User login
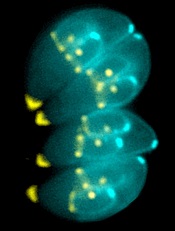
Image by Ke Hu & John Murray
Researchers say they have determined the structure of an enzyme that is vital to the infectious behavior of the parasites that cause toxoplasmosis and malaria.
And this has revealed a potentially druggable target that could prevent the parasites from entering and exiting host cells.
Sebastian Lourido, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues described this work in PNAS.
The researchers noted that the toxoplasmosis-causing parasite Toxoplasma gondii is closely related to the malaria-causing Plasmodium parasites. So research on T gondii can provide insights into Plasmodium’s inner workings.
For this study, Dr Lourido and his colleagues wanted to learn more about calcium-dependent protein kinases (CDPKs), enzymes that are needed for T gondii and related parasites to invade and exit host cells, move, and reproduce.
To investigate CDPKs, the team used single-domain antibody fragments derived from alpacas. Unlike humans, whose antibodies have a heavy chain and a light chain, alpacas create heavy-chain-only antibodies, which can be engineered into even smaller antibody fragments known as nanobodies.
Alpaca nanobodies have a unique shape that allows them to reach into a protein’s nooks and crannies, which are inaccessible to conventional antibodies.
The researchers identified a nanobody against the T gondii enzyme CDPK1 that binds the kinase’s regulatory domain and revealed a previously unappreciated feature of its activation.
The nanobody, called 1B7, stabilizes CDPK1 in a conformation that allowed the researchers to determine the kinase’s structure and describe the nanobody’s interaction with the molecule.
With the structure in hand, the team created long-timescale molecular dynamics simulations of the enzyme, to model the events leading to kinase inactivation.
Structural homology between CDPKs and the calmodulin-dependent kinases (CaMKs) found in humans led to earlier assumptions that both types of enzymes are activated in a similar fashion. But this new work shows otherwise.
A CaMK is activated when a wedge holding it in an inactive state is knocked away. In contrast, Dr Lourido likened a CDPK’s active conformation to a broken arm that must be splinted in two places to maintain its integrity.
When the rigid splint is removed, the kinase loses its structural ability to function. By blocking CDPK1’s regulatory domain, the 1B7 nanobody inhibits the kinase by preventing the enzyme’s “splint” from attaching.
“This work reveals something interesting about this class of enzymes,” Dr Lourido said. “It’s the first time a calcium-regulated kinase has been shown to be activated in this manner. The principle that we identify is really important. We’ve found a new vulnerability within an enzyme that we know is extremely important to this class of parasites, including Plasmodium . . . , and is absent from humans.”
Because humans lack similar kinases, drugs that target CDPKs would not affect host cells.
“The location where 1B7 binds to CDPK1 is a new drug target that people had not considered before,” said study author Jessica Ingram, PhD, also of the Whitehead Institute for Biomedical Research.
“We’d like to do some drug screens in the presence of the nanobody to see if we can find small molecules that bind in the same way. We could also look at other nanobodies against other kinases to see if this is applicable to other parasites and systems.” ![]()

Image by Ke Hu & John Murray
Researchers say they have determined the structure of an enzyme that is vital to the infectious behavior of the parasites that cause toxoplasmosis and malaria.
And this has revealed a potentially druggable target that could prevent the parasites from entering and exiting host cells.
Sebastian Lourido, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues described this work in PNAS.
The researchers noted that the toxoplasmosis-causing parasite Toxoplasma gondii is closely related to the malaria-causing Plasmodium parasites. So research on T gondii can provide insights into Plasmodium’s inner workings.
For this study, Dr Lourido and his colleagues wanted to learn more about calcium-dependent protein kinases (CDPKs), enzymes that are needed for T gondii and related parasites to invade and exit host cells, move, and reproduce.
To investigate CDPKs, the team used single-domain antibody fragments derived from alpacas. Unlike humans, whose antibodies have a heavy chain and a light chain, alpacas create heavy-chain-only antibodies, which can be engineered into even smaller antibody fragments known as nanobodies.
Alpaca nanobodies have a unique shape that allows them to reach into a protein’s nooks and crannies, which are inaccessible to conventional antibodies.
The researchers identified a nanobody against the T gondii enzyme CDPK1 that binds the kinase’s regulatory domain and revealed a previously unappreciated feature of its activation.
The nanobody, called 1B7, stabilizes CDPK1 in a conformation that allowed the researchers to determine the kinase’s structure and describe the nanobody’s interaction with the molecule.
With the structure in hand, the team created long-timescale molecular dynamics simulations of the enzyme, to model the events leading to kinase inactivation.
Structural homology between CDPKs and the calmodulin-dependent kinases (CaMKs) found in humans led to earlier assumptions that both types of enzymes are activated in a similar fashion. But this new work shows otherwise.
A CaMK is activated when a wedge holding it in an inactive state is knocked away. In contrast, Dr Lourido likened a CDPK’s active conformation to a broken arm that must be splinted in two places to maintain its integrity.
When the rigid splint is removed, the kinase loses its structural ability to function. By blocking CDPK1’s regulatory domain, the 1B7 nanobody inhibits the kinase by preventing the enzyme’s “splint” from attaching.
“This work reveals something interesting about this class of enzymes,” Dr Lourido said. “It’s the first time a calcium-regulated kinase has been shown to be activated in this manner. The principle that we identify is really important. We’ve found a new vulnerability within an enzyme that we know is extremely important to this class of parasites, including Plasmodium . . . , and is absent from humans.”
Because humans lack similar kinases, drugs that target CDPKs would not affect host cells.
“The location where 1B7 binds to CDPK1 is a new drug target that people had not considered before,” said study author Jessica Ingram, PhD, also of the Whitehead Institute for Biomedical Research.
“We’d like to do some drug screens in the presence of the nanobody to see if we can find small molecules that bind in the same way. We could also look at other nanobodies against other kinases to see if this is applicable to other parasites and systems.” ![]()

Image by Ke Hu & John Murray
Researchers say they have determined the structure of an enzyme that is vital to the infectious behavior of the parasites that cause toxoplasmosis and malaria.
And this has revealed a potentially druggable target that could prevent the parasites from entering and exiting host cells.
Sebastian Lourido, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues described this work in PNAS.
The researchers noted that the toxoplasmosis-causing parasite Toxoplasma gondii is closely related to the malaria-causing Plasmodium parasites. So research on T gondii can provide insights into Plasmodium’s inner workings.
For this study, Dr Lourido and his colleagues wanted to learn more about calcium-dependent protein kinases (CDPKs), enzymes that are needed for T gondii and related parasites to invade and exit host cells, move, and reproduce.
To investigate CDPKs, the team used single-domain antibody fragments derived from alpacas. Unlike humans, whose antibodies have a heavy chain and a light chain, alpacas create heavy-chain-only antibodies, which can be engineered into even smaller antibody fragments known as nanobodies.
Alpaca nanobodies have a unique shape that allows them to reach into a protein’s nooks and crannies, which are inaccessible to conventional antibodies.
The researchers identified a nanobody against the T gondii enzyme CDPK1 that binds the kinase’s regulatory domain and revealed a previously unappreciated feature of its activation.
The nanobody, called 1B7, stabilizes CDPK1 in a conformation that allowed the researchers to determine the kinase’s structure and describe the nanobody’s interaction with the molecule.
With the structure in hand, the team created long-timescale molecular dynamics simulations of the enzyme, to model the events leading to kinase inactivation.
Structural homology between CDPKs and the calmodulin-dependent kinases (CaMKs) found in humans led to earlier assumptions that both types of enzymes are activated in a similar fashion. But this new work shows otherwise.
A CaMK is activated when a wedge holding it in an inactive state is knocked away. In contrast, Dr Lourido likened a CDPK’s active conformation to a broken arm that must be splinted in two places to maintain its integrity.
When the rigid splint is removed, the kinase loses its structural ability to function. By blocking CDPK1’s regulatory domain, the 1B7 nanobody inhibits the kinase by preventing the enzyme’s “splint” from attaching.
“This work reveals something interesting about this class of enzymes,” Dr Lourido said. “It’s the first time a calcium-regulated kinase has been shown to be activated in this manner. The principle that we identify is really important. We’ve found a new vulnerability within an enzyme that we know is extremely important to this class of parasites, including Plasmodium . . . , and is absent from humans.”
Because humans lack similar kinases, drugs that target CDPKs would not affect host cells.
“The location where 1B7 binds to CDPK1 is a new drug target that people had not considered before,” said study author Jessica Ingram, PhD, also of the Whitehead Institute for Biomedical Research.
“We’d like to do some drug screens in the presence of the nanobody to see if we can find small molecules that bind in the same way. We could also look at other nanobodies against other kinases to see if this is applicable to other parasites and systems.” ![]()