increasing data and expert opinion suggest that CTs performed within 6 hours of onset of classic thunderclap headache in neurologically normal patients are nearly 100% sensitive, obviating the need for LP.11-13 Although another study called these data into question, the methodology did not allow distinction between traumatic taps in patients with incidental aneurysms and true SAH and not all of their “negative” CT scans were truly negative.14
The author’s practice no longer uses LP in patients with classic thunderclap headache who are neurologically normal and whose CT scans are performed within 6 hours of headache onset and are read as negative by an attending-level radiologist. Physicians who follow this method should strictly adhere to each component of the preceding sentence. It is estimated that such an approach will miss as many as one in 600 to 1,000 patients. Because this represents a change in practice that has not yet been incorporated into any published guidelines, if a physician chooses to skip the LP in this circumstance, a discussion with the patient is warranted, the nature of which should focus on the balance of benefits (eg, LP picking up the very rare SAH in this group of patients) versus the harm (eg, side effects of the LP, which the author believes is mostly the increased number of advanced imaging due to traumatic taps and from incidental findings). This introduces the concept of “testing threshold,” an interesting and increasingly important concept for EPs to consider in diagnosing any low-frequency but high-stakes condition.15,16
Newer Diagnostic Strategies
Over the last few years, various alternative workups, including LP-first, magnetic resonance imaging (MRI) only and CT/CT angiography (CTA), have been proposed to replace the standard workup. Various authors have analyzed both the clinical and economic advantages and disadvantages of each modality.17,18
LP-First. The logic for the LP-first approach is because physicians often do not perform the LP in routine practice. A careful neurological examination is critical to ensure that this method is restricted to neurologically normal patients. Some neurologically intact patients with a lesion on CT that does not affect the CSF will be missed with this approach. In addition, if this approach is used, the opening pressure of the CSF must be measured in every case. The advantage is that it spares radiation exposure and forces an LP to be performed.
Primary MRI. The advantage of primary MRI is that current MRI sequences are as sensitive as CT for acute blood, and more sensitive for subacute and chronic blood. There is also no radiation exposure. Furthermore, depending on the sequences, which may include cerebrovascular imaging, one may be able to diagnose other rare causes of thunderclap headache beside SAH or unruptured aneurysm. It is important to note, however, that as with CT, smaller bleeds may appear negative on MRI—thus, spectrum bias exists for both modalities.1 The obvious disadvantage is cost and more importantly, availability in real time. As MRI technology and penetration into routine practice advance, this would become a very reasonable approach.
CT AND CTA. Finally, some have suggested that CT followed by CTA should be the new paradigm in order to avoid LP. These tests are easy for the EP to do and less painful to the patient, but there are unintended consequences to this strategy, including missing CSF-diagnosable conditions, diagnosing incidental aneurysms and other findings (all of which invariably lead to more imaging), and increased radiation and contrast dye exposure.19 The CT/CTA strategy does make sense in patients who cannot undergo LP (eg, patient refusal, unfavorable body habitus, anticoagulation use).
Beyond SAH
For most ED patients with thunderclap headache, CT and LP—the first two steps in the diagnostic workup—are sufficient to diagnose SAH. A meta-analysis of seven studies of neurologically normal subjects with thunderclap headache and normal CT and CSF results (813 patients) found no cases of SAH or occurrence of sudden death during at least 3 months of follow-up.20 Using the statistical worst-case scenario (upper bound of the 95% confidence interval) would be that four of 1,000 patients could have an SAH.
Dissections and CVST
Computed tomography and LP may miss some uncommon conditions associated with thunderclap headache that require some form of advanced imaging (Table 2).3,21 The more common of these include dissections and CVST, which appear to be diagnosed with increasing frequency. Both carotid and vertebral artery dissections can present with isolated head or neck pain without any neurological symptoms or signs during the highly variable phase after the intimal tear has occurred, but before downstream ischemia or infarction occurs.22 Approximately 15% of patients with CVST present with thunderclap headache, and roughly half of patients with CVST will show some abnormality on CT; however, CT venography or MR venography is necessary to confirm the diagnosis.23 These two modalities are probably equivalent in sensitivity.
Other Uncommon Conditions
There is a short list of other uncommon conditions: pituitary apoplexy, cerebellar infarction, and some vascular disorders. Patients with pituitary apoplexy (infarction of the gland usually due to bleeding into a previously undiagnosed adenoma) present with headache, symptoms of endocrine insufficiency, and visual field cuts—classically the bitemporal hemianopia due to the tumor pushing upwards on the optic chiasm.24 Some of these patients will have blood in the CSF, simulating an SAH. Dedicated CT or, preferably, MRI of the sella turcica is diagnostic. Cerebellar infarction can cause thunderclap headache and is generally accompanied by nonspecific symptoms such as vomiting and dizziness.
RCVS AND PRES
Emergency physicians should be aware of two other conditions associated with thunderclap headache: RCVS and posterior reversible encephalopathy syndrome (PRES). Reversible cerebral vasoconstriction syndrome is associated with reversible cerebral arterial spasm.25 Patients often have multiple thunderclap headaches over days to weeks, a pattern which is almost pathognomonic of RCVS. Risk factors include postpartum state, exposure to vasoactive drugs and immunosuppressive agents, catecholamine secreting tumors and others.3,25
In PRES, patients generally present with headache (thunderclap or otherwise), visual symptoms, and seizures.26 Blood pressure is usually, but not invariably, elevated in PRES, which is strongly related to hypertensive encephalopathy. There is also overlap between RCVS and PRES.27
Pregnant and Postpartum Patients
Lastly, one special circumstance merits discussion. Most headaches in pregnant and postpartum women are migraine and tension-related headaches. However EPs should have a very low threshold for advanced imaging in these patients with severe headache, who are at risk for RCVS, PRES, CVST, stroke, and low-pressure headaches.27 Some, but not all, of these conditions are eclampsia-related, and the risk if highest in late pregnancy or in the weeks afterward.
Conclusion
Despite the long differential diagnosis for thunderclap headache, most patients have primary headache disorders. As with many high-risk but low-frequency problems in EM, one must develop an organized diagnostic approach. Ideally, EPs should communicate the clinical situation to their radiology consultants to maximize the information to be acquired by imaging.28 Assuming a normal physical examination, one has to use clues in the history and epidemiological context to decide which patients to work up beyond the standard SAH evaluation.
Dr Edlow is a professor, department of medicine, Harvard Medical School; and vice-chair of emergency medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Unusual Causes of Headache
Allison Tadros MD; Joseph Minardi, MD
In evaluating patients presenting with severe headache, there are unusual and life-threatening etiologies EPs should include in the differential diagnosis.
Providers of acute care have been well educated on the red flags, work up, and treatment of life-threatening causes of headache such as intracranial bleeding and meningitis. However, there are other unusual but important headache etiologies and syndromes of which they also should be aware. For example, one of these conditions, cerebral venous sinus thrombosis (CVST), may have serious morbidity and mortality if not diagnosed and treated promptly. Another, giant cell arteritis (GCA), may lead to permanent blindness if not recognized. Other etiologies, while not quite as serious in nature, are still important to be acquainted with in order to provide relief of patient’s symptoms and appropriate referral. This article discusses the signs, symptoms, workup, and treatment of CVST; occipital neuralgia; short-lasting, unilateral, neuralgiform headache attacks with conjunctival injection and tearing (SUNCT); idiopathic intracranial hypertension (IIH); GCA; and indomethacin-responsive headache syndromes (IRHS).
Cerebral Venous Sinus Thrombosis
Cerebral venous sinus thrombosis (also referred to as cavernous venous thrombosis) represents about 1% of all strokes.1,2 While this condition is thought to be uncommon, no epidemiologic studies are currently available.2 In contrast to other forms of stroke, women and children are more commonly affected, with most patients presenting younger than the age 50 years.3,4 Thrombosis may occur in the cerebral veins, the major sinuses, or both, and lead to brain edema, venous infarction, and intracranial hypertension.1
The majority of patients with CVST have an underlying risk factor for thrombosis (eg, injury, infection). In addition, hypercoagulable states seem to be more commonly found in adults with CVST, whereas infection to the head and neck is a more common risk factor in children.1 Women in the peripartum period also seem to be particularly vulnerable.3,5
Symptoms and Diagnosis
Headache is the most common presenting symptom in patients with CVST, although focal neurologic deficits, seizures, and altered mental status may also be seen.3 The headache is usually described as worsening over days to weeks, but thunderclap headache may also be reported.2 In CVST, headache may be the only presenting symptom, making diagnosis difficult.6 A diagnosis of CVST should be considered in any patient presenting with headache in conjunction with cranial nerve 6 palsy or papilledema.
Workup of a suspected diagnosis of CVST can be challenging. A computed tomography (CT) scan of the brain with contrast may only diagnose 30% of cases.6 A noncontrast CT may be normal, but can show a hyperdensity of the dural sinus or cavernous sinus (dense triangle sign; Figure). Computed tomographic venography and magnetic resonance venography have much higher sensitivities in making the diagnosis.6 In addition to imaging studies, D-dimer elevation will be seen in most patients with this diagnosis, but a normal D-dimer may not exclude the presence of disease.4,7 Anticoagulation is considered first-line treatment even if an intracranial hemorrhage is present as a complication of the CVST.2,7 There may be a role for thrombolytic therapies in patients that clinically deteriorate despite anticoagulation.7
Occipital Neuralgia
Occipital neuralgia is characterized by a paroxysmal, lancinating pain over the area of the greater occipital nerve. It is typically associated with tenderness to palpation and may be described as a dysethesia or hypoesthesia.8,9 In occipital neuralgia, the pain originates in the suboccipital area of the head and radiates to the vertex. Although the exact etiology of the nerve irritation may be unknown, it is hypothesized that the cause could be vascular, muscular, osteogenic, or neurogenic.10 In addition to headache, patients may report blurry vision, orbital pain, nausea, dizziness, tinnitus, and nasal congestion.10 The true epidemiology of occipital neuralgia is unknown, as there are no clear diagnostic criteria or consensus on its definition.11 The pain may be spontaneous or provoked by exposure to cold or certain movements of the neck; it may be elicited by tapping on the occipital nerves (Tinel’s sign). The diagnosis is confirmed when the patient reports transient relief of pain after an occipital anesthetic block.11,12
Short-Lasting Unilateral Neuralgiform Headache Attacks With Conjunctival Injection and Tearing
Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing is a syndrome characterized by brief frequent attacks of stabbing pain in and around the eye, and associated tearing. Attacks may occur a few times a day, but often hundreds of times a day, lasting anywhere from seconds to several minutes.13 The short duration and higher frequency of attacks are useful criteria for differentiating SUNCT from cluster headache. Since SUNCT has some association with pituitary tumors, referral for outpatient magnetic resonance imaging is a reasonable recommendation.14 Small studies have shown some promise in improving symptoms with treatment with carbamazepine, gabapentin, and lamotrigine15,16 Steroids have been used with some success in temporarily aborting symptoms of this syndrome, which may be useful in treating patients presenting to the ED.17,18
Giant Cell Arteritis
Giant cell arteritis, also known as temporal arteritis or cranial arteritis, can be a cause for significant morbidity in those affected, with permanent visual loss being the most important complication. The typical presentation is new-onset focal headache in a patient older than age 50 years. The headache may be accompanied by a wide range of visual symptoms. In fact, visual symptoms with or without headache may be the presenting complaint, requiring clinician vigilance to seek out this diagnosis. Other historical features may include jaw claudication, fever, and symptoms of polymyalgia rheumatica.
The clinical presentation of polymyalgia rheumatica, which is present in up to half of patients, is one of proximal symmetric muscle and joint aches, accompanied by constitutional symptoms. Physical examination may be very nonspecific, but the temporal arteries may be tender with diminished pulses. The most useful laboratory finding is an elevated erythrocyte sedimentation rate, which is classically >50, though there have been documented cases with lower levels. An elevated C-reactive protein may be more predictive of the presence of GCA, but more research is needed in this area.19 A complete blood count may reveal a normochromic, microcytic anemia.
The pathophysiology of GCA involves inflammation of larger arteries, usually of the upper body and cranium. A wide range of vascular complications are possible, including ischemia, aneurysm, and dissection to affected vascular territories. The most common serious complication of GCA is permanent visual loss due to ischemic retinopathy. Ophthalmic involvement occurs in 26% of patients with permanent visual loss in 7% to 14%.
With respect to imaging studies, recent research has suggested that ultrasound with color Doppler techniques may be useful in diagnosing GCA.20,21 Other imaging studies may be needed based on the entire clinical presentation, specifically if involvement of other vascular territories is suspected.
Historically, the most definitive diagnostic test is temporal artery biopsy. It should be emphasized that the diagnosis of GCA is a clinical one in which biopsy, laboratory, and other clinical findings all play a supportive role in the diagnosis.
Treatment consists of high-dose corticosteroids, usually prednisone 60 to 80 mg/d initially. Treatment should not be withheld pending temporal artery biopsy as the findings remain positive for weeks. There is ongoing research into low-dose aspirin as well as other immune-modulating drugs. Ophthalmologic consultation should be sought for patients with visual symptoms and surgical follow-up is necessary for biopsy.22
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension, also known as pseudotumor cerebri or benign IIH, is a disease of abnormal elevated intracranial pressure (ICP) that typically affects overweight women of child-bearing age. The etiology is unclear. Patients present with nonspecific headaches and possible visual complaints that can range from blurring to sudden visual loss. Although IIH is rare in healthy men, those who do develop the condition have a higher risk of permanent vision loss.
The most important clinical finding is papilledema, which should be sought on all patients with a new headache as IIH may lead to progressive blindness.23 A detailed history and physical examination should be performed as well as imaging to investigate for intracranial masses and alternate etiologies of increased ICP. Once other causes are excluded, the diagnosis is established by measuring an elevated opening pressure during lumbar puncture performed with the patient in the lateral position.
Pharmacologic therapy is aimed at decreasing ICP. Acetazolamide is a mainstay of medical therapy. Surgical treatments such as optic nerve sheath fenestration and cerebrospinal fluid diversion procedures may be performed to limit visual loss. Ophthalmologic consultation should always be obtained.24
Indomethacin-Responsive Headache Syndromes
While indomethacin may be effective for many types of headaches, the hemicrania syndromes of paroxysmal hemicrania and hemicrania continua are defined by an absolute, invariable response to indomethacin therapy. In IRHS, headaches are typically unilateral, moderate to severe, last longer than other headache syndromes, and may occur in conjunction with autonomic symptoms.
Paroxysmal hemicrania consists of 5- to 30-minute episodes with pain-free periods. This syndrome, however, may progress to hemicrania continua, which is characterized by longer lasting, chronic headaches with less prominent autonomic symptoms. When considering either of these syndromes, a careful history and physical examination should be performed and other etiologies considered.24-26
Cluster Headache
Cluster headache is characterized clinically by unilateral paroxysmal pain, usually involving the upper half of the face and head. Episodes typically last between 15 to 180 minutes and can recur multiple times per day. To make the diagnosis, at least one local autonomic symptom must be present, such as forehead sweating or redness, conjunctival injection, lacrimation, or nasal congestion or drainage. Other ocular symptoms, such as miosis, ptosis, or lid swelling, may occur. Cluster headache is more common in men, and patients typically are restless or agitated. Laboratory studies and imaging are not typically necessary unless evaluation for other problems is warranted. Abortive treatment should include 100% oxygen by non-rebreather facemask, as well as a 5-HT1 receptor agonist, including metoclopramide, the triptan drugs, or ergotamine alkaloids. Corticosteroids may be effective in terminating a cluster headache cycle, and multiple medications have been used for prophylactic management. There are multiple surgical options available for refractory symptoms. Prevention should focus on a headache diary to identify triggers. Use of alcohol and tobacco products have also been shown to worsen symptoms.24
Conclusion
As patients present to the ED with a wide variety of headaches, EPs should include dangerous and secondary causes highest in the differential diagnosis. However, with a careful history and physical examination, other headache syndromes may be diagnosed with implications that can improve immediate and follow-up treatment, and, in some cases, prevent serious complications—particularly blindness and, though rare, death.
Dr Tadros is an associate professor, department of emergency medicine, West Virginia University, Morgantown. Dr Minardi is an associate professor, department of emergency medicine and medical education, West Virginia University, Morgantown.
- Headache
- Fernandes CL. The fifth vital sign. Fed Pract. 2010;27(10):26-28.
- Cantrill SV, Brown MD, Carlisle RJ et al; American College of Emergency Physicians Opioid Guideline Writing Panel. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525.
- Ho TW, Rodgers A, Bigal ME. Impact of recent prior opioid use on rizatriptan efficacy. A post hoc pooled analysis. Headache. 2009;49(3):395-403.
- Pylkas AM, Bart G. Prescribing controlled substances during a prescription drug epidemic. Neurol Clin Pract. 2014;4(2):99-105.
- Emergency Medicine Today. March 18, 2014. Vast majority of brain scans for headache patients may be unnecessary. http://acep.bulletinhealthcare.com/story.aspx?dt=140318. Accessed June 24, 2014.
- Shute N. Yes, It’s a headache. No, you don’t need a brain scan. NPR Shots. Your Health Web site. March 18, 2014. http://www.npr.org/blogs/health/2014/03/18/291044766/yes-its-a-headache-no-you-dont-need-a-brain-scan. Accessed June 18, 2014.
- Callaghan BC, Kerber KA, Pace RJ, Skolarus LE, Burke JF. Headaches and neuroimaging: high utilization and costs despite guidelines. JAMA Intern Med. 2014;174(5):819-821.
- Schwartz DT. US emergency physicians order too many computed tomography scans-or do they? Ann Emerg Med. 2013;62(5):495-497.
- Diaz M, Braude D, Skipper B. Concordance of historical questions used in risk-stratifying patients with headache. Am J Emerg Med. 2007;25(8):
907-910. - Worst headache of my life [List-serve comment]. mymigraineconnection.com. [currently inactive]
- Schull MJ. Lumbar puncture first: an alternative model for the investigation of lone acute sudden headache. Acad Emerg Med.1999;6(2):131-136.
- Clinch CR. Evaluation of acute headaches in adults. Am Fam Physician. 2001;63(4):685-692.
- Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol. 2003;76(908):532-535.
- Parizel PM, Voormolen M, Van Goethem JW, van den Hauwe L. Headache: when is neuroimaging needed? JBR-BTR. 2007;90(4):268-271.
- De Luca GC, Bartleson JD. When and how to investigate the patient with headache. Semin Neurol. 2010;30(2):131-144.
- Cady RK. Red flags and comfort signs for ominous secondary headaches. Otolaryngol Clin North Am. 2014;47(2):289-299.
- Duncan CW, Watson DP, Stein A; Guideline Development Group. Diagnosis and management of headache in adults: summary of SIGN guideline. BMJ. 2008;a2329.
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43(9):1678-1683.
- Daulaire S, Fine L, Salmon M, et al. Ultrasound assessment of optic disc edema in patients with headache. Am J Emerg Med. 2012;30(8):1654.e1-4.
- Migraine: An Evidence-Based Update
- Vinson DR. Treatment patterns of isolated benign headache in US emergency departments. Ann Emerg Med. 2002;39(3):215-222.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
- Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161(2):327-341.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296(10):1274-1283.
- Lipton RB, Dodick D, Sadovsky R, et al; ID Migraine Validation Study. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61(3):375-382.
- Miner JR, Smith SW, Moore J, Biros M. Sumatriptan for the treatment of undifferentiated primary headaches in the ED. Am J Emerg Med. 2007;25(1):60-64.
- Weinman D, Nicastro O, Akala O, Friedman BW. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
- Akpunonu BE, Mutgi AB, Federman DJ, et al. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: a multicenter study. Ann Emerg Med. 1995;25(4):464-269.
- Taggart E, Doran S, Kokotillo A, Campbell S, Villa-Roel C, Rowe BH. Ketorolac in the treatment of acute migraine: a systematic review. Headache. 2013;53(2):277-287.
- Friedman BW, Corbo J, Lipton RB, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology. 2005;64(3):463-468.
- Talabi S, Masoumi B, Azizkhani R, Esmailian M. Metoclopramide versus sumatriptan for treatment of migraine headache: A randomized clinical trial. J Res Med Sci. 2013;18(8):695-698.
- Kostic MA, Gutierrez FJ, Rieg TS, Moore TS, Gendron RT. A prospective, randomized trial of intravenous prochlorperazine versus subcutaneous sumatriptan in acute migraine therapy in the emergency department. Ann Emerg Med. 2010;56(1):1-6.
- Ashkenazi A, Levin M. Greater occipital nerve block for migraine and other headaches: is it useful? Curr Pain Headache Rep. 2007;11(3):231-235.
- Colman I, Rothney A, Wright SC, Zilkalns B, Rowe BH. Use of narcotic analgesics in the emergency department treatment of migraine headache. Neurology 2004;62(10):1695-1700.
- Vinson DR, Hurtado TR, Vandenberg JT, Banwart L. Variations among emergency departments in the treatment of benign headache. Ann Emerg Med. 2003;41(1):90-97.
- Friedman BW, Kapoor A, Friedman MS, Hochberg ML, Rowe BH. The relative efficacy of meperidine for the treatment of acute migraine: a meta-analysis of randomized controlled trials. Ann Emerg Med. 2008;52(6):705-713.
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168.
- Friedman BW, Hochberg ML, Esses D, et al. Recurrence of primary headache disorders after emergency department discharge: frequency and predictors of poor pain and functional outcomes. Ann Emerg Med. 2008;52(6):696-704.
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ. 2008;336(7657):1359-1361.
- Friedman BW, Solorzano C, Esses D, et al. Treating headache recurrence after emergency department discharge: a randomized controlled trial of naproxen versus sumatriptan. Ann Emerg Med. 2010;56(1):7-17.
: Thunderclap Headache
- Edlow JA, Malek AM, Ogilvy CS. Aneurysmal subarachnoid hemorrhage: update for emergency physicians. J Emerg Med. 2008;34(3):237-251.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med. 2008;52(4):407-436.
- Ducros A, Bousser MG. Thunderclap headache. BMJ. 2013;346:e8557.
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage [see comments]. N Engl J Med. 2000;342(1):29-36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA. 2013;310(12):1248-1255.
- Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291(7):866-869.
- Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke. 2007;38(4):1216-1221.
- Byyny RL, Mower WR, Shum N, Gabayan GZ, Fang S, Baraff LJ. Sensitivity of noncontrast cranial computed tomography for the emergency department diagnosis of subarachnoid hemorrhage. Ann Emerg Med. 2008;51(6) 697-703.
- Kumar S, Goddeau RP, Jr, Selim MH, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology 2010;74(11):893-899.
- Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Arch Pathol Lab Med. 2002;126(4):413-415.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke. 2012;43(8):2115-2119.
- Edlow JA, Fisher J. Diagnosis of subarachnoid hemorrhage: time to change the guidelines? Stroke. 2012;43(8):2031,2032.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
- Mark DG, Hung YY, Offerman SR, et al; Kaiser Permanente CREST Network Investigators. Nontraumatic subarachnoid hemorrhage in the setting of negative cranial computed tomography results: external validation of a clinical and imaging prediction rule. Ann Emerg Med. 2013;62(1):1-10e1.
- Mark DG, Pines JM. The detection of nontraumatic subarachnoid hemorrhage: still a diagnostic challenge. Am J Emerg Med. 2006;24(7):859-863.
- Pines JM, Szyld D. Risk tolerance for the exclusion of potentially life-threatening diseases in the ED. Am J Emerg Med. 2007;25(5):540-544.
- Farzad A, Radin B, Oh JS, et al. Emergency diagnosis of subarachnoid hemorrhage: an evidence-based debate. J Emerg Med. 2013;44(5):1045-1053.
- Ward MJ, Bonomo JB, Adeoye O, Raja AS, Pines JM. Cost-effectiveness of diagnostic strategies for evaluation of suspected subarachnoid hemorrhage in the emergency department. Acad Emerg Med. 2012;19(10):1134-1144.
- Edlow JA. What are the unintended consequences of changing the diagnostic paradigm for subarachnoid hemorrhage after brain computed tomography to computed tomographic angiography in place of lumbar puncture? Acad Emerg Med. 2010;17(9):991-995; discussion 996,997.
- Savitz SI, Levitan EB, Wears R, Edlow JA. Pooled analysis of patients with thunderclap headache evaluated by CT and LP: is angiography necessary in patients with negative evaluations? J Neurol Sci. 2009;276(1-2):123-125.
- Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol. 2006;5(7):621-631.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
- Rajasekaran S, Vanderpump M, Baldeweg S, et al. UK guidelines for the management of pituitary apoplexy. Clinical endocrinology (Oxf). 2011;74(1):9-20.
- Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005-1012.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
- Edlow JA, Caplan LR, O’Brien K, Tibbles CD. Diagnosis of acute neurological emergencies in pregnant and post-partum women. Lancet Neurol 2013;12(2):175-185.
- Mortimer AM, Bradley MD, Stoodley NG, Renowden SA. Thunderclap headache: diagnostic considerations and neuroimaging features. Clin Radiol. 2013;68(3):e101-e113.
: Unusual Causes of Headache
- Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158.
- Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791-1798.
- Sidhom Y, Mansour M, Messelmani M, et al. Cerebral venous thrombosis: clinical features, risk factors, and long-term outcome in a Tunisian cohort. J Stroke Cerebrovasc Dis. 2014;23(6):1291-1295.
- Kosinski CM, Mull M, Schwarz M, et al. Do normal D-dimer levels reliably exclude cerebral sinus thrombosis? Stroke. 2004;35(12):2820-2825.
- Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274-1282.
- Wasay M, Azeemuddin M. Neuroimaging of cerebral venous thrombosis. J Neuroimaging. 005;15(2):118-128.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
- Vincent MB. Headache and neck. Curr Pain Headache Rep. 2011;15(4):324-331.
- Vanelderen P, Lataster A, Levy R, Mekhail N, van Kleef M, Van Zundert J. Occipital neuralgia. Pain Pract. 2010;10(2):137-144.
- Bogduk N. The neck and headaches. Neurol Clin. 2004;22(1):151-171, vii.
- Vanderhoek MD, Hoang HT, Goff B. Ultrasound-guided greater occipital nerve blocks and pulsed radiofrequency ablation for diagnosis and treatment of occipital neuralgia. Anesth Pain Med. 2013;3(2):256-259.
- Young WB. Blocking the greater occipital nerve: utility in headache management. Curr Pain Headache Rep. 2010;14(5):404-408.
- Cohen AS, Matharu MS, Goadsby PJ. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)—a prospective clinical study of SUNCT and SUNA. Brain. 2006;129(Pt 10):2746-2760.
- Chitsantikul P, Becker WJ. SUNCT, SUNA and pituitary tumors: clinical characteristics and treatment. Cephalalgia. 2013;33(3):160-170.
- Williams MH, Broadley SA. SUNCT and SUNA: clinical features and medical treatment. J Clin Neurosci. 2008;15(5):526-534.
- Etemadifar M, Maghzi AH, Ghasemi M, Chitsaz A, Kaji Esfahani M. Efficacy of gabapentin in the treatment of SUNCT syndrome. Cephalalgia. 2008;28(12):1339-1342.
- Trauninger A, Alkonyi B, Kovács N, Komoly S, Pfund Z. Methylprednisolone therapy for short-term prevention of SUNCT syndrome. Cephalalgia. 2010;30(6):735-739.
- de Lourdes Figuerola M, Bruera O, Pozzo MJ, Leston J. SUNCT syndrome responding absolutely to steroids in two cases with different etiologies. J Headache Pain. 2009;10(1):55-57.
- Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012;41(6):866-871.
- Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336-1342.
- Stammler F, Grau C, Schnabel A. Value of colour doppler ultrasonography in relation to clinical pretest probability in giant cell (temporal) arteritis. Dtsch Med Wochenschr. 2009;134(42):2109-2115.
- Hellmann DB. Giant cell arteritis, polymyalgia rheumatica, and Takayasu’s arteritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, eds. Kelley’s Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier Saunders; 2012:1461-1472.
- Pham L, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri). University of Iowa Healthcare Ophthalmology and Visual Sciences Web site. http://webeye.ophth.uiowa.edu/eyeforum/cases/99-pseudotumor-cerebri.htm. Accessed July 1, 2014.
- Garza I, Swanson, JW, Chesire WP Jr, et al. Headache and other craniofacial pain. In: Daroff R, Fenichel GM, Jankovic J, Mazziotta J, eds. Bradley’s Neurology in Clinical Practice. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1732,1736.
- Rosenberg GA. Brain edema and disorders of cerebrospinal fluid circulation. In: Daroff R, Fenichel GM, Jankovic J, Mazziotta J, eds. Bradley’s Neurology in Clinical Practice. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1389-1391.
- Dodick DW. Indomethacin responsive headache syndromes. Curr Pain Headache Rep. 2004;8(1):19-26.
- Headache
- Fernandes CL. The fifth vital sign. Fed Pract. 2010;27(10):26-28.
- Cantrill SV, Brown MD, Carlisle RJ et al; American College of Emergency Physicians Opioid Guideline Writing Panel. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525.
- Ho TW, Rodgers A, Bigal ME. Impact of recent prior opioid use on rizatriptan efficacy. A post hoc pooled analysis. Headache. 2009;49(3):395-403.
- Pylkas AM, Bart G. Prescribing controlled substances during a prescription drug epidemic. Neurol Clin Pract. 2014;4(2):99-105.
- Emergency Medicine Today. March 18, 2014. Vast majority of brain scans for headache patients may be unnecessary. http://acep.bulletinhealthcare.com/story.aspx?dt=140318. Accessed June 24, 2014.
- Shute N. Yes, It’s a headache. No, you don’t need a brain scan. NPR Shots. Your Health Web site. March 18, 2014. http://www.npr.org/blogs/health/2014/03/18/291044766/yes-its-a-headache-no-you-dont-need-a-brain-scan. Accessed June 18, 2014.
- Callaghan BC, Kerber KA, Pace RJ, Skolarus LE, Burke JF. Headaches and neuroimaging: high utilization and costs despite guidelines. JAMA Intern Med. 2014;174(5):819-821.
- Schwartz DT. US emergency physicians order too many computed tomography scans-or do they? Ann Emerg Med. 2013;62(5):495-497.
- Diaz M, Braude D, Skipper B. Concordance of historical questions used in risk-stratifying patients with headache. Am J Emerg Med. 2007;25(8):
907-910. - Worst headache of my life [List-serve comment]. mymigraineconnection.com. [currently inactive]
- Schull MJ. Lumbar puncture first: an alternative model for the investigation of lone acute sudden headache. Acad Emerg Med.1999;6(2):131-136.
- Clinch CR. Evaluation of acute headaches in adults. Am Fam Physician. 2001;63(4):685-692.
- Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol. 2003;76(908):532-535.
- Parizel PM, Voormolen M, Van Goethem JW, van den Hauwe L. Headache: when is neuroimaging needed? JBR-BTR. 2007;90(4):268-271.
- De Luca GC, Bartleson JD. When and how to investigate the patient with headache. Semin Neurol. 2010;30(2):131-144.
- Cady RK. Red flags and comfort signs for ominous secondary headaches. Otolaryngol Clin North Am. 2014;47(2):289-299.
- Duncan CW, Watson DP, Stein A; Guideline Development Group. Diagnosis and management of headache in adults: summary of SIGN guideline. BMJ. 2008;a2329.
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43(9):1678-1683.
- Daulaire S, Fine L, Salmon M, et al. Ultrasound assessment of optic disc edema in patients with headache. Am J Emerg Med. 2012;30(8):1654.e1-4.
- Migraine: An Evidence-Based Update
- Vinson DR. Treatment patterns of isolated benign headache in US emergency departments. Ann Emerg Med. 2002;39(3):215-222.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
- Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161(2):327-341.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296(10):1274-1283.
- Lipton RB, Dodick D, Sadovsky R, et al; ID Migraine Validation Study. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61(3):375-382.
- Miner JR, Smith SW, Moore J, Biros M. Sumatriptan for the treatment of undifferentiated primary headaches in the ED. Am J Emerg Med. 2007;25(1):60-64.
- Weinman D, Nicastro O, Akala O, Friedman BW. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
- Akpunonu BE, Mutgi AB, Federman DJ, et al. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: a multicenter study. Ann Emerg Med. 1995;25(4):464-269.
- Taggart E, Doran S, Kokotillo A, Campbell S, Villa-Roel C, Rowe BH. Ketorolac in the treatment of acute migraine: a systematic review. Headache. 2013;53(2):277-287.
- Friedman BW, Corbo J, Lipton RB, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology. 2005;64(3):463-468.
- Talabi S, Masoumi B, Azizkhani R, Esmailian M. Metoclopramide versus sumatriptan for treatment of migraine headache: A randomized clinical trial. J Res Med Sci. 2013;18(8):695-698.
- Kostic MA, Gutierrez FJ, Rieg TS, Moore TS, Gendron RT. A prospective, randomized trial of intravenous prochlorperazine versus subcutaneous sumatriptan in acute migraine therapy in the emergency department. Ann Emerg Med. 2010;56(1):1-6.
- Ashkenazi A, Levin M. Greater occipital nerve block for migraine and other headaches: is it useful? Curr Pain Headache Rep. 2007;11(3):231-235.
- Colman I, Rothney A, Wright SC, Zilkalns B, Rowe BH. Use of narcotic analgesics in the emergency department treatment of migraine headache. Neurology 2004;62(10):1695-1700.
- Vinson DR, Hurtado TR, Vandenberg JT, Banwart L. Variations among emergency departments in the treatment of benign headache. Ann Emerg Med. 2003;41(1):90-97.
- Friedman BW, Kapoor A, Friedman MS, Hochberg ML, Rowe BH. The relative efficacy of meperidine for the treatment of acute migraine: a meta-analysis of randomized controlled trials. Ann Emerg Med. 2008;52(6):705-713.
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168.
- Friedman BW, Hochberg ML, Esses D, et al. Recurrence of primary headache disorders after emergency department discharge: frequency and predictors of poor pain and functional outcomes. Ann Emerg Med. 2008;52(6):696-704.
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ. 2008;336(7657):1359-1361.
- Friedman BW, Solorzano C, Esses D, et al. Treating headache recurrence after emergency department discharge: a randomized controlled trial of naproxen versus sumatriptan. Ann Emerg Med. 2010;56(1):7-17.
: Thunderclap Headache
- Edlow JA, Malek AM, Ogilvy CS. Aneurysmal subarachnoid hemorrhage: update for emergency physicians. J Emerg Med. 2008;34(3):237-251.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med. 2008;52(4):407-436.
- Ducros A, Bousser MG. Thunderclap headache. BMJ. 2013;346:e8557.
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage [see comments]. N Engl J Med. 2000;342(1):29-36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA. 2013;310(12):1248-1255.
- Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291(7):866-869.
- Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke. 2007;38(4):1216-1221.
- Byyny RL, Mower WR, Shum N, Gabayan GZ, Fang S, Baraff LJ. Sensitivity of noncontrast cranial computed tomography for the emergency department diagnosis of subarachnoid hemorrhage. Ann Emerg Med. 2008;51(6) 697-703.
- Kumar S, Goddeau RP, Jr, Selim MH, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology 2010;74(11):893-899.
- Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Arch Pathol Lab Med. 2002;126(4):413-415.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke. 2012;43(8):2115-2119.
- Edlow JA, Fisher J. Diagnosis of subarachnoid hemorrhage: time to change the guidelines? Stroke. 2012;43(8):2031,2032.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
- Mark DG, Hung YY, Offerman SR, et al; Kaiser Permanente CREST Network Investigators. Nontraumatic subarachnoid hemorrhage in the setting of negative cranial computed tomography results: external validation of a clinical and imaging prediction rule. Ann Emerg Med. 2013;62(1):1-10e1.
- Mark DG, Pines JM. The detection of nontraumatic subarachnoid hemorrhage: still a diagnostic challenge. Am J Emerg Med. 2006;24(7):859-863.
- Pines JM, Szyld D. Risk tolerance for the exclusion of potentially life-threatening diseases in the ED. Am J Emerg Med. 2007;25(5):540-544.
- Farzad A, Radin B, Oh JS, et al. Emergency diagnosis of subarachnoid hemorrhage: an evidence-based debate. J Emerg Med. 2013;44(5):1045-1053.
- Ward MJ, Bonomo JB, Adeoye O, Raja AS, Pines JM. Cost-effectiveness of diagnostic strategies for evaluation of suspected subarachnoid hemorrhage in the emergency department. Acad Emerg Med. 2012;19(10):1134-1144.
- Edlow JA. What are the unintended consequences of changing the diagnostic paradigm for subarachnoid hemorrhage after brain computed tomography to computed tomographic angiography in place of lumbar puncture? Acad Emerg Med. 2010;17(9):991-995; discussion 996,997.
- Savitz SI, Levitan EB, Wears R, Edlow JA. Pooled analysis of patients with thunderclap headache evaluated by CT and LP: is angiography necessary in patients with negative evaluations? J Neurol Sci. 2009;276(1-2):123-125.
- Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol. 2006;5(7):621-631.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
- Rajasekaran S, Vanderpump M, Baldeweg S, et al. UK guidelines for the management of pituitary apoplexy. Clinical endocrinology (Oxf). 2011;74(1):9-20.
- Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005-1012.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
- Edlow JA, Caplan LR, O’Brien K, Tibbles CD. Diagnosis of acute neurological emergencies in pregnant and post-partum women. Lancet Neurol 2013;12(2):175-185.
- Mortimer AM, Bradley MD, Stoodley NG, Renowden SA. Thunderclap headache: diagnostic considerations and neuroimaging features. Clin Radiol. 2013;68(3):e101-e113.
: Unusual Causes of Headache
- Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158.
- Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791-1798.
- Sidhom Y, Mansour M, Messelmani M, et al. Cerebral venous thrombosis: clinical features, risk factors, and long-term outcome in a Tunisian cohort. J Stroke Cerebrovasc Dis. 2014;23(6):1291-1295.
- Kosinski CM, Mull M, Schwarz M, et al. Do normal D-dimer levels reliably exclude cerebral sinus thrombosis? Stroke. 2004;35(12):2820-2825.
- Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274-1282.
- Wasay M, Azeemuddin M. Neuroimaging of cerebral venous thrombosis. J Neuroimaging. 005;15(2):118-128.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
- Vincent MB. Headache and neck. Curr Pain Headache Rep. 2011;15(4):324-331.
- Vanelderen P, Lataster A, Levy R, Mekhail N, van Kleef M, Van Zundert J. Occipital neuralgia. Pain Pract. 2010;10(2):137-144.
- Bogduk N. The neck and headaches. Neurol Clin. 2004;22(1):151-171, vii.
- Vanderhoek MD, Hoang HT, Goff B. Ultrasound-guided greater occipital nerve blocks and pulsed radiofrequency ablation for diagnosis and treatment of occipital neuralgia. Anesth Pain Med. 2013;3(2):256-259.
- Young WB. Blocking the greater occipital nerve: utility in headache management. Curr Pain Headache Rep. 2010;14(5):404-408.
- Cohen AS, Matharu MS, Goadsby PJ. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)—a prospective clinical study of SUNCT and SUNA. Brain. 2006;129(Pt 10):2746-2760.
- Chitsantikul P, Becker WJ. SUNCT, SUNA and pituitary tumors: clinical characteristics and treatment. Cephalalgia. 2013;33(3):160-170.
- Williams MH, Broadley SA. SUNCT and SUNA: clinical features and medical treatment. J Clin Neurosci. 2008;15(5):526-534.
- Etemadifar M, Maghzi AH, Ghasemi M, Chitsaz A, Kaji Esfahani M. Efficacy of gabapentin in the treatment of SUNCT syndrome. Cephalalgia. 2008;28(12):1339-1342.
- Trauninger A, Alkonyi B, Kovács N, Komoly S, Pfund Z. Methylprednisolone therapy for short-term prevention of SUNCT syndrome. Cephalalgia. 2010;30(6):735-739.
- de Lourdes Figuerola M, Bruera O, Pozzo MJ, Leston J. SUNCT syndrome responding absolutely to steroids in two cases with different etiologies. J Headache Pain. 2009;10(1):55-57.
- Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012;41(6):866-871.
- Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336-1342.
- Stammler F, Grau C, Schnabel A. Value of colour doppler ultrasonography in relation to clinical pretest probability in giant cell (temporal) arteritis. Dtsch Med Wochenschr. 2009;134(42):2109-2115.
- Hellmann DB. Giant cell arteritis, polymyalgia rheumatica, and Takayasu’s arteritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, eds. Kelley’s Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier Saunders; 2012:1461-1472.
- Pham L, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri). University of Iowa Healthcare Ophthalmology and Visual Sciences Web site. http://webeye.ophth.uiowa.edu/eyeforum/cases/99-pseudotumor-cerebri.htm. Accessed July 1, 2014.
- Garza I, Swanson, JW, Chesire WP Jr, et al. Headache and other craniofacial pain. In: Daroff R, Fenichel GM, Jankovic J, Mazziotta J, eds. Bradley’s Neurology in Clinical Practice. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1732,1736.
- Rosenberg GA. Brain edema and disorders of cerebrospinal fluid circulation. In: Daroff R, Fenichel GM, Jankovic J, Mazziotta J, eds. Bradley’s Neurology in Clinical Practice. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1389-1391.
- Dodick DW. Indomethacin responsive headache syndromes. Curr Pain Headache Rep. 2004;8(1):19-26.