User login
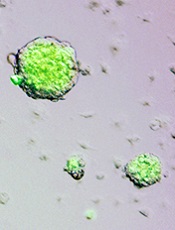
Credit: Haruko Obokata
Researchers say they have developed a novel technique for inducing pluripotency in somatic cells.
Unlike methods for creating induced pluripotent stem cells (iPSCs), the new process—called stimulus-triggered acquisition of pluripotency (STAP)—does not require the introduction of genetic material.
Instead, adult cells must only be injured in order to revert to a pluripotent state.
The investigators tested STAP in preclinical models and reported the results in both a letter and an article published in Nature.
Inspired by plants
Haruko Obokata, PhD, of the RIKEN Center for Developmental Biology in Japan, and her colleagues said this research was inspired by the ability of a plant callus—a node of plant cells created by injuring an existing plant—to grow into a new plant.
The researchers thought this phenomenon suggested that any somatic cell could be de-differentiated through injury.
To find out, they tested cells derived from mice. The team chose hematopoietic cells positive for CD45 because these are lineage-committed, somatic cells that do not express pluripotency-related markers unless they are reprogrammed.
The investigators stressed the cells almost to the point of death by exposing them to various stimuli in vitro, including trauma, a low-oxygen environment, and a low-pH environment.
Within a few days, the cells had recovered from the stressful stimuli by naturally reverting to a pluripotent state. These stem cells were then able to re-differentiate and mature into any type of cell and grow into any type of tissue, depending on the environment into which they were placed.
“It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell,” Dr Obokata said.
She and her colleagues found that the low-pH environment was most effective for inducing pluripotency.
“Once again, Japanese scientists have unexpectedly rewritten the rules on making pluripotent cells from adult cells,” said Chris Mason, MD, PhD, of the University College London in the UK, who was not involved in this research.
“In 2006, [Shinya] Yamanaka used 4 genes [to create iPSCs]. And now, the far simpler and quicker route discovered by Obokata . . . requires only transient exposure of adult cells to an acidic solution.”
Growth in mice
To examine the cells’ growth potential in vivo, Dr Obokata and her colleagues used CD45+ cells from GFP+ mice. The team exposed the cells to a low-pH environment and found that, in the days following the stress, the cells reverted back to a pluripotent state.
These stem cells then began growing in spherical clusters, similar to a plant callus. The researchers introduced the cell clusters into the developing embryo of a non-GFP mouse and found the clusters could create GFP+ tissues in all organs tested, thereby confirming that the cells are pluripotent.
The investigators think these findings raise the possibility that unknown cellular functions activated through external stress may set somatic cells free from their current commitment and permit them to revert to their naïve state.
“Our findings suggest that, somehow, through part of a natural repair process, mature cells turn off some of the epigenetic controls that inhibit expression of certain nuclear genes that result in differentiation,” said study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston.
If this process can be replicated in human cells, researchers might one day be able to use a skin biopsy or blood sample to create stem cells specific to each individual. And this could have implications for treating cancers and other diseases.
The investigators are now testing the STAP technique in human cells. ![]()

Credit: Haruko Obokata
Researchers say they have developed a novel technique for inducing pluripotency in somatic cells.
Unlike methods for creating induced pluripotent stem cells (iPSCs), the new process—called stimulus-triggered acquisition of pluripotency (STAP)—does not require the introduction of genetic material.
Instead, adult cells must only be injured in order to revert to a pluripotent state.
The investigators tested STAP in preclinical models and reported the results in both a letter and an article published in Nature.
Inspired by plants
Haruko Obokata, PhD, of the RIKEN Center for Developmental Biology in Japan, and her colleagues said this research was inspired by the ability of a plant callus—a node of plant cells created by injuring an existing plant—to grow into a new plant.
The researchers thought this phenomenon suggested that any somatic cell could be de-differentiated through injury.
To find out, they tested cells derived from mice. The team chose hematopoietic cells positive for CD45 because these are lineage-committed, somatic cells that do not express pluripotency-related markers unless they are reprogrammed.
The investigators stressed the cells almost to the point of death by exposing them to various stimuli in vitro, including trauma, a low-oxygen environment, and a low-pH environment.
Within a few days, the cells had recovered from the stressful stimuli by naturally reverting to a pluripotent state. These stem cells were then able to re-differentiate and mature into any type of cell and grow into any type of tissue, depending on the environment into which they were placed.
“It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell,” Dr Obokata said.
She and her colleagues found that the low-pH environment was most effective for inducing pluripotency.
“Once again, Japanese scientists have unexpectedly rewritten the rules on making pluripotent cells from adult cells,” said Chris Mason, MD, PhD, of the University College London in the UK, who was not involved in this research.
“In 2006, [Shinya] Yamanaka used 4 genes [to create iPSCs]. And now, the far simpler and quicker route discovered by Obokata . . . requires only transient exposure of adult cells to an acidic solution.”
Growth in mice
To examine the cells’ growth potential in vivo, Dr Obokata and her colleagues used CD45+ cells from GFP+ mice. The team exposed the cells to a low-pH environment and found that, in the days following the stress, the cells reverted back to a pluripotent state.
These stem cells then began growing in spherical clusters, similar to a plant callus. The researchers introduced the cell clusters into the developing embryo of a non-GFP mouse and found the clusters could create GFP+ tissues in all organs tested, thereby confirming that the cells are pluripotent.
The investigators think these findings raise the possibility that unknown cellular functions activated through external stress may set somatic cells free from their current commitment and permit them to revert to their naïve state.
“Our findings suggest that, somehow, through part of a natural repair process, mature cells turn off some of the epigenetic controls that inhibit expression of certain nuclear genes that result in differentiation,” said study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston.
If this process can be replicated in human cells, researchers might one day be able to use a skin biopsy or blood sample to create stem cells specific to each individual. And this could have implications for treating cancers and other diseases.
The investigators are now testing the STAP technique in human cells. ![]()

Credit: Haruko Obokata
Researchers say they have developed a novel technique for inducing pluripotency in somatic cells.
Unlike methods for creating induced pluripotent stem cells (iPSCs), the new process—called stimulus-triggered acquisition of pluripotency (STAP)—does not require the introduction of genetic material.
Instead, adult cells must only be injured in order to revert to a pluripotent state.
The investigators tested STAP in preclinical models and reported the results in both a letter and an article published in Nature.
Inspired by plants
Haruko Obokata, PhD, of the RIKEN Center for Developmental Biology in Japan, and her colleagues said this research was inspired by the ability of a plant callus—a node of plant cells created by injuring an existing plant—to grow into a new plant.
The researchers thought this phenomenon suggested that any somatic cell could be de-differentiated through injury.
To find out, they tested cells derived from mice. The team chose hematopoietic cells positive for CD45 because these are lineage-committed, somatic cells that do not express pluripotency-related markers unless they are reprogrammed.
The investigators stressed the cells almost to the point of death by exposing them to various stimuli in vitro, including trauma, a low-oxygen environment, and a low-pH environment.
Within a few days, the cells had recovered from the stressful stimuli by naturally reverting to a pluripotent state. These stem cells were then able to re-differentiate and mature into any type of cell and grow into any type of tissue, depending on the environment into which they were placed.
“It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell,” Dr Obokata said.
She and her colleagues found that the low-pH environment was most effective for inducing pluripotency.
“Once again, Japanese scientists have unexpectedly rewritten the rules on making pluripotent cells from adult cells,” said Chris Mason, MD, PhD, of the University College London in the UK, who was not involved in this research.
“In 2006, [Shinya] Yamanaka used 4 genes [to create iPSCs]. And now, the far simpler and quicker route discovered by Obokata . . . requires only transient exposure of adult cells to an acidic solution.”
Growth in mice
To examine the cells’ growth potential in vivo, Dr Obokata and her colleagues used CD45+ cells from GFP+ mice. The team exposed the cells to a low-pH environment and found that, in the days following the stress, the cells reverted back to a pluripotent state.
These stem cells then began growing in spherical clusters, similar to a plant callus. The researchers introduced the cell clusters into the developing embryo of a non-GFP mouse and found the clusters could create GFP+ tissues in all organs tested, thereby confirming that the cells are pluripotent.
The investigators think these findings raise the possibility that unknown cellular functions activated through external stress may set somatic cells free from their current commitment and permit them to revert to their naïve state.
“Our findings suggest that, somehow, through part of a natural repair process, mature cells turn off some of the epigenetic controls that inhibit expression of certain nuclear genes that result in differentiation,” said study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston.
If this process can be replicated in human cells, researchers might one day be able to use a skin biopsy or blood sample to create stem cells specific to each individual. And this could have implications for treating cancers and other diseases.
The investigators are now testing the STAP technique in human cells. ![]()