User login
Engineers say they have developed biomimetic bone tissues that could one day provide new bone marrow for patients requiring transplants.
The team created bone tissues with functional bone marrow that can be filled with donor cells and implanted under the skin of mice.
The implant gives donor cells their own space to live and grow without competition, eliminating the need for a conditioning regimen to wipe out the host’s pre-existing cells prior to transplant.
“We’ve made an accessory bone that can separately accommodate donor cells,” explained Shyni Varghese, PhD, of the University of California, San Diego.
“This way, we can keep the host cells and bypass irradiation.”
In mice that received the engineered bone tissue, donor hematopoietic cells survived for at least 6 months and supplied the mice with new blood cells.
“In the future, our work could contribute to improved therapies for bone marrow disease,” said Yu-Ru Vernon Shih, PhD, a researcher in Dr Varghese’s lab.
The researchers noted that these implants would be limited to patients with non-malignant bone marrow diseases, such as aplastic anemia, where there aren’t any cancerous cells that need to be eliminated prior to transplant.
The team described their bone tissue implants in PNAS.
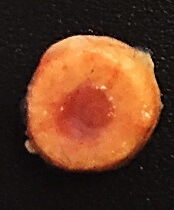
The implants mimic the structure of long bones in the body, consisting of an outer bone compartment and an inner marrow compartment.
The implants are made of a porous hydrogel matrix. The outer matrix contains calcium phosphate minerals. Stem cells grown in this mineralized matrix differentiate into bone-building cells. The inner matrix houses donor stem cells that produce blood cells.
When implanted beneath the skin of mice, the structures matured into bone tissues that have a working blood vessel network and a bone marrow that supplies new blood cells.
After 4 weeks, the implanted marrow contained a mix of host and donor blood cells. This mix was still circulating in the bloodstream after 24 weeks.
The researchers said these findings suggest the implanted marrow is functional, donor cells can grow and survive for long time periods in the presence of host cells, and host and donor cells can travel between the implanted marrow and the host’s circulating blood via the blood vessel network formed in the implanted bone tissue.
In another set of experiments, the researchers took hematopoietic stem cells from the implanted marrow and transplanted them into a second group of mice that had their stem cells destroyed by radiation and drugs. The team found the transplanted cells had diffused into the bloodstream of these mice.
“We did these experiments to show that the bone marrow cells from the engineered bone tissues function similar to native bone,” Dr Shih said.
“We’re working on making this a platform to generate more bone marrow stem cells,” Dr Varghese added. “That would have useful applications for cell transplantations in the clinic.” ![]()
Engineers say they have developed biomimetic bone tissues that could one day provide new bone marrow for patients requiring transplants.
The team created bone tissues with functional bone marrow that can be filled with donor cells and implanted under the skin of mice.
The implant gives donor cells their own space to live and grow without competition, eliminating the need for a conditioning regimen to wipe out the host’s pre-existing cells prior to transplant.
“We’ve made an accessory bone that can separately accommodate donor cells,” explained Shyni Varghese, PhD, of the University of California, San Diego.
“This way, we can keep the host cells and bypass irradiation.”
In mice that received the engineered bone tissue, donor hematopoietic cells survived for at least 6 months and supplied the mice with new blood cells.
“In the future, our work could contribute to improved therapies for bone marrow disease,” said Yu-Ru Vernon Shih, PhD, a researcher in Dr Varghese’s lab.
The researchers noted that these implants would be limited to patients with non-malignant bone marrow diseases, such as aplastic anemia, where there aren’t any cancerous cells that need to be eliminated prior to transplant.
The team described their bone tissue implants in PNAS.
The implants mimic the structure of long bones in the body, consisting of an outer bone compartment and an inner marrow compartment.
The implants are made of a porous hydrogel matrix. The outer matrix contains calcium phosphate minerals. Stem cells grown in this mineralized matrix differentiate into bone-building cells. The inner matrix houses donor stem cells that produce blood cells.
When implanted beneath the skin of mice, the structures matured into bone tissues that have a working blood vessel network and a bone marrow that supplies new blood cells.
After 4 weeks, the implanted marrow contained a mix of host and donor blood cells. This mix was still circulating in the bloodstream after 24 weeks.
The researchers said these findings suggest the implanted marrow is functional, donor cells can grow and survive for long time periods in the presence of host cells, and host and donor cells can travel between the implanted marrow and the host’s circulating blood via the blood vessel network formed in the implanted bone tissue.
In another set of experiments, the researchers took hematopoietic stem cells from the implanted marrow and transplanted them into a second group of mice that had their stem cells destroyed by radiation and drugs. The team found the transplanted cells had diffused into the bloodstream of these mice.
“We did these experiments to show that the bone marrow cells from the engineered bone tissues function similar to native bone,” Dr Shih said.
“We’re working on making this a platform to generate more bone marrow stem cells,” Dr Varghese added. “That would have useful applications for cell transplantations in the clinic.” ![]()
Engineers say they have developed biomimetic bone tissues that could one day provide new bone marrow for patients requiring transplants.
The team created bone tissues with functional bone marrow that can be filled with donor cells and implanted under the skin of mice.
The implant gives donor cells their own space to live and grow without competition, eliminating the need for a conditioning regimen to wipe out the host’s pre-existing cells prior to transplant.
“We’ve made an accessory bone that can separately accommodate donor cells,” explained Shyni Varghese, PhD, of the University of California, San Diego.
“This way, we can keep the host cells and bypass irradiation.”
In mice that received the engineered bone tissue, donor hematopoietic cells survived for at least 6 months and supplied the mice with new blood cells.
“In the future, our work could contribute to improved therapies for bone marrow disease,” said Yu-Ru Vernon Shih, PhD, a researcher in Dr Varghese’s lab.
The researchers noted that these implants would be limited to patients with non-malignant bone marrow diseases, such as aplastic anemia, where there aren’t any cancerous cells that need to be eliminated prior to transplant.
The team described their bone tissue implants in PNAS.
The implants mimic the structure of long bones in the body, consisting of an outer bone compartment and an inner marrow compartment.
The implants are made of a porous hydrogel matrix. The outer matrix contains calcium phosphate minerals. Stem cells grown in this mineralized matrix differentiate into bone-building cells. The inner matrix houses donor stem cells that produce blood cells.
When implanted beneath the skin of mice, the structures matured into bone tissues that have a working blood vessel network and a bone marrow that supplies new blood cells.
After 4 weeks, the implanted marrow contained a mix of host and donor blood cells. This mix was still circulating in the bloodstream after 24 weeks.
The researchers said these findings suggest the implanted marrow is functional, donor cells can grow and survive for long time periods in the presence of host cells, and host and donor cells can travel between the implanted marrow and the host’s circulating blood via the blood vessel network formed in the implanted bone tissue.
In another set of experiments, the researchers took hematopoietic stem cells from the implanted marrow and transplanted them into a second group of mice that had their stem cells destroyed by radiation and drugs. The team found the transplanted cells had diffused into the bloodstream of these mice.
“We did these experiments to show that the bone marrow cells from the engineered bone tissues function similar to native bone,” Dr Shih said.
“We’re working on making this a platform to generate more bone marrow stem cells,” Dr Varghese added. “That would have useful applications for cell transplantations in the clinic.” ![]()